The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Participants
2.3. Inclusion and Exclusion Criteria
2.4. Randomization and Blinding
2.5. Periodontal Clinical Parameters Measurement
2.6. Outcomes
2.7. Treatment
2.8. Statistical Analysis
3. Results
3.1. MANCOVA Analysis
3.1.1. Assumptions
3.1.2. Mixed Model MANCOVA Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Mariano Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Ong, M.M.A. Minimally Invasive Periodontology: A Treatment Philosophy and Suggested Approach. Int. J. Dent. 2021, 2021, 2810264. [Google Scholar] [CrossRef]
- Kaur, G.; Grover, V.; Bhaskar, N.; Kaur, R.K.; Jain, A. Periodontal infectogenomics. Inflamm. Regen. 2018, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, S.A.; Archila, L.; Foroud, T.; Koller, D.; Eckert, G.J.; Kowolik, M.J. The effect of shared genetic and environmental factors on periodontal disease parameters in untreated adult siblings in Guatemala. J. Periodontol. 2002, 73, 1160–1168. [Google Scholar] [CrossRef]
- Taba, M., Jr.; Souza, S.L.; Mariguela, V.C. Periodontal disease: A genetic perspective. Braz. Oral Res. 2012, 26, 32–38. [Google Scholar] [CrossRef]
- Barros, S.P.; Offenbacher, S. Modifiable risk factors in periodontal disease: Epigenetic regulation of gene expression in the inflammatory response. Periodontology 2000 2014, 64, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs. silk sutures in the healing of postextraction sockets: A split mouth study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef] [Green Version]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 176–198. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Carossa, M.; Ripa, M.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Di Santantonio, G.; Lenoci, G.; Barile, G.; et al. Comparative clinical study between zirconium-ceramic and metal-ceramic fixed rehabilitations. Oral Implantol. 2018, 11, 150–160. [Google Scholar]
- Gandhi, K.K.; Pavaskar, R.; Cappetta, E.G.; Drew, H.J. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019, 39, 837–843. [Google Scholar] [CrossRef]
- Grassi, F.R.; Grassi, R.; Rapone, B.; Gianfranco, A.; Balena, A.; Kalemaj, Z. Dimensional changes of buccal bone plate in immediate implants inserted through open flap, open flap and bone grafting, and flapless technique. A CBCT randomized controlled clinical trial. Clin. Oral Implant. Res. 2019, 30, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Grassi, F.R.; Rapone, B.; Scarano Catanzaro, F.; Corsalini, M.; Kalemaj, Z. Effectiveness of computer-assisted anesthetic delivery system (STA™) in dental implant surgery: A prospective study. Oral Implantol. 2017, 10, 381–389. [Google Scholar] [CrossRef]
