Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
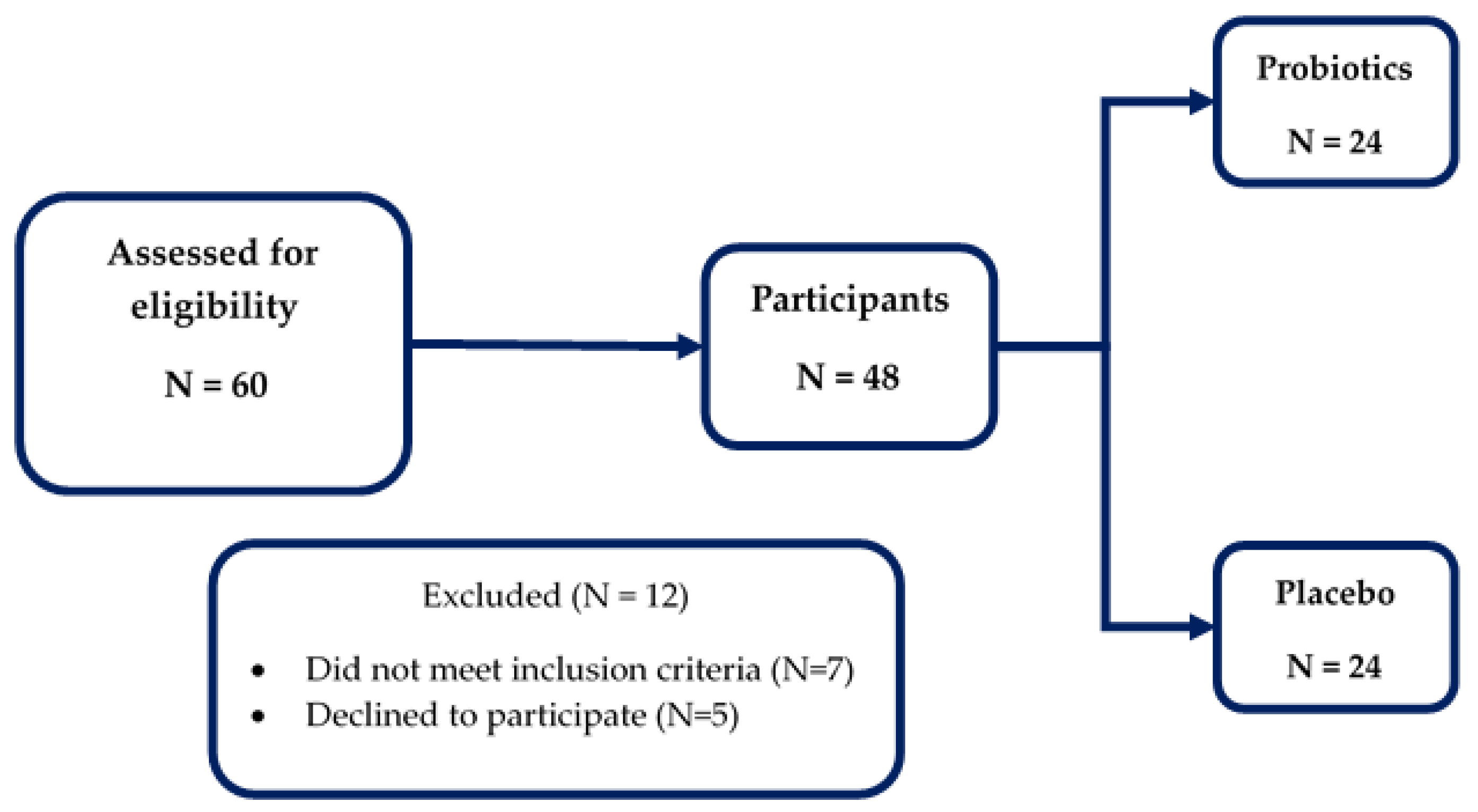
2.1. Study Design and Participants
2.2. Treatment
2.3. Assessments
2.3.1. Clinical Data
2.3.2. Laboratory Data
2.3.3. Statistical Analyses
3. Results
3.1. The Study Participants
3.2. Changes in the Study Parameters within the Group
3.3. Changes in the Study Parameters between the Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dignass, A.U. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Gannon, J.; Walker, W.A. Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 2002, 75, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.K.; Samak, G. Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [PubMed] [Green Version]
- Plaza-Díaz, J.; Solís-Urra, P.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadía-Molina, F.; Álvarez-Mercado, A.I. The gut barrier, intestinal microbiota, and liver disease: Molecular mechanisms and strategies to manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol. Lett. 2010, 309, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making sense of… the microbiome in psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.S. Gut-brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy Sarkar, S.; Mitra Mazumder, P.; Banerjee, S. Probiotics protect against gut dysbiosis associated decline in learning and memory. J. Neuroimmunol. 2020, 348, 577390. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, M.; Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: Towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 2019, 30, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S.; et al. Synbiotic supplementation improves obesity index and metabolic biomarkers in Thai obese adults: A randomized clinical trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Tirawat, Y.; Sivamaruthi, B.S.; Kesika, P.; Thangaleela, S.; Khongtan, S.; Khampithum, N.; Peerajan, S.; Chaiyasut, K.; Sirilun, S.; et al. Effect of Lactobacillus paracasei HII01 supplementation on total cholesterol and on parameters of lipid and carbohydrate metabolism, oxidative stress, inflammation, and digestion in Thai hypercholesterolemic subjects. Appl. Sci. 2021, 11, 4333. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Sequeira, I.R.; Lentle, R.G.; Kruger, M.C.; Hurst, R.D. Standardizing the lactulose mannitol test of gut permeability to minimize error and promote comparability. PLoS ONE 2014, 9, e99256. [Google Scholar] [CrossRef] [Green Version]
