High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
2.3. Preparation of the Reactive Media
2.3.1. Reactive Media with Various [Co + Fe] Concentrations
2.3.2. Reactive Media with [M + Fe] = 50 Mm (M = Co, Fe, Mn)
2.3.3. Washing Steps
2.4. Synthesis Procedure
2.5. Characterizations
3. Results and Discussion
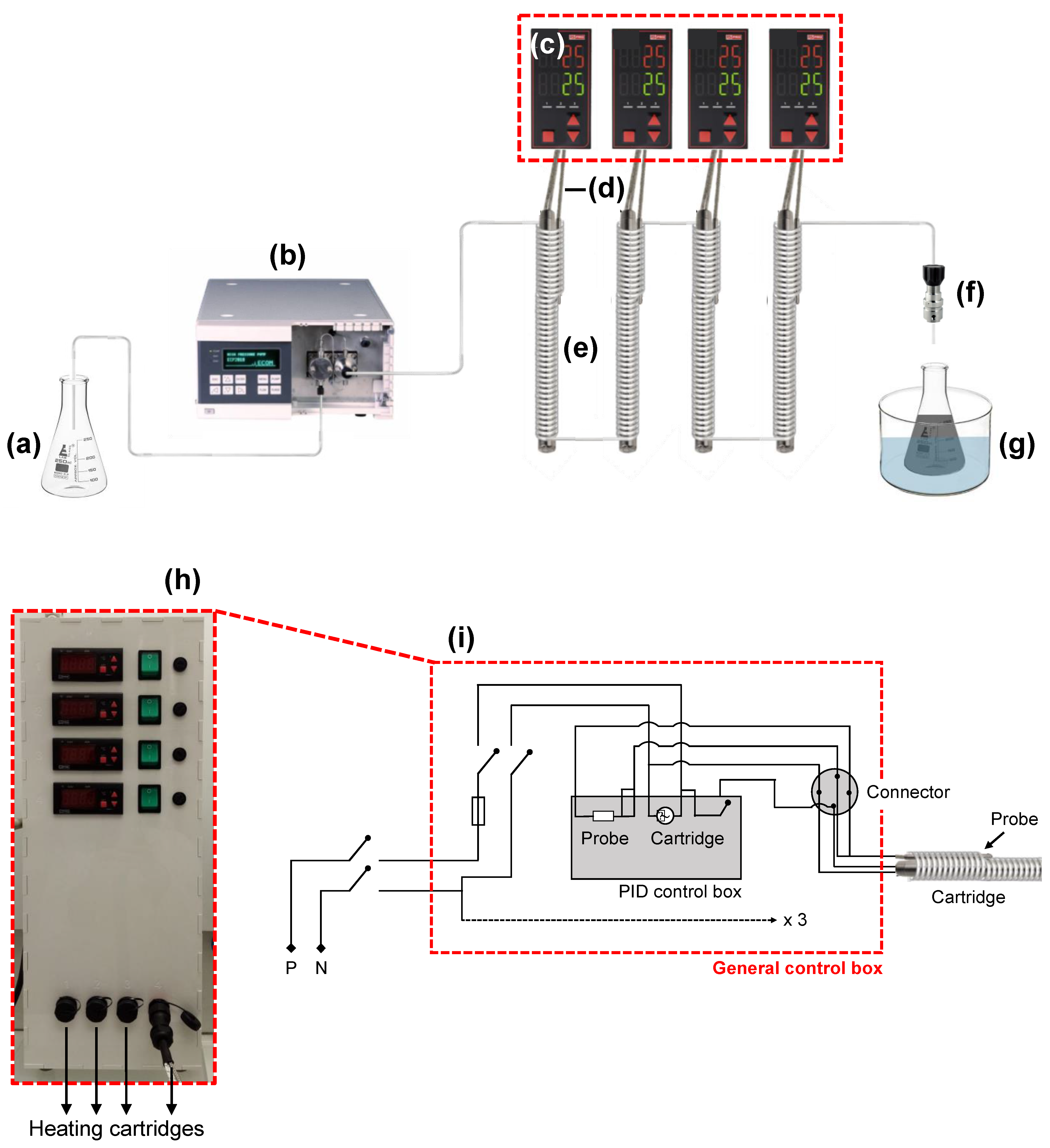
3.1. Special Strengths of the Multi-Parametric Millifluidic Device
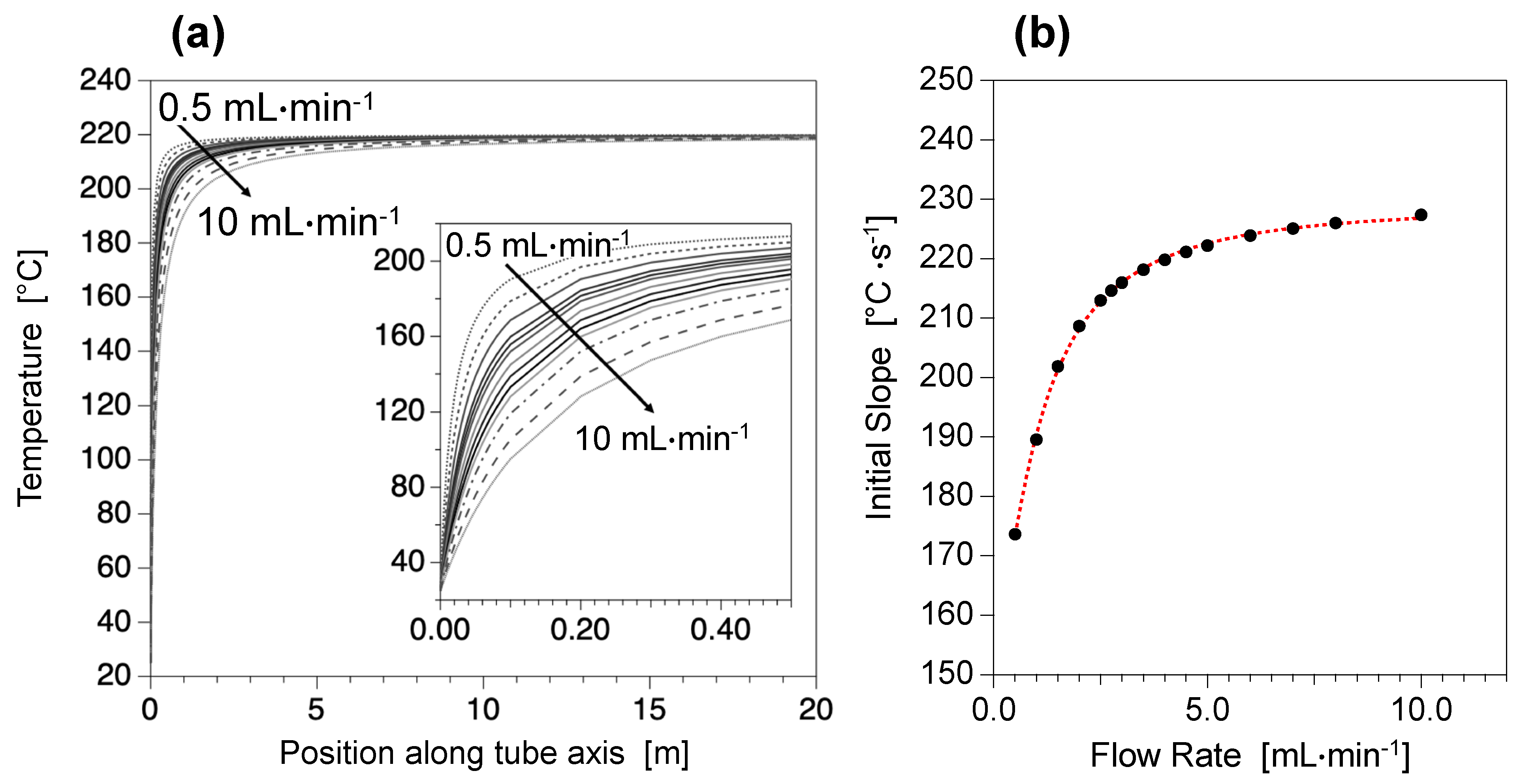
3.2. An Easy and Highly Precise Control of Temperature Conditions
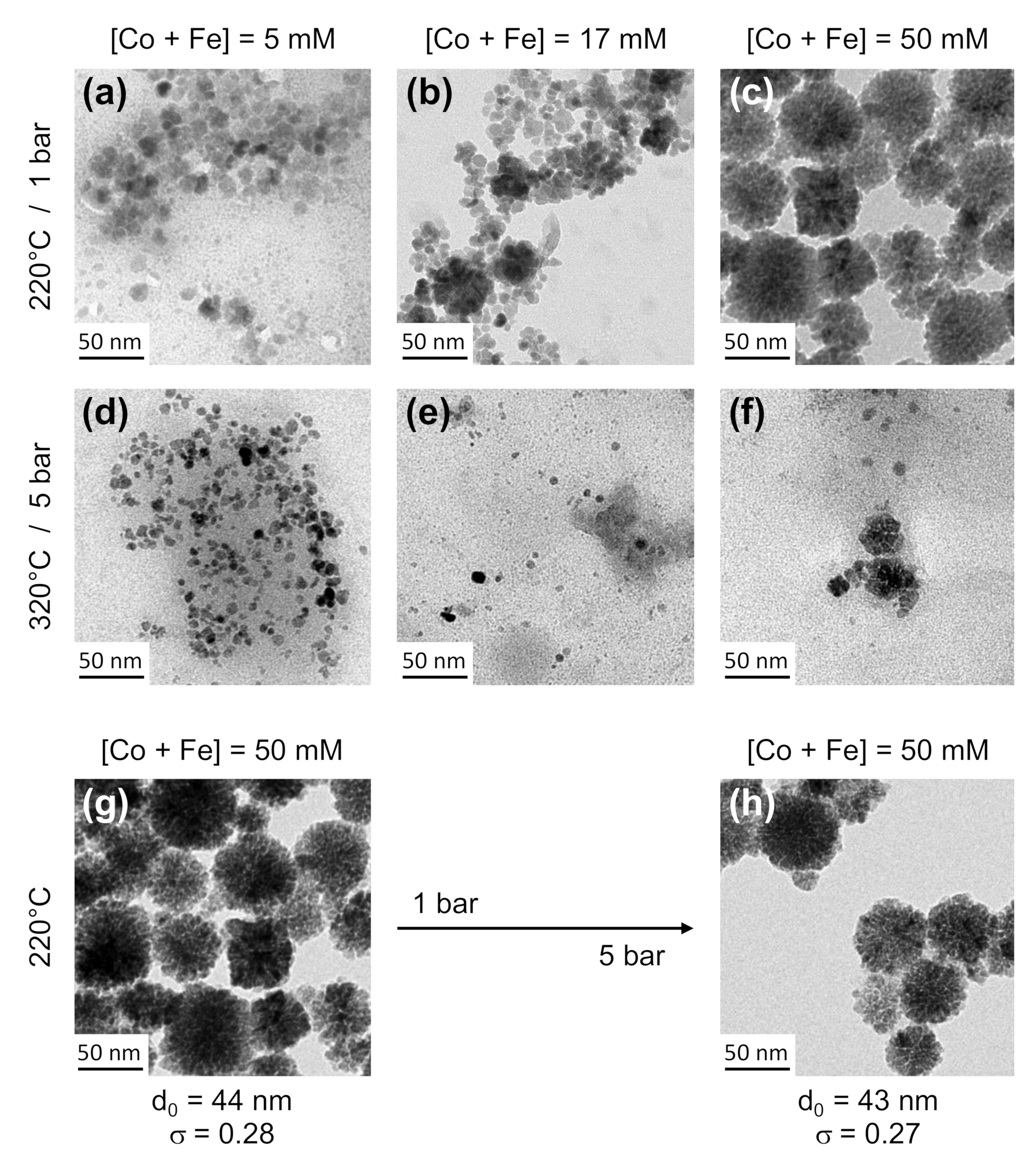
3.3. Optimization of the Synthesis Parameters for Ferrite Nanoflowers Production
3.4. A Millifluidic Device That Offers a Very Good Control over NPs Physico-Chemical Features
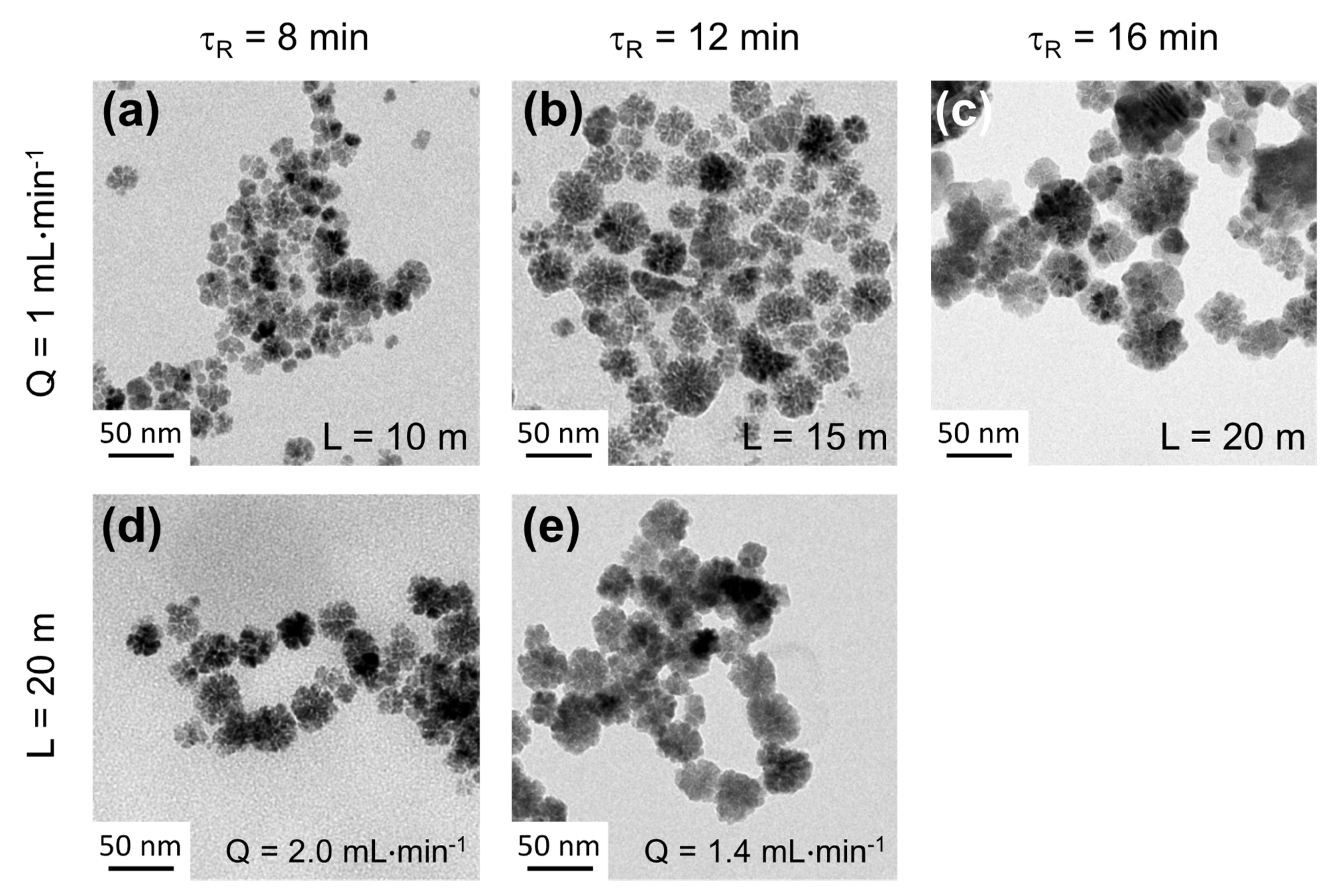
3.5. Towards the Comprehension of the Kinetics of Formation of Fe3O4 and Cofe2O4 NFs
3.6. The Millifluidic Reactor as a Tool to Enhance Chemical Yield and Improve Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duraiswamy, S.; Khan, S.A. Plasmonic Nanoshell Synthesis in Microfluidic Composite Foams. Nano Lett. 2010, 10, 3757–3763. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Sandre, O.; Cabuil, V. Microfluidics in Inorganic Chemistry. Angew. Chem. Int. Ed. 2010, 49, 6268–6286. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of Micro and Nanostructures in Microfluidic Systems. Chem. Soc. Rev. 2010, 39, 1183. [Google Scholar] [CrossRef]
- Phillips, T.W.; Lignos, I.G.; Maceiczyk, R.M.; deMello, A.J.; deMello, J.C. Nanocrystal Synthesis in Microfluidic Reactors: Where Next? Lab Chip 2014, 14, 3172. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Sandre, O.; Cabuil, V. Microfluidic Synthesis of Iron Oxide and Oxyhydroxide Nanoparticles. In Microfluidic Devices in Nanotechnology; Kumar, C.S., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Falsini, S.; Bardi, U.; Abou-Hassan, A.; Ristori, S. Sustainable Strategies for Large-Scale Nanotechnology Manufacturing in the Biomedical Field. Green Chem. 2018, 20, 3897–3907. [Google Scholar] [CrossRef]
