Impacts of Slow-Release Nitrogen Fertilizer Rates on the Morpho-Physiological Traits, Yield, and Nitrogen Use Efficiency of Rice under Different Water Regimes
Abstract
:1. Introduction
2. Materials and Methods
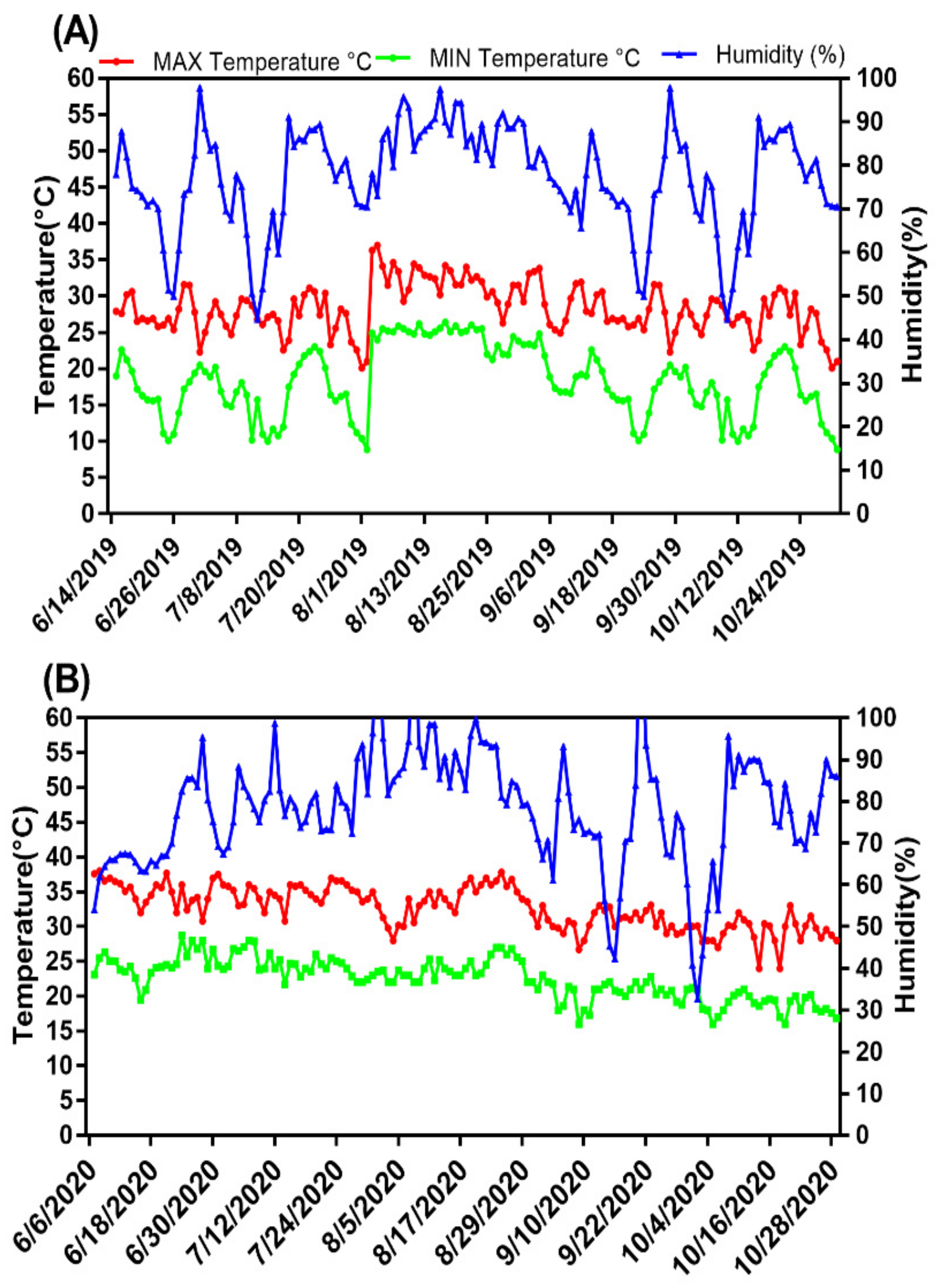
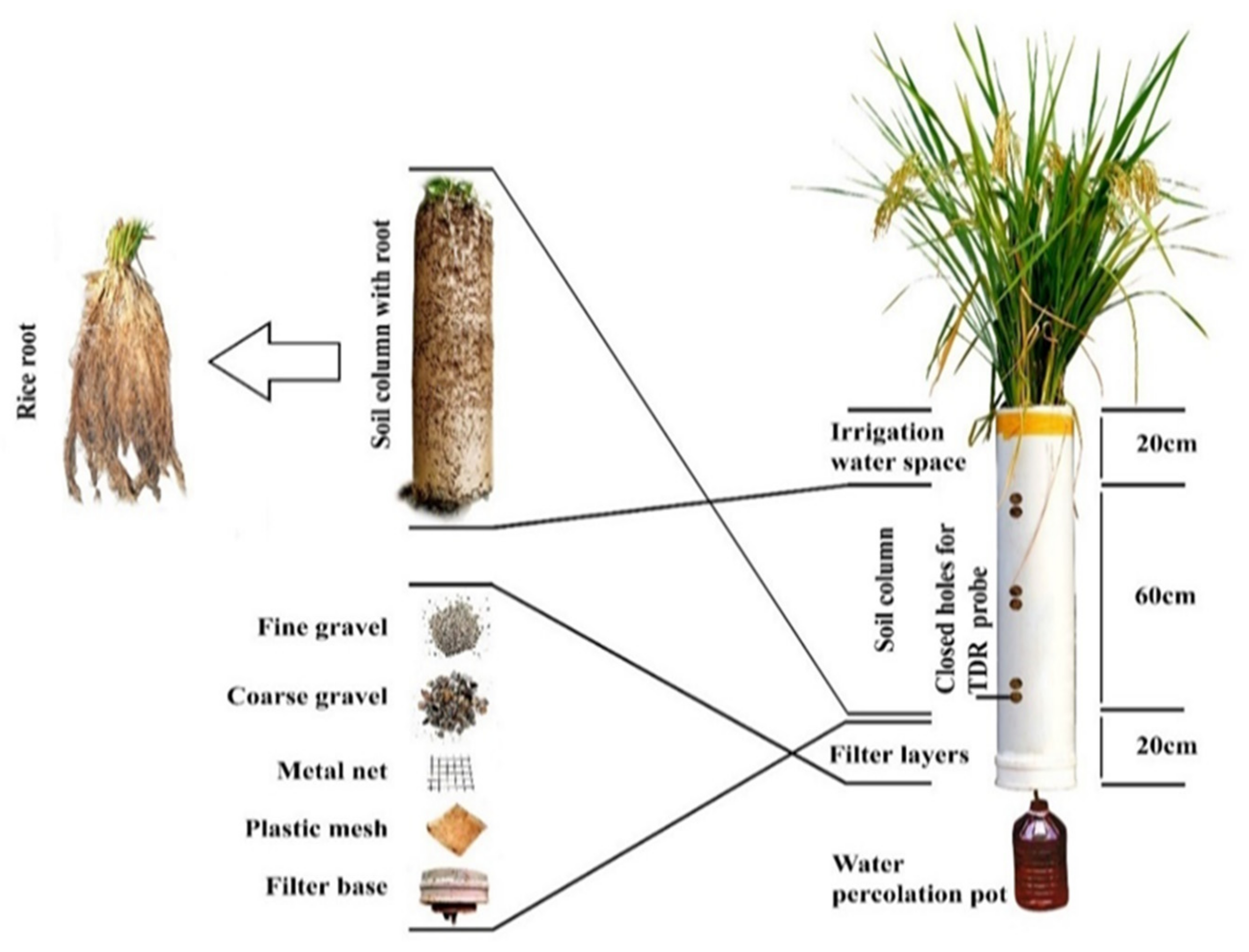
2.1. Experimental Site and Pipe Setup
2.2. Soil Analysis
2.3. Experimental Design and Treatments
2.4. Data Collection
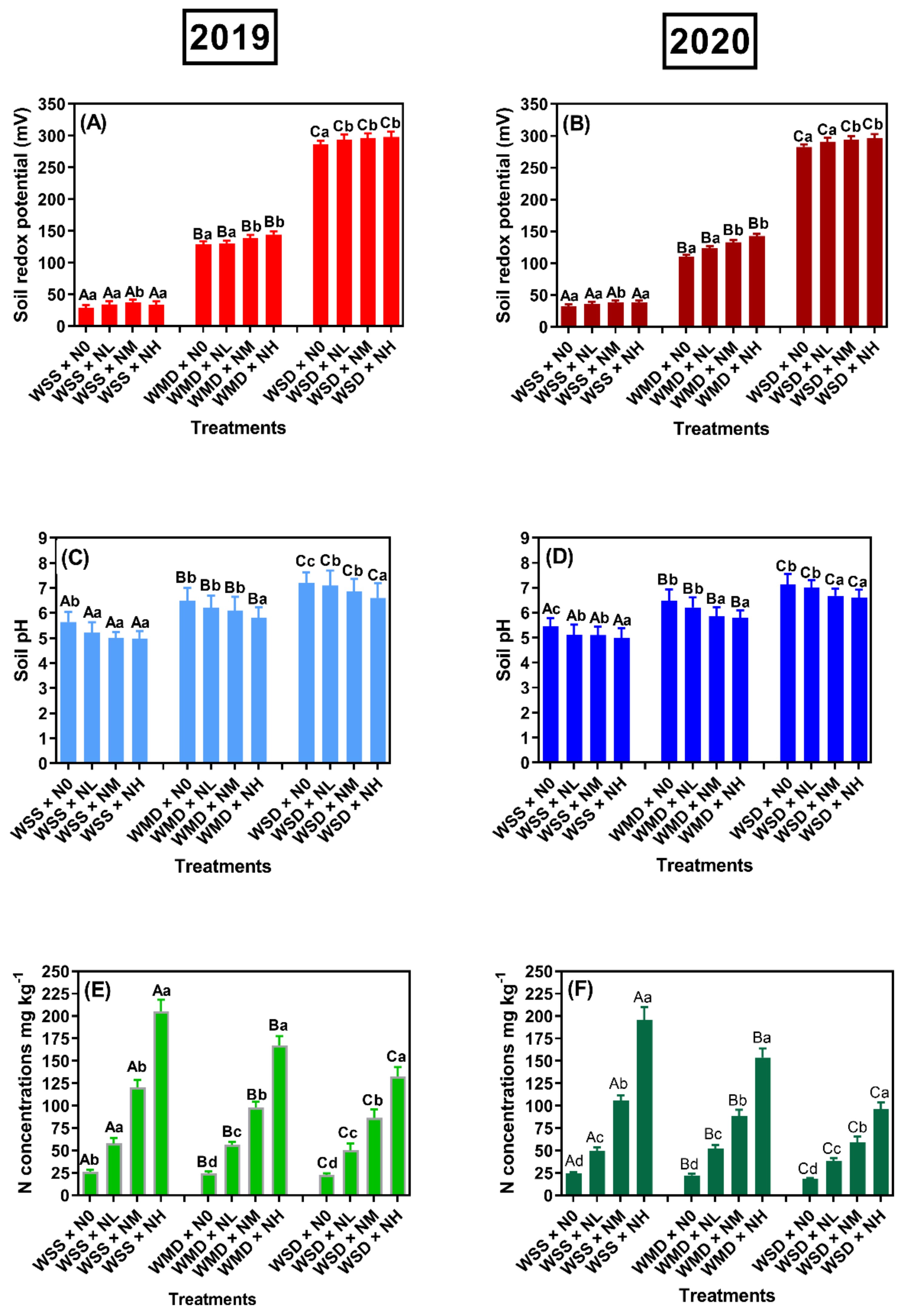
2.4.1. Chemical Characteristics of the Soil in the Root Zone
2.4.2. Measurement of Plant Sampling and Yield Indicators
2.4.3. Root Growth Measurements
2.4.4. Plant Tissues N Concentrations Measurements and the Amount of Nutrient Removed by the Crop
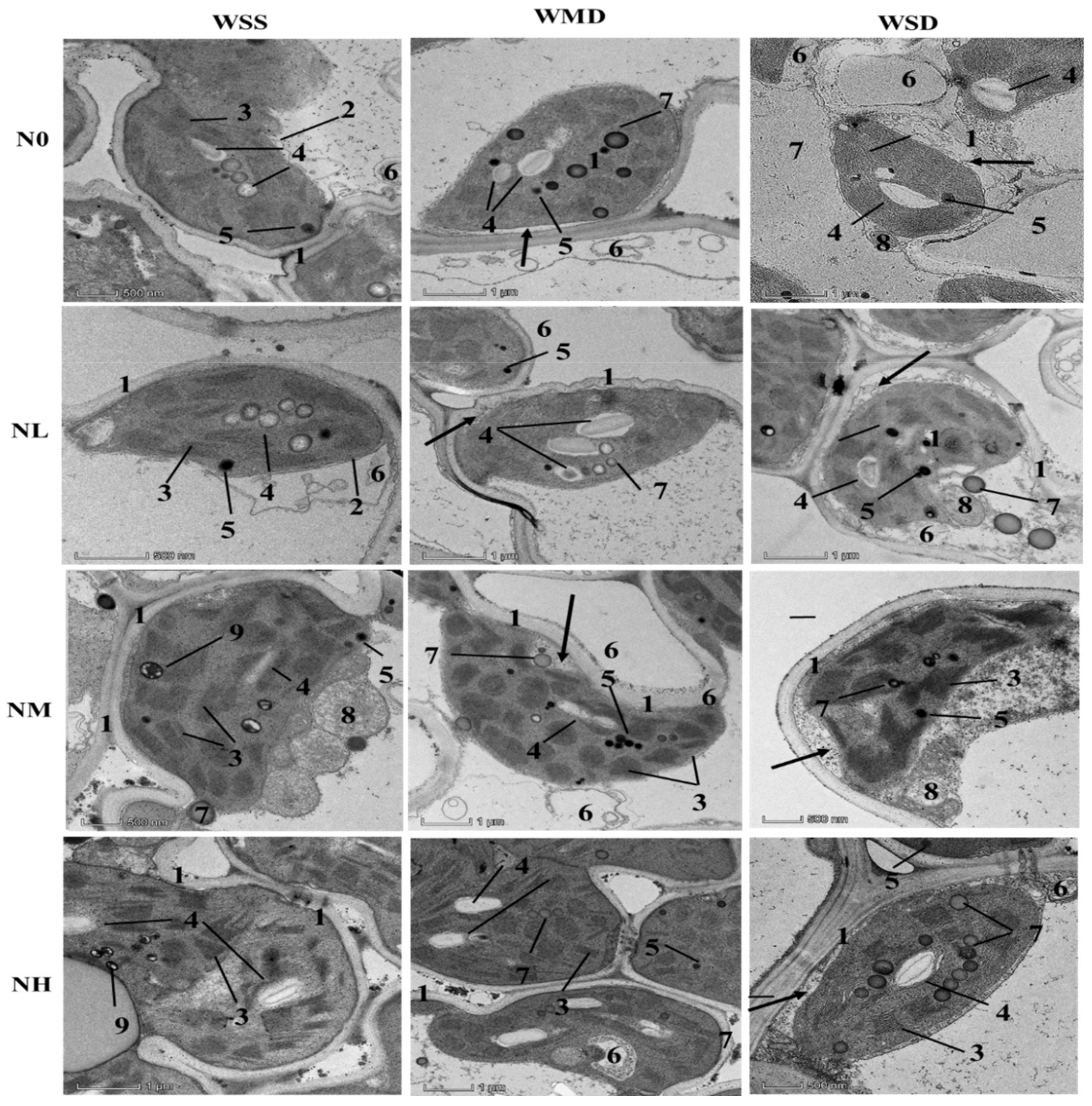
2.4.5. Transmission Electron Microscopy Observation
2.4.6. Determination of the NUE
2.5. Statistical Analysis
3. Results
3.1. Effects of SRNF on the Environmental Chemical Parameters of Soil in the Root Zone under Different AWD Regimes
3.2. Effects of SRNF Application on Rice Root Morphology under Different AWD Regimes
3.3. Effects of SRNF Application on Rice Biomass and Yield Indicators under Different AWD Regimes
3.4. Effects of SRNF Application on Leaf Mesophyll Cell Ultrastructure under Different AWD Regimes
3.5. Effects of SRNF Application on N Tissue Concentration and N Uptake under Different AWD Regimes
3.6. Effects of SRNF Application on NUE under Different AWD Regimes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.-H.; Cao, L.-Y.; Zhuang, J.-Y.; Chen, S.-G.; Zhan, X.-D.; Fan, Y.-Y.; Zhu, D.-F.; Min, S.-K. Super Hybrid Rice Breeding in China: Achievements and Prospects. J. Integr. Plant Biol. 2007, 49, 805–810. [Google Scholar] [CrossRef]
- He, L.; Wu, Q.; Gao, X.; Wu, K. Population Life Tables for the Invasive Fall Armyworm, Spodoptera Frugiperda Fed on Major Oil Crops Planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; et al. Improving Nitrogen Fertilization in Rice by Sitespecific N Management. A Review. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, S.; Zheng, M.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Dry Direct-Seeded Rice as an Alternative to Transplanted-Flooded Rice in Central China. Agron. Sustain. Dev. 2015, 35, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Lampayan, R.M.; Rejesus, R.M.; Singleton, G.R.; Bouman, B.A.M. Adoption and Economics of Alternate Wetting and Drying Water Management for Irrigated Lowland Rice. Field Crops Res. 2015, 170, 95–108. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Lundy, M.E.; Linquist, B.A. Rice Yields and Water Use under Alternate Wetting and Drying Irrigation: A Meta-Analysis. Field Crops Res. 2017, 203, 173–180. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current status, challenges, and opportunities in rice production. In Rice Production Worldwide; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–32. [Google Scholar]
- Linquist, B.A.; Anders, M.M.; Adviento-Borbe, M.A.A.; Chaney, R.L.; Nalley, L.L.; Da Rosa, E.F.F.; Van Kessel, C. Reducing Greenhouse Gas Emissions, Water Use, and Grain Arsenic Levels in Rice Systems. Glob. Chang. Biol. 2015, 21, 407–417. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Guo, X.; Wang, Z.; Chen, S.; Rasoul, G. Effects of Irrigation Water Regime, Soil Clay Content and Their Combination on Growth, Yield, and Water Use Efficiency of Rice Grown in South China. Int. J. Agric. Biol. Eng. 2018, 11, 144–155. [Google Scholar]
