Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons
Abstract
:1. Introduction
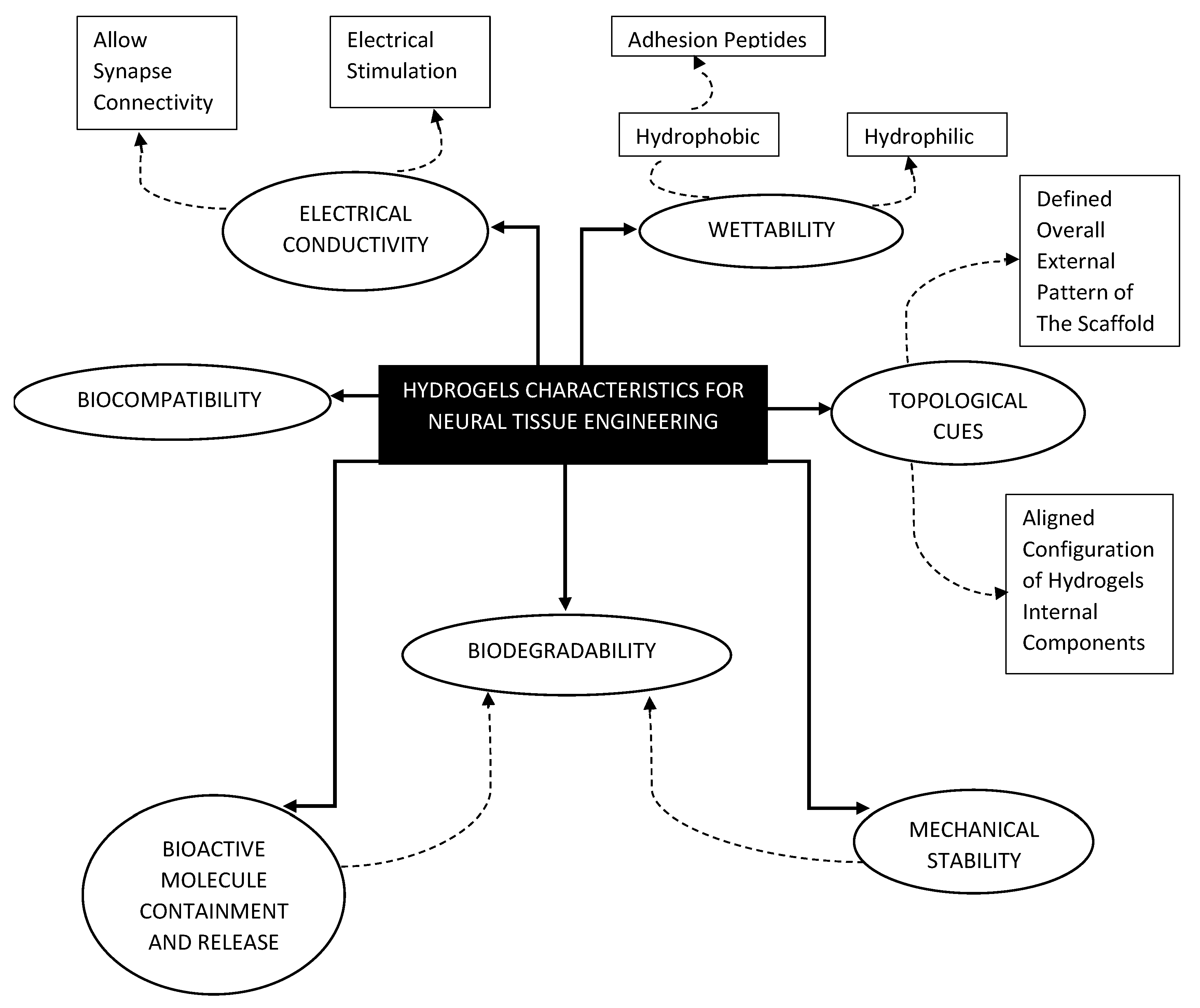
2. The Common Characteristics of Hydrogels Used for NTE
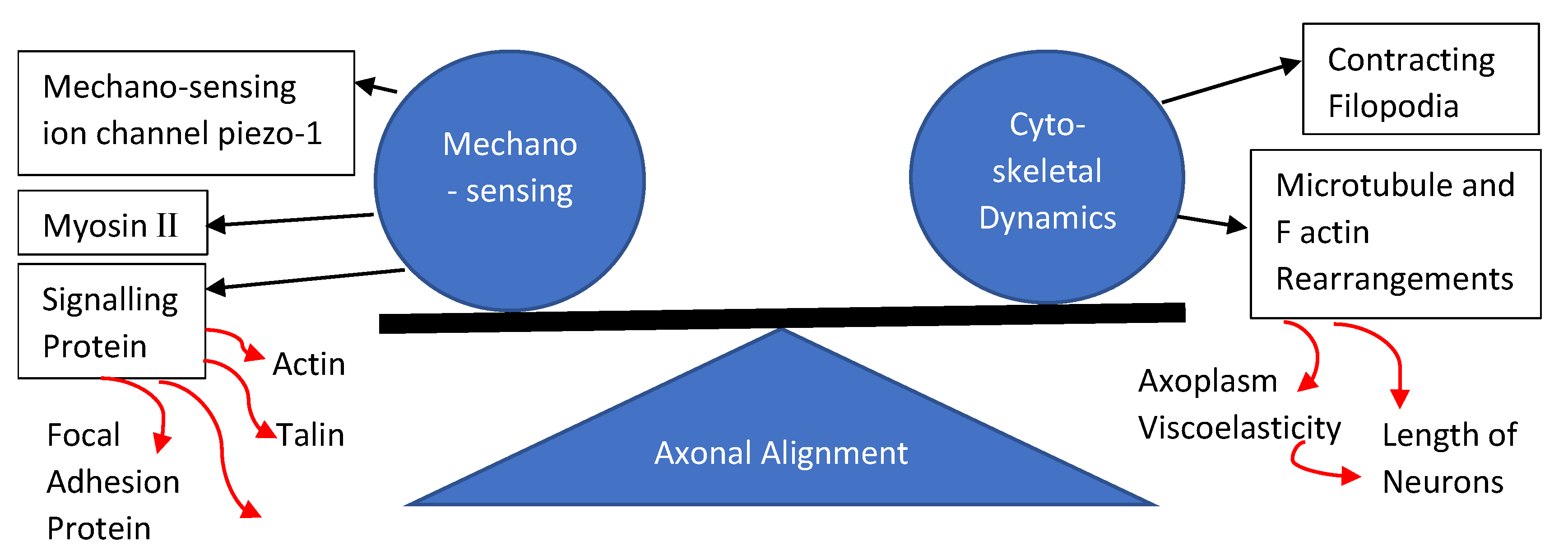
3. Process of Axonal Alignment in Neural Cells
4. Methods of Scaffold Fabrication That Promotes Axonal Alignment
4.1. Electrospinning Technique
4.2. Microfluidic Technique
4.3. 3D Bioprinting
4.4. Magnetic Orientation
4.5. Other Fabrication Methods for Producing Aligned Microstructures within Scaffolds
5. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Van Horne, C.G.; Quintero, J.E.; Gurwell, J.A.; Wagner, R.P.; Slevin, J.T.; Gerhardt, G.A. Implantation of autologous peripheral nerve grafts into the substantia nigra of subjects with idiopathic Parkinson’s disease treated with bilateral STN DBS: A report of safety and feasibility. J. Neurosurg. 2017, 126, 1140–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.U.; Hassan, G.; Rasool, Z. Role of Stem Cells in Treatment of Neurological Disorder. Int. J. Health Sci. 2009, 3, 227–233. [Google Scholar]
- Yoon, S.H.; Shim, Y.S.; Park, Y.H.; Chung, J.K.; Nam, J.H.; Kim, M.O.; Park, H.C.; Park, S.R.; Min, B.; Kim, E.Y.; et al. Complete Spinal Cord Injury Treatment Using Autologous Bone Marrow Cell Transplantation and Bone Marrow Stimulation with Granulocyte Macrophage-Colony Stimulating Factor: Phase I/II Clinical Trial. Stem Cells 2007, 25, 2066–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Koss, K.; Unsworth, L. Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016, 44, 2–15. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Nasr-Esfahani, M.H.; Sadri, S.; Hilderink, J.; van Apeldoorn, A.; Van Blitterswijk, C.A.; Moroni, L. Fabrication, Characterization and Cellular Compatibility of Poly (Hydroxy Alkanoate) Composite Nanofibrous Scaffolds for Nerve Tissue Engineering. PLoS ONE 2013, 8, e57157. [Google Scholar] [CrossRef]
- Saracino, G.A.A.; Cigognini, D.; Silva, D.; Caprini, A.; Gelain, F. Nanomaterials design and tests for neural tissue engineering. Chem. Soc. Rev. 2013, 42, 225–262. [Google Scholar] [CrossRef]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 84, 34–48. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the Field of Electrospinning for Tissue Engineering Applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for stimulating nerve growth. Science 2017, 356, 1010–1011. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-Y.; Wang, W.-T.; Liu, C.-C.; Fu, L.-M. Passive mixers in microfluidic systems: A review. Chem. Eng. J. 2016, 288, 146–160. [Google Scholar] [CrossRef]
