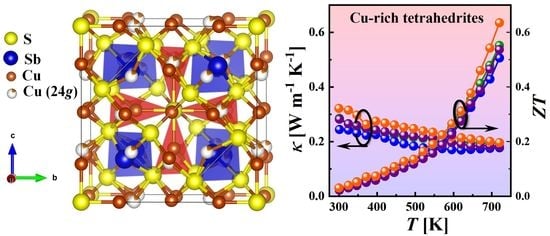
Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Sintering
2.3. Characterization
2.4. Electronic Structure Calculations
3. Results and Discussion
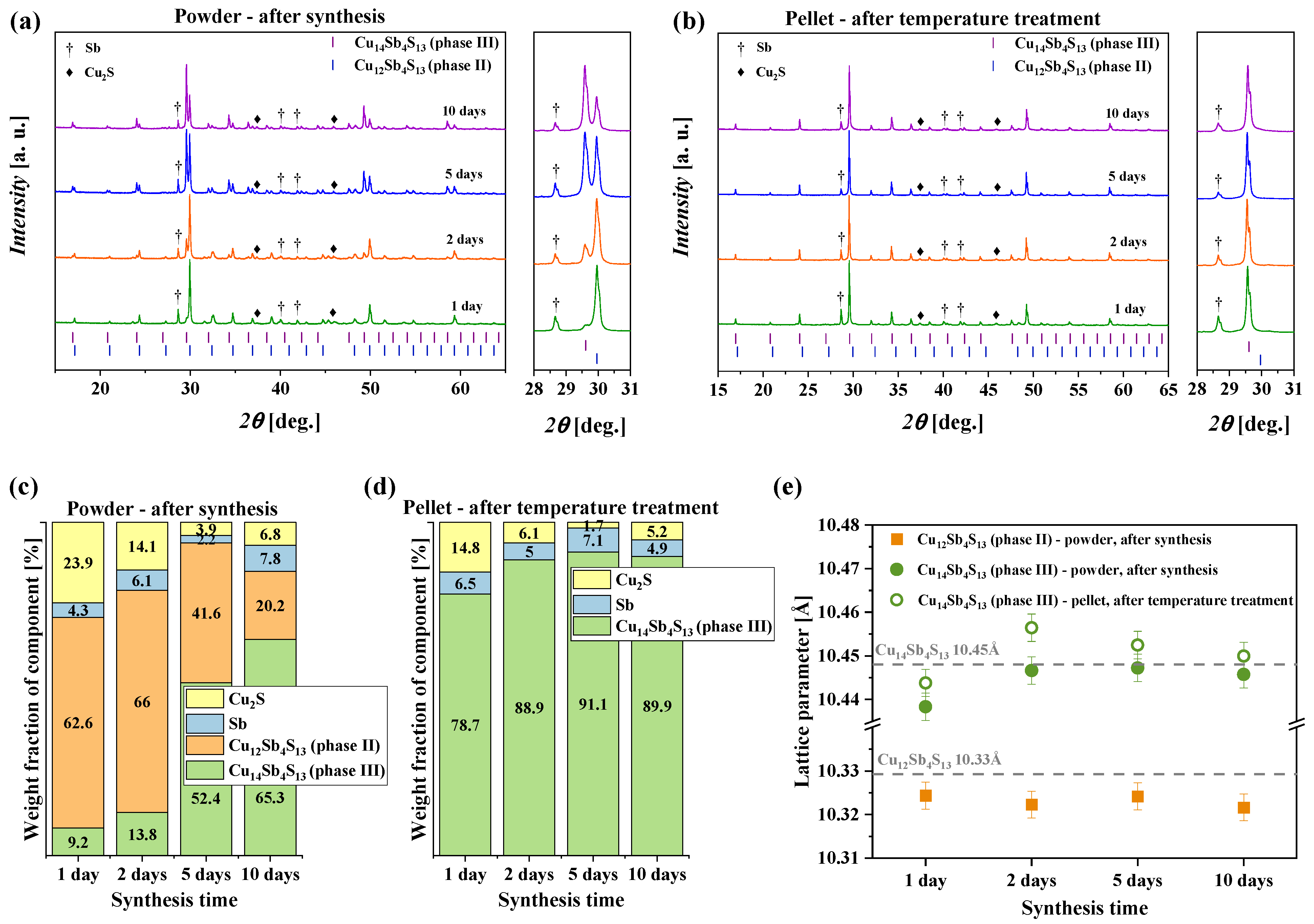
3.1. Structural Analysis
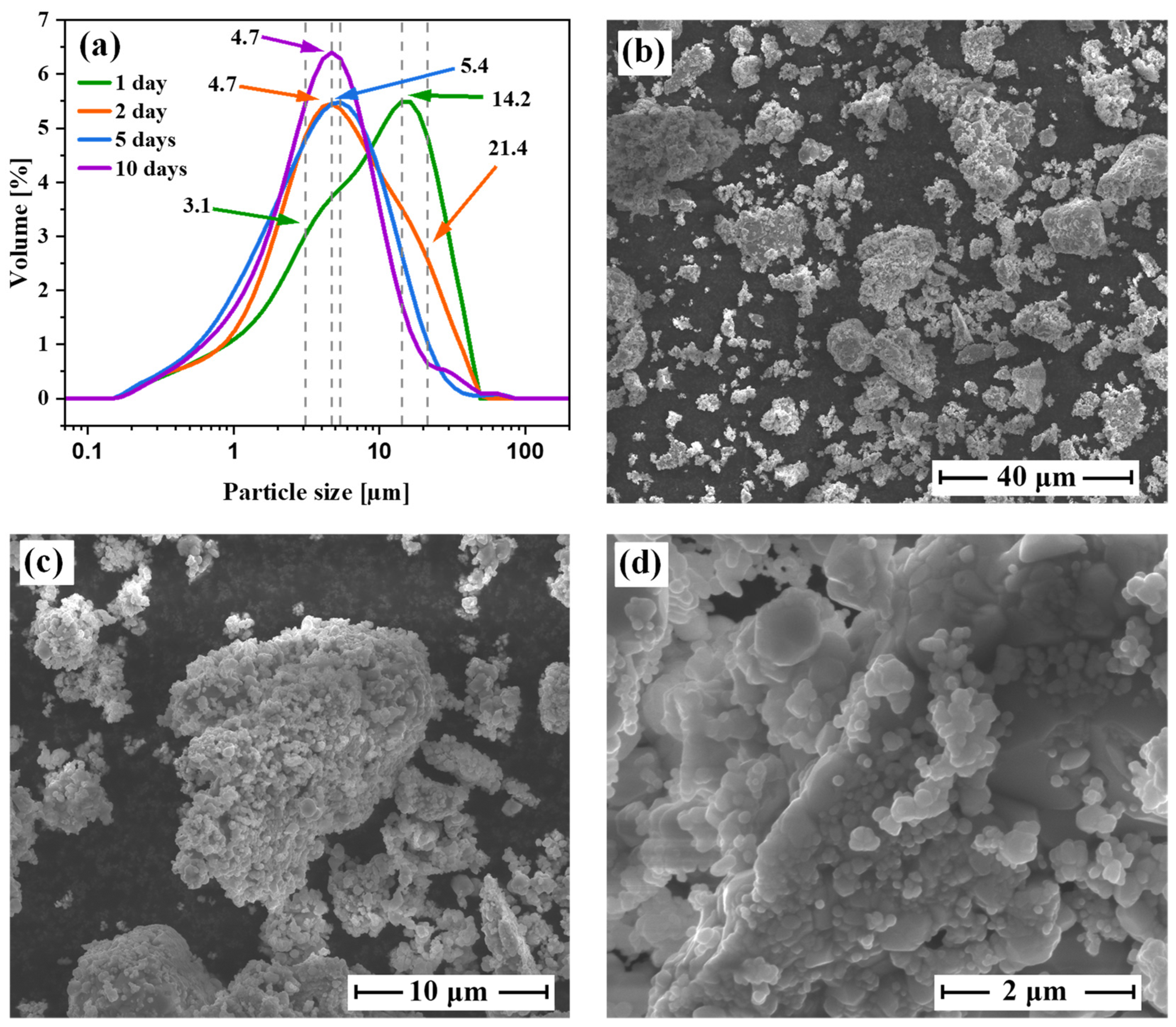
3.2. Microstructural Analysis
3.3. Electronic Structure Calculations
3.4. Electrical and Thermal Transport Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Huang, X.Y.; Bai, S.Q.; Shi, X.; Uher, C.; Chen, L.D. Thermoelectric Devices for Power Generation: Recent Progress and Future Challenges . Adv. Eng. Mater. 2016, 18, 194–213. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 121, ISBN 978-3-642-00715-6. [Google Scholar]
- Shi, X.L.; Zou, J.; Chen, Z.G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Lim, J.R.; Huang, C.-K.; Fleurial, J.-P. Thermoelectric Microdevice Fabricated by a MEMS-like Electrochemical Process. Nat. Mater. 2003, 2, 528–531. [Google Scholar] [CrossRef] [PubMed]
- El Oualid, S.; Kosior, F.; Dauscher, A.; Candolfi, C.; Span, G.; Mehmedovic, E.; Paris, J.; Lenoir, B. Innovative Design of Bismuth-Telluride-Based Thermoelectric Micro-Generators with High Output Power. Energy Environ. Sci. 2020, 13, 3579–3591. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and Old Concepts in Thermoelectric Materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef]
- Wojciechowski, K.T.; Parashchuk, T.; Wiendlocha, B.; Cherniushok, O.; Dashevsky, Z. Highly Efficient N-Type PbTe Developed by Advanced Electronic Structure Engineering. J. Mater. Chem. C 2020, 8, 13270–13285. [Google Scholar] [CrossRef]
- Cherniushok, O.; Cardoso-Gil, R.; Parashchuk, T.; Grin, Y.; Wojciechowski, K.T. Phase Equilibria and Thermoelectric Properties in the Pb–Ga–Te System in the Vicinity of the PbGa6Te10 Phase. Inorg. Chem. 2021, 60, 2771–2782. [Google Scholar] [CrossRef]
- Knura, R.; Parashchuk, T.; Yoshiasa, A.; Wojciechowski, K.T. Origins of Low Lattice Thermal Conductivity of Pb1-XSnxTe Alloys. Dalton Trans. 2021, 50, 4323–4334. [Google Scholar] [CrossRef]
- Horichok, I.V.; Parashchuk, T.O. Point Defects in PbCdTe Solid Solutions. J. Appl. Phys. 2020, 127, 055704. [Google Scholar] [CrossRef]
