Abstract
Mitochondrial dysfunction occurs early in the progression of Alzheimer’s disease. Amyloid-β peptide has deleterious effects on mitochondrial function and contributes to energy failure, respiratory chain impairment, neuronal apoptosis, and generation of reactive oxygen species in Alzheimer’s disease. The mechanisms underlying amyloid-β induced mitochondrial stress remain unclear. Emerging evidence indicates that mitochondrial permeability transition pore is important for maintenance of mitochondrial and neuronal function in aging and neurodegenerative disease. Cyclophilin D (Cyp D) plays a central role in opening mitochondrial permeability transition pore, ultimately leading to cell death. Interaction of amyloid-β with cyclophilin D triggers or enhances the formation of mitochondrial permeability transition pores, consequently exacerbating mitochondrial and neuronal dysfunction, as shown by decreased mitochondrial membrane potential, impaired mitochondrial respiration function, and increased oxidative stress and cytochrome c release. Blockade of cyclophilin D by genetic abrogation or pharmacologic inhibition protects mitochondria and neurons from amyloid-β induced toxicity, suggesting that cyclophilin D dependent mitochondrial transition pore is a therapeutic target for Alzheimer’s disease.
1. Introduction
Amyloid beta (Aβ), a major component of amyloid plaque, is a neurotoxic peptide, the accumulation of which leads to neuronal degeneration relevant to the pathogenesis of Alzheimer’s disease (AD) [1,2,3,4,5]. Aβ accumulates in the extracellular and intracellular compartments, including mitochondria. Notably, recent studies from several independent groups including our laboratory demonstrate the accumulation of Aβ in mitochondria of brains from AD patients and AD mouse models [1,6,7,8,9,10,11,12]. It is known that progressive accumulation of mitochondrial Aβ is significantly related to the mitochondrial and neuronal dysfunction in an Aβ rich environment [1,6,7,11,12,13,14]. Predominant mitochondrial pathological changes in AD include mitochondrial membrane potential dissipation [15,16,17], respiration defect [6,7,18], oxidative stress [1,14,19,20,21], Aβ accumulation in mitochondria [1,5,6,10,11], impaired calcium buffering capacity [22,23,24,25], altered mitochondrial dynamics and trafficking [26,27,28], mtDNA mutation [29,30,31] and mitochondrial permeability transition [7,13,32]. Mitochondria are essential for provision of energy by oxidative phosphorylation; this organelle also modulates intra-neuronal calcium homeostasis necessary to sustain neuronal function and survival. Dysregulation of mitochondrial function leads to synaptic stress, disruption of synaptic transmission, apoptosis and ultimately neuronal death [6,15,33,34,35]. Thus, it is highly important to unravel the mechanism(s) of Aβ-associated mitochondrial alterations to enhance our understanding of the pathophysiological process of AD.
Recent studies emphasize that mitochondrial permeability transition pore (mPTP) is involved in Aβ induced mitochondrial perturbation [7,13,25,32,36,37,38]. The formation of mPTP is closely related to Aβ superimposition and perturbation of mitochondrial structure and function. Inhibition of mPTP formation in an AD animal model and in Aβ-insulted cells results in enhanced protection of neurons from Aβ toxicity and oxidative stress. Here, we review the role of mPTP in mitochondrial pathology relevant to the pathogenesis of AD, particularly related to the involvement of Cyclophilin D (Cyp D) in mPTP.
2. mPTP and Alzheimer’s Disease
Mitochondrial permeability transition, or MPT, is an increase in permeability of the mitochondrial membranes to molecules of less than 1,500 Daltons in molecular weight. MPT results from opening of mitochondrial permeability transition pores, known as MPT pores or mPTP. mPTP is a protein pore that is formed in mitochondrial membranes under certain pathological conditions such as oxidative stress, ischemia, traumatic brain injury and stroke. Induction of the permeability transition pore can lead to mitochondrial swelling and cell death. The deleterious impact of mitochondrial permeability transition on mitochondrial function has long been proposed [39,40,41].
Mitochondria are two-membrane encapsulated organelles with strict regulation of the uptake and release of substances. Disruptions in this regulation lead to mitochondrial and cellular perturbation. For example, the release of cytochrome c from mitochondria triggers a signal transduction cascade and apoptosis. mPTP is one among several factors that interfere with the integrity of mitochondrial membrane. The formation of mPTP in a mitochondrial membrane opens a nonselective portal that results in abnormal exchange of solutes and molecules > 1,500 Daltons between mitochondria and cytoplasm [42].