- Djaman, K.; Mel, V.C.; Diop, L.; Sow, A.; El-Namaky, R.; Manneh, B.; Saito, K.; Futakuchi, K.; Irmak, S. Effects of Alternate Wetting and Drying Irrigation Regime and Nitrogen Fertilizer on Yield and Nitrogen Use Efficiency of Irrigated Rice in the Sahel. Water 2018, 10, 711. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice Root Growth and Nutrient Uptake as Influenced by Organic Manure in Continuously and Alternately Flooded Paddy Soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Cheng, Y.; Chen, W.; Liu, G.; Yang, J. The Effects of Water and Nitrogen on the Roots and Yield of Upland and Paddy Rice. J. Integr. Agric. 2020, 19, 1363–1374. [Google Scholar] [CrossRef]
- Sander, T.; Gerke, H.H. Preferential Flow Patterns in Paddy Fields Using a Dye Tracer. Vadose Zone J. 2007, 6, 105–115. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.; Guo, X.; Elshaikh, N.A.; Khan, N.U.; Oumarou, A.; Rahim, S.F. Impact of Alternative Wetting and Soil Drying and Soil Clay Content on the Morphological and Physiological Traits of Rice Roots and Their Relationships to Yield and Nutrient Use-Efficiency. Agric. Water Manag. 2019, 223, 105706. [Google Scholar] [CrossRef]
- Amanullah; Ullah, H.; Soliman Elshikh, M.; Alwahibi, M.S.; Alkahtani, J.; Muhammad, A.; Khalid, S.; Imran. Nitrogen Contents in Soil, Grains, and Straw of Hybrid Rice Differ When Applied with Different Organic Nitrogen Sources. Agriculture 2020, 10, 386. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Redding, M.; Pratt, C.; Wang, W. Plant Growth Promoting Rhizobacteria Increase the Efficiency of Fertilisers While Reducing Nitrogen Loss. J. Environ. Manag. 2019, 233, 337–341. [Google Scholar] [CrossRef]
- Frye, W. Nitrification Inhibition for Nitrogen Efficiency and Environment Protection. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005; pp. 28–30. [Google Scholar]
- Guo, Y.; Zhang, M.; Liu, Z.; Zhao, C.; Lu, H.; Zheng, L.; Li, Y.C. Applying and Optimizing Water-Soluble, Slow-Release Nitrogen Fertilizers for Water-Saving Agriculture. ACS Omega 2020, 5, 11342–11351. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, X.; Zeng, J.; Liang, K.; Pan, J.; Xin, Y.; Liu, Y.; Hu, X.; Peng, B.; Chen, R.; et al. Improving Grain Yield, Nitrogen Use Efficiency and Radiation Use Efficiency by Dense Planting, with Delayed and Reduced Nitrogen Application, in Double Cropping Rice in South China. J. Integr. Agric. 2021, 20, 565–580. [Google Scholar] [CrossRef]
- Fu, J.; Wang, C.; Chen, X.; Huang, Z.; Chen, D. Classification Research and Types of Slow Controlled Release Fertilizers (SRFs) Used-a Review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. [Google Scholar] [CrossRef]
- Hamoud, Y.A.; Guo, X.; Wang, Z.; Shaghaleh, H.; Chen, S.; Hassan, A.; Bakour, A. Effects of Irrigation Regime and Soil Clay Content and Their Interaction on the Biological Yield, Nitrogen Uptake and Nitrogen-Use Efficiency of Rice Grown in Southern China. Agric. Water Manag. 2019, 213, 934–946. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Alordzinu, K.E.; Jiuhao, L.; Appiah, S.A.; Aasmi, A.A.L.; Blege, P.K.; Afful, E.A. Water Stress Affects the Physio-Morphological Development of Tomato Growth. Afr. J. Agric. Res. 2021, 17, 733–742. [Google Scholar]
