A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA
Abstract
:1. Introduction
2. Results
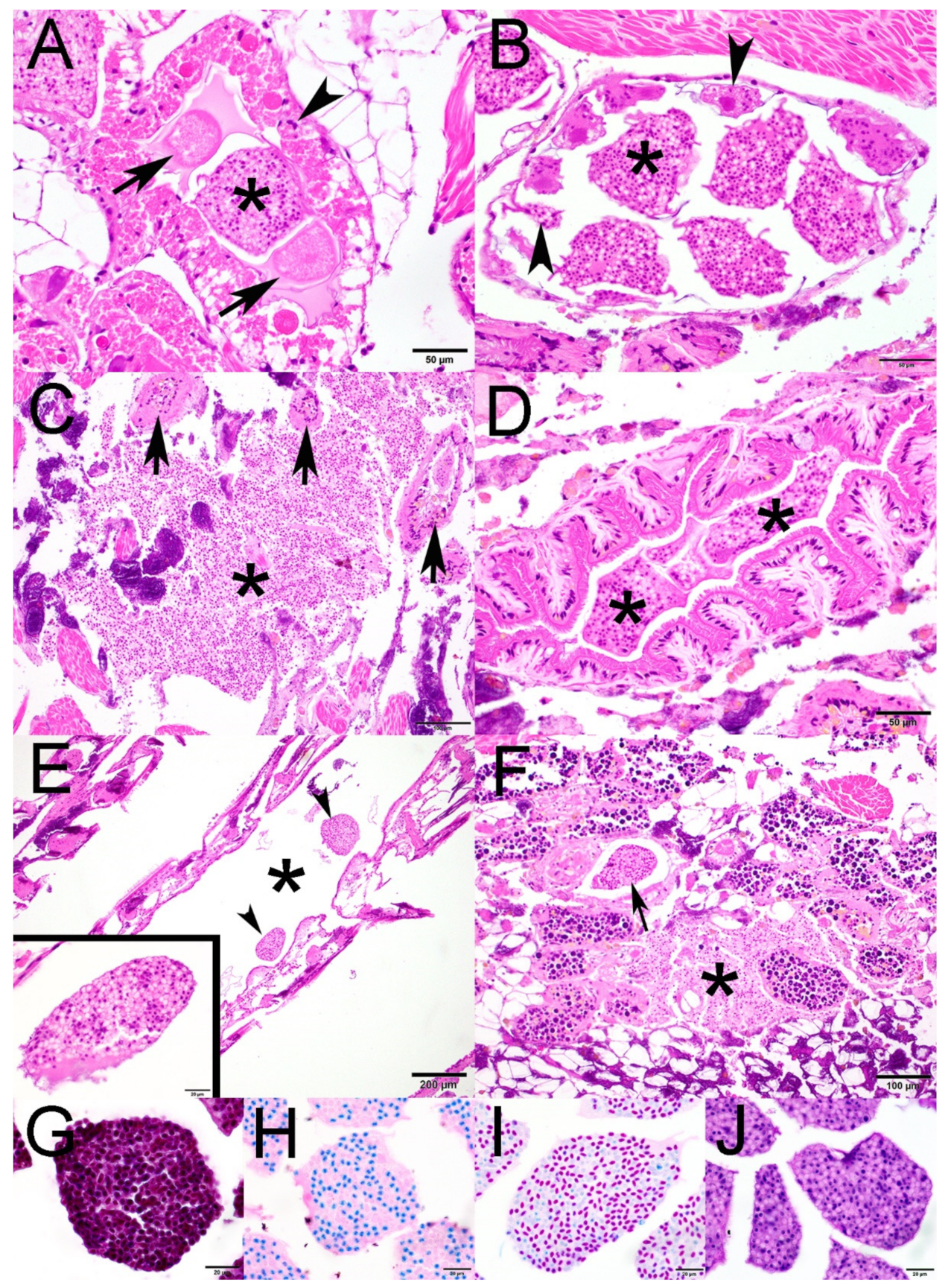
2.1. Histopathology
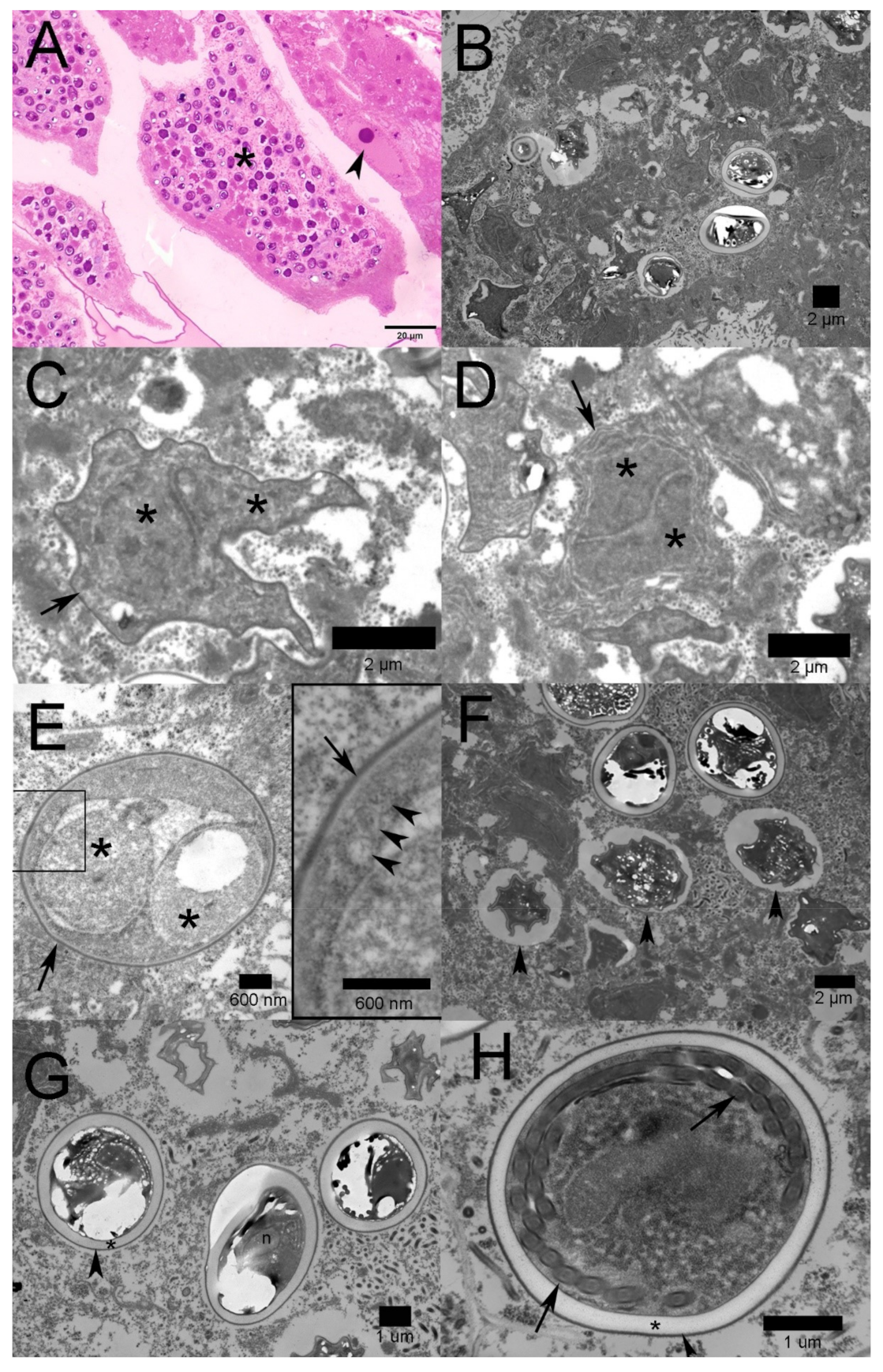
2.2. Transmission Electron Microscopy
2.3. Molecular Identification
2.4. Description
2.4.1. Taxonomic Summary
2.4.2. Remarks
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Field Sampling
4.2. Tissue Processing
4.3. Transmission Electron Microscopy
4.4. DNA Extraction and PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogan, A.E. Global Diversity of Freshwater Mussels (Mollusca, Bivalvia) in Freshwater. Hydrobiologia 2008, 595, 139–147. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of Freshwater Bivalves at the Global Scale: Diversity, Threats and Research Needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Haag, W.R.; Williams, J.D. Biodiversity on the Brink: An Assessment of Conservation Strategies for North American Freshwater Mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Carella, F.; Villari, G.; Maio, N.; De Vico, G. Disease and Disorders of Freshwater Unionid Mussels: A Brief Overview of Recent Studies. Front. Physiol. 2016, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Haag, W.R. The Decline of the North American Mussel Fauna: Chronology and Causes. In North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012; pp. 316–390. [Google Scholar]