- Parashchuk, T.; Horichok, I.; Kosonowski, A.; Cherniushok, O.; Wyzga, P.; Cempura, G.; Kruk, A.; Wojciechowski, K.T. Insight into the Transport Properties and Enhanced Thermoelectric Performance of N-Type Pb1-XSbxTe. J. Alloy. Compd. 2020, 860, 158355. [Google Scholar] [CrossRef]
- Parashchuk, T.; Wiendlocha, B.; Cherniushok, O.; Knura, R.; Wojciechowski, K.T. High Thermoelectric Performance of P-Type PbTe Enabled by the Synergy of Resonance Scattering and Lattice Softening. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef] [PubMed]
- Knura, R.; Parashchuk, T.; Yoshiasa, A.; Wojciechowski, K.T. Evaluation of the Double-Tuned Functionally Graded Thermoelectric Material Approach for the Fabrication of n -Type Leg Based on Pb 0.75 Sn 0.25 Te. Appl. Phys. Lett. 2021, 119, 223902. [Google Scholar] [CrossRef]
- Ben-Yehuda, O.; Shuker, R.; Gelbstein, Y.; Dashevsky, Z.; Dariel, M.P. Highly Textured Bi2Te3-Based Materials for Thermoelectric Energy Conversion. J. Appl. Phys. 2007, 101, 113707. [Google Scholar] [CrossRef]
- Dashevsky, Z.; Skipidarov, S. Investigating the Performance of Bismuth-Antimony Telluride. In Novel Thermoelectric Materials and Device Design Concepts; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–21. ISBN 9783030120573. [Google Scholar]
- Kumar, A.; Bhumla, P.; Parashchuk, T.; Baran, S.; Bhattacharya, S.; Wojciechowski, K.T. Engineering Electronic Structure and Lattice Dynamics to Achieve Enhanced Thermoelectric Performance of Mn-Sb Co-Doped GeTe. Chem. Mater. 2021, 33, 3611–3620. [Google Scholar] [CrossRef]
- Xing, T.; Zhu, C.; Song, Q.; Huang, H.; Xiao, J.; Ren, D.; Shi, M.; Qiu, P.; Shi, X.; Xu, F.; et al. Ultralow Lattice Thermal Conductivity and Superhigh Thermoelectric Figure-of-Merit in (Mg, Bi) Co-Doped GeTe. Adv. Mater. 2021. [Google Scholar] [CrossRef]
- Kosonowski, A.; Kumar, A.; Parashchuk, T.; Cardoso-Gil, R.; Wojciechowski, K.T. Thermal Conductivity of PbTe–CoSb 3 Bulk Polycrystalline Composite: Role of Microstructure and Interface Thermal Resistance. Dalton Trans. 2021, 50, 1261–1273. [Google Scholar] [CrossRef]
- Chetty, R.; Tobola, J.; Klimczyk, P.; Jaworska, L.; Wojciechowski, K.T. Structural, Electronic and Thermal Properties of Te Co4Sb11.75Te0.25. J. Alloy. Compd. 2019, 809, 151477. [Google Scholar] [CrossRef]
