Juglone from Walnut Produces Cardioprotective Effects against Isoproterenol-Induced Myocardial Injury in SD Rats
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Experimental Animals and Housing Conditions
2.3. Cardioprotective Study
2.3.1. Cardioprotective Study of Juglone against the Isoproterenol-Induced MI in Rats
2.3.2. Biochemical Estimations in Serum
2.3.3. Histopathological Examination
2.4. Statistical Analysis
3. Results
3.1. Cardioprotective Effect of Juglone against the Isoproterenol (ISO)-Induced Myocardial Injury in Rats
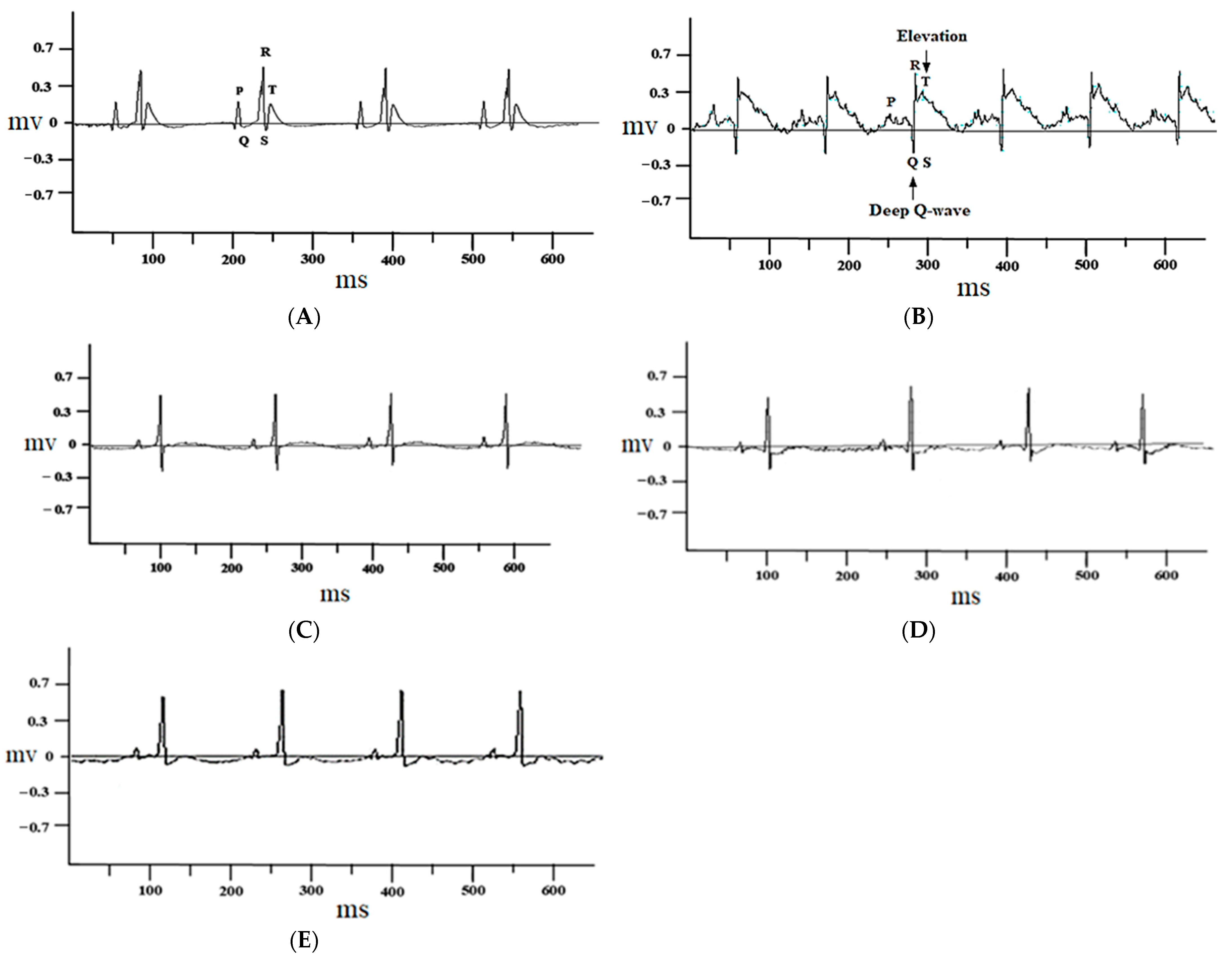
3.1.1. Effect of Juglone on Electrocardiograph (ECG) Parameters
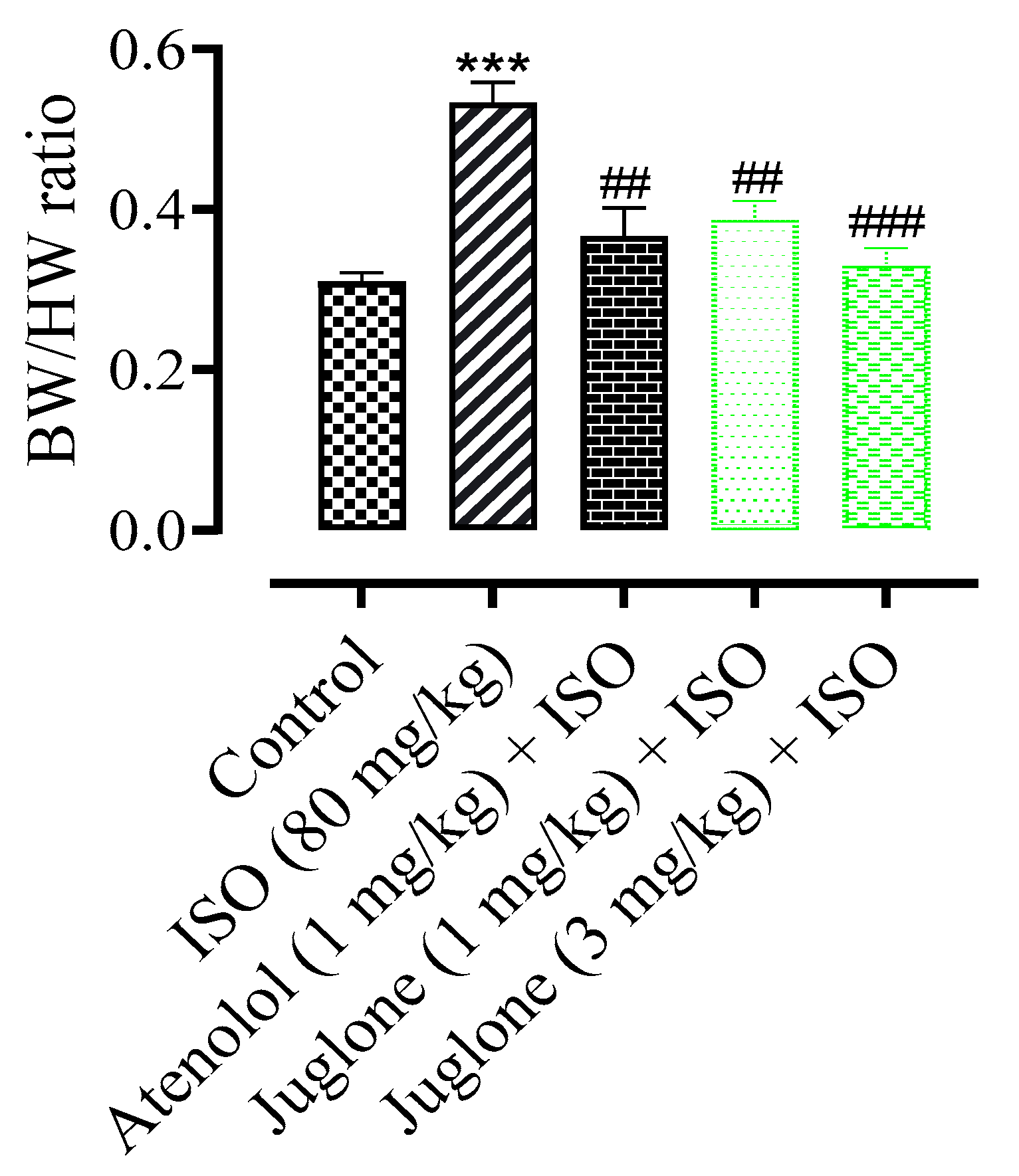
3.1.2. Effect of Juglone on Heart and Body Weight Ratio
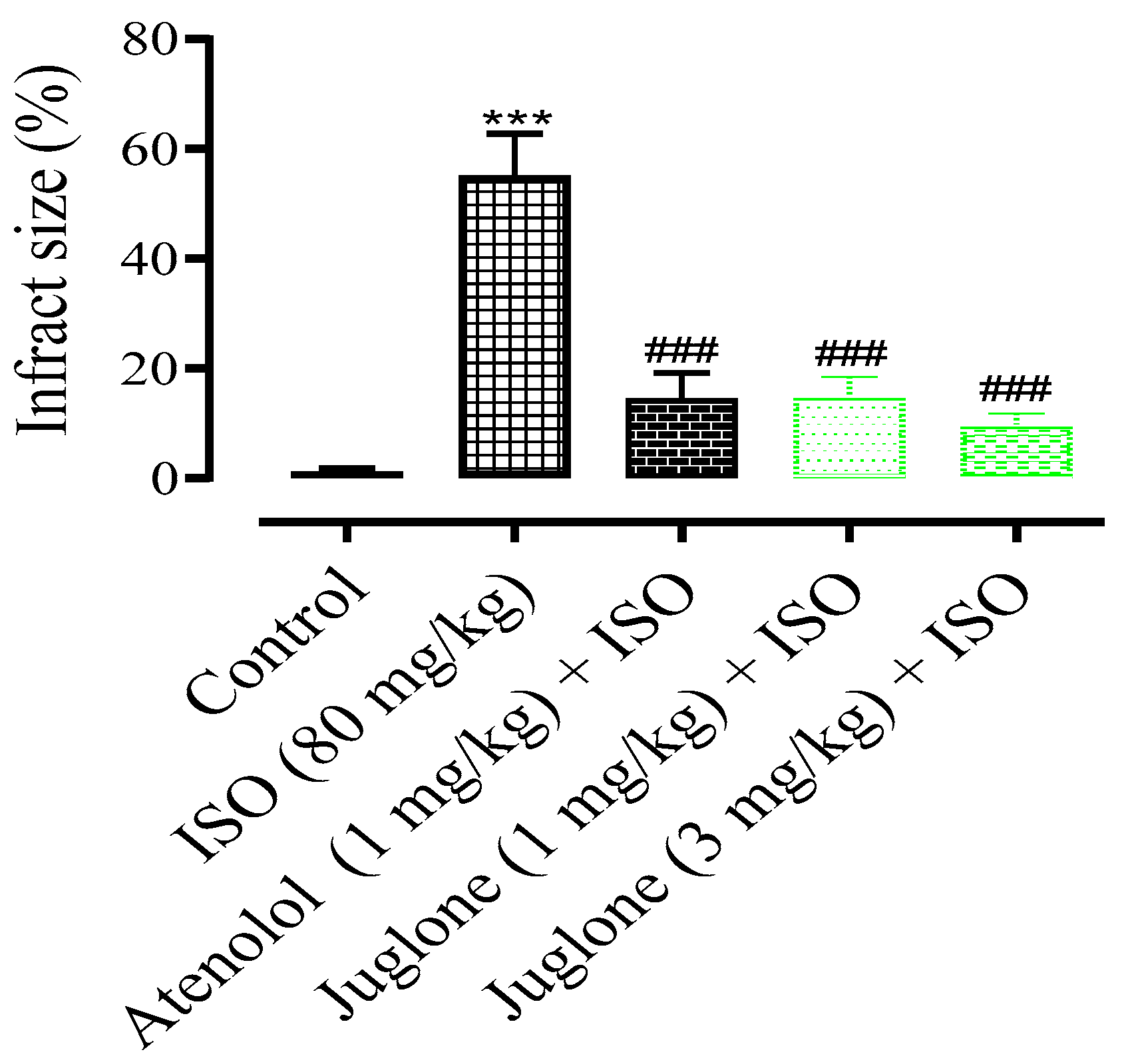
3.1.3. Changes in the Rat Heart Structural Anatomy and Measurement of Myocardial Infarct Size
3.1.4. Effect of Juglone on Cardiac Marker Enzymes
3.1.5. Effect of Juglone on Histological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimokawa, H.; Yasuda, S. Myocardial ischemia: Current concepts and future perspectives. J. Cardiol. 2008, 52, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, R.B.; Reimer, K.A. Factors involved in salvaging ischemic myocardium: Effect of reperfusion of arterial blood. Circulation 1983, 68, 125–136. [Google Scholar]
- World Health Organization (WHO). Cardiovascular Disease. Prevention of Recurrences of Myocardial Infarction and Stroke Study. 2019. Available online: https://www.who.int/cardiovascular_diseases/priorities/secondary_prevention/country/en/index1.html (accessed on 12 December 2019).