Though the exact structure of the mPTP is still unknown, it is postulated that several proteins come together to form the pore, including the outer membrane voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT) located in the mitochondrial inner membrane, and cyp D residing in mitochondrial matrix [43,44,45,46]. A recent study suggests that phosphate carrier (PiC) in mitochondrial inner membrane is also a possible component of mPTP [47]. It is known that formation of mPTP relies on the translocation of cyp D to inner mitochondrial membrane and the intra- mitochondrial perturbations of calcium, phosphate and oxidative stress are strong inducers of the cyp D translocation [39,42,47,48,49]. Mitochondria undergoing mPTP show dissipated membrane potential, perturbed mitochondrial respiration chain, decreased ATP production, increased free radical generation, and disruption of calcium modulation [7,40,50]. Calcium effluxes from mitochondria while cytoplasm solutes flow into mitochondria; thereby causing mitochondrial swelling that in turn leads to ruptures in mitochondrial membrane. Importantly, pre-apoptotic molecules such as cytochrome c are released from the mPTP afflicted mitochondria through these ruptures, which then trigger apoptosis [43,50]. Obviously, mPTP formation is a detrimental process that significantly contributes to mitochondrial and cellular malfunction.
Involvement of mPTP in neurodegeneration has been reported in neurodegenerative diseases, including AD [7], ALS (amyotrophic lateral sclerosis) [51,52], HD (Huntington’s disease) [53] and PD (Parkinson’s disease) [54,55], as evidenced by increased CypD expression, decreased mitochondrial calcium handling capacity and mitochondrial oxidative stress in disease-affected brain regions. In our published studies, we demonstrated that CypD levels were elevated in mitochondria isolated from the hippocampus and temporal pole of AD patients. Increased Cyp D expression is predominantly localized in neurons in these specific areas of AD patients [7]. Given the positive correlation of Cyp D expression to mPTP opening [7,43,52,56], neurons with increased expression of CypD in AD-affected brain regions would be more susceptible to mPTP formation and the resultant consequences. Similarly, AD mice overexpressing amyloid precursor protein (APP) and Aβ (APP mice) demonstrated up-regulation of CypD expression in cortical mitochondria. As expected, cortical mitochondria containing Aβ undergo increased mitochondrial swelling in the presence of calcium. In addition, APP mice demonstrate increased CypD translocation to mitochondrial inner membrane and decreased mitochondrial calcium buffering capacity, suggesting that mitochondria enriched for Aβ environment are susceptible to mPTP formation, which is consistent with increased CypD expression also seen in this strain [7,13].
Transgenic AD mouse models show age-dependent accumulation of cerebral/mitochondrial Aβ as well as neuronal and mitochondrial stress. In APP mice (J-20 line), Aβ accumulation in the brain occurs by 4-5 months and progresses with age. By the age of 10-12 months, there are plentiful amyloid deposits in the brain [57,58]. Consistent with this observation, impaired mPTP function and calcium buffering capacity correlate with age-related Aβ accumulation in APP mouse brain and mitochondria. Cortical mitochondria from transgenic and nonTg mice showed swelling in response to Ca2+, although APP mitochondria show greater swelling compared to nonTg mitochondria at the ages of 12-24 months. Cortical mitochondria of both nonTg and APP mice exhibited an age-dependent increased swelling in response to Ca2+. Similarly, brain mitochondria isolated from APP mice demonstrated an age-related mitochondrial respiration defect, mitochondrial oxidative stress, and decreased ATP production [7,13]. Another study showed that the inhibition of ANT substantially attenuated apoptosis and autophagy in a mouse model for cerebral amyloid angiopathy, lending further credence to the involvement of mPTP in AD [59]. Taken together, these data indicate that mitochondrial permeability transition pore is sensitized in the Aβ milieu.
3. The Interplay of Aβ and mPTP
Aβ has been shown to directly perturb mPTP function. Moreira and colleagues demonstrated that Aβ directly induces mitochondrial swelling, cytochrome c release and mitochondrial membrane potential decrease in isolated brain mitochondria [37,60]. Administration of Aβ 25-35 triggers mPTP formation accompanying mitochondrial oxidative stress [38]. We observed in isolated brain, that the addition of Aβ to mitochondria in the presence of mPTP inducers (e.g. phosphate) enhances mitochondrial swelling in a dose-dependent manner [7]. It has been demonstrated that Aβ treatment significantly sequestrates Cyp D translocation to mitochondrial inner membrane and reduces mitochondrial calcium buffering capacity. These data indicate that Aβ is responsible for mPTP formation.
