Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors
Abstract
:Abbreviations
| AR | adrenoceptor |
| cAMP | cyclic adenosine monophosphate |
| AT1R | Angiotensin receptor 1 |
| Ang II | angiotensin II |
| CNS | Central nervous system |
| C-tail | Carboxyl Terminal Tail |
| CXCR | Chemokine receptor |
| CO-IP | Co-immunoprecipitation |
| DR | Dopamine Receptor |
| ECL | Extracellular loop |
| EP1 | prostaglandin E1 receptor |
| ER | Endoplasmic reticulum |
| ERKs | extracellular signal-regulated kinases |
| EGFR | Epidermal growth factor receptor |
| FSHR | Follicle-stimulating hormone receptor |
| FSK | forskolin |
| GABAR | gamma-aminobutyric acid receptor |
| GPCRs | G-protein coupled receptors |
| GRK | GPCR kinases |
| hSSTR | human somatostatin receptor |
| HA | hemagglutinin |
| HEK-293 | human embryonic kidney-293 |
| ICL | Intracellular loop |
| MAPK | mitogen-activated protein kinase |
| MR | muscarinic receptor |
| NMDAR | N-Methyl-D-aspartate receptor |
| ORs | Opioid Receptors |
| Pb-FRET | Photobleaching-fluorescence resonance energy transfer |
| PKC | Protein kinase C |
| PTX | pertussis toxin |
| RTK | Receptor tyrosine kinase |
| SSTRs | somatostatin receptors |
| TRs | Taste Receptors, V2R, Vasopressin receptor 2, 5-HT, Serotonin Receptor |
| CaSR | Calcium Sensing receptor |
| FCS | Fluorescence correlation spectroscopy |
| PD | Parkinson’s Disease |
1. Introduction
| Method | Receptors | References |
|---|---|---|
| Complementation Assay | Somatostatin receptors 1, 4 and 5 | [21,36] |
| Calcium sensing receptor | [41] | |
| Muscarinic M2 and M3 receptors | [42] | |
| Muscarinic M3/α2 Adrenoceptor | [22] | |
| Dopamine receptor 2/3 | [43] | |
| GABAR1/GABAR2 | [44] | |
| Co-Immunoprecipitation | α,β-Adrenoceptors | [45,46] |
| Dopamine receptors | [47,48] | |
| Opioid receptors | [49,50] | |
| Chemokine receptor 2 | [51] | |
| Somatostatin receptors | [31,32,34,35,36,52] | |
| Somatostatin receptor 2/µ-Opioid receptor | [53] | |
| β-Adrenoceptors/Somatostatin receptor 5 | [37,40] | |
| Somatostatin receptor 2A/3 | [52] | |
| Angiotensin receptor 1/Cannabinoid receptor1 | [54] | |
| β2 Adrenoceptor/Opioid receptor | [55] | |
| GABAR1/GABAR2 | [56,57] | |
| Calcium sensing receptor/Glutamate Receptors | [58] | |
| FRET | α-Adrenoceptors | [45] |
| Thyrotropin receptor | [59] | |
| Neuropeptide Y receptor | [60] | |
| Dopamine receptor 2 | [61] | |
| Chemokine receptor 2 and 5 | [62] | |
| Somatostatin Receptors | [63,64] | |
| Photobleaching-FRET | Somatostatin receptors | [21,31,32,34,36,38] |
| Gonadotrophin-releasing hormone receptors | [65,66] | |
| Somatostatin receptors/Dopamine receptor | [30,35] | |
| Somatostatin receptor 5/β-Adrenoceptors | [37,40] | |
| Somatostatin receptor 4/µ-Opioid receptor | [67] | |
| Somatostatin receptor 4/δ-Opioid receptor | [39] | |
| Somatostatin receptors/EGFRs | [15,16,17] | |
| BRET | β-Adrenoceptors | [68] |
| Thyrotropin-releasing hormone receptor | [69] | |
| Opioid receptors | [70] | |
| Chemokine receptor 4 and 5 | [71] | |
| Adenosine receptor | [72,73] | |
| Oxytocin Receptor | [10] | |
| Vasopressin Receptor | [10] | |
| Adenosine 2a receptor/Dopamine receptor 2 | [73] | |
| Adenosine 2a receptor/Purinergic receptor 2 | [72] | |
| Oxytocin/Vasopressin receptors | [10] | |
| Angiotensin receptor 1/Cannabinoid receptor 1 | [54] | |
| TR-FRET | δ-Opioid receptor | [74] |
| β2 Adrenoceptor/δ Opioid receptors | [74] | |
| Histamine 4 receptor | [75] | |
| GABAR1/GABAR2 | [76] |
2. G-Proteins are Powerful Regulator of GPCR Signaling
| G Protein | Receptor Subtypes | References |
|---|---|---|
| Gi protein | Chemokine Receptor | [82] |
| Opioid Receptor | [83] | |
| Somatostatin Receptor | [84] | |
| Neuropeptide Y Receptor | [85] | |
| Melatonin Receptor | [86] | |
| Cannabinoid Receptor | [87] | |
| Sphingosine-1-phosphate Receptor | [88] | |
| Histamine Receptor | [87] | |
| 5-hydroxytryptamine | [89] | |
| Dopamine Receptor | [87] | |
| Muscarinic Receptor | [90,91] | |
| Formyl-methionyl peptide Receptor | [92] | |
| Gs protein | Vasopressin receptor 2 | [87] |
| Adrenoceptors | [93] | |
| Prostaglandin E receptor subtypes | [94] | |
| 5-hydroxytryptamine receptor subtypes | [89] | |
| Melanocyte-stimulating hormone receptor | [95] | |
| Melanocortin receptor subtypes | [96] | |
| Relaxin receptor subtypes | [97] | |
| Adenosine receptor | [98] | |
| Gq protein | Vasopressin receptor subtypes (V1a and V1b) | [87,99] |
| Muscarinic acetylcholine receptor subtypes | [90] | |
| Gonadotropin-releasing hormone receptor | [100] | |
| P2Y purinoceptor subtypes | [101,102] | |
| Bradykinin receptor subtypes | [103] | |
| Oxytocin receptors subtypes | [99] | |
| Gastrin/cholecystokinin type B receptor | [104] | |
| Neuromedin U Receptor subtypes | [105] | |
| Neurotensin Receptor | [106] | |
| Gi/s proteins | Glycoprotein hormone receptors | [107] |
| β-Adrenoceptors | [108,109] | |
| Gi/q proteins | Platelet activating factor receptor | [87] |
| Sphingolipid (S1P3)/Lysophospholipid receptor (LPA2) | [81] | |
| Galanin receptor 2 | [110] | |
| Endothelin B | [111] | |
| Gq/s proteins | Calcitonin Receptor | [112] |
| Parathyroid hormone receptor | [113] | |
| Cholecystokinin-Areceptor | [87] | |
| Gq/i/s proteins | Prostaglandin E3 receptor | [94] |
| Thyrotropin receptor | [107] | |
| Luteinizing hormone receptor | [87] | |
| Lysophospholipid receptor subtypes | [114,115] |
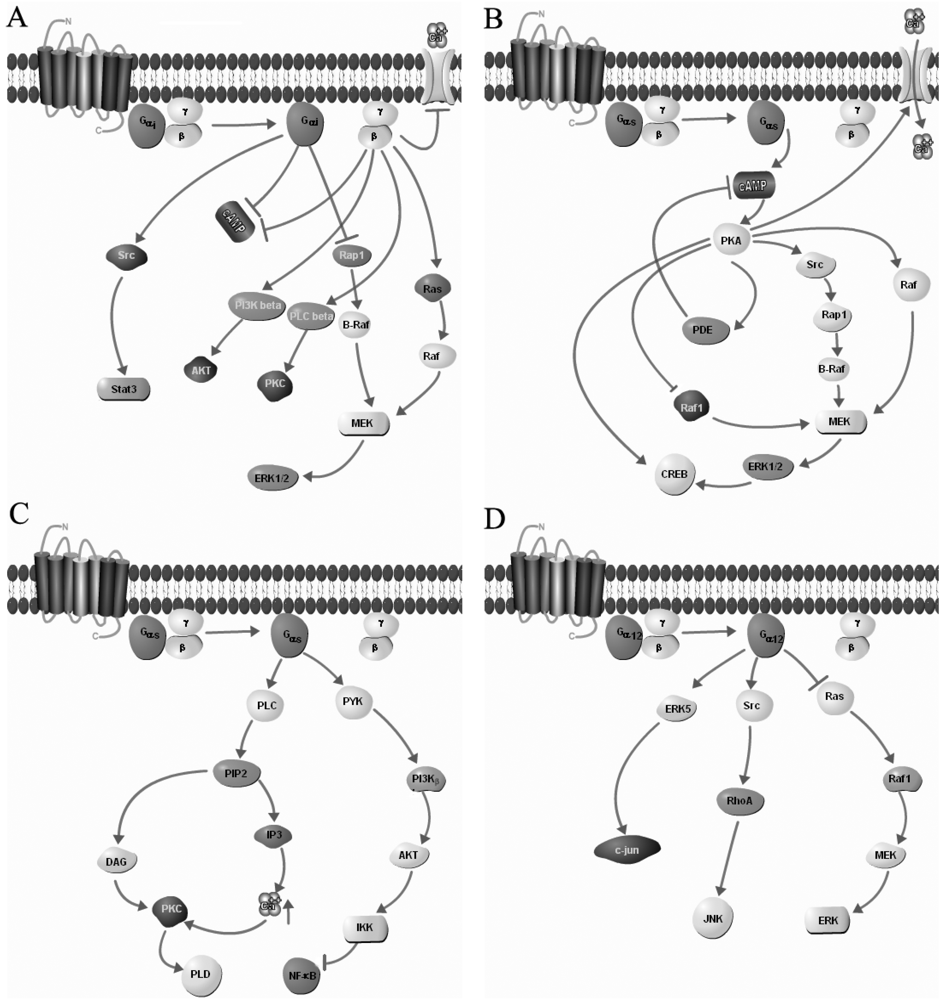
3. Homo- and Heterodimerization of GPCRs
4. Implications of Structural Domains in GPCR Dimerization
5. GPCR Trafficking and Ligand Binding Is Altered upon Dimerization
6. Implication of GPCR Heterodimers in Pathophysiological conditions
7. Implication of Somatostatin Receptors Heterodimerization in Pathological Conditions
8. Conclusions
Acknowledgments
References
- Fredholm, B.B.; Hokfelt, T.; Milligan, G. G-protein-coupled receptors: An update. Acta Physiol. (Oxf.) 2007, 190, 3–7. [Google Scholar] [CrossRef]
- Attwood, T.K.; Findlay, J.B. Fingerprinting G-protein-coupled receptors. Protein Eng. 1994, 7, 195–203. [Google Scholar]
- Kolakowski, L.F., Jr. GCRDb: A G-protein-coupled receptor database. Recept. Channels 1994, 2, 1–7. [Google Scholar]
- Bockaert, J.; Pin, J.P. Molecular tinkering of g protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar]
- Fredriksson, R.; Lagerstrom, M.C.; Lundin, L.G.; Schioth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Fredriksson, R.; Schioth, H.B. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 2005, 67, 1414–1425. [Google Scholar]
