2.1. AAV2/5 Transduction of the β5−/− Mouse Retina
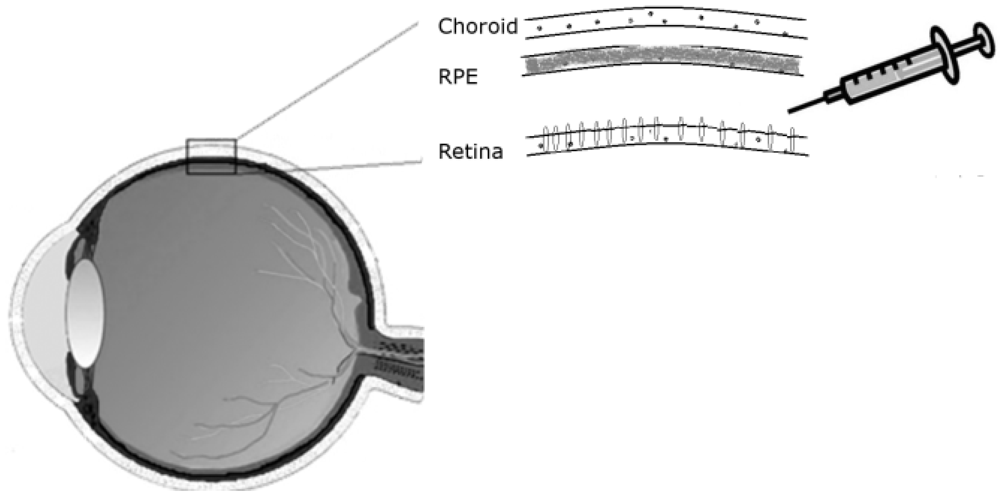
Drugs can be delivered to the retina by injecting them in solution between the retina and the RPE, hence called sub-retinal injection. For administration of treatments targeting the retina’s outer nuclear layer, a sub-retinal mode of delivery is preferable to an intravitreal route, as it allows direct contact with the photoreceptor cells (
Figure 1).
Following subretinal injection the therapeutic solution is expected to be equally distributed between the RPE and the neural retina. However, a therapy that is delivered by an AAV vector may show a preference for RPE transduction, depending in particular on the serotype used. The β5
−/− mouse model does not express functional αvβ5R and shows some defects in the adhesion of the retina to the RPE as well as a marked loss of the circadian peak of phagocytosis and engulfment of shed POS [
7,
8] We examined the transduction pattern of a CMV-driven fluorescent eGFP marker from AAV2/5 following subretinal injection in β5
−/− mice. In contrast to that observed in WT mice, the injected β5
−/− mice show a stronger (
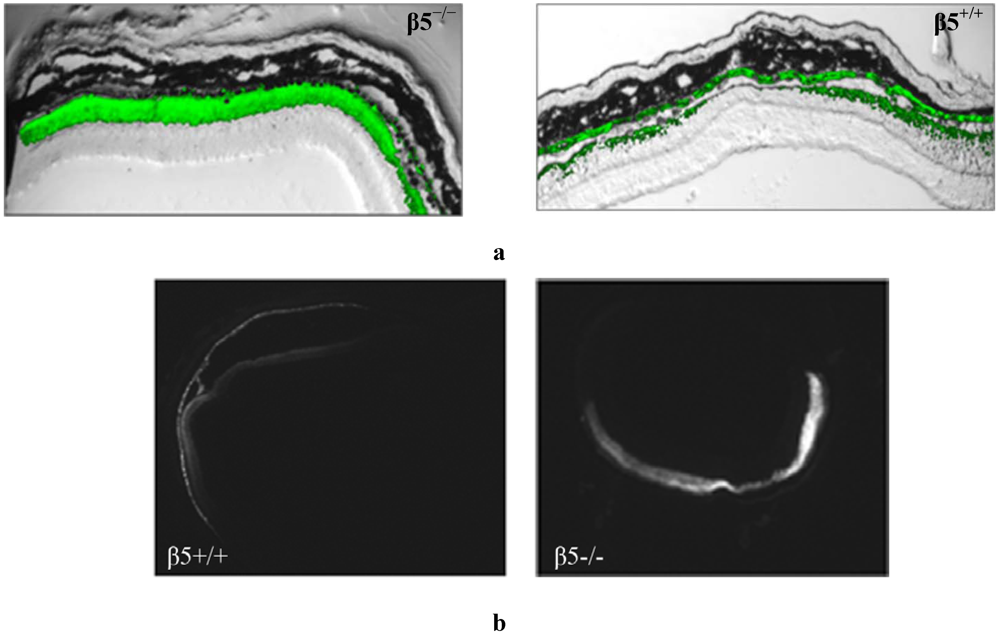
Figure 2a) and more widespread (
Figure 2b) level of retinal fluorescence combined with a reduced level of RPE fluorescence as evident in horizontal depth-matched frozen sections.
