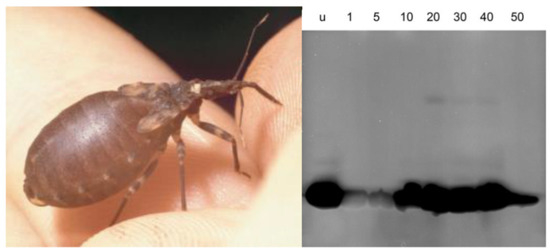
Feeding-Induced Changes of Bacteriolytic Activity and the Pattern of Bacteriolytic Compounds in the Stomach and Small Intestine of the Haematophagous Bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae)
Abstract
:1. Introduction
2. Results
2.1. Weight of Stomach and Small Intestine and Concentration of Soluble Proteins
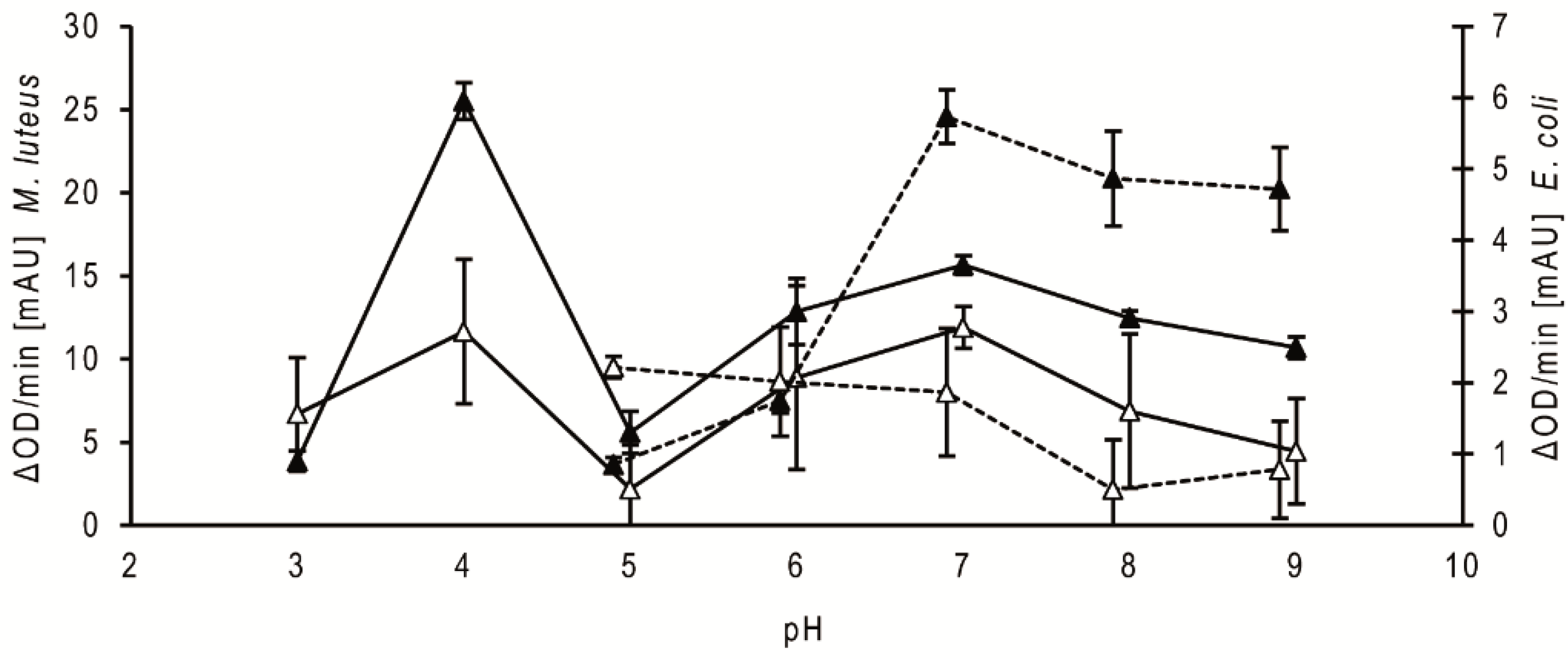
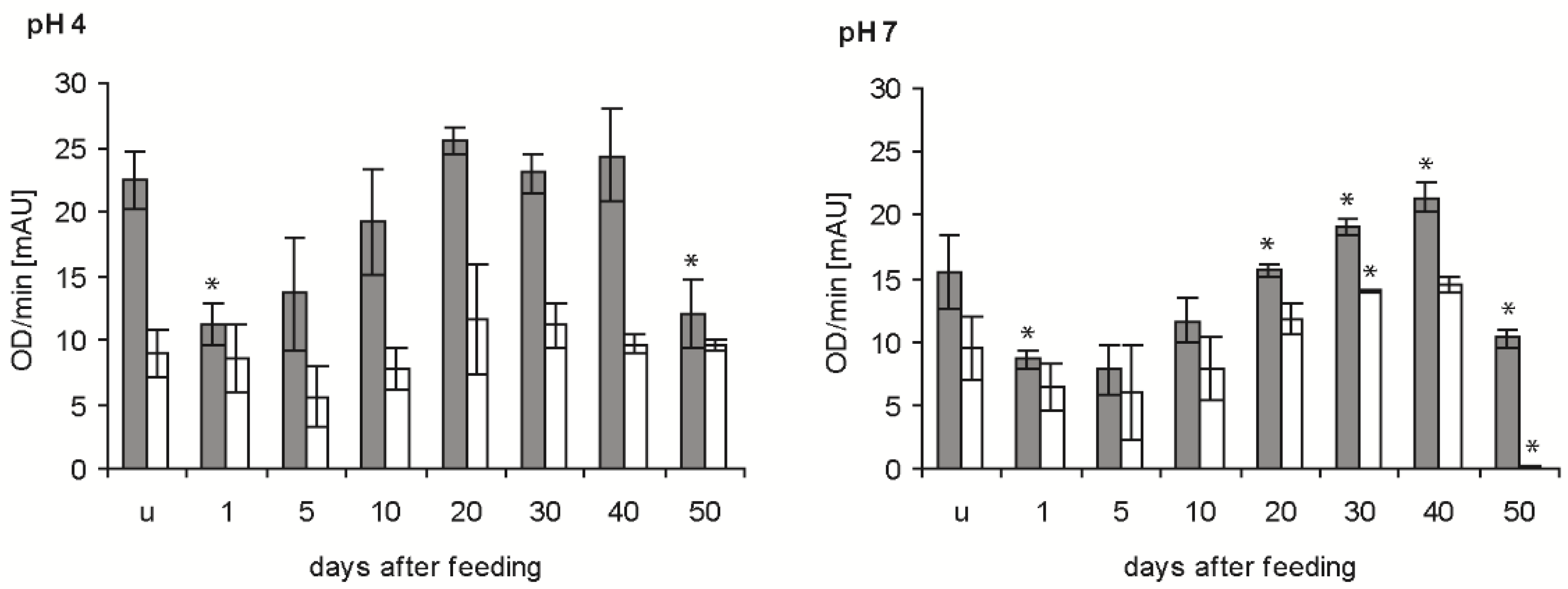
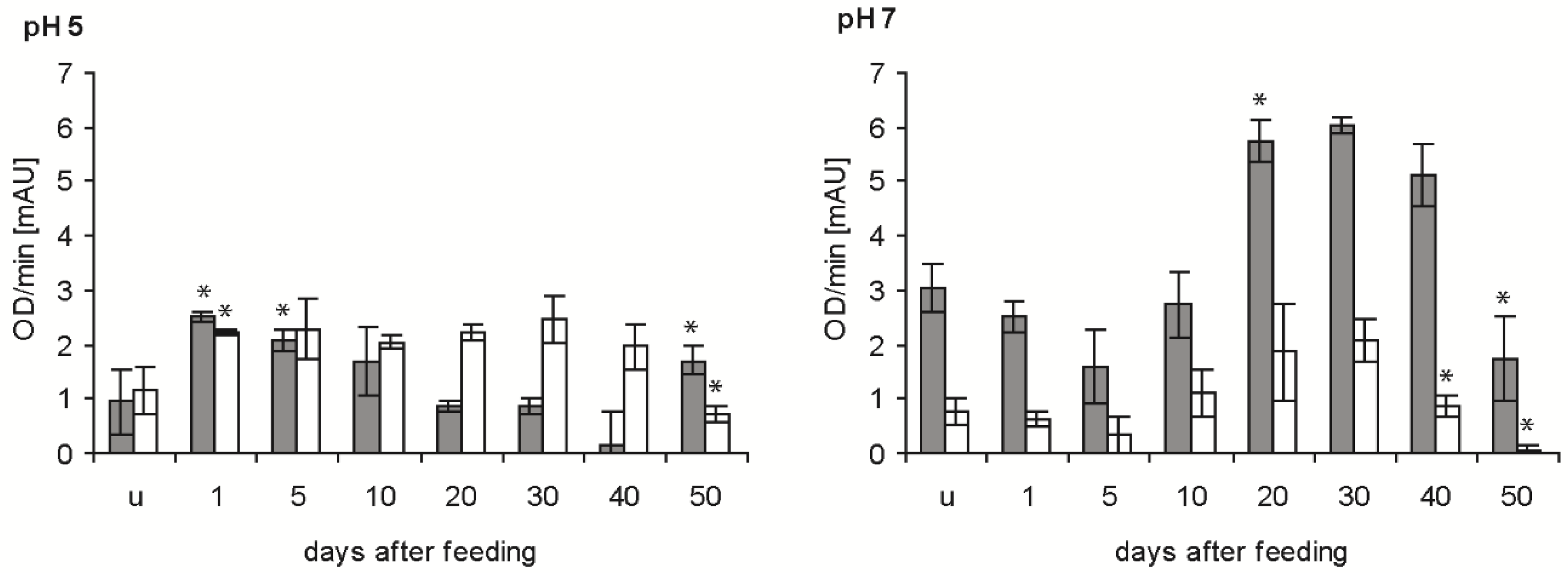
2.2. Bacteriolytic Activity
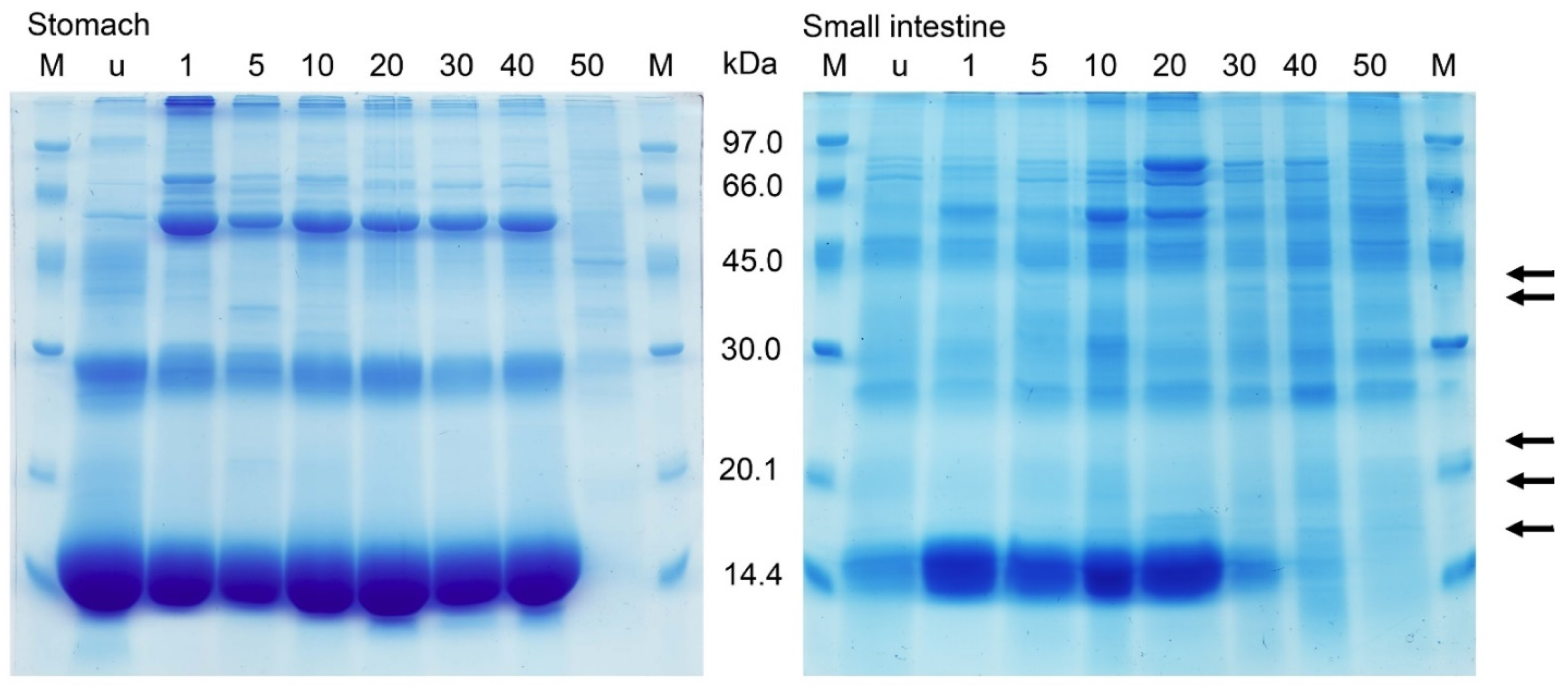
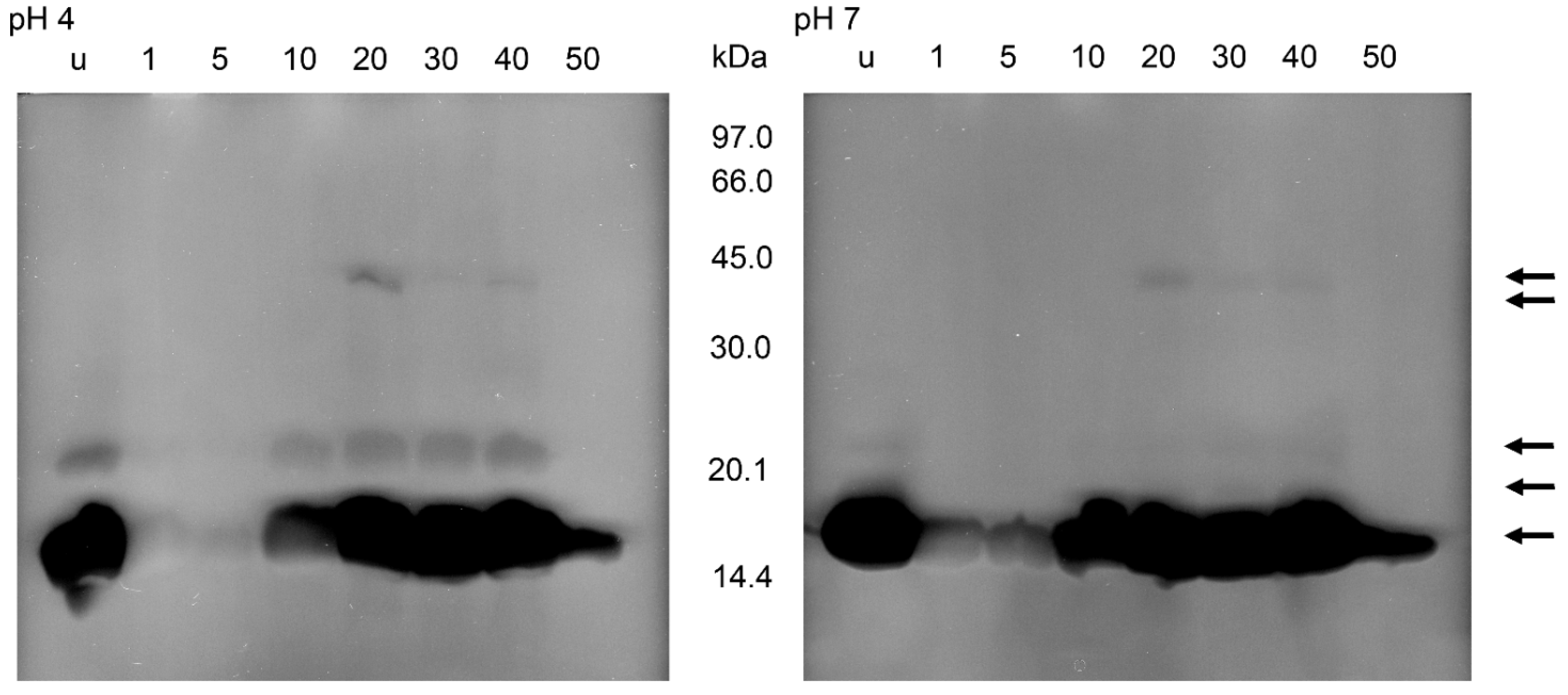
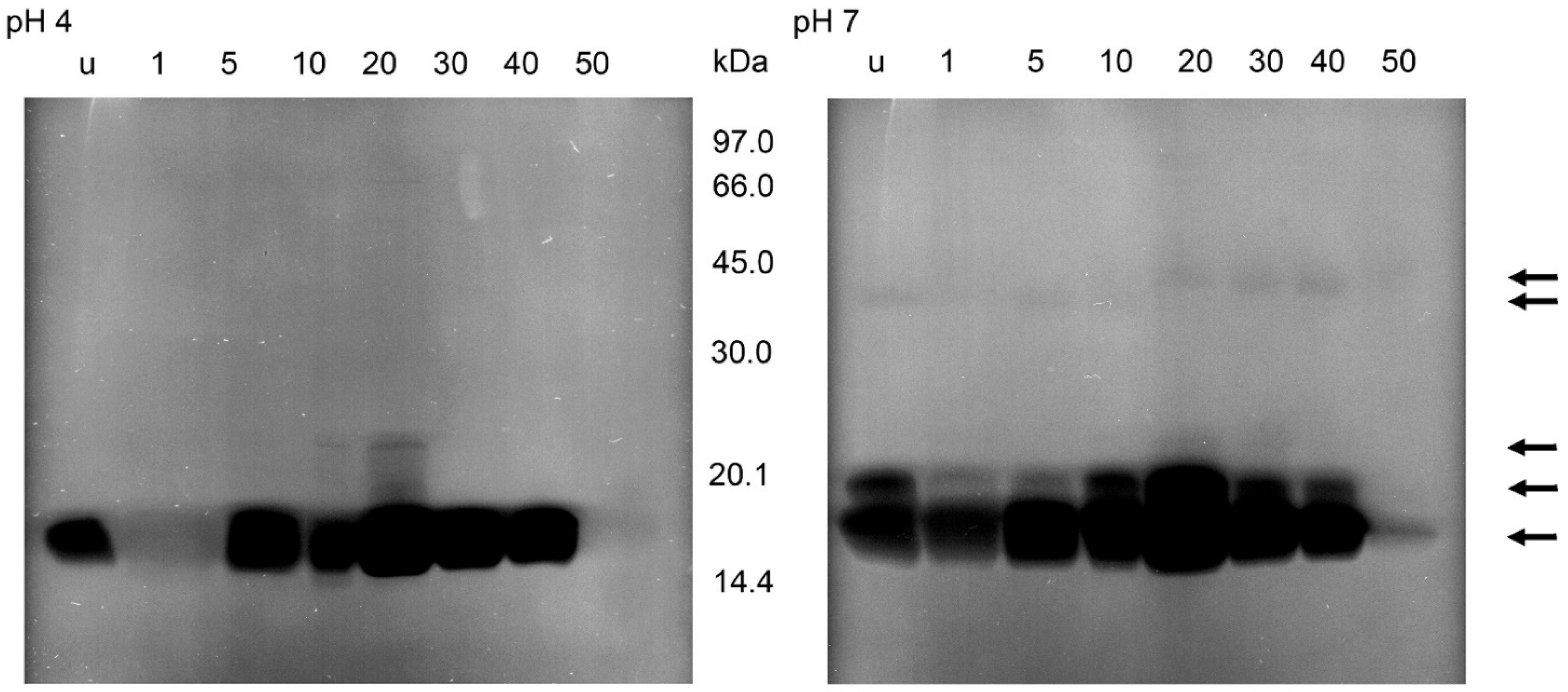
2.3. Protein Banding after Electrophoresis and Bacterial Lysis in Gels
3. Discussion
4. Materials and Methods
4.1. Insect Origin, Maintenance and Sample Preparation
4.2. Determination of Bacteriolytic Activity
4.3. Electrophoresis and Zymography
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balczun, C.; Meiser, C.K.; Schaub, G.A. Triatomines as vectors of American Trypanosomiasis. In Arthropods as Vectors of Emerging Diseases; Parasitology Research Monographs; Mehlhorn, H., Ed.; Springer: Berlin, Germany, 2012; Volume 3, pp. 275–299. [Google Scholar]
- Oliveira, P.L.; Genta, F.A. Blood digestion in triatomine insects. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 265–284. [Google Scholar]
- Lehane, M.J. Managing the blood meal. In Biology of Blood-Sucking Insects; Lehane, M.J., Ed.; Harper Collins Academic: London, UK, 1991; pp. 84–115. [Google Scholar]
- Schaub, G.A. Blood digestion of triatomines and the body louse—A review. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2020, 22, 217–220. [Google Scholar]
- Brecher, G.; Wigglesworth, V.B. The transmission of Actinomyces rhodnii Erikson in Rhodnius prolixus Stål (Hemiptera) and its influence on the growth of the host. Parasitology 1944, 35, 220–224. [Google Scholar] [CrossRef]
