Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells
Abstract
:1. Introduction

2. Experimental Section
2.1. Chemicals, Antibodies and Cell-Lines
2.2. Synthesis of BAM
2.3. Preparation of BAM-Conjugated IgG
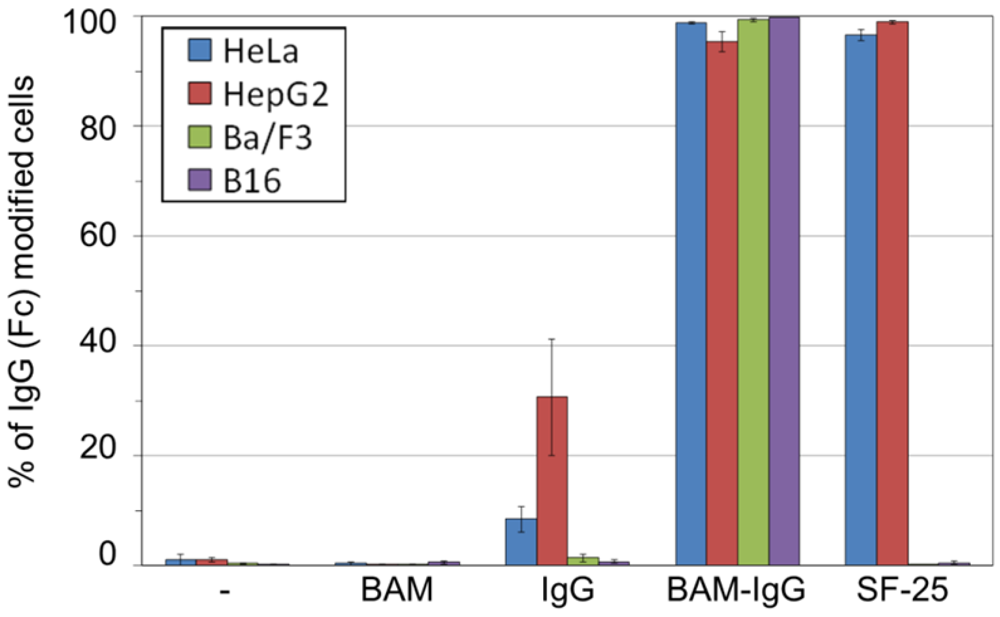
2.4. Incorporation of IgG-BAM Conjugates onto Cell Membrane
2.5. Generation of Bone Marrow-derived DCs
2.6. Phagocytosis of Apoptotic Cancer Cells by DCs
3. Results and Discussion
3.1. Conjugation of IgG and BAM

3.2. Incorporation of IgG-BAM Conjugates onto the Cell Membrane
3.3. Effects of Incorporated IgG-BAM on Phagocytosis by Dendritic Cells