It is inferred that the absence of αvβ5 integrin function is responsible for the altered viral transduction pattern. This loss has both signalling and mechanical consequences for the retina and either or both may be affecting AAV transduction—the loss of αvβ5 receptor function may lessen the possibility of AAV particles reaching the RPE as POS-cargo. At the same time the reduced retinal/RPE adhesion may lead to greater retinal detachment upon subretinal injection. However, while this latter physical change accounts for more viral delivery overall it does not explain why there is greater transduction of retina compared to RPE in β5−/− mice. We therefore focus our investigation of this retina/RPE bias on the signalling effects. We propose that the β5−/− mice may exhibit a skewed AAV retinal/RPE transduction due to changes in subretinal phagocytic signalling and/or due to loss of a putative AAV coreceptor, the β5 integrin.
Figure 2.
Frozen retinal sections taken from mice (n = 10), 5 weeks after sub-retinal injection with AAV2/5(CMV-eGFP). (a) The representative level of fluorescence in a mouse that has no functional expression of the β5 integrinreceptors (β5−/−) compared to WT (β5+/+) are shown at 20X magnification; (b) Comparable nomarski full-field images are shown in 4× magnification.
Figure 2.
Frozen retinal sections taken from mice (n = 10), 5 weeks after sub-retinal injection with AAV2/5(CMV-eGFP). (a) The representative level of fluorescence in a mouse that has no functional expression of the β5 integrinreceptors (β5−/−) compared to WT (β5+/+) are shown at 20X magnification; (b) Comparable nomarski full-field images are shown in 4× magnification.
2.2. Increased Phagocytic Signalling Alters the AAV Transduction Profile Following Subretinal Injection in Wild-Type Mice
The RPE is generally very effectively transduced following subretinal injection of AAV. This is because it is exposed on the apical side to the subretinal space and can receive its share of virus following injection. In addition, it is possible that an AAV having successfully transduced a photoreceptor cell may be lost in shed outer segments before it can be trafficked to the photoreceptor nucleus. Following uptake of these AAV-carrying segments by the RPE, the AAV can escape the lysosomal degradation pathway and be expressed by the RPE cell. Fundamental to the uptake of POS by RPE is the protein Milk Fat Globule-EGF8 (MFG-E8) protein ligand, which binds to apoptotic cells and facilitates their removal through bridging interaction with phagocytes. As well as stimulating phagocytosis the MFG-E8/integrin interaction may havehas important adhesive effects [
9].