- Yang, J.; Ghobadian, S.; Goodrich, P.J.; Montazami, R.; Hashemi, N. Miniaturized biological and electrochemical fuel cells: Challenges and applications. Phys. Chem. Chem. Phys. 2013, 15, 14147–14161. [Google Scholar] [CrossRef] [PubMed]
- Daniele, M.A.; Boyd, D.A.; Adams, A.; Ligler, F.S. Microfluidic Strategies for Design and Assembly of Microfibers and Nanofibers with Tissue Engineering and Regenerative Medicine Applications. Adv. Health Mater. 2015, 4, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ostrovidov, S.; Zhao, Y.; Liang, X.; Kasuya, M.; Kurihara, K.; Nakajima, K.; Bae, H.; Wu, H.; Khademhosseini, A. Microfluidic Spinning of Cell-Responsive Grooved Microfibers. Adv. Funct. Mater. 2015, 25, 2250–2259. [Google Scholar] [CrossRef]
- Abu-Rub, M.T.; Billiar, K.L.; van Es, M.H.; Knight, A.; Rodriguez, B.J.; Zeugolis, D.I.; McMahon, S.; Windebank, A.J.; Pandit, A. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter 2011, 7, 2770–2781. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Sun, J.; Liu, X.; Guo, Z.; He, Y.; Wei, D.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. Wet-spinning fabrication of shear-patterned alginate hydrogel microfibers and the guidance of cell alignment. Regen. Biomater. 2017, 4, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Koppes, R.A.; Park, S.; Hood, T.; Jia, X.; Poorheravi, N.A.; Achyuta, A.H.; Fink, Y.; Anikeeva, P. Thermally drawn fibers as nerve guidance scaffolds. Biomaterials 2016, 81, 27–35. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Lü, S.; Bai, X.; Wu, X.; Gao, C.; Wen, N.; Liu, M. High encapsulation and localized delivery of curcumin from an injectable hydrogel. Mater. Sci. Eng. C 2018, 83, 121–129. [Google Scholar] [CrossRef]
- Han, S.-S.; Yoon, H.Y.; Yhee, J.Y.; Cho, M.O.; Shim, H.-E.; Jeong, J.-E.; Lee, D.-E.; Kim, K.; Guim, H.; Lee, J.H.; et al. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym. Chem. 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Ballios, B.G.; Shoichet, M.S. Injectable hydrogels for central nervous system therapy. Biomed. Mater. 2012, 7, 024101. [Google Scholar] [CrossRef]