- Di Venere, D.; Corsalini, M.; Nardi, G.M.; Laforgia, A.; Grassi, F.R.; Rapone, B.; Pettini, F. Obstructive site localization in patients with Obstructive Sleep Apnea Syndrome: A comparison between otolaryngologic data and cephalometric values. Oral Implantol. 2017, 10, 295–310. [Google Scholar] [CrossRef]
- Gou, H.; Fan, R.; Chen, X.; Li, L.; Wang, X.; Xu, Y.; Svensson, P.; Wang, K. Adjunctive effects of laser therapy on somatosensory function and vasomotor regulation of periodontal tissues in patients with periodontitis: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 1307–1317. [Google Scholar] [CrossRef]
- Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Soares, G.; Teles, R.P.; Fermiano, D.; Duarte, P.M.; Faveri, M. The effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: A 1-year double-blinded, placebocontrolled, randomized clinical trial. J. Clin. Periodontol. 2012, 39, 955–961. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Rapone, B.; Stefanachi, G.; Laforgia, A.; Pettini, F. Evidence of signs and symptoms of Craniomandibular Disorders in Fibromyalgia patients. Open Dent. J. 2017, 11, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Marconcini, S.; Giammarinaro, E.; Cosola, S.; Oldoini, G.; Genovesi, A.; Covani, U. Effects of Non-Surgical Periodontal Treatment on Reactive Oxygen Metabolites and Glycemic Control in Diabetic Patients with Chronic Periodontitis. Antioxidants 2021, 10, 1056. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Limeback, H. The application of ozone in dentistry: A systematic review of literature. J. Dent. 2008, 36, 104–116. [Google Scholar] [CrossRef]
- Cosola, S.; Giammarinaro, E.; Genovesi, A.M.; Pisante, R.; Poli, G.; Covani, U.; Marconcini, S. A short-term study of the effects of ozone irrigation in an orthodontic population with fixed appliances. Eur. J. Paediatr. Dent. 2019, 20, 15–18. [Google Scholar]
- Gupta, G.; Mansi, B. Ozone therapy in periodontics. J. Med. life 2012, 5, 59. [Google Scholar]
- Di Venere, D.; Nardi, G.M.; Lacarbonara, V.; Laforgia, A.; Stefanachi, G.; Corsalini, M.; Grassi, F.R.; Rapone, B.; Pettini, F. Early mandibular canine-lateral incisor transposition: Case Report. Oral Implantol. 2017, 10, 181–189. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef]
- Saini, R. Ozone therapy in dentistry: A strategic review. J. Nat. Sci. Biol. Med. 2011, 2, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, N.; Bocci, V.; Gaggiotti, E. Ozone therapy. Int. J. Artif. Organs 2004, 27, 168–175. [Google Scholar] [CrossRef]