- Uson, L.; Arruebo, M.; Sebastian, V.; Santamaria, J. Single Phase Microreactor for the Continuous, High-Temperature Synthesis of <4 Nm Superparamagnetic Iron Oxide Nanoparticles. Chem. Eng. J. 2018, 340, 66–72. [Google Scholar] [CrossRef]
- Larrea, A.; Sebastian, V.; Ibarra, A.; Arruebo, M.; Santamaria, J. Gas Slug Microfluidics: A Unique Tool for Ultrafast, Highly Controlled Growth of Iron Oxide Nanostructures. Chem. Mater. 2015, 27, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Miller, J.T.; Li, Y.; Nandakumar, K.; Kumar, C.S.S.R. Developing a Millifluidic Platform for the Synthesis of Ultrasmall Nanoclusters: Ultrasmall Copper Nanoclusters as a Case Study. Small 2012, 8, 688–698. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Margarida Cruz, M.; Thanh, N.T.K.; et al. Co-Precipitation Synthesis of Stable Iron Oxide Nanoparticles with NaOH: New Insights and Continuous Production via Flow Chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Volk, A.A.; Epps, R.W.; Abolhasani, M. Accelerated Development of Colloidal Nanomaterials Enabled by Modular Microfluidic Reactors: Toward Autonomous Robotic Experimentation. Adv. Mater. 2021, 33, 2004495. [Google Scholar] [CrossRef]
- Chan, E.M.; Alivisatos, A.P.; Mathies, R.A. High-Temperature Microfluidic Synthesis of CdSe Nanocrystals in Nanoliter Droplets. J. Am. Chem. Soc. 2005, 127, 13854–13861. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Thöming, J.; Bäumer, M. Improving the Quality of Nanoparticle Production by Using a New Biphasic Synthesis in a Slug Flow Microreactor. Chem. Eng. J. 2013, 228, 1083–1091. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; deMello, A.J. The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Sebastian, V.; Smith, C.D.; Jensen, K.F. Shape-Controlled Continuous Synthesis of Metal Nanostructures. Nanoscale 2016, 8, 7534–7543. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, V.; Zaborenko, N.; Gu, L.; Jensen, K.F. Microfluidic Assisted Synthesis of Hybrid Au–Pd Dumbbell-like Nanostructures: Sequential Addition of Reagents and Ultrasonic Radiation. Cryst. Growth Des. 2017, 17, 2700–2710. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y. Highly Efficient and Flexible Preparation of Water-Dispersed Fe3O4 Nanoclusters Using a Micromixer. Particuology 2019, 45, 42–48. [Google Scholar] [CrossRef]