- Rose, J.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J.; Haraszti, T.; De Laporte, L. An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells. Small 2017, 13, 1702207. [Google Scholar] [CrossRef] [PubMed]
- Antman-Passig, M.; Shefi, O. Remote Magnetic Orientation of 3D Collagen Hydrogels for Directed Neuronal Regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef]
- Mujtaba, T.; Mayer-Proschel, M.; Rao, M. A Common Neural Progenitor for the CNS and PNS. Dev. Biol. 1998, 200, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Haile, Y.; Haastert, K.; Cesnulevicius, K.; Stummeyer, K.; Timmer, M.; Berski, S.; Dräger, G.; Gerardy-Schahn, R.; Grothe, C. Culturing of glial and neuronal cells on polysialic acid. Biomaterials 2007, 28, 1163–1173. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [Green Version]
- Bergethon, P.; Trinkaus-Randall, V.; Franzblau, C. Modified hydroxyethylmethacrylate hydrogels as a modelling tool for the study of cell-substratum interactions. J. Cell Sci. 1989, 92, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Chun, I.K.; Li, Y.F.; Kataria, R.; Zhang, Q.; Rosenblatt, J.; Brewster, M.E. Preparation and physicochemical char-acterization of biodegradable nerve guides containing the nerve growth agent sabeluzole. Biomaterials 2005, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 2003, 24, 2871–2880. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Holmstrom, N.A.V.; Lilja, J.A.; Schweinhardt, P.; Hao, J.X.; Spenger, C.; Olson, L. Allodynia limits the use-fulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005, 8, 346–353. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yu, H.; Gao, S.J.; Mao, H.Q.; Leong, K.W.; Wang, S. Polyphosphoester microspheres for sustained release of bio-logically active nerve growth factor. Biomaterials 2002, 23, 3765–3772. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V.; Howe, C.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth Factor Delivery for Tissue Engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Kotwal, A.; Schmidt, C.E. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically con-ducting biomaterials. Biomaterials 2001, 22, 1055–1064. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue ap-plications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically Conductive Biodegradable Polymer Composite for Nerve Regeneration: Electricity-Stimulated Neurite Outgrowth and Axon Regeneration. Artif. Organs 2007, 31, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Reano, R.M.; Pang, S.; Yee, A.F.; Chen, C.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Cao, H.; Liu, T.; Chew, S.Y. The application of nanofibrous scaffolds in neural tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Koci, Z.; Výborný, K.; Dubišová, J.; Vacková, I.; Jäger, A.; Lunov, O.; Jiráková, K.; Kubinova, S. Extracellular Matrix Hydrogel Derived from Human Umbilical Cord as a Scaffold for Neural Tissue Repair and Its Comparison with Extracellular Matrix from Porcine Tissues. Tissue Eng. Part C Methods 2017, 23, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Gnavi, S.; Ruini, F.; Gambarotta, G.; Gioffredi, E.; Chiono, V.; Perroteau, I.; Ciardelli, G. Development and characterization of novel agar and gelatin injectable hydrogel as filler for peripheral nerve guidance channels. J. Tissue Eng. Regen. Med. 2017, 11, 197–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franze, K.; Gerdelmann, J.; Weick, M.; Betz, T.; Pawlizak, S.; Lakadamyali, M.; Bayer, J.; Rillich, K.; Gögler, M.; Lu, Y.-B.; et al. Neurite Branch Retraction Is Caused by a Threshold-Dependent Mechanical Impact. Biophys. J. 2009, 97, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Chanet, S.; Martin, A.C. Mechanical force sensing in tissues. Prog. Mol. Biol. Transl. Sci. 2014, 126, 317–352. [Google Scholar] [CrossRef] [Green Version]
- Kerstein, P.C.; Iv, R.H.N.; Gomez, T.M. Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci. 2015, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.-B.; Franze, K.; Seifert, G.; Steinhauser, C.; Kirchhoff, F.; Wolburg, H.; Guck, J.; Janmey, P.; Wei, E.-Q.; Kas, J.; et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl. Acad. Sci. USA 2006, 103, 17759–17764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.; Guck, J. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Dai Trang, T.L.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [Green Version]
- Gomez, T.M.; Letourneau, P.C. Actin dynamics in growth cone motility and navigation. J. Neurochem. 2014, 129, 221–234. [Google Scholar] [CrossRef]
