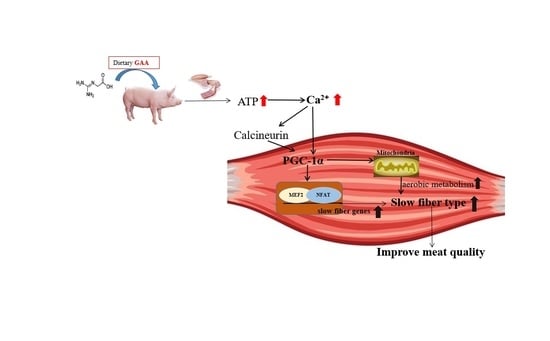
Guanidinoacetic Acid Supplementation Promotes Skeletal Muscle Fiber Type Transformation from Fast-to-Slow-Twitch via Increasing the PPARGC1A Based Mitochondrial Function and CaN/NFAT Pathway in Finishing Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Treatment
2.2. Sample Collection
2.3. Antibodies
2.4. Measurement of Sarcoplasmic Ca2+ Contents
2.5. Measurement of Mitochondrial Membrane Potential, Mitochondrial Respiratory Chain Complex I, and Mitochondrial Respiratory Chain Complex III
2.6. Gene Expression Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Skeletal Muscle Fiber Type Transformation
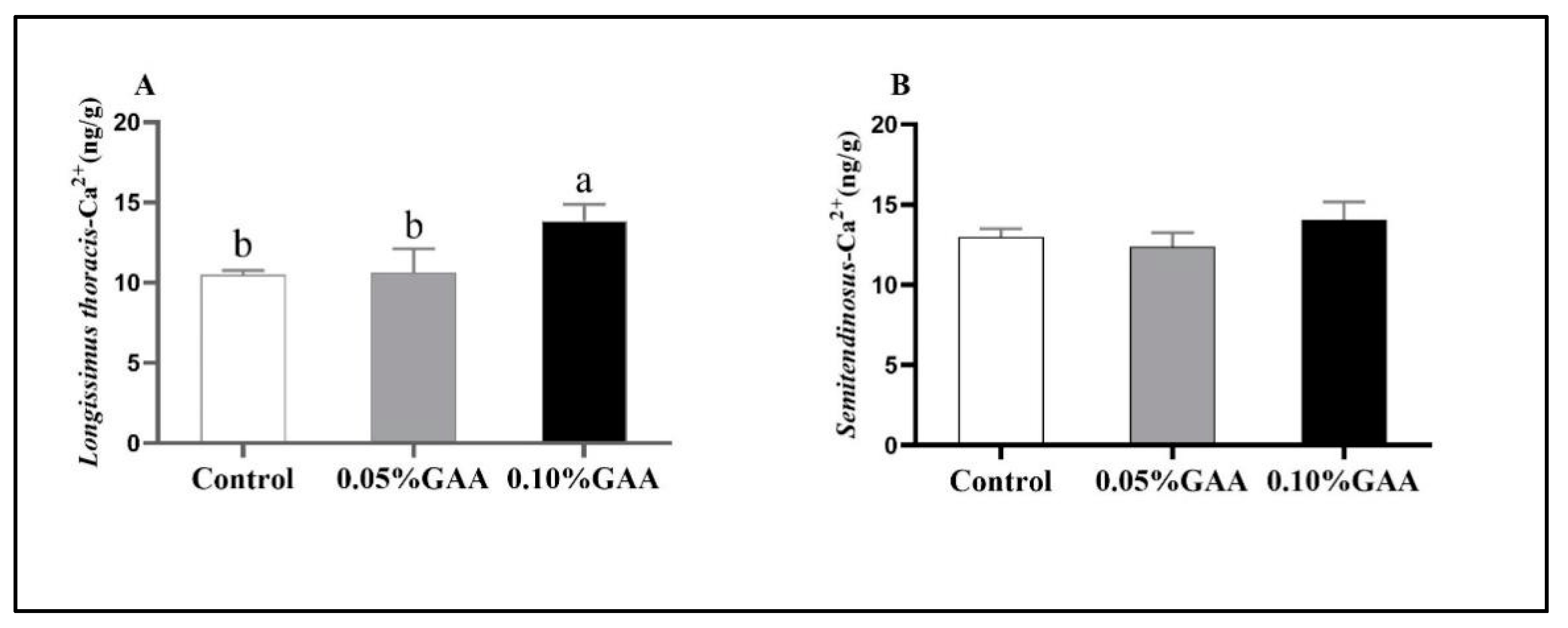
3.2. Sarcoplasmic Ca2+ Contents
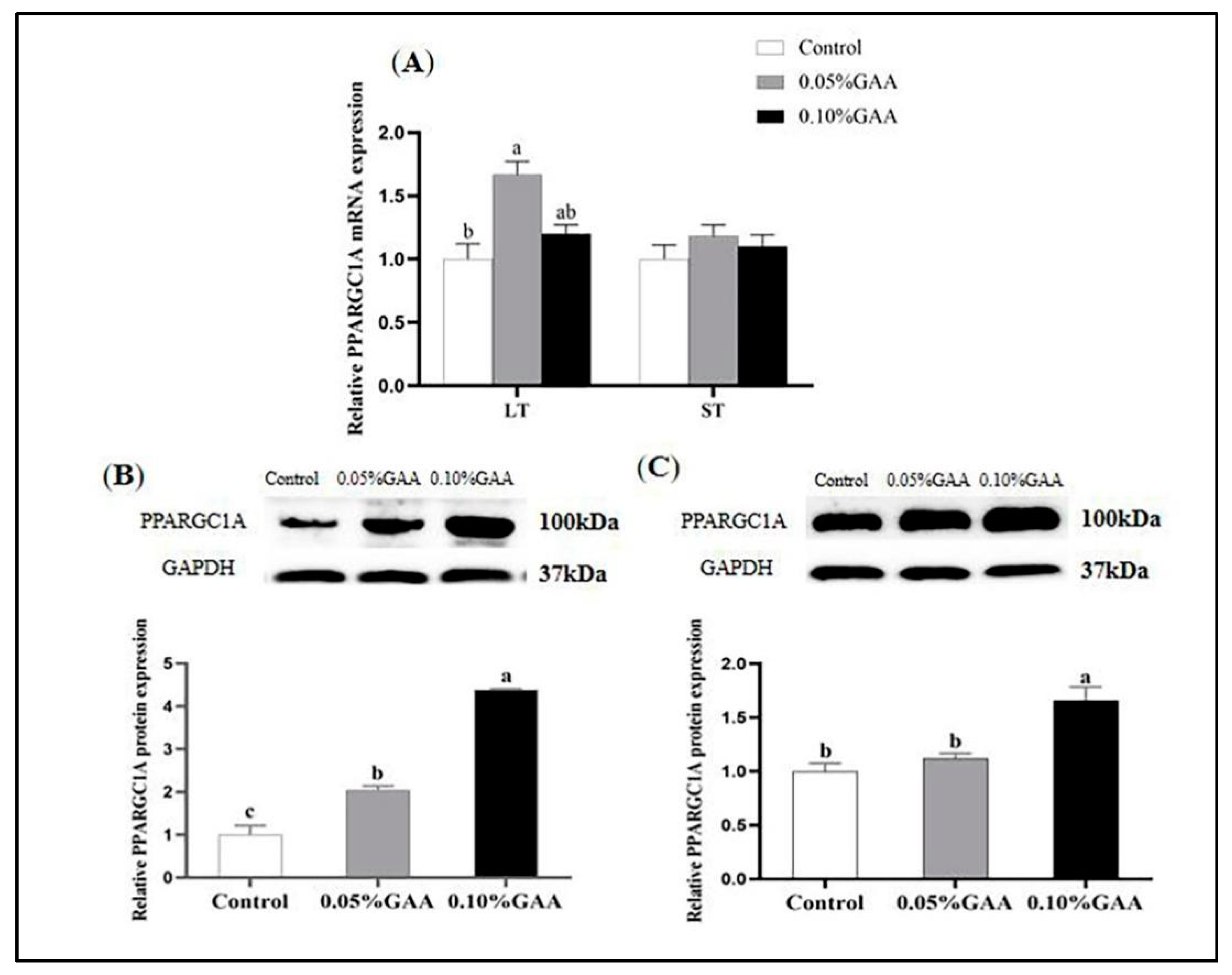
3.3. Gene and Protein Expressions of PPARGC1A
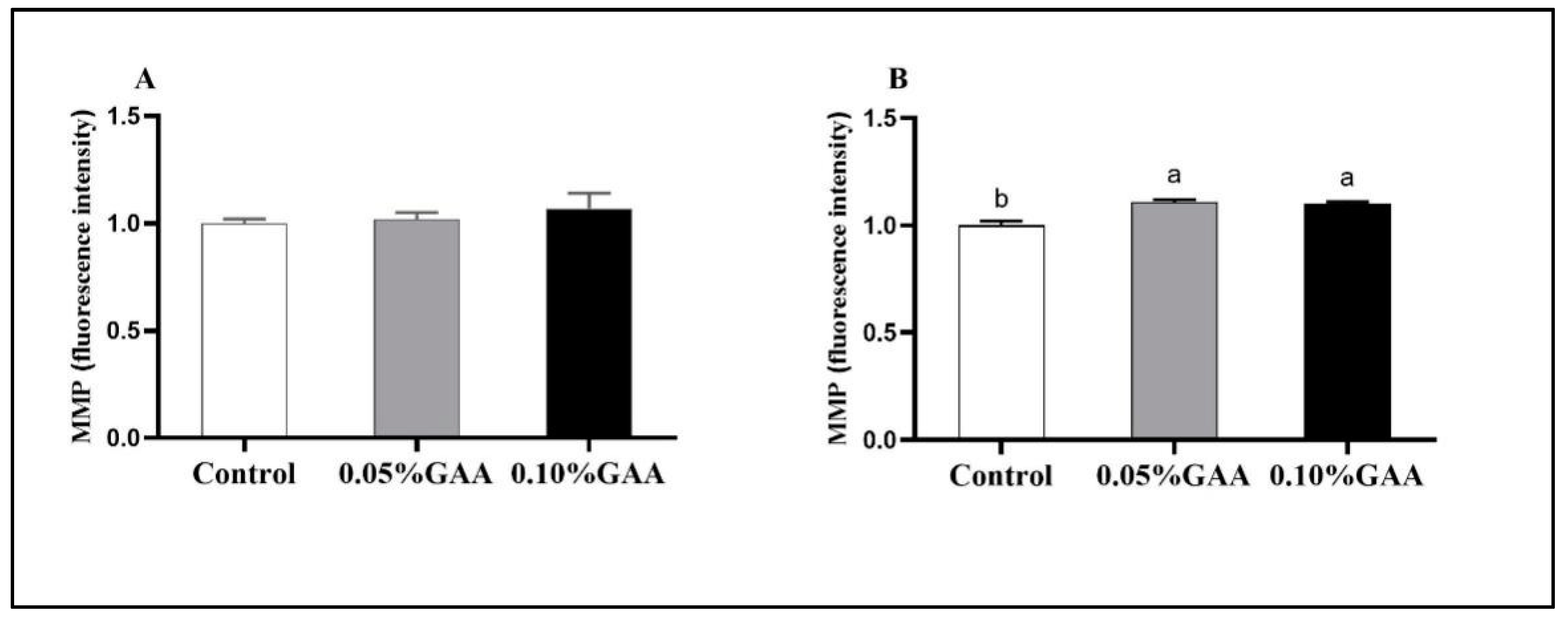
3.4. Indexes Related to Mitochondrial Function
3.5. Related Genes and Protein Expressions of Mitochondrial Synthesis
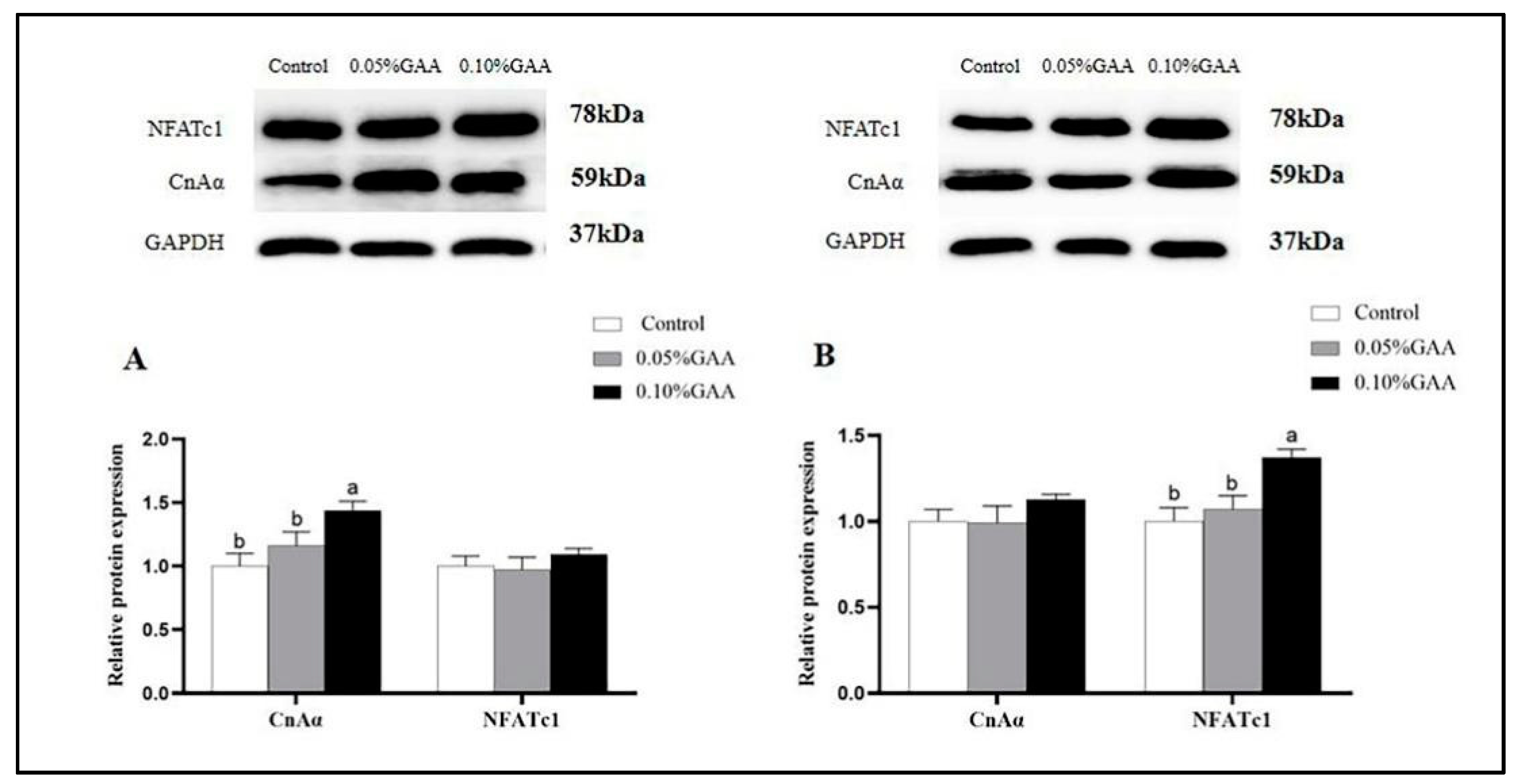
3.6. Protein Expressions of CnAα and NFATc1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefaucheur, L. A second look into fibre typing–relation to meat quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef]
- Talmadge, R.J.; Roy, R.R.; Edgerton, R.V. Muscle fiber types and function. Curr. Opin. Rheumatol. 1993, 5, 695–705. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Men, X.M.; Deng, B.; Xu, Z.W.; Tao, X.; Qi, K.K. Age-related changes and nutritional regulation of myosin heavy-chain composition in longissimus thoracis of commercial pigs. Animal 2013, 7, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Jin, J.P. TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships. Gene 2016, 582, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, Y.; Zheng, P.; Yu, J.; He, J. Grape seed proanthocyanidin extract promotes skeletal muscle fiber type transformation via AMPK signaling pathway. J. Nutr. Biochem. 2020, 84, 108462. [Google Scholar] [CrossRef]