An indirect effect of Aβ on mPTP is due to the ability of Aβ to elevate intra-cellular calcium and free radical levels. Aβ, a peptide cleaved from its precursor protein (APP), causes severe intra-neuronal free radical injury and calcium dysregulation, leading to accelerated neuronal damage [22,61,62,63]. Calcium and free radicals are strong inducers of mPTP and conversely mPTP formation further exacerbates calcium perturbation and oxidative stress. Thus, it is proposed that deregulated neuronal calcium metabolism and accumulation/production of reactive oxygen species (ROS) are possible mechanisms underlying Aβ-induced mPTP formation. Aβ treatment in cells or primary cultured neurons induces oxidative stress, calcium perturbation, increased cobalt quenching of intra-mitochondrial calcein intensity and mitochondrial cytochrome c release, suggesting the involvement of mPTP formation in an Aβ-induced disturbance of calcium and free radical production [60,64,65,66,67,68,69,70]. Thus, Aβ mediates ROS accumulation and stimulates intracellular and intra-mitochondrial calcium accumulation, thereby triggering the formation of mPTP, which, in turn, leads to further mitochondrial calcium efflux and free radical generation from mitochondria.
Mechanistically, we know that Aβ enhances translocation of CypD to mitochondrial inner membrane to trigger mPTP formation and forming Aβ-Cyp D complex. Using co-immunoprecipitation of Aβ and Cyp D, Aβ-Cyp D complex was found in mitochondrial fractions from brains of AD subjects and APP mice as well as in neurons and isolated brain mitochondria exposed to Aβ. These findings indicate the presence of Aβ-CypD interaction in vivo in brain mitochondria. Using surface plasmon resonance (SPR), different species of Aβ, including monomeric and oligomeric Aβ, were found to have high affinity for binding to Cyp D in vitro, confirming interaction of Aβ with Cyp D [7,13]. In addition, a recent report using molecular docking experiments postulates that Aβ binds with ANT [71]. However, we are not aware of any conclusive report regarding interaction of Aβ with ANT.
Although Aβ or oxidative stress could directly or indirectly affect mitochondrial function, such as mPTP formation, enhancement of mPTP formation by Aβ might be due to synergistic action of the two. Given that mPTP is critical for mitochondrial pathology and neuronal dysfunction in the pathogenesis of AD, blocking or limiting mPTP formation holds potential as a therapeutic strategy for AD.
4. Blockage of mPTP Attenuates Aβ-Mediated Neuronal and Mitochondrial Malfunction
Several studies have shown that the blockage of mPTP by either genetic depletion of the mPTP key component, Cyp D or through use of the Cyp D or VDAC inhibitors protects neurons against oxidative stress- or Aβ-induced injury [7,72,73,74]. Genetic depletion of Cyp D decreases mitochondrial swelling induced by calcium. Cyp D deficient cells show less oxidative stress and apoptosis and maintain mitochondrial membrane potential even in the presence of stress inducers [7,43,50]. Further, Cyp D depletion attenuates cardiac ischemia and reperfusion injuries in mice [7,75,76] and ameliorates axonal degeneration and movement disorders in a multiple sclerosis (MS) mouse model [77]. To investigate the protective effect of blockading mPTP by genetic depletion of Cyp D in an Aβ milieu, we generated Cyp D deficient APP mice by crossing APP/Aβ overexpressing mice (APP mice) to Cyp D-deficient mice and then investigated the effect of Cyp D depletion on Aβ-induced toxicity. Cyp D-deficient APP mice preserve mitochondrial function including mitochondrial cytochrome c oxidase activity, mitochondrial respiration control ratio and mitochondrial ATP production. Furthermore, the protective effects of CypD deficiency were observed even in aged AD mice (22–24 months), suggesting that abrogation of CypD results in persistent life-long protection against Aβ toxicity in an Alzheimer’s disease mouse model [7,13]. Cyp D depletion also results in improved synaptic function and spatial learning memory, even in aged 22-24-month-old APP mice.
Pharmaceutical inhibition of Cyp D is another approach to inhibit mPTP formation. There are several known Cyp D inhibitors: cyclosporin A, sanglifehrin A, FK506 and FK1706. Administration of these inhibitors results in significant protection against mPTP-associated mitochondrial pathology in several animal models of neurodegenerative diseases, as follows. Cyclosporin A injection ameliorated the moving disorders in an ALS mouse models [78]. Cyclosporin A, FK506 or FK1706 treatment attenuated the symptoms of MS mouse models [79]. Administration of cyclosporin A or FK506 also had protective effects on HD mouse model [80]; the protective effects of these Cyp D inhibitors are proposed to be, at least in part, due to the inhibition of mPTP formation. Notably, treatment with Cyp D inhibitors at experimental dosages did not show detectable adverse effects in the mice, suggesting probable safety of these drugs in clinical translation.