- Hodson, N.; Dunne, S.M. Using ozone to treat dental caries. J. Esthet. Restor. Dent. 2007, 19, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, D.; Millar, B. Ozone and its use in periodontal treatment. Open J. Stomatol. 2013, 3, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective Face Masks: Effect on the Oxygenation and Heart Rate Status of Oral Surgeons during Surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 2020, 11, 27. [Google Scholar] [CrossRef]
- Hoffman, R.K. Ozone. In Inhibition and Destruction of the Microbial Cell; Hugo, W.B., Ed.; Academic Press: London, UK, 1971; pp. 251–253. [Google Scholar]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine and monochloramine on Cryptosporidium parvuum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yousef, A.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Rapone, B.; Nardi, G.M.; Di Venere, D.; Pettini, F.; Grassi, F.R.; Corsalini, M. Oral hygiene in patients with oral cancer undergoing chemotherapy and/or radiotherapy after prosthesis rehabilitation: Protocol proposal. Oral Implantol. 2016, 9, 90–97. [Google Scholar] [CrossRef]
- Di Venere, D.; Pettini, F.; Nardi, G.M.; Laforgia, A.; Stefanachi, G.; Notaro, V.; Rapone, B.; Grassi, F.R.; Corsalini, M. Correlation between parodontal indexes and orthodontic retainers: Prospective study in a group of 16 patients. Oral Implantol. 2017, 10, 78–86. [Google Scholar] [CrossRef]
- Kalemaj, Z.; Scarano, A.; Valbonetti, L.; Rapone, B.; Grassi, F.R. Bone response to four dental implants with different surface topography: A histologic and histometric study in minipigs. Int. J. Periodontics Restor. Dent. 2016, 36, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Michalowicz, B.S.; Hodges, J.S.; Pihlstrom, B.L. Is change in probing depth a reliable predictor of change in clinical attachment loss? J. Am. Dent. Assoc. 2013, 144, 171–178. [Google Scholar] [CrossRef]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State Art Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, M.; Cantatore, F.; Cagnetta, C.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A.; et al. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Cesarano, F.; Arazzi, M.; Di Liberato, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics 2019, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorusso, F.; Noumbissi, S.; Inchingolo, F.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef]
- Holt, G.R. Declaration of Helsinki—The World’s Document of Conscience and Responsibility. South Med. J. 2014, 107, 407. [Google Scholar] [CrossRef] [PubMed]