- de Boor, J.; Dasgupta, T.; Saparamadu, U.; Müller, E.; Ren, Z.F. Recent Progress in P-Type Thermoelectric Magnesium Silicide Based Solid Solutions. Mater. Today Energy 2017, 4, 105–121. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, Y.K.; Yu, Y.; Byun, S.; Luo, Z.-Z.; Lee, H.; Ge, B.; Lee, Y.-L.; Chen, X.; Lee, J.Y.; et al. Polycrystalline SnSe with a Thermoelectric Figure of Merit Greater than the Single Crystal. Nat. Mater. 2021, 20, 1378–1384. [Google Scholar] [CrossRef]
- Candolfi, C.; Ibrahim, D.; Vaney, J.-B.; Sassi, S.; Masschelein, P.; Dauscher, A.; Lenoir, B. SnSe: Breakthrough or Not Breakthrough? In Novel Thermoelectric Materials and Device Design Concepts; Springer International Publishing: Cham, Switzerland, 2019; pp. 23–46. [Google Scholar]
- Lin, S.; Li, W.; Pei, Y. Thermally Insulative Thermoelectric Argyrodites. Mater. Today 2021, 48, 198–213. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Mallik, R.C. Tetrahedrites as Thermoelectric Materials: An Overview. J. Mater. Chem. C 2015, 3, 12364–12378. [Google Scholar] [CrossRef]
- Candolfi, C.; Bouyrie, Y.; Sassi, S.; Dauscher, A.; Lenoir, B. Tetrahedrites: Prospective Novel Thermoelectric Materials. In Thermoelectrics for Power Generation—A Look at Trends in the Technology; InTech: Winchester, UK, 2016. [Google Scholar]
- Tippireddy, S.; Chetty, R.; Raut, K.K.; Naik, M.H.; Mukharjee, P.K.; Jain, M.; Nath, R.; Wojciechowski, K.; Mallik, R.C. Electronic and Thermoelectric Properties of Zn and Se Double Substituted Tetrahedrite. Phys. Chem. Chem. Phys. 2018, 20, 28667–28677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.-H.; Zhao, L.-D.; Wu, D.; Liu, X.; Zhang, B.-P.; Li, J.-F.; He, J. Low-Cost, Abundant Binary Sulfides as Promising Thermoelectric Materials. Mater. Today 2016, 19. [Google Scholar] [CrossRef]
- Chen, L.-C.; Jiang, B.-B.; Yu, H.; Pang, H.-J.; Su, L.; Shi, X.; Chen, L.-D.; Chen, X.-J. Thermoelectric Properties of Polycrystalline Palladium Sulfide. RSC Adv. 2018, 8, 13154–13158. [Google Scholar] [CrossRef] [Green Version]
- Cherniushok, O.; Parashchuk, T.; Tobola, J.; Luu, S.; Pogodin, A.; Kokhan, O.; Studenyak, I.; Barchiy, I.; Piasecki, M.; Wojciechowski, K.T. Entropy-Induced Multivalley Band Structures Improve Thermoelectric Performance in p-Cu 7 P(S x Se 1- x) 6 Argyrodites. ACS Appl. Mater. Interfaces 2021, 13, 39606–39620. [Google Scholar] [CrossRef]