4. Conclusions
Acknowledgements
References
- Figdor, C.G.; de Vries, I.J.; Lesterhuis, W.J.; Melief, C.J. Dendritic cell immunotherapy: Mapping the way. Nat. Med. 2004, 10, 475–480. [Google Scholar]
- Lu, W.; Wu, X.; Lu, Y.; Guo, W.; Andrieu, J.M. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 2003, 9, 27–32. [Google Scholar]
- Banchereau, J.; Palucka, A.K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 2005, 5, 296–306. [Google Scholar]
- Nestle, F.O.; Farkas, A.; Conrad, C. Dendritic-cell-based therapeutic vaccination against cancer. Curr. Opin. Immunol. 2005, 17, 163–169. [Google Scholar]
- Janikashvili, N.; Larmonier, N.; Katsanis, E. Personal dendritic cell-based tumor immunotherapy. Immunotherapy 2010, 2, 57–68. [Google Scholar]
- Schnurr, M.; Scholz, C.; Rothenfusser, S.; Galambos, P.; Dauer, M.; Röbe, J.; Endres, S.; Eigler, A. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and γδ T cells. Cancer Res. 2002, 62, 2347–2352. [Google Scholar]
- Akiyama, K.; Ebihara, S.; Yada, A.; Matsumura, K.; Aiba, S.; Nukiwa, T.; Takai, T. Targeting apoptotic tumor cells to FcγR provides efficient and versatile vaccination against tumors by dendritic cells. J. Immunol. 2003, 170, 1641–1648. [Google Scholar]
- Galea-Lauri, J.; Darling, D.; Mufti, G.; Harrison, P.; Farzaneh, F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: Evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol. Immunother. 2002, 51, 299–310. [Google Scholar]
- Kotera, Y.; Shimizu, K.; Mulé, J.J. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001, 61, 8105–8109. [Google Scholar]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudt, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 5, 405–411. [Google Scholar]
- Kim, K.D.; Choi, S.C.; Kim, A.; Choc, Y.K.; Choc, I.S.; Lim, J.S. Dendritic cell-tumor coculturing vaccine can induce antitumor immunity through both NK and CTL interaction. Int. Immunopharmacol. 2001, 1, 2117–2129. [Google Scholar]
- Regnault, A.; Lankar, D.; Lacabanne, V.; Rodriguez, A.; Théry, C.; Rescigno, M.; Saito, T.; Verbeek, S.; Bonnerot, C.; Ricciardi-Castagnoli, P.; et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999, 189, 371–380. [Google Scholar]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar]
- Kato, K.; Itoh, C.; Yasukouchi, T.; Nagamune, T. Rapid protein anchoring into the membranes of mammalian cells using oleyl chain and poly(ethylene glycol) derivatives. Biotechnol. Prog. 2004, 20, 897–904. [Google Scholar]
- Chung, H.A.; Tajima, K.; Kato, K.; Matsummoto, N.; Yamamoto, K.; Nagamune, T. Modulating the actions of NK cell-mediated cytotoxicity using lipid-PEG (n) and inhibitory receptor-specific antagonistic peptide conjugates. Biotechnol. Prog. 2005, 21, 1226–1230. [Google Scholar]
- Wang, T.; Leventis, R.; Silvius, J.R. Artificially lipid-anchored proteins can elicit clustering-induced intracellular signaling events in Jurkat T-lymphocytes independent of lipid raft association. J. Biol. Chem. 2005, 280, 22839–22846. [Google Scholar]
- Teramura, Y.; Iwata, H. Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation. Transplantation 2009, 88, 624–630. [Google Scholar]
- Teramura, Y.; Iwata, H. Cell surface modification with polymers for biomedical studies. Soft Matter 2010, 6, 1081–1091. [Google Scholar]
- Ashraf, S.Q.; Umana, P.; Mossner, E.; Ntouroupi, T.; Brunker, P.; Schmidt, C.; Wilding, J.L.; Mortensen, N.J.; Bodmer, W.F. Humanised IgG1 antibody variants targeting membrane-bound carcinoembryonic antigen by antibody-dependent cellular cytotoxicity and phagocytosis. Br. J. Cancer 2009, 101, 1758–1768. [Google Scholar]
- Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: A review. Adv. Drug Deliv. Rev. 2002, 54, 531–545. [Google Scholar]
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. ChemMedChem 2008, 3, 20–53. [Google Scholar]
- Kitamura, K.; Takahashi, T.; Yamaguchi, T.; Noguchi, A.; Noguchi, A.; Takashima, K.-I.; Tsurumi, H.; Inagake, M.; Toyokuni, T.; Hakomori, S.-I. Chemical engineering of the monoclonal antibody A7 by polyethylene glycol for targeting cancer chemotherapy. Cancer Res. 1991, 51, 4310–4315. [Google Scholar]
- Takahashi, H.; Wilson, B.; Ozturk, M.; Motté, P.; Strauss, W.; Isselbacher, K.J.; Wands, J.R. In vivo localization of human colon adenocarcinoma by monoclonal antibody binding to a highly expressed cell surface antigen. Cancer Res. 1988, 48, 6573–6579. [Google Scholar]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose of bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar]
- Suzuki, T.; Kanbara, N.; Tomono, T.; Hayashi, N.; Shinohara, I. Physicochemical and biological properties of poly(ethylene glycol)-coupled immunoglobulin G. Biochim. Biophys. Acta 1984, 788, 248–255. [Google Scholar]
- Koning, G.A.; Morselt, H.W.; Kamps, J.A.; Scherphof, G.L. Uptake and intracellular processing of PEG-liposomes and PEG-immunoliposomes by kupffer cells in vitro. J. Liposome Res. 2001, 11, 195–209. [Google Scholar] [CrossRef]
- Chikh, G.G.; Kong, S.; Bally, M.B.; Meunier, J.C.; Schutze-Redelmeier, M.P. Efficient delivery of Antennapedia homeodomain fused to CTL epitope with liposomes into dendritic cells results in the activation of CD8+ T cells. J. Immunol. 2001, 167, 6462–6470. [Google Scholar]
- Kawamura, K.; Kadowaki, N.; Suzuki, R.; Udagawa, S.; Kasaoka, S.; Utoguchi, N.; Kitawaki, T.; Sugimoto, N.; Okada, N.; Maruyama, K.; et al. Dendritic cells that endocytosed antigen-containing IgG-liposomes elicit effective antitumor immunity. J. Immunother. 2006, 29, 165–174. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tomita, U.; Yamaguchi, S.; Sugimoto, Y.; Takamori, S.; Nagamune, T. Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells. Pharmaceuticals 2012, 5, 405-416. https://doi.org/10.3390/ph5050405
Tomita U, Yamaguchi S, Sugimoto Y, Takamori S, Nagamune T. Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells. Pharmaceuticals. 2012; 5(5):405-416. https://doi.org/10.3390/ph5050405
Chicago/Turabian StyleTomita, Urara, Satoshi Yamaguchi, Yoichiro Sugimoto, Satoshi Takamori, and Teruyuki Nagamune. 2012. "Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells" Pharmaceuticals 5, no. 5: 405-416. https://doi.org/10.3390/ph5050405
APA StyleTomita, U., Yamaguchi, S., Sugimoto, Y., Takamori, S., & Nagamune, T. (2012). Poly(ethylene glycol)-Lipid-Conjugated Antibodies Enhance Dendritic Cell Phagocytosis of Apoptotic Cancer Cells. Pharmaceuticals, 5(5), 405-416. https://doi.org/10.3390/ph5050405