- Luo, P.; Luo, L.; Zhao, W.; Wang, L.; Sun, L.; Wu, H.; Li, Y.; Zhang, R.; Shu, G.; Wang, S.; et al. Dietary thymol supplementation promotes skeletal muscle fibre type switch in longissimus thoracis of finishing pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; He, Y.; Du, H.; Yang, J.; Wan, H. Output Regulation and Function Optimization of Mitochondria in Eukaryotes. Front. Cell Dev. Biol. 2020, 8, 598112. [Google Scholar] [CrossRef]
- Shen, L.Y.; Luo, J.; Lei, H.G.; Jiang, Y.Z.; Bai, L.; Li, M.Z.; Tang, G.Q.; Li, X.W.; Zhang, S.H.; Zhu, L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015, 14, 14366–14378. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biosynthesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011, 110, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Popov, D.V. Adaptation of Skeletal Muscles to Contractile Activity of Varying Duration and Intensity: The Role of PGC-1α. Biochemistry 2018, 83, 613–628. [Google Scholar] [CrossRef]
- Ying, F.; Zhang, L.; Bu, G.; Xiong, Y.; Zuo, B. Muscle fiber-type conversion in the transgenic pigs with overexpression of PGC-1α gene in muscle. Biochem. Biophys. Res. Commun. 2016, 480, 669–674. [Google Scholar] [CrossRef]
- Ducreux, S.; Gregory, P.; Schwaller, B. Inverse regulation of the cytosolic Ca2+ buffer parvalbumin and mitochondrial volume in muscle cells via Sirt1/PGC-1α axis. PLoS ONE 2012, 7, e44837. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tao, S.; Li, X.; Yao, Q. Resistin destroys mitochondrial biosynthesis by inhibiting the PGC-1α/NRF1/TFAM signaling pathway. Biochem. Biophys. Res. Commun. 2018, 504, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, L.; Luo, L.; Wang, L.; Yang, K.; Shu, G.; Wang, S.; Zhu, X.; Gao, P.; Jiang, Q. Ca2+-Calcineurin-NFAT pathway mediates the effect of thymol on oxidative metabolism and fiber-type switch in skeletal muscle. Food Funct. 