Effect of Vacuum Heat Treatment on the Element Diffusion Behavior and Corrosion Resistance of Al2O3-3wt.%TiO2 Coating of Q235 Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Analysis
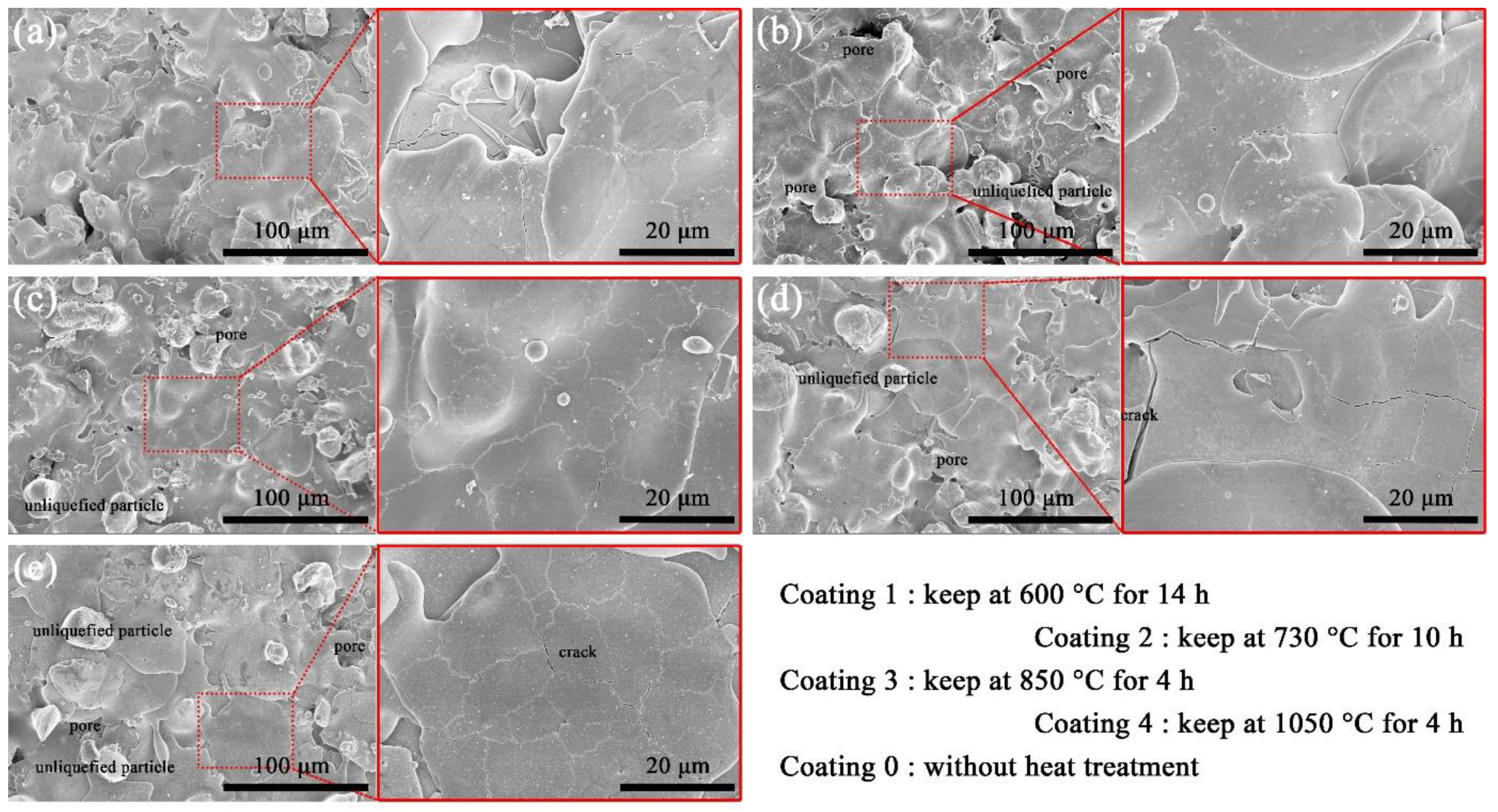
3.1. Micromorphology of the Coating Surface
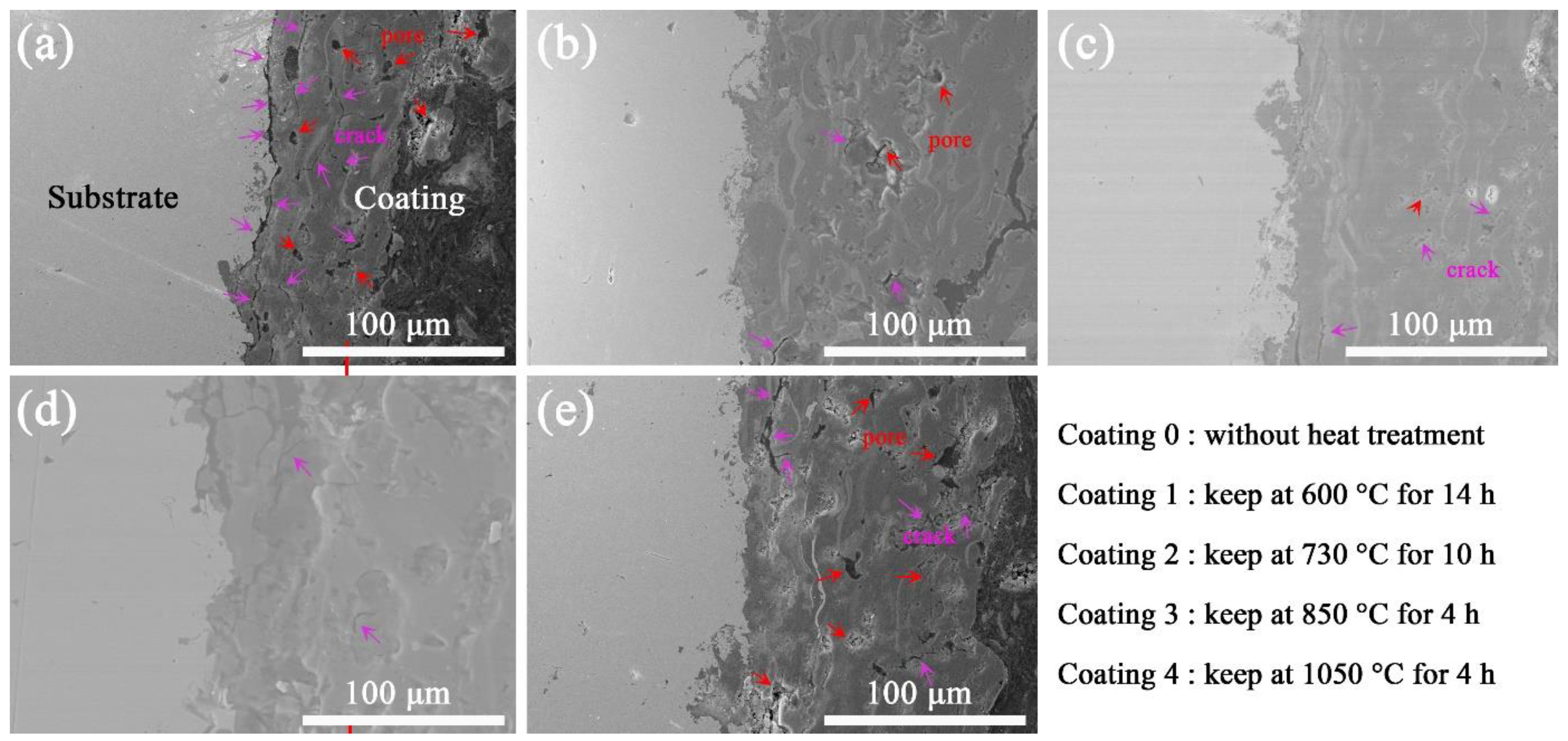
3.2. Interface Bonding between Coating and Substrate
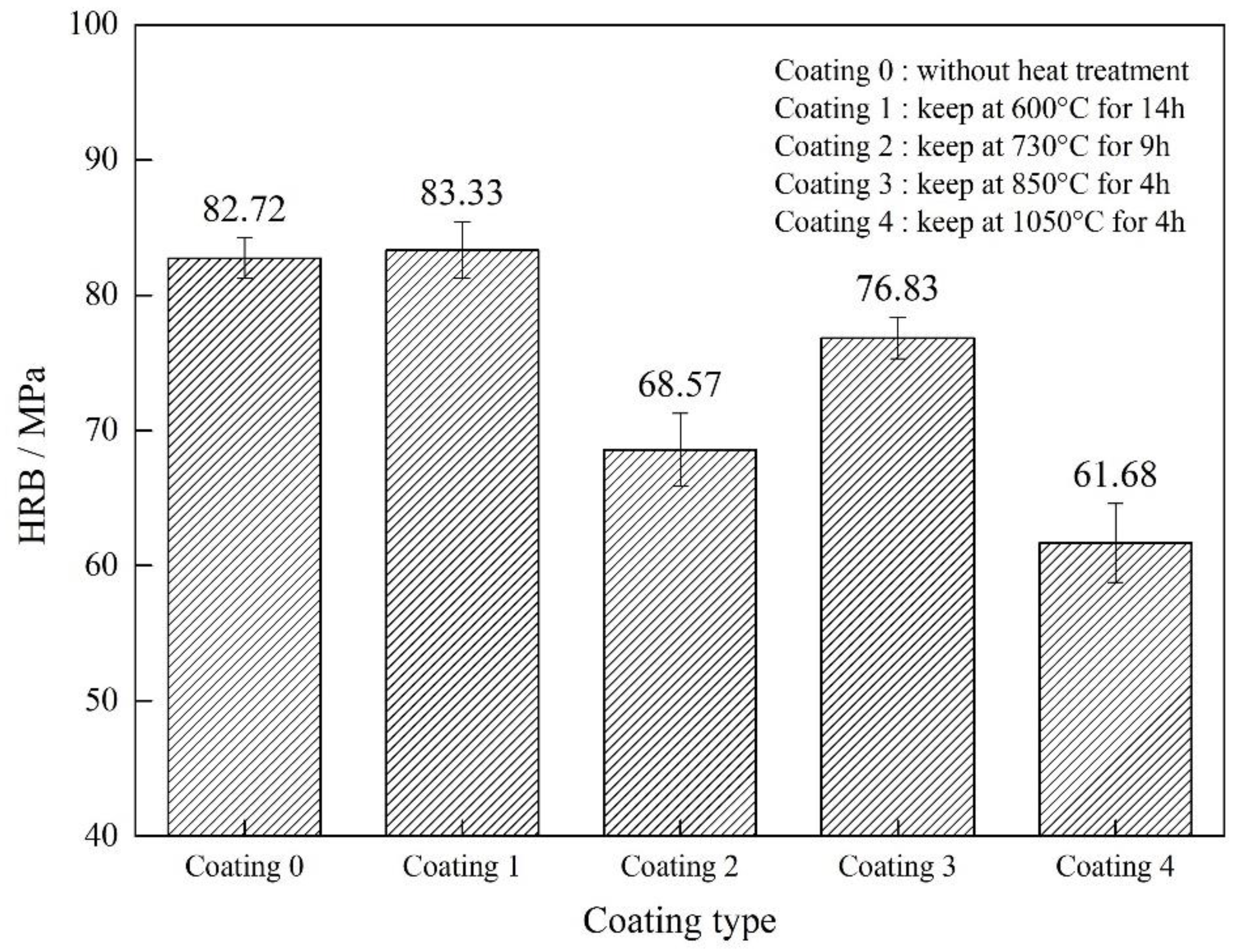
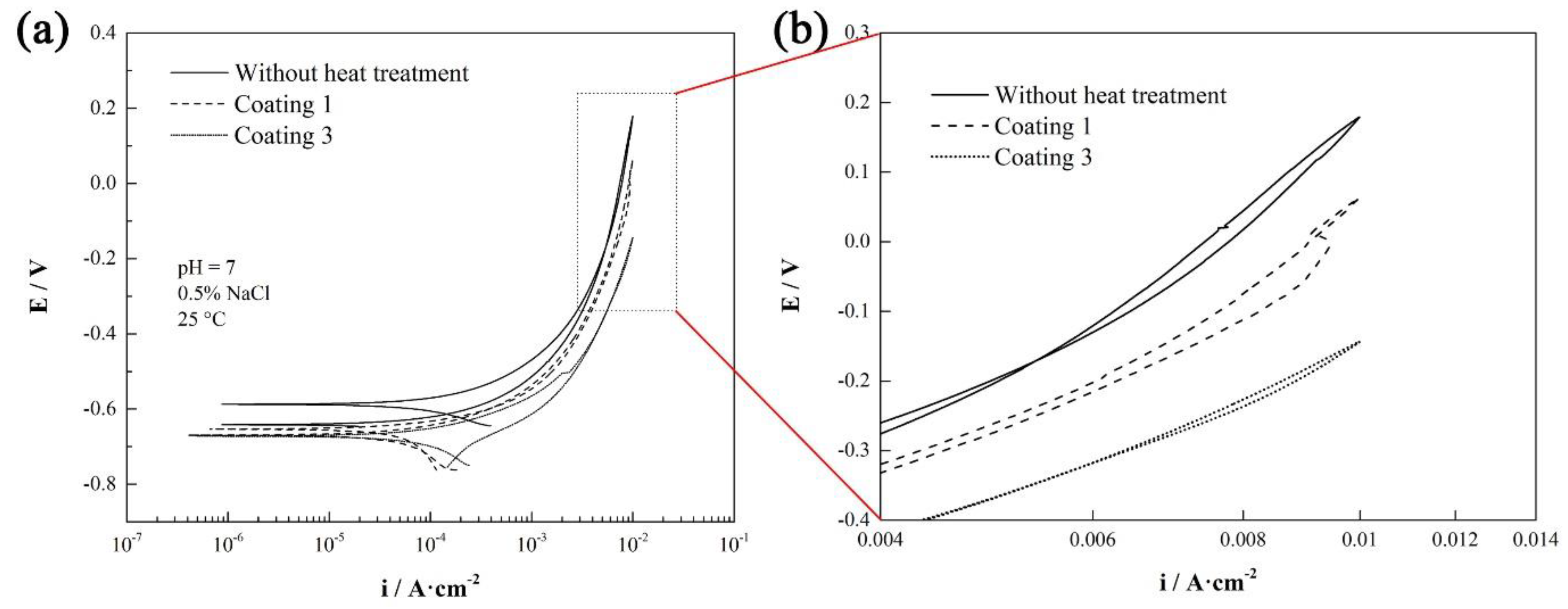
3.3. Surface Hardness and Corrosion Resistance
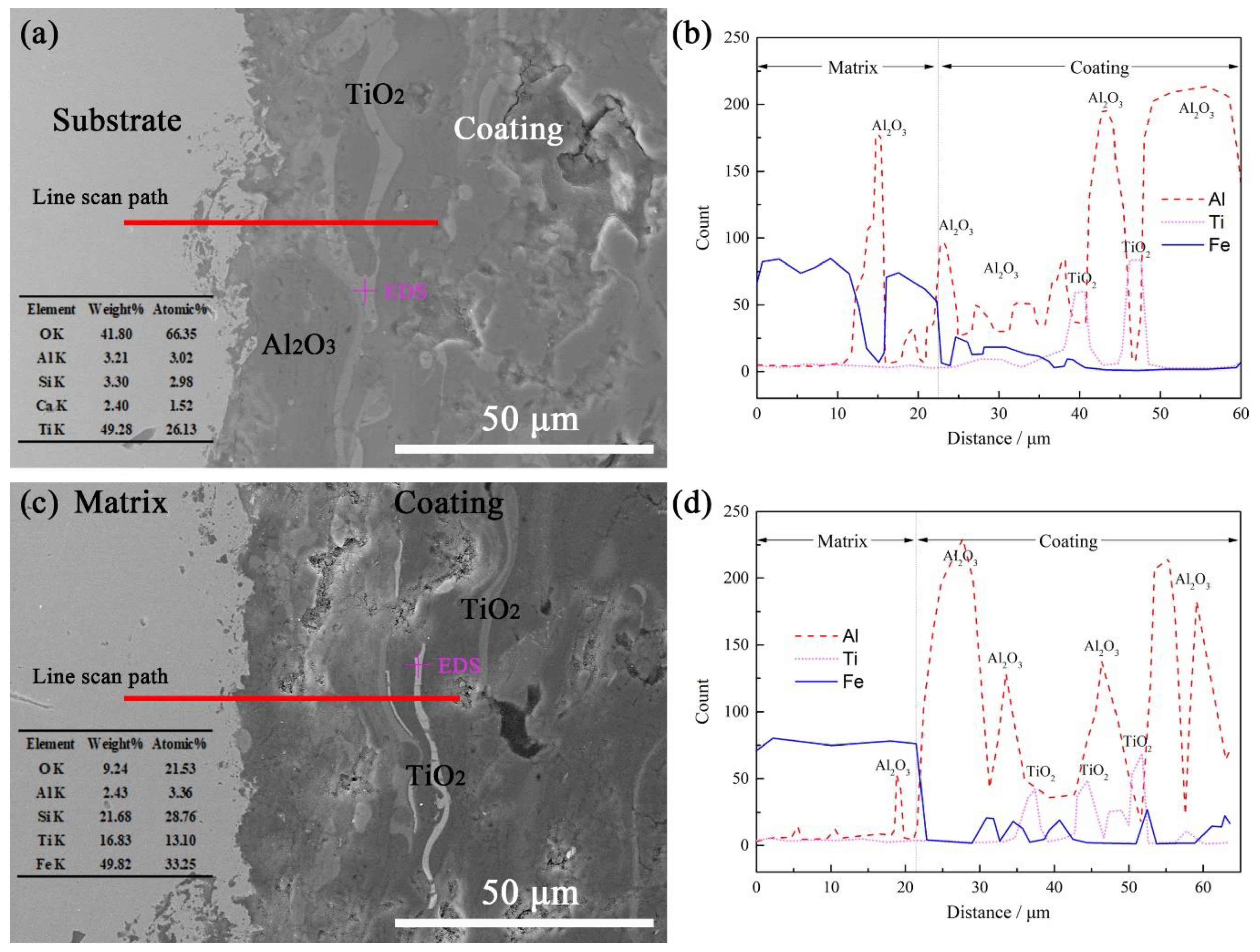
3.4. Interface-Element Distribution
4. Conclusions
- (1)
- By comparing the surface morphology of the coating, new oxide was observed to be produced after vacuum heat treatment. The corrosion products on the coating surface after vacuum heat treatment have less Fe content, and heat treatment effectively hinders corrosion depth.
- (2)
- Vacuum heat treatment can strengthen the interface between the substrate and the coating, and the interface is more closely bonded. The effect of vacuum heat treatment on coating performance is mainly caused by the mutual fusion of Al2O3 and TiO2 in the coating and the fragmentation of the phase in the coating. Vacuum heat treatment improves the surface hardness and corrosion resistance.
- (3)
- Coating performance is improved, while the Q235 performance decreases with an increase in heating temperature and holding time in vacuum heat treatment. Compared with other parameters, the vacuum heat treatment at 600 °C for 