Nandrot
et al. have shown secreted MFG-E8, which localizes to the subretinal space, to be the primary ligand responsible for stimulating synchronized αvβ5 integrin signalling and specific RPE phagocytosis in the mouse retina [
9,
10]. We wished to investigate whether increased levels of this molecule in the subretinal space would lead to more AAV being ‘redistributed’ to the RPE due to an increase in αvβ5 signalling and POS uptake
. A 0.3 mg/mL solution of MFG-E8 (
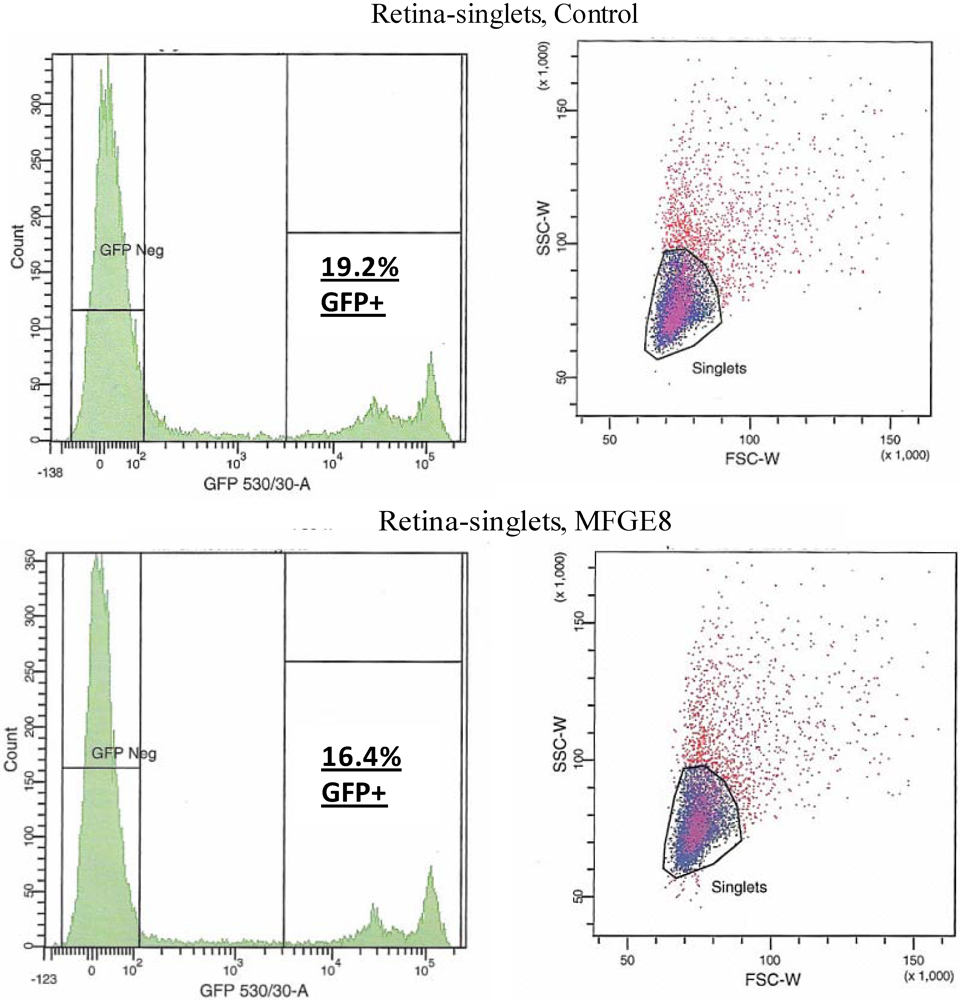
Figure 3) was co-subretinally-injected with AAVs expressing either eGFP (for FACS analysis) or the firefly luciferase gene (for plate-reader analysis) into WT mice. The contralateral eye was injected with PBS and the AAV. By FACS analysis, a modest decrease in the number of fluorescent retinal cells was counted when the αvβ5 ligand MFG-E8, was injected with the viral vector: 16.4% of cells from the MFG-E8 retinas compared to 19.2% from the control retinas (
Figure 4 and
Table 1).