- Hu, H.; Zhuang, H.; Jiang, Y.; Shi, J.; Li, J.; Cai, B.; Han, Z.; Pei, J.; Su, B.; Ge, Z.; et al. Thermoelectric Cu 12 Sb 4 S 13 -Based Synthetic Minerals with a Sublimation-Derived Porous Network. Adv. Mater. 2021, 33, 2103633. [Google Scholar] [CrossRef]
- Long, S.O.; Powell, A.V.; Hull, S.; Orlandi, F.; Tang, C.C.; Supka, A.R.; Fornari, M.; Vaqueiro, P. Jahn–Teller Driven Electronic Instability in Thermoelectric Tetrahedrite. Adv. Funct. Mater. 2020, 30, 1909409. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.; Uher, C. High Performance Thermoelectricity in Earth-Abundant Compounds Based on Natural Mineral Tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Lai, W.; Wang, Y.; Morelli, D.T.; Lu, X. From Bonding Asymmetry to Anharmonic Rattling in Cu 12 Sb 4 S 13 Tetrahedrites: When Lone-Pair Electrons Are Not So Lonely. Adv. Funct. Mater. 2015, 25, 3648–3657. [Google Scholar] [CrossRef]
- Bouyrie, Y.; Candolfi, C.; Ohorodniichuk, V.; Malaman, B.; Dauscher, A.; Tobola, J.; Lenoir, B. Crystal Structure, Electronic Band Structure and High-Temperature Thermoelectric Properties of Te-Substituted Tetrahedrites Cu12Sb4−xTexS13 (0.5 ≤ x ≤ 2.0). J. Mater. Chem. C 2015, 3. [Google Scholar] [CrossRef]
- Sun, F.-H.; Wu, C.-F.; Li, Z.; Pan, Y.; Asfandiyar, A.; Dong, J.; Li, J.-F. Powder Metallurgically Synthesized Cu 12 Sb 4 S 13 Tetrahedrites: Phase Transition and High Thermoelectricity. RSC Adv. 2017, 7, 18909–18916. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wu, H.; Wang, G.; Lu, X.; Zhou, X. High Thermoelectric Performance Balanced by Electrical and Thermal Transport in Tetrahedrites Cu12+Sb4S12Se. Energy Storage Mater. 2018, 13, 127–133. [Google Scholar] [CrossRef]
- Heo, J.; Laurita, G.; Muir, S.; Subramanian, M.A.; Keszler, D.A. Enhanced Thermoelectric Performance of Synthetic Tetrahedrites. Chem. Mater. 2014, 26, 2047–2051. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T. Natural Mineral Tetrahedrite as a Direct Source of Thermoelectric Materials. Phys. Chem. Chem. Phys. 2013, 15, 5762. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Jin, Y.; Tang, K.; Qian, Y. Selective Synthesis and Characterization of Famatinite Nanofibers and Tetrahedrite Nanoflakes. J. Mater. Chem. 2003, 13, 301–303. [Google Scholar] [CrossRef]