- Kotani, A.; Miyaguchi, Y.; Kohama, M.; Ohtsuka, T.; Shiratori, T.; Kusu, F. Determination of short-chain fatty acids in rat and human feces by High-performance liquid chromatography with electrochemical detection. Anal. Sci. 2009, 25, 1007–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef] [PubMed]
- van Krimpen, S.J.; Jansen, F.A.C.; Ottenheim, V.L.; Belzer, C.; van der Ende, M.; van Norren, K. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review-gut permeability as potential treatment target. Nutrients 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.P.; Hsu, C.A.; Hung, W.T.; Chen, M.J. Effects of Lactobacillus paracasei 01 fermented milk beverage on protection of intestinal epithelial cell in vitro. J. Sci. Food Agric. 2016, 96, 2154–2160. [Google Scholar] [CrossRef]
- Saiki, A.; Ishida, Y.; Segawa, S.; Hirota, R.; Nakamura, T.; Kuroda, A. A Lactobacillus mutant capable of accumulating long-chain polyphosphates that enhance intestinal barrier function. Biosci. Biotechnol. Biochem. 2016, 80, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, X.; Hao, S.; Lu, Q.; Zhang, L.; Han, X.; Lu, W. Inhibition of Shigella sonnei-induced epithelial barrier disruption by surface-layer associated proteins of lactobacilli from Chinese fermented food. J. Dairy Sci. 2018, 101, 1834–1842. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kirkland, R.; Grunewald, Z.I.; Sun, Q.; Wicker, L.; de La Serre, C.B. Beneficial effects of non-encapsulated or encapsulated probiotic supplementation on microbiota composition, intestinal barrier functions, inflammatory profiles, and glucose tolerance in high fat fed rats. Nutrients 2019, 11, 1975. [Google Scholar] [CrossRef] [Green Version]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; Desclée de Maredsous, C.; Capronnier, S.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.E.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.; Nighot, P.; Nighot, M.; Haque, M.; Rawat, M.; Ma, T.Y. Lactobacillus acidophilus induces a strain-specific and toll-like receptor 2-dependent enhancement of intestinal epithelial tight junction barrier and protection against intestinal inflammation. Am. J. Pathol. 2021, 191, 872–884. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de Los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: A cross-sectional study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Zhao, S.; Li, D.; Chang, F.; Tian, X.; Huang, G.; Zhu, Z.; Liu, D.; Dou, X.; Li, S.; et al. Nutrient intake is associated with longevity characterization by metabolites and element profiles of healthy centenarians. Nutrients 2016, 8, 564. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Binetti, A.; Gueimonde, M.; Alonso, A.; Garrido, P.; Gonzalez del Rey, C.; de los Reyes-Gavilan, C.G.; Gonzalez, C.; Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G. Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium longum IPLA E44 orally administered to Wistar rats. Int. J. Food Microbiol. 2011, 144, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic Lactobacillus plantarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Van den Abbeele, P.; Basit, A.W.; Dodoo, D.; Chatterjee, R.; Smith, B.; Gaisford, S. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Int. J. Pharm. 2019, 555, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: A randomized clinical trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; El Aidy, S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef] [Green Version]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; De La Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar] [PubMed]
- Kirpich, I.A.; Solovieva, N.V.; Leikhter, S.N.; Shidakova, N.A.; Lebedeva, O.V.; Sidorov, P.I.; Bazhukova, T.A.; Soloviev, A.G.; Barve, S.S.; McClain, C.J.; et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol 2008, 42, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagundes, R.A.B.; Soder, T.F.; Grokoski, K.C.; Benetti, F.; Mendes, R.H. Probiotics in the treatment of chronic kidney disease: A systematic review. J. Bras. Nefrol. 2018, 40, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Jia, Q.; Yang, J.; Jia, R.; Zhang, H. Efficacy of probiotics supplementation on chronic kidney disease: A systematic review and meta-analysis. Kidney Blood Press. Res. 2018, 43, 1623–1635. [Google Scholar] [CrossRef]

| Parameters | Placebo Group (n = 24) | Probiotics Group (n = 24) | p-Value |
|---|---|---|---|
| Age, years | 58.79 ± 1.21 | 61.63 ± 0.84 | 0.061 |
| Male, n (%) Female, n (%) | 7 (29.17) 17 (70.83) | 3 (12.50) 21 (87.50) | 0.286 |
| Smoking | 2 (8.33) | 3 (12.50) | 1.000 |
| Alcoholic | 2 (8.33) | 1 (4.17) | 1.000 |
| Height, cm | 154.07 ± 1.57 | 153.40 ± 1.02 | 0.722 |
| Body mass index, kg/m2 | 25.13 ± 0.66 | 23.95 ± 0.39 | 0.136 |
| BMR, kcal | 1287.96 ± 32.58 | 1220.27 ± 21.49 | 0.090 |
| Body fat, % | 29.06 ± 1.57 | 29.19 ± 2.43 | 0.965 |
| Visceral fat, % | 11.00 ± 0.90 | 9.38 ± 0.52 | 0.129 |
| Muscle, % | 66.03 ± 1.62 | 69.57 ± 1.20 | 0.086 |
| Body age, years | 59.88 ± 1.83 | 60.57 ± 1.03 | 0.747 |
| Arm circumference, cm | 28.87 ± 0.59 | 28.25 ± 2.75 | 0.827 |