14 h can simultaneously improve the surface hardness of AT3 coatings and the corrosion resistance of the coating sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, Y.; Yang, J.; Tian, X.; Gong, C.; Zheng, W.; He, Y.; Gao, Z. Microstructure, adhesion, mechanical and corrosion properties of TiN coatings deposited by high energy pulse-enhanced vacuum arc evaporation. J. Adhes. Sci. Technol. 2020, 34, 1040–1061. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Khlifi, K.; Barhoumi, N.; Larbi, A.B.C. Effect of voltage on microstructure and its influence on corrosion and tribological properties of TiO2 coatings. J. Mater. Res. Technol. 2020, 9, 5293–5303. [Google Scholar] [CrossRef]
- Kania, H.; Saternus, M.; Kudláček, J. Structural aspects of decreasing the corrosion resistance of zinc coating obtained in baths with Al, Ni, and Pb additives. Materials 2020, 13, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, C.; Singh, S.; Pruncu, C.I.; Mishra, V.; Królczyk, G.; Pimenov, D.Y.; Pramanik, A. Surface modification of Ti-6Al-4V alloy by electrical discharge coating process using partially sintered Ti-Nb electrode. Materials 2019, 12, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tameemi, H.A.; Al-Dulaimi, T.; Awe, M.O.; Sharma, S.; Pimenov, D.Y.; Koklu, U.; Giasin, K. Evaluation of cutting-tool coating on the surface roughness and hole dimensional tolerances during drilling of Al6061-T651 alloy. Materials 2021, 14, 1783. [Google Scholar] [CrossRef]
