From Fertilisation to Implantation in Mammalian Pregnancy—Modulation of Early Human Reproduction by the Endocannabinoid System
Abstract
:1. Introduction
2. The Endocannabinoid System
2.1. Synthesis
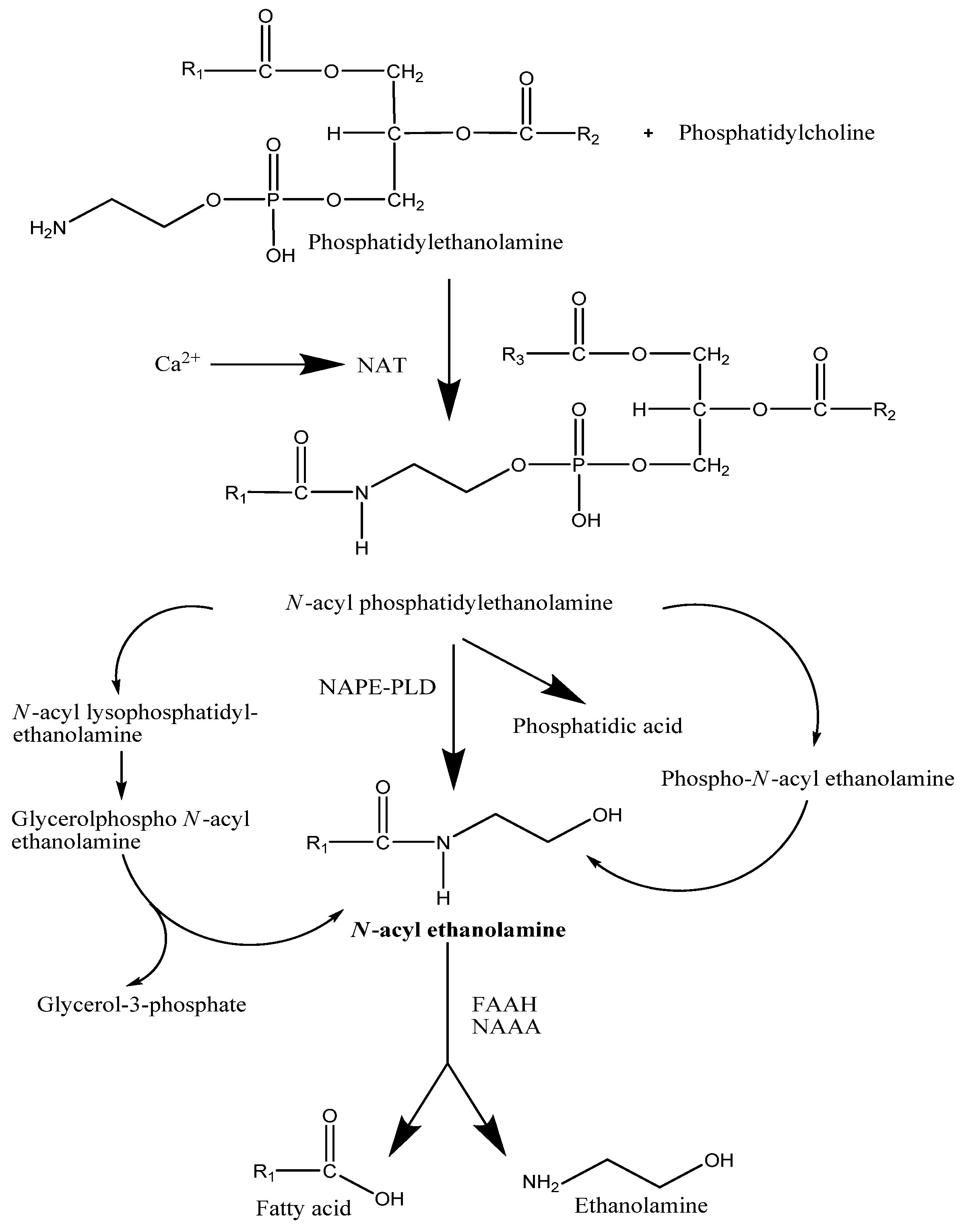
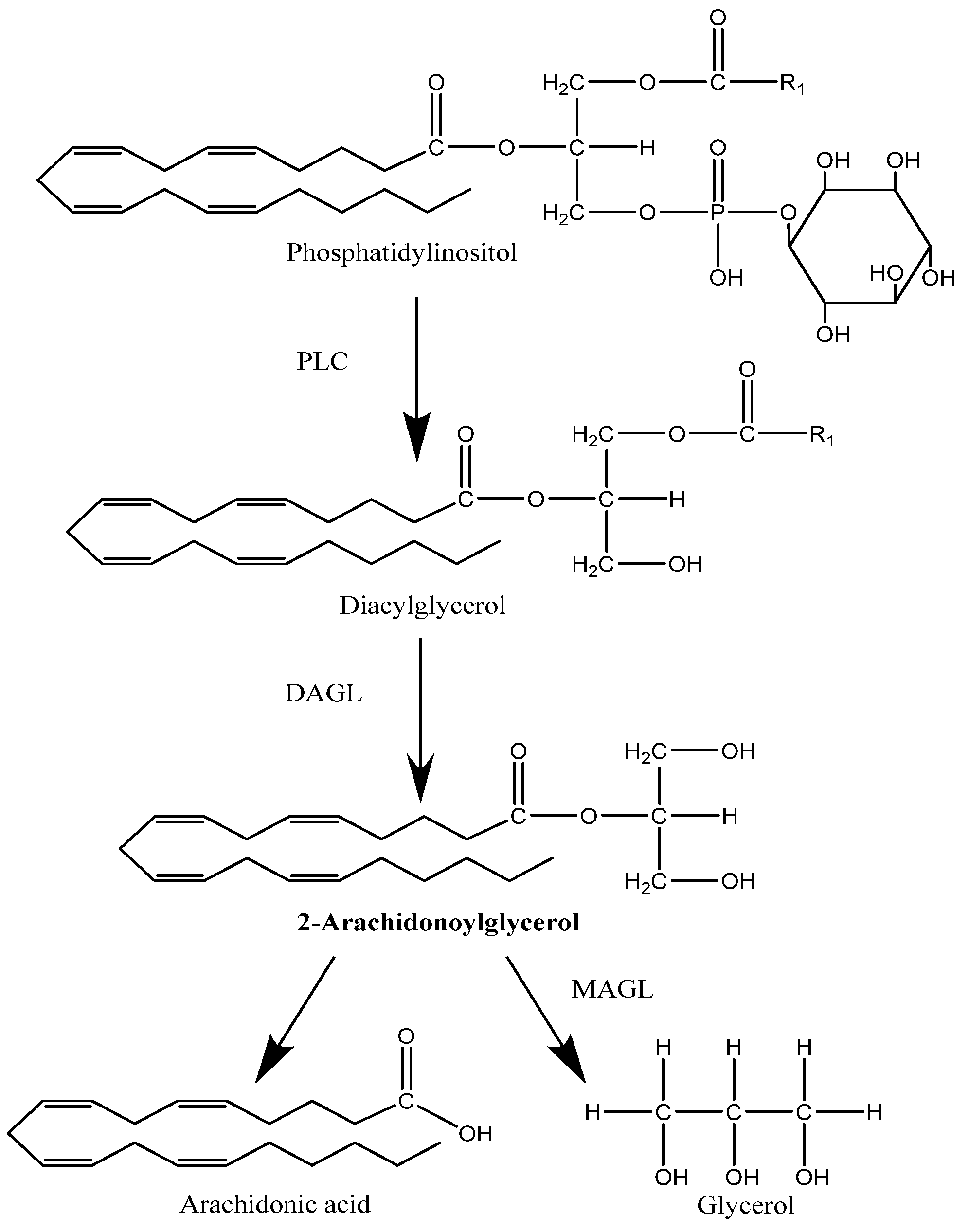
2.2. Degradation
2.3. Cannabinoid Receptors and Signal Transduction
3. Gametogenesis
3.1. Oogenesis
3.2. Spermatogenesis and Fertilisation
3.3. Blastocyst Development
3.4. Oviductal Transport
3.5. Implantation
3.6. Early Pregnancy Maintenance
3.7. Endocannabinoids, Immunomodulation and Pregnancy
4. Conclusions
References
- Park, B.; McPartland, J.M.; Glass, M. Cannabis, cannabinoids and reproduction. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 189–197. [Google Scholar]
- Evers, J.L. Female subfertility. Lancet 2002, 360, 151–159. [Google Scholar]
- Regan, L.; Braude, P.R.; Trembath, P.L. Influence of past reproductive performance on risk of spontaneous abortion. BMJ 1989, 299, 541–545. [Google Scholar]
- Wenger, T.; Croix, D.; Tramu, G.; Leonardelli, J. Effects of delta-9-tetrahydrocannabinol on pregnancy, puberty, and the neuroendocrine system. In Marijuana/Cannabinoids: Neurobiology and Neurophysiology; Murphy, L., Bartke, A., Eds.; CRC Press: Boca Raton, FL USA, 1992. [Google Scholar]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Mahony, M.C.; Giuffrida, A.; Picone, R.P.; Makriyannis, A. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol. Reprod. Devel. 2002, 63, 376–387. [Google Scholar]
- Abel, E.L. Infertility increases when alcohol and marijuana are combined. Teratology 1985, 31, 35–40. [Google Scholar]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [PubMed]
- Wenger, T.; Fragkakis, G.; Giannikou, P.; Probonas, K.; Yiannikakis, N. Effects of anandamide on gestation in pregnant rats. Life Sci. 1997, 60, 2361–2371. [Google Scholar]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar]
- Rodriguez de Fonseca, F.; Del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol 2005, 40, 2–14. [Google Scholar]
- Di Marzo, V.; Petrosino, S. Endocannabinoids and their levels in health and disease. Curr. Opin. Lipid 2007, 18, 129–140. [Google Scholar]
- Sipe, J.C.; Scott, T.M.; Murray, S.; Harismendy, O.; Simon, G.M.; Cravatt, B.F.; Waalen, J. Biomarkers of Endocannabinoid System Activation in Severe Obesity. PLoS One 2010, 5, e8792. [Google Scholar]
