A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Digestion of EBN
2.3. Dissolution Rate and Degree of Hydrolysis
2.4. Molecular Weight Distribution
2.5. Determination of Sialic Acid
2.6. In Vitro Cytotoxicity Assay
2.7. Cellular Antioxidant Activity
2.8. Hydrogen-Peroxide-Induced Cell Oxidative Damage
2.9. Intracellular Tyrosinase Activity Assay
2.10. Contribution Rate of Component
2.11. Data Analysis
3. Results and Discussion
3.1. Analysis of the Physicochemical Properties of Digested EBN
3.1.1. Dissolution Rate and Degree of Hydrolysis
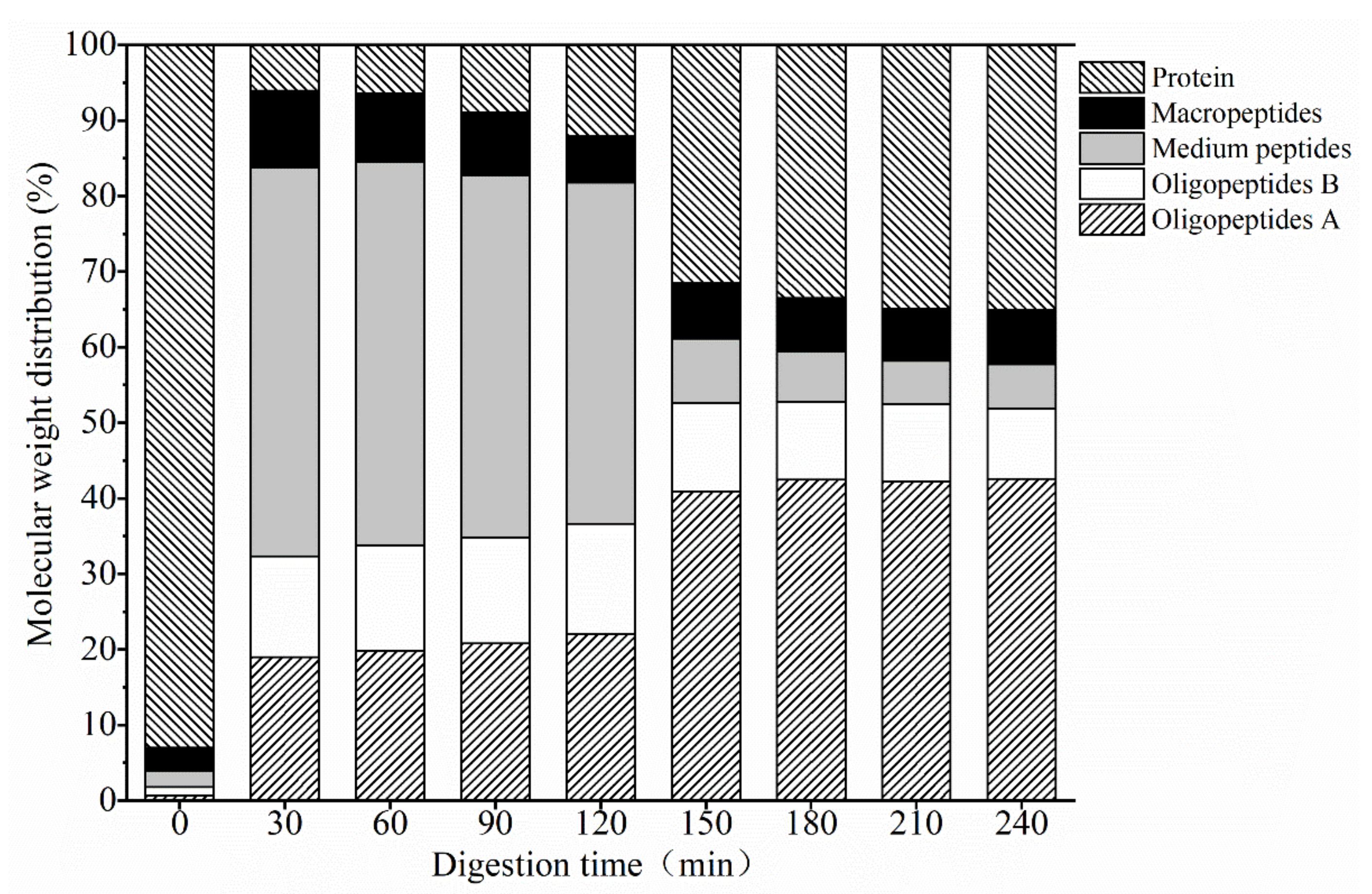
3.1.2. Mw Distribution
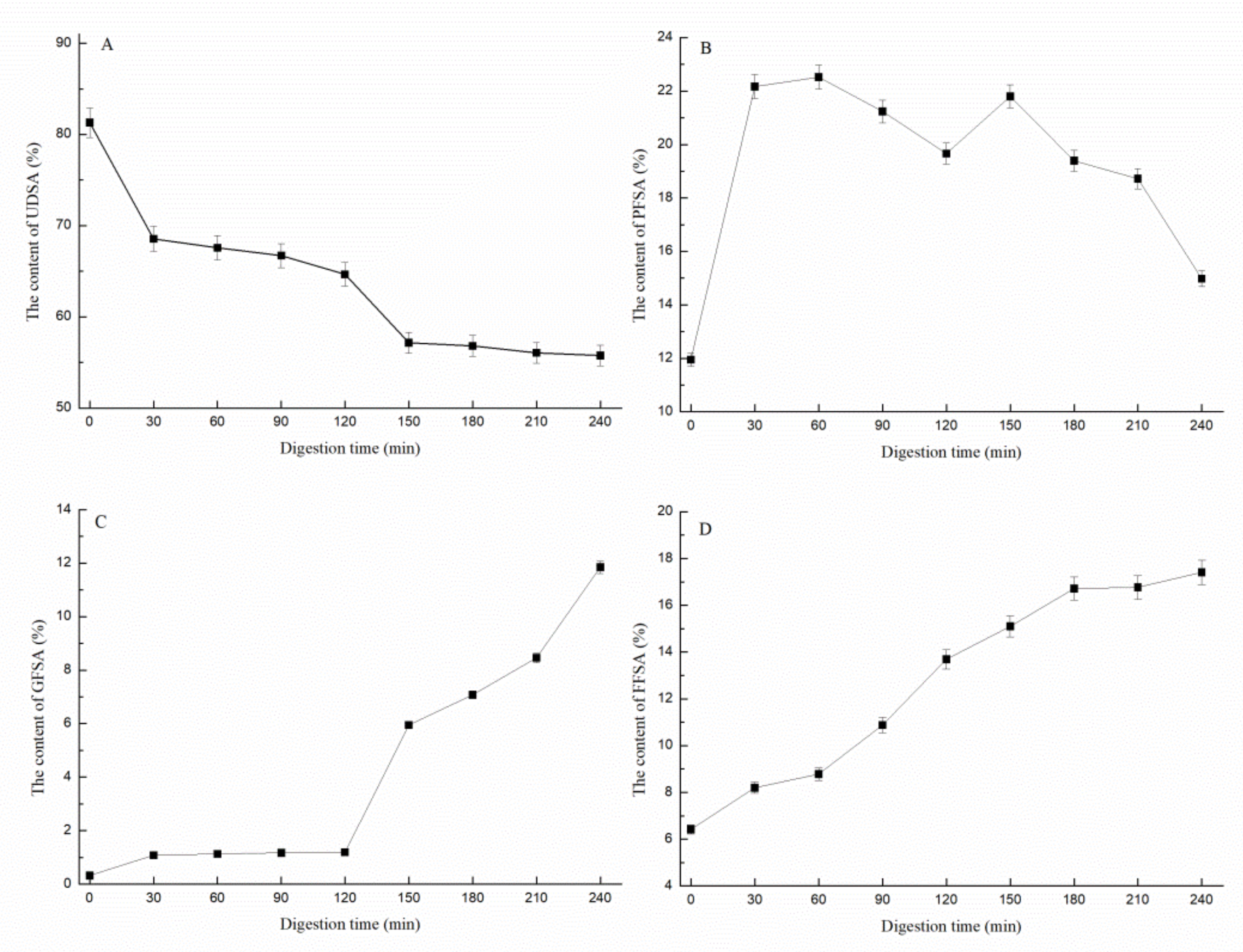
3.1.3. Determination of Sialic Acid
3.2. Whitening Activity of Digested EBN
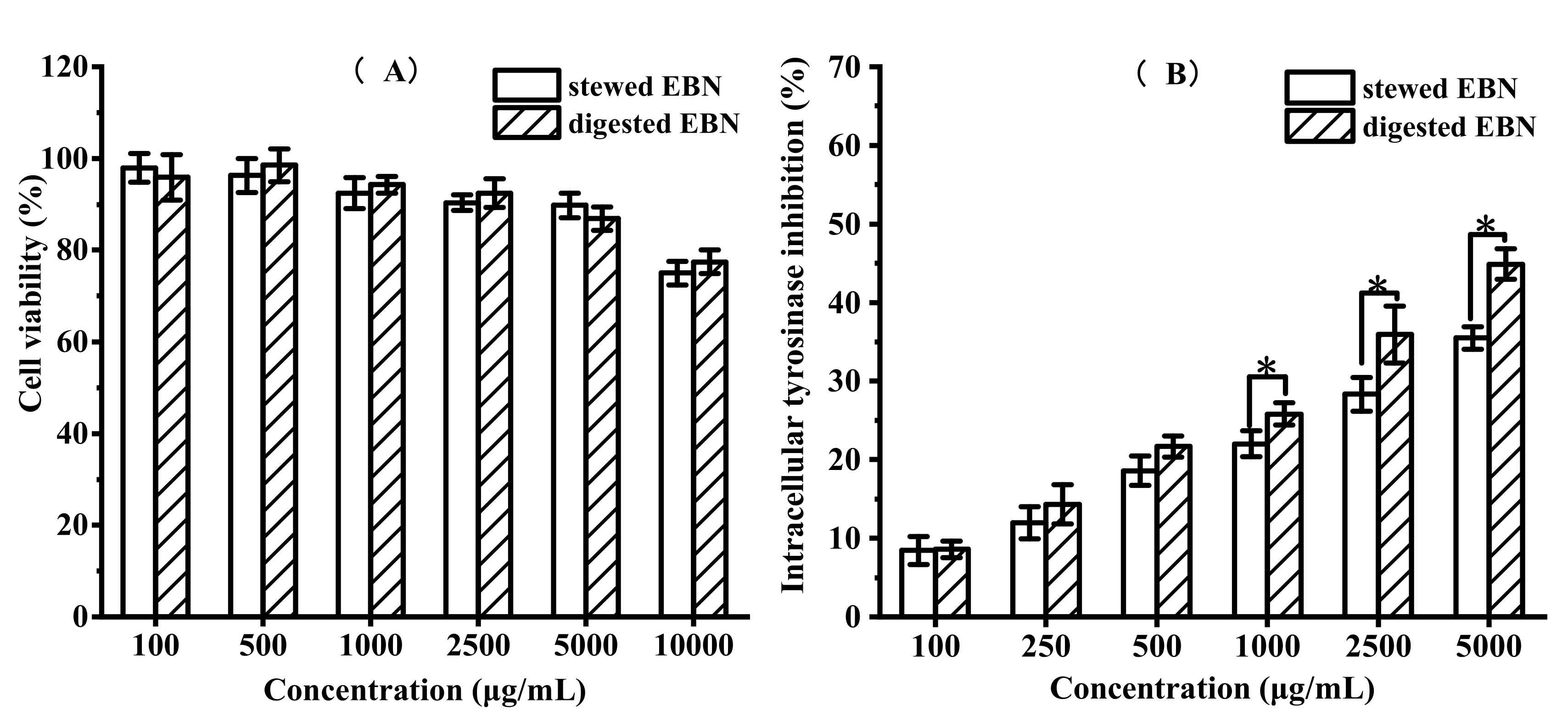
3.2.1. In Vitro Cytotoxicity and Protective Effect of Digested EBN on HepG2 Cell Viability
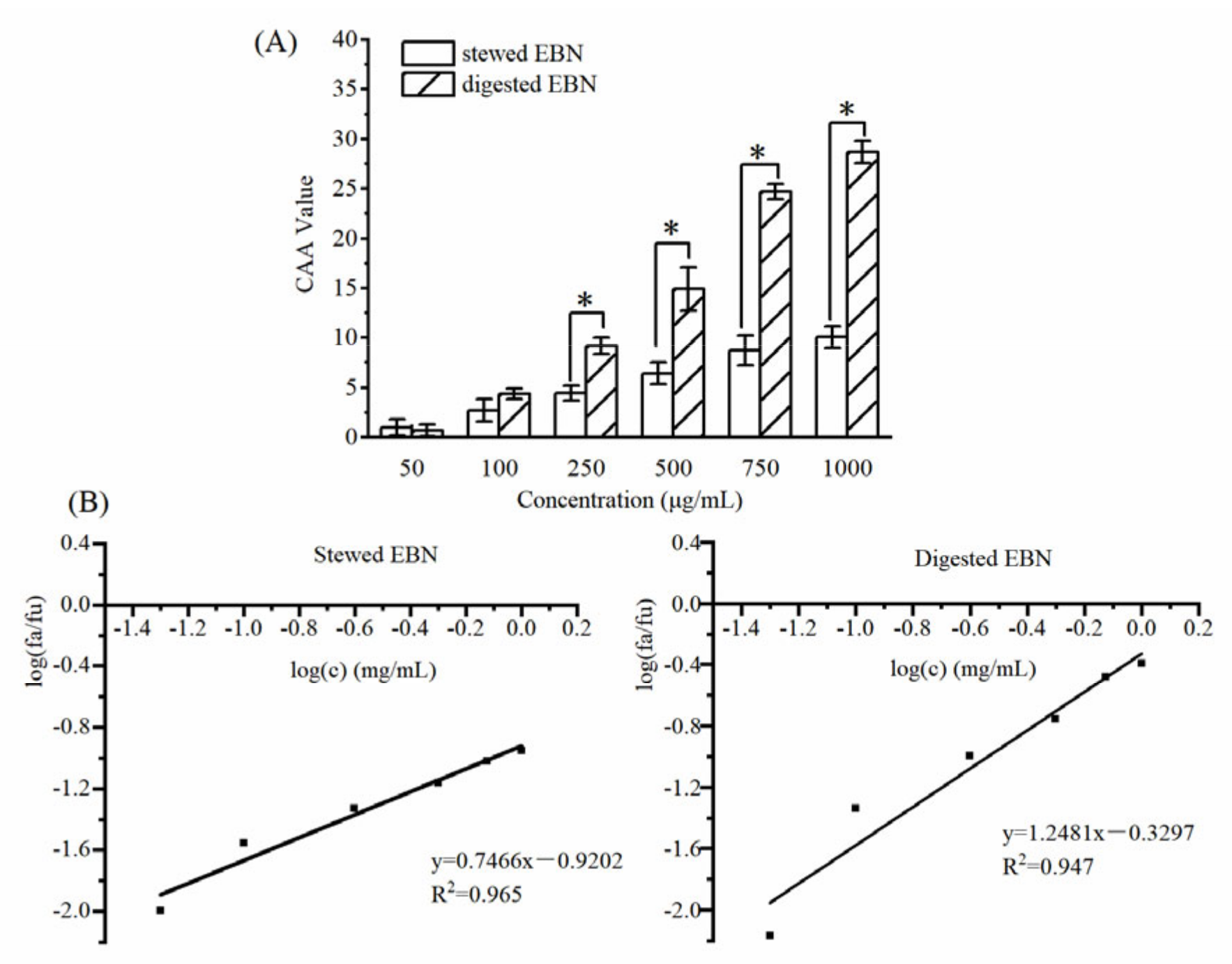
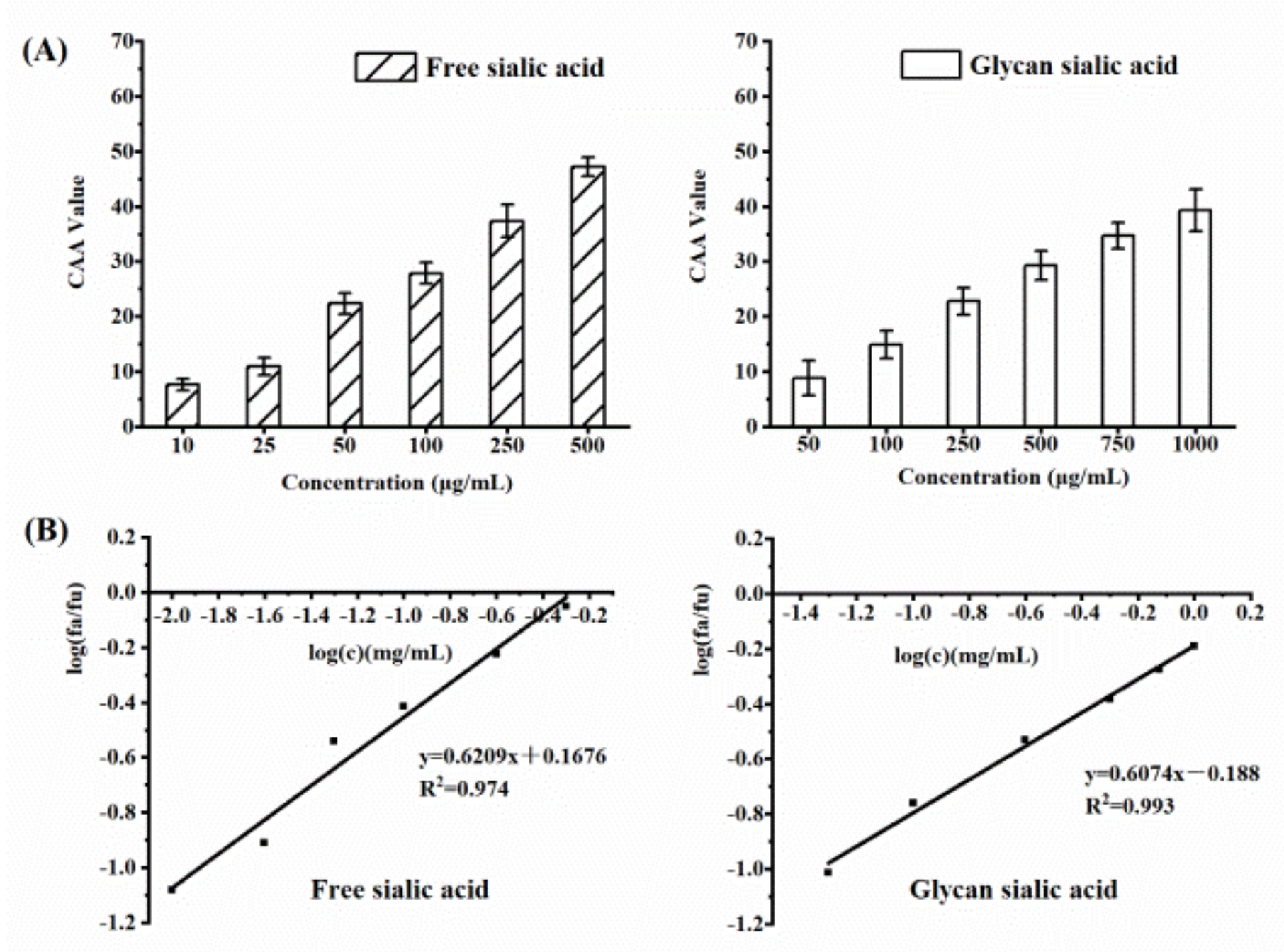
3.2.2. CAA of Digested EBN
3.2.3. In Vitro Cytotoxicity and Intracellular Tyrosinase Inhibition of Digested EBN on B16 Cell Viability
3.3. Mechanism Analysis of Whitening Activity