- Mühlemann, H.R.; Son, S. Gingival sulcus bleeding—A leading symptom in initial gingivitis. Helv. Odontol. Acta 1971, 15, 107–113. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Field, P.A.; Wilcox, R.R. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav. Res. Ther. 2017, 98, 19–38. [Google Scholar] [CrossRef]
- Osborne, W.J.; Waters, E. Four assumptions of multiple regression that researchers should always test. Pract. Assess. Res. Eval. 2002, 8, 2. [Google Scholar]
- Newton, R.R.; Rudestam, K.E. Your Statistical Consultant, 2nd ed.; Sage Publications, Inc.: Newbury Park, CA, USA, 2013. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 4th ed.; Guilford Publications: New York City, NY, USA, 2015. [Google Scholar]
- Butera, A.; Gallo, S.; Maiorani, C.; Preda, C.; Chiesa, A.; Esposito, F.; Pascadopoli, M.; Scribante, A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021, 11, 8586. [Google Scholar] [CrossRef]
- Preda, C.; Butera, A.; Pelle, S.; Pautasso, E.; Chiesa, A.; Esposito, F.; Oldoini, G.; Scribante, A.; Genovesi, A.M.; Cosola, S. The Efficacy of Powered Oscillating Heads vs. Powered Sonic Action Heads Toothbrushes to Maintain Periodontal and Peri-Implant Health: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1468. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Luongo, C.; Capodanno, P.; Giordano, C.; Scafuro, M.A.; Siniscalco, D.; Lettieri, B.; Rossi, F.; Maglione, S.; Berrino, L. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharmacol. 2008, 603, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Claesson, R.; van Dijken, J.W. Antibacterial effect of ozone on cariogenic bacterial species. J. Dent. 2009, 37, 449–453. [Google Scholar] [CrossRef]
- Di Filippo, C.; Cervone, C.; Rossi, C.; Di Ronza, C.; Marfella, R.; Capodanno, P.; Luongo, C.; Rossi, F.; D’Amico, M. Antiarrhythmic effect of acute oxygen-ozone administration to rats. Eur. J. Pharmacol. 2010, 629, 89–95. [Google Scholar] [CrossRef]
- Stoker, G. Ozone in chronic middle ear deafness. Lancet 1902, 160, 1187–1188. [Google Scholar] [CrossRef] [Green Version]
- Oldoini, G.; Ricci Frabattista, G.; Saragoni, M.; Cosola, S.; Giammarinaro, E.; Genovesi, A.M.; Marconcini, S. Ozone Therapy for Oral Palatal Ulcer in a Leukaemic Patient. Eur. J. Case Rep. Intern. Med. 2020, 7, 001406. [Google Scholar]
- Baysan, A.; Whiley, R.; Lynch, E. Antimicrobial effects of a novel ozone generating device on microorganisms associated with primary root carious lesion in vitro. Caries Res. 2000, 34, 498–501. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in older Subjects (MOSES). PLoS ONE 2019, 14, e0222601. [Google Scholar]
- Boch, T.; Tennert, C.; Vach, K.; Al-Ahmad, A.; Hellwig, E.; Polydorou, O. Effect of gaseous ozone on Enterococcus faecalis biofilm-an in vitro study. Clin. Oral Investig. 2016, 20, 1733–1739. [Google Scholar] [CrossRef]