- Jafar, T.H.; Islam, M.; Poulter, N.; Hatcher, J.; Schmid, C.H.; Levey, A.S.; Chaturvedi, N. Children in South Asia Have Higher Body Mass–Adjusted Blood Pressure Levels Than White Children in the United States A Comparative Study. Circulation 2005, 111, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Saleem, F.; Hassali, A.A.; Shafie, A.A. Hypertension in Pakistan: Time to take some serious action. Br. J. Gen. Pract. 2010, 60, 449–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegstad, A.C.; Ytrehus, K.; Myklebust, R.; Jørgensen, L. Ultrastructural changes in the myocardial myocytic mitochondria: Crucial step in the development of oxygen radical-induced damage in isolated rat hearts? Basic Res. Cardiol. 1994, 89, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, RA209–RA219. [Google Scholar] [PubMed]
- Wu, J.; Hecker, J.G.; Chiamvimonvat, N. Antioxidant enzyme gene transfer for ischemic diseases. Adv. Drug Deliv. Rev. 2009, 61, 351–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, P.C.; Ribas, F.F.; Serrano, C.V.; Hueb, W. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J. Thorac. Dis. 2019, 11, 1005–1015. [Google Scholar] [CrossRef]
- Baker, J.E.; Su, J.; Fu, X.; Hsu, A.; Gross, G.J.; Tweddell, J.S.; Hogg, N. Nitrite confers protection against myocardial infarction: Role of xanthine oxidoreductase, NADPH oxidase and KATP channels. J. Mol. Cell. Cardiol. 2007, 43, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Goodman, L.S.; Gillman, A.; Laurence, L.B.; Lazo, J.S. Goodman and Gillman the Pharmacological Basis of Therapeutics, 13th ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2018; pp. 823–872. [Google Scholar]
- Talukder, M.A.H.; Zweier, J.L.; Periasamy, M. Targeting calcium transport in ischaemic heart disease. Cardiovasc. Res. 2009, 84, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Ahn, J.K.; Shin, K.-A.; Kim, C.-H.; Lee, Y.-H.; Park, K.-M. Correlation of Cardiac Markers and Biomarkers With Blood Pressure of Middle-Aged Marathon Runners. J. Clin. Hypertens. 2015, 17, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Akila, P.; Vennila, L. Chlorogenic Acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in Vivo Study. Biomed. Pharm. 2016, 84, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alexandria Med. J. 2018, 4, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction. Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Kubler, W.; Haass, M. Cardioprotection: Definition, classification, and fundamental principles. Heart 1996, 75, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Egred, M.; Shaw, S.; Mohammad, B.; Waitt, P.; Rodrigues, E. Under-use of beta-blockers in patients with ischemic heart disease and concomitant chronic obstructive pulmonary disease. QJM Int. J. Med. 2005, 98, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Gilani, A.H. Studies on blood pressure lowering, vasodilator and cardiac suppressant activities of Vitex negundo: Involvement of K+ channel activation and Ca++ channel blockade. Int. J. Pharmcol. 2015, 11, 137–142. [Google Scholar] [CrossRef]
