Pretreatment of Garlic Oil Extracts Hampers Epithelial Damage in Cell Culture Model of Peptic Ulcer Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
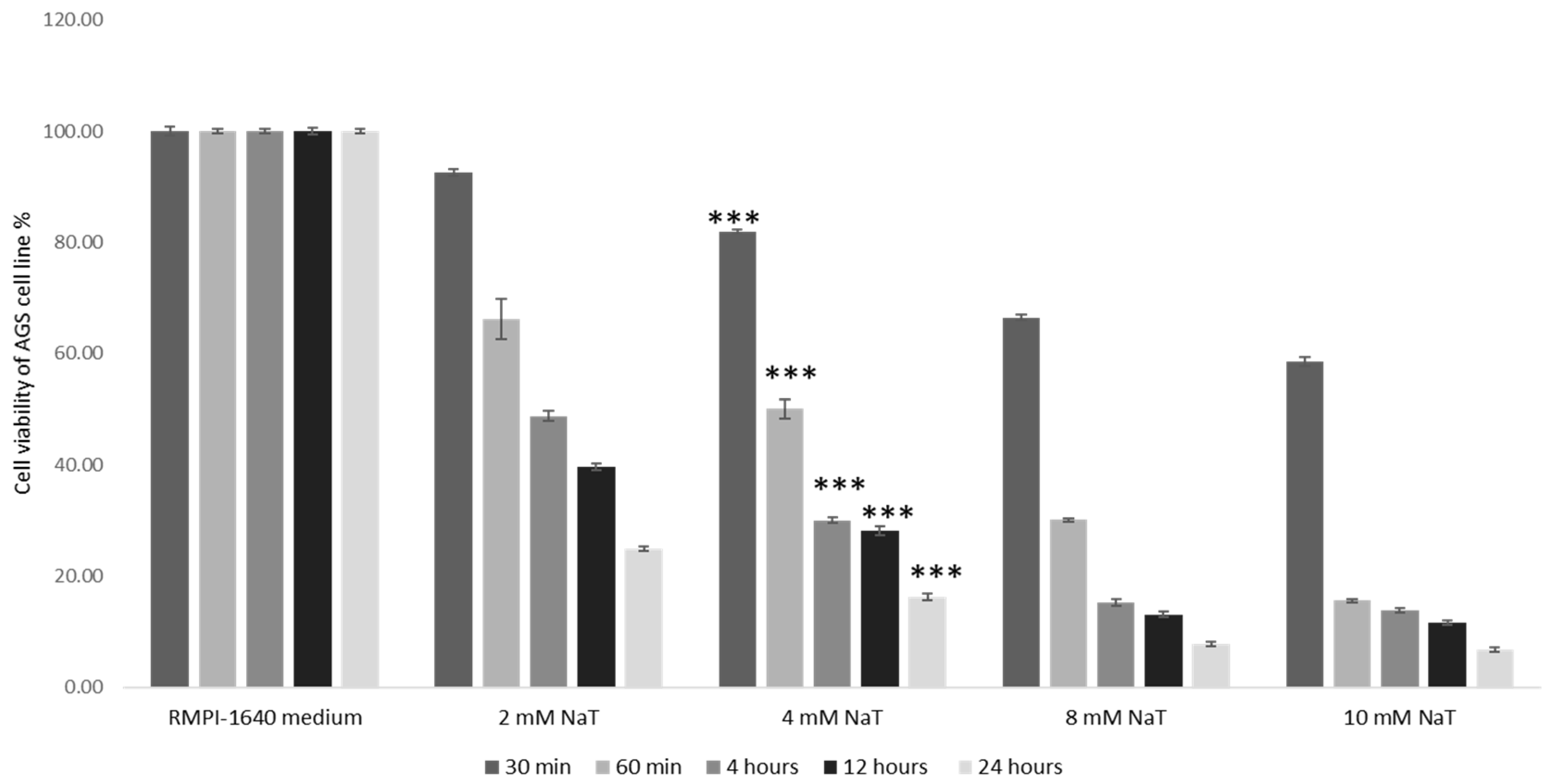
2.2. Sodium Taurocholate-Induced Damage to AGS Cells
2.3. Measurement of the Gastroprotective Effect of Garlic Extracts (GE) in a Cell Culture Model of Peptic Ulcer Disease
2.4. Measurement of Cellular Glutathione (GSH) Concentration
2.5. Measurement of Prostaglandin E2 (PGE2) Concentration
2.6. Total RNA Isolation and Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.7. Visualization of the F-Actin Cytoskelet with Rhodamine Phalloidin Stain
2.8. Statistical Analyses
3. Results
3.1. Establishment of the Cell Culture Model of Peptic Ulcer Disease and Assessment of the Toxic Effect of Sodium Taurocholate (NaT)
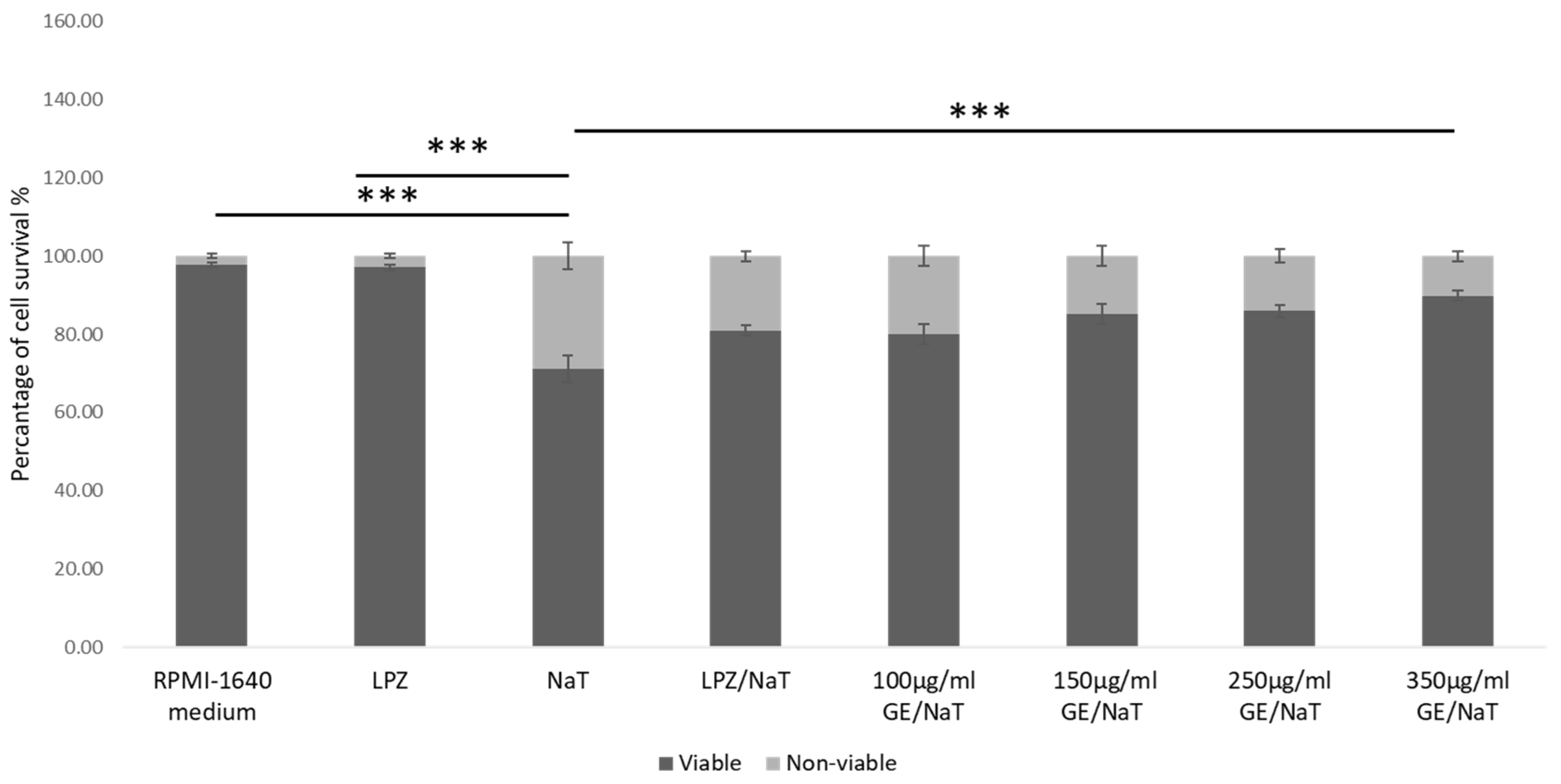
3.2. Gastroprotective Effect of Garlic Extracts (GE) in a Cell Culture Model of Peptic Ulcer Disease
3.3. Measurement of Cellular Glutathione (GSH) Concentration in a Cell Culture Model of Peptic Ulcer Disease
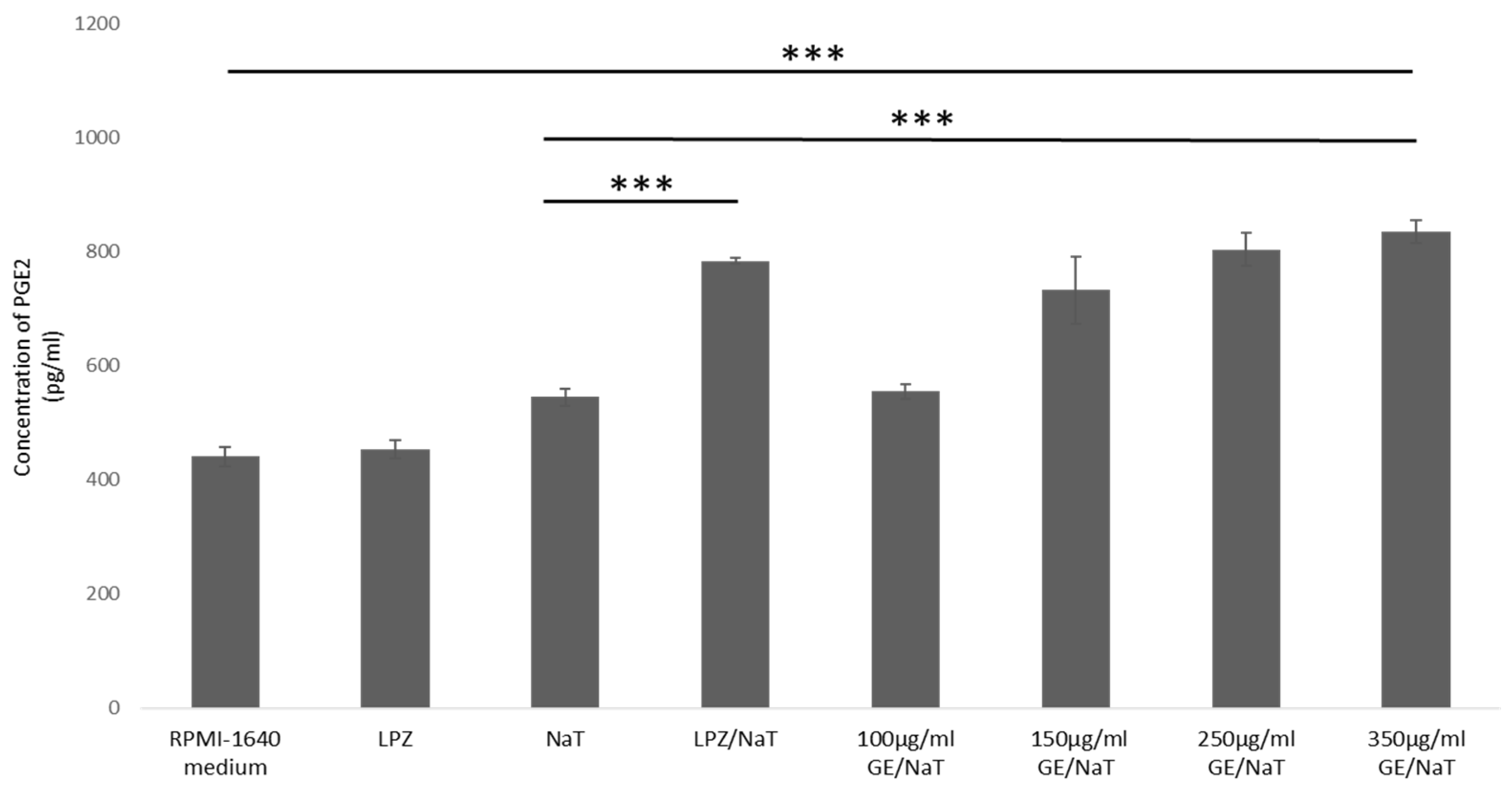
3.4. Measurement of Prostaglandin E2 (PGE2) Concentration in a Cell Culture Model of Peptic Ulcer Disease
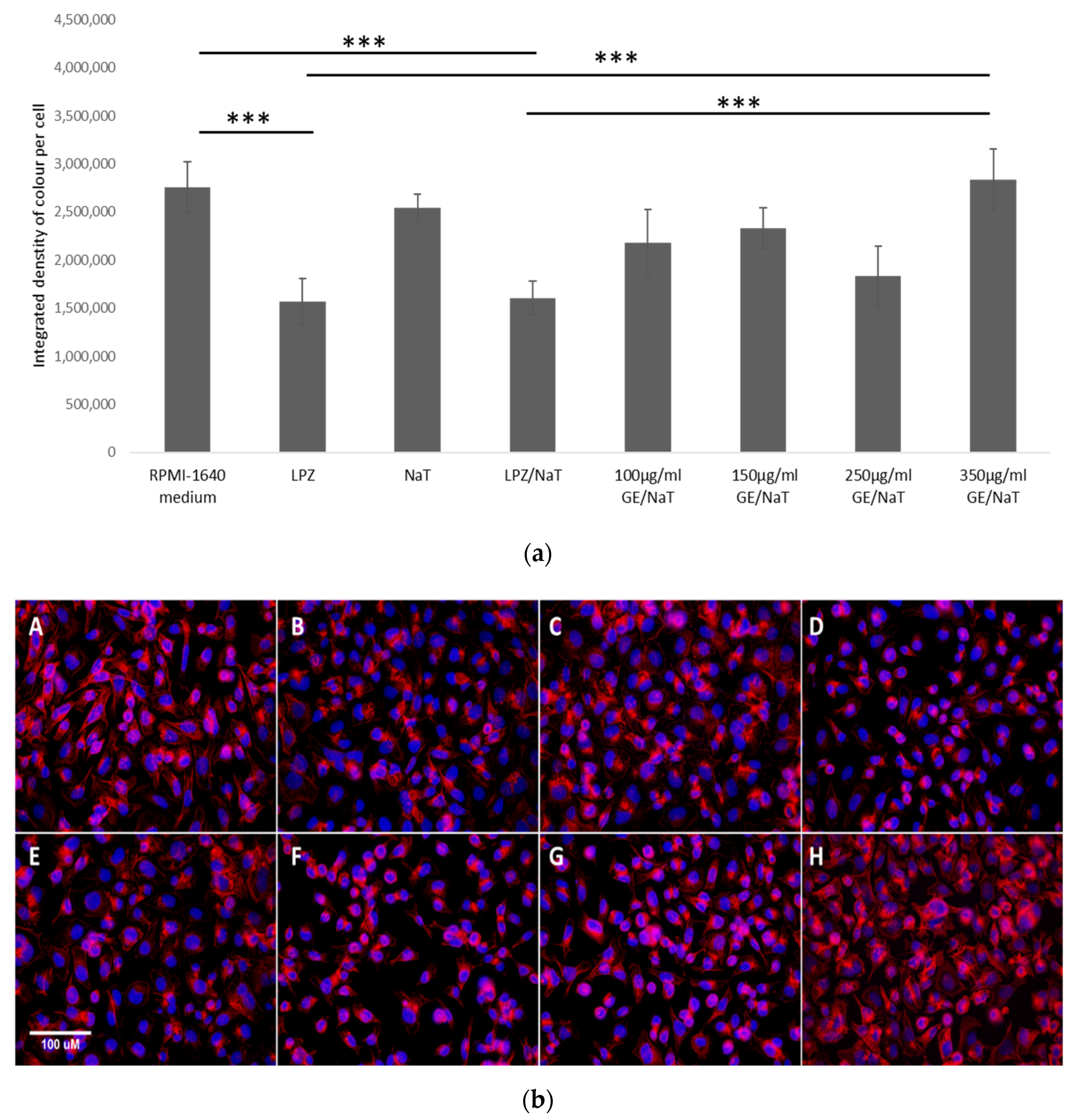
3.5. Visualization and Quantification of the F-Actin Cytoskeleton with Rhodamine Phalloidin Stain in a Cell Culture Model of Peptic Ulcer Disease
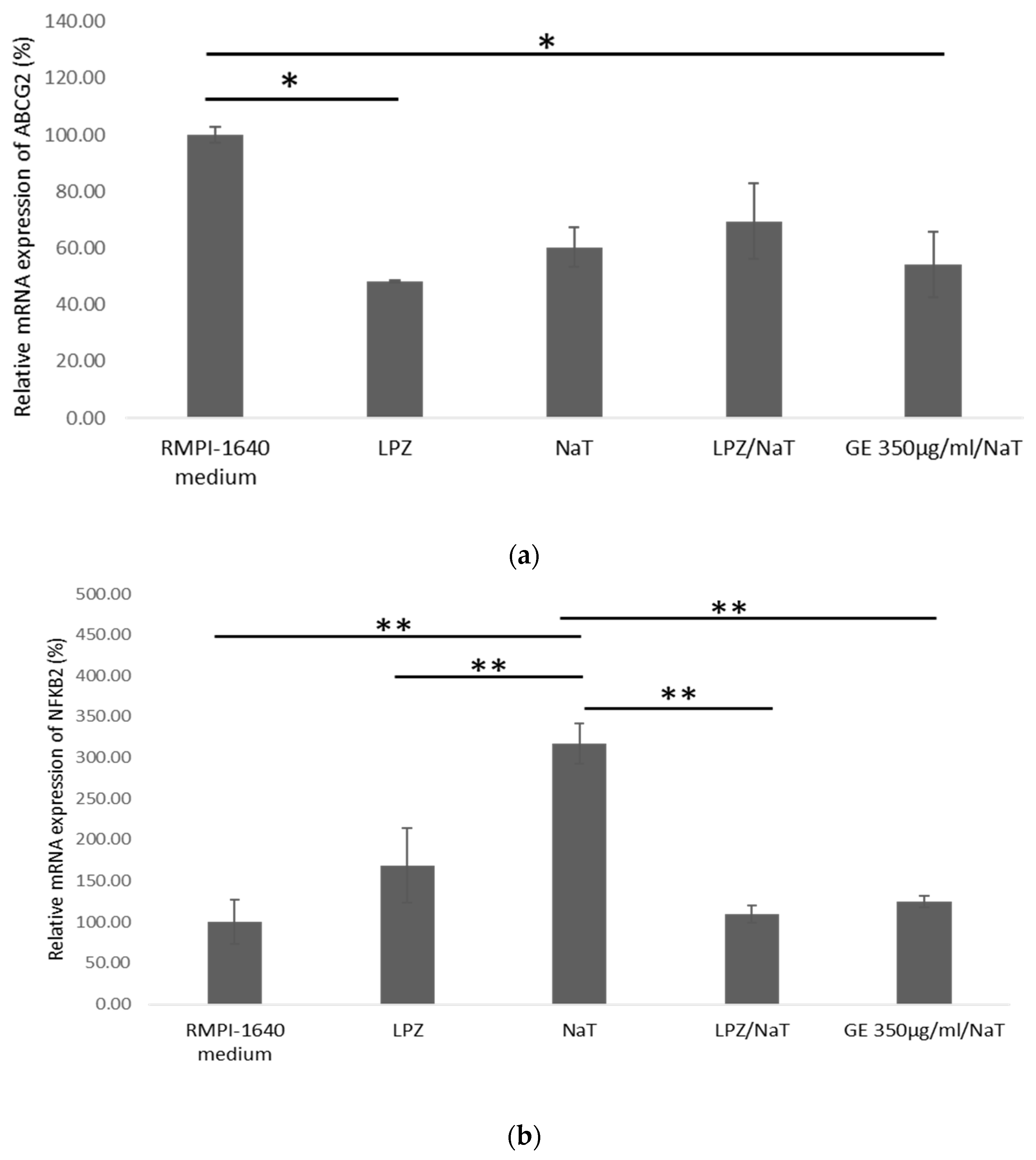
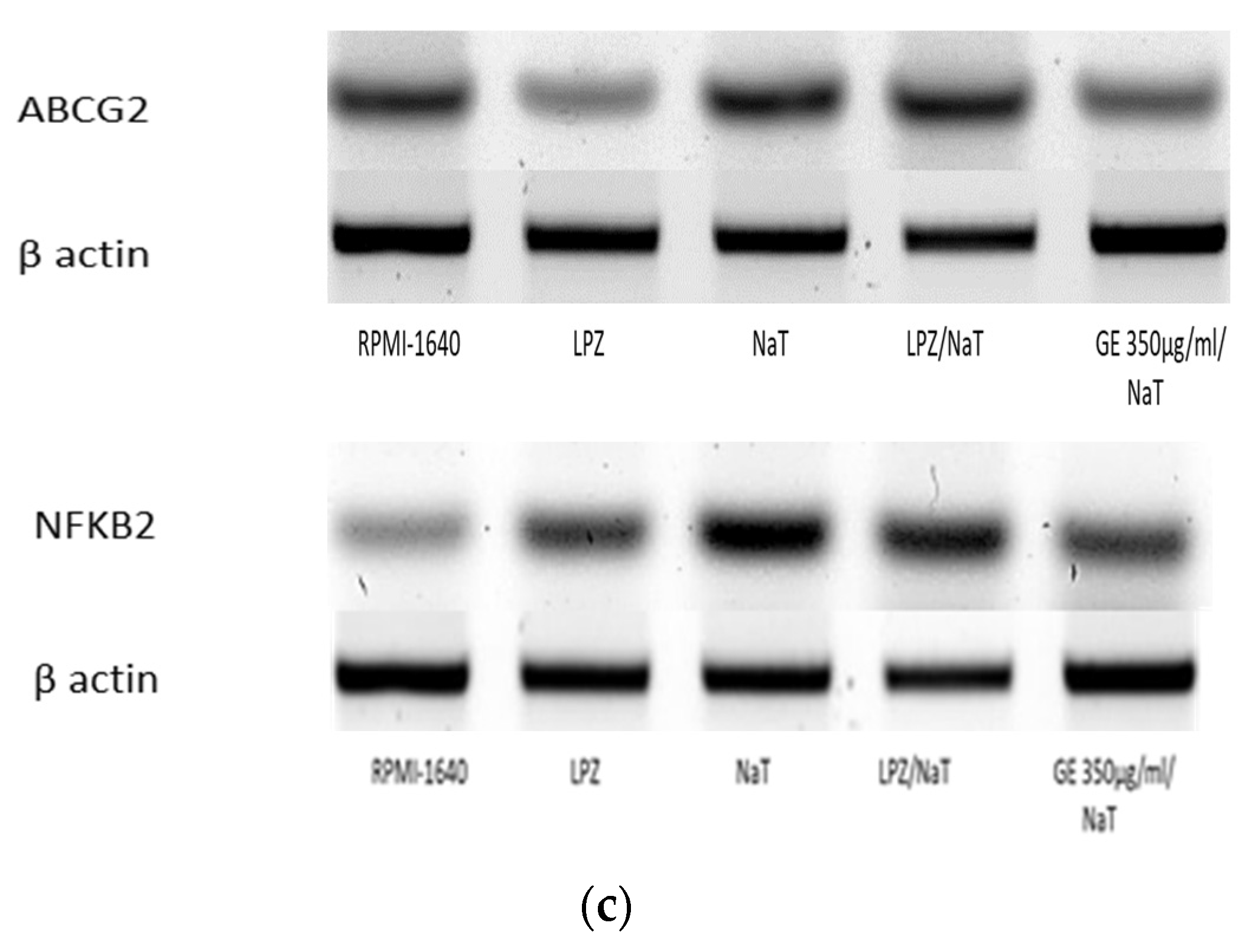
3.6. Expression of ABCG2 and NFκB2 in a Cell Culture Model of Peptic Ulcer Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCG2 | ATP-binding cassette, sub-family G, member 2 |