- Case, P.D.; Bird, P.S.; Kahler, W.A.; George, R.; Walsh, L.J. Treatment of Root Canal Biofilms of Enterococcus faecalis with Ozone Gas and Passive Ultrasound Activation. J. Endod. 2012, 38, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, Z.; Oskaybas, M.N.; Alkan, A.B.; Cakmak, O. The effects of ozone therapy on periodontal therapy: A randomized placebo-controlled clinical trial. Oral Dis. 2019, 25, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Seydanur Dengizek, E.; Serkan, D.; Abubekir, E.; Aysun Bay, K.; Onder, O.; Arife, C. Evaluating clinical and laboratory effects of ozone in non-surgical periodontal treatment: A randomized controlled trial. J. Appl. Oral Sci. 2019, 27, e20180108. [Google Scholar] [CrossRef] [PubMed]
- Isler, S.C.; Unsal, B.; Soysal, F.; Ozcan, G.; Peker, E.; Karaca, I.R. The effects of ozone therapy as an adjunct to the surgical treatment of peri-implantitis. J. Periodontal Implant Sci. 2018, 48, 136–151. [Google Scholar] [CrossRef] [PubMed]

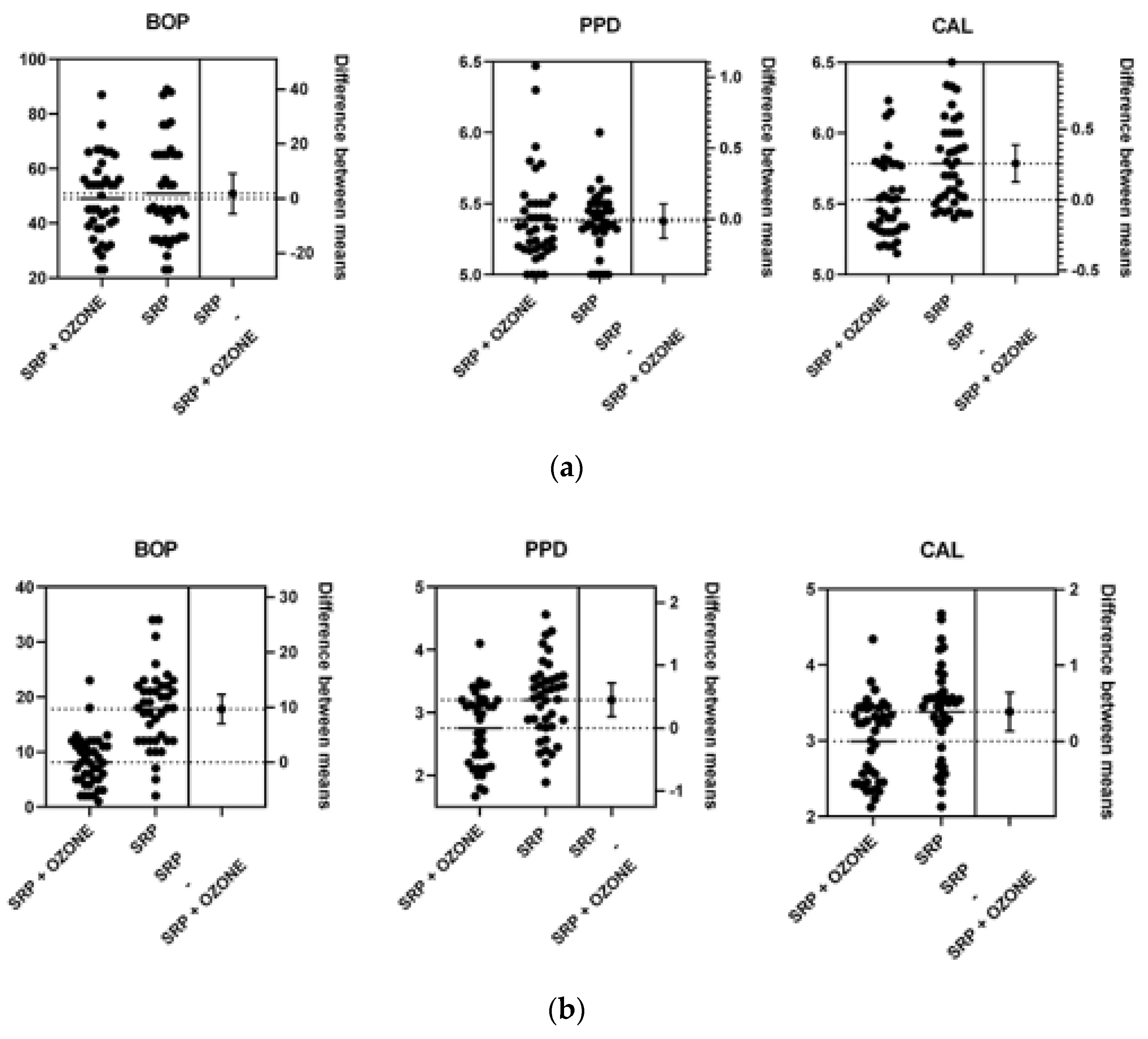
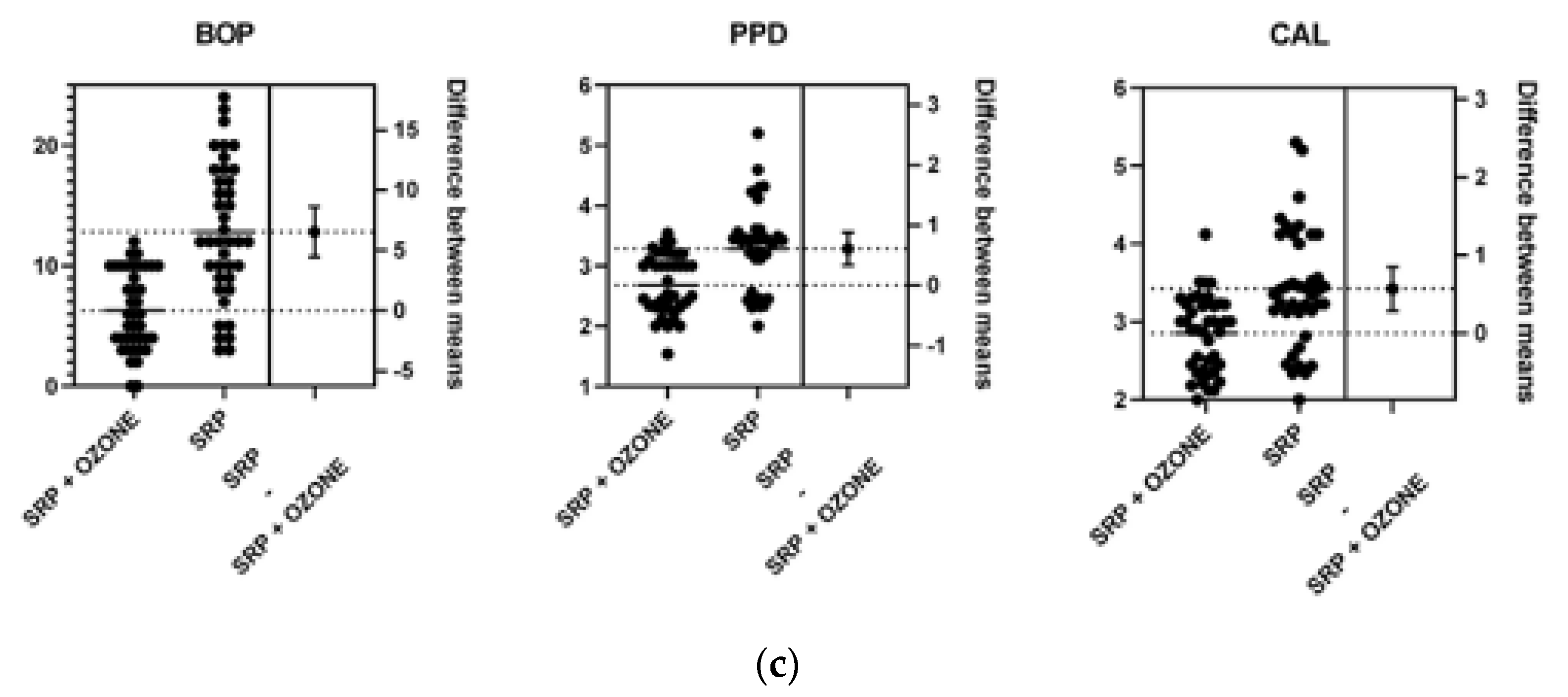

| Group A * | Group B ** | |
|---|---|---|
| Age (mean ± SD) | 51.62 ± 9.56 | 49.88 ± 10.54 |
| Sex | M 87% F 13% | M 78% F 22% |
| Prevalence of Moderate Periodontitis (%) | 78 | 83 |
| Prevalence of Severe Periodontitis (%) | 22 | 17 |
| PPD (mm) Group A * | PPD (mm) Group B ** | p Value | CAL (mm) Group A | CAL (mm) Group B | p Value | BOP (%) Group A | BOP (%) Group B | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 5.39 | 5.37 | 0.81 | 5.53 | 5.78 | <0.05 | 49 | 50.83 | 0.62 |
| Std. Deviation | 0.31 | 0.2 | - | 0.27 | 0.3 | - | 14.74 | 18.11 | - |
| PPD (mm) Group A * | PPD (mm) Group B ** | p Value | CAL (mm) Group A | CAL (mm) Group B | p Value | BOP (%) Group A | BOP (%) Group B | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 2.75 | 3.2 | < 0.001 | 2.99 | 3.38 | < 0.003 | 8.12 | 17.78 | < 0.0001 |
| Median | 2.93 | 3.25 | - | 3.18 | 3.47 | - | 8 | 18 | - |
| Std. Deviation | 0.59 | 0.6 | - | 0.53 | 0.6 | - | 4.6 | 7.05 | - |
| PPD (mm) Group A * | PPD (mm) Group B ** | p Value | CAL (mm) Group A | CAL (mm) Group B | p Value | BOP (%) Group A | BOP (%) Group B | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 2.67 | 3.28 | <0.0001 | 2.85 | 3.42 | <0.0001 | 6.27 | 12.83 | <0.0001 |
| Median | 2.52 | 3.41 | - | 2.94 | 3.37 | - | 6 | 12 | - |
| Std. Deviation | 0.48 | 0.71 | - | 0.48 | 0.75 | - | 3.32 | 5.7 | - |
| PPD (mm) 3 Months | PPD (mm) 6 Months | CAL (mm) 3 Months | CAL (mm) 6 Months | BOP (%) 3 Months | BOP (%) 6 Months | |
|---|---|---|---|---|---|---|