- Afsheen, N.; Khalil-ur-Rehman; Jahan, N.; Ijaz, M.; Manzoor, A.; Khan, K.M.; Hina, S. Cardioprotective and Metabolomic Profiling of Selected Medicinal Plants against Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 9819360. [Google Scholar] [CrossRef] [Green Version]
- Patwardhan, B.; Vaidya, A.D.B.; Chorghade, M. Ayurveda and natural products drug discovery. Curr. Sci. 2004, 86, 789–799. [Google Scholar]
- Lahlou, M. The success of natural products in drug discovery. Pharm. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, K.; Rahman, S.; Ali, M.; Raisuddin, S. In vitro antioxidant activity of Juglans regia L. bark extract and its protective effect on cyclophosphamide-induced urotoxicity in mice. Redox. Rep. 2006, 11, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, G.; Li, D.; Meng, H.; Zhu, Z.; Zhao, Y.; Qi, Y.; Zhang, X. Walnut (Juglans regia L.) Kernel Extracts Protect Against Isoproterenol-Induced Myocardial Infarction in Rats. Rejuvenation Res. 2018, 22, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.A.; Reischauer, C. About a new organic body in the fruit shells of Juglans regia. Arch. Pharm. 1857, 141, 67. [Google Scholar]
- Stickney, J.; Hoy, P. Toxic action of black walnut. Trans. Wis. State Hort. Soc. 1881, 11, 166–167. [Google Scholar]
- Auyong, T.K.; Westfall, B.A.; Russell, R.L. Pharmacological aspects of juglone. Toxicon 1963, 1, 235–239. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Shah, A.J.; Roberts, R. Mechanisms mediating the vasodilatory effects of juglone in porcine isolated coronary artery. Eur. J. Pharmacol. 2020, 866, 172815. [Google Scholar] [CrossRef]
- Ahmad, T.; Khan, T.; Alamgeer; Shah, A.J. Juglone as antihypertensive agent acts through multiple vascular mechanisms. Clin. Exp. Hypertens. 2020, 42, 335–344. [Google Scholar] [CrossRef]
- Clark, A.M.; Jurgens, T.M.; Huord, C.D. Antimicrobial activity of juglone. Phytother. Res. 1990, 4, 11–14. [Google Scholar] [CrossRef]
- Drage, S.; Engelmeier, D.; Bachmann, G.; Sessitsch, A.; Mitter, B.; Hadacek, F. Combining microdilution with MicroResp™: Microbial substrate utilization, antimicrobial susceptibility and respiration. J. Microbiol. Methods 2012, 88, 399–412. [Google Scholar] [CrossRef]
- Bhargava, U.C.; Westfall, B.A. Antitumor activity of Juglans nigra (black walnut) extractives. J. Pharm. Sci. 1968, 57, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Yu, X.-F.; Qu, S.-C.; Zhang, R.; Qu, X.-R.; Chen, Y.-P.; Ma, X.-Y.; Sui, D.-Y. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. Eur. J. Pharmacol. 2010, 645, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, C.; Wang, W.; Yao, L.P.; Zhang, J.; Zhang, S.D.; Li, J.; Fang, S.H.; Fu, Y.J. Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2018, 116, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zakavi, F.; Hagh, L.G.; Daraeighadikolaei, A.; Sheikh, A.F.; Daraeighadikolaei, A.; Shooshtari, Z.L. Antibacterial Effect of Juglans Regia Bark against Oral Pathologic Bacteria. Int. J. Dent. 2013, 2013, 854765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chobot, V.; Hadacek, F. Milieu-dependent pro-and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. J. Chem. Ecol. 2009, 35, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R. A DFT study on the radical scavenging activity of juglone and its derivatives. J. Mol. Struct. THEOCHEM 2010, 939, 9–13. [Google Scholar] [CrossRef]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Wang, S.; Tian, S.; Yang, F.; Yang, H.; Yang, X.; Du, G. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2009, 615, 125–132. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.-H.; Yang, Q.; Wang, S.-W.; Zhang, B.-L.; Wang, J.-B.; Cao, W.; Bi, L.-L.; Sun, J.-Y.; Miao, S.; et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 2012, 7, e48872. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.D.; Anubolu, H.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S. Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats: Possible involvement of mitochondrial dysfunction and apoptosis. Life Sci. 2014, 107, 59–67. [Google Scholar] [CrossRef]
- Ahmed, S.; Gul, S.; Ze Jaafar, H.; Moga, M.; Zia-Ul-Haq, M.; Dima, L. Anti-platelet effects of nimesulide in isoproterenol-induced myocardial ischaemia and infarction in rabbits. Acta Cardiol. 2015, 70, 401–408. [Google Scholar] [CrossRef]
- Ho, D.; Yan, L.; Iwatsubo, K.; Vatner, D.E.; Vatner, S.F. Modulation of β-adrenergic receptor signaling in heart failure and longevity: Targeting adenylyl cyclase type 5. Heart Fail Rev. 2010, 15, 495–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wexler, B.C. Myocardial infarction in young vs old male rats: Pathophysiologic changes. Am. Heart J. 1978, 96, 70–80. [Google Scholar] [CrossRef]
- Sambhi, M.P.; White, F.N. The electrocardiogram of the normal and hypertensive rat. Circ. Res. 1960, 8, 129–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef]
- Rajadurai, M.; Prince, P.S. Preventive effect of naringin on cardiac mitochondrial enzymes during isoproterenol-induced myocardial infarction in rats: A transmission electron microscopic study. J. Biochem. Mol. Toxicol. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Alpert, J.S.; Thygesen, K.; Antman, E.; Bassand, J.P. Myocardial infarction redefined—A consensus document of The Joint European Society of Cardiology/American 36(3 College of Cardiology Committee for the redefinition of myocardial infarction). J. Am. Coll. Cardiol. 2000, 36, 959–969. [Google Scholar]
- Suchalatha, S.; Devi, C.S.S. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J. Exp. Biol. 2004, 42, 174–178. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Anandan, R.; Kumar, S.H.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef]