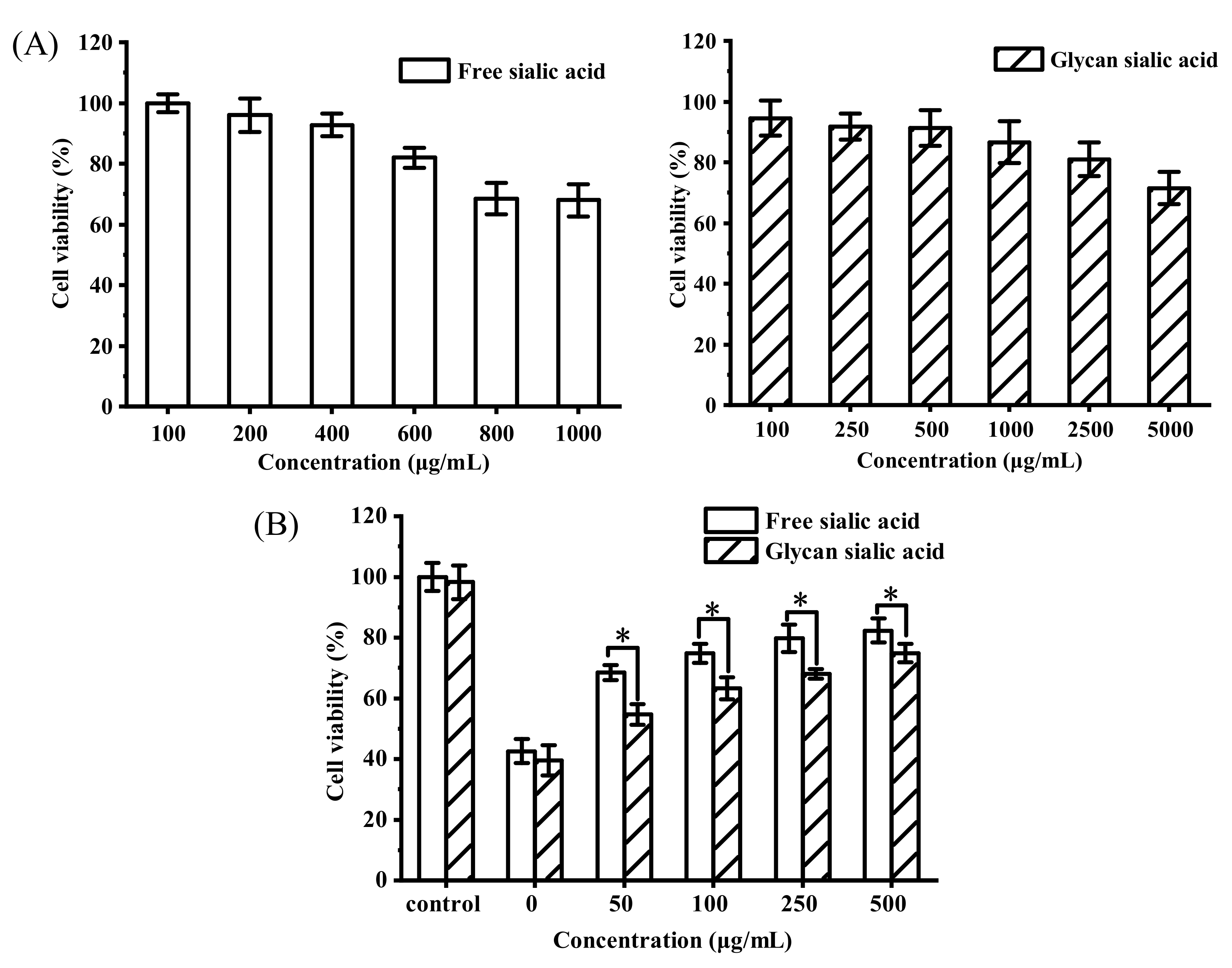
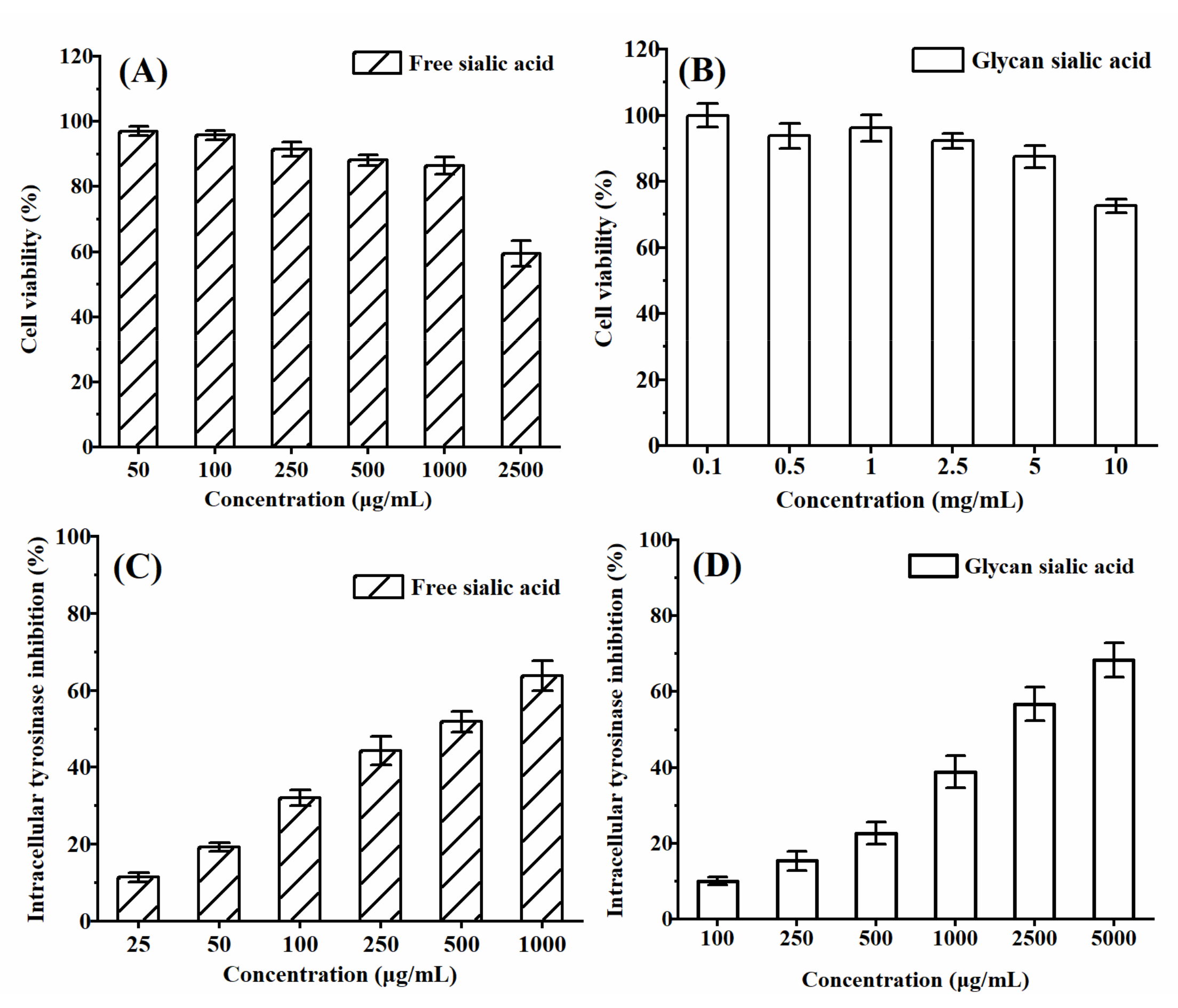
3.3.1. In Vitro Cytotoxicity and Protective Effect of Sialic Acid on HepG2 Cell Viability
3.3.2. CAA of Sialic Acid
3.3.3. In Vitro Cytotoxicity and Intracellular Tyrosinase Inhibition of Sialic Acid on B16 Cell Viability
3.3.4. Analysis of Activity Contribution Rate of Sialic Acid and Protein Components in Digested EBN
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, F.; Liu, D. Sketch of the edible bird’s nest and its important bioactivities. Food Res. Int. 2012, 48, 559–567. [Google Scholar] [CrossRef]
- Daud, N.A.; Mohamad Yusop, S.; Babji, A.S.; Lim, S.J.; Sarbini, S.R.; Hui Yan, T. Edible Bird’s Nest: Physicochemical Properties, Production, and Application of Bioactive Extracts and Glycopeptides. Food Rev. Int. 2019, 37, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Marcone, M.F. Characterization of the edible bird’s nest the “Caviar of the East”. Food Res. Int. 2005, 38, 1125–1134. [Google Scholar] [CrossRef]