- Hu, W.; Zhu, H.; Hu, J.; Li, B.; Qiu, C. Influence of vanadium microalloying on microstructure and property of laser-cladded martensitic stainless steel coating. Materials 2020, 13, 826. [Google Scholar] [CrossRef] [Green Version]
- Çelik, İ. Structure and surface properties of Al2O3–TiO2 ceramic coated AZ31 magnesium alloy. Ceram. Int. 2016, 42, 13659–13663. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Tian, W.; Yan, D.-R.; Zhang, J.-X.; Wang, L. Influence of composite powders’ microstructure on the microstructure and properties of Al2O3–TiO2 coatings fabricated by plasma spraying. Mater. Des. 2015, 65, 814–822. [Google Scholar] [CrossRef]
- Ghazali, M.; Forghani, S.; Hassanuddin, N.; Muchtar, A.; Daud, A. Comparative wear study of plasma sprayed TiO2 and Al2O3–TiO2 on mild steels. Tribol. Int. 2016, 93, 681–686. [Google Scholar] [CrossRef]
- Luo, H.; Song, P.; Khan, A.; Feng, J.; Zang, J.; Xiong, X.; Lü, J.; Lu, J. Alternant phase distribution and wear mechanical properties of an Al2O3-40 wt% TiO2 composite coating. Ceram. Int. 2017, 43, 7295–7304. [Google Scholar] [CrossRef]
- Żórawski, W.; Góral, A.; Bokuvka, O.; Lityńska-Dobrzyńska, L.; Berent, K. Microstructure and tribological properties of nanostructured and conventional plasma sprayed alumina–titania coatings. Surf. Coat. Technol. 2015, 268, 190–197. [Google Scholar] [CrossRef]
