Dendritic Cell Regulation by Cannabinoid-Based Drugs
Abstract
:1. Introduction
2. The Endocannabinoid System in Immunity and Inflammation
3. Cannabinoids and Modulation of Dendritic Cell Function
| Source | Functions affected/receptors involved | References |
|---|---|---|
| In vivo | ||
| Tissue resident DC, murine | 2-AG-induced migration from periphery to draining lymph nodes, CB2-mediated. | [57] |
| Spleen DC, murine | THC induced apoptosis, CB1- and CB2-mediated. | [58] |
| Ex vivo | ||
| Spleen DC, murine | Reduced MHC-II expression and T cell stimulatory capacity, CB1-mediated. | [24] |
| Spleen DC, murine | Increased cell-mediated immunity in response to low levels of anandamide. | [59] |
| In vitro | ||
| Bone marrow-derived DC, murine | Reduced MHC-II expression and T cell stimulatory capacity, CB1-mediated. | [24] |
| Bone marrow-derived DC, murine | 2-AG-induced migration, CB2-mediated. | [57] |
| Monocyte derived DC, human | T helper cell type 2-polarized response, CB2-mediated. | [60] |
| Bone marrow-derived DC, murine | THC stimulation induces T helper cell type 1-polarized response and inhibits upregulation of CD86, CD4, and MHC class II. | [27] |
| Bone marrow-derived DC, murine | DC capacity to kill intracellular Legionella pneumophila is unaffected by THC-stimulation. | [27] |
| Bone marrow-derived DC, murine | THC induced apoptosis, CB1- and CB2-mediated. | [58] |
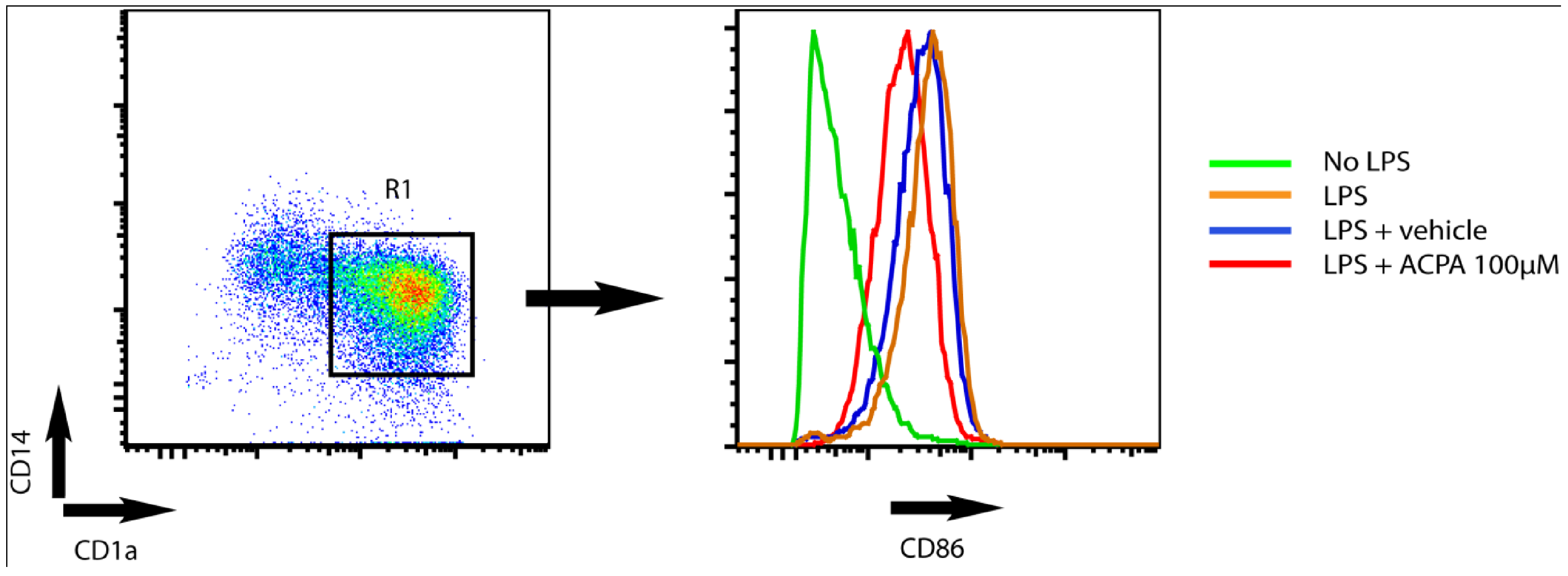
4. Potassium Channel Function as a Target for Cannabinoid Receptor-mediated Modulation of Dendritic Cell Function
5. Prospects of Using Cannabinoid-Based Drugs in Immune Therapy
| Disorder | Cannabinoid | Action | Reference |
|---|---|---|---|
| Lung cancer | THC | Inhibition of tumor growth, metastasis and vascularisation of A549 xenografts with possible involvement of attenuated EGF downstream signalling | [78] |
| Glioma | WIN 55,212-2, THC | CB receptor-mediated apoptosis by accumulation of ceramide and Raf1/ERK activation | [79] |
| Breast cancer | Cannabidiol (CBD) | Reduced tumor aggressiveness by decreasing Id-1 expression | [80] |
| Pancreatic cancer | THC | Iincreased apoptosis by CB2-mediated ceramide-dependent upregulation of stress protein p8 | [81] |
| Melanoma | WIN 55,212-2, JWH-133 | Inhibition of cell growth, partially owing to cell cycle arrest in G1-S phase by inhibition of Akt | [82] |
| ALS | Cannabinol (CBN) | Delays disease onset, but no affect on survival in SOD1 mouse model of ALS | [83] |
| Prostate cancer | WIN-55,212-2 | Inhibition of cell growth (cell cycle arrest in G0-G1 phase) and induction of apoptosis by ERK1/2 activation | [84] |
| Colitis/IBD (chemically induced) | HU-210 | Decrease colonic inflammation mediated through CB1receptor | [85] |
| Cancer | HU-331 | CB1/CB2-independent inhibition of topoisomerase II | [86] |
| Myocardial I/R injury | WIN 55,212-2 | CB2-mediated protection, parallel with lower levels of IL-1b and CXCL8 | [35] |
| Breast cancer | JWH-133, WIN 55,212-2 | CB1/CB2 mediated inhibition of cell proliferation and migration | [87] |
| Lymphoma/leukemia | HU-210, THC, JHW-015 | Partial CB2-mediated apoptosis | [88] |
6. Concluding Remarks
Abbreviations
| ACPA | arachidonylcyclopropylamide; |
| AEA | arachidonylethanolamide; |
| 7AAD | 7-aminoactinomycin; |
| CB1 | cannabinoid receptor 1; |
| CB2 | cannabinoid receptor 2; |
| COX-2 | cyclooxygenase-2; |
| CXCL | CXC-chemokine ligand; |
| CGRP | calcitonin gene-related peptide; |
| DC | dendritic cells; |
| FAAH | fatty acid amide hydrolase; |