- Mitchison, T.; Cramer, L. Actin-Based Cell Motility and Cell Locomotion. Cell 1996, 84, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Insall, R.H.; Machesky, L.M. Actin Dynamics at the Leading Edge: From Simple Machinery to Complex Networks. Dev. Cell 2009, 17, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, N.A.; Burnette, D.T.; Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006, 8, 216–226. [Google Scholar] [CrossRef]
- Dent, E.W.; Gupton, S.L.; Gertler, F.B. The Growth Cone Cytoskeleton in Axon Outgrowth and Guidance. Cold Spring Harb. Perspect. Biol. 2011, 3, a001800. [Google Scholar] [CrossRef] [Green Version]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [Green Version]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Reznik, S.N.; Yarin, A.L.; Theron, A.; Zussman, E. Transient and steady shapes of droplets attached to a surface in a strong electric field. J. Fluid Mech. 2004, 516, 349–377. [Google Scholar] [CrossRef] [Green Version]
- Hohman, M.M.; Shin, M.; Rutledge, G.C.; Brenner, M.P. Electrospinning and electrically forced jets. I. Stability theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef] [Green Version]
- Ulrica, E.J.; Eitan, N.; Per Fredrik, J. Tailor-Made Electrospun Culture Scaffolds Control Human Neural Progenitor Cell Be-havior: Studies on Cellular Migration and Phenotypic Differentiation. J. Biomater. Nanobiotechnol. 2017, 8, 1–21. [Google Scholar]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, M.; Lee, D.; Heo, D.N.; Lim, H.-N.; Kwon, I.K. Fabrication and design of bioactive agent coated, highly-aligned electrospun matrices for nerve tissue engineering: Preparation, characterization and application. Appl. Surf. Sci. 2017, 424, 359–367. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, srep42212. [Google Scholar] [CrossRef] [Green Version]
- Vimal, S.K.; Ahamad, N.; Katti, D.S. A simple method for fabrication of electrospun fibers with controlled degree of alignment having potential for nerve regeneration applications. Mater. Sci. Eng. C 2016, 63, 616–627. [Google Scholar] [CrossRef]
- Yao, S.; Liu, X.; Yu, S.; Wang, X.; Zhang, S.; Wu, Q.; Sun, X.; Mao, H. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale 2016, 8, 10252–10265. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Yao, S.; Mao, H.-Q.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef]
