Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions
Abstract
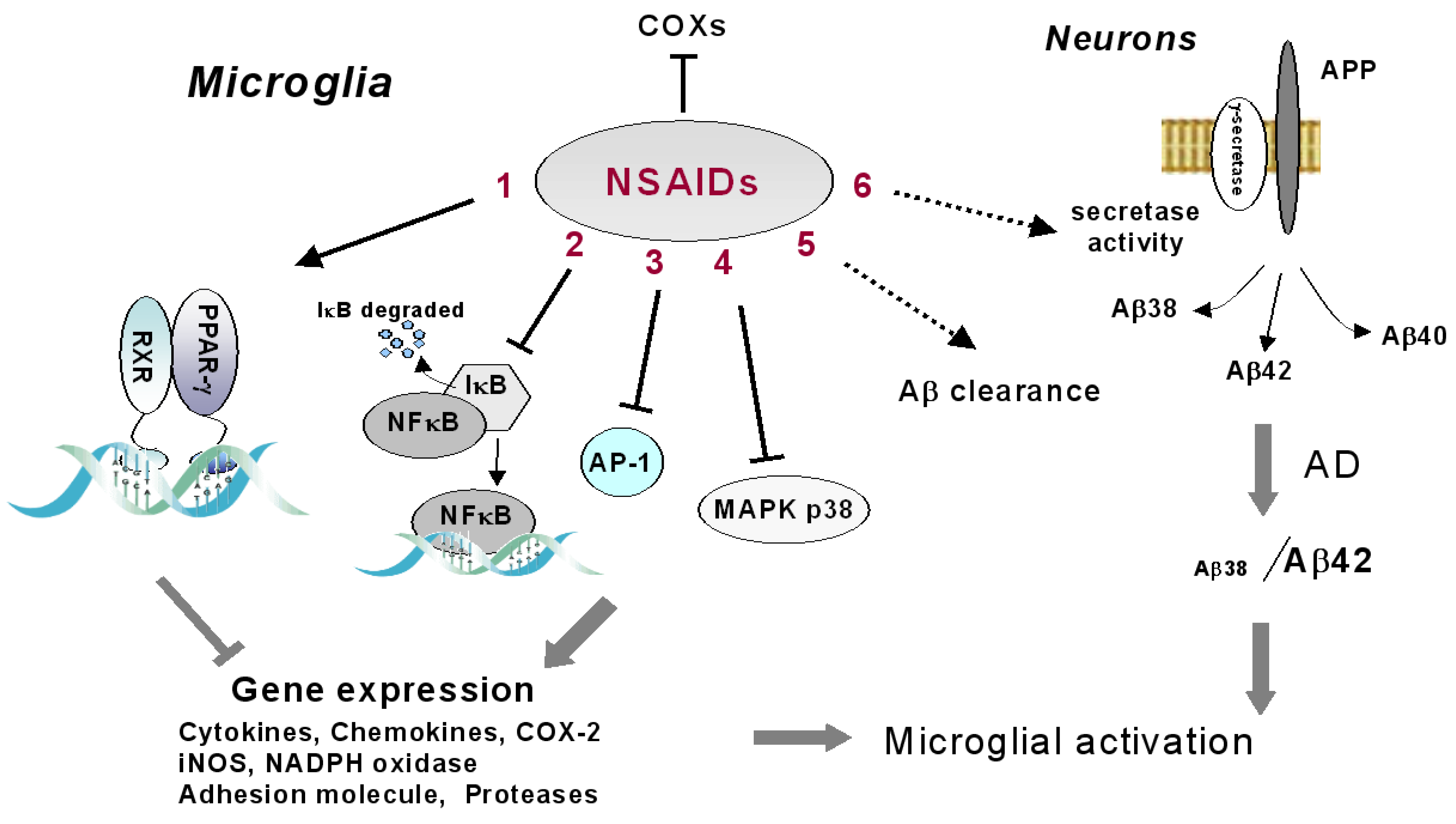
1. Introduction
2. Microglia, PG Synthesis and NSAIDs
| Drugs | IC50 |
|---|---|
| Indomethacin | 1 nM |
| NS-398 | 2.5 nM |
| Flurbiprofen | 0.1µM |
| Piroxicam | 0.1µM |
| Paracetamol | 7.6 µM |
| ASA | 10 µM |
3. NSAID-Dependent Activation of Peroxisome Proliferator Activated Receptor-γ in Microglial Cells
4. Alternative Molecular Targets of NSAIDs
5. Nitric Oxide- and Hydrogen Sulfide-Releasing NSAIDs
6. Natural Anti-Inflammatory Drugs
4. Conclusions
Acknowledgements
References
- Minghetti, L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 2005, 18, 315–321. [Google Scholar]
- Ajmone-Cat, M.A.; Cacci, E.; Minghetti, L. Brain inflammation and the neuronal fate: From neurogenesis to neurodegeneration. In Neurovascular Medicine, 1st; Maiese, K., Ed.; Oxford University Press: Oxford, UK, 2009; pp. 319–344. [Google Scholar]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar]
- Schilling, M.; Besselmann, M.; Leonhard, C.; Mueller, M.; Ringelstein, E.B.; Kiefer, R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: A study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2003, 183, 25–33. [Google Scholar]
- Schroeter, M.; Jander, S.; Huitinga, I.; Witte, O.W.; Stoll, G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke 1997, 28, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Komine-Kobayashi, M.; Mochizuki, H.; Yamada, M.; Furuya, T.; Migita, M.; Shimada, T.; Mizuno, Y.; Urabe, T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience 2003, 117, 531–539. [Google Scholar]
- Giulian, D.; Haverkamp, L.J.; Li, J.; Karshin, W.L.; Yu, J.; Tom, D.; Li, X.; Kirkpatrick, J.B. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 1995, 27, 119–137. [Google Scholar]
- Zhang, Z.; Chopp, M.; Powers, C. Temporal profile of microglial response following transient (2 h) middle cerebral artery occlusion. Brain Res. 1997, 744, 189–198. [Google Scholar]
- Lalancette-Hebert, M.; Gowing, G.; Simard, A.; Weng, Y.C.; Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007, 27, 2596–2605. [Google Scholar]
- Klegeris, A.; McGeer, E.G.; McGeer, P.L. Therapeutic approaches to inflammation in neurodegenerative disease. Curr. Opin. Neurol. 2007, 20, 351–357. [Google Scholar]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009, 65, 732–741. [Google Scholar]
- Müller, N.; Schwarz, M.J. COX-2 inhibition in schizophrenia and major depression. Curr. Pharm. Des. 2008, 14, 1452–1465. [Google Scholar]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2007, 57, 67–81. [Google Scholar]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Progr. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef]
- Ek, M.; Engblom, D.; Saha, S.; Blomqvist, A.; Jakobsson, P.J.; Ericsson-Dahlstrand, A. Inflammatory response: Pathway across the blood-brain barrier. Nature 2001, 410, 430–431. [Google Scholar]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar]
