Color, Starch Digestibility, and In Vitro Fermentation of Roasted Highland Barley Flour with Different Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sand Roasting and Milling
2.3. Particle Size Analysis
2.4. Flour Analysis
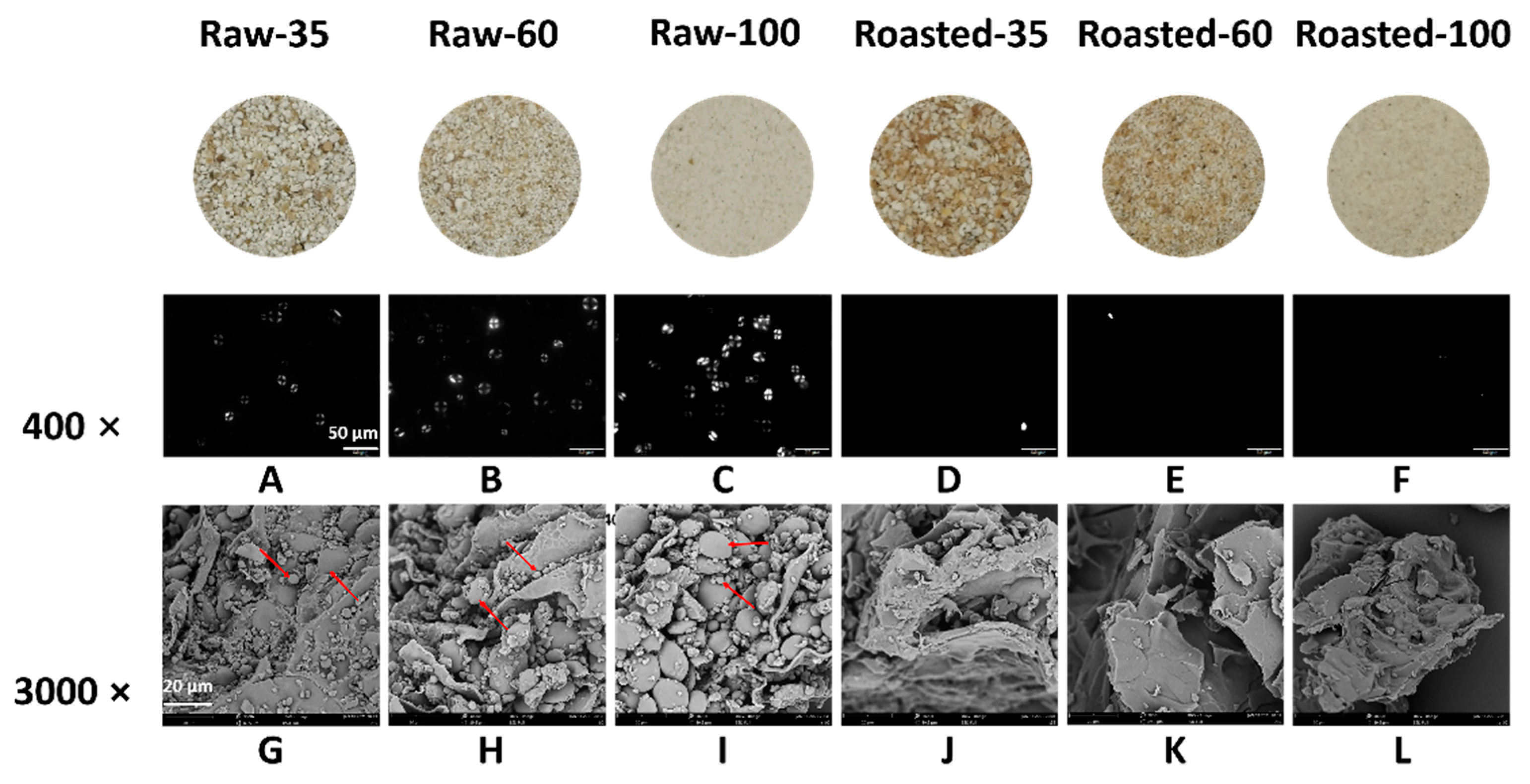
2.5. Microscope Observation
2.6. Laser Confocal Micro-Raman Spectroscopy
2.7. X-ray Diffraction (XRD)
2.8. Differential Scanning Calorimetry (DSC)
2.9. In Vitro Simulated Saliva–Gastrointestinal Digestion
2.10. In Vitro Simulated Fermentation
2.11. Determination of pH and Short-Chain Fatty Acids
2.12. Data Analysis
3. Results and Discussion
3.1. Physiochemical Analysis
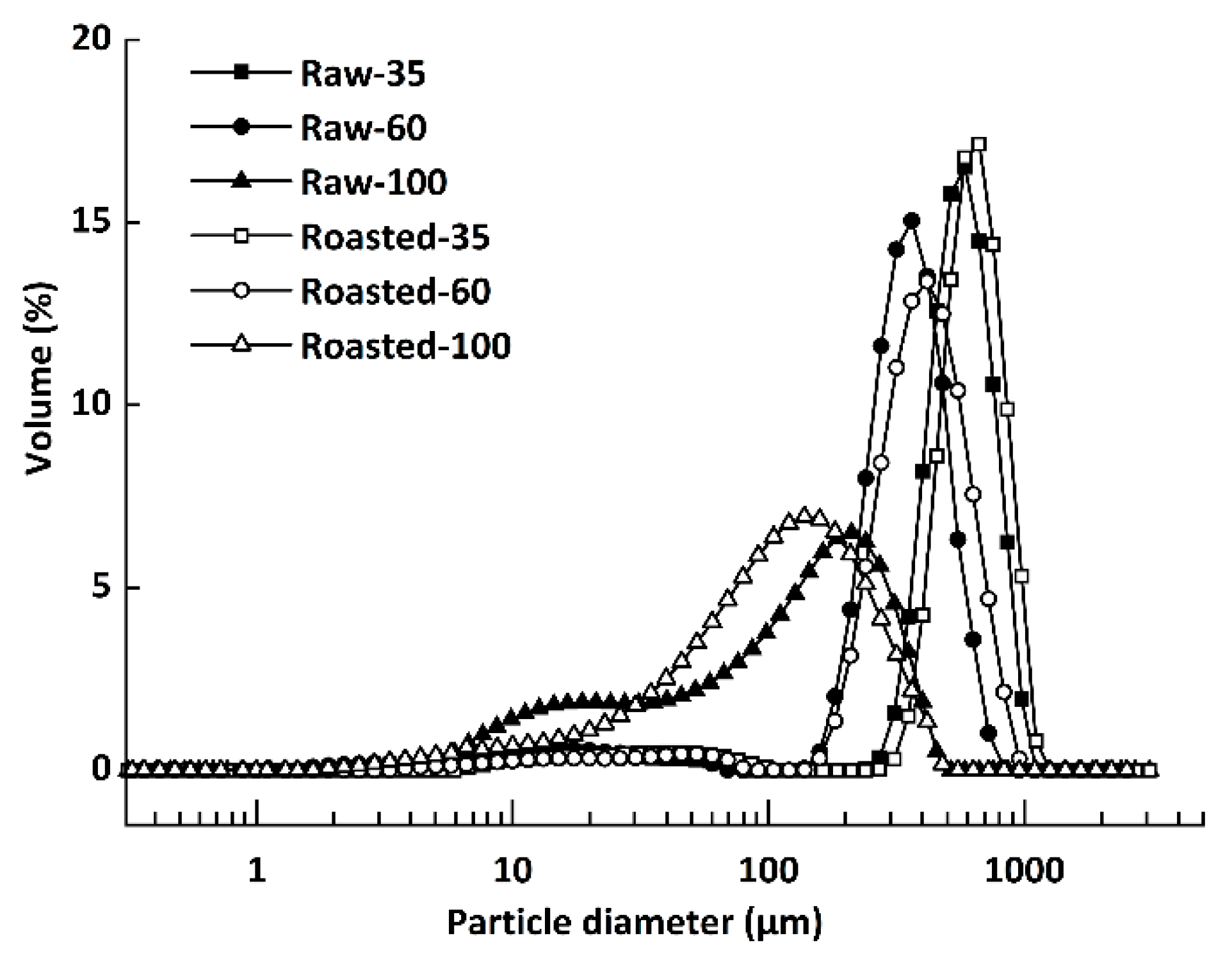
3.1.1. Particle Size Analysis, Morphological Analysis, and Color Values
3.1.2. Proximate Analysis
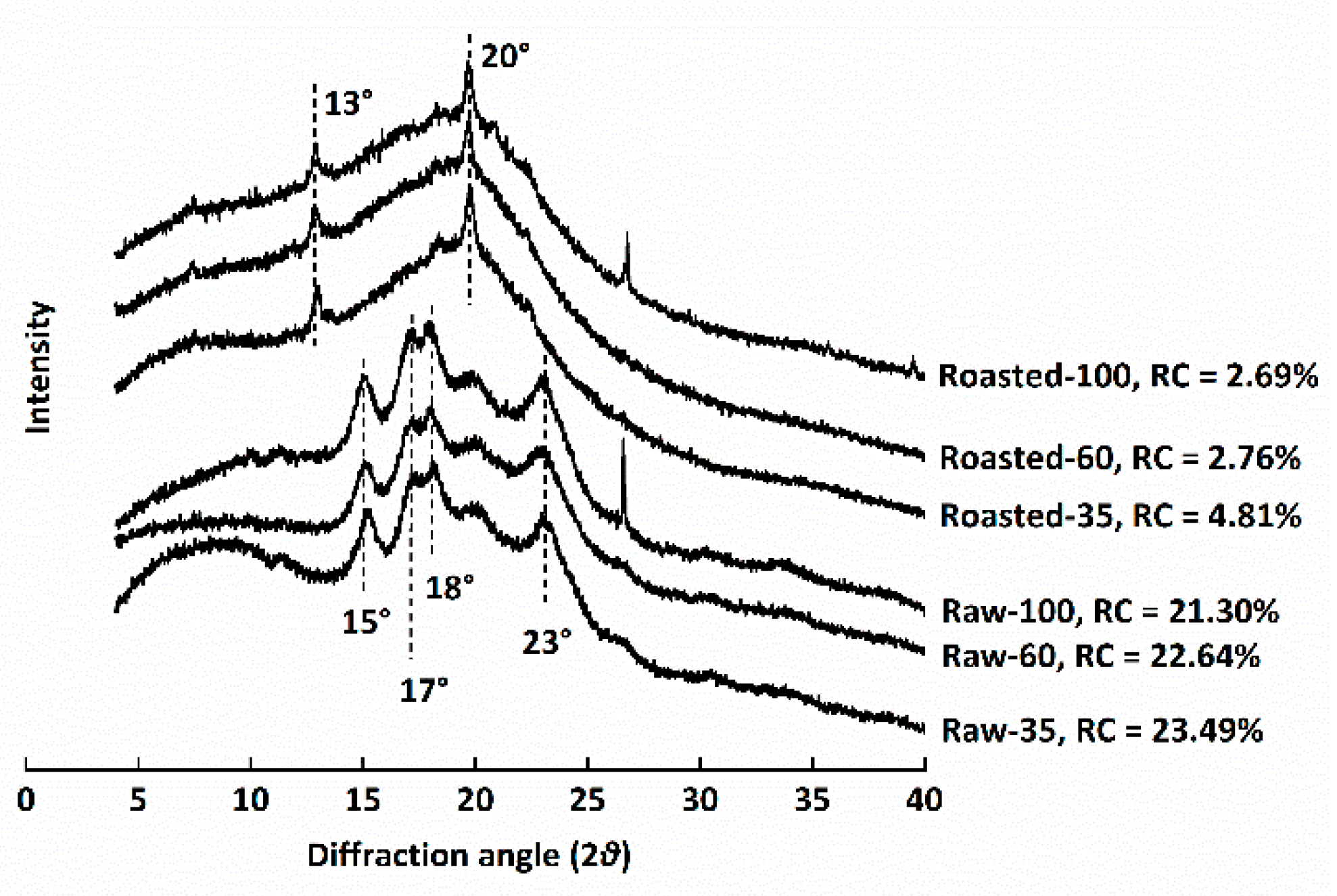
3.1.3. Short-Range Ordered Molecular Structure and Crystalline Structure
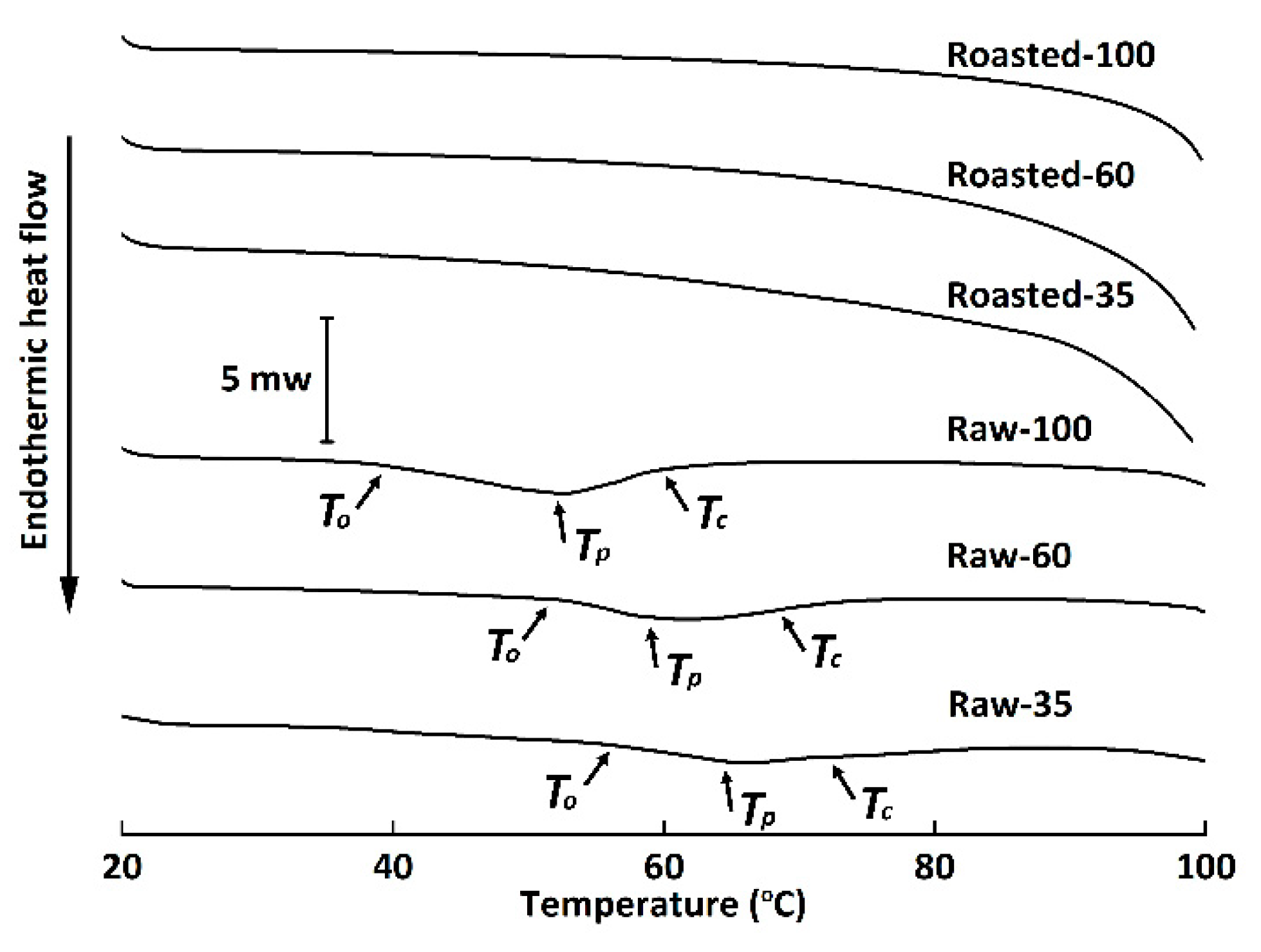
3.2. Thermal Properties
3.3. Digestion and Fermentation Properties
3.3.1. Digestion
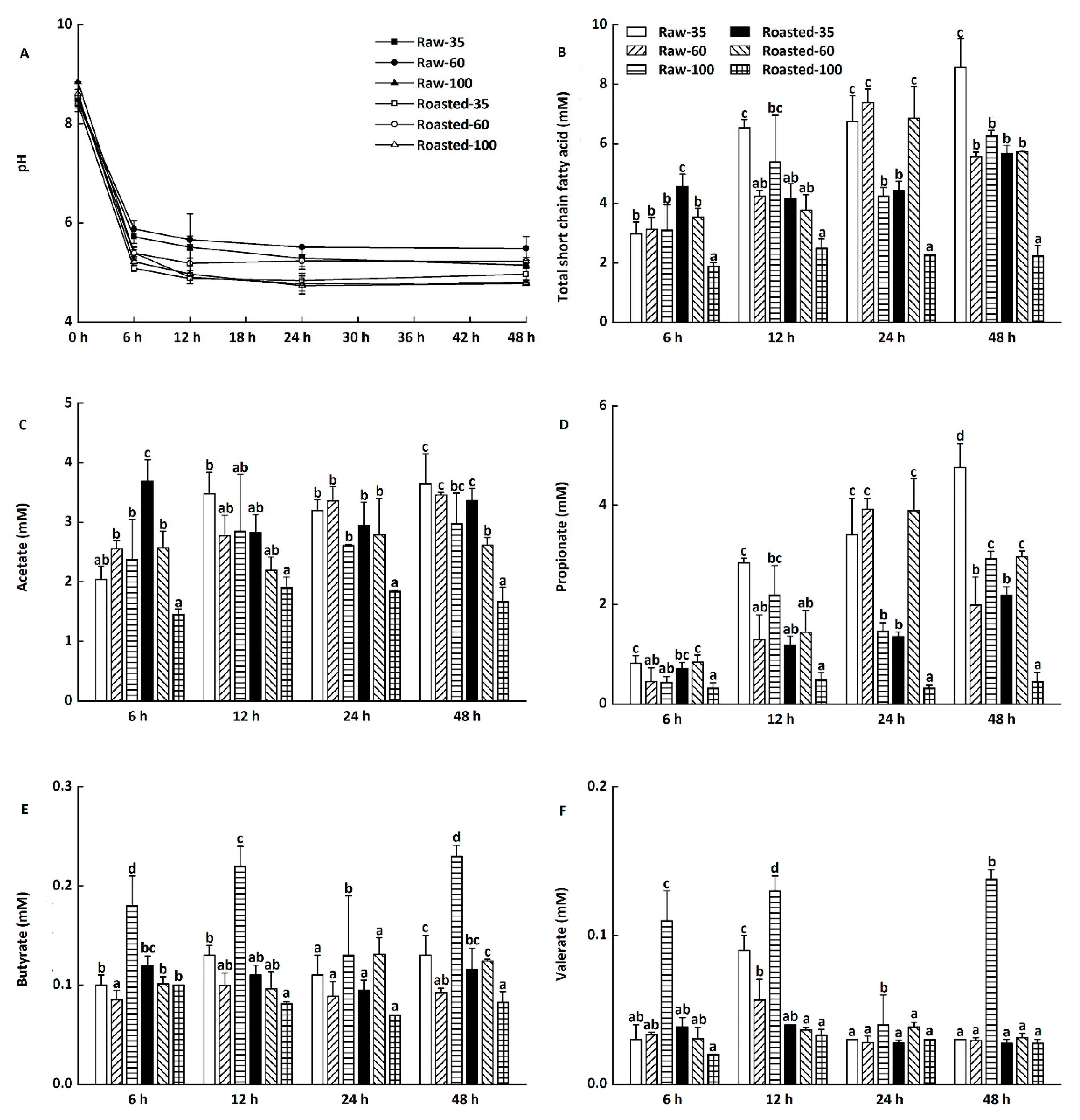
3.3.2. Fermentation
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, Z.; Jiang, H.; Zha, F.; Manthey, F.; Rao, J.; Chen, B. Toward a comprehensive understanding of ultracentrifugal milling on the physicochemical properties and aromatic profile of yellow pea flour. Food Chem. 2021, 345, 128760. [Google Scholar] [CrossRef]
- Martin-Garcia, B.; Pasini, F.; Verardo, V.; Gomez-Caravaca, A.M.; Marconi, E.; Caboni, M.F. Distribution of free and bound phenolic compounds in buckwheat milling fractions. Foods 2019, 8, 670. [Google Scholar] [CrossRef] [Green Version]