- Schaub, G.A. Intestinal bacteria/mutualistic symbionts of triatomines—A review. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2020, 22, 191–194. [Google Scholar]
- Eichler, S.; Schaub, G.A. The effects of aposymbiosis and of an infection with Blastocrithidia triatomae (Trypanosomatidae) on the tracheal system of the reduviid bugs Rhodnius prolixus and Triatoma infestans. J. Insect Physiol. 1998, 44, 131–140. [Google Scholar] [CrossRef]
- Vallejo, G.A.; Guhl, F.; Schaub, G.A. Triatominae-Trypanosoma cruzi/T. rangeli: Vector-parasite interactions. Acta Trop. 2009, 110, 137–147. [Google Scholar] [CrossRef]
- Kollien, A.H.; Fechner, S.; Waniek, P.J.; Schaub, G.A. Isolation and characterization of a cDNA encoding for a lysozyme from the gut of the reduviid bug Triatoma infestans. Arch. Insect Biochem. Physiol. 2003, 53, 134–145. [Google Scholar] [CrossRef]
- Müller, U.; Vogel, P.; Alber, G.; Schaub, G.A. The innate immune system of mammals and insects. In Trends in Innate Immunity; Contributions to Microbiology; Egesten, A., Schmidt, A., Herwald, H., Eds.; Karger: Basel, Switzerland, 2008; Volume 15, pp. 21–44. [Google Scholar]
- Guarneri, A.A.; Schaub, G.A. Interaction of triatomines, trypanosomes and microbiota. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 345–386. [Google Scholar]
- Eichler, S.; Schaub, G.A. Development of symbionts in triatomine bugs and the effects of infections with trypanosomatids. Exp. Parasitol. 2002, 100, 17–27. [Google Scholar] [CrossRef]
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [Green Version]