- Tanioka, T.; Nakatani, Y.; Semmyo, N.; Murakami, M.; Kudo, I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000, 275, 32775–32782. [Google Scholar]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Lee, C.A.; Goulding, E.H.; Kluckman, K.D.; Kim, H.S.; Smithies, O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 1995, 83, 483–492. [Google Scholar]
- Choi, S.H.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar]
- Teeling, J.L.; Cunningham, C.; Newman, T.A.; Perry, V.H. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav. Immun. 2010, 24, 409–419. [Google Scholar]
- Minghetti, L. Role of COX-2 in inflammatory and degenerative brain diseases. Subcell. Biochem. 2007, 42, 127–141. [Google Scholar]
- Choi, S.H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar]
- Breder, C.D.; Dewitt, D.; Kraig, R.P. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995, 355, 296–315. [Google Scholar]
- Kaufmann, W.E.; Worley, P.F.; Pegg, J.; Bremer, M.; Isakson, P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons as postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. USA 1996, 93, 2317–2321. [Google Scholar]
- Minghetti, L.; Levi, G. Induction of prostanoid biosynthesis by bacterial lipopolysaccharide and isoproterenol in rat microglial cultures. J. Neurochem. 1995, 65, 2690–2698. [Google Scholar]
- Fiebich, B.L.; Biber, K.; Lieb, K.; van Calker, D.; Berger, M.; Bauer, J.; Gebicke-Haerter, P.J. Cyclooxygenase-2 expression in rat microglia is induced by adenosine A2a-receptors. Glia 1996, 18, 152–160. [Google Scholar]
- Slepko, N.; Minghetti, L.; Polazzi, E.; Nicolini, A.; Levi, G. Reorientation of prostanoid production accompanies "activation" of adult microglial cells in culture. J. Neurosci. Res. 1997, 49, 292–300. [Google Scholar]
- Walsh, D.T.; Perry, V.H.; Minghetti, L. Cyclooxygenase-2 is highly expressed in microglial-like cells in a murine model of prion disease. Glia 2000, 29, 392–396. [Google Scholar]
- Greco, A.; Ajmone-Cat, M.A.; Nicolini, A.; Sciulli, M.G.; Minghetti, L. Paracetamol effectively reduces prostaglandin E2 synthesis in brain macrophages by inhibiting enzymatic activity of cyclooxygenase but not phospholipase and prostaglandin E synthase. J. Neurosci. Res. 2003, 71, 844–852. [Google Scholar]
- De Oliveira, A.C.; Candelario-Jalil, E.; Bhatia, H.S.; Lieb, K.; Hüll, M.; Fiebich, B.L. Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: Evidence for uncoupled regulation of mPGES-1 and COX-2. Glia 2008, 56, 844–855. [Google Scholar]
- Anderson, B.J. Paracetamol (Acetaminophen): Mechanisms of action. Paediatr. Anaesth. 2008, 18, 915–921. [Google Scholar]
- Hirrlinger, J.; Gutterer, J.M.; Kussmaul, L.; Hamprecht, B.; Dringen, R. Microglial cells in culture express a prominent glutathione system for the defense against reactive oxygen species. Dev. Neurosci. 2000, 22, 384–392. [Google Scholar]