2019, 10, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Benefits and drawbacks of guanidinoacetic acid as a possible treatment to replenish cerebral creatine in AGAT deficiency. Nutr. Neurosci. 2019, 22, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Edison, E.E.; Brosnan, M.E.; Meyer, C.; Brosnan, J.T. Creatine synthesis: Production of guanidinoacetate by the rat and human kidney in vivo. Am. J. Physiol. Ren. Physiol. 2007, 293, 1799–1804. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, J.L.; Xing, T.; Zhang, L.; Gao, F. Effects of guanidinoacetic acid and complex antioxidant supplementation on growth performance, meat quality, and antioxidant function of broiler chickens. J. Sci. Food Agric. 2020, 101, 3961–3968. [Google Scholar] [CrossRef]
- Volek, J.S.; Duncan, N.D.; Mazzetti, S.A.; Staron, R.S.; Putukian, M.; Gómez, A.L.; Pearson, D.R.; Fink, W.J.; Kraemer, W.J. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med. Sci. Sports Exerc. 1999, 31, 1147–1156. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, C.; Hu, S.; Li, B.; Zeng, X.; Yin, J. Dietary guanidinoacetic acid supplementation improved carcass characteristics, meat quality and muscle fibre traits in growing-finishing gilts. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1454–1461. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Tian, X.; Fan, X.; Shi, Y.; Zhang, W.; Hou, Q.; Zhou, G. Comparison of Activity, Expression, and S-Nitrosylation of Calcium Transfer Proteins between Pale, Soft, and Exudative and Red, Firm, and Non-exudative Pork during Post-Mortem Aging. J. Agric. Food Chem. 2019, 67, 3242–3248. [Google Scholar] [CrossRef]
- Serrano, A.L.; Petrie, J.L.; Rivero, J.L.; Hermanson, J.W. Myosin isoforms and muscle fiber characteristics in equine gluteus medius muscle. Anat. Rec. 1996, 244, 444–451. [Google Scholar] [CrossRef]