- Millar, R.P.; Newton, C.L. The year in G protein-coupled receptor research. Mol. Endocrinol. 2010, 24, 261–274. [Google Scholar]
- Milligan, G. G protein-coupled receptor dimerization: Function and ligand pharmacology. Mol. Pharmacol. 2004, 66, 1–7. [Google Scholar]
- Pagano, A.; Rovelli, G.; Mosbacher, J.; Lohmann, T.; Duthey, B.; Stauffer, D.; Ristig, D.; Schuler, V.; Meigel, I.; Lampert, C.; et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(B) receptors. J. Neurosci. 2001, 21, 1189–1202. [Google Scholar]
- Terrillon, S.; Durroux, T.; Mouillac, B.; Breit, A.; Ayoub, M.A.; Taulan, M.; Jockers, R.; Barberis, C.; Bouvier, M. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol. Endocrinol. 2003, 17, 677–691. [Google Scholar]
- Pidasheva, S.; Grant, M.; Canaff, L.; Ercan, O.; Kumar, U.; Hendy, G.N. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: Biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum. Mol. Genet. 2006, 15, 2200–2209. [Google Scholar]
- Monyer, H.; Sprengel, R.; Schoepfer, R.; Herb, A.; Higuchi, M.; Lomeli, H.; Burnashev, N.; Sakmann, B.; Seeburg, P.H. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science 1992, 256, 1217–1221. [Google Scholar]
- Watt, H.L.; Kharmate, G.; Kumar, U. Biology of somatostatin in breast cancer. Mol. Cell. Endocrinol. 2008, 286, 251–261. [Google Scholar]
- Earp, H.S.; Dawson, T.L.; Li, X.; Yu, H. Heterodimerization and functional interaction between EGF receptor family members: A new signaling paradigm with implications for breast cancer research. Breast Cancer Res. Treat. 1995, 35, 115–132. [Google Scholar]
- Kharmate, G.; Rajput, P.S.; Watt, H.L.; Somvanshi, R.K.; Chaudhari, N.; Qiu, X.; Kumar, U. Dissociation of epidermal growth factor receptor and ErbB2 heterodimers in the presence of somatostatin receptor 5 modulate signaling pathways. Endocrinology 2011, 152, 931–945. [Google Scholar]
- Watt, H.L.; Kharmate, G.D.; Kumar, U. Somatostatin receptors 1 and 5 heterodimerize with epidermal growth factor receptor: Agonist-dependent modulation of the downstream MAPK signalling pathway in breast cancer cells. Cell. Signal. 2009, 21, 428–439. [Google Scholar]
- Kharmate, G.; Rajput, P.S.; Watt, H.L.; Somvanshi, R.K.; Chaudhari, N.; Qiu, X.; Kumar, U. Role of somatostatin receptor 1 and 5 on epidermal growth factor receptor mediated signaling. Biochim. Biophys. Acta 2011, 1813, 1172–1189. [Google Scholar]
- Milligan, G.; Bouvier, M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005, 272, 2914–2925. [Google Scholar]
- Thomas, P.; Smart, T.G. Hek293 cell line: A vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar]
- Sarramegna, V.; Talmont, F.; Demange, P.; Milon, A. Heterologous expression of G-protein-coupled receptors: Comparison of expression systems from the standpoint of large-scale production and purification. Cell. Mol. Life Sci. 2003, 60, 1529–1546. [Google Scholar]
- Rocheville, M.; Lange, D.C.; Kumar, U.; Sasi, R.; Patel, R.C.; Patel, Y.C. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 2000, 275, 7862–7869. [Google Scholar]
- Maggio, R.; Vogel, Z.; Wess, J. Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “Cross-talk” between G-protein-linked receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 3103–3107. [Google Scholar]
- Monnot, C.; Bihoreau, C.; Conchon, S.; Curnow, K.M.; Corvol, P.; Clauser, E. Polar residues in the transmembrane domains of the type 1 angiotensin ii receptor are required for binding and coupling. Reconstitution of the binding site by co-expression of two deficient mutants. J. Biol. Chem. 1996, 271, 1507–1513. [Google Scholar]
- Osuga, Y.; Hayashi, M.; Kudo, M.; Conti, M.; Kobilka, B.; Hsueh, A.J. Co-expression of defective luteinizing hormone receptor fragments partially reconstitutes ligand-induced signal generation. J. Biol. Chem. 1997, 272, 25006–25012. [Google Scholar]
- Schoneberg, T.; Sandig, V.; Wess, J.; Gudermann, T.; Schultz, G. Reconstitution of mutant V2 vasopressin receptors by adenovirus-mediated gene transfer. Molecular basis and clinical implication. J. Clin. Invest. 1997, 100, 1547–1556. [Google Scholar] [CrossRef]
- Schoneberg, T.; Yun, J.; Wenkert, D.; Wess, J. Functional rescue of mutant V2 vasopressin receptors causing nephrogenic diabetes insipidus by a co-expressed receptor polypeptide. EMBO J. 1996, 15, 1283–1291. [Google Scholar]
- Schulz, A.; Grosse, R.; Schultz, G.; Gudermann, T.; Schoneberg, T. Structural implication for receptor oligomerization from functional reconstitution studies of mutant V2 vasopressin receptors. J. Biol. Chem. 2000, 275, 2381–2389. [Google Scholar]
- Hebert, T.E.; Moffett, S.; Morello, J.P.; Loisel, T.P.; Bichet, D.G.; Barret, C.; Bouvier, M. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 1996, 271, 16384–16392. [Google Scholar]
- Casado, V.; Cortes, A.; Mallol, J.; Perez-Capote, K.; Ferre, S.; Lluis, C.; Franco, R.; Canela, E.I. GPCR homomers and heteromers: A better choice as targets for drug development than GPCR monomers? Pharmacol. Ther. 2009, 124, 248–257. [Google Scholar] [CrossRef]
- Baragli, A.; Alturaihi, H.; Watt, H.L.; Abdallah, A.; Kumar, U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell. Signal. 2007, 19, 2304–2316. [Google Scholar]
- Grant, M.; Alturaihi, H.; Jaquet, P.; Collier, B.; Kumar, U. Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol. Endocrinol. 2008, 22, 2278–2292. [Google Scholar]
- Grant, M.; Collier, B.; Kumar, U. Agonist-dependent dissociation of human somatostatin receptor 2 dimers: A role in receptor trafficking. J. Biol. Chem. 2004, 279, 36179–36183. [Google Scholar]
- Grant, M.; Kumar, U. The role of G-proteins in the dimerisation of human somatostatin receptor types 2 and 5. Regul. Pept. 2010, 159, 3–8. [Google Scholar]
- Grant, M.; Patel, R.C.; Kumar, U. The role of subtype-specific ligand binding and the C-tail domain in dimer formation of human somatostatin receptors. J. Biol. Chem. 2004, 279, 38636–38643. [Google Scholar]
- Rocheville, M.; Lange, D.C.; Kumar, U.; Patel, S.C.; Patel, R.C.; Patel, Y.C. Receptors for dopamine and somatostatin: Formation of hetero-oligomers with enhanced functional activity. Science 2000, 288, 154–157. [Google Scholar]
- Somvanshi, R.K.; Billova, S.; Kharmate, G.; Rajput, P.S.; Kumar, U. C-tail mediated modulation of somatostatin receptor type-4 homo- and heterodimerizations and signaling. Cell. Signal. 2009, 21, 1396–1414. [Google Scholar] [CrossRef]