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ratcliffe, N.A.; Azambuja, P.; Garcia, E.S. Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS ONE 2012, 7, e36591. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.T. On a bactericidal principle present in the alimentary canal of insects and arachnids. Parasitology 1926, 18, 238–252. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Pereira, M.E.A. Midgut glycosidases of Rhodnius prolixus. Insect Biochem. 1984, 14, 103–108. [Google Scholar] [CrossRef]
- Waniek, P.J.; Mendonça-Lima, L.; Menezes, G.B.; Jansen, A.M.; Araújo, C.A.C. Recombinant expression and characterization of a lysozyme from the midgut of Triatoma brasiliensis (Hemiptera, Reduviidae) in comparison with intestinal muramidase activity. Physiol. Entomol. 2009, 34, 309–317. [Google Scholar] [CrossRef]
- Araújo, C.A.C.; Waniek, P.J.; Stock, P.; Mayer, C.; Jansen, A.M.; Schaub, G.A. Sequence characterization and expression patterns of defensin and lysozyme encoding genes from the gut of the reduviid bug Triatoma brasiliensis. Insect Biochem. Mol. Biol. 2006, 36, 547–560. [Google Scholar] [CrossRef]
- Ursic-Bedoya, R.J.; Nazzari, H.; Cooper, D.; Triana, O.; Wolff, M.; Lowenberger, C. Identification and characterization of two novel lysozymes from Rhodnius prolixus, a vector of Chagas’ disease. J. Insect Physiol. 2008, 54, 593–603. [Google Scholar] [CrossRef]
- Balczun, C.; Knorr, E.; Topal, H.; Meiser, C.K.; Kollien, A.H.; Schaub, G.A. Sequence characterization of an unusual lysozyme gene expressed in the intestinal tract of the reduviid bug Triatoma infestans (Insecta). Parasitol. Res. 2008, 102, 229–232. [Google Scholar] [CrossRef]
- Ursic-Bedoya, R.J.; Lowenberger, C.A. Rhodnius prolixus: Identification of immune-related genes up-regulated in response to pathogens and parasites using suppressive subtractive hybridization. Dev. Comp. Immunol. 2007, 31, 109–120. [Google Scholar] [CrossRef]