- Rajadurai, M.; Prince, P.S. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology 2007, 230, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.S.; Naik, S.R. Cardioprotective activity of Ginkgo biloba phytosomes in isoproterenol-induced myocardial necrosis in rats: A biochemical and histoarchitectural evaluation. Exp. Toxicol. Pathol. 2008, 60, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Prince, P.S.M. Protective effects of sinapic acid on cardiac hypertrophy, dyslipidemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 699, 213–218. [Google Scholar] [CrossRef]
- Takeda, S.; Yamashita, A.; Maeda, K.; Maeda, Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 2003, 424, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Body, R.; McDowell, G.; Carley, S.; Wibberley, C.; Ferguson, J.; Mackway-Jones, K. A FABP-ulous “rule out” strategy? Heart fatty acid binding protein and troponin for rapid exclusion of acute myocardial infarction. Resuscitation 2011, 82, 1041–1046. [Google Scholar]
- Roos, A.; Hellgren, A.; Rafatnia, F.; Hammarsten, O.; Ljung, R.; Carlsson, A.C.; Holzmann, M.J. Investigations, findings, and follow-up in patients with chest pain and elevated high-sensitivity cardiac troponin T levels but no myocardial infarction. Int. J. Cardiol. 2017, 232, 111–116. [Google Scholar] [CrossRef]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, R.P.; Stefanello, S.T.; Mauriz, J.L.; Gonzalez-Gallego, J.; Soares, F.A.A. Creatine and the Liver: Metabolism and Possible Interactions. Mini Rev. Med. Chem. 2016, 16, 12–18. [Google Scholar] [CrossRef]
- Karras, D.J.; Kane, D.L. Serum markers in the emergency department diagnosis of acute myocardial infarction. Emerg. Med. Clin. N. Am. 2001, 19, 321–337. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef] [Green Version]
- Peer, P.A.; Trivedi, P.C.; Nigade, P.B.; Ghaisas, M.M.; Deshpande, A.D. Cardioprotective effect of Azadirachta indica A. Juss. On isoprenaline induced myocardial infarction in rats. Int. J. Cardiol. 2008, 126, 123–126. [Google Scholar] [PubMed]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Agress, C.M.; Jacobs, H.I.; Glassner, H.F.; Lederer, M.A.; Clark, W.G.; Wroblewski, F.; Karmen, A.; Ladue, J.S. Serum transaminase levels in experimental myocardial infarction. Circulation 1955, 11, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Zhang, J.; Wen, J.; Ming, Q.; Zhang, J.; Xu, Y. Correlation of serum alanine aminotransferase and aspartate aminotransferase with coronary heart disease. Int. J. Clin. Exp. Med. 2015, 8, 4399–4404. [Google Scholar] [PubMed]
- Padmanabhan, M.; Prince, P.S.M. Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: A histopathological study. Toxicology 2006, 224, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Vidyasagar, A.; Jacobson, L.; Acun, Z.; Esnault, S.; Hullett, D.; Malter, J.S.; Djamali, A. The Pin 1 inhibitor juglone attenuates kidney fibrogenesis via Pin 1-independent mechanisms in the unilateral ureteral occlusion model. Fibrogenesis Tissue Repair. 2010, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Bendary, E.; Francis, R.R.; Ali HM, G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Rona, G. Catecholamine cardiotoxicity. J. Mol. Cell. Cardiol. 1985, 17, 291–306. [Google Scholar] [CrossRef]