The effect of Cyp D inhibitors on Aβ toxicity has also been investigated. We and other groups have demonstrated that cyclosporin A significantly inhibited apoptosis and production of oxidative stress induced by Aβ accumulation [7,13,32,81]. Cyclosporin A treatment attenuated Aβ- induced mitochondrial swelling and increased mitochondrial calcium buffering capacity. In addition, the addition of cyclosporin A to the hippocampal CA1 region completely rescued Aβ-induced long term potentiation (LTP) reduction. These data indicate that pharmaceutical inhibition of Cyp D is a potential strategy to protect neurons from Aβ toxicity [7]. It remains to be determined whether the protective effects of CypD inhibitors are present in animal model studies.
VDAC is another key component of mPTP. A recent report using a VDAC inhibitor, cholest-4-en-3-one oxime (TRO19622), showed results of significantly extended lifespan as well as attenuation of symptoms in G93A SOD1 ALS mice [82]. TRO19622 has not yet been tested in AD mouse or cell models. 4,4’-Diisothiocyanatostilbene-2,2’-disulfonic acid (DIDS) is a VDAC blocker and has been shown to protect cells from VDAC -potentiated cell apoptosis [73]. Small and his colleagues demonstrated that DIDS protected against neurotoxicity induced by Aβ25-35 or staurosporine on primary cultured neurons as evidenced by significantly less cell death upon the application of DIDS. These findings implicate that inhibiting VDAC-mediated mPTP might be a potential therapeutic option for the protection of neurodegeneration in AD [74].
In summary, interventions affecting mPTP formation such as genetic Cyp D depletion or use of Cyp D or VDAC inhibitors have been proven experimentally to be effective in counteracting the detrimental effects of Aβ or oxidative stress on mitochondrial and neuronal perturbation, suggesting that targeting mPTP may result in the rescue of neurons from Aβ-induced damage.
5. Conclusions
We reviewed here the recent studies eliciting the involvement of mPTP in the pathogenesis of AD and the effects of inhibiting mPTP on mitochondrial and neuronal dysfunction. The interplay of Aβ with mPTP may be a novel mechanism underlying Aβ-associated mitochondrial pathology. CypD-dependent mitochondrial permeability transition contributes significantly to Aβ-induced neuronal and mitochondrial injury relevant to the pathogenesis of Alzheimer’s disease. In an Aβ-rich environment, Aβ gains access to the mitochondrial matrix by the translocase of the outer membrane (TOM) machinery [10] or an as yet unknown mechanism, and forms a complex with CypD, promoting its translocation to the inner mitochondrial membrane and formation of mPTP. In addition, CypD-Aβ interaction enhances generation of ROS and triggers signal transduction. These events eventually lead to cell death relevant to the AD pathogenesis (Figure 1). Thus, decreasing CypD dependent mPTP formation through pharmacologic inhibition on cyp D is an important therapeutic target for prevention and treatment of Alzheimer disease and other neurodegenerative diseases. There is also potential benefit in the development of new inhibitors of other mitochondrial transition pore components (e.g. VDAC) as therapeutic approaches to treat AD and other diseases.
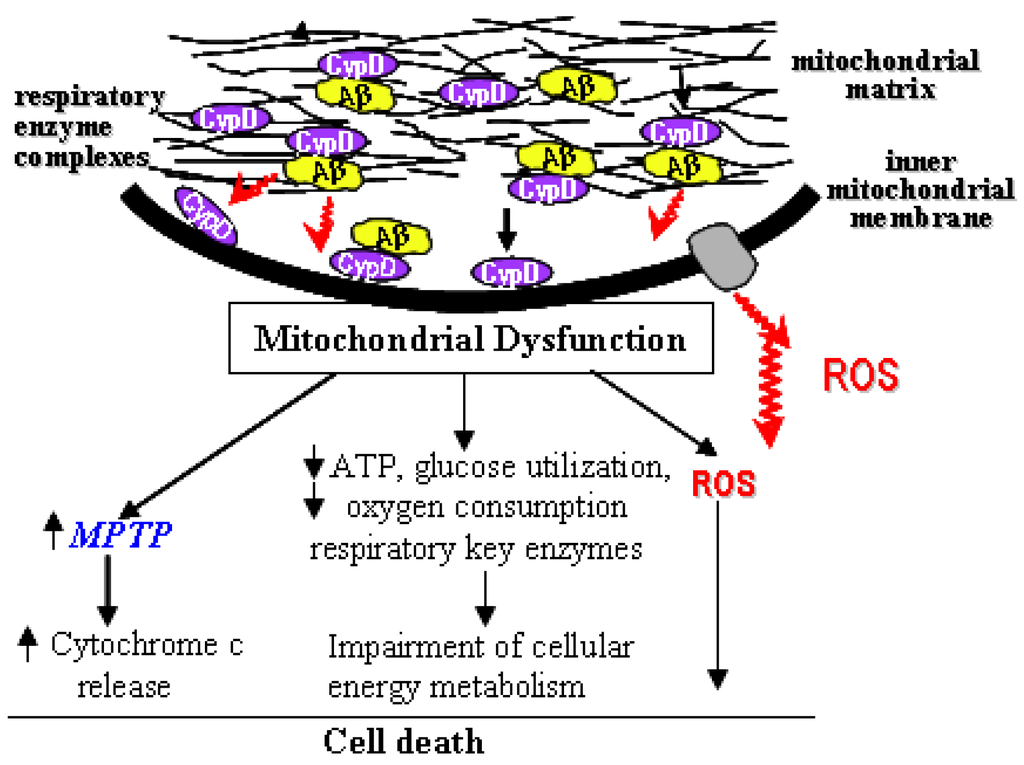
Figure 1.