| AGS | Adenocarcinoma epithelial gastric cell line |
| BSA | Bovine serum albumin |
| CCl | Tetrachloride |
| DAPI | 4,6-diamidino-2-phenylindole |
| DATS | Diallyl trisulfide |
| FBS | Fetal bovine serum |
| GE | Garlic oil extracts |
| GI | Gastrointestinal |
| GSH | Glutathione |
| H. pylori | Helicobacter pylori |
| IFN-γ | Interferon gamma |
| IL-17 | Interleukin 17 |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| LPZ | Lansoprazole |
| MIP-1 | Macrophage Inflammatory Protein 2 |
| MTT | 3-(4, 5-dimethyl-2-thiazolyl) -2, 5-diphenyl-2H- tetrazolium bromide |
| NaT | Sodium taurocholate |
| NFκB2 | Nuclear factor kappa B subunit 2 |
| NSAID | Non-steroidal anti-inflammatory drugs |
| PBS | Phosphate buffered saline |
| PG | Prostaglandin |
| PGE2 | Prostaglandin E2 |
| PPI | Proton pump inhibitors |
| PUD | Peptic ulcer disease |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted |
| ROS | Reactive oxygen species |
| SAC | S-allyl-cysteine |
| SOD | Superoxide dismutases |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β1 | Transforming growth factor beta 1 |
| tTG | Transglutaminase |
| TIMP-1 | Tissue metalloproteinase inhibitor |
| SAC | S-allyl-cysteine |
| α-SMA | α-smooth muscle actin |
References
- Ardalani, H.; Hadipanah, A.; Sahebkar, A. Medicinal Plants in the Treatment of Peptic Ulcer Disease: A Review. Mini Rev. Med. Chem. 2020, 20, 662–702. [Google Scholar] [CrossRef]
- Tsoi, A.H.; Garg, M.; Tsoi, E.H. Peptic ulcer disease: An unusual presentation of a common problem. Gastroenterology 2021. [Google Scholar] [CrossRef] [PubMed]
- Serafim, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef]