- Starliper, C.E. Pathogens and Diseases of Freshwater Mussels in the United States: Studies on Bacterial Transmission and Depuration. Bridging America and Russia with Shared Perspectives on Aquatic Animal Health. In Proceedings of the Third Bilateral Conference between Russia and the United States, Shepherdstown, WV, USA, 12–20 July 2009; Cipriano, R.C., Bruckner, A.W., Shchelkunov, I.S., Eds.; Khaled bin Sultan Living Oceans Foundation: Landover, MD, USA, 2011; pp. 47–55. [Google Scholar]
- Downing, J.A.; Van Meter, P.; Woolnough, D.A. Suspects and Evidence: A Review of the Causes of Extirpation and Decline in Freshwater Mussels. Anim. Biodivers. Conserv. 2010, 33.2, 151–185. [Google Scholar]
- Haag, W.R. Reassessing Enigmatic Mussel Declines in the United States. Freshw. Mollusk Biol. Conserv. 2019, 22, 43–60. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Brunner, C.J. Infectious Diseases of Freshwater Mussels and Other Freshwater Bivalve Mollusks. Rev. Fish. Sci. 2009, 17, 425–467. [Google Scholar] [CrossRef]
- McElwain, A. Are Parasites and Diseases Contributing to the Decline of Freshwater Mussels (Bivalvia, Unionida)? Freshw. Mollusk Biol. Conserv. 2019, 22, 85–89. [Google Scholar] [CrossRef]
- McElwain, A.; Warren, M.B.; Pereira, F.B.; Ksepka, S.P.; Bullard, S.A. Pathobiology and First Report of Larval Nematodes (Ascaridomorpha sp.) Infecting Freshwater Mussels Villosa nebulosa (Unonidae), Including an Inventory of Nematode Infections in Freshwater and Marine Bivalves. Int. J. Parasitol. Parasites Wildl. 2019, 10, 41–58. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, X.; Li, J. Advances of the Studies on Diseases of Hyriopsis cumingii and Its Control. J. Shanghai Fish. 2005, 14, 313–318. (In Chinese) [Google Scholar]