- Martín-García, B.; Verardo, V.; Diaz de Cerio, E.; Razola-Díaz, M.D.C.; Messia, M.C.; Marconi, E.; Gómez-Caravaca, A.M. Air classification as a useful technology to obtain phenolics-enriched buckwheat flour fractions. LWT 2021, 150, 111893. [Google Scholar] [CrossRef]
- Roman, L.; Gomez, M.; Li, C.; Hamaker, B.R.; Martinez, M.M. Biophysical features of cereal endosperm that decrease starch digestibility. Carbohydr. Polym. 2017, 165, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandal, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Li, L.; Hao, C.; Zheng, X.; Bian, K.; Zhang, J.; Wang, X. Effects of different milling processes on whole wheat flour quality and performance in steamed bread making. LWT 2015, 62, 310–318. [Google Scholar] [CrossRef]
- Al-Rabadi, G.J.; Torley, P.J.; Williams, B.A.; Bryden, W.L.; Gidley, M.J. Particle size heterogeneity in milled barley and sorghum grains: Effects on physico-chemical properties and starch digestibility. J. Cereal Sci. 2012, 56, 396403. [Google Scholar] [CrossRef]
- Farooq, A.M.; Li, C.; Chen, S.; Fu, X.; Zhang, B.; Huang, Q. Particle size affects structural and in vitro digestion properties of cooked rice flours. Int. J. Biol. Macromol. 2018, 118, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.H.; Grundy, M.M.; Grassby, T.; Vasilopoulou, D.; Frost, G.S.; Butterworth, P.J.; Berry, S.E.; Sanderson, J.; Ellis, P.R. Manipulation of starch bioaccessibility in wheat endosperm to regulate starch digestion, postprandial glycemia, insulinemia, and gut hormone responses: A randomized controlled trial in healthy ileostomy participants. Am. J. Clin. Nutr. 2015, 102, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Hera, E.; Talegon, M.; Caballero, P.; Gomez, M. Influence of maize flour particle size on gluten-free breadmaking. J. Sci. Food Agric. 