- Somvanshi, R.K.; War, S.A.; Chaudhari, N.; Qiu, X.; Kumar, U. Receptor specific crosstalk and modulation of signaling upon heterodimerization between beta(1)-adrenergic receptor and somatostatin receptor-5. Cell. Signal. 2011, 23, 794–811. [Google Scholar]
- War, S.A.; Somvanshi, R.K.; Kumar, U. Somatostatin receptor-3 mediated intracellular signaling and apoptosis is regulated by its cytoplasmic terminal. Biochim. Biophys. Acta 2011, 1813, 390–402. [Google Scholar]
- Somvanshi, R.K.; Chaudhari, N.; Bonnett, J.; Kharmate, G.; Rajput, P.S.; Qiu, X.; Cairns, B.E.; Kumar, U. Somatostatin receptor-4 and delta-opioid receptor heterodimerization modulates signaling pathways mediating analgesic responses. In 2010 Neuroscience Meeting, San Diego, CA, USA, 13-17 November, 2010.
- Somvanshi, R.K.; Chaudhari, N.; Qiu, X.; Kumar, U. Heterodimerization of beta2 adrenergic receptor and somatostatin receptor 5: Implications in modulation of signaling pathway. J. Mol. Signal. 2011, 6, 9. [Google Scholar]
- Bai, M.; Trivedi, S.; Kifor, O.; Quinn, S.J.; Brown, E.M. Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function. Proc. Natl. Acad. Sci. USA 1999, 96, 2834–2839. [Google Scholar]
- Maggio, R.; Barbier, P.; Colelli, A.; Salvadori, F.; Demontis, G.; Corsini, G.U. G protein-linked receptors: Pharmacological evidence for the formation of heterodimers. J. Pharmacol. Exp. Ther. 1999, 291, 251–257. [Google Scholar]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar]
- White, J.H.; Wise, A.; Main, M.J.; Green, A.; Fraser, N.J.; Disney, G.H.; Barnes, A.A.; Emson, P.; Foord, S.M.; Marshall, F.H. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998, 396, 679–682. [Google Scholar]
- Stanasila, L.; Perez, J.B.; Vogel, H.; Cotecchia, S. Oligomerization of the alpha 1a- and alpha 1b-adrenergic receptor subtypes. Potential implications in receptor internalization. J. Biol. Chem. 2003, 278, 40239–40251. [Google Scholar]
- Angers, S.; Salahpour, A.; Joly, E.; Hilairet, S.; Chelsky, D.; Dennis, M.; Bouvier, M. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc. Natl. Acad. Sci. USA 2000, 97, 3684–3689. [Google Scholar]
- Lee, S.P.; O’Dowd, B.F.; Ng, G.Y.; Varghese, G.; Akil, H.; Mansour, A.; Nguyen, T.; George, S.R. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol. Pharmacol. 2000, 58, 120–128. [Google Scholar]
- George, S.R.; Lee, S.P.; Varghese, G.; Zeman, P.R.; Seeman, P.; Ng, G.Y.; O’Dowd, B.F. A transmembrane domain-derived peptide inhibits D1 dopamine receptor function without affecting receptor oligomerization. J. Biol. Chem. 1998, 273, 30244–30248. [Google Scholar]
- George, S.R.; Fan, T.; Xie, Z.; Tse, R.; Tam, V.; Varghese, G.; O’Dowd, B.F. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000, 275, 26128–26135. [Google Scholar]
- Gomes, I.; Jordan, B.A.; Gupta, A.; Trapaidze, N.; Nagy, V.; Devi, L.A. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J. Neurosci. 2000, 20, RC110. [Google Scholar]
- Trettel, F.; di Bartolomeo, S.; Lauro, C.; Catalano, M.; Ciotti, M.T.; Limatola, C. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 2003, 278, 40980–40988. [Google Scholar]
- Pfeiffer, M.; Koch, T.; Schroder, H.; Klutzny, M.; Kirscht, S.; Kreienkamp, H.J.; Hollt, V.; Schulz, S. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of SST(3) receptor function by heterodimerization with SST(2A). J. Biol. Chem. 2001, 276, 14027–14036. [Google Scholar]
- Pfeiffer, M.; Koch, T.; Schroder, H.; Laugsch, M.; Hollt, V.; Schulz, S. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 2002, 277, 19762–19772. [Google Scholar]
- Rozenfeld, R.; Gupta, A.; Gagnidze, K.; Lim, M.P.; Gomes, I.; Lee-Ramos, D.; Nieto, N.; Devi, L.A. AT1R-CBR heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011, 30, 2350–2363. [Google Scholar]
- Jordan, B.A.; Trapaidze, N.; Gomes, I.; Nivarthi, R.; Devi, L.A. Oligomerization of opioid receptors with beta 2-adrenergic receptors: A role in trafficking and mitogen-activated protein kinase activation. Proc. Natl. Acad. Sci. USA 2001, 98, 343–348. [Google Scholar]
- Jones, K.A.; Borowsky, B.; Tamm, J.A.; Craig, D.A.; Durkin, M.M.; Dai, M.; Yao, W.J.; Johnson, M.; Gunwaldsen, C.; Huang, L.Y.; et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 1998, 396, 674–679. [Google Scholar]
- Kaupmann, K.; Malitschek, B.; Schuler, V.; Heid, J.; Froestl, W.; Beck, P.; Mosbacher, J.; Bischoff, S.; Kulik, A.; Shigemoto, R.; et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 1998, 396, 683–687. [Google Scholar]
- Gama, L.; Wilt, S.G.; Breitwieser, G.E. Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J. Biol. Chem. 2001, 276, 39053–39059. [Google Scholar]
- Latif, R.; Graves, P.; Davies, T.F. Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J. Biol. Chem. 2002, 277, 45059–45067. [Google Scholar]
- Dinger, M.C.; Bader, J.E.; Kobor, A.D.; Kretzschmar, A.K.; Beck-Sickinger, A.G. Homodimerization of neuropeptide Y receptors investigated by fluorescence resonance energy transfer in living cells. J. Biol. Chem. 2003, 278, 10562–10571. [Google Scholar]
- Wurch, T.; Matsumoto, A.; Pauwels, P.J. Agonist-independent and -dependent oligomerization of dopamine D(2) receptors by fusion to fluorescent proteins. FEBS Lett. 2001, 507, 109–113. [Google Scholar]
- Mellado, M.; Rodriguez-Frade, J.M.; Vila-Coro, A.J.; Fernandez, S.; Martin de Ana, A.; Jones, D.R.; Toran, J.L.; Martinez, A.C. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001, 20, 2497–2507. [Google Scholar]
- Duran-Prado, M.; Bucharles, C.; Gonzalez, B.J.; Vazquez-Martinez, R.; Martinez-Fuentes, A.J.; Garcia-Navarro, S.; Rhodes, S.J.; Vaudry, H.; Malagon, M.M.; Castano, J.P. Porcine somatostatin receptor 2 displays typical pharmacological SST2 features but unique dynamics of homodimerization and internalization. Endocrinology 2007, 148, 411–421. [Google Scholar]
- Duran-Prado, M.; Saveanu, A.; Luque, R.M.; Gahete, M.D.; Gracia-Navarro, F.; Jaquet, P.; Dufour, H.; Malagon, M.M.; Culler, M.D.; Barlier, A.; et al. A potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogs. J. Clin. Endocrinol. Metab. 2011, 95, 2497–2502. [Google Scholar]
- Horvat, R.D.; Roess, D.A.; Nelson, S.E.; Barisas, B.G.; Clay, C.M. Binding of agonist but not antagonist leads to fluorescence resonance energy transfer between intrinsically fluorescent gonadotropin-releasing hormone receptors. Mol. Endocrinol. 2001, 15, 695–703. [Google Scholar]