- Bao, G.; Liu, X.; Wang, J.; Hu, J.; Shi, B.; Li, S.; Luo, Y. Effects of Slaughter Age on Myosin Heavy Chain Isoforms, Muscle Fibers, Fatty Acids, and Meat Quality in Longissimus Thoracis Muscle of Tibetan Sheep. Front. Vet. Sci. 2021, 8, 689589. [Google Scholar] [CrossRef] [PubMed]
- Reiser, P.J. Current understanding of conventional and novel co-expression patterns of mammalian sarcomeric myosin heavy chains and light chains. Arch. Biochem. Biophys. 2019, 662, 129–133. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; López-Ordaz, R.; Hernández-García, P.A. Productive Performance, Carcass Traits, and Meat Quality in Finishing Lambs Supplemented with a Polyherbal Mixture. Agriculture 2021, 11, 942. [Google Scholar] [CrossRef]
- Muroya, S.; Nakajima, I.; Chikuni, K. Amino acid sequences of multiple fast and slow troponin T isoforms expressed in adult bovine skeletal muscles. J. Anim. Sci. 2003, 81, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef]
- Russell, A.P.; Feilchenfeldt, J.; Schreiber, S.; Praz, M.; Crettenand, A.; Gobelet, C.; Meier, C.A.; Bell, D.R.; Kralli, A.; Giacobino, J.P.; et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 2003, 52, 2874–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.K.; Park, J.H.; Baek, Y.Y.; Won, M.H.; Jeoung, D.; Lee, H.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca2+ channel-mediated PGC-1α/ERRα activation. Biochem. Biophys. Res. Commun. 2016, 479, 297–304. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- He, D.T.; Gai, X.R.; Yang, L.B.; Li, J.T.; Lai, W.Q.; Sun, X.L.; Zhang, L.Y. Effects of guanidinoacetic acid on growth performance, creatine and energy metabolism, and carcass characteristics in growing-finishing pigs. J. Anim. Sci. 2018, 96, 3264–3273. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Venhoff, N.; Lebrecht, D.; Pfeifer, D.; Venhoff, A.C.; Bissé, E.; Kirschner, J.; Walker, U.A. Muscle-fiber transdifferentiation in an experimental model of respiratory chain myopathy. Arthritis Res. Ther. 2012, 14, R233. [Google Scholar] [CrossRef] [Green Version]