- Ribeiro, J.M.C.; Genta, F.A.; Sorgine, M.H.F.; Raquel Logullo, R.; Mesquita, R.D.; Paiva-Silva, G.O.; Majerowicz, D.; Medeiros, M.; Koerich, L.; Terra, W.R.; et al. An insight into the transcriptome of the digestive tract of the bloodsucking bug, Rhodnius prolixus. PLoS Negl. Trop. Dis. 2014, 8, e2594. [Google Scholar] [CrossRef] [Green Version]
- Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A.; Gutiérrez-Cabrera, A.E.; Rojas-Wastavino, G.E.; Bucio-Torres, M.I.; Cabrera-Bravo, M. Immune defence mechanisms of triatomines against bacteria, viruses, fungi and parasites. Bull. Entomol. Res. 2015, 105, 523–532. [Google Scholar] [CrossRef]
- Soares, T.S.; Buarque, D.S.; Queiroz, B.R.; Gomes, C.M.; Braz, G.R.C.; Araújo, R.N.; Pereira, M.H.; Guarneri, A.A.; Tanaka, A.S. A Kazal-type inhibitor is modulated by Trypanosoma cruzi to control microbiota inside the anterior midgut of Rhodnius prolixus. Biochimie 2015, 112, 41–48. [Google Scholar] [CrossRef]
- Buarque, D.S.; Gomes, C.M.; Araújo, R.N.; Pereira, M.H.; Ferreira, R.C.; Guarneri, A.A.; Tanaka, A.S. A new antimicrobial protein from the anterior midgut of Triatoma infestans mediates Trypanosoma cruzi establishment by controlling the microbiota. Biochimie 2016, 123, 138–143. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Lowenberger, C. The innate immune system of kissing bugs, vectors of Chagas disease. Dev. Comp. Immunol. 2019, 98, 119–128. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Lowenberger, C. Immune system of triatomines. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Springer Nature: New York, NY, USA, 2021; pp. 307–344. [Google Scholar]
- Meiser, C.K.; Piechura, H.; Werner, T.; Dittmeyer-Schäfer, S.; Meyer, H.E.; Warscheid, B.; Schaub, G.A.; Balczun, C. Kazal-type inhibitors in the stomach of Panstrongylus megistus (Triatominae, Reduviidae). Insect Biochem. Mol. Biol. 2010, 40, 345–353. [Google Scholar] [CrossRef]
