Functionalized Core/Shell Gold-Palladium Bimetallic Nanoparticles in Transferrin-Targeted Dual-Drug Delivery in a Cervical Cancer Cell Model
Abstract
1. Introduction
2. Results
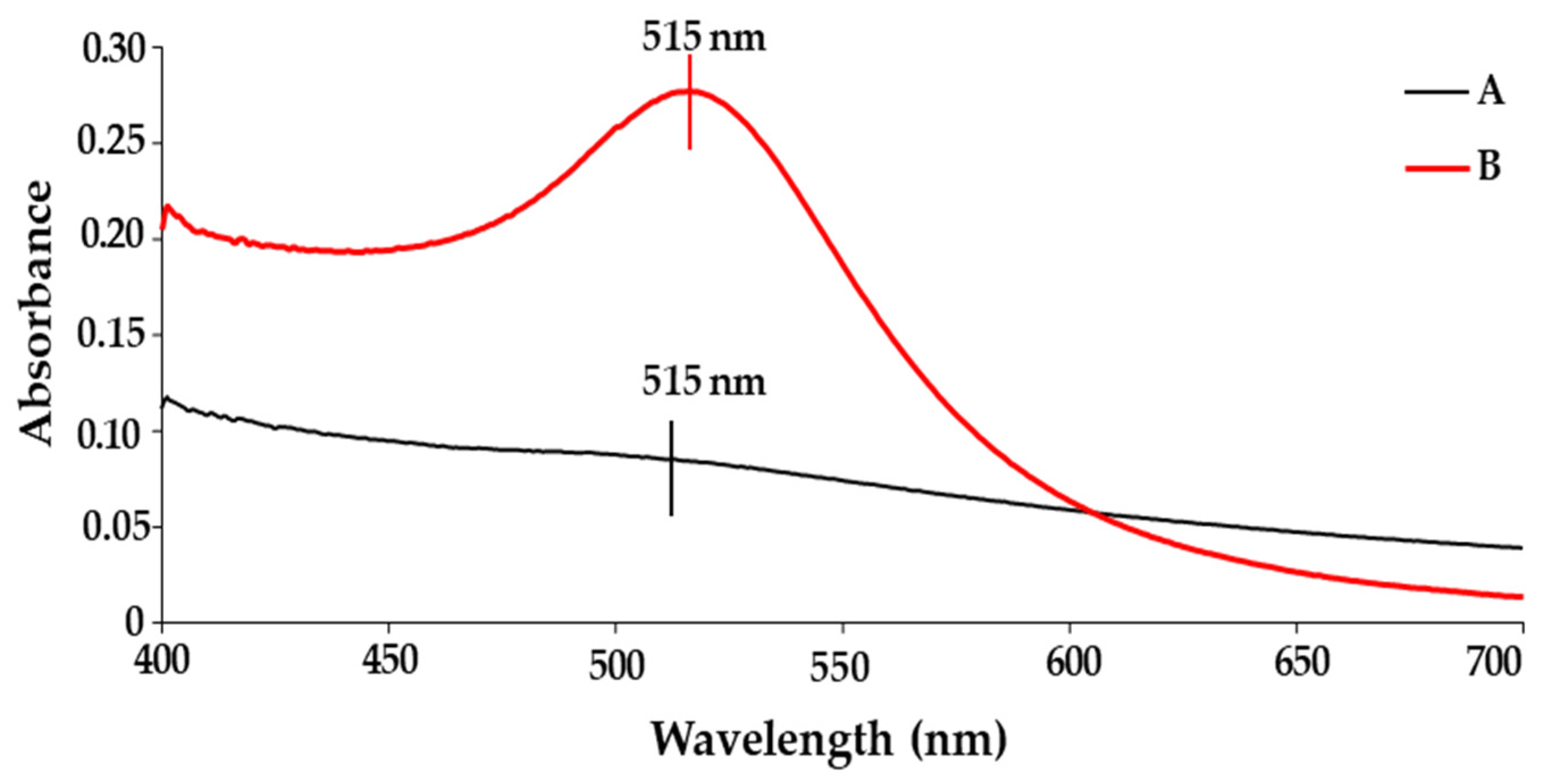