- Shen, J.; Li, C.; Mi, G.; Li, L.; Yuan, L.; Jiang, R.; Zhang, F. Maximizing Root/Rhizosphere Efficiency to Improve Crop Productivity and Nutrient Use Efficiency in Intensive Agriculture of China. J. Exp. Bot. 2013, 64, 1181–1192. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Sun, H.; Taghizadeh-Hesary, F. How Does Climate Change Affect Rice Yield in China? Agriculture 2020, 10, 441. [Google Scholar] [CrossRef]
- Lin, Y.; Huabin, Z.; Jianxia, L.; Hui, H.; Huang, H. Current Situation and Prospect of Rice Water-Saving Irrigation Technology in China. Chin. J. Ecol. 2014, 33, 1381–1387. [Google Scholar]
- Gupta, P.K.; Gupta, P.K.; Gupta, P.K. Soil, Plant, Water and Fertilizer Analysis; Agro Botanica: Jodhpur, India, 1999. [Google Scholar]
- Marx, E.S.; Hart, J.; Stevens, R.G. Soil Test Interpretation Guide; EC 1478; Oregon State University: Corvallis, OR, USA, 1999. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil Anal. Part 3 Chem. Methods 2018, 5, 961–1010. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Sims, J.R.; Jackson, G.D. Rapid Analysis of Soil Nitrate with Chromotropic Acid. Soil Sci. Soc. Am. J. 1971, 35, 603–606. [Google Scholar] [CrossRef]
- Henriksen, A.; Selmer-Olsen, A.R. Automatic Methods for Determining Nitrate and Nitrite in Water and Soil Extracts. Analyst 1970, 95, 514–518. [Google Scholar] [CrossRef]
- Dhyan, S.; Chhonkar, P.K.; Dwivedi, B.S. Manual on Soil, Plant and Water Analysis; Westville Publishing House: New Delhi, India, 2005. [Google Scholar]
- Tang, S.H.; Xu, P.Z.; Chen, J.S.; Ai, S.Y.; Zhang, F.B.; Huang, Y. Effects of Single Basal Application of Controlled-Release Fertilizer on Root Activity and Nutrient Absorption of Rice (Oryza satava L.). Plant Nutr. Fert Sci. 2007, 3, 586–596. [Google Scholar]
- Cavalca, L.; Zanchi, R.; Corsini, A.; Colombo, M.; Romagnoli, C.; Canzi, E.; Andreoni, V. Arsenic-Resistant Bacteria Associated with Roots of the Wild Cirsium arvense (L.) Plant from an Arsenic Polluted Soil, and Screening of Potential Plant Growth-Promoting Characteristics. Syst. Appl. Microbiol. 2010, 33, 154–164. [Google Scholar] [CrossRef]
- Tennant, D. A Test of a Modified Line Intersect Method of Estimating Root Length. J. Ecol. 1975, 63, 995–1001. [Google Scholar] [CrossRef]
- Pang, W.; Crow, W.T.; Luc, J.E.; Mcsorley, R.; Kruse, J.K. Comparison of Water Displacement and WinRHIZO Software for Plant Root Parameter Assessment. Plant Dis. 2011, 95, 1308–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Zhang, X.Z.; Tan, G.; Huang, Y. Experimental Technology of Plant Physiology; Liaoning Science and Technology Press: Shenyang, China, 1994; pp. 123–126. [Google Scholar]
- Novamsky, I.; Van Eck, R.; Van Schouwenburg, C.H.; Walinga, I. Total Nitrogen Determination in Plant Material by Means of the Indophenol-Blue Method. Neth. J. Agric. Sci. 1974, 22, 3–5. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and Their Combination by Regulating Cellular and Molecular Signals in Rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oi, T.; Enomoto, S.; Nakao, T.; Arai, S.; Yamane, K.; Taniguchi, M. Three-Dimensional Intracellular Structure of a Whole Rice Mesophyll Cell Observed with FIB-SEM. Ann. Bot. 2017, 120, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Deng, F.; Li, W.; Wang, L.; Hu, H.; Liao, S.; Pu, S.-L.; Tao, Y.-F.; Li, G.-H.; Ren, W.-J. Effect of Controlled-Release Fertilizers on Leaf Characteristics, Grain Yield, and Nitrogen Use Efficiency of Machine-Transplanted Rice in Southwest China. Arch. Agron. Soil Sci. 2020, 67, 1739–1753. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Redox Potential and PH as Major Drivers of Fertility in Submerged Rice Soils: A Conceptual Framework for Management. Commun. Soil Sci. Plant Anal. 2015, 46, 1597–1606. [Google Scholar] [CrossRef]