- Cornea, A.; Janovick, J.A.; Maya-Nunez, G.; Conn, P.M. Gonadotropin-releasing hormone receptor microaggregation. Rate monitored by fluorescence resonance energy transfer. J. Biol. Chem. 2001, 276, 2153–2158. [Google Scholar]
- Somvanshi, R.K.; Chaudhari, N.; Yang, B.; Sastry, B.R.; Kumar, U. Somatostatin receptor-4 and μ-opioid receptor interaction modulates receptor coupling, signaling and excitatory postsynaptic currents. In 2011 Neuroscience meeting, Washington, DC, USA, 12-16 November 2011.
- Lavoie, C.; Mercier, J.F.; Salahpour, A.; Umapathy, D.; Breit, A.; Villeneuve, L.R.; Zhu, W.Z.; Xiao, R.P.; Lakatta, E.G.; Bouvier, M.; et al. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J. Biol. Chem. 2002, 277, 35402–35410. [Google Scholar]
- Hanyaloglu, A.C.; Seeber, R.M.; Kohout, T.A.; Lefkowitz, R.J.; Eidne, K.A. Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes. Differential regulation of beta-arrestins 1 and 2. J. Biol. Chem. 2002, 277, 50422–50430. [Google Scholar]
- Gomes, I.; Filipovska, J.; Jordan, B.A.; Devi, L.A. Oligomerization of opioid receptors. Methods 2002, 27, 358–365. [Google Scholar]
- Issafras, H.; Angers, S.; Bulenger, S.; Blanpain, C.; Parmentier, M.; Labbe-Jullie, C.; Bouvier, M.; Marullo, S. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J. Biol. Chem. 2002, 277, 34666–34673. [Google Scholar]
- Yoshioka, K.; Saitoh, O.; Nakata, H. Agonist-promoted heteromeric oligomerization between adenosine A(1) and P2Y(1) receptors in living cells. FEBS Lett. 2002, 523, 147–151. [Google Scholar]
- Kamiya, T.; Saitoh, O.; Yoshioka, K.; Nakata, H. Oligomerization of adenosine A2A and dopamine D2 receptors in living cells. Biochem. Biophys. Res. Commun. 2003, 306, 544–549. [Google Scholar]
- McVey, M.; Ramsay, D.; Kellett, E.; Rees, S.; Wilson, S.; Pope, A.J.; Milligan, G. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. The human delta-opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J. Biol. Chem. 2001, 276, 14092–14099. [Google Scholar]
- van Rijn, R.M.; Chazot, P.L.; Shenton, F.C.; Sansuk, K.; Bakker, R.A.; Leurs, R. Oligomerization of recombinant and endogenously expressed human histamine H(4) receptors. Mol. Pharmacol. 2006, 70, 604–615. [Google Scholar]
- Maurel, D.; Comps-Agrar, L.; Brock, C.; Rives, M.L.; Bourrier, E.; Ayoub, M.A.; Bazin, H.; Tinel, N.; Durroux, T.; Prezeau, L.; et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat. Methods 2008, 5, 561–567. [Google Scholar]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The gtpase superfamily: Conserved structure and molecular mechanism. Nature 1991, 349, 117–127. [Google Scholar]
- Hamm, H.E. The many faces of G protein signaling. J. Biol. Chem. 1998, 273, 669–672. [Google Scholar]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar]
- Rodbell, M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature 1980, 284, 17–22. [Google Scholar]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar]
- Murphy, P.M. International union of pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002, 54, 227–229. [Google Scholar] [CrossRef]
- Law, P.Y.; Wong, Y.H.; Loh, H.H. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 389–430. [Google Scholar]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar]
- Michel, M.C.; Beck-Sickinger, A.; Cox, H.; Doods, H.N.; Herzog, H.; Larhammar, D.; Quirion, R.; Schwartz, T.; Westfall, T. XVI. International union of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998, 50, 143–150. [Google Scholar]
- Dubocovich, M.L.; Masana, M.I.; Benloucif, S. Molecular pharmacology and function of melatonin receptor subtypes. Adv. Exp. Med. Biol. 1999, 460, 181–190. [Google Scholar]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to receptors and channels (GRAC), 5th edition. Br. J. Pharmacol. 2011, 164, S1–S324. [Google Scholar]
- Contos, J.J.; Ishii, I.; Fukushima, N.; Kingsbury, M.A.; Ye, X.; Kawamura, S.; Brown, J.H.; Chun, J. Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: Signaling deficits without obvious phenotypic abnormality attributable to lpa2. Mol. Cell. Biol. 2002, 22, 6921–6929. [Google Scholar]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar]
- Caulfield, M.P. Muscarinic receptors—Characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar]
- Le, Y.; Murphy, P.M.; Wang, J.M. Formyl-peptide receptors revisited. Trends Immunol. 2002, 23, 541–548. [Google Scholar]
- Xiao, R.P.; Avdonin, P.; Zhou, Y.Y.; Cheng, H.; Akhter, S.A.; Eschenhagen, T.; Lefkowitz, R.J.; Koch, W.J.; Lakatta, E.G. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999, 84, 43–52. [Google Scholar]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar]
- Kim, I.S.; Kim, E.R.; Nam, H.J.; Chin, M.O.; Moon, Y.H.; Oh, M.R.; Yeo, U.C.; Song, S.M.; Kim, J.S.; Uhm, M.R.; et al. Activating mutation of GSα in mccune-albright syndrome causes skin pigmentation by tyrosinase gene activation on affected melanocytes. Horm. Res. 1999, 52, 235–240. [Google Scholar]
- Wikberg, J.E. Melanocortin receptors: Perspectives for novel drugs. Eur. J. Pharmacol. 1999, 375, 295–310. [Google Scholar]
- Bathgate, R.A.; Samuel, C.S.; Burazin, T.C.; Gundlach, A.L.; Tregear, G.W. Relaxin: New peptides, receptors and novel actions. Trends Endocrinol. Metab. 2003, 14, 207–213. [Google Scholar]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Hermosilla, R. Vasopressin/Oxytocin; Springer: Berlin, Germany, 2004; pp. 957–961. [Google Scholar]
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Dubyak, G.R.; Harden, T.K.; Jacobson, K.A.; Schwabe, U.; Williams, M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997, 18, 79–82. [Google Scholar]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Leeb-Lundberg, L.M.; Marceau, F.; Muller-Esterl, W.; Pettibone, D.J.; Zuraw, B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005, 57, 27–77. [Google Scholar] [CrossRef]
- Shulkes, A.; Baldwin, G.S. Biology of gut cholecystokinin and gastrin receptors. Clin. Exp. Pharmacol. Physiol. 1997, 24, 209–216. [Google Scholar]
- Ohki-Hamazaki, H. Neuromedin B. Prog. Neurobiol. 2000, 62, 297–312. [Google Scholar]
- Vincent, J.P.; Mazella, J.; Kitabgi, P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar]
- Vassart, G.; Pardo, L.; Costagliola, S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem. Sci. 2004, 29, 119–126. [Google Scholar]
- Martin, N.P.; Whalen, E.J.; Zamah, M.A.; Pierce, K.L.; Lefkowitz, R.J. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell. Signal. 2004, 16, 1397–1403. [Google Scholar]