- Lei, Z.; Tiao-Yi, X.; Jie, H.; Liang-Ying, D.; Xiao-Yan, L. Histopathological Examination of Bivalve Mussel Hyriopsis cumingii Lea Artificially Infected by Virus. Acta Hydrobiol. Sin. 2011, 35, 666–671. [Google Scholar] [CrossRef]
- Brian, J.I.; Ollard, I.S.; Aldridge, D.C. Don’t Move a Mussel? Parasite and Disease Risk in Conservation Action. Conserv. Lett. 2021, 14, e12799. [Google Scholar] [CrossRef]
- Han, B.; Takvorian, P.M.; Weiss, L.M. Invasion of Host Cells by Microsporidia. Front. Microbiol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, L.S. Laboratory Identification of the Microsporidia. J. Clin. Microbiol. 2002, 40, 1892–1901. [Google Scholar] [CrossRef] [Green Version]
- Stentiford, G.D.; Dunn, A.M. Microsporidia in Aquatic Invertebrates. In Microsporidia: Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2014; pp. 579–604. [Google Scholar]
- Kohler, S.L.; Wiley, M.J. Parasite-Induced Collapse of Populations of a Dominant Grazer in Michigan Streams. Oikos 1992, 65, 443–449. [Google Scholar] [CrossRef]
- Knowles, S.; Leis, E.M.; Richard, J.C.; Cole, R.; Agbalog, R.E.; Putnam, J.G.; Goldberg, T.L.; Waller, D.L. A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA: U.S. Geological Survey Data Release. Available online: https://doi.org/10.5066/P9U4RYE1 (accessed on 27 October 2021).