- Nakamura, T.; Qian, G.; Berndt, C.C. Effects of pores on mechanical properties of plasma-sprayed ceramic coatings. J. Am. Ceram. Soc. 2000, 83, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Sure, J.; Shankar, A.R.; Upadhyay, B.; Mudali, U.K. Microstructural characterization of plasma sprayed Al2O3-40 wt.% TiO2 coatings on high density graphite with different post-treatments. Surf. Coat. Technol. 2012, 206, 4741–4749. [Google Scholar] [CrossRef]
- Luo, H.; Song, P.; Khan, A.; Feng, J.; Zang, J.; Xiong, X.; Lü, J.; Lu, J. Effects of the metal-ceramic transition region on the mechanical properties and crack propagation behavior of an Al2O3-40 wt% TiO2 coating. Surf. Coat. Technol. 2017, 321, 200–212. [Google Scholar] [CrossRef]
- Kang, J.; Xu, B.; Wang, H.; Wang, C. Influence of spraying parameters on the microstructure and properties of plasma-sprayed Al2O3/40% TiO2 coating. Phys. Procedia 2013, 50, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-C.; Yan, D.-R.; Yong, Y.; Dong, Y.-C.; He, J.-N.; Zhang, J.-X. Phase evolution of plasma sprayed Al2O3−13% TiO2 coatings derived from nanocrystalline powders. Trans. Nonferrous Met. Soc. China 2013, 23, 2951–2956. [Google Scholar] [CrossRef]
- Shen, G.; Chen, Y.; Lin, C. Corrosion protection of 316 L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Celik, E.; Ozdemir, I.; Avci, E.; Tsunekawa, Y. Corrosion behaviour of plasma sprayed coatings. Surf. Coat. Technol. 2005, 193, 297–302. [Google Scholar] [CrossRef]
- Jia, S.-k.; Yong, Z.; Xu, J.-y.; Jing, W.; Lei, Y. Effect of TiO2 content on properties of Al2O3 thermal barrier coatings by plasma spraying. Trans. Nonferrous Met. Soc. China 2015, 25, 175–183. [Google Scholar] [CrossRef]