- Whitten, M.; Sun, F.; Tew, I.; Schaub, G.A.; Soukou, C.; Nappi, A.; Ratcliffe, N. Differential modulation of Rhodnius prolixus nitric oxide activities following challenge with Trypanosoma rangeli, T. cruzi and bacterial cell wall components. Insect Biochem. Mol. Biol. 2007, 37, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins: Antibacterial peptides from honeybees. EMBO J. 1989, 8, 2387–2391. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Hetru, C. Insect defensins: Inducible antibacterial peptides. Immunol. Today 1992, 13, 411–415. [Google Scholar] [CrossRef]
- Balczun, C.; Siemanowski, J.; Pausch, J.K.; Helling, S.; Marcus, K.; Stephan, C.; Meyer, H.E.; Schneider, T.; Cizmowski, C.; Oldenburg, M.; et al. Intestinal aspartate proteases TiCatD and TiCatD2 of the haematophagous bug Triatoma infestans (Reduviidae): Sequence characterisation, expression pattern and characterisation of proteolytic activity. Insect Biochem. Mol. Biol. 2012, 42, 240–250. [Google Scholar] [CrossRef]
- Cociancich, S.; Dupont, A.; Hegy, G.; Lanot, R.; Holder, F.; Hetru, C.; Hoffmann, J.A.; Bulet, P. Novel inducible antibacterial peptides from a hemipteran insect, the sap-sucking bug Pyrrhocoris apterus. Biochem. J. 1994, 300, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Feder, D.; Mello, C.B.; Garcia, E.S.; Azambuja, P. Immune responses in Rhodnius prolixus: Influence of nutrition and ecdysone. J. Insect Physiol. 1997, 43, 513–519. [Google Scholar] [CrossRef]
- Schneider, M.; Dorn, A. Differential infectivity of two Pseudomonas species and the immune response in the milkweed bug, Oncopeltus fasciatus (Insecta: Hemiptera). J. Invertebr. Pathol. 2001, 78, 135–140. [Google Scholar] [CrossRef]