- De Brito, B.B.; da Silva, F.A.F.; de Melo, F.F. Role of polymorphisms in genes that encode cytokines and Helicobacter pylori virulence factors in gastric carcinogenesis. World J. Clin. Oncol. 2018, 9, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Watanabe, T.; Tanigawa, T.; Tominaga, K.; Fujiwara, Y.; Morimoto, K. Quality of ulcer healing in gastrointestinal tract: Its pathophysiology and clinical relevance. World J. Gastroenterol. 2012, 18, 4811–4822. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, Y.; Li, Q.; Sun, L.; Chen, R.; Lai, X.; Lai, Z.; Yuan, E.; Sun, S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules 2020, 25, 4626. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef]
- Bi, W.-P.; Man, H.-B.; Man, M.-Q. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. 2014, 20, 17020–17028. [Google Scholar] [CrossRef]
- Theoduloz, C.; Pertino, M.W.; Schmeda-Hirschmann, G. Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells. Molecules 2014, 19, 581–594. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Lee, H.; Hong, S.H.; Park, C.; Park, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Salagacka-Kubiak, A.; Zebrowska, M.; Wosiak, A.; Balcerczak, M.; Mirowski, M.; Balcerczak, E. ABCG2 in peptic ulcer: Gene expression and mutation analysis. J. Appl. Genet. 2015, 57, 335–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gruhlke, M.C.; Antelmann, H.; Bernhardt, J.; Kloubert, V.; Rink, L.; Slusarenko, A.J. The human allicin-proteome: S-thioallylation of proteins by the garlic defence substance allicin and its biological effects. Free Radic. Biol. Med. 2018, 131, 144–153. [Google Scholar] [CrossRef]