2.1. UV-Visible (UV-Vis) Spectroscopy Studies
2.2. ICP-OES and FTIR Analysis
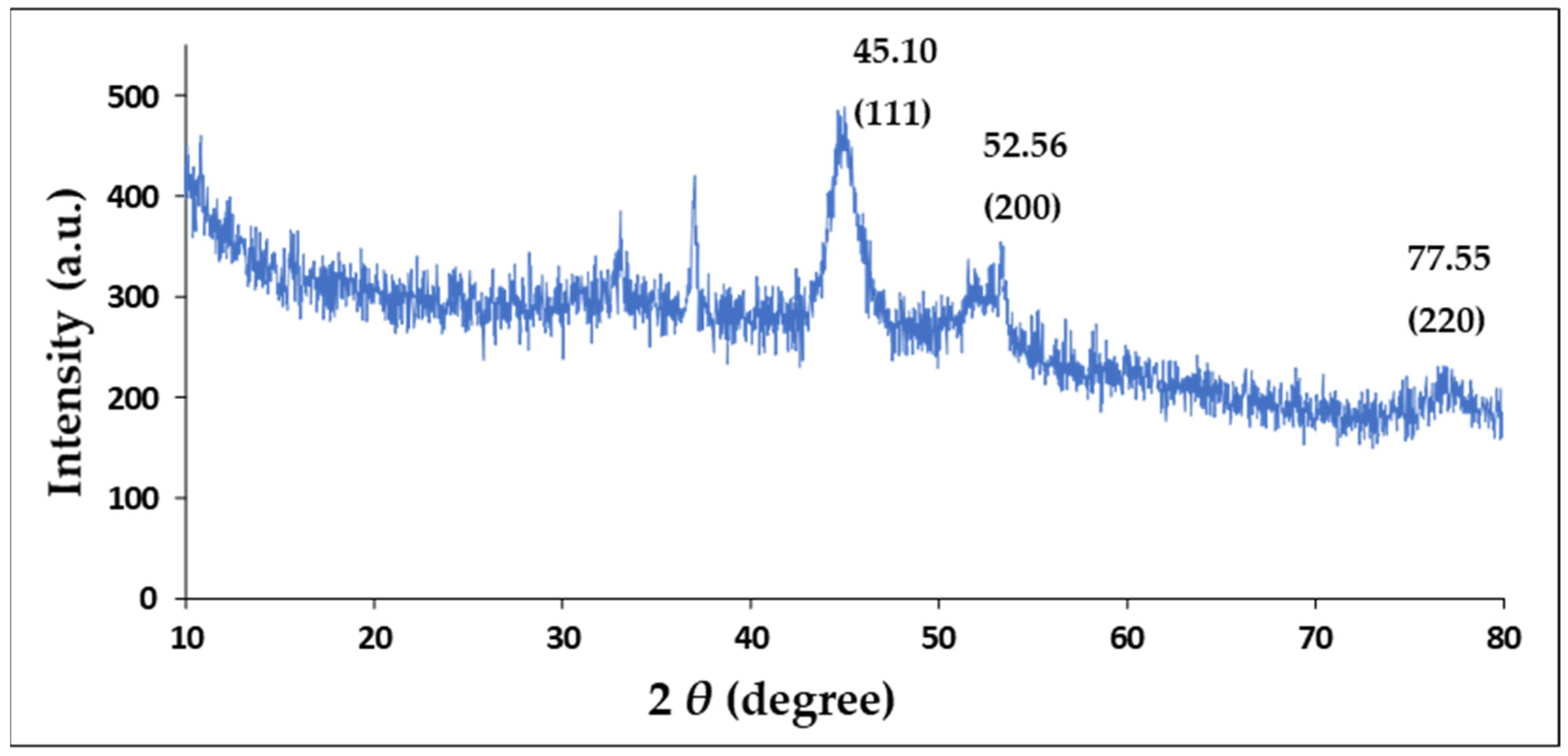
2.3. X-Ray Diffraction (XRD)
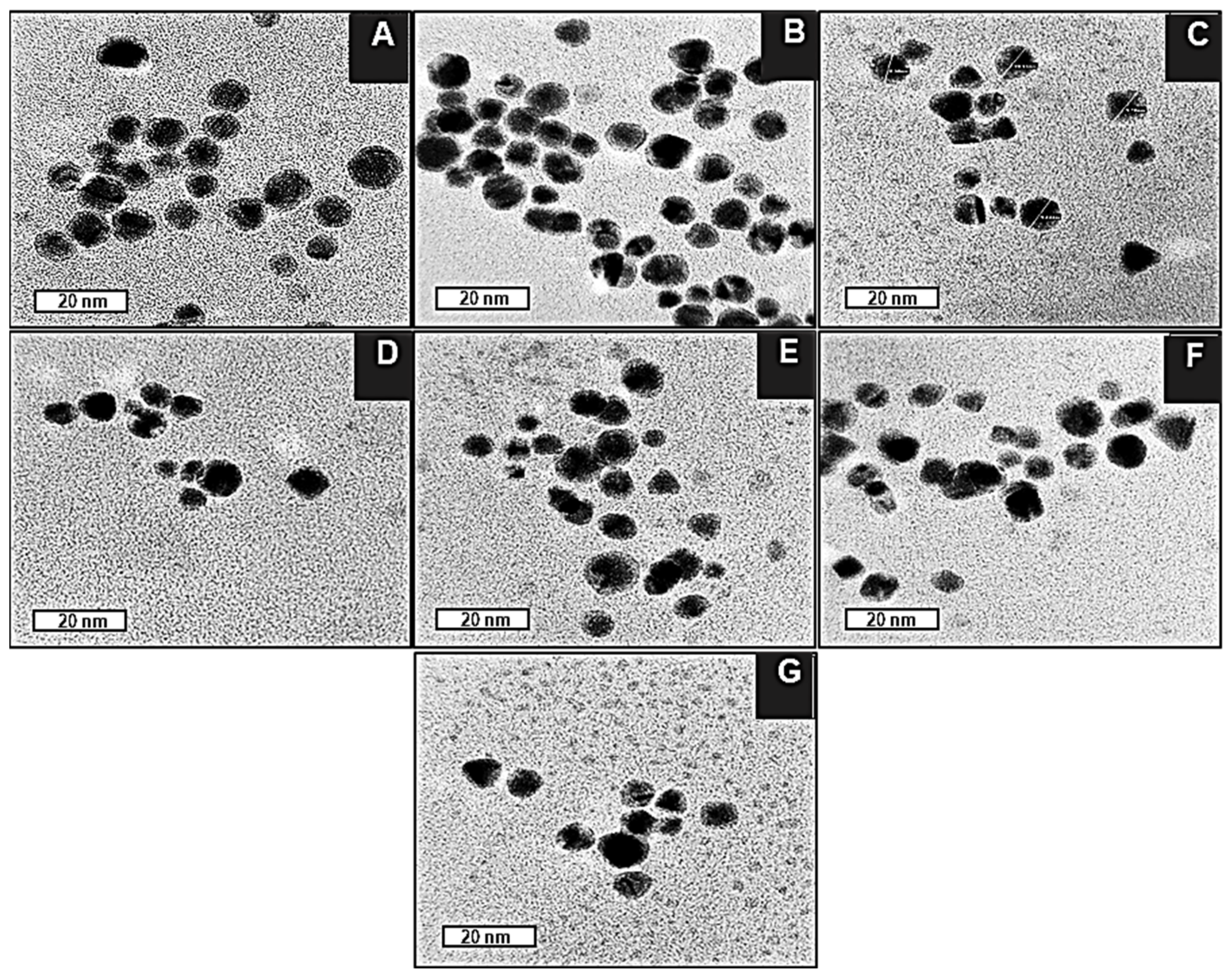