- Toby, B.H.; von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Bansil, A.; Kaprzyk, S.; Tobola, J. Applications of Multiple Scattering Theory in Material Science. Mater. Res. Soc. 1992, 505. [Google Scholar]
- Stopa, T.; Kaprzyk, S.; Tobo, A.J. Linear Aspects of the Korringa–Kohn–Rostoker Formalism. J. Phys. Condens. Matter 2004, 16, 4921–4933. [Google Scholar] [CrossRef]
- Bansil, A.; Kaprzyk, S.; Mijnarends, P.E.; Toboła, J. Electronic Structure and Magnetism of Fe3-XVxX (X = Si, Ga, and Al) Alloys by the KKR-CPA Method. Phys. Rev. B 1999, 60, 13396–13412. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Kaprzyk, S.; Bansil, A. Green’s Function and a Generalized Lloyd Formula for the Density of States in Disordered Muffin-Tin Alloys. Phys. Rev. B 1990, 42, 7358–7362. [Google Scholar] [CrossRef]
- Wuensch, B.J. The Crystal Structure of Tetrahedrite, Cu12Sb4S13. Z. Für Krist. 1964, 119, 437–453. [Google Scholar] [CrossRef]
- Vaqueiro, P.; Guélou, G.; Kaltzoglou, A.; Smith, R.I.; Barbier, T.; Guilmeau, E.; Powell, A.V. The Influence of Mobile Copper Ions on the Glass-Like Thermal Conductivity of Copper-Rich Tetrahedrites. Chem. Mater. 2017, 29, 4080–4090. [Google Scholar] [CrossRef]
- Ghassemi, N.; Lu, X.; Tian, Y.; Conant, E.; Yan, Y.; Zhou, X.; Ross, J.H. Structure Change and Rattling Dynamics in Cu12 Sb4 S13 Tetrahedrite: An NMR Study. ACS Appl. Mater. Interfaces 2018, 10, 36010–36017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Liu, M.; Wang, J.; Sun, K.; Yang, Y.; Hu, B.; Xu, J.; Su, T.; Du, B. Preparation and Thermoelectric Performance of Tetrahedrite-like Cubic Cu3SbS3 Compound. J. Mater. Sci. Mater. Electron. 2021, 32, 10789–10802. [Google Scholar] [CrossRef]
- Mashadiyeva, L.F.; Mammadli, P.R.; Babanly, D.M.; Ashirov, G.M.; Shevelkov, A.V.; Yusibov, Y.A. Solid-Phase Equilibria in the Cu-Sb-S System and Thermodynamic Properties of Copper-Antimony Sulfides. JOM 2021, 73, 1522–1530. [Google Scholar] [CrossRef]
- Skinner, B.J.; Luce, F.D.; Makovicky, E. Studies of the Sulfosalts of Copper III. Phases and Phase Relations in the System Cu-Sb-S. Econ. Geol. 1972, 67, 924–938. [Google Scholar] [CrossRef]
- Machatsehki, F. XII. Präzisionsmessungen Der Gitterkonstanten Verschiedener Fahlerze. Z. Für Krist.–Cryst. Mater. 1928, 68, 204–222. [Google Scholar] [CrossRef]