- Rosenblat, M.; Aviram, M. Macrophage glutathione content and glutathione peroxidase activity are inversely related to cell-mediated oxidation of LDL: In vitro and in vivo studies. Free Radic. Biol. Med. 1998, 24, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Parepally, J.M.; Mandula, H.; Smith, Q.R. Brain uptake of nonsteroidal anti-inflammatory drugs: Ibuprofen, flurbiprofen, and indomethacin. Pharm. Res. 2006, 23, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Courad, J.P.; Besse, D.; Delchambre, C.; Hanoun, N.; Hamon, M.; Eschalier, A.; Caussade, F.; Cloarec, A. Acetaminophen distribution in the rat central nervous system. Life Sci. 2001, 69, 1455–1464. [Google Scholar]
- Bernareggi, A. The pharmacokinetic profile of nimesulide in healthy volunteers. Drugs 1993, 46, 64–72. [Google Scholar]
- Day, R.O.; Brooks, P.M. Variations in response to non-steroidal anti-inflammatory drugs. Br. J. Clin. Pharmacol. 1987, 23, 655–658. [Google Scholar]
- Bernardo, A.; Minghetti, L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr. Pharm. Des. 2006, 12, 93–109. [Google Scholar]
- Bernardo, A.; Minghetti, L. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. 2008, 2008, 864140. [Google Scholar] [PubMed]
- Bernardo, A.; Levi, G.; Minghetti, L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur. J. Neurosci. 2000, 12, 2215–2223. [Google Scholar]
- Bernardo, A.; Ajmone-Cat, M.A.; Levi, G.; Minghetti, L. 15-deoxy-delta12,14-prostaglandin J2 regulates the functional state and the survival of microglial cells through multiple molecular mechanisms. J. Neurochem. 2003, 87, 742–751. [Google Scholar]
- Lleo, A.; Galea, E.; Sastre, M. Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol. Life Sci. 2007, 64, 1403–1418. [Google Scholar]
- Imbimbo, B.P. An update on the efficacy of non-steroidal anti-inflammatory drugs in Alzheimer's disease. Expert Opin. Investig. Drugs 2009, 18, 1147–1168. [Google Scholar]
- Kurkowska-Jastrzebska, I.; Babiuch, M.; Joniec, I.; Przybylkowski, A.; Czlonkowski, A.; Czlonkowska, A. Indomethacin protects against neurodegeneration caused by MPTP intoxication in mice. Int. Immunopharmacol. 2002, 2, 1213–1218. [Google Scholar]
- Esposito, E.; Di Matteo, V.; Benigno, A.; Pierucci, M.; Crescimanno, G.; Di Giovanni, G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp. Neurol. 2007, 205, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I.; Pfeilschifter, J.; Geisslinger, G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001, 15, 2057–2072. [Google Scholar]
- Cho, J.Y. Immunomodulatory effect of nonsteroidal anti-inflammatory drugs (NSAIDs) at the clinically available doses. Arch. Pharm. Res. 2007, 30, 64–74. [Google Scholar]
- Heneka, M.T.; O'Banion, M.K. Inflammatory processes in Alzheimer's disease. J. Neuroimmunol. 2007, 184, 69–91. [Google Scholar]
- Koenigsknecht-Talboo, J.; Landreth, G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005, 25, 8240–8249. [Google Scholar]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; Cole, G.M. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [PubMed]
- Strohmeyer, R.; Kovelowski, C.J.; Mastroeni, D.; Leonard, B.; Grover, A.; Rogers, J. Microglial responses to amyloid beta peptide opsonization and indomethacin treatment. J. Neuroinflam. 2005, 2, 18. [Google Scholar]