- Vávra, J.; Ronny Larsson, J.I. Structure of Microsporidia. In Microsporidia: Pathogens of Opportunity; Weiss, L.M., Becnel, J.J., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2014; pp. 1–70. [Google Scholar]
- Hoffman, G.L.; Williams, E.H. Subkingdom Protozoa (Kingdom Protista). In Parasites of North American Freshwater Fishes; Cornell University Press: Ithaca, NY, USA, 1999; pp. 21–91. [Google Scholar]
- Sprague, V.; Becnel, J.J.; Hazard, E.I. Taxonomy of Phylum Microspora. Crit. Rev. Microbiol. 1992, 18, 285–395. [Google Scholar] [CrossRef]
- Matos, E.; Matos, P.; Azevedo, C. Observations on the Intracytoplasmic Microsporidian Steinhausia mytilovum, a Parasite of Mussel (Mytella guyanensis) Oocytes from the Amazon River Estuary. J. Morphol. Sci. 2005, 22, 183–186. [Google Scholar]
- Sprague, V.; Ormières, R.; Manier, J.F. Creation of a New Genus and a New Family in the Microsporida. J. Invertebr. Pathol. 1972, 20, 228–231. [Google Scholar] [CrossRef]
- Kalavati, C.; Narasimhamurti, C.C. Steinhausia spraguei n. sp. a Microsporidian Parasite of the Excretory Cells Found in the Fluid from Renal Appendages of Sepia elliptica. Rivista Parassit 1977, 38, 271–275. [Google Scholar]
- Farley, C.A. Neoplasms in Estuarine Mollusks and Approaches to Ascertain Causes. Ann. N. Y. Acad. Sci. 1977, 298, 225–232. [Google Scholar] [CrossRef]
- Villalba, A.; Carballal, M.J.; López, M.C. Pathologic Conditions of Three Carpet Shell Clam Species of Galicia (NW. of Spain). In Proceedings of the Proceedings of World Aquaculture ’93—From discovery to commercialization, Torremolinos, Spain, 26–28 May 1993; Carrillo, M., Ed.; European Aquaculture Society: Oostende, Belgium, 1993; p. 85. [Google Scholar]
- Anderson, T.; Hine, P.; Lester, R. A Steinhausia-like Infection in the Ovocytes of Sydney Rock Oysters Saccostrea commercialis. Dis. Aquat. Organ. 1995, 22, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Carballal, M.J.; Iglesias, D.; Santamarina, J.; Ferro-Soto, B.; Villalba, A. Parasites and Pathologic Conditions of the Cockle Cerastoderma edule Populations of the Coast of Galicia (NW Spain). J. Invertebr. Pathol. 2001, 78, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Comtet, T.; Garcia, C.; Le Coguic, Y.; Joly, J. Infection of the Cockle Cerastoderma edule in the Baie Des Veys (France) by the Microsporidian Parasite Steinhausia sp. Dis. Aquat. Organ. 2003, 57, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.D.; Scardua, M.P.; Vieira, C.B.; Alves, A.C.; Dungan, C.F. Survey of Pathologies in Crassostrea gasar (Adanson, 1757) Oysters from Cultured and Wild Populations in the São Francisco Estuary, Sergipe, Northeast Brazil. J. Shellfish Res. 2015, 34, 289–296. [Google Scholar] [CrossRef]