- Husson, O. Redox Potential (Eh) and PH as Drivers of Soil/Plant/Microorganism Systems: A Transdisciplinary Overview Pointing to Integrative Opportunities for Agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Halder, D.; Wegner, L.; Brüggenwirth, L.; Schaller, J.; Martin, M.; Said-Pullicino, D.; Romani, M.; Planer-Friedrich, B. Redox Dependence of Thioarsenate Occurrence in Paddy Soils and the Rice Rhizosphere. Environ. Sci. Technol. 2020, 54, 3940–3950. [Google Scholar] [CrossRef]
- Marin, A.R.; Masscheleyn, P.H.; Patrick, W.H. Soil Redox-PH Stability of Arsenic Species and Its Influence on Arsenic Uptake by Rice. Plant Soil 1993, 152, 245–253. [Google Scholar] [CrossRef]
- Ren, X.; Chen, F.; Ma, T.; Hu, Y. Soil Quality Characteristics as Affected by Continuous Rice Cultivation and Changes in Cropping Systems in South China. Agriculture 2020, 10, 443. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Begg, C.B.M.; Solivas, J.L. The Chemistry of the Lowland Rice Rhizosphere. Plant Soil 1993, 155, 83–86. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial Interactions in the Rhizosphere: Beneficial Influences of Plant Growth-Promoting Rhizobacteria on Nutrient Acquisition Process. A Review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Bandaogo, A.; Bidjokazo, F.; Youl, S.; Safo, E.; Abaidoo, R.; Andrews, O. Effect of Fertilizer Deep Placement with Urea Supergranule on Nitrogen Use Efficiency of Irrigated Rice in Sourou Valley (Burkina Faso). Nutr. Cycl. Agroecosystems 2015, 102, 79–89. [Google Scholar] [CrossRef]
- Guo, C.; Li, P.; Lu, J.; Ren, T.; Cong, R.; Li, X. Application of Controlled-Release Urea in Rice: Reducing Environmental Risk While Increasing Grain Yield and Improving Nitrogen Use Efficiency. Commun. Soil Sci. Plant Anal. 2016, 47, 1176–1183. [Google Scholar] [CrossRef]
- Xu, X.; He, P.; Pampolino, M.F.; Qiu, S.; Zhao, S.; Zhou, W. Spatial Variation of Yield Response and Fertilizer Requirements on Regional Scale for Irrigated Rice in China. Sci. Rep. 2019, 9, 3589. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, S.; Zhang, Y.; Li, T.; Ge, H.; Xia, S.; Gu, J.; Zhang, H.; Lü, B.; Wu, X.; et al. Rice Root Morphological and Physiological Traits Interaction with Rhizosphere Soil and Its Effect on Methane Emissions in Paddy Fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Xie, X.; Machikowa, T.; Wonprasaid, S. Fertigation Based on a Nutrient Balance Model for Cassava Production in Two Different Textured Soils. Plant Prod. Sci. 2020, 23, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating Materials for Slow Release of Nitrogen from Urea Fertilizer: A Review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Hua, Z.; Mei, S.; Wang, P.; Wang, S. Comparing Ammonia Volatilization between Conventional and Slow-Release Nitrogen Fertilizers in Paddy Fields in the Taihu Lake Region. Environ. Sci. Pollut. Res. 2020, 27, 8386–8394. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Guo, N.; Li, Y.; Jian, S.; Yu, K. Determining the Canopy Water Stress for Spring Wheat Using Canopy Hyperspectral Reflectance Data in Loess Plateau Semiarid Regions. Lett. Spectrosc. 2015, 48, 492–498. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping Maize Water Stress Based on UAV Multispectral Remote Sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef] [Green Version]