- Kato-Negishi, M.; Onoe, H.; Ito, A.; Takeuchi, S. Rod-Shaped Neural Units for Aligned 3D Neural Network Connection. Adv. Healthc. Mater. 2017, 6, 1700143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.; Na, S.; Jang, J.M.; Kim, J.; Jeon, N.L. Engineering-Aligned 3D Neural Circuit in Microfluidic Device. Adv. Healthc. Mater. 2016, 5, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Patel, B.B.; Dzuilko, A.K.; Montazami, R.; Sakaguchi, D.S.; Hashemi, N. Polycaprolactone Microfibrous Scaffolds to Navigate Neural Stem Cells. Biomacromolecules 2016, 17, 3287–3297. [Google Scholar] [CrossRef] [Green Version]
- Tachizawa, S.; Takahashi, H.; Kim, Y.-J.; Odawara, A.; Pauty, J.; Ikeuchi, Y.; Suzuki, I.; Kikuchi, A.; Matsunaga, Y.T. Bundle Gel Fibers with a Tunable Microenvironment for in Vitro Neuron Cell Guiding. ACS Appl. Mater. Interfaces 2017, 9, 43250–43257. [Google Scholar] [CrossRef] [PubMed]
- Haynl, C.; Hofmann, E.; Pawar, K.; Förster, S.; Scheibel, T. Microfluidics-Produced Collagen Fibers Show Extraordinary Mechanical Properties. Nano Lett. 2016, 16, 5917–5922. [Google Scholar] [CrossRef]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Zhu, W.; O’Brien, C.; O’Brien, J.R.; Zhang, L.G. 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine 2014, 9, 859–875. [Google Scholar] [CrossRef]
- Knowlton, S.; Cho, Y.; Li, X.-J.; Khademhosseini, A.; Tasoglu, S. Utilizing stem cells for three-dimensional neural tissue engineering. Biomater. Sci. 2016, 4, 768–784. [Google Scholar] [CrossRef]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. (Daniel) Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Kuss, M.; Kong, Y.; Nie, F.; Liu, X.; Liu, B.; Dunaevsky, A.; Fayad, P.; Duan, B.; Li, X. 3D Printed Hydrogels with Aligned Microchannels to Guide Neural Stem Cell Migration. ACS Biomater. Sci. Eng. 2021, 7, 690–700. [Google Scholar] [CrossRef]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a Highly Aligned Neural Scaffold via a Table Top Stereolithography 3D Printing and Electrospinning. Tissue Eng. Part A 2017, 23, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sanz, B.; Sanchez, A.A.; Tangey, B.; Gilmore, K.; Yue, Z.; Liu, X.; Wallace, G. Light Cross-Linkable Marine Collagen for Coaxial Printing of a 3D Model of Neuromuscular Junction Formation. Biomedicines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.J.; Alvarez, Z.; Goldberger, J.E.; Boekhoven, J.; Kessler, J.A.; Kuhn, H.G.; Stupp, S.I. A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells. Acta Biomater. 2016, 37, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