Cyclophilin D-Aβ interaction: implications for mitochondrial function.
Acknowledgements
This study is supported by National Institutes of Health Grant PO1AG17490 and the Alzheimer’s Association.
References and Notes
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; Trinchese, F.; Liu, S.; Gunn-Moore, F.; Lue, L.F.; Walker, D.G.; Kuppusamy, P.; Zewier, Z.L.; Arancio, O.; Stern, D.; Yan, S.S.; Wu, H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 2004, 304, 448–452. [Google Scholar]
- Selkoe, D.J. Alzheimer's disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar]
- Cummings, B.J.; Pike, C.J.; Shankle, R.; Cotman, C.W. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiol. Aging 1996, 17, 921–933. [Google Scholar]
- Gu, Z.; Liu, W.; Yan, Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J. Biol. Chem. 2009, 284, 10639–10649. [Google Scholar] [PubMed]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N.; Nagai, T.; Mizoguchi, H.; Ibi, D.; Hori, O.; Ogawa, S.; Stern, D.M.; Yamada, K.; Yan, S.S. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar]
- Caspersen, C.; Wang, N.; Yao, J.; Sosunov, A.; Chen, X.; Lustbader, J.W.; Xu, H.W.; Stern, D.; McKhann, G.; Yan, S.D. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005, 19, 2040–2041. [Google Scholar]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; Sosunov, A.A.; McKhann, G.M.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; Gunn-Moore, F.J.; Vonsattel, J.P.; Arancio, O.; Chen, J.X.; Yan, S.D. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar]
- Wang, X.; Su, B.; Perry, G.; Smith, M.A.; Zhu, X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radical Biol. Med. 2007, 43, 1569–1573. [Google Scholar]
- Eckert, A.; Hauptmann, S.; Scherping, I.; Rhein, V.; Muller-Spahn, F.; Gotz, J.; Muller, W.E. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neuro-Degenerative Dis. 2008, 5, 157–159. [Google Scholar] [CrossRef]
- Hansson Petersen, C.A.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.F.; Alafuzoff, I.; Leinonen, V.; Ito, A.; Winblad, B.; Glaser, E.; Ankarcrona, M. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2008, 105, 13145–13150. [Google Scholar]
- Manczak, M.; Anekonda, T.S.; Henson, E.; Park, B.S.; Quinn, J.; Reddy, P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006, 15, 1437–1449. [Google Scholar]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar]
- Du, H.; Guo, L.; Zhang, W.; Rydzewska, M.; Yan, S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging 2009. [Google Scholar]
- Takuma, K.; Yao, J.; Huang, J.; Xu, H.; Chen, X.; Luddy, J.; Trillat, A.C.; Stern, D.M.; Arancio, O.; Yan, S.S. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005, 19, 597–598. [Google Scholar]
- Cardoso, S.M.; Santana, I.; Swerdlow, R.H.; Oliveira, C.R. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J. Neurochem. 2004, 89, 1417–1426. [Google Scholar]
- Hauptmann, S.; Scherping, I.; Drose, S.; Brandt, U.; Schulz, K.L.; Jendrach, M.; Leuner, K.; Eckert, A.; Muller, W.E. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol. Aging 2009, 30, 1574–1586. [Google Scholar]
- Qiao, H.; Koya, R.C.; Nakagawa, K.; Tanaka, H.; Fujita, H.; Takimoto, M.; Kuzumaki, N. Inhibition of Alzheimer's amyloid-beta peptide-induced reduction of mitochondrial membrane potential and neurotoxicity by gelsolin. Neurobiol. Aging 2005, 26, 849–855. [Google Scholar]
- Casley, C.S.; Canevari, L.; Land, J.M.; Clark, J.B.; Sharpe, M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002, 80, 91–100. [Google Scholar]
- Swerdlow, R.H.; Parks, J.K.; Cassarino, D.S.; Binder, D.R.; Bennett, J.P., Jr.; Di Iorio, G.; Golbe, L.I.; Parker, W.D., Jr. Biochemical analysis of cybrids expressing mitochondrial DNA from Contursi kindred Parkinson's subjects. Exp. Neurol. 2001, 169, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Wille, E.; Stack, C.; Calingasan, N.Y.; Beal, M.F.; Lin, M.T. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009, 23, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Galvan, V.; Lange, M.B.; Tang, H.; Sowell, R.A.; Spilman, P.; Fombonne, J.; Gorostiza, O.; Zhang, J.; Sultana, R.; Bredesen, D.E. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic. Biol. Med. 48, 136–144. [PubMed]