- Kondhia, A.; Tabien, R.E.; Ibrahim, A. Evaluation and Selection of High Biomass Rice (Oryza sativa L.) for Drought Tolerance. Am. J. Plant Sci. 2015, 6, 1962. [Google Scholar] [CrossRef] [Green Version]
- Sriphirom, P.; Chidthaisong, A.; Towprayoon, S. Effect of Alternate Wetting and Drying Water Management on Rice Cultivation with Low Emissions and Low Water Used during Wet and Dry Season. J. Clean. Prod. 2019, 223, 980–988. [Google Scholar] [CrossRef]
- Mishra, A.; Uphoff, N. Morphological and Physiological Responses of Rice Roots and Shoots to Varying Water Regimes and Soil Microbial Densities. Arch. Agron. Soil Sci. 2013, 59, 705–731. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Schaffer, B.; Davies, F.S.; Muñoz-Carpena, R. Characterization of Soil-Water Retention of a Very Gravelly Loam Soil Varied with Determination Method. Soil Sci. 2006, 171, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Kotula, L.; Ranathunge, K.; Steudle, E. Apoplastic Barriers Effectively Block Oxygen Permeability across Outer Cell Layers of Rice Roots under Deoxygenated Conditions: Roles of Apoplastic Pores and of Respiration. New Phytol. 2009, 184, 909–917. [Google Scholar] [CrossRef]
- Shiono, K.; Yamauchi, T.; Yamazaki, S.; Mohanty, B.; Malik, A.I.; Nagamura, Y.; Nishizawa, N.K.; Tsutsumi, N.; Colmer, T.D.; Nakazono, M. Microarray Analysis of Laser-Microdissected Tissues Indicates the Biosynthesis of Suberin in the Outer Part of Roots during Formation of a Barrier to Radial Oxygen Loss in Rice (Oryza sativa). J. Exp. Bot. 2014, 65, 4795–4806. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lv, J.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Patience, B.E. Effect of Slow-Release Fertilizer on Soil Fertility and Growth and Quality of Wintering Chinese Chives (Allium tuberm Rottler Ex Spreng.) in Greenhouses. Sci. Rep. 2021, 11, 8070. [Google Scholar] [CrossRef]
- Hou, P.; Xue, L.; He, S.; Zhou, Y.; Li, G.; Feng, Y.; Yang, L.; Xue, L. Controlled-Release Fertilizer Affects Growth and Yield of Japonica Rice. J. Anim. Plant Sci. 2021, 31, 97–102. [Google Scholar]
| Parameters | Values |
|---|---|
| Soil texture | Loam |
| Sand (%) | 33.53 ± 1.7 |
| Silt (%) | 41.6 ± 0.28 |
| Clay (%) | 24.87 ± 0.45 |
| Bulk density (g cm−3) | 1.2 ± 0.09 |
| Particle density (g cm−3) | 2.32 ± 0.03 |
| Total soil porosity % | 54.71 ± 3.57 |
| Soil pH | 6.1 ± 0.11 |
| Soil organic matter (g kg−1) | 12.96 ± 1.36 |
| Total Nitrogen (g kg−1) | 0.6 ± 0.02 |
| Total Phosphorus (g kg−1) | 0.47 ± 0.01 |
| Total Potassium (g kg−1) | 14.92 ± 0.12 |
| Available nitrogen (mg kg−1) | 32.15 ± 0.15 |
| Available Phosphorus (mg kg−1) | 2.85 ± 0.26 |
| Exchangeable potassium (mg kg−1) | 165.63 ± 17.2 |
| Treatment | Root Growth Morphology Indicator | |||||||
|---|---|---|---|---|---|---|---|---|
| RWD (g cm−3) | RLD (mm cm−3) | TSA (m2 hill−1) | RDW (g hill−1) | |||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| WSS × N0 | 0.52 ± 0.02 Ac | 0.54 ± 0.03 Ac | 10.37 ± 1.23 Ac | 10.72 ± 1.8 Ac | 62.82 ± 7.89 Ac | 64.96 ± 5.5 Ac | 6.57 ± 0.25 Ab | 6.77 ± 0.32 Ac |
| WSS × NL | 0.60 ± 0.03 Ab | 0.61 ± 0.02 Ab | 11.75 ± 1.07 Ab | 12.1 ± 0.61 Ab | 72.20 ± 4.68 Ab | 74.36 ± 8.1 Ab | 7.57 ± 0.35 Ab | 7.63 ± 0.28 Ab |
| WSS × NM | 0.64 ± 0.07 Ab | 0.68 ± 0.11 Ab | 11.51 ± 2.35 Ab | 11.85 ± 0.58 Aa | 75.93 ± 11.25 Ab | 78.16 ± 10.38 Ab | 8.10 ± 0.83 Aa | 8.57 ± 1.32 Ab |