- Rathore, N.; John, S.; Kale, M.; Bhatnagar, D. Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol. Res. 1998, 38, 297–303. [Google Scholar] [CrossRef]
- Ji, L.L.; Stratman, F.W.; Lardy, H.A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch. Biochem. Biophys. 1988, 263, 150–160. [Google Scholar] [CrossRef]
- Joukar, S.; Ebrahimi, S.; Khazaei, M.; Bashiri, A.; Shakibi, M.R.; Naderi, V.; Shahouzehi, B.; Alasvand, M. Co-administration of walnut (Juglans regia) prevents systemic hypertension induced by long-term use of dexamethasone: A promising strategy for steroid consumers. Pharm Biol. 2016, 55, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfraind, T. Antioxidant effects and the therapeutic mode of action of calcium channel blockers in hypertension and atherosclerosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2259–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.-J.; Mu, D.; Jiang, M.-D.; Zheng, S.-M.; Zhang, Y.; He, S.; Weng, M.; Zeng, W.-Z. Hepatoprotective effect of juglone on dimethylnitrosamine-induced liver fibrosis and its effect on hepatic antioxidant defence and the expression levels of α-SMA and collagen III. Mol. Med. Rep. 2015, 12, 4095–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]

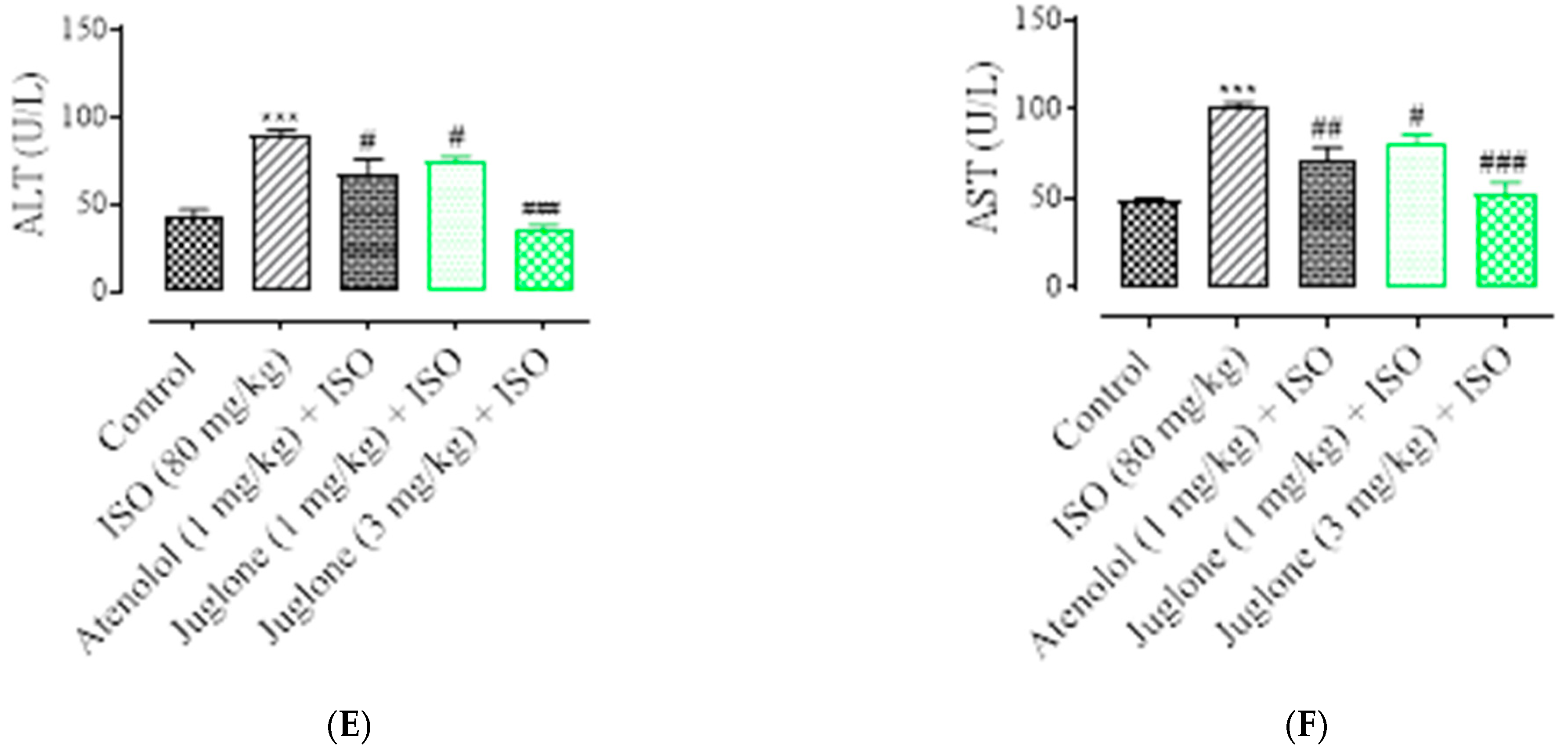
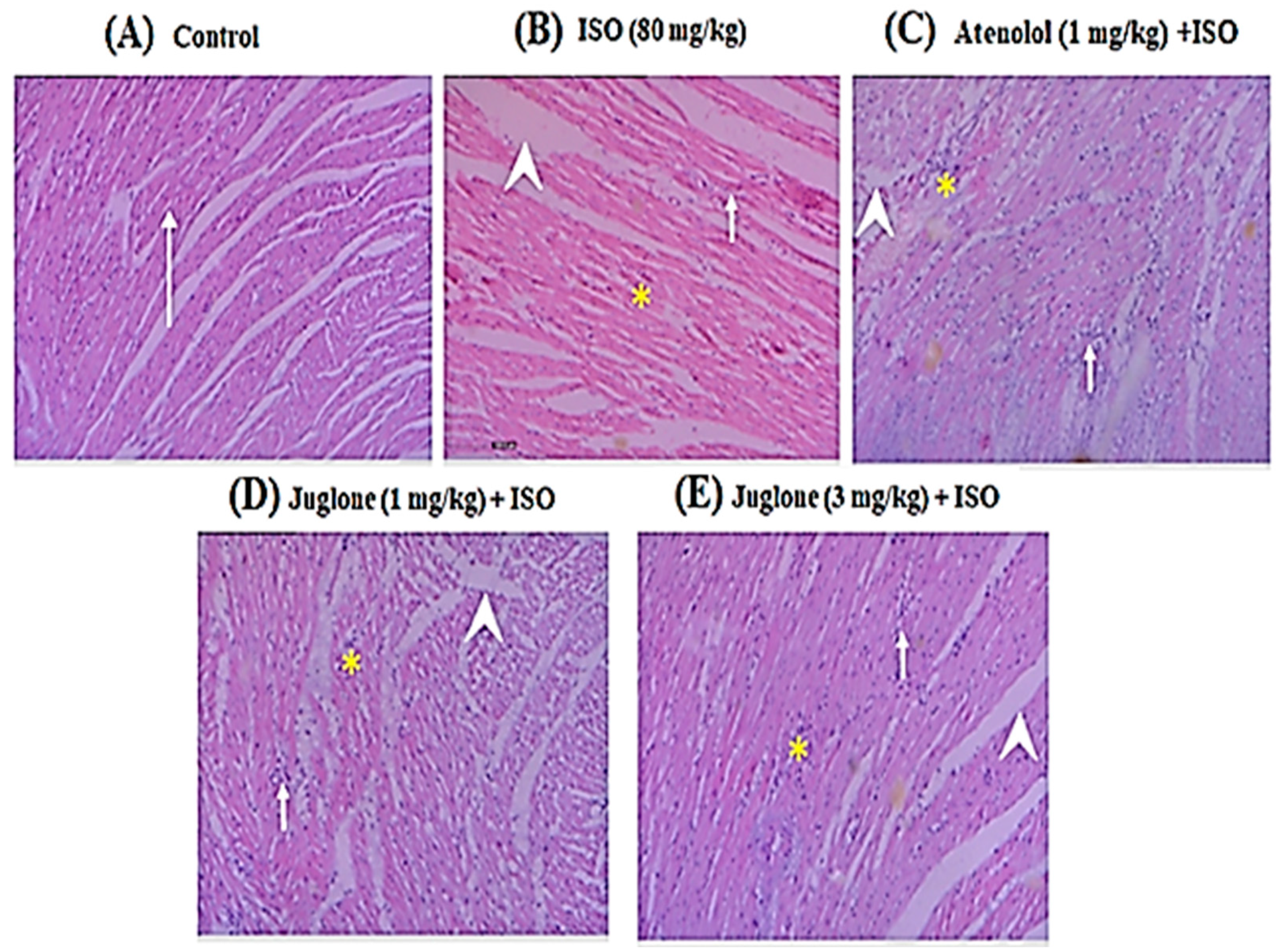
| Groups | Inflammation | Edema | Necrosis |
|---|---|---|---|
| Control | - | - | - |
| ISO (80 mg/kg) | +++ | +++ | +++ |
| Atenolol (1 mg/kg) + ISO | + | + | + |
| Juglone (1 mg/kg) + ISO | + | ++ | + |
| Juglone (3 mg/kg) + ISO | + | + | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.; Khan, T.; Tabassum, T.; Alqahtani, Y.S.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Alasmary, M.Y.; Almedhesh, S.A.; Shah, A.J. Juglone from Walnut Produces Cardioprotective Effects against Isoproterenol-Induced Myocardial Injury in SD Rats. Curr. Issues Mol. Biol. 2022, 44, 3180-3193. https://doi.org/10.3390/cimb44070220
Ahmad T, Khan T, Tabassum T, Alqahtani YS, Mahnashi MH, Alyami BA, Alqarni AO, Alasmary MY, Almedhesh SA, Shah AJ. Juglone from Walnut Produces Cardioprotective Effects against Isoproterenol-Induced Myocardial Injury in SD Rats. Current Issues in Molecular Biology. 2022; 44(7):3180-3193. https://doi.org/10.3390/cimb44070220
Chicago/Turabian StyleAhmad, Taseer, Taous Khan, Tahira Tabassum, Yahya S. Alqahtani, Mater H. Mahnashi, Bandar A. Alyami, Ali O. Alqarni, Mohammed Y. Alasmary, Sultan A. Almedhesh, and Abdul Jabbar Shah. 2022. "Juglone from Walnut Produces Cardioprotective Effects against Isoproterenol-Induced Myocardial Injury in SD Rats" Current Issues in Molecular Biology 44, no. 7: 3180-3193. https://doi.org/10.3390/cimb44070220
APA StyleAhmad, T., Khan, T., Tabassum, T., Alqahtani, Y. S., Mahnashi, M. H., Alyami, B. A., Alqarni, A. O., Alasmary, M. Y., Almedhesh, S. A., & Shah, A. J. (2022). Juglone from Walnut Produces Cardioprotective Effects against Isoproterenol-Induced Myocardial Injury in SD Rats. Current Issues in Molecular Biology, 44(7), 3180-3193. https://doi.org/10.3390/cimb44070220