- Pfitzner, A.; Evain, M.; Petricek, V. Cu12Sb4S13: A Temperature-Dependent Structure Investigation. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 337–345. [Google Scholar] [CrossRef]
- Makovicky, E.; Skinner, B.J. Studies of the Sulfosalts of Copper. VII. Crystal Structures of the Exsolution Products Cu12.3Sb4S13 and Cu13.8Sb4S13 of Unsubstituted Synthetic Tetrahedrite. Can. Mineral. 1979, 17, 619–634. [Google Scholar]
- Zhu, C.; Chen, Q.; Ming, H.; Qin, X.; Yang, Y.; Zhang, J.; Peng, D.; Chen, T.; Li, D.; Kawazoe, Y. Improved Thermoelectric Performance of Cu 12 Sb 4 S 13 through Gd-Substitution Induced Enhancement of Electronic Density of States and Phonon Scattering. ACS Appl. Mater. Interfaces 2021, 13, 25092–25101. [Google Scholar] [CrossRef]
- Du, B.; Zhang, R.; Liu, M.; Chen, K.; Zhang, H.; Reece, M.J. Crystal Structure and Improved Thermoelectric Performance of Iron Stabilized Cubic Cu3SbS3 Compound. J. Mater. Chem. C 2019, 7, 394–404. [Google Scholar] [CrossRef]
- Bullett, D.W.; Dawson, W.G. Bonding Relationships in Some Ternary and Quarternary Phosphide and Tetrahedrite Structures: (Ag6M4P12)M6’, Cu12+xSb4S13 and Cu14-xSb4S13, Ln6Ni6P17. J. Phys. C Solid State Phys. 1986, 19, 5837–5847. [Google Scholar] [CrossRef]
- Ghassemi, N.; Tian, Y.; Lu, X.; Yan, Y.; Zhou, X.; Ross, J.H. Copper-Ion Dynamics and Phase Segregation in Cu-Rich Tetrahedrite: An NMR Study. J. Phys. Chem. C 2020, 124, 3973–3979. [Google Scholar] [CrossRef] [Green Version]
- Weldert, K.S.; Zeier, W.G.; Day, T.W.; Panthöfer, M.; Snyder, G.J.; Tremel, W. Thermoelectric Transport in Cu7PSe6 with High Copper Ionic Mobility. J. Am. Chem. Soc. 2014, 136, 12035–12040. [Google Scholar] [CrossRef] [PubMed]
- Guélou, G.; Powell, A.; Smith, R.I.; Vaqueiro, P. The impact of manganese substitution on the structure and properties of tetrahedrite. J. Appl. Phys. 2019, 126, 045107. [Google Scholar] [CrossRef]
- Yan, Y.; Li, N.; Wang, G.; Xiong, Q.; Fan, L.; Jiang, P.; Lu, X.; Wang, G.; Zhou, X. Achieving High Average Power Factor in Tetrahedrite Cu12Sb4S13 via Regulating Electron-Phonon Coupling Strength. Mater. Today Phys. 2022, 22, 100590. [Google Scholar] [CrossRef]
- Kwak, S.-G.; Lee, G.-E.; Kim, I.-H. Effects of Se Doping on Thermoelectric Properties of Tetrahedrite Cu12Sb4S13−zSez. Electron. Mater. Lett. 2021, 17, 164–171. [Google Scholar] [CrossRef]