- Zhu, W.Z.; Zheng, M.; Koch, W.J.; Lefkowitz, R.J.; Kobilka, B.K.; Xiao, R.P. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 1607–1612. [Google Scholar]
- Walker, M.W.; Smith, K.E. Galanin Receptors; Springer: Berlin, Germany, 2004; pp. 378–383. [Google Scholar]
- Davenport, A.P. International union of pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol. Rev. 2002, 54, 219–226. [Google Scholar] [CrossRef]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International union of pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef]
- Gardella, T.J.; Juppner, H. Molecular properties of the PTH/PTHrP receptor. Trends. Endocrinol. Metab. 2001, 12, 210–217. [Google Scholar]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar]
- Meyer zu Heringdorf, D.; Jakobs, K.H. Lysophospholipid receptors: Signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta 2007, 1768, 923–940. [Google Scholar]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar]
- Horn, F.; van der Wenden, E.M.; Oliveira, L.; AP, I.J.; Vriend, G. Receptors coupling to G proteins: Is there a signal behind the sequence? Proteins 2000, 41, 448–459. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Sheffler, D.J.; Roth, B.L. G-protein-coupled receptors at a glance. J. Cell Sci. 2003, 116, 4867–4869. [Google Scholar]
- Brodde, O.E. Beta 1- and beta 2-adrenoceptors in the human heart: Properties, function, and alterations in chronic heart failure. Pharmacol. Rev. 1991, 43, 203–242. [Google Scholar]
- Brodde, O.E. Beta-adrenoceptors in cardiac disease. Pharmacol. Ther. 1993, 60, 405–430. [Google Scholar]
- Rockman, H.A.; Koch, W.J.; Lefkowitz, R.J. Seven-transmembrane-spanning receptors and heart function. Nature 2002, 415, 206–212. [Google Scholar]
- Cruciani, R.A.; Dvorkin, B.; Morris, S.A.; Crain, S.M.; Makman, M.H. Direct coupling of opioid receptors to both stimulatory and inhibitory guanine nucleotide-binding proteins in F-11 neuroblastoma-sensory neuron hybrid cells. Proc. Natl. Acad. Sci. USA 1993, 90, 3019–3023. [Google Scholar]
- Ramirez, J.L.; Gracia-Navarro, F.; Garcia-Navarro, S.; Torronteras, R.; Malagon, M.M.; Castano, J.P. Somatostatin stimulates GH secretion in two porcine somatotrope subpopulations through a camp-dependent pathway. Endocrinology 2002, 143, 889–897. [Google Scholar] [Green Version]
- Ben-Shlomo, A.; Melmed, S. Pituitary somatostatin receptor signaling. Trends Endocrinol. Metab. 2010, 21, 123–133. [Google Scholar] [Green Version]
- Pei, L.; Li, S.; Wang, M.; Diwan, M.; Anisman, H.; Fletcher, P.J.; Nobrega, J.N.; Liu, F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat. Med. 2010, 16, 1393–1395. [Google Scholar] [Green Version]
- Protein lounge. Available online: http://www.proteinlounge.com/ (accessed on 18 April 2012).
- Fraser, C.M.; Venter, J.C. The size of the mammalian lung beta 2-adrenergic receptor as determined by target size analysis and immunoaffinity chromatography. Biochem. Biophys. Res. Commun. 1982, 109, 21–29. [Google Scholar] [Green Version]
- Paglin, S.; Jamieson, J.D. Covalent crosslinking of angiotensin II to its binding sites in rat adrenal membranes. Proc. Natl. Acad. Sci. USA 1982, 79, 3739–3743. [Google Scholar] [Green Version]
- Hebert, T.E.; Bouvier, M. Structural and functional aspects of G protein-coupled receptor oligomerization. Biochem. Cell Biol. 1998, 76, 1–11. [Google Scholar] [Green Version]
- Marshall, F.H.; Jones, K.A.; Kaupmann, K.; Bettler, B. Gabab receptors—The first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [Google Scholar] [Green Version]
- Mohler, H.; Fritschy, J.M. Gabab receptors make it to the top—As dimers. Trends Pharmacol. Sci. 1999, 20, 87–89. [Google Scholar] [Green Version]
- Zhao, G.Q.; Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Erlenbach, I.; Ryba, N.J.; Zuker, C.S. The receptors for mammalian sweet and umami taste. Cell 2003, 115, 255–266. [Google Scholar] [Green Version]
- Jordan, B.A.; Devi, L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 1999, 399, 697–700. [Google Scholar] [Green Version]
- Cvejic, S.; Devi, L.A. Dimerization of the delta opioid receptor: Implication for a role in receptor internalization. J. Biol. Chem. 1997, 272, 26959–26964. [Google Scholar] [Green Version]
- Videau, C.; Hochgeschwender, U.; Kreienkamp, H.J.; Brennan, M.B.; Viollet, C.; Richter, D.; Epelbaum, J. Characterisation of [125I]-Tyr0dtrp8-somatostatin binding in sst1- to sst4- and SRIF-gene-invalidated mouse brain. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 562–571. [Google Scholar] [Green Version]
- Viollet, C.; Lepousez, G.; Loudes, C.; Videau, C.; Simon, A.; Epelbaum, J. Somatostatinergic systems in brain: Networks and functions. Mol. Cell. Endocrinol. 2008, 286, 75–87. [Google Scholar] [Green Version]
- Olias, G.; Viollet, C.; Kusserow, H.; Epelbaum, J.; Meyerhof, W. Regulation and function of somatostatin receptors. J. Neurochem. 2004, 89, 1057–1091. [Google Scholar] [Green Version]
- Ramirez, J.L.; Mouchantaf, R.; Kumar, U.; Otero Corchon, V.; Rubinstein, M.; Low, M.J.; Patel, Y.C. Brain somatostatin receptors are up-regulated in somatostatin-deficient mice. Mol. Endocrinol. 2002, 16, 1951–1963. [Google Scholar] [Green Version]
- Tallent, M.K.; Siggins, G.R. Somatostatin acts in CA1 and CA3 to reduce hippocampal epileptiform activity. J. Neurophysiol. 1999, 81, 1626–1635. [Google Scholar] [Green Version]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [Green Version]
- Cervia, D.; Nunn, C.; Fehlmann, D.; Langenegger, D.; Schuepbach, E.; Hoyer, D. Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs. Br. J. Pharmacol. 2003, 139, 109–121. [Google Scholar] [Green Version]
- Patel, R.C.; Kumar, U.; Lamb, D.C.; Eid, J.S.; Rocheville, M.; Grant, M.; Rani, A.; Hazlett, T.; Patel, S.C.; Gratton, E.; et al. Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3294–3299. [Google Scholar] [Green Version]
- Viollet, C.; Bodenant, C.; Prunotto, C.; Roosterman, D.; Schaefer, J.; Meyerhof, W.; Epelbaum, J.; Vaudry, H.; Leroux, P. Differential expression of multiple somatostatin receptors in the rat cerebellum during development. J. Neurochem. 1997, 68, 2263–2272. [Google Scholar] [Green Version]
- Gines, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casado, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611. [Google Scholar] [Green Version]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef]