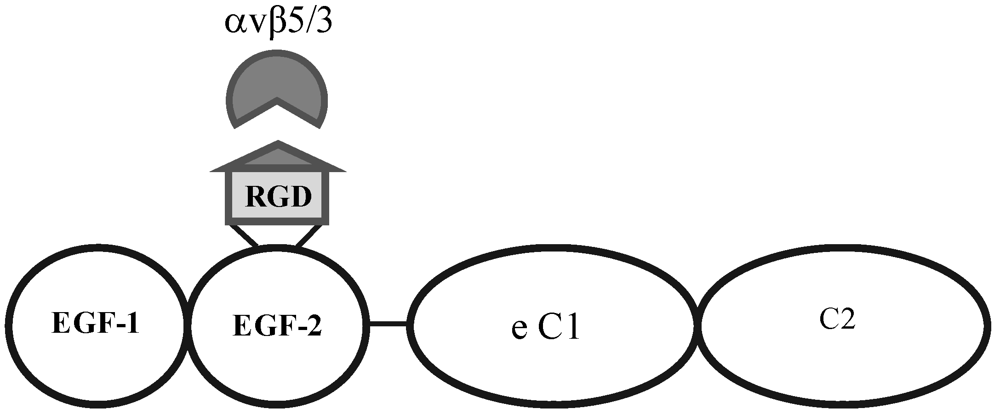
Figure 3.
Structural motifs of MFG-E8, containing two EGF-like domains, the second of which carries the integrin-binding motif. This protein is the signalling ligand for the αvβ5-integrin receptor in the retina.
Figure 3.
Structural motifs of MFG-E8, containing two EGF-like domains, the second of which carries the integrin-binding motif. This protein is the signalling ligand for the αvβ5-integrin receptor in the retina.
Figure 4.
FACS analysis was used to quantify the level of transduction achieved by AAV in the presence of MFG-E8. Four weeks following co-subretinal injection of MFG-E8 with AAV2/5(eGFP) in adult mice (n = 10), retinas were dissociated and cells counted. 19.2% of retinal cells are positive for eGFP in the control PBS pool compared to 16.4% eGFP-positive retinal cells in the test MFG-E8 pool.
Figure 4.
FACS analysis was used to quantify the level of transduction achieved by AAV in the presence of MFG-E8. Four weeks following co-subretinal injection of MFG-E8 with AAV2/5(eGFP) in adult mice (n = 10), retinas were dissociated and cells counted. 19.2% of retinal cells are positive for eGFP in the control PBS pool compared to 16.4% eGFP-positive retinal cells in the test MFG-E8 pool.
Table 1.
Cell numbers from FACS analysis of AAV subretinal transduction with increased levels of MFG-E8. The number of cells that are transduced (GFP +ve) or not (GFP −ve) by virus expressing eGFP are shown.
Table 1.
Cell numbers from FACS analysis of AAV subretinal transduction with increased levels of MFG-E8. The number of cells that are transduced (GFP +ve) or not (GFP −ve) by virus expressing eGFP are shown.