- Lemière, J.; Valentino, F.; Campillo, C.; Sykes, C. How cellular membrane properties are affected by the actin cytoskeleton. Biochimie 2016, 130, 33–40. [Google Scholar] [CrossRef]
- Fritzsche, M. Self-organizing actin patterns shape cytoskeletal cortex organization. Commun. Integr. Biol. 2017, 10, e1303591. [Google Scholar] [CrossRef][Green Version]
- Ávila, F.; Theoduloz, C.; López-Alarcón, C.; Dorta, E.; Schmeda-Hirschmann, G. Cytoprotective Mechanisms Mediated by Polyphenols from Chilean Native Berries against Free Radical-Induced Damage on AGS Cells. Oxidative Med. Cell. Longev. 2017, 2017, 9808520. [Google Scholar] [CrossRef]
- Volarevic, M.; Wu, C.H.; Smolic, R.; Andorfer, J.H.; Wu, G.Y. A Novel G418 Conjugate Results In Targeted Selection of Genetically Protected Hepatocytes without Bystander Toxicity. Bioconjug. Chem. 2007, 18, 1965–1971. [Google Scholar] [CrossRef]
- Kizivat, T.; Smolić, M.; Marić, I.; Levak, M.T.; Smolić, R.; Čurčić, I.B.; Kuna, L.; Mihaljević, I.; Včev, A.; Tucak-Zorić, S. Antioxidant Pre-Treatment Reduces the Toxic Effects of Oxalate on Renal Epithelial Cells in a Cell Culture Model of Urolithiasis. Int. J. Environ. Res. Public Health 2017, 14, 109. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kumar, V.M.; Henley, A.K.; Nelson, C.J.; Indumati, O.; Rao, Y.P.; Rajanna, S.; Rajanna, B. Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ. Sci. Pollut. Res. 2016, 24, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Carrión, I.B.; Pertino, M.W.; Valenzuela, D.; Schmeda-Hirschmann, G. Potential Gastroprotective Effect of Novel Cyperenoic Acid/Quinone