- Lee, G.-E.; Kim, I.-H. Effects of Zn/Bi Double Doping on the Charge Transport and Thermoelectric Properties of Tetrahedrites Cu12−xZnxSb4−yBiyS13. J. Electron. Mater. 2020, 49, 2768–2774. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Kim, I.-H. Charge Transport and Thermoelectric Properties of Sn-Doped Tetrahedrites Cu12Sb4-YSnyS13. Korean J. Met. Mater. 2021, 59, 724–731. [Google Scholar] [CrossRef]
- Joffe, A.F.; Stil’bans, L.S. Physical Problems of Thermoelectricity. Rep. Prog. Phys. 1959, 22, 306. [Google Scholar] [CrossRef]
- Morelli, D.T.; Caillat, T.; Fleurial, J.-P.; Borshchevsky, A.; Vandersande, J.; Chen, B.; Uher, C. Low-Temperature Transport Properties of p-Type CoSb3. Phys. Rev. B 1995, 51, 9622–9628. [Google Scholar] [CrossRef]
- el Akkad, F.; Mansour, B.; Hendeya, T. Electrical and Thermoelectric Properties of Cu2Se and Cu2S. Mater. Res. Bull. 1981, 16. [Google Scholar] [CrossRef]
- Nieroda, P.; Leszczyński, J.; Mikuła, A.; Mars, K.; Kruszewski, M.J.; Koleżyński, A. Thermoelectric Properties of Cu2S Obtained by High Temperature Synthesis and Sintered by IHP Method. Ceram. Int. 2020, 46, 25460–25466. [Google Scholar] [CrossRef]
- Levinsky, P.; Candolfi, C.; Dauscher, A.; Tobola, J.; Hejtmánek, J.; Lenoir, B. Thermoelectric Properties of the Tetrahedrite–Tennantite Solid Solutions Cu 12 Sb 4−x As x S 13 and Cu10Co2Sb4−yAsyS13 (0 ≤ x, y ≤ 4). Phys. Chem. Chem. Phys. 2019, 21, 4547–4555. [Google Scholar] [CrossRef]
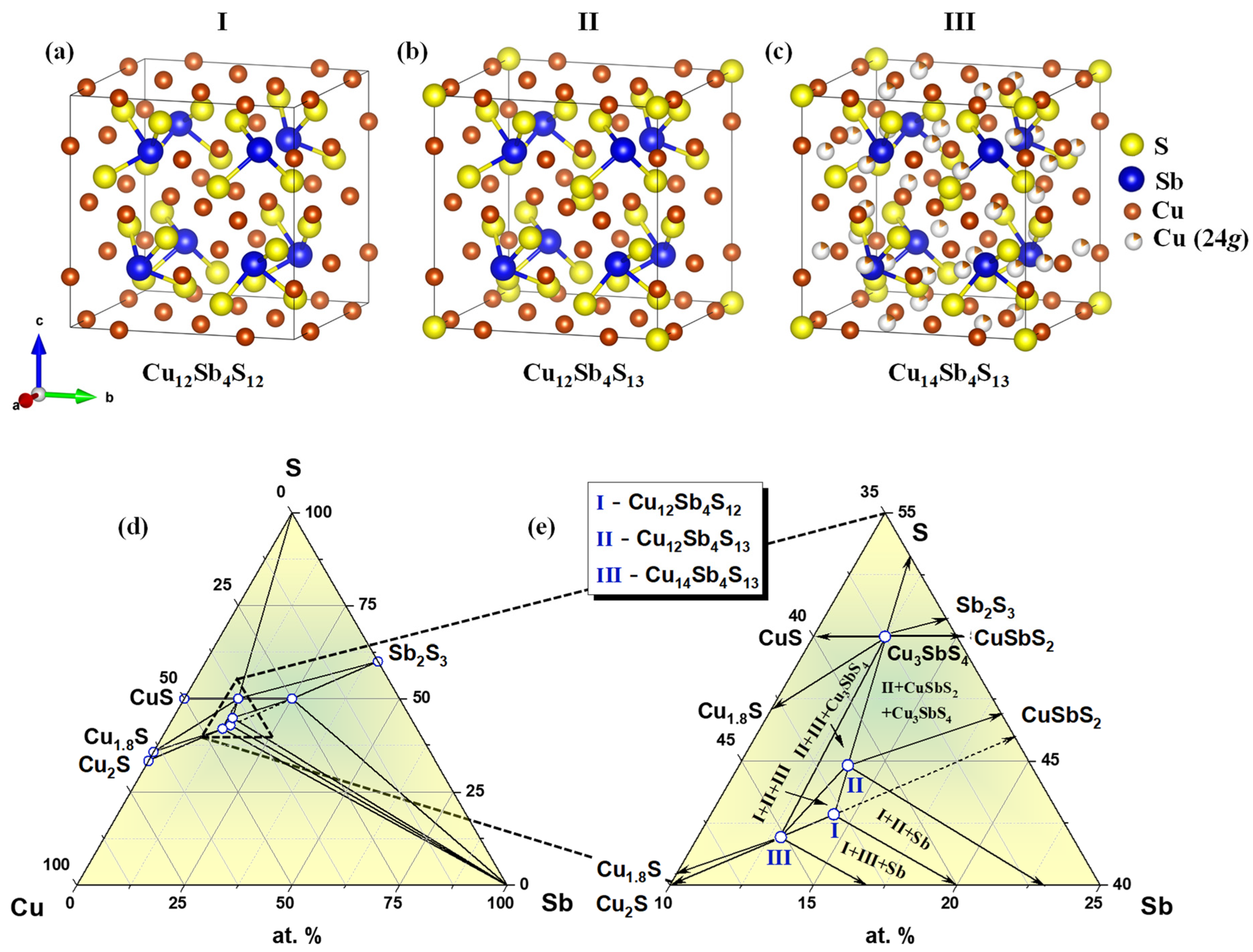
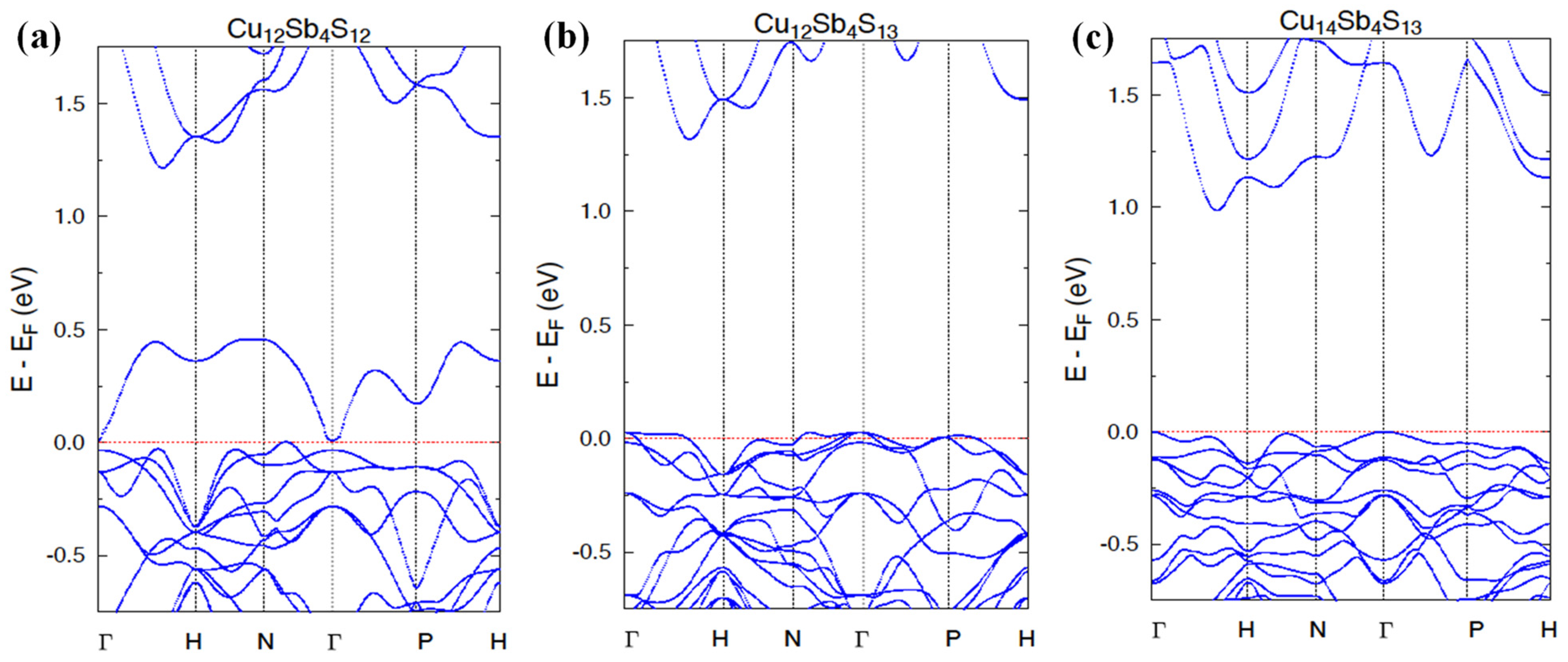
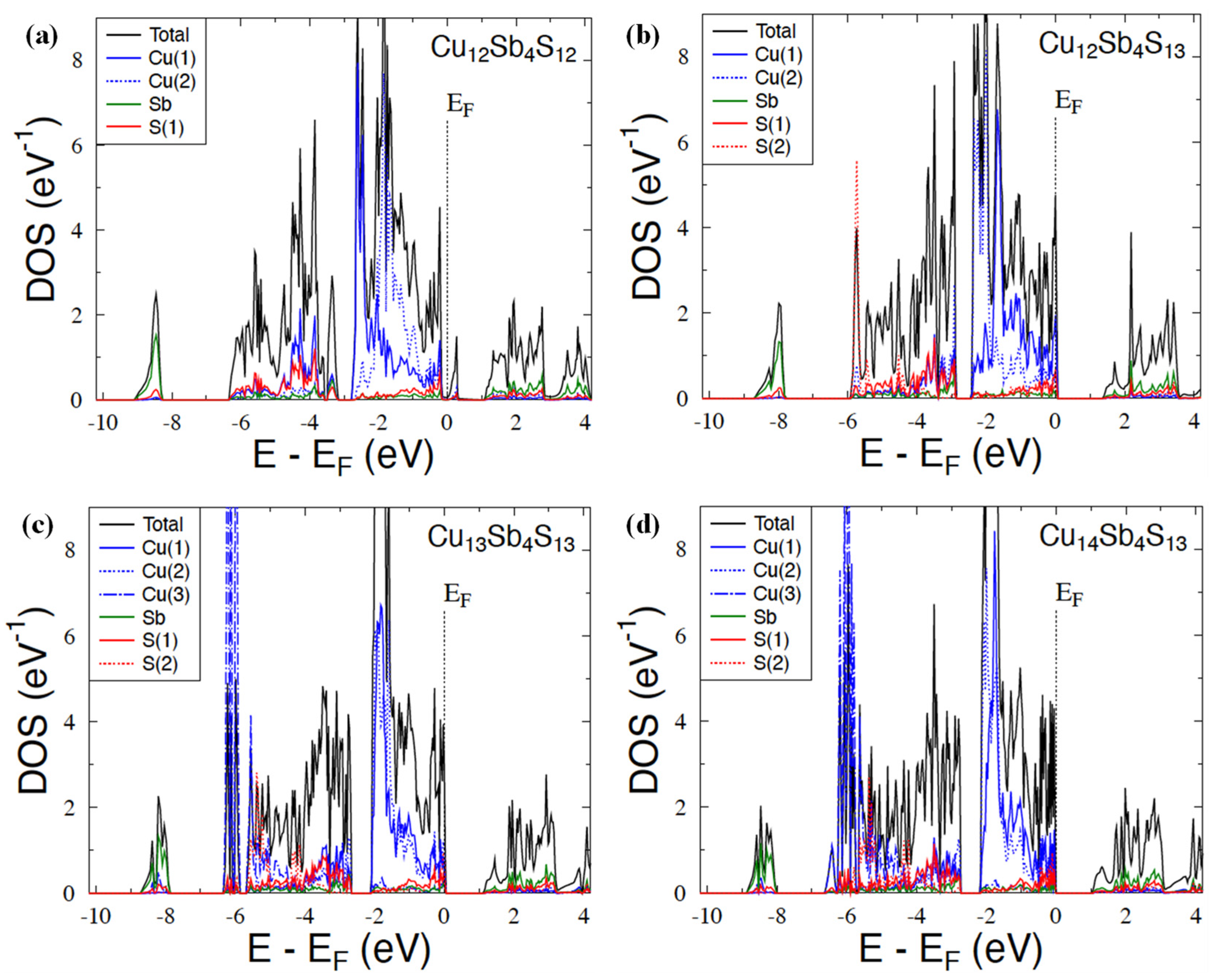

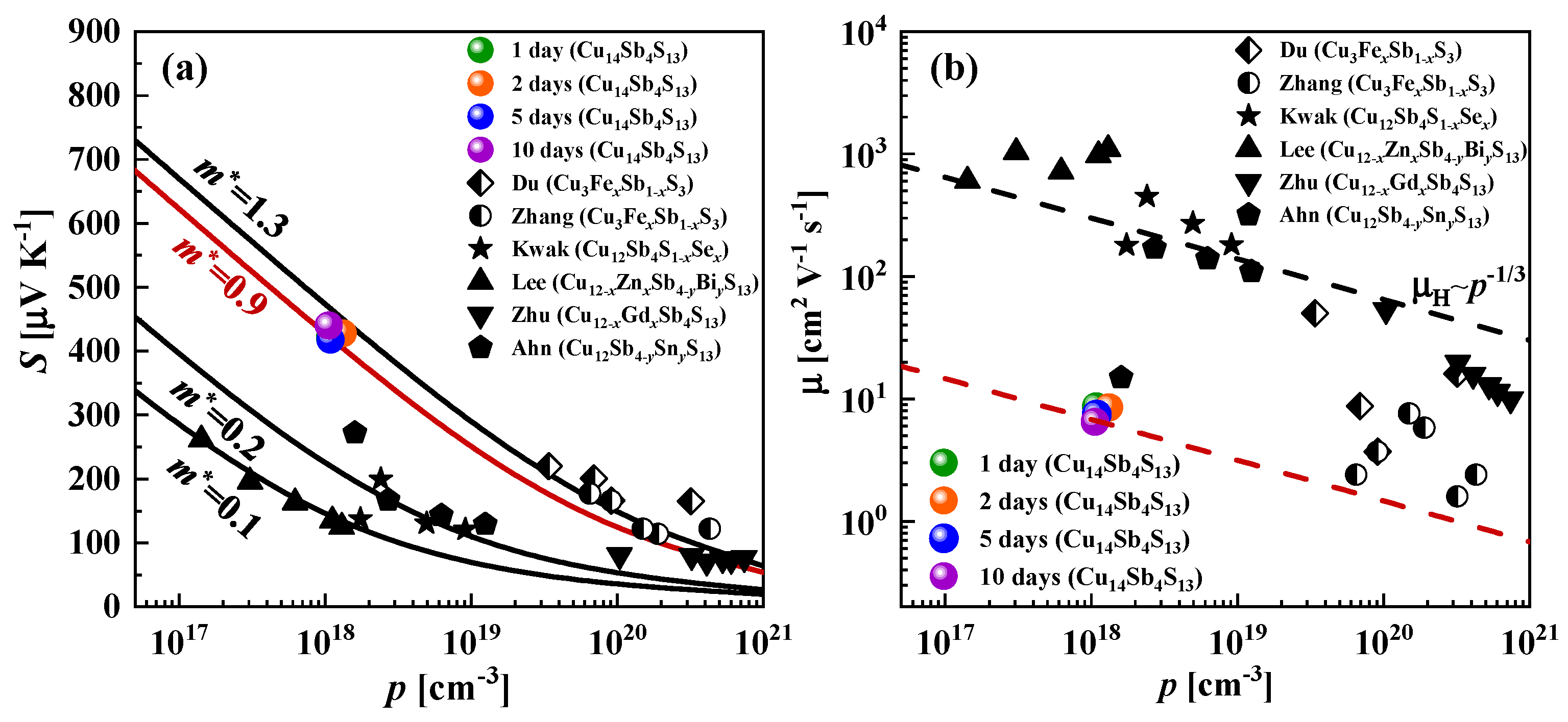
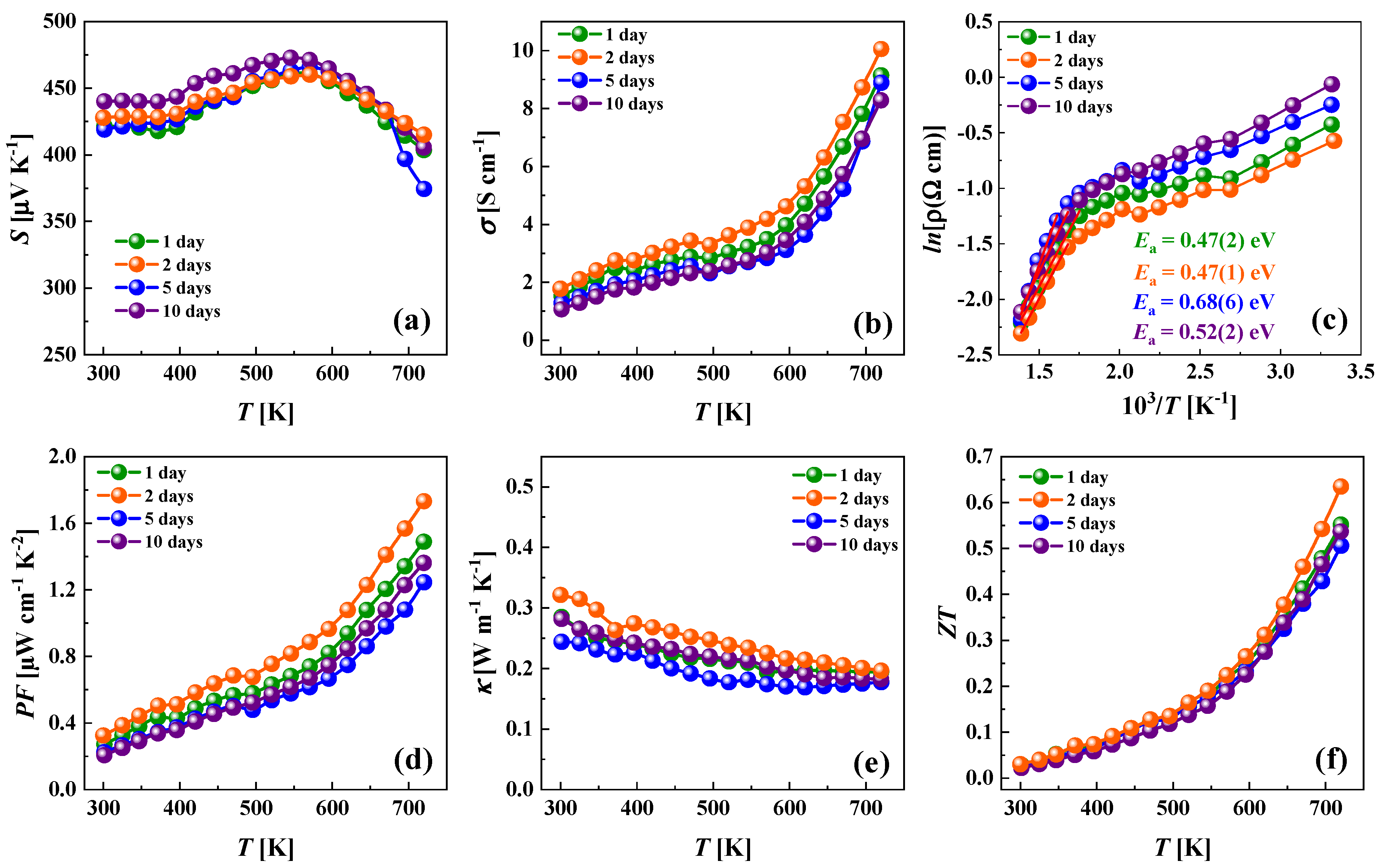
| Nominal Composition | Cu12Sb4S12 (Phase I) | Cu12Sb4S13 (Phase II) | Cu14Sb4S13 (Phase III) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Space group | I-43m (No. 217) | ||||||||||||
| a/Å | 10.240 | 10.329 | 10.448 | ||||||||||
| Unit cell volume/Å3 | 1073.74 | 1102.08 | 1140.51 | ||||||||||
| Z | 2 | 2 | 2 | ||||||||||
| Atom | Site | x | y | z | SOF | x | y | z | SOF | x | y | z | SOF |
| Cu(1) | 12d | 0.2500 | 0.5000 | 0.0000 | 1 | 0.2500 | 0.5000 | 0.0000 | 1 | 0.2500 | 0.5000 | 0.0000 | 1 |
| Cu(2) | 12e | 0.2500 | 0.0000 | 0.0000 | 1 | 0.2150 | 0.0000 | 0.0000 | 1 | 0.2157 | 0.0000 | 0.0000 | 1 |