| Waist circumference, cm | 86.46 ± 1.74 | 84.77 ± 1.23 | 0.433 |
| Hip circumference, cm | 97.32 ± 1.23 | 94.63 ± 1.11 | 0.112 |
| Parameters | Placebo (n = 24) | p-Value | Probiotics (n = 24) | p-Value | ||
|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | |||
| Body mass index, kg/m2 | 25.13 ± 0.66 | 24.53 ± 1.41 | 0.611 | 23.95 ± 0.39 | 23.38 ± 0.34 | 0.010 * |
| BMR, kcal | 1287.96 ± 32.58 | 1280.05 ± 28.70 | 0.604 | 1220.27 ± 21.49 | 1233.36 ± 19.21 | 0.043 * |
| Body fat, % | 29.06 ± 1.57 | 32.41 ± 1.08 | <0.001 * | 29.19 ± 2.43 | 27.72 ± 1.03 | 0.563 |
| Visceral fat, % | 11.00 ± 0.90 | 11.48 ± 0.53 | 0.397 | 9.38 ± 0.52 | 9.14 ± 0.48 | 0.234 |
| Muscle, % | 66.03 ± 1.62 | 62.91 ± 0.61 | 0.079 | 69.57 ± 1.20 | 70.36 ± 1.26 | 0.188 |
| Body age, years | 59.88 ± 1.83 | 60.75 ± 1.21 | 0.544 | 60.57 ± 1.03 | 60.96 ± 0.94 | 0.464 |
| Arm circumference, cm | 28.87 ± 0.59 | 28.92 ± 0.61 | 0.883 | 28.25 ± 2.75 | 26.87 ± 0.48 | 0.610 |
| Waist circumference, cm | 86.46 ± 1.74 | 88.00 ± 1.60 | 0.099 | 84.77 ± 1.23 | 81.99 ± 1.26 | 0.011 * |
| Hip circumference, cm | 97.32 ± 1.23 | 99.20 ± 1.25 | 0.064 | 94.63 ± 1.11 | 87.87 ± 3.49 | 0.049 * |
| BUN, mg/dL | 13.07 ± 0.58 | 13.30 ± 0.58 | 0.622 | 13.81 ± 0.87 | 13.63 ± 0.99 | 0.811 |
| Creatinine, mg/dL | 0.81 ± 0.03 | 0.82 ± 0.03 | 0.177 | 0.87 ± 0.04 | 0.83 ± 0.03 | 0.013 * |
| AST, IU/L | 23.96 ± 1.85 | 27.57 ± 3.57 | 0.352 | 21.60 ± 1.29 | 19.70 ± 1.00 | 0.013 * |
| ALT, IU/L | 20.58 ± 1.68 | 23.42 ± 3.36 | 0.840 | 19.35 ± 1.67 | 16.25 ± 1.71 | 0.002 * |
| Total cholesterol, mg/dL | 215.57 ± 8.48 | 206.35 ± 10.27 | 0.234 | 226.35 ± 9.66 | 217.80 ± 8.02 | 0.229 |
| HDL-cholesterol, mg/dL | 51.61 ± 1.76 | 48.22 ± 2.12 | 0.074 | 53.25 ± 2.86 | 56.65 ± 2.78 | <0.001 * |
| Triglyceride, mg/dL | 141.52 ± 11.81 | 157.17 ± 13.80 | 0.330 | 163.55 ± 21.36 | 147.40 ± 20.35 | 0.332 |
| LDL-cholesterol, mg/dL | 136.57 ± 8.02 | 130.10 ± 8.62 | 0.367 | 145.46 ± 7.46 | 126.60 ± 6.83 | 0.001 * |
| FBS, mg/dL | 99.87 ± 5.85 | 107.09 ± 6.84 | 0.130 | 106.53 ± 8.03 | 98.79 ± 7.79 | 0.021 * |
| IgA, ng/mL | 739.44 ± 80.41 | 790.20 ± 79.52 | 0.200 | 881.79 ± 50.35 | 1172.34 ± 50.53 | <0.001 * |
| LPS, pg/mL | 112.62 ± 16.22 | 94.14 ± 10.97 | 0.114 | 99.08 ± 5.10 | 39.82 ± 4.76 | 0.001 * |
| hsCRP, ml/L | 0.0087 ± 0.0014 | 0.0141 ± 0.0017 | 0.059 | 0.0117 ± 0.0046 | 0.0060 ± 0.0020 | 0.201 |
| Lactulose–Mannitol ratio | 0.156 ± 0.026 | 0.113 ± 0.017 | 0.052 | 0.222 ± 0.036 | 0.047 ± 0.004 | <0.001 * |
| Lactulose | 0.1292 ± 0.0248 | 0.0789 ± 0.0165 | 0.002 * | 0.0023 ± 0.0003 | 0.0013 ± 0.0002 | 0.006 * |