| IL-12 | interleukine-12; |
| KV-channel | voltage-gated potassium channel; |
| LPS | lipopolysaccharide; |
| MHC-II | MHC class II molecules; |
| NGS | normal goat serum; |
| PPARγ | peroxisome-proliferative-activated receptor-γ ; |
| PTX | pertussis toxin; |
| RT | room temperature; |
| SP | substance P; |
| TEA | tetraethyl-ammonium; |
| TNF | tumour-necrosis factor; |
| THC | delta-9-tetrahydrocannabinol; |
| VEGF | vascular endothelium growth factor. |
References
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [Green Version]
- McAllister, S.D.; Glass, M. CB(1) and CB(2) receptor-mediated signalling: A focus on endocannabinoids. Prostaglandins Leukot Essent Fatty Acids 2002, 66, 161–171. [Google Scholar] [Green Version]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids 2002, 66, 101–121. [Google Scholar] [Green Version]
- Blednov, Y.A.; Stoffel, M.; Alva, H.; Harris, R.A. A pervasive mechanism for analgesia: Activation of GIRK2 channels. Proc. Natl. Acad. Sci. USA 2003, 100, 277–282. [Google Scholar] [Green Version]
- Guo, J.; Ikeda, S.R. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 2004, 65, 665–674. [Google Scholar] [Green Version]
- Khasabova, I.A.; Harding-Rose, C.; Simone, D.A.; Seybold, V.S. Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons. J. Neurosci. 2004, 24, 1744–1753. [Google Scholar] [Green Version]
- Schweitzer, P. Cannabinoids decrease the K(+) M-current in hippocampal CA1 neurons. J. Neurosci. 2000, 20, 51–58. [Google Scholar] [Green Version]
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997, 78, 43–50. [Google Scholar] [Green Version]
- Arevalo-Martin, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 2003, 23, 2511–2516. [Google Scholar] [Green Version]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J. Clin. Invest. 2003, 111, 1231–1240. [Google Scholar] [Green Version]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [Green Version]
- Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483. [Google Scholar] [Green Version]
- Cella, M.; Engering, A.; Pinet, V.; Pieters, J.; Lanzavecchia, A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 1997, 388, 782–787. [Google Scholar] [Green Version]
- Pierre, P.; Turley, S.J.; Gatti, E.; Hull, M.; Meltzer, J.; Mirza, A.; Inaba, K.; Steinman, R.M.; Mellman, I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 1997, 388, 787–792. [Google Scholar] [PubMed][Green Version]
- Shin, J.S.; Ebersold, M.; Pypaert, M.; Delamarre, L.; Hartley, A.; Mellman, I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 2006, 444, 115–118. [Google Scholar] [Green Version]
- Turley, S.J.; Inaba, K.; Garrett, W.S.; Ebersold, M.; Unternaehrer, J.; Steinman, R.M.; Mellman, I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 2000, 288, 522–527. [Google Scholar] [Green Version]
- Wilson, N.S.; El-Sukkari, D.; Villadangos, J.A. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 2004, 103, 2187–2195. [Google Scholar] [Green Version]
- Inaba, K.; Witmer-Pack, M.; Inaba, M.; Hathcock, K.S.; Sakuta, H.; Azuma, M.; Yagita, H.; Okumura, K.; Linsley, P.S.; Ikehara, S.; Muramatsu, S.; Hodes, R.J.; Steinman, R.M. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 1994, 180, 1849–1860. [Google Scholar] [Green Version]
- Larsen, C.P.; Ritchie, S.C.; Pearson, T.C.; Linsley, P.S.; Lowry, R.P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J. Exp. Med. 1992, 176, 1215–1220. [Google Scholar] [Green Version]