- Vieira, C.S.; Waniek, P.J.; Mattos, D.P.; Castro, D.P.; Mello, C.B.; Ratcliffe, N.A.; Garcia, E.S.; Azambuja, P. Humoral responses in Rhodnius prolixus: Bacterial feeding induces differential patterns of antibacterial activity and enhances mRNA levels of antimicrobial peptides in the midgut. Parasites Vectors 2014, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Waniek, P.J.; Castro, H.C.; Sathler, P.C.; Miceli, L.; Jansen, A.M.; Araújo, C.A.C. Two novel defensin-encoding genes of the Chagas disease vector Triatoma brasiliensis (Reduviidae, Triatominae): Gene expression and peptide-structure modeling. J. Insect Physiol. 2009, 55, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.V.; Munks, R.J.L.; Lehane, S.M.; Lehane, M.J. Association of midgut defensin with a novel serine protease in the blood-sucking fly Stomoxys calcitrans. Insect Mol. Biol. 2002, 11, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Ursic-Bedoya, R.J.; Buchhop, J.; Joy, J.B.; Durvasula, R.; Lowenberger, C. Prolixicin: A novel antimicrobial peptide isolated from Rhodnius prolixus with differential activity against bacteria and Trypanosoma cruzi. Insect Mol. Biol. 2011, 20, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Sophianopoulos, A.J.; van Holde, K.E. Evidence for dimerization of lysozyme in alkaline solution. J. Biol. Chem. 1961, 236, PC82–PC83. [Google Scholar] [CrossRef]
- Ermakova, E. Lysozyme dimerization: Brownian dynamics simulation. J. Mol. Model. 2005, 12, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.R.; Vekilov, P.G.; Rosenberger, F. Heterogeneity determination and purification of commercial hen egg-white lysozyme. Acta Crystallogr. D 1996, 52, 776–784. [Google Scholar] [CrossRef]
- Espinoza-Fuentes, F.P.; Terra, W.R. Physiological adaptations for digesting bacteria. Water fluxes and distribution of digestive enzymes in Musca domestica larval midgut. Insect Biochem. 1987, 6, 809–817. [Google Scholar] [CrossRef]