- Sagristà, E.; Bozzo, M.G.; Bigas, M.; Poquet, M.; Durfort, M. Developmental Cycle and Ultrastructure of Steinhausia mytilovum, a Microsporidian Parasite of Oocytes of the Mussel, Mytilus galloprovincialis (Mollusca, Bivalvia). Eur. J. Protistol. 1998, 34, 58–68. [Google Scholar] [CrossRef]
- Léger, L.; Hollande, A. Sur Un Nouveau Protiste À Facies De Chytridiopsis, Parasite Des Ovules De L’huître. CR Séances Soc. Biol. Paris 1917, 80, 61–64. [Google Scholar]
- Cunningham, A.A.; Daszak, P. Extinction of a Species of Land Snail Due to Infection with a Microsporidian Parasite. Conserv. Biol. 1998, 12, 1139–1141. [Google Scholar] [CrossRef]
- Cope, W.G.; Bergeron, C.M.; Archambault, J.M.; Jones, J.W.; Beaty, B.; Lazaro, P.R.; Shea, D.; Callihan, J.L.; Rogers, J.J. Understanding the Influence of Multiple Pollutant Stressors on the Decline of Freshwater Mussels in a Biodiversity Hotspot. Sci. Total Environ. 2021, 773, 144757. [Google Scholar] [CrossRef]
- Richard, J. Clinch River Mussel Die-Off. Ellipsaria 2018, 20, 1–3. [Google Scholar]
- Sanders, J.; Lawrence, C.; Nichols, D.; Brubaker, J.; Peterson, T.; Murray, K.; Kent, M. Pleistophora hyphessobryconis (Microsporidia) Infecting Zebrafish Danio rerio in Research Facilities. Dis. Aquat. Organ. 2010, 91, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.L.; Watral, V.; Kent, M.L. Microsporidiosis in Zebrafish Research Facilities. ILAR J. 2012, 53, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Ebert, D. The Ecological Interactions Between a Microsporidian Parasite and Its Host Daphnia magna. J. Anim. Ecol. 1995, 64, 361–369. [Google Scholar] [CrossRef]
- Dunn, A.M.; Smith, J.E. Microsporidian Life Cycles and Diversity: The Relationship between Virulence and Transmission. Microbes Infect. 2001, 3, 381–388. [Google Scholar] [CrossRef]
- Richard, J.C.; Leis, E.; Dunn, C.D.; Agbalog, R.; Waller, D.; Knowles, S.; Putnam, J.; Goldberg, T.L. Mass Mortality in Freshwater Mussels (Actinonaias pectorosa) in the Clinch River, USA, Linked to a Novel Densovirus. Sci. Rep. 2020, 10, 14498. [Google Scholar] [CrossRef]
- Richard, J.C.; Campbell, L.J.; Leis, E.M.; Agbalog, R.E.; Dunn, C.D.; Waller, D.L.; Knowles, S.; Putnam, J.G.; Goldberg, T.L. Mussel Mass Mortality and the Microbiome: Evidence for Shifts in the Bacterial Microbiome of a Declining Freshwater Bivalve. Microorganisms 2021, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Leis, E.; Erickson, S.; Waller, D.; Richard, J.; Goldberg, T. A Comparison of Bacteria Cultured from Unionid Mussel Hemolymph Between Stable Populations in the Upper Mississippi River Basin and Populations Affected by a Mortality Event in the Clinch River. Freshw. Mollusk Biol. Conserv. 2019, 22, 70–80. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