- Reis e Sousa, C.; Hieny, S.; Scharton-Kersten, T.; Jankovic, D.; Charest, H.; Germain, R.N.; Sher, A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997, 186, 1819–1829. [Google Scholar] [Green Version]
- Whelan, M.; Harnett, M.M.; Houston, K.M.; Patel, V.; Harnett, W.; Rigley, K.P. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 2000, 164, 6453–6460. [Google Scholar] [Green Version]
- Kapsenberg, M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [Green Version]
- Hackstein, H.; Thomson, A.W. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004, 4, 24–34. [Google Scholar] [Green Version]
- Wacnik, P.W.; Luhr, K.M.; Hill, R.H.; Ljunggren, H.G.; Kristensson, K.; Svensson, M. Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J. Immunol. 2008, 181, 3057–3066. [Google Scholar] [Green Version]
- Matias, I.; Pochard, P.; Orlando, P.; Salzet, M.; Pestel, J.; Di Marzo, V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur. J. Biochem. 2002, 269, 3771–3778. [Google Scholar] [Green Version]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar] [Green Version]
- Lu, T.; Newton, C.; Perkins, I.; Friedman, H.; Klein, T.W. Cannabinoid treatment suppresses the T-helper cell-polarizing function of mouse dendritic cells stimulated with Legionella pneumophila infection. J. Pharmacol. Exp. Ther. 2006, 319, 269–276. [Google Scholar] [PubMed][Green Version]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [Green Version]
- Di Marzo, V.; Bisogno, T.; De Petrocellis, L.; Melck, D.; Orlando, P.; Wagner, J.A.; Kunos, G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur. J. Biochem. 1999, 264, 258–267. [Google Scholar] [Green Version]
- Maccarrone, M.; Bari, M.; Battista, N.; Finazzi-Agro, A. Endocannabinoid degradation, endotoxic shock and inflammation. Curr. Drug Targets Inflamm. Allergy 2002, 1, 53–63. [Google Scholar] [Green Version]
- Liu, J.; Li, H.; Burstein, S.H.; Zurier, R.B.; Chen, J.D. Activation and binding of peroxisome proliferator-activated receptor gamma by synthetic cannabinoid ajulemic acid. Mol. Pharmacol. 2003, 63, 983–992. [Google Scholar] [Green Version]
- Berdyshev, E.V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids 2000, 108, 169–190. [Google Scholar] [Green Version]
- Klein, T.W.; Lane, B.; Newton, C.A.; Friedman, H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol. Med. 2000, 225, 1–8. [Google Scholar] [Green Version]
- Smith, S.R.; Terminelli, C.; Denhardt, G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000, 293, 136–150. [Google Scholar] [Green Version]
- Di Filippo, C.; Rossi, F.; Rossi, S.; D'Amico, M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. J. Leukoc. Biol. 2004, 75, 453–459. [Google Scholar] [Green Version]
- Klein, T.W.; Newton, C.A.; Nakachi, N.; Friedman, H. Delta 9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J. Immunol. 2000, 164, 6461–6466. [Google Scholar] [Green Version]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [Green Version]
- Derocq, J.M.; Jbilo, O.; Bouaboula, M.; Segui, M.; Clere, C.; Casellas, P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. Possible involvement of the CB2 receptor in cell differentiation. J. Biol. Chem. 2000, 275, 15621–15628. [Google Scholar] [PubMed][Green Version]
- Kishimoto, S.; Kobayashi, Y.; Oka, S.; Gokoh, M.; Waku, K.; Sugiura, T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J. Biochem. 2004, 135, 517–524. [Google Scholar] [Green Version]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; Ullrich, O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 2006, 49, 67–79. [Google Scholar] [Green Version]