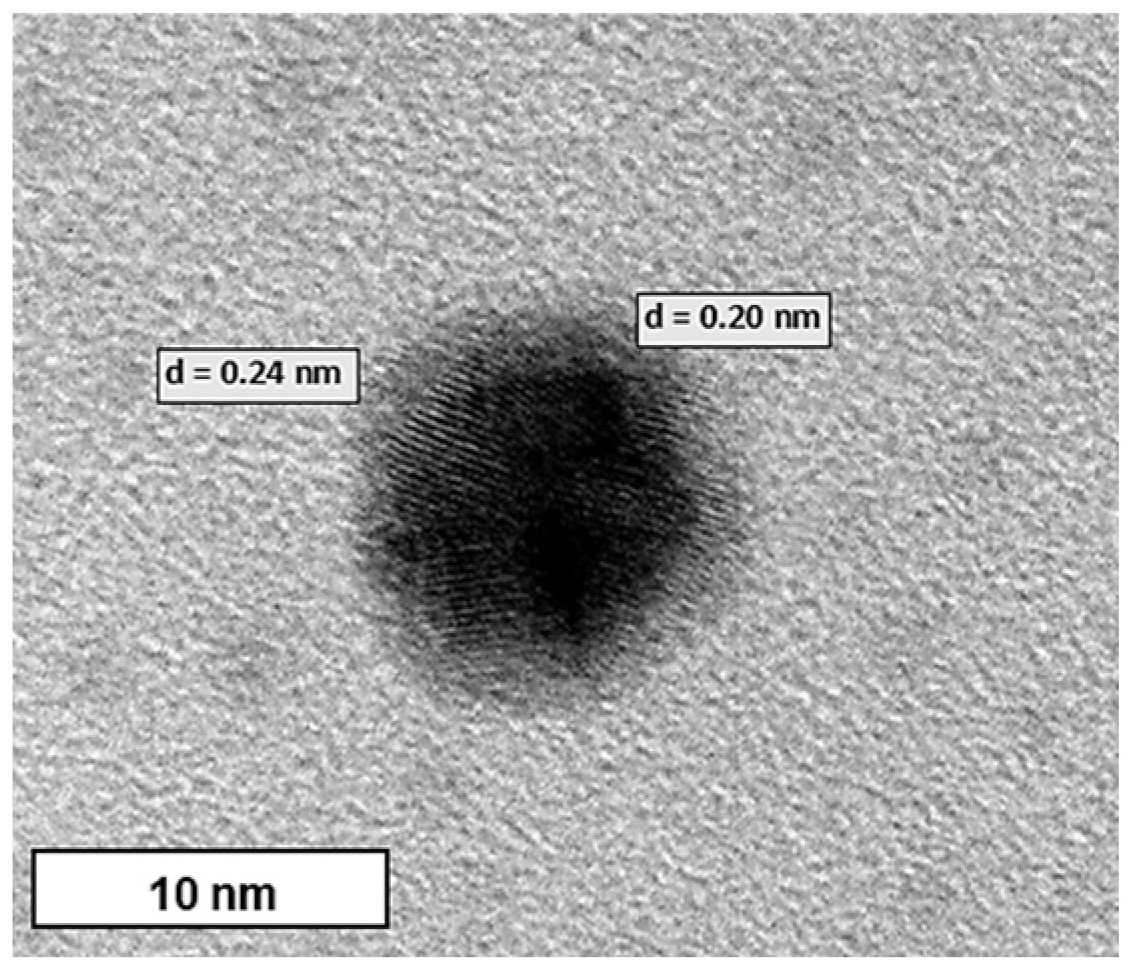
2.4. Transmission Electron Microscopy (TEM)
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