- Lemos, F.J.; Terra, W. Digestion of bacteria and the role of midgut lysozyme in some insect larvae. Comp. Biochem. Physiol. B 1991, 100, 265–268. [Google Scholar] [CrossRef]
- Borges, E.C.; Machado, E.M.M.; Garcia, E.S.; Azambuja, P. Trypanosoma cruzi: Effects of infection on cathepsin D activity in the midgut of Rhodnius prolixus. Exp. Parasitol. 2006, 112, 130–133. [Google Scholar] [CrossRef]
- Buarque, D.S.; Braz, G.R.C.; Martins, R.M.; Tanaka-Azevedo, A.M.; Gomes, C.M.; Oliveira, F.A.A.; Schenkman, S.; Tanaka, A.S. Differential expression profiles in the midgut of Triatoma infestans infected with Trypanosoma cruzi. PLoS ONE 2013, 8, e61203. [Google Scholar]
- Schaub, G.A. An update on the knowledge of parasite-vector interactions of Chagas disease. Res. Rep. Trop. Med. 2021, 12, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, V.B. Symbiotic bacteria in a blood-sucking insect, Rhodnius prolixus Stål (Hemiptera, Triatomidae). Parasitology 1936, 28, 284–289. [Google Scholar] [CrossRef]
- Baines, S. The role of the symbiotic bacteria in the nutrition of Rhodnius prolixus (Hemiptera). J. Exp. Biol. 1956, 33, 533–541. [Google Scholar] [CrossRef]
- Schaub, G.A. Trypanosoma cruzi: Quantitative studies of development of two strains in small intestine and rectum of the vector Triatoma infestans. Exp. Parasitol. 1989, 68, 260–273. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. Trypanosoma cruzi in the rectum of the bug Triatoma infestans: Effects of blood ingestion by the starved vector. Am. J. Trop. Med. Hyg. 1998, 59, 166–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zor, T.; Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. Universal buffer solution and the dissociation constant of veronal. J. Chem. Soc. 1931, 198, 1456–1462. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cytryńska, M.; Zdybicka-Barabas, A.; Jabłoński, P.; Jakubowicz, T. Detection of antibacterial polypeptide activity in situ after sodium dodecyl sulfate-polyacryl-amide gel electrophoresis. Anal. Biochem. 