| QA, ng/mL | 28.38 ± 1.83 | 26.49 ± 1.21 | 0.513 | 29.84 ± 0.87 | 19.47 ± 0.83 | <0.001 * |
| 5-HIAA, mg/L | 3.17 ± 1.12 | 4.94 ± 1.85 | 0.463 | 8.04 ± 2.06 | 8.73 ± 1.38 | 0.551 |
| QA/5-HIAA ratio | 0.0145 ± 0.0038 | 0.0092 ± 0.0025 | 0.463 | 0.0056 ± 0.0009 | 0.0036 ± 0.0007 | 0.121 |
| Lactic acid, mmol/g sample | 232.96 ± 144.52 | 78.58 ± 22.84 | 0.593 | 48.22 ± 8.79 | 96.74 ± 23.06 | 0.066 |
| Acetic acid, mmol/g sample | 45.11 ± 0.20 | 37.95 ± 1.40 | 0.180 | 37.07 ± 5.25 | 26.94 ± 5.66 | 0.128 |
| Propionic, mmol/g sample | 413.81 ± 74.29 | 694.21 ± 216.16 | 0.225 | 411.97 ± 28.18 | 682.59 ± 90.31 | 0.012 * |
| Butyric acid, mmol/g sample | 5.67 ± 1.02 | 7.47 ± 2.27 | 0.913 | 14.62 ± 5.74 | 63.45 ± 15.60 | 0.046 * |
| Parameters | Baseline—12 Weeks | p-Value | |
|---|---|---|---|
| Placebo (n = 24) | Probiotics (n = 24) | ||
| Body mass index, kg/m2 | −0.59 | −0.57 | <0.001 * |
| BMR, kcal | −7.91 | 13.09 | 0.518 |
| Body fat, % | 3.35 | −1.47 | 0.016 * |
| Visceral fat, % | 0.48 | −0.24 | 0.621 |
| Muscle, % | −3.13 | 0.80 | 0.022 * |
| Body age, years | 0.88 | 0.39 | 0.324 |
| Arm circumference, cm | 0.05 | −1.38 | 0.137 |
| Waist circumference, cm | 1.55 | −2.78 | 0.001 * |
| Hip circumference, cm | 1.88 | −6.77 | 0.001 * |
| BUN, mg/dL | 0.23 | −0.18 | 0.752 |
| Creatinine, mg/dL | 0.02 | −0.04 | 0.001 * |
| AST, IU/L | 3.61 | −1.90 | 0.024 * |
| ALT, IU/L | 2.84 | −3.10 | 0.055 |
| Total cholesterol, mg/dL | −9.22 | −8.55 | 0.670 |
| HDL-cholesterol, mg/dL | −3.39 | 3.40 | 0.001 * |
| Triglyceride, mg/dL | 15.65 | −16.15 | 0.154 |
| LDL-cholesterol, mg/dL | −6.46 | −18.86 | 0.173 |
| FBS, mg/dL | 7.22 | −7.74 | 0.001 * |
| IgA, ng/mL | 50.76 | 290.55 | <0.001 * |
| LPS, pg/mL | −18.48 | −59.26 | 0.001 * |
| hsCRP, ml/L | 0.005 | −0.006 | 0.029 * |
| Lactulose–Mannitol ratio | −0.04 | −0.18 | 0.001 * |
| Lactulose | −0.0502 | −0.0010 | 0.025 * |
| QA, ng/mL | −1.89 | −10.36 | 0.008 * |
| 5-HIAA, mg/L | 1.77 | 0.69 | 0.837 |
| QA/5-HIAA ratio | −0.005 | −0.002 | 0.461 |
| Lactic acid, mmol/g sample | −154.38 | 48.53 | 0.079 |
| Acetic acid, mmol/g sample | −7.16 | −10.12 | 0.558 |
| Propionic, mmol/g sample | 280.40 | 270.62 | 0.965 |
| Butyric acid, mmol/g sample | 1.79 | 48.83 | 0.014 * |
| Parameter | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Body mass index, kg/m2 | −0.86 | −4.35 to 2.62 | 0.612 |
| BMR, kcal | −9.38 | −35.21 to 16.45 | 0.458 |
| Body fat, % | −3.65 | −4.76 to −2.54 | <0.001 * |
| Visceral fat, % | −0.84 | −1.41 to −0.28 | 0.006 * |