Encapsulation Efficiency
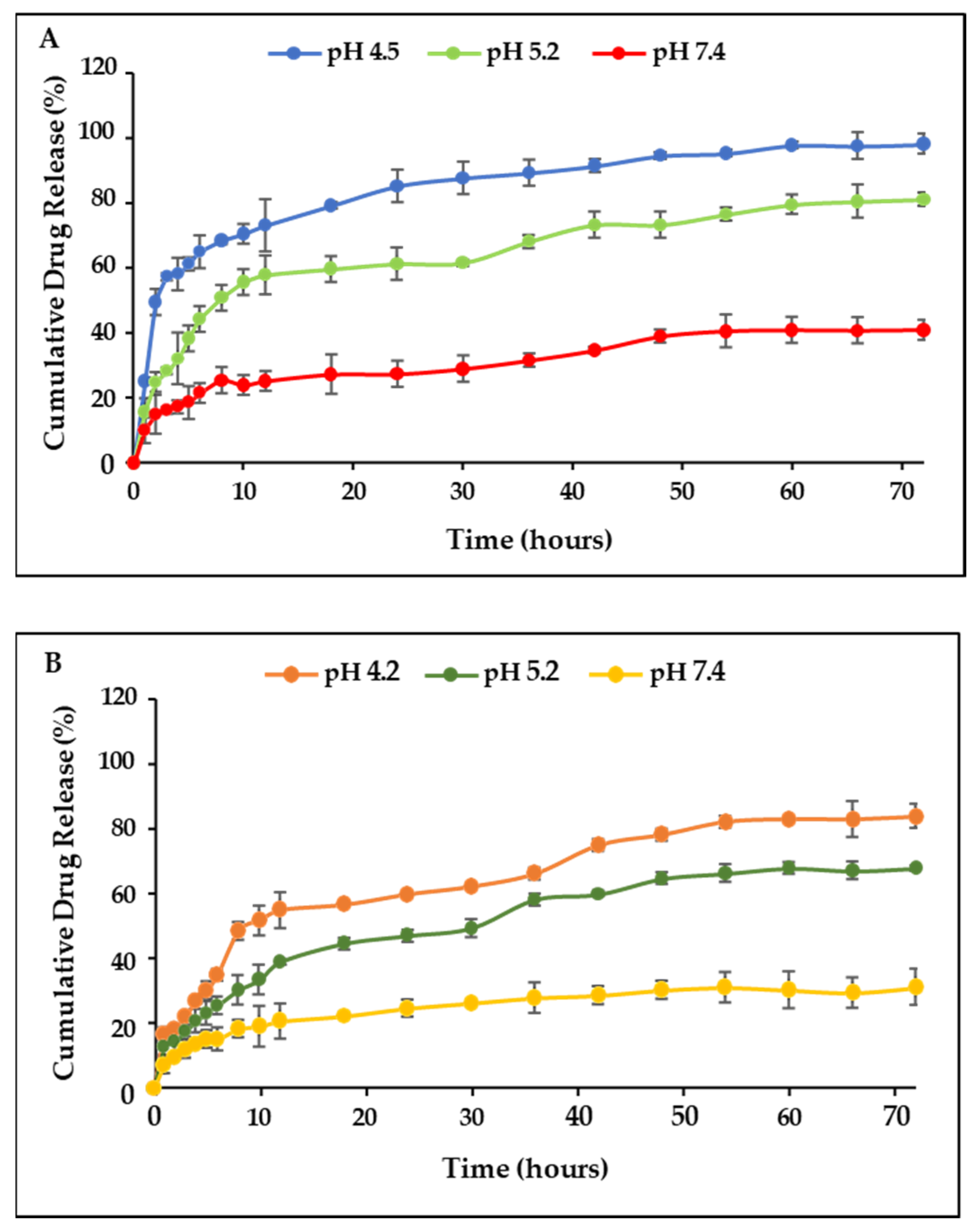