- Pello, O.M.; Martinez-Munoz, L.; Parrillas, V.; Serrano, A.; Rodriguez-Frade, J.M.; Toro, M.J.; Lucas, P.; Monterrubio, M.; Martinez, A.C.; Mellado, M. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur. J. Immunol. 2008, 38, 537–549. [Google Scholar]
- Rodriguez-Frade, J.M.; del Real, G.; Serrano, A.; Hernanz-Falcon, P.; Soriano, S.F.; Vila-Coro, A.J.; de Ana, A.M.; Lucas, P.; Prieto, I.; Martinez, A.C.; et al. Blocking HIV-1 infection via CCR5 and CXCR4 receptors by acting in trans on the CCR2 chemokine receptor. EMBO J. 2004, 23, 66–76. [Google Scholar]
- McGraw, D.W.; Mihlbachler, K.A.; Schwarb, M.R.; Rahman, F.F.; Small, K.M.; Almoosa, K.F.; Liggett, S.B. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J. Clin. Invest. 2006, 116, 1400–1409. [Google Scholar]
- Vila-Coro, A.J.; Rodriguez-Frade, J.M.; Martin De Ana, A.; Moreno-Ortiz, M.C.; Martinez, A.C.; Mellado, M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999, 13, 1699–1710. [Google Scholar]
- Berglund, M.M.; Schober, D.A.; Esterman, M.A.; Gehlert, D.R. Neuropeptide Y Y4 receptor homodimers dissociate upon agonist stimulation. J. Pharmacol. Exp. Ther. 2003, 307, 1120–1126. [Google Scholar]
- Latif, R.; Graves, P.; Davies, T.F. Oligomerization of the human thyrotropin receptor: Fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 2001, 276, 45217–45224. [Google Scholar]
- Ayoub, M.A.; Couturier, C.; Lucas-Meunier, E.; Angers, S.; Fossier, P.; Bouvier, M.; Jockers, R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002, 277, 21522–21528. [Google Scholar]
- Perron, A.; Chen, Z.G.; Gingras, D.; Dupre, D.J.; Stankova, J.; Rola-Pleszczynski, M. Agonist-independent desensitization and internalization of the human platelet-activating factor receptor by coumermycin-gyrase B-induced dimerization. J. Biol. Chem. 2003, 278, 27956–27965. [Google Scholar]
- Song, G.J.; Hinkle, P.M. Regulated dimerization of the thyrotropin-releasing hormone receptor affects receptor trafficking but not signaling. Mol. Endocrinol. 2005, 19, 2859–2870. [Google Scholar]
- Margeta-Mitrovic, M.; Jan, Y.N.; Jan, L.Y. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 14649–14654. [Google Scholar]
- Green, S.A.; Turki, J.; Innis, M.; Liggett, S.B. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry 1994, 33, 9414–9419. [Google Scholar]
- Lappalainen, J.; Zhang, L.; Dean, M.; Oz, M.; Ozaki, N.; Yu, D.H.; Virkkunen, M.; Weight, F.; Linnoila, M.; Goldman, D. Identification, expression, and pharmacology of a Cys23-Ser23 substitution in the human 5-HT2c receptor gene (HTR2C). Genomics 1995, 27, 274–279. [Google Scholar]
- Bond, C.; LaForge, K.S.; Tian, M.; Melia, D.; Zhang, S.; Borg, L.; Gong, J.; Schluger, J.; Strong, J.A.; Leal, S.M.; et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA 1998, 95, 9608–9613. [Google Scholar]
- Rotondo, A.; Nielsen, D.A.; Nakhai, B.; Hulihan-Giblin, B.; Bolos, A.; Goldman, D. Agonist-promoted down-regulation and functional desensitization in two naturally occurring variants of the human serotonin 1A receptor. Neuropsychopharmacology 1997, 17, 18–26. [Google Scholar]
- Kunishima, N.; Shimada, Y.; Tsuji, Y.; Sato, T.; Yamamoto, M.; Kumasaka, T.; Nakanishi, S.; Jingami, H.; Morikawa, K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 2000, 407, 971–977. [Google Scholar]
- Bai, M.; Trivedi, S.; Brown, E.M. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 1998, 273, 23605–23610. [Google Scholar]
- AbdAlla, S.; Zaki, E.; Lother, H.; Quitterer, U. Involvement of the amino terminus of the B(2) receptor in agonist-induced receptor dimerization. J. Biol. Chem. 1999, 274, 26079–26084. [Google Scholar]
- Zhang, Z.; Sun, S.; Quinn, S.J.; Brown, E.M.; Bai, M. The extracellular calcium-sensing receptor dimerizes through multiple types of intermolecular interactions. J. Biol. Chem. 2001, 276, 5316–5322. [Google Scholar]
- Ray, K.; Hauschild, B.C. Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J. Biol. Chem. 2000, 275, 34245–34251. [Google Scholar]
- Kuner, R.; Kohr, G.; Grunewald, S.; Eisenhardt, G.; Bach, A.; Kornau, H.C. Role of heteromer formation in GABAB receptor function. Science 1999, 283, 74–77. [Google Scholar]
- Rana, B.K.; Shiina, T.; Insel, P.A. Genetic variations and polymorphisms of g protein-coupled receptors: Functional and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 593–624. [Google Scholar]
- Liu, I.S.; Seeman, P.; Sanyal, S.; Ulpian, C.; Rodgers-Johnson, P.E.; Serjeant, G.R.; van Tol, H.H. Dopamine D4 receptor variant in africans, D4valine194glycine, is insensitive to dopamine and clozapine: Report of a homozygous individual. Am. J. Med. Genet. 1996, 61, 277–282. [Google Scholar] [CrossRef]
- Kotlar, T.J.; Young, R.H.; Albanese, C.; Crowley, W.F., Jr.; Scully, R.E.; Jameson, J.L. A mutation in the follicle-stimulating hormone receptor occurs frequently in human ovarian sex cord tumors. J. Clin. Endocrinol. Metab. 1997, 82, 1020–1026. [Google Scholar]
- Fotiadis, D.; Liang, Y.; Filipek, S.; Saperstein, D.A.; Engel, A.; Palczewski, K. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature 2003, 421, 127–128. [Google Scholar]
- Carrillo, J.J.; Pediani, J.; Milligan, G. Dimers of class a G protein-coupled receptors function via agonist-mediated trans-activation of associated g proteins. J. Biol. Chem. 2003, 278, 42578–42587. [Google Scholar]
- Yoshioka, K.; Saitoh, O.; Nakata, H. Heteromeric association creates a P2Y-like adenosine receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7617–7622. [Google Scholar]
- AbdAlla, S.; Lother, H.; Quitterer, U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 2000, 407, 94–98. [Google Scholar]
- Mellado, M.; Rodriguez-Frade, J.M.; Vila-Coro, A.J.; de Ana, A.M.; Martinez, A.C. Chemokine control of HIV-1 infection. Nature 1999, 400, 723–724. [Google Scholar]
- Lee, B.; Doranz, B.J.; Rana, S.; Yi, Y.; Mellado, M.; Frade, J.M.; Martinez, A.C.; O’Brien, S.J.; Dean, M.; Collman, R.G.; et al. Influence of the CCR2-V64I polymorphism on human immunodeficiency virus type 1 coreceptor activity and on chemokine receptor function of CCR2b, CCR3, CCR5, and CXCR4. J. Virol. 1998, 72, 7450–7458. [Google Scholar]
- Barki-Harrington, L.; Luttrell, L.M.; Rockman, H.A. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: A functional role for receptor-receptor interaction in vivo. Circulation 2003, 108, 1611–1618. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Bookout, A.L.; Wang, G.; Lamb, M.E.; Leeb-Lundberg, L.M.; Daaka, Y. Requirement for direct cross-talk between B1 and B2 kinin receptors for the proliferation of androgen-insensitive prostate cancer PC3 cells. Biochem. J. 2003, 371, 581–587. [Google Scholar]