- Dalle Carbonare, M.; Del Giudice, E.; Stecca, A.; Colavito, D.; Fabris, M.; D'Arrigo, A.; Bernardini, D.; Dam, M.; Leon, A. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: A neglected story. J. Neuroendocrinol. 2008, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Fonseca, F.; Navarro, M.; Gomez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodriguez, E.; Giuffrida, A.; LoVerme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature. 2001, 414, 209–212. [Google Scholar] [PubMed]
- Lambert, D.M.; Vandevoorde, S.; Jonsson, K.O.; Fowler, C.J. The palmitoylethanolamide family: A new class of anti-inflammatory agents? Curr. Med. Chem. 2002, 9, 663–674. [Google Scholar] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar]
- Schmid, H.H. Pathways and mechanisms of N-acylethanolamine biosynthesis: Can anandamide be generated selectively? Chem. Phys. Lipids 2000, 108, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar]
- Jin, X.H.; Uyama, T.; Wang, J.; Okamoto, Y.; Tonai, T.; Ueda, N. cDNA cloning and characterization of human and mouse ca(2+)-independent phosphatidylethanolamine N-acyltransferases. Biochim. Biophys. Acta. 2009, 1791, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yamano, Y.; Asano, A.; Ohyama, K.; Ohta, M.; Nishio, R.; Morishima, I. Expression of the ha-ras suppressor family member 5 gene in the maturing rat testis. Biosci. Biotechnol. Biochem. 2008, 72, 1360–1363. [Google Scholar]
- Wang, J.; Okamoto, Y.; Morishita, J.; Tsuboi, K.; Miyatake, A.; Ueda, N. Functional analysis of the purified anandamide-generating phospholipase D as a member of the metallo-beta-lactamase family. J. Biol. Chem. 2006, 281, 12325–31235. [Google Scholar]
- Ueda, N.; Liu, Q.; Yamanaka, K. Marked activation of the N-acylphosphatidylethanolamine-hydrolyzing phosphodiesterase by divalent cations. Biochim. Biophys. Acta 2001, 1532, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Saghatelian, A.; Simon, G.M.; Cravatt, B.F. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 2006, 45, 4720–4726. [Google Scholar]
- Liu, J.; Wang, L.; Harvey-White, J.; Huang, B.X.; Kim, H.Y.; Luquet, S.; Palmiter, R.D.; Krystal, G.; Rai, R.; Mahadena, A.; et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signalling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar]
- Mechoulam, R.; Deutsch, D.G. Toward an anandamide transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 17541–17542. [Google Scholar]
- Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. 2008, 7, 489–503. [Google Scholar]
- Gong, Y.Z.; Everett, E.T.; Schwartz, D.A.; Norris, J.S.; Wilson, F.A. Molecular cloning, tissue distribution, and expression of a 14-kDa bile acid-binding protein from rat ileal cytosol. Proc. Natl. Acad. Sci. USA 1994, 91, 4741–4745. [Google Scholar]