2013, 93, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Lee, S.M.; Shim, J.-H.; Yoo, S.-H.; Lee, S. Effect of dry- and wet-milled rice flours on the quality attributes of gluten-free dough and noodles. J. Food Eng. 2013, 116, 213–217. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Starch digestibility and bioactivity of high altitude hulless barley. Food Chem. 2016, 194, 561–568. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhong, S.; Tang, Y.; Chen, L. Understanding the nutritional functions of thermally-processed whole grain highland barley in vitro and in vivo. Food Chem. 2020, 310, 125979. [Google Scholar] [CrossRef]
- Deng, N.; He, Z.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. Highland barley whole grain (Hordeum vulgare L.) ameliorates hyperlipidemia by modulating cecal microbiota, miRNAs, and AMPK pathways in leptin receptor-deficient db/db mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, S.; Li, S.; Pang, X.; Sun, J.; Zhu, X.; Lv, F.; Lu, Z.; Li, X. β-Glucan extracted from highland barley alleviates dextran sulfate sodium-induced ulcerative colitis in C57BL/6J mice. Molecules 2021, 26, 5812. [Google Scholar] [CrossRef]
- Zhao, B.; Shang, J.; Wang, L.; Liu, L.; Tong, L.; Zhou, X.; Wang, S.; Zhang, Y.; Zhou, S. Evaluation of ingredient mixing procedure on quality characteristics of noodles enriched with half hulless barley flour. Int. J. Food Sci. Technol. 2020, 55, 3350–3360. [Google Scholar] [CrossRef]
- Cakir, E.; Arici, M.; Durak, M.Z. Effect of starter culture sourdough prepared with Lactobacilli and Saccharomyces cerevisiae on the quality of hull-less barley-wheat bread. LWT 2021, 152, 112230. [Google Scholar] [CrossRef]