- James, M.; Revia, R.A.; Stephen, Z.; Zhang, M. Microfluidic Synthesis of Iron Oxide Nanoparticles. Nanomaterials 2020, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, C.D.; Choi, J.W.; Chung, B.G. Droplet-Based Synthesis of Homogeneous Magnetic Iron Oxide Nanoparticles. Beilstein J. Nanotechnol. 2018, 9, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Frenz, L.; El Harrak, A.; Pauly, M.; Bégin-Colin, S.; Griffiths, A.D.; Baret, J.-C. Droplet-Based Microreactors for the Synthesis of Magnetic Iron Oxide Nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 6817–6820. [Google Scholar] [CrossRef]
- Abou Hassan, A.; Sandre, O.; Cabuil, V.; Tabeling, P. Synthesis of Iron Oxide Nanoparticles in a Microfluidic Device: Preliminary Results in a Coaxial Flow Millichannel. Chem. Commun. 2008, 15, 1783. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep Continuous-Flow Microsynthesis of Magnetic and Fluorescent γ-Fe2O3 @SiO2 Core/Shell Nanoparticles. Angew. Chem. 2009, 121, 7316–7319. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Neveu, S.; Dupuis, V.; Cabuil, V. Synthesis of Cobalt Ferrite Nanoparticles in Continuous-Flow Microreactors. RSC Adv. 2012, 2, 11263. [Google Scholar] [CrossRef]
- Mahin, J.; Franck, C.O.; Fanslau, L.; Patra, H.K.; Mantle, M.D.; Fruk, L.; Torrente-Murciano, L. Green, Scalable, Low Cost and Reproducible Flow Synthesis of Biocompatible PEG-Functionalized Iron Oxide Nanoparticles. React. Chem. Eng. 2021, 6, 1961–1973. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Famiani, S.; Pucciarelli, M.; Lettieri, P.; Thanh, N.T.K.; Gavriilidis, A. Continuous Production of Iron Oxide Nanoparticles via Fast and Economical High Temperature Synthesis. React. Chem. Eng. 2020, 5, 1474–1483. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous Synthesis of Iron Oxide (Fe3O4) Nanoparticles via Thermal Decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Panepinto, A.; Socoliuc, V.; Vekas, L.; Muller, R.N.; Laurent, S. Influence of Experimental Parameters of a Continuous Flow Process on the Properties of Very Small Iron Oxide Nanoparticles (VSION) Designed for T1-Weighted Magnetic Resonance Imaging (MRI). Nanomaterials 2020, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Lévy, M.; Bacri, J.-C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shah, N.; Huffman, G.P. Size Dependence of Magnetic Parameters and Surface Disorder in Magnetite Nanoparticles. J. Appl. Phys. 2009, 105, 07B501. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Espinosa, A.; del Puerto Morales, M.; Bessais, L.; Zehani, K.; Antić, B.; Dojcinović, B. Iron Oxide Nanoflower–Based Screen Print Electrode for Enhancement Removal of Organic Dye Using Electrochemical Approach. Electrocatalysis 2019, 10, 663–671. [Google Scholar] [CrossRef]
- Han, J.; Luo, P.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Construction of Magnetic Nanoflower Biocatalytic System with Enhanced Enzymatic Performance by Biomineralization and Its Application for Bisphenol A Removal. J. Hazard. Mater. 2019, 380, 120901. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.; Serrano-Pertierra, E.; Salvador, M.; Martínez-García, J.; Piñeiro, Y.; Yañez-Vilar, S.; Gónzalez-Gómez, M.; Rivas, J.; Rivas, M.; Blanco-López, M. Carbon-Coated Superparamagnetic Nanoflowers for Biosensors Based on Lateral Flow Immunoassays. Biosensors 2020, 10, 80. [Google Scholar] [CrossRef]
- Urraca, J.L.; Cortés-Llanos, B.; Aroca, C.; de la Presa, P.; Pérez, L.; Moreno-Bondi, M.C. Magnetic Field-Induced Polymerization of Molecularly Imprinted Polymers. J. Phys. Chem. C 2018, 122, 10189–10196. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Ognjanović, M.; Radović, M.; Mirković, M.; Prijović, Ž.; del Puerto Morales, M.; Čeh, M.; Vranješ-Đurić, S.; Antić, B. 99m Tc-, 90 Y-, and 177 Lu-Labeled Iron Oxide Nanoflowers Designed for Potential Use in Dual Magnetic Hyperthermia/Radionuclide Cancer Therapy and Diagnosis. ACS Appl. Mater. Interfaces 2019, 11, 41109–41117. [Google Scholar] [CrossRef]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.E.; Abou-Hassan, A.; Hémadi, M.; Luciani, N.; Wilhelm, C. Magnetic Nanoparticles in Regenerative Medicine: What of Their Fate and Impact in Stem Cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]
- Hu, F.; MacRenaris, K.W.; Waters, E.A.; Schultz-Sikma, E.A.; Eckermann, A.L.; Meade, T.J. Highly Dispersible, Superparamagnetic Magnetite Nanoflowers for Magnetic Resonance Imaging. Chem. Commun. 2010, 46, 73–75. [Google Scholar] [CrossRef]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron Oxide Monocrystalline Nanoflowers for Highly Efficient Magnetic Hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Shaw, S.K.; Kailashiya, J.; Gangwar, A.; Alla, S.K.; Gupta, S.K.; Prajapat, C.L.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. γ-Fe2O3 Nanoflowers as Efficient Magnetic Hyperthermia and Photothermal Agent. Appl. Surf. Sci. 2021, 560, 150025. [Google Scholar] [CrossRef]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; Chen, Y.; O’Connor, C.J.; Goloverda, G.; Kolesnichenko, V.L. Synthesis of Variable-Sized Nanocrystals of Fe3O4 with High Surface Reactivity. Inorg. Chem. 2002, 41, 6137–6146. [Google Scholar] [CrossRef]
- Hemery, G.; Keyes, A.C., Jr.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning Sizes, Morphologies, and Magnetic Properties of Monocore versus Multicore Iron Oxide Nanoparticles through the Controlled Addition of Water in the Polyol Synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef]
- Gavilán, H.; Kowalski, A.; Heinke, D.; Sugunan, A.; Sommertune, J.; Varón, M.; Bogart, L.K.; Posth, O.; Zeng, L.; González-Alonso, D.; et al. Colloidal Flower-Shaped Iron Oxide Nanoparticles: Synthesis Strategies and Coatings. Part. Part. Syst. Charact. 2017, 34, 1700094. [Google Scholar] [CrossRef]