| Population | # Events | % Total |
|---|
| | Retina, Control | |
| All events | 20,000 | |
| Live singlet cells | 9,344 | 100 |
| GFP −ve | 6,840 | 34.2 |
| GFP +ve | 1,792 | 19.2 |
| | Retina, MFG-E8 | |
| All events | 20,000 | |
| Live singlet cells | 8,683 | 100 |
| GFP −ve | 6,871 | 34.4 |
| GFP +ve | 1,420 | 16.4 |
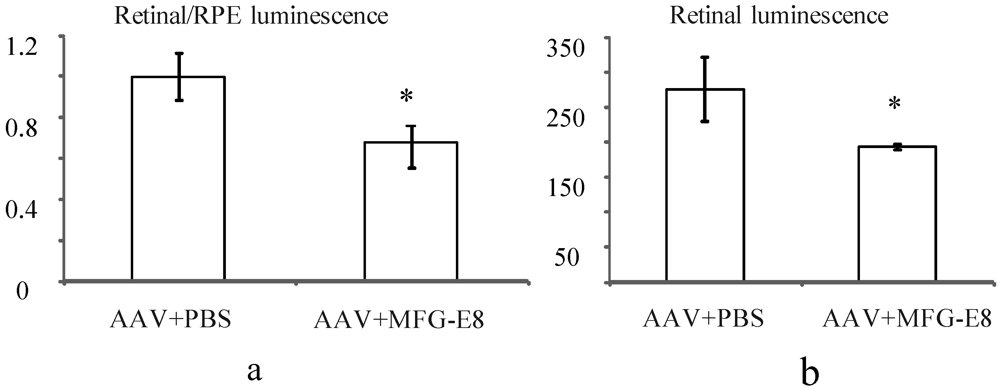
In order to account for potential variability in injection between mice, the transduction ratio for an AAV expressing firefly luciferase, (retina luminescence/RPE luminescence), was tested. An AAV vector expressing firefly luciferase was coinjected with PBS or MFG-E8 in the alternate eyes of nine CD1 mice. This outbred albino strain was used as they have no pigment in the RPE, which may otherwise prevent luminescence readings from the RPE fraction. Moreover they do not have a retinal degeneration (
rd) mutation as do many other outbred mouse strains. A luciferase assay was used to determine the relative luminescence of an RPE fraction compared to a retinal fraction from each individual mouse and the average ratios plotted (
Figure 5a). An average ratio of 1 was determined for the AAV-luciferase + PBS injected mice. However when the MFG-E8 ligand was coinjected with the luciferase-expressing AAV, the retina/RPE transduction ratio dropped to 0.68 (
p = 0.03,
Figure 5a). Similarly, the absolute retinal levels of luciferase expression also show decreased AAV transduction when coinjected with MFG-E8 (
Figure 5b) suggesting an RPE bias for transduction when MFG-E8 level was increased in the subretinal space. This supports the hypothesis that triggering αvβ5-integrin-induced phagocytosis may reduce the level of AAV-mediated expression in the neural retina.
Figure 5.
(a) Effect of MFG-E8 on the luminescence profile (retinal luminescence)/(RPE luminescence), following co-subretinal injection with AAV-luciferase in adult mice (n = 9), p = 0.03. Overall the ratio of retina to RPE transduction is reduced when MFG-E8 is present in the viral adjuvant. The retinal luminescence (b) shows the absolute reduction in retinal transduction due to MFG-E8, p = 0.04.
Figure 5.
(a) Effect of MFG-E8 on the luminescence profile (retinal luminescence)/(RPE luminescence), following co-subretinal injection with AAV-luciferase in adult mice (n = 9), p = 0.03. Overall the ratio of retina to RPE transduction is reduced when MFG-E8 is present in the viral adjuvant. The retinal luminescence (b) shows the absolute reduction in retinal transduction due to MFG-E8, p = 0.04.
2.3. Blocking the αvβ5 Integrin Coreceptor Alters AAV Uptake Following Subretinal Injection in Wild-Type Mice
The broad tissue tropism characteristic of AAV may be attributed to the range of ubiquitously expressed receptors and coreceptors which the virus can recruit for transduction. Heparin Sulfate Proteoglycan (HSPG) was originally identified as the primary attachment receptor of AAV2 [
11], since then several coreceptors necessary for cell entry have been identified. Amongst these, are the integrins which have been found to be involved in the attachment, entry and uncoating of AAV [
11]. In addition to important roles in cell-cell adhesion and cell signalling the αvβ5 integrin receptor has been shown to serve as a coreceptor for adenovirus and has been suggested to serve as coreceptor for some serotypes of AAV, in particular for AAV serotype 1 [
12]. It is a possibility that the absence of expression of the αvβ5 integrin on the RPE apical surface of the β5
−/− mouse may increase the utilisation of alternative coreceptors by the virus, such as FGFR1, HGF and PDGF, which may be more prominently expressed on the retinal side. MFG-E8 can serve as a bridging protein via its Arginine-Glycine-Aspartic Acid (RGD) motif between αvβ5 integrin receptors at the RPE surface and spent POS in the subretinal space [
13]. The RGD domain of the adenovirus is shown to interact with the integrin receptor and for this reason we tested whether the RGD peptide may be used to saturate the receptor in WT mice. This would have the effect of effectively blocking transduction of the RPE at times of AAV injection thus redirecting the virus to alternative receptors on the adjacent retinal cells. In theory this should lead to similar transduction characteristics as those of the β5
−/− mouse. AAV-eGFP was cosubretinally injected with RGD peptide in one eye and a control RAD peptide in the contralateral eye of adult WT mice. After 5 weeks the mice were euthanized and retinal fluorescence levels compared by FACS analysis (n = 10).