- Martínez-Subirà, M.; Romero, M.P.; Puig, E.; Macià, A.; Romagosa, I.; Moralejo, M. Purple, high β-glucan, hulless barley as valuable ingredient for functional food. LWT 2020, 131, 109582. [Google Scholar] [CrossRef]
- Deng, X.Q.; Pan, Z.F.; Li, Q.; Deng, G.B.; Long, H.; Tashi, N.; Zhao, Y.; Yu, M.Q. Nutritional components, in vitro digestibility, and textural properties of cookies made from whole hull-less barley. Cereal Chem. 2019, 97, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Narwal, S.; Kumar, D.; Sheoran, S.; Verma, R.P.S.; Gupta, R.K. Hulless barley as a promising source to improve the nutritional quality of wheat products. J. Food Sci. 2017, 54, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Kaur, M. Quantification of phenolic acids and antioxidant potential of wheat rusks as influenced by partial replacement with barley flour. J. Food Sci. 2020, 57, 3782–3791. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhang, C.; Sun-Waterhouse, D.; Li, B.-S.; Li, D.-D. Chiffon cakes made using wheat flour with/without substitution by highland barley powder or mung bean flour: Correlations among ingredient heat absorption enthalpy, batter rheology, and cake porosity. Food Bioproc. Technol. 2019, 12, 1232–1243. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. Available online: https://pubmed.ncbi.nlm.nih.gov/1330528/ (accessed on 25 November 2021).
- Srivastava, A.; Greenspan, P.; Hartle, D.K.; Hargrove, J.L.; Amarowicz, R.; Pegg, R.B. Antioxidant and anti-inflammatory activities of polyphenolics from Southeastern, U.S. range blackberry cultivars. J. Agric. Food Chem. 2010, 58, 6102–6109. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Zhang, X.; Copeland, L.; Wang, S. Retrogradation enthalpy does not always reflect the retrogradation behavior of gelatinized starch. Sci. Rep. 2016, 6, 20965. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Sun, Y.; Ye, H.; Hu, B.; Zeng, X. Influences of structures of galactooligosaccharides and fructooligosaccharides on the fermentation in vitro by human intestinal microbiota. J. Funct. 2015, 13, 158–168. [Google Scholar] [CrossRef]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef]
- Protonotariou, S.; Drakos, A.; Evageliou, V.; Ritzoulis, C.; Mandala, I. Sieving fractionation and jet mill micronization affect the functional properties of wheat flour. J. Food Eng. 2014, 134, 24–29. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019, 295, 138–146. [Google Scholar] [CrossRef]
- Sakhare, S.D.; Inamdar, A.A.; Soumya, C.; Indrani, D.; Rao, G.V. Effect of flour particle size on microstructural, rheological and physico-sensory characteristics of bread and south Indian parotta. J. Food Sci. 2014, 51, 4108–4113. [Google Scholar] [CrossRef] [Green Version]
- Dutta, H.; Mahanta, C.L.; Singh, V.; Das, B.B.; Rahman, N. Physical, physicochemical and nutritional characteristics of Bhoja chaul, a traditional ready-to-eat dry heat parboiled rice product processed by an improvised soaking technique. Food Chem. 2016, 191, 52–62. [Google Scholar] [CrossRef]
- Guo, P.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. Effects of particle size and water content during cooking on the physicochemical properties and in vitro starch digestibility of milled durum wheat grains. Food Hydrocoll. 2018, 77, 445–453. [Google Scholar] [CrossRef]
- Zhou, W.; Song, J.; Zhang, B.; Zhao, L.; Hu, Z.; Wang, K. The impacts of particle size on starch structural characteristics and oil-binding ability of rice flour subjected to dry heating treatment. Carbohydr. Polym. 2019, 223, 115053. [Google Scholar] [CrossRef]