- Farhat, S.; Ouar, N.; Hosni, M.; Hinkov, I.; Mercone, S.; Schoenstein, F.; Jouini, N. Scale-Up of the Polyol Process for Nanomaterial Synthesis. Mater. Sci. Chem. Eng. 2014, 2, 1–11. [Google Scholar] [CrossRef][Green Version]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A.A. Novel Flow Injection Synthesis of Iron Oxide Nanoparticles with Narrow Size Distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- Ibrahim, A.M. Synthesis and Characterization of Nano-Sized Cobalt Ferrite Prepared via Polyol Method Using Conventional and Microwave Heating Techniques. J. Alloys Compd. 2010, 506, 201–204. [Google Scholar] [CrossRef]
- Lin, X.Z.; Terepka, A.D.; Yang, H. Synthesis of Silver Nanoparticles in a Continuous Flow Tubular Microreactor. Nano Lett. 2004, 4, 2227–2232. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite–Maghemite Nanoparticles in the 5–15 Nm Range: Correlating the Core–Shell Composition and the Surface Structure to the Magnetic Properties. A Total Scattering Study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Bender, P.; Fock, J.; Frandsen, C.; Hansen, M.F.; Balceris, C.; Ludwig, F.; Posth, O.; Wetterskog, E.; Bogart, L.K.; Southern, P.; et al. Relating Magnetic Properties and High Hyperthermia Performance of Iron Oxide Nanoflowers. J. Phys. Chem. C 2018, 122, 3068–3077. [Google Scholar] [CrossRef]
- Kolhatkar, A.; Jamison, A.; Litvinov, D.; Willson, R.; Lee, T. Tuning the Magnetic Properties of Nanoparticles. IJMS 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Espinosa, D.; Carlsson, L.B.; Neto, A.M.F.; Alves, S. Influence of Nanoparticle Size on the Nonlinear Optical Properties of Magnetite Ferrofluids. Phys. Rev. E 2013, 88, 032302. [Google Scholar] [CrossRef] [PubMed]
- Fotukian, S.M.; Barati, A.; Soleymani, M.; Alizadeh, A.M. Solvothermal Synthesis of CuFe2O4 and Fe3O4 Nanoparticles with High Heating Efficiency for Magnetic Hyperthermia Application. J. Alloy. Compd. 2020, 816, 152548. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, W.; Yao, T.; Liu, Q.; Xie, Y.; Wei, S. XAFS in Dilute Magnetic Semiconductors. Dalton Trans. 2013, 42, 13779. [Google Scholar] [CrossRef]