Derivatives in Human Cell Cultures. Planta Med. 2012, 78, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, H.; Hiraishi, H.; Ota, S.; Yoshida, H.; Ivey, K.J.; Terano, A.; Sugimoto, T. Protective role of intracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology 1990, 98, 1452–1459. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Theoduloz, C.; Sánchez, M.; Razmilic, I.; Schmeda-Hirschmann, G. Gastroprotective and ulcer-healing effect of new solidagenone derivatives in human cell cultures. Life Sci. 2005, 77, 2193–2205. [Google Scholar] [CrossRef]
- Rodrigues, C.; Percival, S.S. Immunomodulatory Effects of Glutathione, Garlic Derivatives, and Hydrogen Sulfide. Nutrients 2019, 11, 295. [Google Scholar] [CrossRef]
- Stagos, D.; Amoutzias, G.; Matakos, A.; Spyrou, A.; Tsatsakis, A.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.; El-Bahrawy, H.A.; Selim, H.M. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition 2016, 32, 849–854. [Google Scholar] [CrossRef]
- Natale, G.; Lazzeri, G.; Lubrano, V.; Colucci, R.; Vassalle, C.; Fornai, M.; Blandizzi, C.; Del Tacca, M. Mechanisms of gastroprotection by lansoprazole pretreatment against experimentally induced injury in rats: Role of mucosal oxidative damage and sulfhydryl compounds. Toxicol. Appl. Pharmacol. 2004, 195, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Takahashi, M.; Yoshiura, K.; Hata, Y.; Kawabe, T.; Terano, A.; Omata, M. Antiulcer drugs and gastric prostaglandin E2: An in vitro study. J. Clin. Gastroenterol. 1993, 17 (Suppl. 1), S15–S21. [Google Scholar] [CrossRef]