| p value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| T value | 3.35 | 4.43 | 3.06 | 4.02 | 7.23 | 6.27 |
| Df | 78 | 78 | 78 | 78 | 78 | 78 |
| Differences between the means ± SEM | 0.45 ± 0.13 | 0.6 ± 0.13 | 0.39 ± 0.12 | 0.57 ± 0.14 | 9.65 ± 1.33 | 6.55 ± 1.04 |
| CI 95% | 0.18 to 0.71 | 0.33 to 0.88 | 0.13 to 0.64 | 0.28 to 0.85 | 6.99 to 12.3 | 4.47 to 8.62 |
| R | 0.12 | 0.2 | 0,1 | 0.17 | 0.4 | 0.33 |
| F | 1.058 | 2.14 | 1.3 | 2.36 | 2.34 | 2.95 |
| p value | 0.86 | 0.01 | 0.4 | 0.008 | 0.009 | 0.001 |
| Source | df | SS | MS | F | p | η2p |
|---|---|---|---|---|---|---|
| Between-Subjects | ||||||
| Treatment | 1 | 8.76 | 8.76 | 23.28 | <0.001 | 0.24 |
| Stage_of_disease | 1 | 1.78 | 1.78 | 4.73 | 0.033 | 0.06 |
| Age | 1 | 2.90 | 2.90 | 7.69 | 0.007 | 0.09 |
| sex | 1 | 0.92 | 0.92 | 2.43 | 0.123 | 0.03 |
| Residuals | 75 | 28.24 | 0.38 | |||
| Within-Subjects | ||||||
| Time Factor | 2 | 4.41 | 2.20 | 8.11 | <0.001 | 0.10 |
| Treatment:Time Factor | 2 | 13.82 | 6.91 | 25.45 | <0.001 | 0.25 |
| Stage_of_disease:Time Factor | 2 | 0.99 | 0.49 | 1.82 | 0.166 | 0.02 |
| Age:Time Factor | 2 | 0.40 | 0.20 | 0.73 | 0.482 | 0.01 |
| sex:Time Factor | 2 | 0.71 | 0.36 | 1.32 | 0.271 | 0.02 |
| Time Factor Residuals | 150 | 40.74 | 0.27 | |||
| Dv Factor | 1 | 0.23 | 0.23 | 0.86 | 0.358 | 0.01 |
| Treatment:Dv Factor | 1 | 2.38 | 2.38 | 8.74 | 0.004 | 0.10 |
| Stage_of_disease:Dv Factor | 1 | 0.07 | 0.07 | 0.26 | 0.611 | 0.00 |
| Age:Dv Factor | 1 | 0.04 | 0.04 | 0.15 | 0.698 | 0.00 |
| sex:Dv Factor | 1 | 0.13 | 0.13 | 0.47 | 0.494 | 0.01 |
| Dv Factor Residuals | 75 | 20.43 | 0.27 | |||
| Time Factor:Dv Factor | 2 | 0.11 | 0.06 | 0.20 | 0.778 | 0.00 |
| Treatment:Time Factor:Dv Factor | 2 | 5.43 | 2.71 | 9.57 | <0.001 | 0.11 |
| Stage_of_disease:Time Factor:Dv Factor | 2 | 0.01 | 0.01 | 0.03 | 0.955 | 0.00 |
| Age:Time Factor:Dv Factor | 2 | 0.24 | 0.12 | 0.42 | 0.617 | 0.01 |
| sex:Time Factor:Dv Factor | 2 | 0.73 | 0.37 | 1.29 | 0.276 | 0.02 |
| Time Factor:Dv Factor Residuals | 150 | 42.56 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. https://doi.org/10.3390/ijerph19020985
Rapone B, Ferrara E, Santacroce L, Topi S, Gnoni A, Dipalma G, Mancini A, Di Domenico M, Tartaglia GM, Scarano A, et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(2):985. https://doi.org/10.3390/ijerph19020985
Chicago/Turabian StyleRapone, Biagio, Elisabetta Ferrara, Luigi Santacroce, Skender Topi, Antonio Gnoni, Gianna Dipalma, Antonio Mancini, Marina Di Domenico, Gianluca Martino Tartaglia, Antonio Scarano, and et al. 2022. "The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 2: 985. https://doi.org/10.3390/ijerph19020985