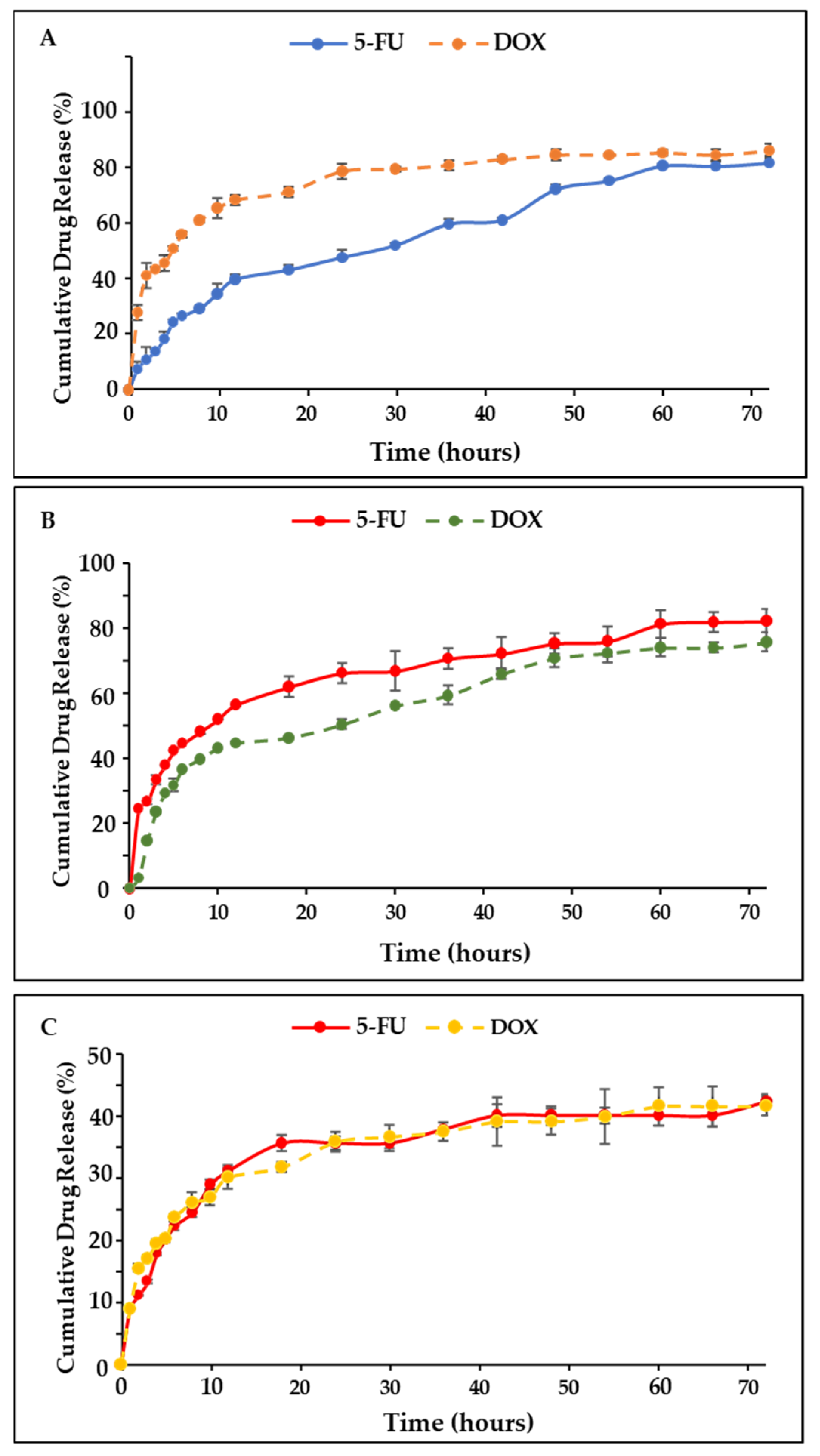
2.7. Drug Release and Kinetics Studies
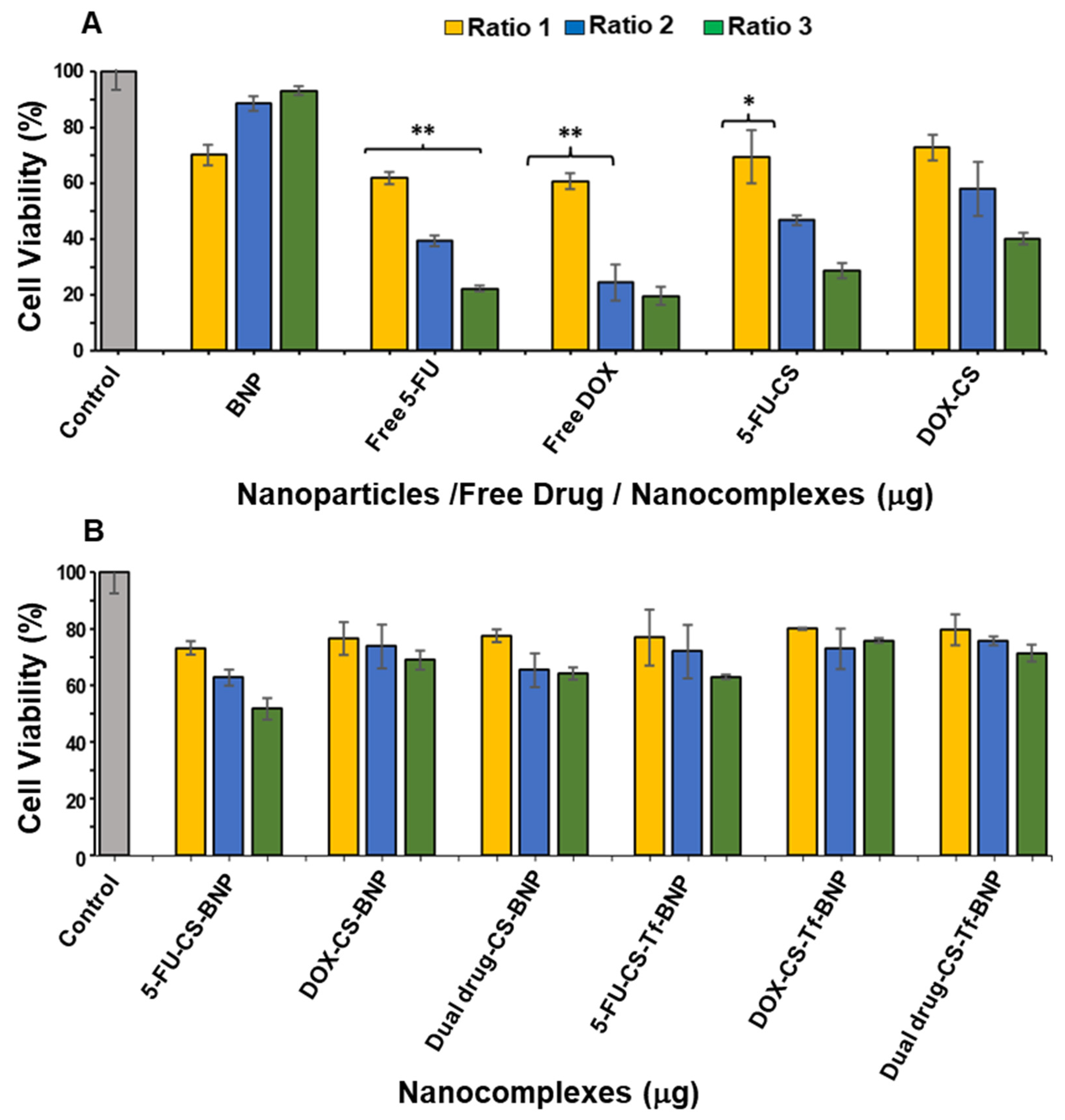