- Klegeris, A.; McGeer, P.L. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr. Alzheimer Res. 2005, 2, 355–365. [Google Scholar]
- Furst, S.M.; Komocsar, W.J.; Khan, K.N.; White, K.L., Jr; Peachee, V.L.; Mennear, J.H. Screening new drugs for immunotoxic potential: I. Assessment of the effects of conventional nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors on in vitro and in vivo phagocytic activity. J. Immunotoxicol. 2005, 1, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Persaud-Sawin, D.A.; Banach, L.; Harry, G.J. Raft aggregation with specific receptor recruitment is required for microglial phagocytosis of Aβ42. Glia 2009, 57, 320–335. [Google Scholar]
- Ajmone-Cat, M.A.; Cacci, E.; Minghetti, L. Non steroidal anti-inflammatory drugs and neurogenesis in the adult mammalian brain. Curr. Pharm. Des. 2008, 14, 1435–1442. [Google Scholar]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [PubMed]
- Li, H.; Hortmann, M.; Daiber, A.; Oelze, M.; Ostad, M.A.; Schwarz, P.M.; Xu, H.; Xia, N.; Kleschyov, A.L.; Mang, C.; Warnholtz, A.; Münzel, T.; Förstermann, U. Cyclooxygenase 2-selective and nonselective nonsteroidal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. J. Pharmacol. Exp. Ther. 2008, 326, 745–753. [Google Scholar]
- Wilkinson, B.L.; Landreth, G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J. Neuroinflam. 2006, 3, 30. [Google Scholar] [Green Version]
- Elsisi, N.S.; Darling-Reed, S.; Lee, E.Y.; Oriaku, E.T.; Soliman, K.F. Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci. Lett. 2005, 375, 91–96. [Google Scholar]
- Kopp, E.; Ghosh, S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar]
- Sagi, S.A.; Weggen, S.; Eriksen, J.; Golde, T.E.; Koo, E.H. The non-cyclooxygenase targets of non-steroidal anti-inflammatory drugs, lipoxygenases, peroxisome proliferator-activated receptor, inhibitor of kappa B kinase, and NF kappaB, do not reduce amyloid beta 42 production. J. Biol. Chem. 2003, 278, 31825–31830. [Google Scholar] [PubMed]
- Niederberger, E.; Tegeder, I.; Schäfer, C.; Seegel, M.; Grösch, S.; Geisslinger, G. Opposite effects of rofecoxib on nuclear factor-kappaB and activating protein-1 activation. J. Pharmacol. Exp. Ther. 2003, 304, 1153–1160. [Google Scholar]
- Rossner, S.; Sastre, M.; Bourne, K.; Lichtenthaler, S.F. Transcriptional and translational regulation of BACE1 expression—Implications for Alzheimer's disease. Prog. Neurobiol. 2006, 79, 95–111. [Google Scholar]
- Mémet, S. NF-kappaB functions in the nervous system: From development to disease. Biochem. Pharmacol. 2006, 72, 1180–1195. [Google Scholar]
- O'Neill, L.A.; Kaltschmidt, C. NF-kappa B: A crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997, 20, 252–258. [Google Scholar]
- Murono, S.; Yoshizaki, T.; Sato, H.; Takeshita, H.; Furukawa, M.; Pagano, J.S. Aspirin inhibits tumor cell invasiveness induced by Epstein-Barr virus latent membrane protein 1 through suppression of matrix metalloproteinase-9 expression. Cancer Res. 2000, 60, 2555–2561. [Google Scholar]