- Rasbach, K.A.; Gupta, R.K.; Ruas, J.L.; Wu, J.; Naseri, E.; Estall, J.L.; Spiegelman, B.M. PGC-1-alpha regulates a HIF-2-alpha-dependent switch in skeletal muscle fiber types. Proc. Natl. Acad. Sci. USA 2010, 107, 21866–21871. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Pessemesse, L.; Grandemange, S.; Seyer, P.; Gueguen, N.; Baris, O.; Lepourry, L.; Cabello, G.; Wrutniak-Cabello, C. Overexpression of the mitochondrial T3 receptor p43 induces a shift in skeletal muscle fiber types. PLoS ONE 2008, 3, e2501. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. Mitochondrial Membrane Potential (ΔΨ) Fluctuations Associated with the Metabolic States of Mitochondria. Methods Mol. Biol. 2018, 1782, 109–119. [Google Scholar] [CrossRef]
- Bagkos, G.; Koufopoulos, K.; Piperi, C. A new model for mitochondrial membrane potential production and storage. Med. Hypotheses 2014, 83, 175–181. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef] [Green Version]
- Mulder, H. Transcribing β-cell mitochondria in health and disease. Mol. Metab. 2017, 6, 1040–1051. [Google Scholar] [CrossRef]
- Wenz, T. Regulation of mitochondrial biosynthesis and PGC-1α under cellular stress. Mitochondrion 2013, 13, 134–142. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.; Li, F.; Tang, C.; Yin, D. Calcineurin-NFAT Signaling and Neurotrophins Control Transformation of Myosin Heavy Chain Isoforms in Rat Soleus Muscle in Response to Aerobic Treadmill Training. J. Sports Sci. Med. 2014, 13, 934–944. [Google Scholar]
- Parsons, S.A.; Wilkins, B.J.; Bueno, O.F.; Molkentin, J.D. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol. Cell. Biol. 2003, 23, 4331–4343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts-Wilson, T.K.; Reddy, R.N.; Bailey, J.L.; Zheng, B.; Ordas, R.; Gooch, J.L.; Price, S.R. Calcineurin signaling and PGC-1alpha expression are suppressed during muscle atrophy due to diabetes. Biochim. Biophys. Acta 2010, 1803, 960–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Rothermel, B.; Kanatous, S.; Rosenberg, P.; Naya, F.J.; Shelton, J.M.; Hutcheson, K.A.; DiMaio, J.M.; Olson, E.N.; Bassel-Duby, R.; et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001, 20, 6414–6423. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Lu, Y.; Zou, T.; Wang, Z.; Yang, J.; Li, L.; Guo, X.; He, Q.; Chen, L.; You, J. Dietary guanidinoacetic acid improves the growth performance and skeletal muscle development of finishing pigs through changing myogenic gene expression and myofibre characteristics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1875–1883. [Google Scholar] [CrossRef] [PubMed]



| Composition | % | Nutrient Level | % |
|---|---|---|---|
| Maize | 72 | Digestible energy (MJ kg−1) | 13.91 |
| Soybean meal | 20 | Crude protein | 15.81 |
| Wheat bran | 4 | Calcium | 0.88 |
| Dicalcium phosphate | 1.4 | Total phosphorus | 0.57 |
| Limestone | 1.05 | Lysine | 0.98 |
| Lysine·HCl | 0.2 | Methionine | 0.23 |
| Sodium chloride | 0.35 | Threonine | 0.58 |
| Premix A | 1 | Tryptophan | 0.18 |
| Total | 100 |
| Genes | Prime Sequences | Product Lengths | GenBank Numbers |
|---|---|---|---|
| PPARGC1A | F:AAGGATGCGCTCTCGTTCAA | 184 | XM_021100442.1 |
| R:TGAACGTGATCTGGCGCAC | |||
| TFAM | F:GAAAGTCAGGAGCGGACCTC | 289 | NM_001130211 |
| R:TTGTACACCTGCCAGTCTGC | |||
| NRF1 | F:TCCTTCATCTCCTGAAGACACC | 156 | XM_021079000.1 |
| R:ACATGAGGCCGTTTCCGTTT | |||
| SIRT1 | F:AGGTTTGAAGAATGTTGCCTGC | 256 | NM_001145750 |
| R:TGGACTCTGGCAAGTTCCAC | |||
| TFB1M | F:ATGGAACGCACTGGGAAACT | 175 | NM_001128475.1 |
| R:TTTCTGACGTGTGCCTGAG | |||
| GAPDH | F: CCCGCCAACATCAAATGGG | 175 | NM_001206359.1 |
| R: ACTTCTCATGGTTCACGCCC |
| Item | Control | 0.05%GAA | 0.10%GAA | SEM | p-Value |
|---|---|---|---|---|---|