- De Olinda, T.; Lemos, T.; Machado, L.; Rao, V.; Santos, F. Quebrachitol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide and K+ATP channels. Phytomedicine 2008, 15, 327–333. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.-S.; Maghrabi, I.A. Diosmin Protects against Ethanol-Induced Gastric Injury in Rats: Novel Anti-Ulcer Actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Zhi, W.; Zhang, H.; He, Z.; Wang, Y.; Liu, F.; Niu, X.; Zhang, X. The gastroprotective effect of nobiletin against ethanol-induced acute gastric lesions in mice: Impact on oxidative stress and inflammation. Immunopharmacol. Immunotoxicol. 2017, 9, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Bidel, S.; Mustonen, H.; Khalighi-Sikaroudi, G.; Lehtonen, E.; Puolakkainen, P.; Kiviluoto, T.; Kivilaakso, E. Effect of the ulcerogenic agents ethanol, acetylsalicylic acid and taurocholate on actin cytoskeleton and cell motility in cultured rat gastric mucosal cells. World J. Gastroenterol. 2005, 11, 4032–4039. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Kojovic, D.; Cressman, A.; Piquette-Miller, M. Maternal bacterial infections impact expression of drug transporters in human placenta. Int. Immunopharmacol. 2015, 26, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Diestra, J.E.; Scheffer, G.L.; Català, I.; Maliepaard, M.; Schellens, J.H.M.; Scheper, R.J.; Germà-Lluch, J.R.; Izquierdo, M.A. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J. Pathol. 2002, 198, 213–219. [Google Scholar] [CrossRef]
- Lou, H.; Kaplowitz, N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J. Biol. Chem. 2007, 282, 29470–29481. [Google Scholar] [CrossRef]