- Yiming, Y.; Lyckfeldt, O.; Tricoire, A.; Lundström, D.; Klement, U. Microstructure of plasma sprayed Al2O3-3wt% TiO2 coating using freeze granulated powder. J. Mater. Sci. Chem. Eng. 2016, 4, 8–14. [Google Scholar]
- Stevanović, D.; Dimitrijević, S.P.; Kamberović, Ž.; Parežanin, V.; Dimitrijević, S.B. Improvement of the corrosion characteristics of 1.4713 ferritic steel in 0.1 M sulfuric acid by heat treatment and Al2O3–TiO2 coating. Mater. Corros. 2021, 72, 1010–1020. [Google Scholar] [CrossRef]
- Toma, F.-L.; Stahr, C.; Berger, L.-M.; Saaro, S.; Herrmann, M.; Deska, D.; Michael, G. Corrosion resistance of APS-and HVOF-sprayed coatings in the Al2O3-TiO2 system. J. Therm. Spray Technol. 2010, 19, 137–147. [Google Scholar] [CrossRef]
- Merl, D.K.; Panjan, P.; Čekada, M.; Maček, M. The corrosion behavior of Cr-(C, N) PVD hard coatings deposited on various substrates. Electrochim. Acta 2004, 49, 1527–1533. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Gao, Y.; Guo, H.; Zheng, Q.; Kang, Y.; Zhao, S. Influence of Heat Treatment on Corrosion–Wear Behavior of Ni-Based Coating in Artificial Seawater. J. Mater. Eng. Perform. 2019, 28, 7828–7834. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Lai, X.; Zheng, X.; Jia, R.; Yuan, X. Influence of different heat treatment temperatures on the microstructure, corrosion, and mechanical properties behavior of Fe-based amorphous/nanocrystalline coatings. Coatings 2019, 9, 858. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yan, D.; Li, S.; Lu, C. The effect of heat treatment on the electrochemical corrosion behavior of reactive plasma-sprayed TiN coatings. Appl. Surf. Sci. 2011, 257, 10078–10083. [Google Scholar] [CrossRef]
- Chen, K.; Song, P.; Li, C.; Lu, J. Influence of microstructure on hardness of plasma sprayed Al2O3–TiO2–MgO coatings with interface diffusion by heat treatment. Mater Res Express 2017, 4, 126402. [Google Scholar] [CrossRef]
- Chen, K.; Song, P.; Li, C.; Zhou, Y.; He, X.; Yu, X.; Lu, J. Influence of heat treatment on alternant-layer structure and mechanical properties of Al2O3-TiO2-MgO coatings. Ceram. Int. 2018, 44, 13727–13735. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Shi, Y.; Shao, Y.; Gu, C. Combined effect of heat treatment and sealing on the corrosion resistance of reactive plasma sprayed TiNx/TiOy coatings. Ceram. Int. 2019, 45, 24545–24553. [Google Scholar] [CrossRef]
- Hunger, A.; Carl, G.; Gebhardt, A.; Rüssel, C. Ultra-high thermal expansion glass–ceramics in the system MgO/Al2O3/TiO2/ZrO2/SiO2 by volume crystallization of cristobalite. J. Non-Cryst. Solids 2008, 354, 5402–5407. [Google Scholar] [CrossRef]


| Voltage/V | Elective Current/A | Argon Pressure/Mpa | Spray Distance/mm | Argon Pressure/(kg·cm−2) | Powder Delivery/(L·h−1) | Powderfeeding Voltage/V |
|---|---|---|---|---|---|---|
| 27 | 580 | 0.7 | 100 | 100 | 300 | 3 |
| Sample Name | Heating Temperature/°C | Holding Time/h |