- Kuzmin, A.; Chaboy, J. EXAFS and XANES Analysis of Oxides at the Nanoscale. IUCrJ 2014, 1, 571–589. [Google Scholar] [CrossRef]
- Fdez-Gubieda, M.L.; García-Prieto, A.; Alonso, J.; Meneghini, C. X-Ray Absorption Fine Structure Spectroscopy in Fe Oxides and Oxyhydroxides. In Iron Oxides; Faivre, D., Ed.; Wiley: Weinheim, Germany, 2016; pp. 397–422. [Google Scholar]
- Šutka, A.; Lagzdina, S.; Käämbre, T.; Pärna, R.; Kisand, V.; Kleperis, J.; Maiorov, M.; Kikas, A.; Kuusik, I.; Jakovlevs, D. Study of the Structural Phase Transformation of Iron Oxide Nanoparticles from an Fe2+ Ion Source by Precipitation under Various Synthesis Parameters and Temperatures. Mater. Chem. Phys. 2015, 150, 473–479. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.-E.; Brown, G.E.; Martin, F. Oxidation State and Coordination of Fe in Minerals: An Fe K- XANES Spectroscopic Study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Petit, P.-E.; Farges, F.; Wilke, M.; Solé, V.A. Determination of the Iron Oxidation State in Earth Materials Using XANES Pre-Edge Information. J. Synchrotron Rad. 2001, 8, 952–954. [Google Scholar] [CrossRef]
- Caruntu, D.; Remond, Y.; Chou, N.H.; Jun, M.-J.; Caruntu, G.; He, J.; Goloverda, G.; O’Connor, C.; Kolesnichenko, V. Reactivity of 3d Transition Metal Cations in Diethylene Glycol Solutions. Synthesis of Transition Metal Ferrites with the Structure of Discrete Nanoparticles Complexed with Long-Chain Carboxylate Anions. Inorg. Chem. 2002, 41, 6137–6146. [Google Scholar] [CrossRef]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.F.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P.; et al. Formation Mechanism of Maghemite Nanoflowers Synthesized by a Polyol-Mediated Process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef]
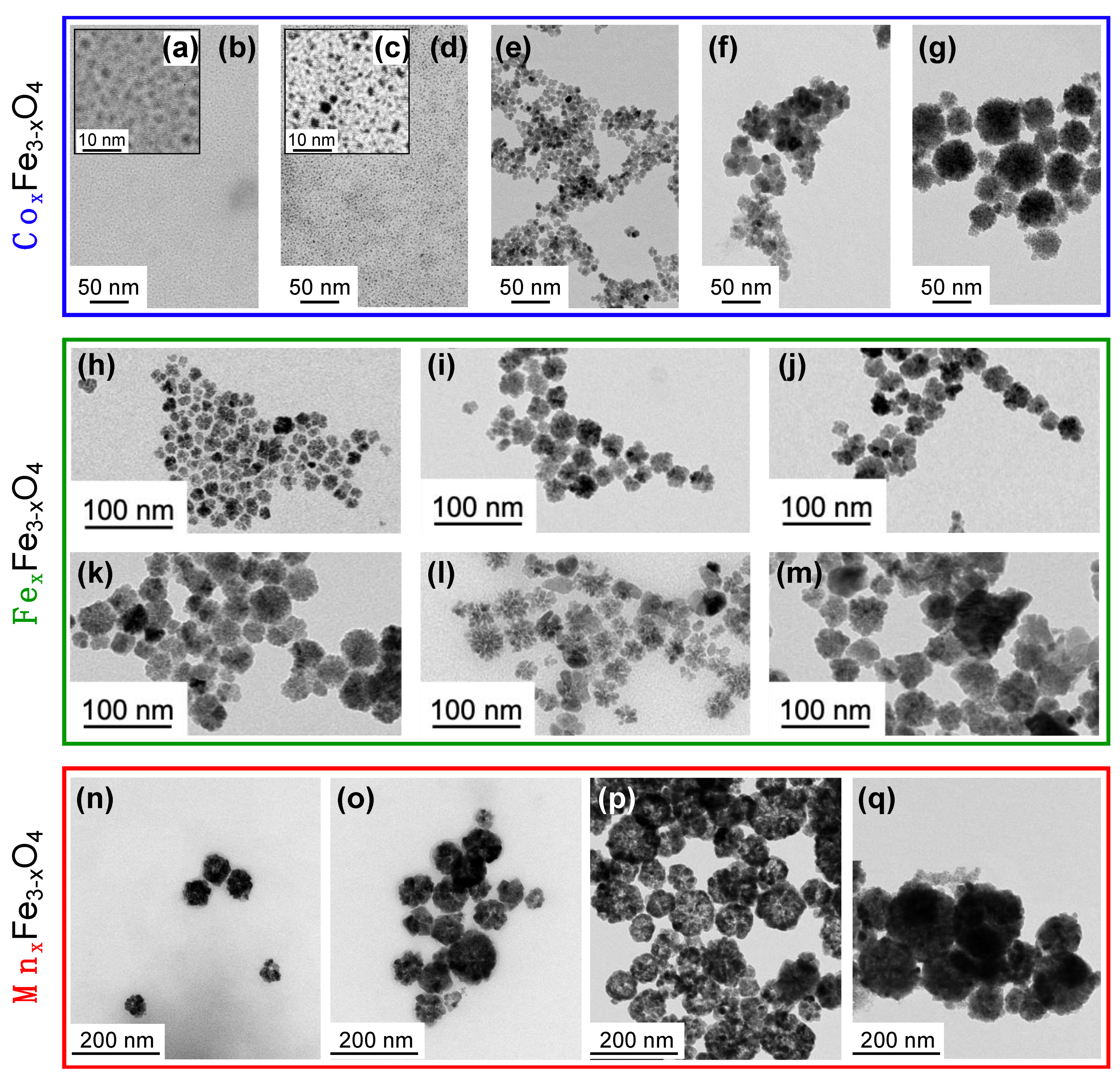

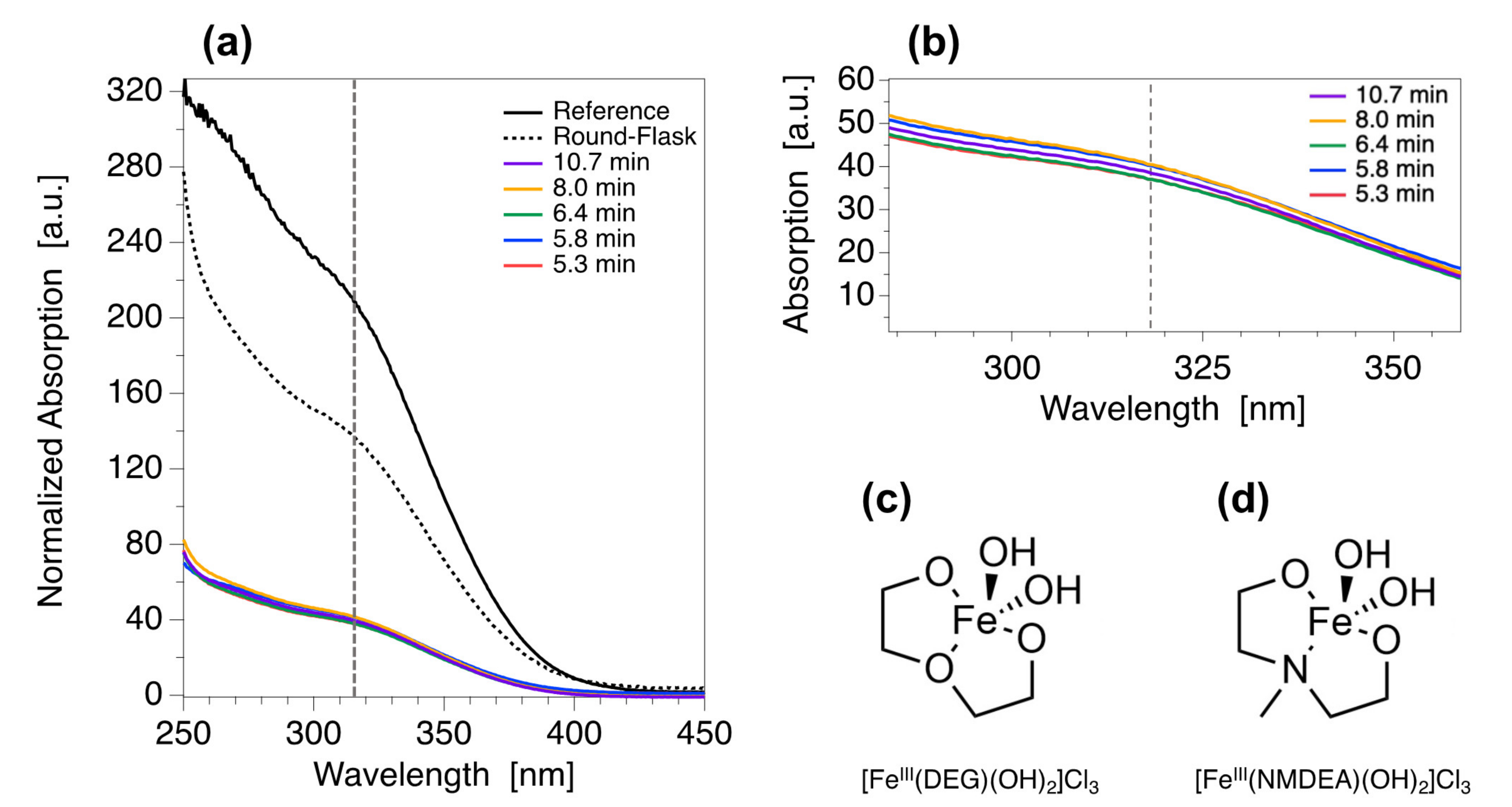
| Element | Sample | τR (min) | d0 (nm) | σ | R0 | RF |
|---|---|---|---|---|---|---|
| Co | A–B | 8 | 1.8 | 0.22 | 0.5 | – |
| C–D | 16 | 2.5 | 0.23 | – | ||
| E | 27 | 9.9 | 0.21 | 0.49 * | ||