2001, 299, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Hardt, M.; Guo, Y.; Henderson, G.; Laine, R.A. Zymogram with Remazol brilliant blue-labeled Micrococcus lysodeikticus cells for the detection of lysozymes: Example of a new lysozyme activity in Formosan termite defense secretions. Anal. Biochem. 2003, 312, 73–76. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meiser, C.K.; Pausch, J.K.; Schaub, G.A. Feeding-Induced Changes of Bacteriolytic Activity and the Pattern of Bacteriolytic Compounds in the Stomach and Small Intestine of the Haematophagous Bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae). Parasitologia 2022, 2, 13-26. https://doi.org/10.3390/parasitologia2010002
Meiser CK, Pausch JK, Schaub GA. Feeding-Induced Changes of Bacteriolytic Activity and the Pattern of Bacteriolytic Compounds in the Stomach and Small Intestine of the Haematophagous Bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae). Parasitologia. 2022; 2(1):13-26. https://doi.org/10.3390/parasitologia2010002
Chicago/Turabian StyleMeiser, Christian K., Jennifer K. Pausch, and Günter A. Schaub. 2022. "Feeding-Induced Changes of Bacteriolytic Activity and the Pattern of Bacteriolytic Compounds in the Stomach and Small Intestine of the Haematophagous Bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae)" Parasitologia 2, no. 1: 13-26. https://doi.org/10.3390/parasitologia2010002
APA StyleMeiser, C. K., Pausch, J. K., & Schaub, G. A. (2022). Feeding-Induced Changes of Bacteriolytic Activity and the Pattern of Bacteriolytic Compounds in the Stomach and Small Intestine of the Haematophagous Bug Triatoma infestans (Klug, 1834) (Reduviidae, Triatominae). Parasitologia, 2(1), 13-26. https://doi.org/10.3390/parasitologia2010002