| WSS × NH | 0.78 ± 0.03 Aa | 0.83 ± 0.03 Aa | 12.98 ± 2.92 Aa | 13.38 ± 1 Aa | 95.86 ± 15.63 Aa | 98.78 ± 1.1 Aa | 9.81 ± 0.33 Aa | 10.37 ± 0.40 Aa |
| WMD × N0 | 0.49 ± 0.01 Bb | 0.54 ± 0.04 Bc | 7.89 ± 1.85 Bb | 8.25 ± 0.34 Bc | 56.25 ± 7.59 Bb | 58.68 ± 2.6 Bc | 6.19 ± 0.19 Bc | 6.8 ± 0.46 Bc |
| WMD × NL | 0.57 ± 0.06 Bb | 0.63 ± 0.08 Bb | 8.92 ± 1.46 Bb | 9.37 ± 0.42 Bb | 64.30 ± 9.55 Bc | 67.57 ± 2.98 Bb | 6.91 ± 0.75 Bb | 7.87 ± 0.94 Bb |
| WMD × NM | 0.55 ± 0.08 Bb | 0.59 ± 0.07 Bb | 10.58 ± 2.2 Ba | 11.03 ± 0.49 Ba | 73.44 ± 10.21 Ba | 76.58 ± 4.21 Bb | 7.10 ± 0.97 Bb | 7.4 ± 0.87 Bb |
| WMD × NH | 0.73 ± 0.02 Ba | 0.71 ± 0.05 Ba | 11.79 ± 1.41 Ba | 12.19 ± 0.03 Ba | 90.94 ± 7.83 Ba | 93.97 ± 2.81 Ba | 9.17 ± 0.20 Ba | 8.97 ± 0.65 Ba |
| WSD × N0 | 0.48 ± 0.02 Cc | 0.51 ± 0.03 Cc | 5.32 ± 3.57 Cc | 7.25 ± 1.32 Cc | 58.39 ± 9.17 Cb | 56.44 ± 8.68 Cc | 6.00 ± 0.26 Cb | 6.4 ± 0.35 Cc |
| WSD × NL | 0.49 ± 0.02 Ca | 0.56 ± 0.02 Cb | 7.09 ± 1.87 Cb | 8.59 ± 0.57 Cb | 65.26 ± 4.57 Cb | 74.79 ± 8.61 Cb | 6.13 ± 0.23 Ca | 7 ± 0.2 Cb |
| WSD × NM | 0.53 ± 0.02 Cb | 0.59 ± 0.05 Cb | 8.19 ± 2.02 Ca | 9.25 ± 0.19 Ca | 80.76 ± 5.43 Ca | 68.38 ± 3.13 Cb | 6.71 ± 0.29 Ca | 7.37 ± 0.65 Cb |
| WSD × NH | 0.54 ± 0.06 Cb | 0.52 ± 0.03 Ca | 8.82 ± 1.15 Ca | 7.43 ± 1.14 Ca | 88.17 ± 11.72 Ca | 61.24 ± 6.5 Ca | 6.98 ± 0.72 Ca | 6.53 ± 0.42 Ca |
| ANOVA | ||||||||
| Water regime | *** | *** | *** | *** | *** | *** | *** | *** |
| N rate | *** | *** | *** | *** | ** | *** | ** | *** |
| Water × N rate | * | ns | ns | ns | ns | ns | ns | *** |
| Water regime × Year | ns | ns | ns | ns | ns | ns | ns | ns |
| N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns |
| Water regime × N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | Number of Panicles (Panicle hill−1) | Panicle Length (cm) | Grain Filling Rate (%) | Grain Yield (g hill−1) | Total Biomass (g hill−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| WSS × N0 | 28.00 ± 1 Ac | 29.7 ± 1.5 Ad | 17.3 ± 1.4 Ab | 20.3 ± 0.6 Ac | 70.5 ± 3.2 Ac | 74.92 ± 2.8 Ac | 50.1 ± 2.7 Ac | 56.67 ± 5.3 Ad | 99.4 ± 5.2 Ac | 100.37 ± 9.5 Ad |
| WSS × NL | 33.08 ± 1 Ab | 34.3 ± 1.1 Ac | 21.17 ± 1.8 Aa | 20.7 ± 1.2 Ab | 76.1 ± 2.2 Ab | 77.84 ± 2.0 Ab | 70.6 ± 3.4 Ab | 94.83 ± 3.5 Ac | 139.6 ± 1.8 Ab | 164.87 ± 7.2 Ac |
| WSS × NM | 34.67 ± 1.5 Aa | 36.7 ± 1.5 Ab | 22.05 ± 1.5 Aa | 23.7 ± 0.6 Aa | 84.5 ± 4.1 Aa | 84.95 ± 5.9 Aa | 79.9 ± 2.2 Aa | 104.2 ± 3.5 Ab | 159.0 ± 3.2 Aa | 191.97 ± 4.8 Ab |
| WSS × NH | 38.00 ± 2 Aa | 41.00 ± 1.5 Aa | 24.16 ± 0.8 Aa | 25.7 ± 1.5 Aa | 90.6 ± 1.3 Aa | 91.35 ± 5.3 Aa | 90.7 ± 1.5 Aa | 112.1 ± 4.3 Aa | 185.4 ± 2.4 Aa | 242.23 ± 3.9 Aa |
| WMD × N0 | 23.67 ± 2.1 Bc | 25.7 ± 1.5 Bd | 15.67 ± 0.4 Bb | 17.3 ± 2.5 Bc | 65.9 ± 3.3 Bb | 68.12 ± 1.3 Bc | 41.9 ± 2.7 Bb | 48.37 ± 1.9 Bd | 83.2 ± 2.3 Bb | 84 ± 2.3 Bd |
| WMD × NL | 29.10 ± 1 Bb | 32 ± 3 Bc | 19.59 ± 0.7 Ba | 20.3 ± 2.1 Bb | 72.8 ± 1.4 Bb | 75.55 ± 1.7 Bb | 66.0 ± 2.2 Bb | 65.57 ± 3.7 Bc | 131.1 ± 2.1 Bb | 125.1 ± 7.4 Bc |
| WMD × NM | 31.34 ± 2.1 Ba | 33 ± 3.5 Bb | 21.10 ± 1.3 Ba | 23.7 ± 0.6 Ba | 75.1 ± 1.5 Ba | 77.22 ± 0.6 Ba | 71.1 ± 2.1 Ba | 83.87 ± 3.5 Bb | 139.3 ± 2.9 Ba | 146.43 ± 9.7 Bb |
| WMD × NH | 35.31 ± 1.5 Ba | 36.7 ± 2.1 Ba | 22.83 ± 1.0 Ba | 24.2 ± 2.0 Ba | 82.3 ± 2.2 Ba | 79.49 ± 8.3 Ba | 86.8 ± 1.4 Ba | 92.71 ± 2.1 Ba | 164.2±1.4 Ba | 200.3 ± 6.4 Ba |
| WSD × N0 | 21.00 ± 2.5 Cc | 23.7 ± 4.5 Cd | 14.05 ± 0.8 Cb | 16 ± 2 Cc | 63.2 ± 3.5 Cc | 65.05 ± 3.8 Cc | 34.7 ± 1.8 Cc | 40.47 ± 3.1 Cd | 65.5 ± 1.8 Cc | 65.17 ± 1.6 Cd |