- Niederberger, E.; Tegeder, I.; Vetter, G.; Schmidtko, A.; Schmidt, H.; Euchenhofer, C.; Bräutigam, L.; Grösch, S.; Geisslinger, G. Celecoxib loses its anti-inflammatory efficacy at high doses through activation of NF-kappaB. FASEB J. 2001, 15, 1622–1624. [Google Scholar]
- Rossi Paccani, S.; Boncristiano, M.; Baldari, C.T. Molecular mechanisms underlying suppression of lymphocyte responses by nonsteroidal antiinflammatory drugs. Cell. Mol. Life Sci. 2003, 60, 1071–1083. [Google Scholar]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. [Google Scholar]
- Scali, C.; Giovannini, M.G.; Prosperi, C.; Bellucci, A.; Pepeu, G.; Casamenti, F. The selective cyclooxygenase-2 inhibitor rofecoxib suppresses brain inflammation and protects cholinergic neurons from excitotoxic degeneration in vivo. Neuroscience 2003, 117, 909–919. [Google Scholar]
- Ross, J.S.; Madigan, D.; Hill, K.P.; Egilman, D.S.; Wang, Y.; Krumholz, H.M. Pooled analysis of rofecoxib placebo-controlled clinical trial data lessons for postmarket pharmaceutical safety surveillance. Arch.Intern. Med. 2009, 169, 1976–1985. [Google Scholar]
- Burgaud, J.L.; Ongini, E.; Del Soldato, P. Nitric oxide-releasing drugs: A novel class of effective and safe therapeutic agents. Ann. N. Y. Acad. Sci. 2002, 962, 360–371. [Google Scholar]
- Keeble, J.E.; Moore, P.K. Pharmacology and potential therapeutic applications of nitric oxide-releasing non-steroidal anti-inflammatory and related nitric oxide-donating drugs. Br. J. Pharmacol. 2002, 137, 295–310. [Google Scholar]
- Bertrand, V.; Guimbaud, R.; Sogni, P.; Lamrani, A.; Mauprivez, C.; Giroud, J.P.; Couturier, D.; Chauvelot-Moachon, L.; Chaussade, S. Role of tumor necrosis factor-α and inducible nitric oxide synthase in the prevention of nitro-flurbiprofen small intestine toxicity. Eur. J. Pharmacol. 1998, 356, 245–253. [Google Scholar]
- Johal, K.; Hanson, J.P. Opposite effects of flurbiprofen and the nitroxybutylester of flurbiprofen on apoptosis in cultured guinea pig gastric mucous cells. Br. J. Pharmacol. 2000, 130, 811–818. [Google Scholar]
- Ajmone-Cat, M.A.; Nicolini, A.; Minghetti, L. Differential effects of the nonsteroidal antiinflammatory drug flurbiprofen and its nitric oxide-releasing derivative, nitroflurbiprofen, on prostaglandin E2, interleukin-1β, and nitric oxide synthesis by activated microglia. J. Neurosci. Res. 2001, 66, 715–722. [Google Scholar] [PubMed]
- Fiorucci, S.; Santucci, L.; Cirino, G.; Mencarelli, A.; Familiari, L.; Del Soldato, P.; Morelli, A. IL-1β converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal antiinflammatory drugs. J. Immunol. 2000, 165, 5245–5254. [Google Scholar]
- Bernardo, A.; Ajmone-Cat, M.A.; Gasparini, L.; Ongini, E.; Minghetti, L. Nuclear receptor peroxisome proliferator-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J. Neurochem. 2005, 92, 895–903. [Google Scholar]
- Bernardo, A.; Gasparini, L.; Ongini, E.; Minghetti, L. Dynamic regulation of microglial functions by the non-steroidal anti-inflammatory drug NCX 2216: Implications for chronic treatments of neurodegenerative diseases. Neurobiol. Dis. 2006, 22, 25–32. [Google Scholar]