- AbdAlla, S.; Lother, H.; El Massiery, A.; Quitterer, U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 2001, 7, 1003–1009. [Google Scholar]
- Hansen, J.L.; Hansen, J.T.; Speerschneider, T.; Lyngso, C.; Erikstrup, N.; Burstein, E.S.; Weiner, D.M.; Walther, T.; Makita, N.; Iiri, T.; et al. Lack of evidence for AT1R/B2R heterodimerization in COS-7, HEK293, and NIH3T3 cells: How common is the AT1R/B2R heterodimer? J. Biol. Chem. 2009, 284, 1831–1839. [Google Scholar]
- Quitterer, U.; Lother, H.; Abdalla, S. AT1 receptor heterodimers and angiotensin II responsiveness in preeclampsia. Semin. Nephrol. 2004, 24, 115–119. [Google Scholar]
- AbdAlla, S.; Abdel-Baset, A.; Lother, H.; El Massiery, A.; Quitterer, U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J. Mol. Neurosci. 2005, 26, 185–192. [Google Scholar]
- Gonzalez-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; Lopez-Gimenez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2008, 452, 93–97. [Google Scholar]
- Sealfon, S.C.; Gonzalez-Maeso, J. Receptor pair for schizophrenia. Pediatr. Res. 2008, 64, 1. [Google Scholar]
- Dalrymple, M.B.; Pfleger, K.D.; Eidne, K.A. G protein-coupled receptor dimers: Functional consequences, disease states and drug targets. Pharmacol. Ther. 2008, 118, 359–371. [Google Scholar]
- Blandini, F. Adenosine receptors and l-dopa-induced dyskinesia in Parkinson’s disease: Potential targets for a new therapeutic approach. Exp. Neurol. 2003, 184, 556–560. [Google Scholar]
- Fuxe, K.; Ferre, S.; Canals, M.; Torvinen, M.; Terasmaa, A.; Marcellino, D.; Goldberg, S.R.; Staines, W.; Jacobsen, K.X.; Lluis, C.; et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005, 26, 209–220. [Google Scholar]
- Fuxe, K.; Marcellino, D.; Genedani, S.; Agnati, L. Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson’s disease. Mov. Disord. 2007, 22, 1990–2017. [Google Scholar]
- Kanda, T.; Jackson, M.J.; Smith, L.A.; Pearce, R.K.; Nakamura, J.; Kase, H.; Kuwana, Y.; Jenner, P. Adenosine A2A antagonist: A novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann. Neurol. 1998, 43, 507–513. [Google Scholar]
- Kumar, U.; Grant, M. Somatostatin and somatostatin receptors. Results Probl. Cell Differ. 2010, 50, 137–184. [Google Scholar]
- Kumar, U. Cross-talk and modulation of signaling between somatostatin and growth factor receptors. Endocrine 2011, 40, 168–180. [Google Scholar]
- Lamberts, S.W.; de Herder, W.W.; Hofland, L.J. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol. Metab. 2002, 13, 451–457. [Google Scholar]
- Hofland, L.J. Somatostatin and somatostatin receptors in cushing’s disease. Mol. Cell. Endocrinol. 2008, 286, 199–205. [Google Scholar]
- Hofland, L.J.; Feelders, R.A.; de Herder, W.W.; Lamberts, S.W. Pituitary tumours: The sst/D2 receptors as molecular targets. Mol. Cell. Endocrinol. 2010, 326, 89–98. [Google Scholar]
- Hofland, L.J.; Lamberts, S.W. Somatostatin receptor subtype expression in human tumors. Ann. Oncol. 2001, 12, S31–S36. [Google Scholar]
- Gatto, F.; Hofland, L.J. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr. Relat. Cancer 2011, 18, R233–R251. [Google Scholar]
- Jaquet, P.; Gunz, G.; Saveanu, A.; Dufour, H.; Taylor, J.; Dong, J.; Kim, S.; Moreau, J.P.; Enjalbert, A.; Culler, M.D. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur. J. Endocrinol. 2005, 153, 135–141. [Google Scholar]
- Saveanu, A.; Gunz, G.; Guillen, S.; Dufour, H.; Culler, M.D.; Jaquet, P. Somatostatin and dopamine-somatostatin multiple ligands directed towards somatostatin and dopamine receptors in pituitary adenomas. Neuroendocrinology 2006, 83, 258–263. [Google Scholar]
- Saveanu, A.; Jaquet, P.; Brue, T.; Barlier, A. Relevance of coexpression of somatostatin and dopamine D2 receptors in pituitary adenomas. Mol. Cell. Endocrinol. 2008, 286, 206–213. [Google Scholar]
- Saveanu, A.; Lavaque, E.; Gunz, G.; Barlier, A.; Kim, S.; Taylor, J.E.; Culler, M.D.; Enjalbert, A.; Jaquet, P. Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J. Clin. Endocrinol. Metab. 2002, 87, 5545–5552. [Google Scholar]
- Ben-Shlomo, A.; Melmed, S. Somatostatin agonists for treatment of acromegaly. Mol. Cell. Endocrinol. 2008, 286, 192–198. [Google Scholar]
- Plockinger, U.; Albrecht, S.; Mawrin, C.; Saeger, W.; Buchfelder, M.; Petersenn, S.; Schulz, S. Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J. Clin. Endocrinol. Metab. 2008, 93, 1203–1210. [Google Scholar]
- Bronstein, M.D. Acromegaly: Molecular expression of somatostatin receptor subtypes and treatment outcome. Front. Horm. Res. 2006, 35, 129–134. [Google Scholar]
- Duran-Prado, M.; Gahete, M.D.; Martinez-Fuentes, A.J.; Luque, R.M.; Quintero, A.; Webb, S.M.; Benito-Lopez, P.; Leal, A.; Schulz, S.; Gracia-Navarro, F.; et al. Identification and characterizationof two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J. Clin. Endocrinol. Metab. 2009, 94, 2634–2643. [Google Scholar]
- Duran-Prado, M.; Malagon, M.M.; Gracia-Navarro, F.; Castano, J.P. Dimerization of G protein-coupled receptors: New avenues for somatostatin receptor signalling, control and functioning. Mol. Cell. Endocrinol. 2008, 286, 63–68. [Google Scholar]
- Duran-Prado, M.; Gahete, M.D.; Hergueta-Redondo, M.; Martinez-Fuentes, A.J.; Cordoba-Chacon, J.; Palacios, J.; Gracia-Navarro, F.; Moreno-Bueno, G.; Malagon, M.M.; Luque, R.M.;et al. The new truncated somatostatin receptor variant sst5TMD4 is associated to poor prognosis in breast cancer and increases malignancy in MCF-7 cells. Oncogene 2011. [Google Scholar]
- Bootsma, A.H.; van Eijck, C.; Schouten, K.K.; Reubi, J.C.; Waser, B.; Foekens, J.A.; van Pel, R.; Zwarthoff, E.C.; Lamberts, S.W.; de Klein, A. Somatostatin receptor-positive primary breast tumors: Genetic, patient and tumor characteristics. Int. J. Cancer 1993, 54, 357–362. [Google Scholar]
- Fekete, M.; Wittliff, J.L.; Schally, A.V. Characteristics and distribution of receptors for [d-trp6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J. Clin. Lab. Anal. 1989, 3, 137–147. [Google Scholar] [CrossRef]
- Prevost, G.; Hosford, D.; Thomas, F. Receptors for somatostatin and somatostatin analogues in human breast tumors. Ann. NY Acad. Sci. 1994, 733, 147–154. [Google Scholar]
- Prevost, G.; Lanson, M.; Thomas, F.; Veber, N.; Gonzalez, W.; Beaupain, R.; Starzec, A.; Bogden, A. Molecular heterogeneity of somatostatin analogue BIM-23014C receptors in human breast carcinoma cells using the chemical cross-linking assay. Cancer Res. 1992, 52, 843–850. [Google Scholar]