- Ferreiro, E.; Oliveira, C.R.; Pereira, C.M. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008, 30, 331–342. [Google Scholar]
- Reddy, P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp. Neurol. 2009, 218, 286–292. [Google Scholar]
- Suen, K.C.; Lin, K.F.; Elyaman, W.; So, K.F.; Chang, R.C.; Hugon, J. Reduction of calcium release from the endoplasmic reticulum could only provide partial neuroprotection against beta-amyloid peptide toxicity. J. Neurochem. 2003, 87, 1413–1426. [Google Scholar]
- Sanz-Blasco, S.; Valero, R.A.; Rodriguez-Crespo, I.; Villalobos, C.; Nunez, L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 2008, 3, e2718. [Google Scholar]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar]
- Wang, X.; Su, B.; Siedlak, S.L.; Moreira, P.I.; Fujioka, H.; Wang, Y.; Casadesus, G.; Zhu, X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 19318–19323. [Google Scholar]
- Rui, Y.; Tiwari, P.; Xie, Z.; Zheng, J.Q. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J. Neurosci. 2006, 26, 10480–10487. [Google Scholar]
- Mancuso, M.; Orsucci, D.; Siciliano, G.; Murri, L. Mitochondria, mitochondrial DNA and Alzheimer's disease. What comes first? Curr. Alzheimer Res. 2008, 5, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondrial DNA--related mitochondrial dysfunction in neurodegenerative diseases. Arch. Pathol. Lab. Med. 2002, 126, 271–280. [Google Scholar]
- Bozner, P.; Grishko, V.; LeDoux, S.P.; Wilson, G.L.; Chyan, Y.C.; Pappolla, M.A. The amyloid beta protein induces oxidative damage of mitochondrial DNA. J. Neuropathol. Exp. Neurol. 1997, 56, 1356–1362. [Google Scholar]
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Rego, A.C.; Oliveira, C. Effect of amyloid beta-peptide on permeability transition pore: a comparative study. J. Neurosci. Res. 2002, 69, 257–267. [Google Scholar]
- Billups, B.; Forsythe, I.D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 2002, 22, 5840–5847. [Google Scholar]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar]
- Li, G.; Zou, L.Y.; Cao, C.M.; Yang, E.S. Coenzyme Q10 protects SHSY5Y neuronal cells from beta amyloid toxicity and oxygen-glucose deprivation by inhibiting the opening of the mitochondrial permeability transition pore. Biofactors 2005, 25, 97–107. [Google Scholar]
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Oliveira, C. Amyloid beta-peptide promotes permeability transition pore in brain mitochondria. Biosci. Rep. 2001, 21, 789–800. [Google Scholar]
- Shevtzova, E.F.; Kireeva, E.G.; Bachurin, S.O. Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull. Exp. Biol. Med. 2001, 132, 1173–1176. [Google Scholar]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.; Gerasimenko, O.V. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar]
- Halestrap, A. Biochemistry: a pore way to die. Nature 2005, 434, 578–579. [Google Scholar]
- Kowaltowski, A.J.; Castilho, R.F.; Vercesi, A.E. Opening of the mitochondrial permeability transition pore by uncoupling or inorganic phosphate in the presence of Ca2+ is dependent on mitochondrial-generated reactive oxygen species. FEBS Lett. 1996, 378, 150–152. [Google Scholar]
- Clarke, S.J.; Khaliulin, I.; Das, M.; Parker, J.E.; Heesom, K.J.; Halestrap, A.P. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ. Res. 2008, 102, 1082–1090. [Google Scholar]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; Robbins, J.; Molkentin, J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar]
- Connern, C.P.; Halestrap, A.P. Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2+]. Biochemistry 1996, 35, 8172–8180. [Google Scholar]
- Zheng, Y.; Shi, Y.; Tian, C.; Jiang, C.; Jin, H.; Chen, J.; Almasan, A.; Tang, H.; Chen, Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene 2004, 23, 1239–1247. [Google Scholar]