- Ahmed, J.; Taher, A.; Mulla, M.Z.; Al-Hazza, A.; Luciano, G. Effect of sieve particle size on functional, thermal, rheological and pasting properties of Indian and Turkish lentil flour. J. Food Eng. 2016, 186, 34–41. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Hu, X. Isolated pea resistant starch substrates with different structural features modulate the production of short-chain fatty acids and metabolism of microbiota in anaerobic fermentation in vitro. J. Agric. Food Chem. 2021, 69, 5392–5404. [Google Scholar] [CrossRef]
- Schlormann, W.; Atanasov, J.; Lorkowski, S.; Dawczynski, C.; Glei, M. Study on chemopreventive effects of raw and roasted beta-glucan-rich waxy winter barley using an in vitro human colon digestion model. Food Funct. 2020, 11, 2626–2638. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. In vitro fermentation of oat flours from typical and high beta-glucan oat lines. J. Agric. Food Chem. 2009, 57, 7529–7536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, J.; Watanabe, K.; Taira, S.; Kasubuchi, M.; Li, X.; Irie, J.; Itoh, H.; Kimura, I. Barley beta-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS ONE 2018, 13, e0196579. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Raw-35 | Raw-60 | Raw-100 | Roasted-35 | Roasted-60 | Roasted-100 | |
|---|---|---|---|---|---|---|
| % of wet basis | ||||||
| Moisture | 10.7 ± 0.1 b | 10.9 ± 0.2 b | 10.9 ± 0.0 b | 5.2 ± 0.9 a | 5.7 ± 0.9 a | 5.1 ± 0.2 a |
| % of dry basis | ||||||
| Crude lipid | 3.9 ± 0.6 a | 4.0 ± 0.0 a | 4.7 ± 0.2 a | 4.1 ± 0.3 a | 4.4 ± 0.3 a | 3.9 ± 0.2 a |