- Lambrecht, B.N. Immunologists getting nervous: Neuropeptides, dendritic cells and T cell activation. Respir. Res. 2001, 2, 133–138. [Google Scholar] [Green Version]
- Luger, T.A. Neuromediators--a crucial component of the skin immune system. J. Dermatol. Sci. 2002, 30, 87–93. [Google Scholar] [Green Version]
- Downing, J.E.; Miyan, J.A. Neural immunoregulation: Emerging roles for nerves in immune homeostasis and disease. Immunol. Today 2000, 21, 281–289. [Google Scholar] [Green Version]
- Tournier, J.N.; Hellmann, A.Q. Neuro-immune connections: Evidence for a neuro-immunological synapse. Trends Immunol. 2003, 24, 114–115. [Google Scholar] [Green Version]
- Langerhans, P. Über die nerven der menschlichen haut. Virchows Arch. Pathol. Anat. Physiol. 1868, 44, 325–337. [Google Scholar] [Green Version]
- Egan, C.L.; Viglione-Schneck, M.J.; Walsh, L.J.; Green, B.; Trojanowski, J.Q.; Whitaker-Menezes, D.; Murphy, G.F. Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J. Cutan. Pathol. 1998, 25, 20–29. [Google Scholar] [Green Version]
- Gaudillere, A.; Misery, L.; Souchier, C.; Claudy, A.; Schmitt, D. Intimate associations between PGP9.5-positive nerve fibres and Langerhans cells. Br. J. Dermatol. 1996, 135, 343–344. [Google Scholar] [Green Version]
- Hosoi, J.; Murphy, G.F.; Egan, C.L.; Lerner, E.A.; Grabbe, S.; Asahina, A.; Granstein, R.D. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature 1993, 363, 159–163. [Google Scholar] [Green Version]
- Muller, T. Intraepidermal free nerve fiber endings in the hairless skin of the rat as revealed by the zinc iodide-osmium tetroxide technique. Histol. Histopathol. 2000, 15, 493–498. [Google Scholar] [Green Version]
- Markus, A.M.; Kockerling, F.; Neuhuber, W.L. Close anatomical relationships between nerve fibers and MHC class II-expressing dendritic cells in the rat liver and extrahepatic bile duct. Histochem. Cell Biol. 1998, 109, 409–415. [Google Scholar] [Green Version]
- Ahluwalia, J.; Yaqoob, M.; Urban, L.; Bevan, S.; Nagy, I. Activation of capsaicin-sensitive primary sensory neurones induces anandamide production and release. J. Neurochem. 2003, 84, 585–591. [Google Scholar] [Green Version]
- Friedman, M.; Cepero, M.L.; Klein, T.; Friedman, H. Suppressive effect of delta 9-tetrahydrocannabinol in vitro on phagocytosis by murine macrophages. Proc. Soc. Exp. Biol. Med. 1986, 182, 225–228. [Google Scholar] [PubMed][Green Version]
- Coffey, R.G.; Yamamoto, Y.; Snella, E.; Pross, S. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochem. Pharmacol. 1996, 52, 743–751. [Google Scholar] [Green Version]
- McCoy, K.L.; Gainey, D.; Cabral, G.A. delta 9-Tetrahydrocannabinol modulates antigen processing by macrophages. J. Pharmacol. Exp. Ther. 1995, 273, 1216–1223. [Google Scholar] [Green Version]
- Watzl, B.; Scuderi, P.; Watson, R.R. Influence of marijuana components (THC and CBD) on human mononuclear cell cytokine secretion in vitro. Adv. Exp. Med. Biol. 1991, 288, 63–70. [Google Scholar] [Green Version]
- Nakano, Y.; Pross, S.H.; Friedman, H. Modulation of interleukin 2 activity by delta 9-tetrahydrocannabinol after stimulation with concanavalin A, phytohemagglutinin, or anti-CD3 antibody. Proc. Soc. Exp. Biol. Med. 1992, 201, 165–168. [Google Scholar] [Green Version]
- Maestroni, G.J. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004, 18, 1914–1916. [Google Scholar] [Green Version]