| Cu(3) | 24g | - | - | - | - | - | - | - | - | 0.2851 | 0.2851 | 0.0102 | 0.167 |
| Sb | 8c | 0.2780 | 0.2780 | 0.2780 | 1 | 0.2682 | 0.2682 | 0.2682 | 1 | 0.2663 | 0.2663 | 0.2663 | 1 |
| S(1) | 24g | 0.1250 | 0.1250 | 0.3750 | 1 | 0.1152 | 0.1152 | 0.3609 | 1 | 0.1137 | 0.1137 | 0.3613 | 1 |
| S(2) | 2a | - | - | - | - | 0.0000 | 0.0000 | 0.0000 | 1 | 0.0000 | 0.0000 | 0.0000 | 1 |
| Cu12Sb4S13 | Cu13Sb4S13 | Cu14Sb4S13 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔE (eV) | Cu(1) | Cu(2) | Cu(3) | ΔE (eV) | Cu(1) | Cu(2) | Cu(3) | ΔE (eV) | Cu(1) | Cu(2) | Cu(3) |
| 0 | 6 | 6 | 0 | 0 | 6 | 6 | 1 | 0 | 6 | 6 | 2 |
| +0.20 | 5 | 6 | 1 | +0.39 | 5 | 6 | 2 | +0.29 | 4 | 6 | 3 |
| +0.19 | 6 | 5 | 1 | +0.40 | 6 | 5 | 2 | +0.32 | 6 | 4 | 3 |
| Sample | S, μVK−1 | σ, Scm−1 | κL, Wm−1K−1 | p, cm−3 | µ, cm2V−1s−1 | m*/me |
|---|---|---|---|---|---|---|
| 1 day (Phase III) | 421 | 1.53 | 0.28 | 1.08 × 1018 | 8.7 | 0.9 |
| 2 days (Phase III) | 428 | 1.78 | 0.32 | 1.32 × 1018 | 8.5 | 0.9 |
| 5 days (Phase III) | 418 | 1.28 | 0.24 | 1.09 × 1018 | 7.4 | 0.9 |
| 10 days (Phase III) | 440 | 1.07 | 0.28 | 1.06 × 1018 | 6.5 | 0.9 |
| Cu12Sb4S12 (Phase I) [57] | 166 | 5.4 | 0.30 | 9.1 × 1019 | 3.7 | 0.68 |
| Cu12Sb4S13 (Phase II) [56] | 71 | 1030 | 0.85 | 4.1 × 1020 | 15.7 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazakowny, K.; Kosonowski, A.; Lis, A.; Cherniushok, O.; Parashchuk, T.; Tobola, J.; Wojciechowski, K.T. Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis. Materials 2022, 15, 849. https://doi.org/10.3390/ma15030849
Zazakowny K, Kosonowski A, Lis A, Cherniushok O, Parashchuk T, Tobola J, Wojciechowski KT. Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis. Materials. 2022; 15(3):849. https://doi.org/10.3390/ma15030849
Chicago/Turabian StyleZazakowny, Karolina, Artur Kosonowski, Adrianna Lis, Oleksandr Cherniushok, Taras Parashchuk, Janusz Tobola, and Krzysztof T. Wojciechowski. 2022. "Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis" Materials 15, no. 3: 849. https://doi.org/10.3390/ma15030849
APA StyleZazakowny, K., Kosonowski, A., Lis, A., Cherniushok, O., Parashchuk, T., Tobola, J., & Wojciechowski, K. T. (2022). Phase Analysis and Thermoelectric Properties of Cu-Rich Tetrahedrite Prepared by Solvothermal Synthesis. Materials, 15(3), 849. https://doi.org/10.3390/ma15030849