| Muscle, % | 4.23 | 1.83 to 6.62 | 0.001 * |
| Body age, years | −2.31 | −4.07 to −0.54 | 0.012 * |
| Arm circumference, cm | −2.35 | −3.99 to −0.70 | 0.007 * |
| Waist circumference, cm | −3.74 | −7.07 to −0.42 | 0.029 * |
| Hip circumference, cm | −5.47 | −9.96 to −0.97 | 0.019 * |
| BUN, mg/dL | −0.75 | −2.89 to 1.38 | 0.477 |
| Creatinine, mg/dL | −0.04 | −0.076 to −0.003 | 0.033 * |
| AST, IU/L | −7.96 | −16.60 to 0.67 | 0.069 |
| ALT, IU/L | −8.27 | −15.56 to −0.99 | 0.028 * |
| Total cholesterol, mg/dL | 6.65 | −15.51 to 28.80 | 0.546 |
| HDL-cholesterol, mg/dL | 7.62 | 2.80 to 12.44 | 0.003 * |
| Triglyceride, mg/dL | −18.13 | −52.47 to 16.22 | 0.290 |
| LDL-cholesterol, mg/dL | −1.42 | −19.92 to 17.09 | 0.877 |
| FBS, mg/dL | −13.63 | −25.72 to −1.54 | 0.028 * |
| IgA, ng/mL | 230.18 | 76.39 to 383.98 | 0.005 * |
| LPS, pg/mL | −58.03 | −82.59 to −33.46 | <0.001 * |
| hsCRP, ml/L | −0.008 | −0.020 to 0.004 | 0.147 |
| Lactulose–Mannitol ratio | −0.08 | −0.12 to −0.04 | 0.001 * |
| Lactulose | −0.004 | −0.030 to 0.021 | 0.733 |
| QA, ng/mL | −6.97 | −10.17 to −3.77 | 0.001 * |
| 5-HIAA, mg/L | 3.43 | −3.81 to 10.68 | 0.307 |
| QA/5-HIAA ratio | −0.01 | −0.02 to −0.01 | 0.002 * |
| Lactic acid, mmol/g sample | 60.65 | −279.10 to 400.41 | 0.610 |
| Acetic acid, mmol/g sample | −9.85 | −89.82 to 70.12 | 0.649 |
| Propionic, mmol/g sample | −19.43 | −466.59 to 427.72 | 0.925 |
| Butyric acid, mmol/g sample | 47.79 | 14.54 to 81.04 | 0.008 * |
| Parameter | Risk Difference | 95% CI | p-Value |
|---|---|---|---|
| Leaky gut | −0.48 | −0.79 to −0.18 | 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. https://doi.org/10.3390/foods11030268
Chaiyasut C, Sivamaruthi BS, Lailerd N, Sirilun S, Khongtan S, Fukngoen P, Peerajan S, Saelee M, Chaiyasut K, Kesika P, et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods. 2022; 11(3):268. https://doi.org/10.3390/foods11030268
Chicago/Turabian StyleChaiyasut, Chaiyavat, Bhagavathi Sundaram Sivamaruthi, Narissara Lailerd, Sasithorn Sirilun, Suchanat Khongtan, Pranom Fukngoen, Sartjin Peerajan, Manee Saelee, Khontaros Chaiyasut, Periyanaina Kesika, and et al. 2022. "Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial" Foods 11, no. 3: 268. https://doi.org/10.3390/foods11030268
APA StyleChaiyasut, C., Sivamaruthi, B. S., Lailerd, N., Sirilun, S., Khongtan, S., Fukngoen, P., Peerajan, S., Saelee, M., Chaiyasut, K., Kesika, P., & Sittiprapaporn, P. (2022). Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods, 11(3), 268. https://doi.org/10.3390/foods11030268