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: Novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 2004, 173, 2373–2382. [Google Scholar] [Green Version]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Costa-Pinto, F.A.; Palermo-Neto, J. Anandamide prior to sensitization increases cell-mediated immunity in mice. Int. Immunopharmacol. 2010, 10, 431–439. [Google Scholar] [Green Version]
- Yuan, M.; Kiertscher, S.M.; Cheng, Q.; Zoumalan, R.L.; Tashkin, D.P.; Roth, M.D. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J. Neuroimmunol. 2002, 133, 124–131. [Google Scholar] [Green Version]
- Bouma, G.; Burns, S.; Thrasher, A.J. Impaired T-cell priming in vivo resulting from dysfunction of WASp-deficient dendritic cells. Blood 2007, 110, 4278–4284. [Google Scholar] [Green Version]
- Ramer, R.; Hinz, B. Cyclooxygenase-2 and tissue inhibitor of matrix metalloproteinases-1 confer the antimigratory effect of cannabinoids on human trabecular meshwork cells. Biochem. Pharmacol. 2010. [Google Scholar]
- Fischer, H.G.; Eder, C. Voltage-gated K+ currents of mouse dendritic cells. FEBS Lett. 1995, 373, 127–130. [Google Scholar] [Green Version]
- George Chandy, K.; Wulff, H.; Beeton, C.; Pennington, M.; Gutman, G.A.; Cahalan, M.D. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004, 25, 280–289. [Google Scholar] [Green Version]
- Panyi, G.; Gaspar, R.; Krasznai, Z.; ter Horst, J.J.; Ameloot, M.; Aszalos, A.; Steels, P.; Damjanovich, S. Immunosuppressors inhibit voltage-gated potassium channels in human peripheral blood lymphocytes. Biochem. Biophys. Res. Commun. 1996, 221, 254–258. [Google Scholar] [Green Version]
- Cahalan, M.D.; Chandy, K.G. Ion channels in the immune system as targets for immunosuppression. Curr. Opin. Biotechnol. 1997, 8, 749–756. [Google Scholar] [Green Version]
- Mullen, K.M.; Rozycka, M.; Rus, H.; Hu, L.; Cudrici, C.; Zafranskaia, E.; Pennington, M.W.; Johns, D.C.; Judge, S.I.; Calabresi, P.A. Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann. Neurol. 2006, 60, 118–127. [Google Scholar] [Green Version]
- Conforti, L.; Petrovic, M.; Mohammad, D.; Lee, S.; Ma, Q.; Barone, S.; Filipovich, A.H. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: A possible role in T cell proliferation. J. Immunol. 2003, 170, 695–702. [Google Scholar] [PubMed][Green Version]
- Shah, K.; Tom Blake, J.; Huang, C.; Fischer, P.; Koo, G.C. Immunosuppressive effects of a Kv1.3 inhibitor. Cell Immunol. 2003, 221, 100–106. [Google Scholar] [Green Version]
- Garcia-Arencibia, M.; Gonzalez, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [Green Version]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [Green Version]
- Centonze, D.; Finazzi-Agro, A.; Bernardi, G.; Maccarrone, M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol. Sci. 2007, 28, 180–187. [Google Scholar] [Green Version]
- Baker, D.; Pryce, G. The therapeutic potential of cannabis in multiple sclerosis. Expert Opin. Investig. Drugs 2003, 12, 561–567. [Google Scholar] [Green Version]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [Green Version]
- Kunos, G.; Pacher, P. Cannabinoids cool the intestine. Nat. Med. 2004, 10, 678–679. [Google Scholar] [Green Version]
- Mechoulam, R.; Panikashvili, D.; Shohami, E. Cannabinoids and brain injury: Therapeutic implications. Trends Mol. Med. 2002, 8, 58–61. [Google Scholar] [Green Version]
- Baldwin, G.C.; Tashkin, D.P.; Buckley, D.M.; Park, A.N.; Dubinett, S.M.; Roth, M.D. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir. Crit. Care Med. 1997, 156, 1606–1613. [Google Scholar] [Green Version]