| Crude protein | 7.4 ± 0.2 b | 7.0 ± 0.2 b | 6.7 ± 0.3 a | 7.5 ± 0.3 b | 7.4 ± 0.7 b | 6.5 ± 0.0 a |
| Total starch | 56.5 ± 0.9 a | 60.3 ± 2.1 bc | 67.0 ± 1.4 d | 53.5 ± 1.4 a | 55.2 ± 1.1 a | 62.6 ± 2.7 c |
| RDS | 0.0 ± 0.0 a | 1.5 ± 0.1 ab | 4.1 ± 2.4 bc | 6.1 ± 0.1 c | 15.0 ± 2.3 d | 21.1 ± 0.9 e |
| SDS | 2.8 ± 0.2 a | 6.7 ± 0.5 b | 13.3 ± 2.0 c | 25.7 ± 1.1 d | 24.4 ± 0.2 d | 30.6 ± 1.3 e |
| RS | 53.7 ± 0.2 e | 52.0 ± 0.7 e | 49.6 ± 0.4 d | 21.7 ± 0.9 c | 15.8 ± 2.0 b | 10.9 ± 0.4 a |
| TDF | 24.6 ± 0.1 b | 24.2 ± 0.9 b | 15.8 ± 0.8 a | 23.7 ± 0.5 b | 25.1 ± 0.4 b | 17.9 ± 1.8 a |
| SDF | 7.9 ± 0.1 a | 8.8 ± 1.2 a | 6.8 ± 0.5 a | 7.7 ± 0.5 a | 8.2 ± 1.3 a | 8.8 ± 1.9 a |
| IDF | 16.7 ± 0.3 c | 15.4 ± 0.3 a | 9.0 ± 0.3 a | 16.1 ± 0.5 bc | 16.8 ± 1.0 c | 9.1 ± 0.2 a |
| β-glucan | 5.5 ± 0.3 c | 5.4 ± 0.4 c | 2.8 ± 0.1 a | 4.9 ± 0.1 b | 4.7 ± 0.2 b | 4.5 ± 0.2 bc |
| Polyphenol (mg/100 g dry basis) | ||||||
| TP | 262.6 ± 1.6 e | 169.7 ± 13.0 c | 148.7 ± 5.0 b | 231.0 ± 12.5 d | 174.3 ± 4.1 c | 122.5 ± 4.2 a |
| FP | 191.5 ± 1.1 e | 121.9 ± 11.0 c | 96.1 ± 6.3 b | 170.8 ± 10.7 d | 123.0 ± 2.7 c | 76.5 ± 4.3 a |
| BP | 71.1 ± 0.4 b | 47.8 ± 2.0 a | 52.6 ± 1.8a | 60.2 ± 12.3 ab | 51.3 ± 1.5 a | 46.0 ± 0.1 a |
| Color values | ||||||
| Lightness (L*) | 86.3 ± 1.3 c | 94.1 ± 1.2 d | 94.7 ± 0.2 d | 77.1 ± 1.1a | 79.6 ± 0.4 b | 86.8 ± 0.1 c |
| Redness (a*) | 3.7 ± 0.2 b | 3.0 ± 0.1a | 2.9 ± 0.1 a | 6.5 ± 0.3 d | 6.2 ± 0.3 d | 4.4 ± 0.1 c |
| Yellowness (b*) | 11.2 ± 0.2 b | 10.0 ± 0.4 a | 9.9 ± 0.0 a | 17.0 ± 0.6 d | 17.0 ± 0.2 d | 14.3 ± 0.1 c |
| Highland Barley Flour Samples | FWHM of the Band at 480 cm−1 |
|---|---|
| Raw-35 | 17.41 ± 0.43 ab |
| Raw-60 | 17.38 ± 0.27 ab |
| Raw-100 | 16.57 ± 0.44 a |
| Roasted-35 | 18.10 ± 1.80 bc |