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar]
- Larqué, E.; Krauss-Etschmann, S.; Campoy, C.; Hartl, D.; Linde, J.; Klingler, M.; Demmelmair, H.; Caño, A.; Gil, A.; Bondy, B.; Koletzko, B. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am. J. Clin. Nutr. 2006, 84, 853–861. [Google Scholar] [PubMed]
- Nixon, B.; MacIntyre, D.A.; Mitchell, L.A.; Gibbs, G.M.; O’Bryan, M.; Aitken, R.J. The identification of mouse sperm-specific-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 2006, 74, 275–287. [Google Scholar]
- Yamamoto, T.; Yamamoto, A.; Watanabe, M.; Matsu,o, T.; Yamazaki, N.; Kataoka, M.; Terada, H.; Shinohara, Y. Classification of FABP isoforms and tissues based on quantitative evaluation of transcript levels of these isoforms in various rat tissues. Biotechnol. Lett. 2009, 31, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Sun, Y.X.; Okamoto, Y.; Araki, N.; Tonai, T.; Ueda, N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J. Biol. Chem. 2005, 280, 1082–1092. [Google Scholar]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar]
- Dinh, T.P.; Kathuria, S.; Piomelli, D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol. Pharmacol. 2004, 66, 1260–1264. [Google Scholar]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar]
- Pan, B.; Wang, W.; Long, J.Z.; Sun, D.; Hillard, C.J.; Cravatt, B.F.; Liu, Q.S. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signalling. J. Pharmacol. Exp. Ther. 2009, 331, 591–597. [Google Scholar]
- Habayeb, O.M.H.; Bell, S.C.; Konje, J.C. Endogenous Cannabinoids: Metabolism and their role in reproduction. Life Sci. 2002, 70, 1963–1977. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar]
- Idris, A.I.; van Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Cacciola, G.; Chianese, R.; Chioccarelli, T.; Fasano, S. Cb1 activity in male reproduction: Mammalian and non-mammalian animal models. Vit. Horm. 2009, 81, 367–387. [Google Scholar]
- Maccarrone, M. Endocannabinoids and reproductive endocrinology. Curr. Opin. Investig. Drugs. 2009, 10, 305–310. [Google Scholar]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Abbas, M.S.; Habiba, M.A.; Konje, J.C. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem. Cell Biol. 2010, 133, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, T.H.; Shin, Y.K.; Lee, C.S.; Park, M.; Song, J.H. Anandamide suppression of Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005, 1062, 39–47. [Google Scholar] [PubMed]