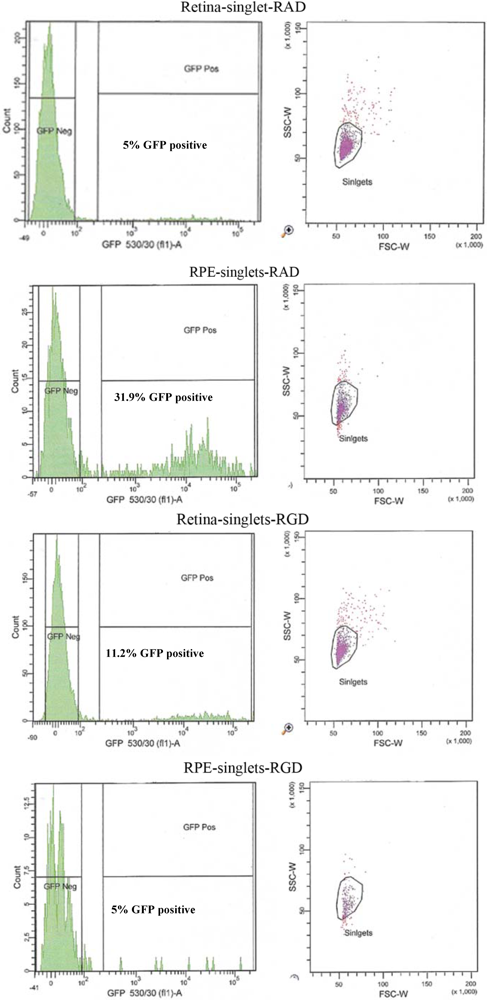
An altered pattern of transduction was found in the cell counts of the dissociated RPE and retinal cells from 10 eyes used for FACS analysis. In fact, total transduction was reduced for RGD-injected versus RAD-injected retinas; however, the retinal-specific eGFP expression of the RGD cells was increased 4.8-fold while the RPE-specific eGFP expression of RGD cells was reduced by approximately 6-fold (
Figure 6 and
Table 2). In order to verify the extent to which this RPE “blocking” by RGD peptide might enhance retinal transduction by AAV, we used an AAV expressing the firefly luciferase gene for injection into albino CD1 mouse eyes which lack of pigment in the RPE facilitating the luminescence readouts from the RPE cell lysates.
Figure 6.
FACS analysis of the effect of peptides on the cellular transduction pattern following co-subretinal injection with AAV2/5 (CMV-eGFP) in adult mice. Only 5% of retinal cells coinjected with RAD peptide control are positive for eGFP compared to 11.2% of eGFP-positive retinal cells coinjected with RGD. By contrast RPE cells from RAD-injected mice are 31.9% positive for eGFP compared to only 2.7% eGFP-positive RPE cells in the RGD pool.
Figure 6.
FACS analysis of the effect of peptides on the cellular transduction pattern following co-subretinal injection with AAV2/5 (CMV-eGFP) in adult mice. Only 5% of retinal cells coinjected with RAD peptide control are positive for eGFP compared to 11.2% of eGFP-positive retinal cells coinjected with RGD. By contrast RPE cells from RAD-injected mice are 31.9% positive for eGFP compared to only 2.7% eGFP-positive RPE cells in the RGD pool.