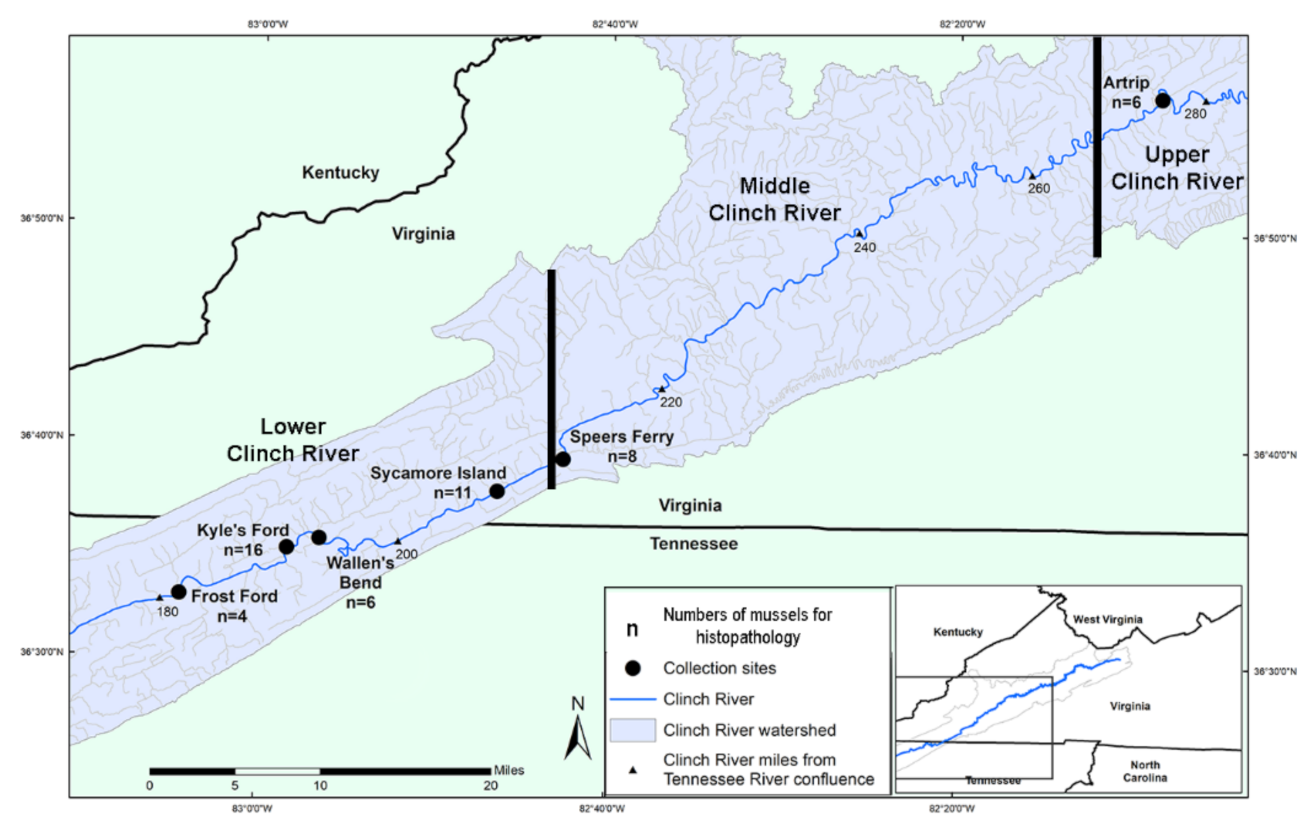
| State | Collection Location | Collection Date | Number of Males (M), Females (F), Undetermined (U) | Infected A. pectorosa | Prevalence in Female A. pectorosa | Infected A. ligamentina |
|---|---|---|---|---|---|---|
| VA | Artrip | 26 September 2018 | 2M 1F | 1/3 | 100% (4/4) | NA |
| 24 October 2018 | 0M 3F | 3/3 | NA | |||
| Speer’s Ferry | 26 September 2018 | 1M 2F | 3/3 | 100% (4/4) | NA | |
| 24 October 2018 | 2M 2F 1U | 2/4 | 0/1 | |||
| Sycamore Island | 16 August 2018 | 3M 1F | 1/2 | 75% (3/4) | 0/2 | |
| 26 September 2018 | 1M 1F | 0/2 | NA | |||
| 25 October 2018 | 3M 2F | 2/4 | 0/1 | |||
| TN | Wallen Bend | 16 August 2018 | 1M 3F | 1/2 | 25% (1/4) | 0/2 |
| 26 September 2018 | 1M 1F | 0/2 | NA | |||
| Kyle’s Ford | 16 August 2018 | 1M 3F | 1/2 | 56% (5/9) | 0/2 | |
| 26 September 2018 | 3M 2F | 1/5 | NA | |||
| 25 October 2018 | 0M 6F 1U | 3/7 | NA | |||
| Frost Ford | 23 August 2018 | 3M 1F | 0/2 | 0% (0/1) | 0/2 | |
| Totals | 21M 28F 2U | 18/41 | 65% (17/26) | 0/10 |
| Orders | PolyBed 812 | Acetone | Time | Temp. |
|---|---|---|---|---|
| 1 | 25% | 75% | 60 min | RT |
| 2 | 50% | 50% | 60 min | RT |
| 3 | 75% | 25% | Overnight | RT |
| 4 | 100% | 0% | 2 × 45 min | 60 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knowles, S.; Leis, E.M.; Richard, J.C.; Cole, R.; Agbalog, R.E.; Putnam, J.G.; Goldberg, T.L.; Waller, D.L. A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA. Parasitologia 2022, 2, 1-12. https://doi.org/10.3390/parasitologia2010001
Knowles S, Leis EM, Richard JC, Cole R, Agbalog RE, Putnam JG, Goldberg TL, Waller DL. A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA. Parasitologia. 2022; 2(1):1-12. https://doi.org/10.3390/parasitologia2010001
Chicago/Turabian StyleKnowles, Susan, Eric M. Leis, Jordan C. Richard, Rebecca Cole, Rose E. Agbalog, Joel G. Putnam, Tony L. Goldberg, and Diane L. Waller. 2022. "A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA" Parasitologia 2, no. 1: 1-12. https://doi.org/10.3390/parasitologia2010001
APA StyleKnowles, S., Leis, E. M., Richard, J. C., Cole, R., Agbalog, R. E., Putnam, J. G., Goldberg, T. L., & Waller, D. L. (2022). A Novel Gonadotropic Microsporidian Parasite (Microsporidium clinchi n. sp.) Infecting a Declining Population of Pheasantshell Mussels (Actinonaias pectorosa) (Unioinidae) from the Clinch River, USA. Parasitologia, 2(1), 1-12. https://doi.org/10.3390/parasitologia2010001