- Maingret, F.; Patel, A.J.; Lazdunski, M.; Honore, E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001, 20, 47–54. [Google Scholar]
- Godlewski, G.; Offertaler, L.; Wagner, J.A.; Kunos, G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009, 89, 105–111. [Google Scholar]
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christiensen, M.; et al. De-orphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- O'Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Taylor, H.S. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 2009, 27, 62–79. [Google Scholar]
- Toth, A.; Blumberg, P.M.; Boczan, J. Anandamide and the vanilloid receptor (TRPV1). Vit.Horm. 2009, 81, 389–419. [Google Scholar]
- Alger, B.E. Retrograde signalling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar]
- Buznikov, G.A.; Nikitina, L.A.; Bezuglov, V.V.; Francisco, M.E.Y.; Bohsen, G.; Obispo-Peak, I.N.; Peterson, R.E; Weiss, E.R.; Schuel, H.; Temple, B.R.S.; Morrow, A.L.; Lauder, J.M. A putative ‘pre-nervous’ endocannabinoid system in early Echinoderm development. Dev. Neurosci. 2010, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Pasquariello, N.; Di Tommaso, M.; Maccarrone, M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J. Neuroendocrinol. 2008, 20, 82–89. [Google Scholar]
- Taylor, A.H.; Ang, C.; Bell, S.C.; Konje, J.C. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum. Reprod. Update 2007, 13, 501–513. [Google Scholar]
- Schuel, H. Tuning the oviduct to the anandamide tone. J. Clin. Invest. 2006, 116, 2087–2090. [Google Scholar]
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Dubois, R.N.; Dey, S.K. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat. Med. 2004, 10, 1074–1080. [Google Scholar]
- Sun, X.; Dey, S.K. Aspects of endocannabinoid signaling in periimplantation biology. Mol. Cell Endocrinol. 2008, 286, S3–S11. [Google Scholar]
- Paria, B.C.; Ma, W.; Andrenyak, D.M.; Schmid, P.C.; Schmid, H.H.; Moody, D.E.; Deng, H.; Makriyannis, A.; Dey, S.K. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol. Reprod. 1998, 58, 1490–1495. [Google Scholar]
- Paria, B.C.; Das, S.K.; Dey, S.K. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc. Natl. Acad. Sci. USA 1995, 92, 9460–9464. [Google Scholar]
- Taylor, A.H.; Amoako, A.A.; Bambang, K.; Karasu, T.; Gebeh, A.; Lam, P.M.; Marzcylo, T.H.M.; Konje, J.C. Endocannabinoids and pregnancy. Clin. Chim. Acta 2010, 411, 921–930. [Google Scholar]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and function of the endocannabinoid system in the human ovary. PLoS One 2009, 4, e4579. [Google Scholar]