| F | 35 | 23.7 | 0.26 | 0.46 * | ||
| G | 40 | 43.6 | 0.27 | 0.47 * | ||
| Fe | H | 5.3 | 20.1 | 0.21 | 0.5 | 0.49 † |
| I | 5.8 | 23.6 | 0.22 | 0.48 † | ||
| J | 6.4 | 30.6 | 0.19 | – | ||
| K | 8 | 33.7 | 0.24 | 0.49 † | ||
| L | 11 | 35.2 | 0.22 | 0.47 † | ||
| M | 16 | 36.2 | 0.24 | – | ||
| Mn | N | 2 | 60.3 | 0.22 | 0.25 | 0.23 * |
| O | 4 | 76.6 | 0.19 | 0.22 * | ||
| P | 8 | 87.5 | 0.26 | 0.22 * | ||
| Q | 16 | 104 | 0.23 | 0.23 * |
| Sample | τR (min) | Q (mL∙min−1) | Φ (%) |
|---|---|---|---|
| Round-Flask | 60 | – | 34.2 |
| H | 5.3 | 3.0 | 81.7 |
| I | 5.8 | 2.75 | 80.2 |
| J | 6.4 | 2.5 | 81.8 |
| K | 8 | 2.0 | 80.1 |
| L | 11 | 1.5 | 81.0 |
| τR (min) | Sample | d0 (nm) | σ | <d0> (nm) | <σ> |
|---|---|---|---|---|---|
| 5.3 | H1 | 20.5 | 0.23 | 20.1 ± 0.9 | 0.21 ± 0.02 |
| H2 | 20.6 | 0.20 | |||
| H3 | 19.1 | 0.20 | |||
| 5.8 | I1 | 22.9 | 0.21 | 23.6 ± 0.6 | 0.22 ± 0.01 |
| I2 | 23.8 | 0.23 | |||
| I3 | 24.2 | 0.21 | |||
| 6.4 | J1 | 31.1 | 0.17 | 30.6 ± 0.7 | 0.19 ± 0.03 |
| J2 | 30.1 | 0.22 | |||
| 8 | K1 | 35.3 | 0.26 | 33.7 ± 1.6 | 0.24 ± 0.02 |
| K2 | 32.1 | 0.22 | |||
| 11 | L1 | 36.0 | 0.25 | 35.2 ± 0.8 | 0.22 ± 0.03 |
| L2 | 34.4 | 0.19 | |||
| 16 | M1 | 36.0 | 0.22 | 36.2 ± 0.2 | 0.24 ± 0.02 |
| M2 | 36.4 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuit, E.; Neveu, S.; Abou-Hassan, A. High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device. Nanomaterials 2022, 12, 119. https://doi.org/10.3390/nano12010119
Bertuit E, Neveu S, Abou-Hassan A. High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device. Nanomaterials. 2022; 12(1):119. https://doi.org/10.3390/nano12010119
Chicago/Turabian StyleBertuit, Enzo, Sophie Neveu, and Ali Abou-Hassan. 2022. "High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device" Nanomaterials 12, no. 1: 119. https://doi.org/10.3390/nano12010119
APA StyleBertuit, E., Neveu, S., & Abou-Hassan, A. (2022). High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device. Nanomaterials, 12(1), 119. https://doi.org/10.3390/nano12010119