- Pestana, C.R.; Silva, C.H.; Pardo-Andreu, G.L.; Rodrigues, F.P.; Santos, A.C.; Uyemura, S.A.; Curti, C. Ca(2+) binding to c-state of adenine nucleotide translocase (ANT)-surrounding cardiolipins enhances (ANT)-Cys(56) relative mobility: a computational-based mitochondrial permeability transition study. Biochim. Biophys. Acta 2009, 1787, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Varanyuwatana, P.; Halestrap, A.P. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008, 283, 26312–26323. [Google Scholar]
- Li, V.; Brustovetsky, T.; Brustovetsky, N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp. Neurol. 2009, 218, 171–182. [Google Scholar]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar]
- Schinzel, A.C.; Takeuchi, O.; Huang, Z.; Fisher, J.K.; Zhou, Z.; Rubens, J.; Hetz, C.; Danial, N.N.; Moskowitz, M.A.; Korsmeyer, S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA 2005, 102, 12005–12010. [Google Scholar]
- Martin, L.J.; Gertz, B.; Pan, Y.; Price, A.C.; Molkentin, J.D.; Chang, Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp. Neurol. 2009, 218, 333–346. [Google Scholar]
- Karlsson, J.; Fong, K.S.; Hansson, M.J.; Elmer, E.; Csiszar, K.; Keep, M.F. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosurg. 2004, 101, 128–137. [Google Scholar]
- Brustovetsky, N.; Brustovetsky, T.; Purl, K.J.; Capano, M.; Crompton, M.; Dubinsky, J.M. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J. Neurosci. 2003, 23, 4858–4867. [Google Scholar]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; Duchen, M.R.; Abramov, A.Y. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 2009, 33, 627–638. [Google Scholar]
- Wang, H.L.; Chou, A.H.; Yeh, T.H.; Li, A.H.; Chen, Y.L.; Kuo, Y.L.; Tsai, S.R.; Yu, S.T. PINK1 mutants associated with recessive Parkinson's disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol. Dis. 2007, 28, 216–226. [Google Scholar]
- Brown, M.R.; Sullivan, P.G.; Geddes, J.W. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 2006, 281, 11658–11668. [Google Scholar]
- Rockenstein, E.M.; McConlogue, L.; Tan, H.; Power, M.; Masliah, E.; Mucke, L. Levels and alternative splicing of amyloid beta protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. J. Biol. Chem. 1995, 270, 28257–28267. [Google Scholar]
- Mucke, L.; Masliah, E.; Johnson, W.B.; Ruppe, M.D.; Alford, M.; Rockenstein, E.M.; Forss-Petter, S.; Pietropaolo, M.; Mallory, M.; Abraham, C.R. Synaptotrophic effects of human amyloid beta protein precursors in the cortex of transgenic mice. Brain Res. 1994, 666, 151–167. [Google Scholar]
- Soskic, V.; Klemm, M.; Proikas-Cezanne, T.; Schwall, G.P.; Poznanovic, S.; Stegmann, W.; Groebe, K.; Zengerling, H.; Schoepf, R.; Burnet, M.; Schrattenholz, A. A connection between the mitochondrial permeability transition pore, autophagy, and cerebral amyloidogenesis. J. Proteome Res. 2008, 7, 2262–2269. [Google Scholar] [PubMed]
- Moreira, A.E.; Hueb, W.A.; Soares, P.R.; Meneghetti, J.C.; Jorge, M.C.; Chalela, W.A.; Martinez Filho, E.E.; Oliveira, S.A.; Jatene, F.B.; Ramires, J.A. Comparative study between the therapeutic effects of surgical myocardial revascularization and coronary angioplasty in equivalent ischemic situations: analysis through myocardial scintigraphy with 99mTc-Sestamibi. Arq. Bras. Cardiol. 2005, 85, 92–99. [Google Scholar]
- Chin, J.H.; Tse, F.W.; Harris, K.; Jhamandas, J.H. Beta-amyloid enhances intracellular calcium rises mediated by repeated activation of intracellular calcium stores and nicotinic receptors in acutely dissociated rat basal forebrain neurons. Brain Cell Biol. 2006, 35, 173–186. [Google Scholar]
- Brewer, G.J.; Lim, A.; Capps, N.G.; Torricelli, J.R. Age-related calcium changes, oxyradical damage, caspase activation and nuclear condensation in hippocampal neurons in response to glutamate and beta-amyloid. Exp. Gerontol. 2005, 40, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Schoneich, C.; Pogocki, D.; Hug, G.L.; Bobrowski, K. Free radical reactions of methionine in peptides: mechanisms relevant to beta-amyloid oxidation and Alzheimer's disease. J. Am. Chem. Soc. 2003, 125, 13700–13713. [Google Scholar]