- Barrett, K.E.; Ganong, W.F. Ganong's Review of Medical Physiology, 23rd ed; McGraw-Hill Medical: New York, NY, USA, 2009; p. 714. [Google Scholar]
- Chabbert Buffet, N.; Djakoure, C.; Maitre, S.C.; Bouchard, P. Regulation of the human menstrual cycle. Front Neuroendocrinol. 1998, 19, 151–186. [Google Scholar]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Hum. Reprod. 2009, 24, 1989–1998. [Google Scholar]
- Carreau, S.; Silandre, D.; Bois, C.; Bouraima, H.; Galeraud-Denis, I.; Delalande, C. Estrogens: a new player in spermatogenesis. Folia Histochem. Cytobiol. 2007, 45, S5–S10. [Google Scholar]
- Sofikitis, N.; Giotitsas, N.; Tsounapi, P.; Baltogiannis, D.; Giannakis, D.; Pardalidis, N. Hormonal regulation of spermatogenesis and spermiogenesis. J. Steroid. Biochem. Mol. Biol. 2008, 109, 323–330. [Google Scholar]
- Sriraman, V.; Rao, A.J. FSH, the neglected sibling: evidence for its role in regulation of spermatogenesis and Leydig cell function. Indian J. Exp. Biol. 2005, 43, 993–1000. [Google Scholar]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Mahony, M.C.; Giuffrida, A.; Picone, R.P.; Makriyannis, A. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol. Reprod. Dev. 2002, 63, 376–387. [Google Scholar]
- Maccarrone, M.; Cecconi, S.; Rossi, G.; Battista, N.; Pauselli, R.; Finazzi-Agro, A. Anandamide activity and degradation are regulated by early postnatal aging and follicle-stimulating hormone in mouse Sertoli cells. Endocrinology 2003, 144, 20–28. [Google Scholar]
- Rossi, G.; Gasperi, V.; Paro, R.; Barsacchi, D.; Cecconi, S.; Maccarrone, M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology 2007, 148, 1431–1439. [Google Scholar]
- Grimaldi, P.; Orlando, P.; Di Siena, S.; Lolicato, F.; Petrosino, S.; Bisogno, T.; Geremia, R.; De Petrocellis, L.; Di Marzo, V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 11131–11136. [Google Scholar]
- Gervasi, M.G.; Rapanelli, M.; Ribeiro, M.L.; Farina, M.; Billi, S.; Franchi, A.M.; Martinez, S. P. The endocannabinoid system in bull sperm and bovine oviductal epithelium: role of anandamide in sperm-oviduct interaction. Reproduction 2009, 137, 403–414. [Google Scholar]
- Grimaldi, P.; Rossi, G.; Catanzaro, G; Maccarrone, M. Modulation of the endocannabinoid enzyme fatty acid amide hydrolase by follicle-stimulating hormone. Vit. Horm. 2009, 81, 231–261. [Google Scholar] [CrossRef]
- Aquila, S.; Guido, C.; Santoro, A.; Perrotta, I.; Laezza, C.; Bifulco, M.; Sebastiano, A. Anat. Rec. 2010, 293, 298–309.