- Quek, M.C.; Chin, N.L.; Yusof, Y.A.; Law, C.L.; Tan, S.W. Characterization of edible bird’s nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res. Int. 2018, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Haghani, A.; Mehrbod, P.; Safi, N.; Aminuddin, N.A.; Bahadoran, A.; Omar, A.R.; Ideris, A. In vitro and in vivo mechanism of immunomodulatory and antiviral activity of Edible Bird’s Nest (EBN) against influenza A virus (IAV) infection. J. Ethnopharmacol. 2016, 185, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.Y.; Chang, L.S.; Mat Nasir, N.A.; Babji, A.S.; Lim, S.J. Evaluation of physicochemical properties, amino acid profile and bioactivities of edible Bird’s nest hydrolysate as affected by drying methods. LWT 2020, 131, 109777. [Google Scholar] [CrossRef]
- Zeng, W.W.; Lai, L.S. Anti-melanization effects and inhibitory kinetics of tyrosinase of bird’s nest fern (Asplenium australasicum) frond extracts on melanoma and human skin. J. Biosci. Bioeng. 2019, 127, 738–743. [Google Scholar] [CrossRef]
- Ling, A.J.W.; Chang, L.S.; Babji, A.S.; Latip, J.; Koketsu, M.; Lim, S.J. Review of sialic acid’s biochemistry, sources, extraction and functions with special reference to edible bird’s nest. Food Chem. 2022, 367, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Lai, X.T.; Liu, X.Q.; Li, Y.; Li, B.F.; Huang, X.L.; Zhang, Q.L.; Chen, W.; Lin, L.; Yang, G.W. Competitive Enzyme-Linked Immunoassay for Sialoglycoprotein of Edible Bird’s Nest in Food and Cosmetics. J. Agric. Food Chem. 2012, 60, 3580–3585. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.G. The Quality Assurance of Edible Bird’s Nest: Removal of Nitrite Contamination and Identification of an Indicative Chemical Marker. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China, 2013. [Google Scholar]
- Ochiai, A.; Tanaka, S.; Imai, Y.; Yoshida, H.; Kanaoka, T.; Tanaka, T.; Taniguchi, M. New tyrosinase inhibitory decapeptide: Molecular insights into the role of tyrosine residues. J. Biosci. Bioeng. 2016, 121, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.L.; Wong, Z.C.F.; Lam, K.Y.C.; Cheng, L.K.W.; Zhang, L.M.; Lin, H.; Dong, T.T.; Tsim, K.W.K. Edible Bird’s Nest, an Asian Health Food Supplement, Possesses Skin Lightening Activities: Identification of N-Acetylneuraminic Acid as Active Ingredient. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babji, A.S.; EttySyarmila, I.K.; Nur ‘Aliah, D.; Nurul Nadia, M.; Hadi Akbar, D.; Norrakiah, A.S.; Ghassem, M.; Najafian, L.; Salma, M.Y. Assessment on bioactive components of hydrolysed edible bird nest. Int. Food Res. J. 2018, 25, 1936–1941. [Google Scholar]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.C.F.; Chan, G.K.L.; Wu, K.Q.Y.; Poon, K.K.M.; Chen, Y.C.; Dong, T.T.X.; Tsim, K.W.K. Complete digestion of edible bird’s nest releases free N-acetylneuraminic acid and small peptides: An efficient method to improve functional properties. Food Funct. 2018, 9, 5139–5149. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Halaban, R.; Patton, R.S.; Cheng, E.; Svedine, S.; Trombetta, E.S.; Wahl, M.L.; Ariyan, S.; Hebert, D.N. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J. Biol. Chem. 2002, 277, 14821–14828. [Google Scholar] [CrossRef] [Green Version]
- Luisi, G.; Stefanucci, A.; Zengin, G.; Dimmito, M.P.; Mollica, A. Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions. Antioxidants 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses Ⅰ: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Zhao, X.; Guan, P. Sample Preparation for HPLC Determination of Free and Oligosaccharides-Bound Sialic Acid in Bovine Colostrum. Adv. Mater. Res. 2013, 641–642, 882. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, L.; Shi, Y.G.; Akhtar, M.; Chen, J.S.; Ettelaie, R. Emulsifying and emulsion stabilizing properties of soy protein hydrolysates, covalently bonded to polysaccharides: The impact of enzyme choice and the degree of hydrolysis. Food Hydrocoll. 2021, 113, 15. [Google Scholar] [CrossRef]
- Wang, B.B.; Yu, Z.; Yokoyama, W.; Chiou, B.S.; Chen, M.S.; Liu, F.; Zhong, F. Collagen peptides with DPP-IV inhibitory activity from sheep skin and their stability to in vitro gastrointestinal digestion. Food Biosci. 2021, 42, 11. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.-M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.-K.; Luo, X.; Zhang, X.-W. Bioactivity-Guided Isolation of Cytotoxic Phenanthrenes from Spiranthessinensis. J. Agric. Food Chem. 2019, 67, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complementary Altern. Med. 2014, 14, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhang, R.-J.; Chen, Y.-M.; Ye, X.; Chen, Q.-X.; Shen, D.-Y.; Wang, Q. Synthesis of caffeic acid ester morpholines and their activation effects on tyrosinase. Process Biochem. 2017, 62, 91–98. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, P.; Zheng, X.; Hamzah, S.S.; Zeng, H.; Zhang, Y.; Hu, J.J.L. Effect of dynamic high pressure microfluidization on the solubility properties and structure profiles of proteins in water-insoluble fraction of edible bird’s nests. LWT 2020, 132, 109923. [Google Scholar] [CrossRef]
- Guo, L.; Lu, L.; Yin, M.; Yang, R.; Zhang, Z.; Zhao, W. Valorization of refractory keratinous waste using a new and sustainable bio-catalysis. Chem. Eng. J. 2020, 397, 125420. [Google Scholar] [CrossRef]
- Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Lv, S.; Jiang, S.; Lu, J.; Lin, L. Radical scavenging activities of peptide from Asian clam (Corbicula fluminea) and its protective effects on oxidative damage induced by hydrogen peroxide in HepG2 cells. J. Food Biochem. 2020, 44, e13146. [Google Scholar] [CrossRef] [PubMed]


| Sample | Free Sialic Acid | Glycan Sialic Acid | Digested EBN |
|---|---|---|---|