| WSD × NL | 26.56 ± 3.1 Cb | 27 ± 4 Cc | 15.63 ± 0.8 Ca | 19 ± 1 Cb | 72.9 ± 1.1 Cb | 73.01 ± 1.5 Cb | 63.8 ± 3.2 Cb | 52.53 ± 1.1 Cc | 125.4 ± 3.2 Cb | 108.63 ± 4.6 Cc |
| WSD × NM | 28.43 ± 2.5 Ca | 31.3 ± 4.1 Cb | 18.27 ± 1.5 Ca | 20.5 ± 0.5 Ca | 71.9 ± 1.0 Ca | 74.62 ± 2.4 Ca | 67.9 ± 2.3 Ca | 63.73 ± 3.2 Cb | 136.9 ± 2.3 Ca | 127.1 ± 6.2 Cb |
| WSD × NH | 28.29 ± 2.5 Ca | 30 ± 3 Ca | 19.75 ± 1.7 Ca | 18.5 ± 0.9 Ca | 79.1 ± 2.8 Ca | 75.89 ± 2.1 Ca | 78.1 ± 1.8 Ca | 78.33 ± 2.1 Ca | 146.6 ± 2.7 Ca | 141.27 ± 6.0 Ca |
| ANOVA | ||||||||||
| Water regime | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| N rate | *** | *** | *** | *** | ** | *** | *** | *** | ** | *** |
| Water × N rate | ns | ns | ** | *** | ns | ns | ns | ns | ns | ns |
| Water regime × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Water regime × N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | N Concentration (%) | N Accumulation (mg hill−1) | NTU (mg hill−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NCG | NCS | NAG | NAS | (NAG+NAS) | ||||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| WSS × N0 | 1.02 ± 0.05 Ac | 1.28 ± 0.1 Ad | 0.57 ± 0.04 Ab | 0.63 ± 0.03 Ac | 534.6 ± 36.3 Ad | 682.79 ± 42.0 Ad | 363.0 ± 40.2 Ac | 459.97 ± 52.5 Ad | 897.6 ± 73.5 Ad | 1142.75 ± 88.5 Ad |
| WSS × NL | 1.68 ± 0.06 Ab | 1.81 ± 0.2 AC | 0.71 ± 0.05 Aa | 0.75 ± 0.04 Ac | 1112 ± 81.8 Ac | 1206.77 ± 178 Ac | 507.6 ± 23.8 Ab | 666.69 ± 30.7 Ac | 1619.3 ± 99.6 Ac | 1873.46 ± 205.8 Ac |
| WSS × NM | 1.97 ± 0.10 Ab | 2.0 ± 0.1 Ab | 0.74 ± 0.09 Aa | 0.82 ± 0.03 Ab | 1421 ± 53.3 Ab | 1454.86 ± 41.4 Ab | 559.2 ± 53.7 Ab | 751.75 ± 66.8 Ab | 1980.0 ± 58.9 Ab | 2206.61 ± 25.8 Ab |
| WSS × NH | 2.32 ± 0.13 Aa | 2.43 ± 0.2 Aa | 0.79 ± 0.11 Aa | 1.07 ± 0.07 Aa | 1919.7 ± 10 Aa | 1953.27 ± 94.9 Aa | 714.5 ± 70.7 Aa | 953.95 ± 69.8 Aa | 2634.2 ± 141 Aa | 2907.22 ± 133.5 Aa |
| WMD × N0 | 0.70 ± 0.05 Bb | 0.97 ± 0.1 Bd | 0.52 ± 0.04 Bb | 0.81 ± 0.13 Bc | 310.6 ± 41.1 Bd | 431.66 ± 48.7 Bd | 322.1 ± 49.6 Bc | 391.00 ± 40.3 Bd | 632.7 ± 76.4 Bc | 822.66 ± 74.6 Bd |
| WMD × NL | 1.48 ± 0.08 Ba | 1.53 ± 0.1 Bc | 0.57 ± 0.06 Bb | 0.72 ± 0.07 Bc | 857.8 ± 26.5 Bc | 895.42 ± 23.7 Bc | 387.3±45.2 Bb | 541.95±52.5 Bc | 1245.1±79.9 Bc | 1437.37 ± 74.5 Bc |
| WMD × NM | 1.65 ± 0.03 Ba | 1.7 ± 0.1 Bb | 0.63 ± 0.03 Ba | 0.77 ± 0.00 Bb | 1044 ± 42.5 Bb | 1081.18 ± 48.4 Bb | 445.8 ± 39.7 Bb | 606.88 ± 60.0 Bb | 1490.1 ± 93.5 Bb | 1688.06 ± 106.5 Bb |
| WMD × NH | 1.87 ± 0.05 Ba | 1.93 ± 0.1 Ba | 0.72 ± 0.03 Ba | 1.00 ± 0.04 Ba | 1479.2 ± 47 Ba | 1534.45 ± 47.9 Ba | 565.0 ± 46.5 Ba | 750.31 ± 63.1 Ba | 2044.2 ± 32.2 Ba | 2284.76 ± 109.9 Ba |
| WSD × N0 | 0.40 ± 0.03 Cc | 0.66 ± 0.1 Cd | 0.22 ± 0.02 Cb | 0.46 ± 0.01 Cc | 147.9 ± 17.9 Cd | 244.27 ± 22.7 Cd | 115.3 ± 14.5 Cc | 172.10 ± 24.0 Cd | 263.1 ± 54.8 Cd | 416.38 ± 46.7 Ad |
| WSD × NL | 0.85 ± 0.05 Cb | 0.98 ± 0.1 Cc | 0.33 ± 0.05 Cb | 0.66 ± 0.06 Cc | 476.7 ± 58.1 Cc | 550.24 ± 60.7 Cc | 213.0 ± 30.7 Cb | 371.03 ± 36.5 Cc | 689.8 ± 43.4 Cc | 921.279 ± 76.3 Ac |
| WSD × NM | 0.96 ± 0.06 Cb | 1.08 ± 0.0 Cb | 0.51 ± 0.04 Ca | 0.84 ± 0.04 Cb | 578.2 ± 13.3 Cb | 653.61 ± 12.8 Cb | 365.1 ± 36.4 Ca | 535.82 ± 45.3 Cb | 943.3 ± 21.2 Cb | 1189.44 ± 41.0 Ad |
| WSD × NH | 1.08 ± 0.06 Ca | 1.20 ± 0.1 Ca | 0.53 ± 0.05 Ca | 0.86 ± 0.04 Ca | 756.2 ± 61.1 Ca | 846.58 ± 62.1 Ca | 374.7 ± 7.1 Ca | 542.88 ± 21.0 Ca | 1130.9 ± 67.7 Ca | 1389.46 ± 74.1 Aa |