- Aquila, S.; Guido, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Baffa, M.F.; Ando, S.; Bifulco, M. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br. J. Pharamacol. 2010, 159, 831–841. [Google Scholar]
- Aquila, S.; Guido, C.; Laezza, C.; Santoro, A.; Pezzi, V.; Panza, S.; Ando, S.; Bifulco, M. A new role of anandamide in human sperm: Focus on metabolism. J. Cell Physiol. 2009, 221, 147–153. [Google Scholar] [PubMed]
- Cobellis, G.; Ricci, G.; Cacciola, G.; Orlando, P.; Petrosino, S.; Cascio, M.G.; Bisogno, T.; De Petrocellis, L.; Chioccarelli, T.; Altucci, L.; Fasano, S.; Meccariello, R.; Pierantoni, R.; Ledent, C.; Di Marzo, V. A gradient of 2-arachonoylglycerol regulates mouse epididymal sperm start-up. Biol.Reprod. 2010, 82, 451–458. [Google Scholar]
- Maccarrone, M.; Barboni, B.; Paradisi, A.; Bernabo, N.; Gasperi, V.; Pistilli, M.G.; Fezza, F.; Lucidi, P.; Mattioli, M. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capactitation and acrosome reaction. J. Cell Sci. 2005, 118, 4393–4404. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, F.; Barbonetti, A.; Vassallo, M.R.; Rapino, C.; Antonangelo, C.; Pasquariello, N.; Catanzaro, G.; Barboni, B.; Maccarrone, M. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 2009, 150, 4692–4700. [Google Scholar]
- Rossato, M.; Ion Popa, F.; Ferigo, M.; Clari, G.; Foresta, C. Human sperm express cannabinoid receptor CB1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J. Clin. Endocrinol. Metab. 2005, 90, 984–991. [Google Scholar] [PubMed]
- Wang, H.; Xie, H.; Guo, Y.; Zhang, H.; Takahashi, T.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Cravatt, B.F.; Dey, S.K. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Invest. 2006, 116, 2122–2131. [Google Scholar]
- Schuel, H.; Burkman, L.J. A tale of two cells: Endocannabinoid-signaling regulates functions of neurons and sperm. Biol. Reprod. 2005, 73, 1078–1086. [Google Scholar]
- Meizel, S. The sperm, a neuron with a tail:’neuronal’ receptors in mammalian sperm. Biol. Rev. 2004, 79, 713–732. [Google Scholar]
- Bray, C.; Son, J.-H.; Meizel, S. A nicotinic acetylcholine receptor is involved in the acrosome reaction of human sperm initiated by recombinant human ZP3. Biol. Reprod. 2002, 67, 782–788. [Google Scholar]
- Bray, C.; Son, J.-H.; Kumar, P.; Harris, J.D.; Meizel, S. A role for the human sperm glycine receptor/Cl- channel in the acrosome reaction initiated by recombinant human ZP3. Biol. Reprod. 2002, 66, 91–97. [Google Scholar]
- Wang, H.; Matsumoto, H.; Guo, Y.; Paria, B.C.; Roberts, R.L.; Dey, S.K. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 14914–14919. [Google Scholar]
- Paria, B.C.; Song, H.; Wang, X.; Schmid, P.C.; Krebsbach, R.J.; Schmid, H.H.; Bonner, T.I.; Zimmer, A.; Dey, S.K. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J. Biol. Chem. 2001, 276, 20523–20528. [Google Scholar]
- Maccarrone, M.; DeFelici, M.; Klinger, F.G.; Battista, N.; Fezza, F.; Dainese, E.; Siracusa, G.; Finazzi-Agro, A. Mouse blastocysts release a lipid which activates anandamide hydrolase in intact uterus. Mol. Hum. Reprod. 2004, 10, 215–221. [Google Scholar]
- Lazzarin, N.; Valensise, H.; Bari, M.; Ubaldi, F.; Battista, N.; Finazzi-Agro, A.; Maccarrone, M. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol. Endocrinol. 2004, 18, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Phillips, J.A., 3rd; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.; Simon, C.; Dey, S.K.; Critchley, H.O. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS One 2008, 3, e3969. [Google Scholar] [PubMed]
- Maccarrone, M. Endocannabinoids: Friends and foes of reproduction. Prog. Lipid Res. 2009, 48, 344–354. [Google Scholar]
- Battista, N.; Bari, M.; Rapino, C.; Trasatti, F.; D'Agostino, A.; Maccarrone, M. Regulation of female fertility by the endocannabinoid system. Hum. Fertil. 2007, 10, 207–216. [Google Scholar]