| longissimus thoracis | |||||
| mitochondrial respiratory chain complex I | |||||
| (U mg−1 protein) | 0.63 | 0.65 | 0.98 | 0.08 | 0.181 |
| mitochondrial respiratory chain complex III | |||||
| (U mg−1 protein) | 0.23 b | 0.48 a | 0.51 a | 0.05 | 0.009 |
| semitendinosus | |||||
| mitochondrial respiratory chain complex I | |||||
| (U mg−1 protein) | 0.62 b | 0.81 b | 1.36 a | 0.12 | 0.006 |
| mitochondrial respiratory chain complex III | |||||
| (U mg−1 protein) | 0.21 | 0.28 | 0.22 | 0.02 | 0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, J.; Zhang, L.; Xing, T.; Jiang, Y.; Gao, F. Guanidinoacetic Acid Supplementation Promotes Skeletal Muscle Fiber Type Transformation from Fast-to-Slow-Twitch via Increasing the PPARGC1A Based Mitochondrial Function and CaN/NFAT Pathway in Finishing Pigs. Agriculture 2022, 12, 87. https://doi.org/10.3390/agriculture12010087
Li J, Li J, Zhang L, Xing T, Jiang Y, Gao F. Guanidinoacetic Acid Supplementation Promotes Skeletal Muscle Fiber Type Transformation from Fast-to-Slow-Twitch via Increasing the PPARGC1A Based Mitochondrial Function and CaN/NFAT Pathway in Finishing Pigs. Agriculture. 2022; 12(1):87. https://doi.org/10.3390/agriculture12010087
Chicago/Turabian StyleLi, Jingzheng, Jiaolong Li, Lin Zhang, Tong Xing, Yun Jiang, and Feng Gao. 2022. "Guanidinoacetic Acid Supplementation Promotes Skeletal Muscle Fiber Type Transformation from Fast-to-Slow-Twitch via Increasing the PPARGC1A Based Mitochondrial Function and CaN/NFAT Pathway in Finishing Pigs" Agriculture 12, no. 1: 87. https://doi.org/10.3390/agriculture12010087
APA StyleLi, J., Li, J., Zhang, L., Xing, T., Jiang, Y., & Gao, F. (2022). Guanidinoacetic Acid Supplementation Promotes Skeletal Muscle Fiber Type Transformation from Fast-to-Slow-Twitch via Increasing the PPARGC1A Based Mitochondrial Function and CaN/NFAT Pathway in Finishing Pigs. Agriculture, 12(1), 87. https://doi.org/10.3390/agriculture12010087