| Antioxidant activity EC50(mg/mL) | 0.54 | 2.04 | 1.84 |
| Tyrosinase inhibitory activity EC50(mg/mL) | 0.4 | 1.89 | 7.22 |
| Component | Free Sialic acid | Glycan Sialic acid | Protein Component |
| Contribution rate of antioxidant activity (%) | 11.97 | 2.16 | 85.87 |
| Contribution rate of tyrosinase inhibitory activity (%) | 63.43 | 9.14 | 27.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Lian, J.; Liu, X.; Zou, F.; Wang, X.; Chen, M. A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein. Gels 2022, 8, 24. https://doi.org/10.3390/gels8010024
Fan Q, Lian J, Liu X, Zou F, Wang X, Chen M. A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein. Gels. 2022; 8(1):24. https://doi.org/10.3390/gels8010024
Chicago/Turabian StyleFan, Qunyan, Jianmei Lian, Xuncai Liu, Fengyang Zou, Xin Wang, and Maoshen Chen. 2022. "A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein" Gels 8, no. 1: 24. https://doi.org/10.3390/gels8010024
APA StyleFan, Q., Lian, J., Liu, X., Zou, F., Wang, X., & Chen, M. (2022). A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein. Gels, 8(1), 24. https://doi.org/10.3390/gels8010024