- Geng, Z.; Rong, Y.; Lau, B.H. S-Allyl Cysteine Inhibits Activation of Nuclear Factor Kappa B in Human T Cells. Free Radic. Biol. Med. 1997, 23, 345–350. [Google Scholar] [CrossRef]
- Schäfer, G.; Kaschula, C.H. The Immunomodulation and Anti-Inflammatory Effects of Garlic Organosulfur Compounds in Cancer Chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Marta, N.; Agnieszka, W.; Jacek, P.; Jeleń, A.; Adrian, K.; Dagmara, S.-K.; Sałagacka-Kubiak, A.; Balcerczak, E. NFκB2 gene expression in patients with peptic ulcer diseases and gastric cancer. Mol. Biol. Rep. 2020, 47, 2015–2021. [Google Scholar] [CrossRef]
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-J. The Garlic Preparation as an Alternative Way for Gastroprotection: From Bench to Clinic. Gut Liver 2016, 10, 321–322. [Google Scholar] [CrossRef]
- Brancaccio, M.; Russo, M.; Masullo, M.; Palumbo, A.; Russo, G.L.; Castellano, I. Sulfur-containing histidine compounds inhibit γ-glutamyl transpeptidase activity in human cancer cells. J. Biol. Chem. 2019, 294, 14603–14614. [Google Scholar] [CrossRef]
- Milito, A.; Brancaccio, M.; D’Argenio, G.; Castellano, I. Natural Sulfur-Containing Compounds: An Alternative Therapeutic Strategy against Liver Fibrosis. Cells 2019, 8, 1356. [Google Scholar] [CrossRef]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic Effect of Marine Ovothiol in an In Vivo Model of Liver Fibrosis. Oxidative Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Amoruso, D.C.; Mazzone, G.; Vitaglione, P.; Romano, A.; Ribecco, M.T.; D’Armiento, M.R.; Mezza, E.; Morisco, F.; Fogliano, V.; et al. Garlic extract prevents CCl4-induced liver fibrosis in rats: The role of tissue transglutaminase. Dig. Liver Dis. 2010, 42, 571–577. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Mazzone, G.; Ribecco, M.T.S.; Lembo, V.; Vitaglione, P.; Guarino, M.; Morisco, F.; Napolitano, M.; Fogliano, V.; Caporaso, N. Garlic extract attenuating rat liver fibrosis by inhibiting TGF-β1. Clin. Nutr. 2012, 32, 252–258. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuna, L.; Zjalic, M.; Kizivat, T.; Roguljic, H.; Nincevic, V.; Omanovic Kolaric, T.; Wu, C.H.; Vcev, A.; Smolic, M.; Smolic, R. Pretreatment of Garlic Oil Extracts Hampers Epithelial Damage in Cell Culture Model of Peptic Ulcer Disease. Medicina 2022, 58, 91. https://doi.org/10.3390/medicina58010091
Kuna L, Zjalic M, Kizivat T, Roguljic H, Nincevic V, Omanovic Kolaric T, Wu CH, Vcev A, Smolic M, Smolic R. Pretreatment of Garlic Oil Extracts Hampers Epithelial Damage in Cell Culture Model of Peptic Ulcer Disease. Medicina. 2022; 58(1):91. https://doi.org/10.3390/medicina58010091
Chicago/Turabian StyleKuna, Lucija, Milorad Zjalic, Tomislav Kizivat, Hrvoje Roguljic, Vjera Nincevic, Tea Omanovic Kolaric, Catherine H. Wu, Aleksandar Vcev, Martina Smolic, and Robert Smolic. 2022. "Pretreatment of Garlic Oil Extracts Hampers Epithelial Damage in Cell Culture Model of Peptic Ulcer Disease" Medicina 58, no. 1: 91. https://doi.org/10.3390/medicina58010091
APA StyleKuna, L., Zjalic, M., Kizivat, T., Roguljic, H., Nincevic, V., Omanovic Kolaric, T., Wu, C. H., Vcev, A., Smolic, M., & Smolic, R. (2022). Pretreatment of Garlic Oil Extracts Hampers Epithelial Damage in Cell Culture Model of Peptic Ulcer Disease. Medicina, 58(1), 91. https://doi.org/10.3390/medicina58010091