- Wang, H.; Xie, H.; Sun, X.; Kingsley, P.J.; Marnett, L.J.; Cravatt, B.F.; Dey, S.K. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins Other Lipid Mediat. 2007, 83, 62–74. [Google Scholar]
- Paria, B.C.; Lim, H.; Wang, X.N.; Liehr, J.; Das, S.K.; Dey, S.K. Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology 1998, 139, 5235–5246. [Google Scholar]
- Guo, Y.; Wang, H.; Okamoto, Y.; Ueda, N.; Kingsley, P.J.; Marnett, L.J.; Schmid, H.H.; Das, S.K.; Dey, S.K. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. J. Biol. Chem. 2005, 280, 23429–23432. [Google Scholar] [PubMed]
- Schmid, P.C.; Paria, B.C.; Krebsbach, R.J.; Schmid, H.H.; Dey, S.K. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 1997, 94, 4188–4192. [Google Scholar]
- Maccarrone, M.; DeFelici, M.; Klinger, F.G.; Battista, N.; Fezza, F.; Dainese, E.; Siracusa, G.; Finazzi-Agro, A. Mouse blastocysts release a lipid which activates anandamide hydrolase in intact uterus. Mol. Hum. Reprod. 2004, 10, 215–221. [Google Scholar]
- Wang, J.; Paria, B.C.; Dey, S.K.; Armant, D.R. Stage-specific excitation of cannabinoid receptor exhibits differential effects on mouse embryonic development. Biol. Reprod. 1999, 60, 839–844. [Google Scholar] [PubMed]
- Yang, Z.M.; Paria, B.C.; Dey, S.K. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol. Reprod. 1996, 55, 756–761. [Google Scholar]
- Turco, M.Y.; Matsukawa, K.; Czernik, M.; Gasperi, V.; Battista, N.; Della Salda, L.; Scapolo, P.A.; Loi, P.; Maccarrone, M.; Ptak, G. High levels of anandamide, an endogenous cannabinoid, block the growth of sheep preimplantation embryos by inducing apoptosis and reversible arrest of cell proliferation. Hum. Reprod. 2008, 23, 2331–2338. [Google Scholar] [PubMed]
- Paria, B.C.; Dey, S.K. Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem. Phys Lipids 2000, 108, 211–220. [Google Scholar]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar]
- Geber, W.F.; Schramm, L.C. Effect of marihuana extract on fetal hamsters and rabbits. Toxicol. Appl. Pharmacol. 1969, 14, 276–282. [Google Scholar]
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agro, A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J. Immun. 2001, 166, 7183–7189. [Google Scholar]
- Maccarrone, M.; Bari, M.; Di Rienzo, M.; Finazzi-Agro, A.; Rossi, A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J. Biol. Chem. 2003, 278, 32726–32732. [Google Scholar] [PubMed]
- Maccarrone, M.; Bisogno, T.; Valensise, H.; Lazzarin, N.; Fezza, F.; Manna, C.; Di Marzo, V.; Finazzi-Agro, A. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol. Hum. Reprod. 2002, 8, 188–195. [Google Scholar]
- Habayeb, O.M.; Taylor, A.H.; Finney, M.; Evans, M.D.; Konje, J.C. Plasma anandamide concentration and pregnancy outcome in women with threatened miscarriage. JAMA 2008, 299, 1135–1136. [Google Scholar]
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agro, A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 2000, 355, 1326–1329. [Google Scholar]
- Trabucco, E.; Acone, G.; Marenna, A.; Pierantoni, R.; Cacciola, G.; Chioccarelli, T.; Mackie, K.; Fasano, S.; Colacurci, N.; Meccariello, R. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta 2009, 30, 516–522. [Google Scholar]
- Habayeb, O.M.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology 2008, 149, 5052–5060. [Google Scholar]
- Helliwell, R.J.; Chamley, L.W.; Blake-Palmer, K.; Mitchell, M.D.; Wu, J.; Kearn, C.S.; Glass, M. Characterization of the endocannabinoid system in early human pregnancy. J. Clin. Endocrinol. Metab. 2004, 89, 5168–5174. [Google Scholar]
- Maccarrone, M.; Finazzi-Agro, A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003, 10, 946–955. [Google Scholar]
- Habayeb, O.M.; Taylor, A.H.; Evans, M.D.; Cooke, M.S.; Taylor, D.J.; Bell, S.C.; Konje, J.C. Plasma levels of the endocannabinoid anandamide in women-a potential role in pregnancy maintenance and labor? J. Clin. Endocrinol. Metab. 2004, 89, 5482–5487. [Google Scholar] [PubMed]