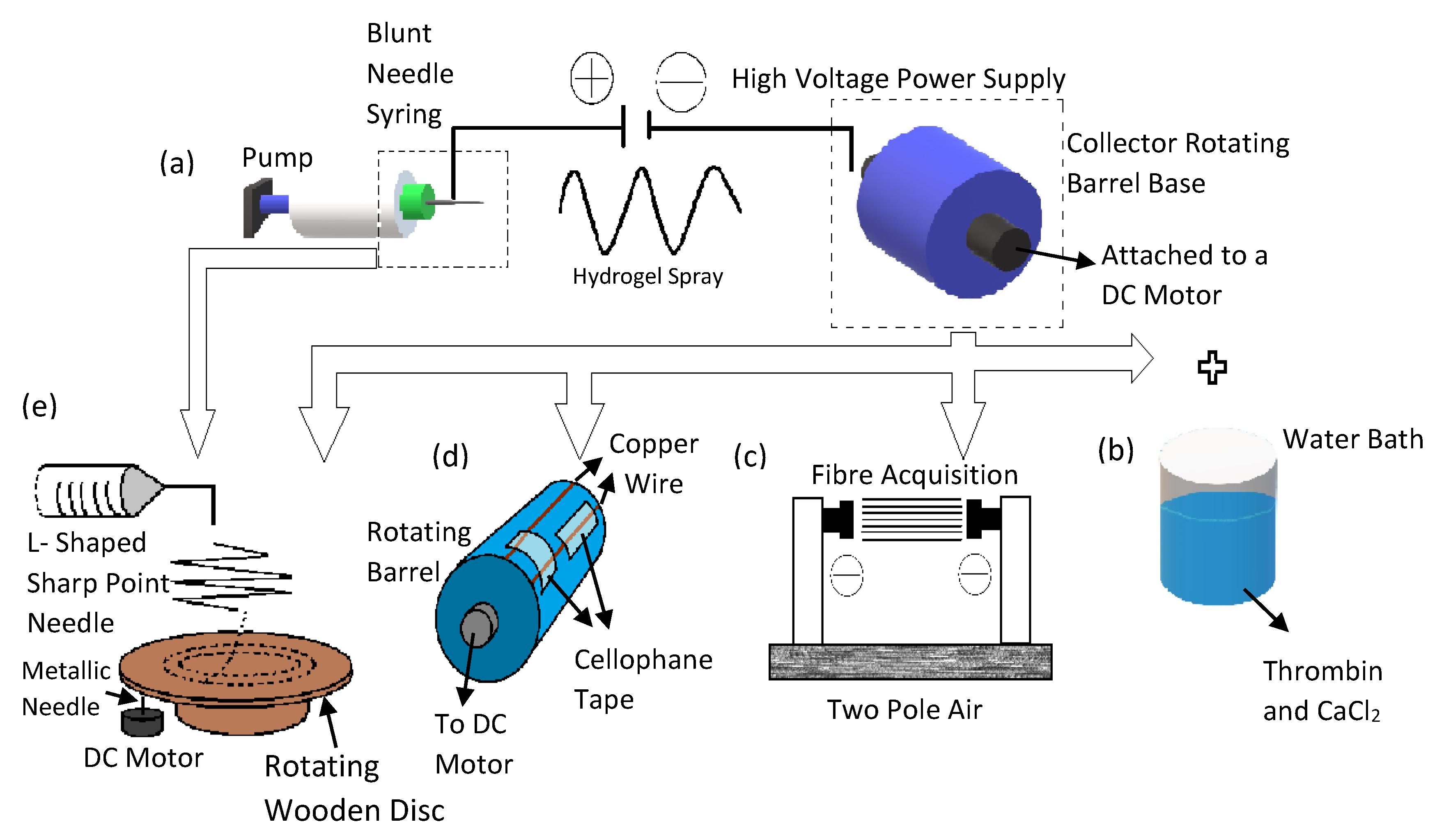
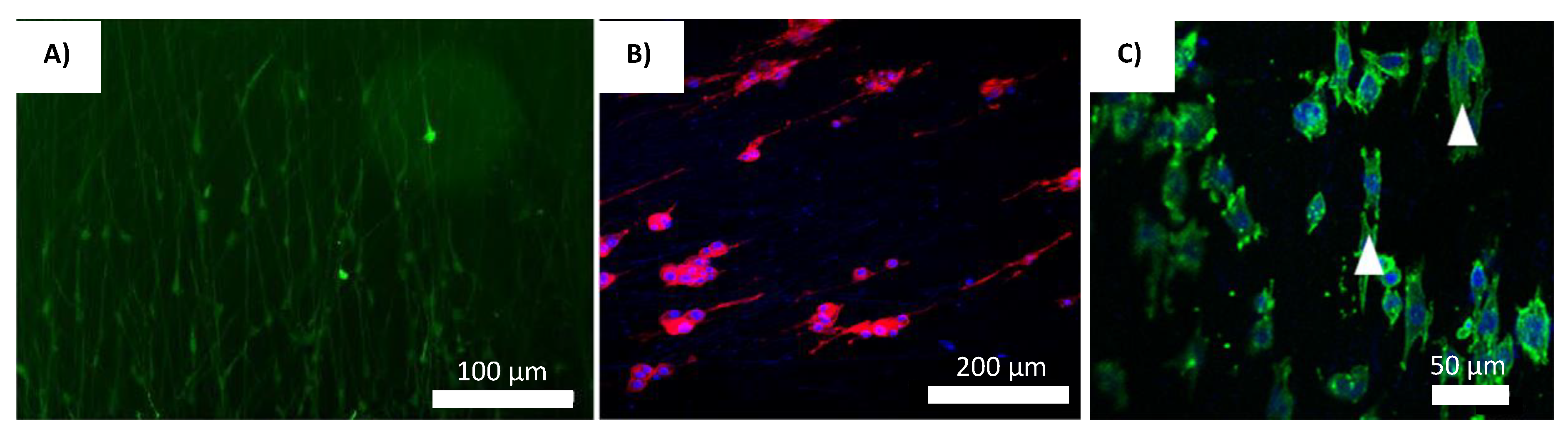
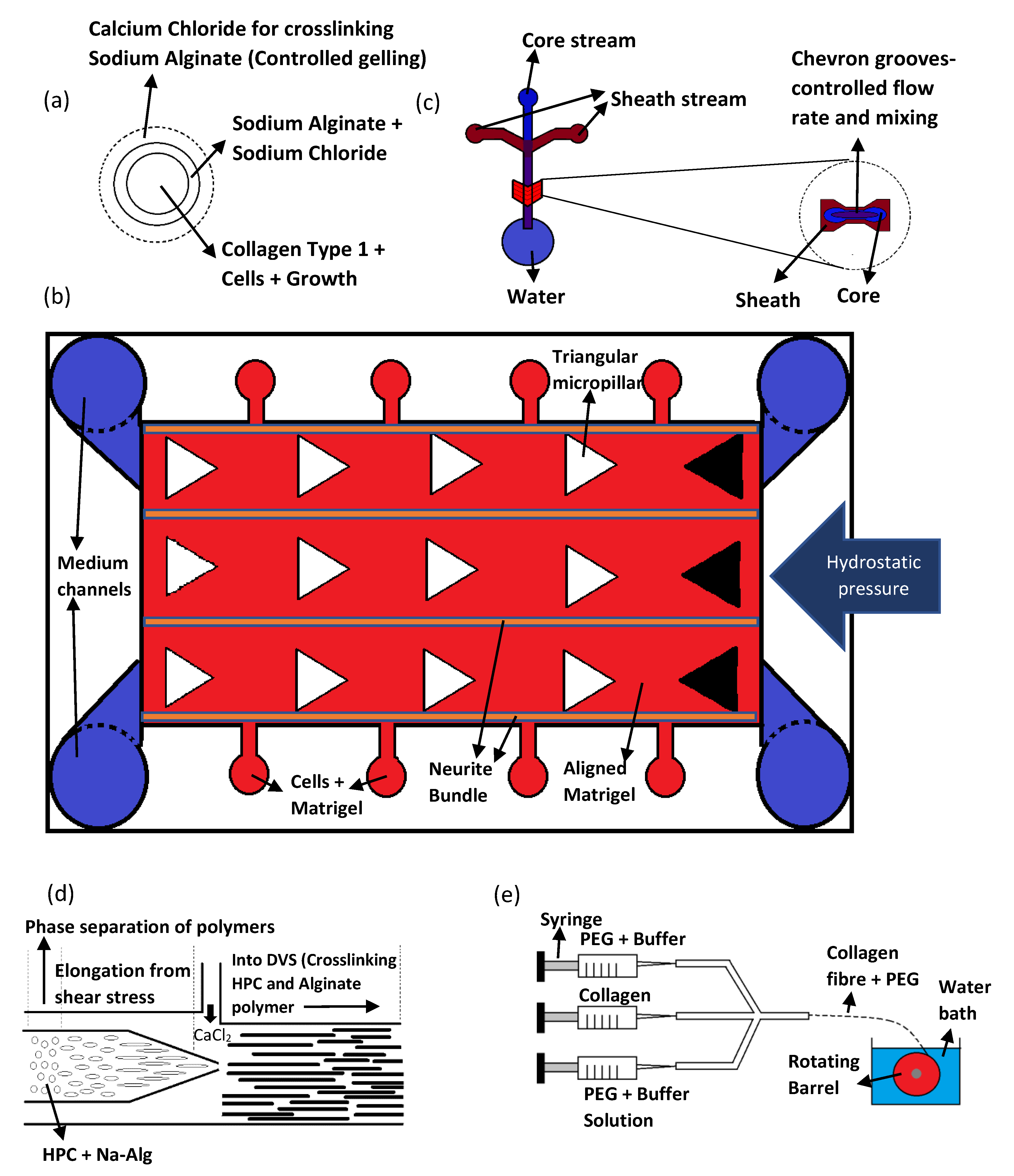
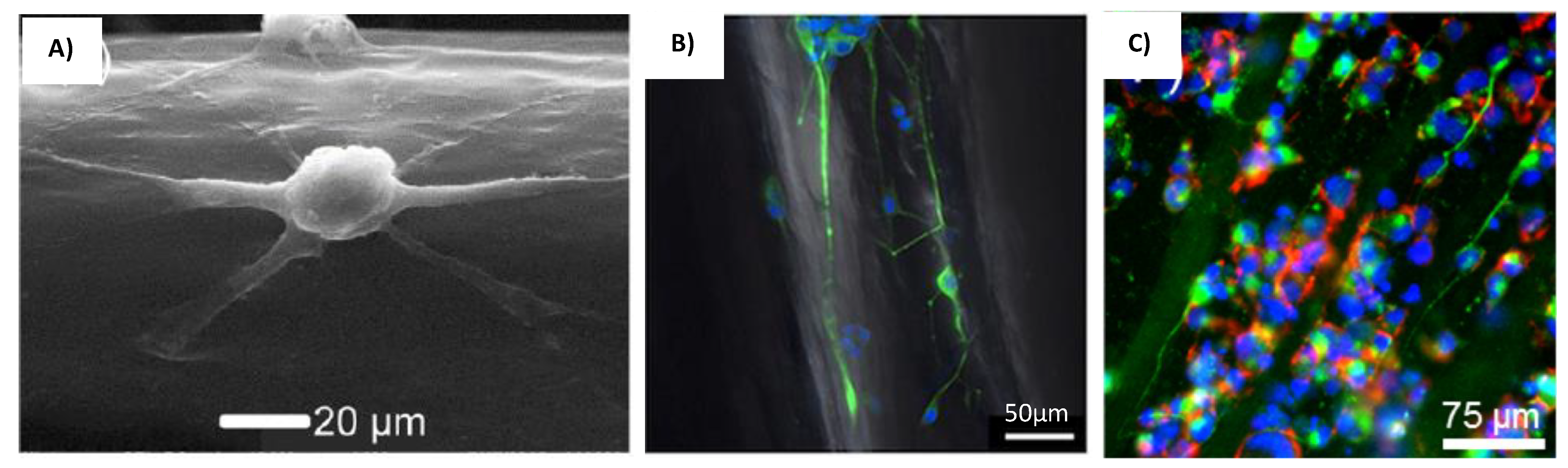

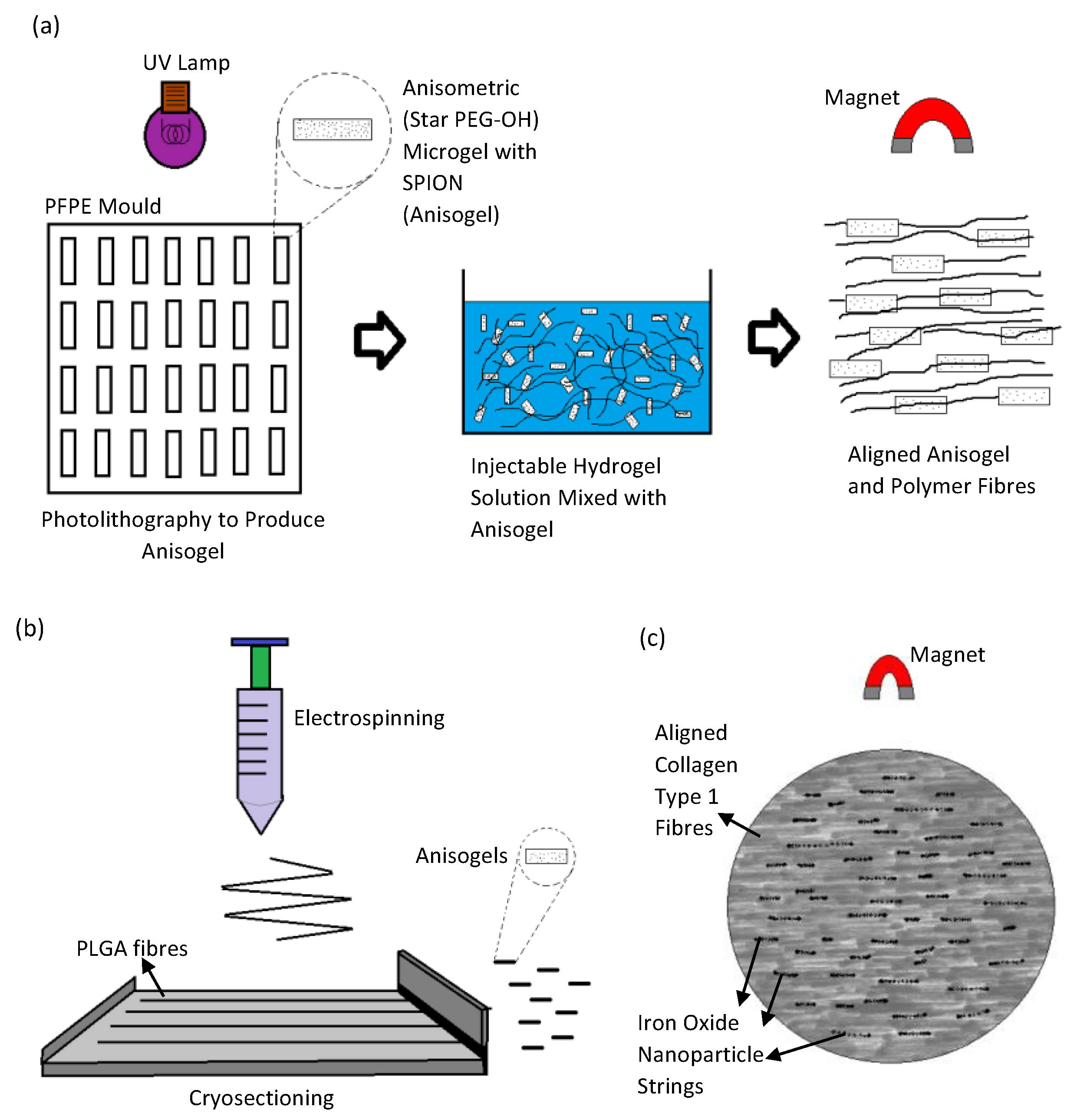


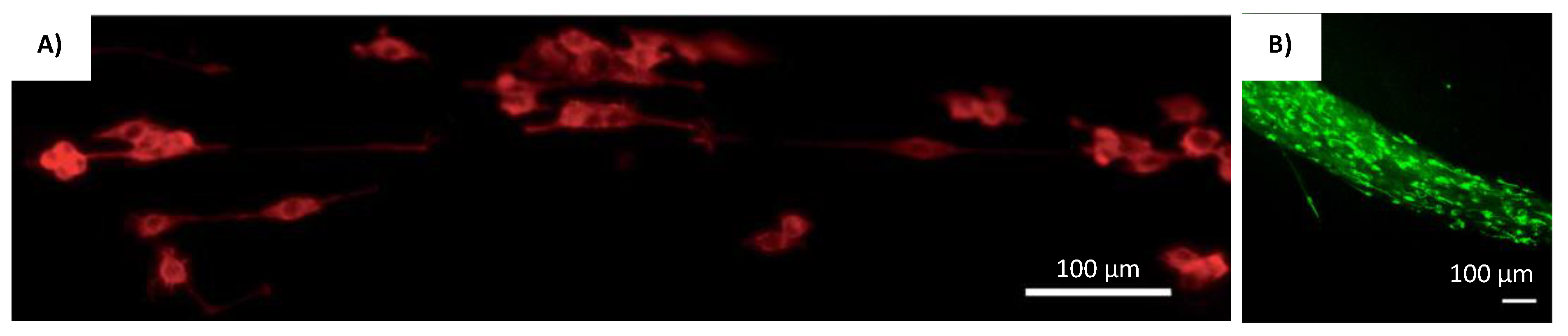
| Method/Mechanism that Creates Fiber Alignment | Materials Used | Cells Used | Results/Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| The orientated fibers were produced by applying high voltage power supply to blunt needles at two different locations | Poly-L-lactic acid (PLLA) fibrous scaffold | Human neural progenitor cell (HNPC) |
|
| [64] |
| PLLA nanofibers-coated graphene oxide (GO) |
|
| [65] | |
| Standard electrospinning setup with the addition of an aluminum foil-coated rotating mandrel to collect fibers jetting out of the blunt needle | Thermoplastic polycarbonate urethane (PCU) coated with Poly-l-Lysine (PLL) and Poly-l-Ornithine (PLO), | Dental pulp stem cells (DPSC) |
| [66] | |
| Wet spinning process which includes a rotating collector water bath containing calcium chloride (CaCl2) and thrombin | Fibrin | Schwann cells |
| [70] | |
| Wet spinning process which includes a rotating collector water bath containing calcium chloride (CaCl2) and thrombin | Fibrin |
|
| [69] | |
| Two pole air gap electrospinning technique | Poly (ε-caprolactone-co-ethyl ethylene phosphate) (PCLEEP) | In Vivo studies—incised portion of adult female Sprague Dawley rats C5 spinal cord |
| [67] | |
| Standard electrospinning set up with a conductive rotating collector with cellophane tape adhered horizontally and vertically onto copper wires of the collector | Poly(lactic-co-glycolic acid)(PLGA) and Poly Urethane (PU) |
|
| [12] | |
| Standard electrospinning set with an L shaped sharp point needle to direct the polymer solution downward onto a rotating wooden disc | Polystyrene mixed with tetrahydrofuran (THF) and dimethylformamide (DMF) | Human astrocytoma cells (U373) |
| [68] |
| Method/Mechanism that Creates Fiber/Microstructure Alignment | Materials Used | Cells Used | Results/Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Core-Collagen Type 1 Sheath-Sodium Alginate | Cortical rod-shaped neural units (cortical units), Hippocampal rod-shaped neural units (hippocampal units), and mNSC rod-shaped neural units (mNSC units) |