- Prevost, G.; Provost, P.; Salle, V.; Lanson, M.; Thomas, F. A cross-linking assay allows the detection of receptors for the somatostatin analogue, lanreotide in human breast tumours. Eur. J. Cancer 1993, 29A, 1589–1592. [Google Scholar]
- Foekens, J.A.; Portengen, H.; van Putten, W.L.; Trapman, A.M.; Reubi, J.C.; Alexieva-Figusch, J.; Klijn, J.G. Prognostic value of receptors for insulin-like growth factor 1, somatostatin, and epidermal growth factor in human breast cancer. Cancer Res. 1989, 49, 7002–7009. [Google Scholar]
- Foekens, J.A.; van Putten, W.L.; Portengen, H.; Rodenburg, C.J.; Reubi, J.C.; Berns, P.M.; Henzen-Logmans, S.C.; van der Burg, M.E.; Alexieva-Figusch, J.; Klijn, J.G. Prognostic value of ps2 protein and receptors for epidermal growth factor (EGF-R), insulin-like growth factor-1 (IGF-1-R) and somatostatin (SS-R) in patients with breast and ovarian cancer. J. Steroid. Biochem. Mol. Biol. 1990, 37, 815–821. [Google Scholar]
- Kumar, U.; Grigorakis, S.I.; Watt, H.L.; Sasi, R.; Snell, L.; Watson, P.; Chaudhari, S. Somatostatin receptors in primary human breast cancer: Quantitative analysis of mRNA for subtypes 1-5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res. Treat. 2005, 92, 175–186. [Google Scholar]
- Nelson, J.; Cremin, M.; Murphy, R.F. Synthesis of somatostatin by breast cancer cells and their inhibition by exogenous somatostatin and sandostatin. Br. J. Cancer 1989, 59, 739–742. [Google Scholar]
- Pagliacci, M.C.; Tognellini, R.; Grignani, F.; Nicoletti, I. Inhibition of human breast cancer cell (MCF-7) growth in vitro by the somatostatin analog SMS 201-995: Effects on cell cycle parameters and apoptotic cell death. Endocrinology 1991, 129, 2555–2562. [Google Scholar] [CrossRef]
- Prevost, G.; Foehrle, E.; Thomas, F.; Pihan, I.; Veber, N.; Starzec, A.; Israel, L. Growth of human breast cancer cell lines is inhibited by the somatostatin analog Bim23014. Endocrinology 1991, 129, 323–329. [Google Scholar]
- Setyono-Han, B.; Henkelman, M.S.; Foekens, J.A.; Klijn, G.M. Direct inhibitory effects of somatostatin (analogues) on the growth of human breast cancer cells. Cancer Res. 1987, 47, 1566–1570. [Google Scholar]
- Weber, C.; Merriam, L.; Koschitzky, T.; Karp, F.; Benson, M.; Forde, K.; LoGerfo, P. Inhibition of growth of human breast carcinomas in vivo by somatostatin analog SMS 201-995: Treatment of nude mouse xenografts. Surgery 1989, 106, 416–422. [Google Scholar]
- Weckbecker, G.; Liu, R.; Tolcsvai, L.; Bruns, C. Antiproliferative effects of the somatostatin analogue octreotide (SMS 201-995) on Zr-75-1 human breast cancer cells in vivo and in vitro. Cancer Res. 1992, 52, 4973–4978. [Google Scholar]
- Weckbecker, G.; Tolcsvai, L.; Liu, R.; Bruns, C. Preclinical studies on the anticancer activity of the somatostatin analogue octreotide (SMS 201-995). Metabolism 1992, 41, 99–103. [Google Scholar]
- Weckbecker, G.; Tolcsvai, L.; Stolz, B.; Pollak, M.; Bruns, C. Somatostatin analogue octreotide enhances the antineoplastic effects of tamoxifen and ovariectomy on 7,12-dimethylbenz(alpha)anthracene-induced rat mammary carcinomas. Cancer Res. 1994, 54, 6334–6337. [Google Scholar]
- van op den Bosch, J.; Torfs, P.; de Winter, B.Y.; de Man, J.G.; Pelckmans, P.A.; van Marck, E.; Grundy, D.; van Nassauw, L.; Timmermans, J.P. Effect of genetic SSTR4 ablation on inflammatory peptide and receptor expression in the non-inflamed and inflamed murine intestine. J. Cell. Mol. Med. 2009, 13, 3283–3295. [Google Scholar]
- van op den Bosch, J.; van Nassauw, L.; Lantermann, K.; van Marck, E.; Timmermans, J.P. Effect of intestinal inflammation on the cell-specific expression of somatostatin receptor subtypes in the murine ileum. Neurogastroenterol. Motil. 2007, 19, 596–606. [Google Scholar]
- Betoin, F.; Ardid, D.; Herbet, A.; Aumaitre, O.; Kemeny, J.L.; Duchene-Marullaz, P.; Lavarenne, J.; Eschalier, A. Evidence for a central long-lasting antinociceptive effect of vapreotide, an analog of somatostatin, involving an opioidergic mechanism. J. Pharmacol. Exp. Ther. 1994, 269, 7–14. [Google Scholar]
- Tashev, R.; Belcheva, S.; Milenov, K.; Belcheva, I. Antinociceptive effect of somatostatin microinjected into caudate putamen. Peptides 2001, 22, 1079–1083. [Google Scholar]
- Bell, J.R.; Young, M.R.; Masterman, S.C.; Morris, A.; Mattick, R.P.; Bammer, G. A pilot study of naltrexone-accelerated detoxification in opioid dependence. Med. J. Aust. 1999, 171, 26–30. [Google Scholar]
- Maurer, R.; Gaehwiler, B.H.; Buescher, H.H.; Hill, R.C.; Roemer, D. Opiate antagonistic properties of an octapeptide somatostatin analog. Proc. Natl. Acad. Sci. USA 1982, 79, 4815–4817. [Google Scholar]
- Mihm, M.J.; Amann, D.M.; Schanbacher, B.L.; Altschuld, R.A.; Bauer, J.A.; Hoyt, K.R. Cardiac dysfunction in the R6/2 mouse model of huntington’s disease. Neurobiol. Dis. 2007, 25, 297–308. [Google Scholar]
- Colao, A.; Marzullo, P.; di Somma, C.; Lombardi, G. Growth hormone and the heart. Clin. Endocrinol. (Oxf.) 2001, 54, 137–154. [Google Scholar] [CrossRef]
- Niehoff, D.L.; Mudge, A.W. Somatostatin alters beta-adrenergic receptor-effector coupling in cultured rat astrocytes. EMBO J. 1985, 4, 317–321. [Google Scholar]
- Mayor, F., Jr.; Benovic, J.L.; Caron, M.G.; Lefkowitz, R.J. Somatostatin induces translocation of the beta-adrenergic receptor kinase and desensitizes somatostatin receptors in S49 lymphoma cells. J. Biol. Chem. 1987, 262, 6468–6471. [Google Scholar]
- Somvanshi, R.K.; Qiu, X.; Kumar, U. Isoproterenol induced hypertrophy and associated signaling pathways are modulated by somatostatin in H9c2 cells. Int. J. Cardiol. 2012, in press. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Somvanshi, R.K.; Kumar, U. Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors. Pharmaceuticals 2012, 5, 417-446. https://doi.org/10.3390/ph5050417
Somvanshi RK, Kumar U. Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors. Pharmaceuticals. 2012; 5(5):417-446. https://doi.org/10.3390/ph5050417
Chicago/Turabian StyleSomvanshi, Rishi K., and Ujendra Kumar. 2012. "Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors" Pharmaceuticals 5, no. 5: 417-446. https://doi.org/10.3390/ph5050417
APA StyleSomvanshi, R. K., & Kumar, U. (2012). Pathophysiology of GPCR Homo- and Heterodimerization: Special Emphasis on Somatostatin Receptors. Pharmaceuticals, 5(5), 417-446. https://doi.org/10.3390/ph5050417