- Fonseca, B. M.; Correia-da-Silva, G.; Taylor, A. H.; Lam, P. M. W.; Marczylo, T. H.; Konje, J. C.; Bell, S. C.; Teixeira, N. A. N-Acylethanolamine levels and expression of their metabolizing enzymes during pregnancy. Endocrinology 2010, 151, 3965–3974. [Google Scholar] [PubMed]
- Piccini, M. P. T-Cell Cytokines in Pregnancy. Am. J. Reprod. Immunol. 2002, 47, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Piccini, M.P.; Beloni, L.; Livi, C.; Maggi, E.; Scarselli, G.; Romagnani, S. Defective production of both leukaemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998, 4, 1020–1024. [Google Scholar]
- Tapia, A.; Salamonsen, L.A.; Manuelpillai, U.; Dimitriadis, E. Leukaemia inhibitory factor promotes human first trimester extravillous trophoblast adhesion to extracellular matrix and secretion of tissue inhibitor metalloproteinases-1 and -2. Hum. Reprod. 2008, 23, 1724–1732. [Google Scholar]
- Pestonjamasp, V.K.; Burstein, S.H. Anandamide synthesis is induced by arachidonate mobilizing agonists in cells of the immune system. Biochim. Biophys. Acta 1998, 1394, 249–260. [Google Scholar] [PubMed]
- Bisogno, T.; Maurelli, S.; Melck, D.; De Petrocellis, L.; Di Marzo, V. Biosynthesis, uptake and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997, 272, 3315–3323. [Google Scholar]
- Di Marzo, V.; De Petrocellis, L.; Sepe, N.; Buono, A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem. J. 1996, 316, 977–984. [Google Scholar]
- Smith, S.R.; Terminelli, C.; Denhardt, G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharm. Exp. Ther. 2000, 293, 136–150. [Google Scholar]
- Newton, C.A.; Klein, T.W.; Friedman, H. Secondary immunity to Legionella Pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect. Immun. 1994, 62, 4015–4020. [Google Scholar]
- Maccarrone, M.; De Petrocellis, L.; Bari, M.; Fezza, F.; Salvati, S.; Di Marzo, V.; Finazzi-Agro, A. Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch. Biochem. Biophys. 2001, 393, 321–328. [Google Scholar]
- Ross, R.A.; Brockie, H.C.; Pertwee, R.G. Inhibition of nitric oxide production in RAW264. 7 macrophages by cannabinoids and palmitoylethanolamide. Eur. J. Pharm. 2000, 81, 715–723. [Google Scholar]
- Ravinet Trillou, C.; Arnone, M.; Delgorge, C.; Gonalons, N.; Keane, P.; Maffrand, J.P.; Soubrie, P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet induced obese mice. Am. J. Physiol. 2003, 284, 345–353. [Google Scholar]
- Arnone, M.; Maruani, J.; Chaperon, F.; Thiebot, M.H.; Poncelet, M.; Soubrie, P.; Le Fur, G. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology 1997, 132, 104–106. [Google Scholar]
- Simiand, J.; Keane, M.; Keane, P.E.; Soubrie, P. SR141716 a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol. 1998, 9, 179–181. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bambang, K.N.; Karasu, T.; Gebeh, A.; Taylor, A.H.; Marczylo, T.H.; Lam, P.; Willets, J.M.; Konje, J.C. From Fertilisation to Implantation in Mammalian Pregnancy—Modulation of Early Human Reproduction by the Endocannabinoid System. Pharmaceuticals 2010, 3, 2910-2929. https://doi.org/10.3390/ph3092910
Bambang KN, Karasu T, Gebeh A, Taylor AH, Marczylo TH, Lam P, Willets JM, Konje JC. From Fertilisation to Implantation in Mammalian Pregnancy—Modulation of Early Human Reproduction by the Endocannabinoid System. Pharmaceuticals. 2010; 3(9):2910-2929. https://doi.org/10.3390/ph3092910
Chicago/Turabian StyleBambang, Katerina N., Tulay Karasu, Alpha Gebeh, Anthony H. Taylor, Timothy H. Marczylo, Patricia Lam, Jonathon M. Willets, and Justin C. Konje. 2010. "From Fertilisation to Implantation in Mammalian Pregnancy—Modulation of Early Human Reproduction by the Endocannabinoid System" Pharmaceuticals 3, no. 9: 2910-2929. https://doi.org/10.3390/ph3092910
APA StyleBambang, K. N., Karasu, T., Gebeh, A., Taylor, A. H., Marczylo, T. H., Lam, P., Willets, J. M., & Konje, J. C. (2010). From Fertilisation to Implantation in Mammalian Pregnancy—Modulation of Early Human Reproduction by the Endocannabinoid System. Pharmaceuticals, 3(9), 2910-2929. https://doi.org/10.3390/ph3092910