- Morais Cardoso, S.; Swerdlow, R.H.; Oliveira, C.R. Induction of cytochrome c-mediated apoptosis by amyloid beta 25-35 requires functional mitochondria. Brain Res. 2002, 931, 117–125. [Google Scholar]
- Celsi, F.; Svedberg, M.; Unger, C.; Cotman, C.W.; Carri, M.T.; Ottersen, O.P.; Nordberg, A.; Torp, R. Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol. Dis. 2007, 26, 342–352. [Google Scholar]
- Montiel, T.; Quiroz-Baez, R.; Massieu, L.; Arias, C. Role of oxidative stress on beta-amyloid neurotoxicity elicited during impairment of energy metabolism in the hippocampus: protection by antioxidants. Exp. Neurol. 2006, 200, 496–508. [Google Scholar]
- Parks, J.K.; Smith, T.S.; Trimmer, P.A.; Bennett, J.P., Jr.; Parker, W.D., Jr. Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J. Neurochem. 2001, 76, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Lee, J.P.; Kim, E.M.; Chang, K.A.; Park, C.H.; Jeong, S.J.; Wittendorp, M.C.; Seo, J.H.; Choi, S.H.; Suh, Y.H. Amyloid beta peptide induces cytochrome C release from isolated mitochondria. Neuroreport 2002, 13, 1989–1993. [Google Scholar]
- Rodrigues, C.M.; Sola, S.; Brito, M.A.; Brondino, C.D.; Brites, D.; Moura, J.J. Amyloid beta-peptide disrupts mitochondrial membrane lipid and protein structure: protective role of tauroursodeoxycholate. Biochem. Biophys. Res. Commun. 2001, 281, 468–474. [Google Scholar]
- Zhang, S.; Zhang, Z.; Sandhu, G.; Ma, X.; Yang, X.; Geiger, J.D.; Kong, J. Evidence of oxidative stress-induced BNIP3 expression in amyloid beta neurotoxicity. Brain Res. 2007, 1138, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Suman, S.; Chandna, S.; Das, T.K. Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer's disease. Bioinformation 2009, 3, 440–445. [Google Scholar]
- Vlachos, P.; Nyman, U.; Hajji, N.; Joseph, B. The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ. 2007, 14, 1497–1507. [Google Scholar]
- Vale, C.; Nicolaou, K.C.; Frederick, M.O.; Vieytes, M.R.; Botana, L.M. Cell volume decrease as a link between azaspiracid-induced cytotoxicity and c-Jun-N-terminal kinase activation in cultured neurons. Toxicol Sci. 113, 158–168. [PubMed]
- Xia, Z.; Tauskela, J.; Small, D.L. Disulfonic stilbenes prevent beta-amyloid (25-35) neuronal toxicity in rat cortical cultures. Neurosci. Lett. 2003, 340, 53–56. [Google Scholar]
- Clarke, S.J.; McStay, G.P.; Halestrap, A.P. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 2002, 277, 34793–34799. [Google Scholar]
- Halestrap, A.P.; Connern, C.P.; Griffiths, E.J.; Kerr, P.M. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol. Cell Biochem 1997, 174, 167–172. [Google Scholar]
- Forte, M.; Gold, B.G.; Marracci, G.; Chaudhary, P.; Basso, E.; Johnsen, D.; Yu, X.; Fowlkes, J.; Rahder, M.; Stem, K.; Bernardi, P.; Bourdette, D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7558–7563. [Google Scholar]
- Keep, M.; Elmer, E.; Fong, K.S.; Csiszar, K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001, 894, 327–331. [Google Scholar]
- Gold, B.G.; Voda, J.; Yu, X.; McKeon, G.; Bourdette, D.N. FK506 and a nonimmunosuppressant derivative reduce axonal and myelin damage in experimental autoimmune encephalomyelitis: neuroimmunophilin ligand-mediated neuroprotection in a model of multiple sclerosis. J. Neurosci. Res. 2004, 77, 367–377. [Google Scholar]
- Kumar, P.; Kumar, A. Neuroprotective effect of cyclosporine and FK506 against 3-nitropropionic acid induced cognitive dysfunction and glutathione redox in rat: possible role of nitric oxide. Neurosci. Res. 2009, 63, 302–314. [Google Scholar]
- Van Den Heuvel, C.; Donkin, J.J.; Finnie, J.W.; Blumbergs, P.C.; Kuchel, T.; Koszyca, B.; Manavis, J.; Jones, N.R.; Reilly, P.L.; Vink, R. Downregulation of amyloid precursor protein (APP) expression following post-traumatic cyclosporin-A administration. J. Neurotrauma 2004, 21, 1562–1572. [Google Scholar]
- Bordet, T.; Buisson, B.; Michaud, M.; Drouot, C.; Galea, P.; Delaage, P.; Akentieva, N.P.; Evers, A.S.; Covey, D.F.; Ostuni, M.A.; Lacapere, J.J.; Massaad, C.; Schumacher, M.; Steidl, E.M.; Maux, D.; Delaage, M.; Henderson, C.E.; Pruss, R.M. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).