|
| [71] |
| Matrigel | Rat cortical neurons prepared from a Sprague–Dawley embryonic rat |
| [72] | |
| PCL (Polycaprolactone) and PEG (Polyethylene glycol) | Adult hippocampal progenitor cells (AHPCs) isolated from adult Fischer 344 rats |
| [73] | |
| Hydroxypropyl cellulose (HPC) and sodium alginate (Na-Alg) |
|
| [74] | |
| Collagen Type 1 PEG | NG108-15 neuronal cell line |
| [75] |
| Method/Mechanism That Creates Fiber/Microstructure Alignment | Materials Used | Cells Used | Results/Advantages | Disadvantages | References |
|---|---|---|---|---|---|
|
| Schwann cells |
|
| [79] |
|
| Human neural stem/progenitor cells (NSCs) |
| [80] | |
|
| Neural stem cell (NSC) |
| [81] | |
|
| NSC-34 (motor neuron-like cells), Primary myoblast cells |
| [82] |
| Method/Mechanism that Creates Fiber/Microstructure Alignment | Materials Used | Cells Used | Results/Advantages | Disadvantages | References |
|---|---|---|---|---|---|
|
|
|
|
| [26] |
|
|
|
| [27] | |
| Collagen, Iron Oxide Nanoparticles | Leech Neuronal Cells, PC12 cells |
| [28] |
| Method/Mechanism that Creates Fiber/Microstructure Alignment | Materials Used | Cells Used | Results/Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Collagen Type 1, polytetrafluoroethylene (PTFE) tubes with two stainless steel plates running along its length |
|
|
| [17] |
| biomimetic amphiphile (PA) derived from ECM glycoprotein Tenascin-C | P19 embryonal carcinoma cells |
| [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirkkunan, D.; Pingguan-Murphy, B.; Muhamad, F. Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons. Gels 2022, 8, 25. https://doi.org/10.3390/gels8010025
Sirkkunan D, Pingguan-Murphy B, Muhamad F. Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons. Gels. 2022; 8(1):25. https://doi.org/10.3390/gels8010025
Chicago/Turabian StyleSirkkunan, Devindraan, Belinda Pingguan-Murphy, and Farina Muhamad. 2022. "Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons" Gels 8, no. 1: 25. https://doi.org/10.3390/gels8010025
APA StyleSirkkunan, D., Pingguan-Murphy, B., & Muhamad, F. (2022). Directing Axonal Growth: A Review on the Fabrication of Fibrous Scaffolds That Promotes the Orientation of Axons. Gels, 8(1), 25. https://doi.org/10.3390/gels8010025