| Roasted-60 | 18.28 ± 0.59 c |
| Roasted-100 | 18.88 ± 0.89 c |
| Parameter | PS | FWHM | Crys | ΔH | RDS | SDS | RS | SCFAs | Acetate | Propionate | Butyrate | Valerate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | 1.00 | |||||||||||
| FWHM | −0.02 | 1.00 | ||||||||||
| Crys | 0.94 * | 0.14 | 1.00 | |||||||||
| ΔH | −0.66 | −0.56 | −0.63 | 1.00 | ||||||||
| RDS | 0.89 * | 0.39 | −0.84 | 0.24 | 1.00 | |||||||
| SDS | −0.83 | −0.30 | −0.71 | 0.79 | 0.57 | 1.00 | ||||||
| RS | 0.93 * | −0.32 | 0.86 | −0.34 | −0.99 ** | −0.65 | 1.00 | |||||
| SCFAs | 0.80 | −0.35 | 0.56 | −0.54 | −0.71 | −0.73 | 0.76 | 1.00 | ||||
| Acetate | 0.92 * | −0.31 | 0.83 | −0.33 | 0.98 ** | −0.70 | 0.99 ** | 0.77 | 1.00 | |||
| Propionate | 0.66 | −0.26 | 0.39 | −0.60 | −0.48 | −0.69 | 0.56 | 0.96 ** | 0.57 | 1.00 | ||
| Butyrate | −0.22 | −0.88 * | −0.38 | 0.47 | −0.04 | 0.49 | −0.02 | 0.23 | −0.05 | 0.26 | 1.00 | |
| Valerate | −0.56 | −0.76 | −0.63 | 0.71 | 0.25 | 0.78 | −0.33 | −0.16 | −0.36 | −0.11 | 0.92 * | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Ming, J.; Zhao, G.; Lei, L. Color, Starch Digestibility, and In Vitro Fermentation of Roasted Highland Barley Flour with Different Fractions. Foods 2022, 11, 287. https://doi.org/10.3390/foods11030287
Zhao Z, Ming J, Zhao G, Lei L. Color, Starch Digestibility, and In Vitro Fermentation of Roasted Highland Barley Flour with Different Fractions. Foods. 2022; 11(3):287. https://doi.org/10.3390/foods11030287
Chicago/Turabian StyleZhao, Zixuan, Jian Ming, Guohua Zhao, and Lin Lei. 2022. "Color, Starch Digestibility, and In Vitro Fermentation of Roasted Highland Barley Flour with Different Fractions" Foods 11, no. 3: 287. https://doi.org/10.3390/foods11030287