Table 2.
Cell numbers from FACS analysis of AAV subretinal transduction with increased levels of MFG-E8. The number of cells that are transduced (GFP +ve) or not (GFP –ve) by virus expressing eGFP are shown.
Table 2.
Cell numbers from FACS analysis of AAV subretinal transduction with increased levels of MFG-E8. The number of cells that are transduced (GFP +ve) or not (GFP –ve) by virus expressing eGFP are shown.
| Population | # Events | % Total | # Events | % Total |
|---|
| | Retina RAD | | RPE RAD | |
| Live singlet cells | 4,297 | 100 | 864 | 100 |
| GFP −ve | 4,035 | 93.9 | 562 | 65 |
| GFP +ve | 215 | 5 | 276 | 31.9 |
| | Retina RGD | | RPE RGD | |
| Live singlet cells | 3,743 | 100 | 314 | 100 |
| GFP −ve | 3,219 | 86 | 298 | 95 |
| GFP +ve | 429 | 11 | 8 | 5 |
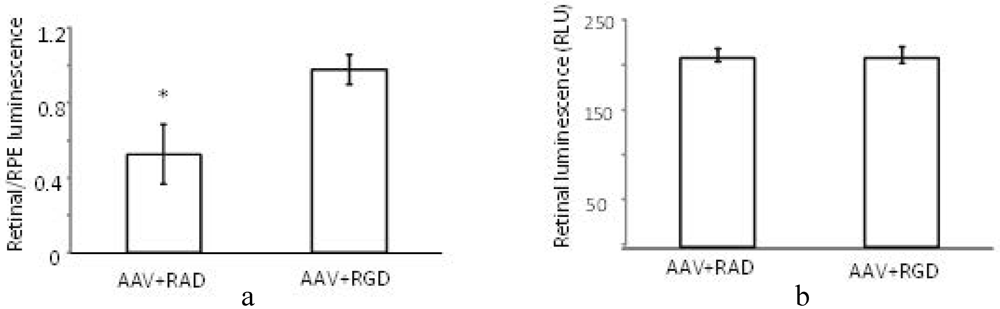
The (retinal luminescence)/(RPE luminescence) ratio is significantly higher where AAV (CMV-luciferase) was coinjected with RGD peptide compared to the control RAD (
Figure 7a). This implies that the RPE-bias evident in the control injection virus is less pronounced in the presence of the αvβ5 integrin blocking RGD peptide. As we are interested in the ratio of AAV mediated expression in the retina versus the RPE the variability in the success of subretinal injections is of less concern. However, it is nonetheless worth noting that the absolute levels of luminescence in the retina are found to be equivalent between RAD and RGD retinas (
Figure 7b). Thus, through FACS analysis and luciferase reporter assay we have determined that the RGD domain alone can block the RPE uptake, presumably through saturation of the integrin receptor used for cell entry
. However in contrast to the FACS study, the luciferase assay does suggest that this blocking effect may not necessarily enhance retinal transduction. This would imply that the viral transduction pattern of the β5
−/− mouse is not due to virus/receptor interactions.
Figure 7.
Effect of peptides on luminescence profile following co-subretinal injection with AAV2/1(CMV-luciferase) in WT mice (n = 8). While the ratio (retinal luminescence)/(RPE luminscence) is higher for RGD co-injected mice, (a, p = 0.017), the absolute retinal luminescence (b) suggests that the RPE-blocking effect is not improving retinal transduction.
Figure 7.
Effect of peptides on luminescence profile following co-subretinal injection with AAV2/1(CMV-luciferase) in WT mice (n = 8). While the ratio (retinal luminescence)/(RPE luminscence) is higher for RGD co-injected mice, (a, p = 0.017), the absolute retinal luminescence (b) suggests that the RPE-blocking effect is not improving retinal transduction.