- Preet, A.; Ganju, R.K.; Groopman, J.E. Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [Green Version]
- Galve-Roperh, I.; Sanchez, C.; Cortes, M.L.; Gomez del Pulgar, T.; Izquierdo, M.; Guzman, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [Green Version]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [Green Version]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzman, M.; Velasco, G.; Iovanna, J.L. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [Green Version]
- Blazquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernandez-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzman, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [Green Version]
- Weydt, P.; Hong, S.; Witting, A.; Moller, T.; Stella, N.; Kliot, M. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph. Lateral Scler. 2005, 6, 182–184. [Google Scholar] [Green Version]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [Green Version]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Invest. 2004, 113, 1202–1209. [Google Scholar] [Green Version]
- Kogan, N.M.; Schlesinger, M.; Priel, E.; Rabinowitz, R.; Berenshtein, E.; Chevion, M.; Mechoulam, R. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 2007, 6, 173–183. [Google Scholar] [Green Version]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [Green Version]
- McKallip, R.J.; Lombard, C.; Fisher, M.; Martin, B.R.; Ryu, S.; Grant, S.; Nagarkatti, P.S.; Nagarkatti, M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002, 100, 627–634. [Google Scholar] [Green Version]
- Berdyshev, E.V.; Boichot, E.; Germain, N.; Allain, N.; Anger, J.P.; Lagente, V. Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 1997, 330, 231–240. [Google Scholar] [Green Version]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [Green Version]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [Green Version]
- Volpert, O.V.; Fong, T.; Koch, A.E.; Peterson, J.D.; Waltenbaugh, C.; Tepper, R.I.; Bouck, N.P. Inhibition of angiogenesis by interleukin 4. J. Exp. Med. 1998, 188, 1039–1046. [Google Scholar] [Green Version]
- Matsuda, A.; Fukuda, S.; Matsumoto, K.; Saito, H. Th1/Th2 cytokines reciprocally regulate in vitro pulmonary angiogenesis via CXC chemokine synthesis. Am. J. Respir. Cell. Mol. Biol. 2008, 38, 168–175. [Google Scholar] [Green Version]
- Sun, H.; Chung, W.C.; Ryu, S.H.; Ju, Z.; Tran, H.T.; Kim, E.; Kurie, J.M.; Koo, J.S. Cyclic AMP-responsive element binding protein- and nuclear factor-kappaB-regulated CXC chemokine gene expression in lung carcinogenesis. Cancer Prev. Res. (Phila Pa) 2008, 1, 316–328. [Google Scholar] [CrossRef]
- Lee, M.; Yang, K.H.; Kaminski, N.E. Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J. Pharmacol. Exp. Ther. 1995, 275, 529–536. [Google Scholar]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Svensson, M.; Chen, P.; Hammarfjord, O. Dendritic Cell Regulation by Cannabinoid-Based Drugs. Pharmaceuticals 2010, 3, 2733-2750. https://doi.org/10.3390/ph3082733
Svensson M, Chen P, Hammarfjord O. Dendritic Cell Regulation by Cannabinoid-Based Drugs. Pharmaceuticals. 2010; 3(8):2733-2750. https://doi.org/10.3390/ph3082733
Chicago/Turabian StyleSvensson, Mattias, Puran Chen, and Oscar Hammarfjord. 2010. "Dendritic Cell Regulation by Cannabinoid-Based Drugs" Pharmaceuticals 3, no. 8: 2733-2750. https://doi.org/10.3390/ph3082733
APA StyleSvensson, M., Chen, P., & Hammarfjord, O. (2010). Dendritic Cell Regulation by Cannabinoid-Based Drugs. Pharmaceuticals, 3(8), 2733-2750. https://doi.org/10.3390/ph3082733