|---|---|---|
| Coating 0 | Without heat treatment | |
| Coating 1 | 600 | 14 |
| Coating 2 | 730 | 10 |
| Coating 3 | 850 | 4 |
| Coating 4 | 1050 | 4 |
| Samples | Ecorr (Volts) | Icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| coating 0 | −0.58737 | 3.246 × 10−4 | 3.8174 |
| coating 1 | −0.66956 | 1.997 × 10−4 | 2.3491 |
| coating 3 | −0.67271 | 2.331 × 10−4 | 2.6239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, G.; Wang, X.; Zhang, X.; Zhang, J.; Cheng, J. Effect of Vacuum Heat Treatment on the Element Diffusion Behavior and Corrosion Resistance of Al2O3-3wt.%TiO2 Coating of Q235 Steel. Materials 2022, 15, 848. https://doi.org/10.3390/ma15030848
Ma Y, Liu G, Wang X, Zhang X, Zhang J, Cheng J. Effect of Vacuum Heat Treatment on the Element Diffusion Behavior and Corrosion Resistance of Al2O3-3wt.%TiO2 Coating of Q235 Steel. Materials. 2022; 15(3):848. https://doi.org/10.3390/ma15030848
Chicago/Turabian StyleMa, Yulin, Guang Liu, Xinyu Wang, Xupeng Zhang, Jun Zhang, and Jun Cheng. 2022. "Effect of Vacuum Heat Treatment on the Element Diffusion Behavior and Corrosion Resistance of Al2O3-3wt.%TiO2 Coating of Q235 Steel" Materials 15, no. 3: 848. https://doi.org/10.3390/ma15030848
APA StyleMa, Y., Liu, G., Wang, X., Zhang, X., Zhang, J., & Cheng, J. (2022). Effect of Vacuum Heat Treatment on the Element Diffusion Behavior and Corrosion Resistance of Al2O3-3wt.%TiO2 Coating of Q235 Steel. Materials, 15(3), 848. https://doi.org/10.3390/ma15030848