| ANOVA | ||||||||||
| Water regime | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| N fertilizer rate | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Water × N rate | *** | *** | ** | *** | *** | *** | ** | *** | *** | *** |
| Water regime × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Water regime × N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | NUE Indicators | |||||||
|---|---|---|---|---|---|---|---|---|
| NAE (%) | NARE (%) | NPFP (g g−1) | NHI (%) | |||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| WSS × NL | 37.3 ± 1.31 Aa | 39.5 ± 1.50 Aa | 63.1 ± 4.33 Aa | 67.61 ± 4.95 Aa | 39.9 ± 1.50 Aa | 41.13 ± 1.94 Aa | 76.6 ± 1.39 Aa | 77.43 ± 1.72 Aa |
| WSS × NM | 22.0 ± 0.55 Ab | 22.8 ± 0.53 Ab | 54.4 ± 1.91 Ab | 56.25 ± 2.70 Ab | 24.5 ± 0.55 Ab | 25.2 ± 0.80 Ab | 71.8 ± 2.20 Aa | 73.2 ± 2.62 Ab |
| WSS × NH | 17.4 ± 0.2 Ac | 18.07 ± 0.31 Ac | 52.8 ± 3.00 Ab | 54.99 ± 3.49 Ac | 19.0 ± 0.20 Ac | 21.03 ± 0.86 Ac | 68.6 ± 0.94 Ab | 69.97 ± 0.67 Ac |
| WMD × NL | 32.9 ± 1.2 Ba | 32.4 ± 1.83 Ba | 59.1 ± 230 Ba | 61.28 ± 3.18 Ba | 37.3 ± 0.98 Ba | 38.1 ± 1.01 Ba | 72.4 ± 0.81 Ba | 73.5 ± 1.10 Aa |
| WMD × NM | 19.3 ± 0.9 Bb | 20.37 ± 1.06 Bb | 44.9 ± 2.42 Bb | 46.52 ± 2.73 Bb | 21.8 ± 0.73 Bb | 22.87 ± 0.12 Bb | 70.0 ± 0.73 Ba | 71.23 ± 0.75 Ab |
| WMD × NH | 16.5 ± 0.3 Bb | 17.4 ± 0.35 Bc | 41.4 ± 1.01 Bb | 44.45 ± 0.73 Bc | 18.2 ± 0.25 Bb | 19.33 ± 0.61 Bc | 69.6 ± 0.34 Bb | 70.13 ± 0.70 Ac |
| WSD × NL | 31.5 ± 1.7 Ca | 32.5 ± 0.98 Ca | 38.6 ± 1.78 Ca | 41.53 ± 3.05 Ca | 36.0 ± 1.46 Ca | 37.4 ± 2.12 Ca | 69.0 ± 3.91 Ca | 70.44 ± 4.80 Ba |
| WSD × NM | 18.4 ± 0.7 Cb | 19.07 ± 1.21 Cb | 31.6 ± 1.10 Cb | 33.84 ± 1.50 Cb | 20.8 ± 0.57 Cb | 21.67 ± 0.61 Cb | 66.8 ± 1.19 Ca | 68.07 ± 1.79 Bb |
| WSD × NH | 14.7 ± 0.6 Cb | 14.87 ± 1.42 Cc | 26.7 ± 1.04 Cc | 28.65 ± 1.61 Cc | 16.4 ± 0.46 Cc | 17.13 ± 0.70 Cc | 61.3 ± 2.46 Cb | 63.13 ± 1.90 Bc |
| ANOVA | ||||||||
| Water regime | *** | *** | ** | *** | *** | *** | *** | *** |
| N rate | *** | *** | *** | *** | *** | *** | ** | ** |
| Water × N rate | ns | ns | ns | ns | ns | ns | ns | ns |
| Water regime × Year | ns | ns | ns | ns | ns | ns | ns | ns |
| N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns |
| Water regime × N rate × Year | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL Aasmi, A.; Li, J.; Hamoud, Y.A.; Lan, Y.; Alordzinu, K.E.; Appiah, S.A.; Shaghaleh, H.; Sheteiwy, M.; Wang, H.; Qiao, S.; et al. Impacts of Slow-Release Nitrogen Fertilizer Rates on the Morpho-Physiological Traits, Yield, and Nitrogen Use Efficiency of Rice under Different Water Regimes. Agriculture 2022, 12, 86. https://doi.org/10.3390/agriculture12010086
AL Aasmi A, Li J, Hamoud YA, Lan Y, Alordzinu KE, Appiah SA, Shaghaleh H, Sheteiwy M, Wang H, Qiao S, et al. Impacts of Slow-Release Nitrogen Fertilizer Rates on the Morpho-Physiological Traits, Yield, and Nitrogen Use Efficiency of Rice under Different Water Regimes. Agriculture. 2022; 12(1):86. https://doi.org/10.3390/agriculture12010086
Chicago/Turabian StyleAL Aasmi, Alaa, Jiuhao Li, Yousef Alhaj Hamoud, Yubin Lan, Kelvin Edom Alordzinu, Sadick Amoakohene Appiah, Hiba Shaghaleh, Mohamed Sheteiwy, Hao Wang, Songyang Qiao, and et al. 2022. "Impacts of Slow-Release Nitrogen Fertilizer Rates on the Morpho-Physiological Traits, Yield, and Nitrogen Use Efficiency of Rice under Different Water Regimes" Agriculture 12, no. 1: 86. https://doi.org/10.3390/agriculture12010086