- Jantzen, P.T.; Connor, K.E.; DiCarlo, G.; Wenk, G.L.; Wallace, J.L.; Rojiani, A.M.; Coppola, D.; Morgan, D.; Gordon, M.N. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 2002, 22, 2246–2254. [Google Scholar]
- Van Groen, T.; Kadish, I. Transgenic AD model mice, effects of potential anti-AD treatments on inflammation and pathology. Brain Res. Rev. 2005, 48, 370–378. [Google Scholar] [CrossRef]
- Wallace, J.L. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol. Sci. 2007, 28, 501–505. [Google Scholar]
- Sparatore, A.; Perrino, E.; Tazzari, V.; Giustarini, D.; Rossi, R.; Rossoni, G.; Erdman, K.; Schr€oder, H.; Del Soldato, P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radic. Biol. Med. 2009, 46, 586–592. [Google Scholar]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar]
- Hu, L.F.; Wong, P.T.; Moore, P.K.; Bian, J.S. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J. Neurochem. 2007, 100, 1121–1128. [Google Scholar]
- Lee, M.; Sparatore, A.; Del Soldato, P.; McGeer, E.; McGeer, P.L. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia 2010, 58, 103–113. [Google Scholar]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite “scavenger?”. J. Neurochem. 2004, 90, 765–768. [Google Scholar] [PubMed]
- Choi, Y.; Lee, M.K.; Lim, S.Y.; Sung, S.H.; Kim, Y.C. Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br. J. Pharmacol. 2009, 156, 933–940. [Google Scholar]
- Zhou, H.Y.; Shin, E.M.; Guo, L.Y.; Youn, U.J.; Bae, K.; Kang, S.S.; Zou, L.B.; Kim, Y.S. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur. J. Pharmacol. 2008, 586, 340–349. [Google Scholar] [PubMed]
- Jin, D.Q.; Lim, C.S.; Hwang, J.K.; Ha, I.; Han, J.S. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1264–1269. [Google Scholar]
- Ma, J.; Hwang, Y.K.; Cho, W.H.; Han, S.H.; Hwang, J.K.; Han, J.S. Macelignan attenuates activations of mitogen-activated protein kinases and nuclear factor kappa B induced by lipopolysaccharide in microglial cells. Biol. Pharm. Bull. 2009, 32, 1085–1090. [Google Scholar]
- Zhou, Y.; Dial, E.J.; Doyen, R.; Lichtenberger, L.M. Effect of indomethacin on bile acid-phospholipid interactions: Implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G722–G731. [Google Scholar]
- Kashfi, K.; Rigas, B. Non-COX-2 targets and cancer: Expanding the molecular target repertoire of chemoprevention. Biochem. Pharmacol. 2005, 70, 969–986. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949-1965. https://doi.org/10.3390/ph3061949
Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals. 2010; 3(6):1949-1965. https://doi.org/10.3390/ph3061949
Chicago/Turabian StyleAjmone-Cat, Maria Antonietta, Antonietta Bernardo, Anita Greco, and Luisa Minghetti. 2010. "Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions" Pharmaceuticals 3, no. 6: 1949-1965. https://doi.org/10.3390/ph3061949
APA StyleAjmone-Cat, M. A., Bernardo, A., Greco, A., & Minghetti, L. (2010). Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals, 3(6), 1949-1965. https://doi.org/10.3390/ph3061949