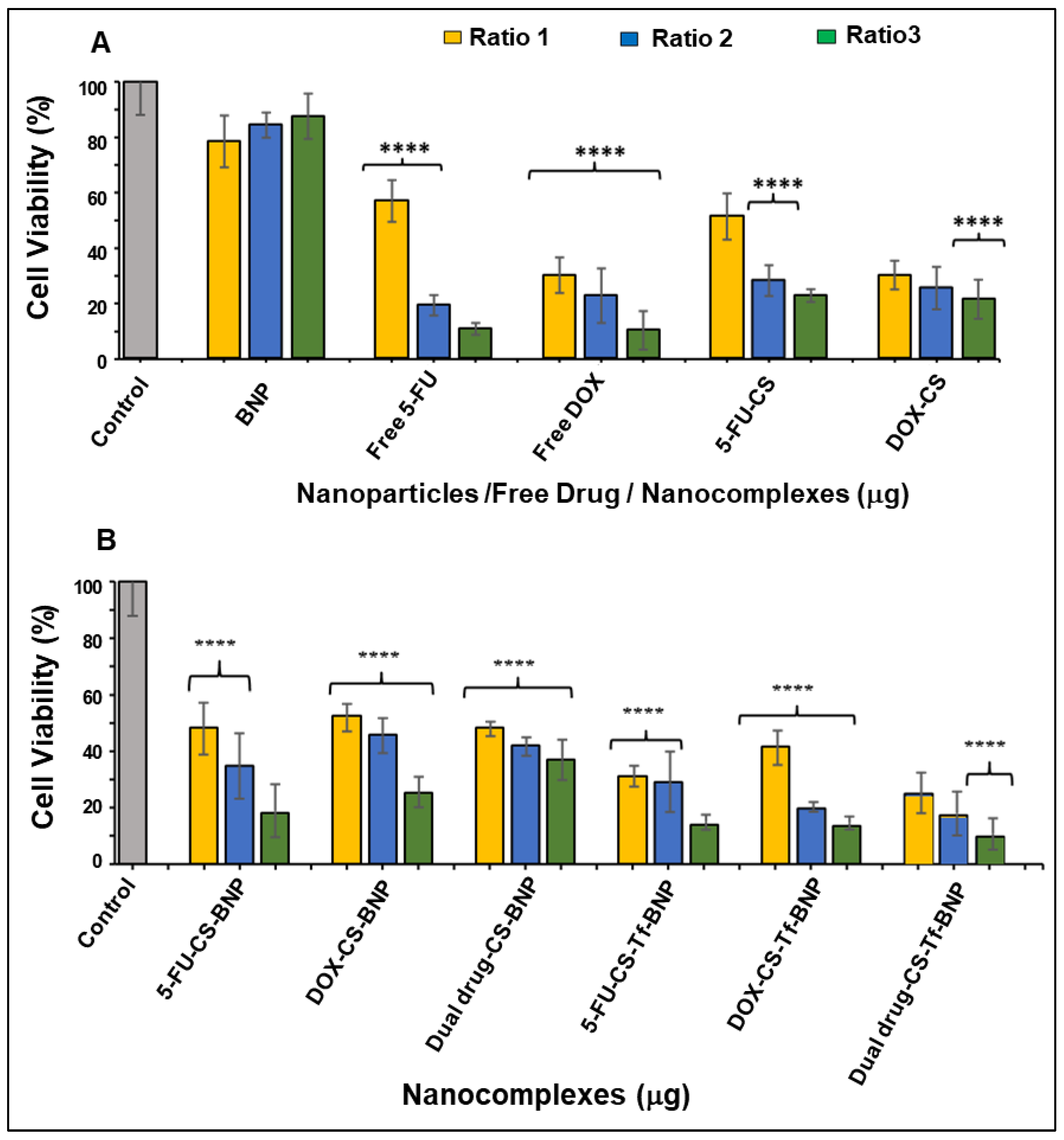
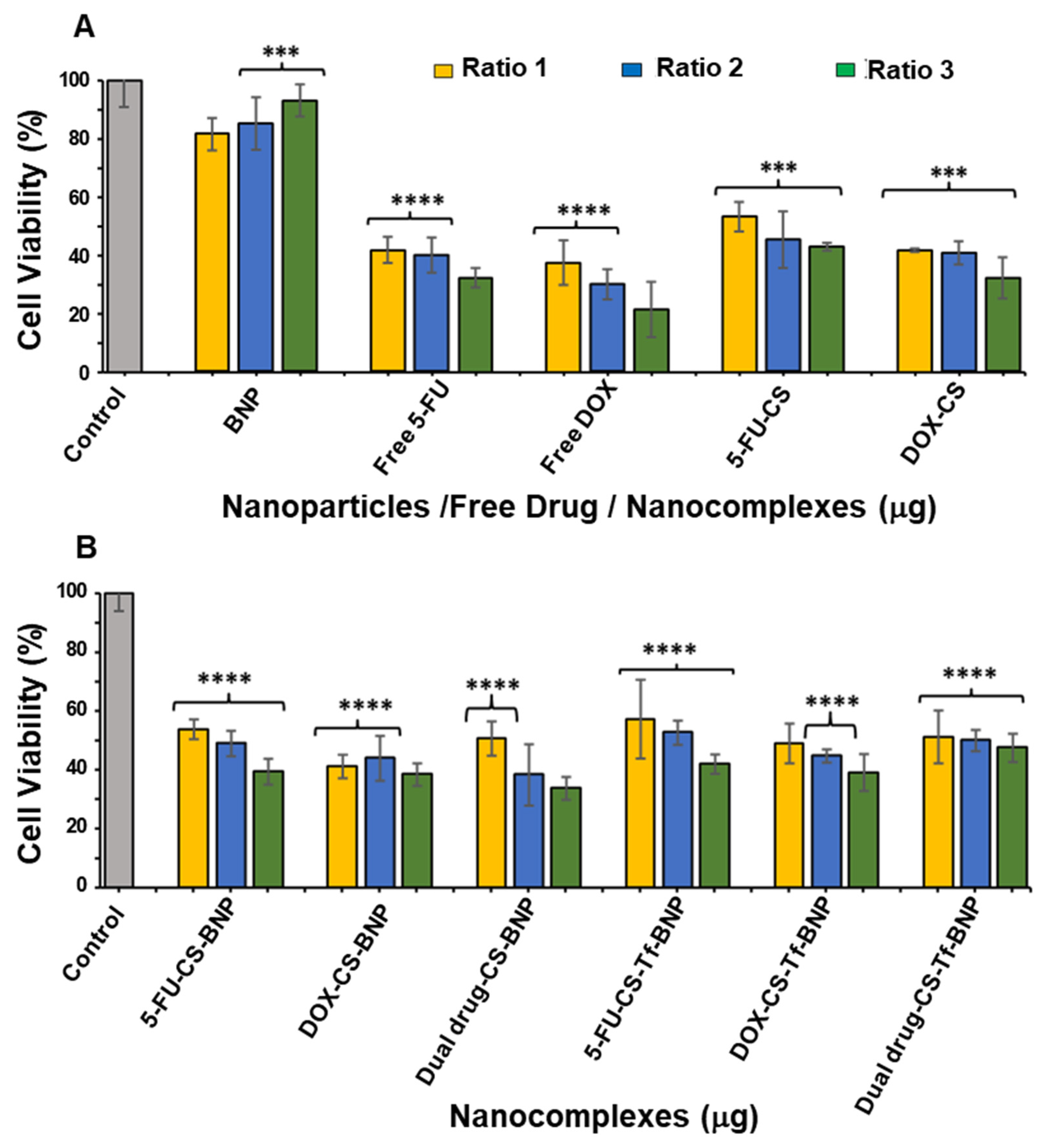
2.8. In Vitro Cytotoxicity
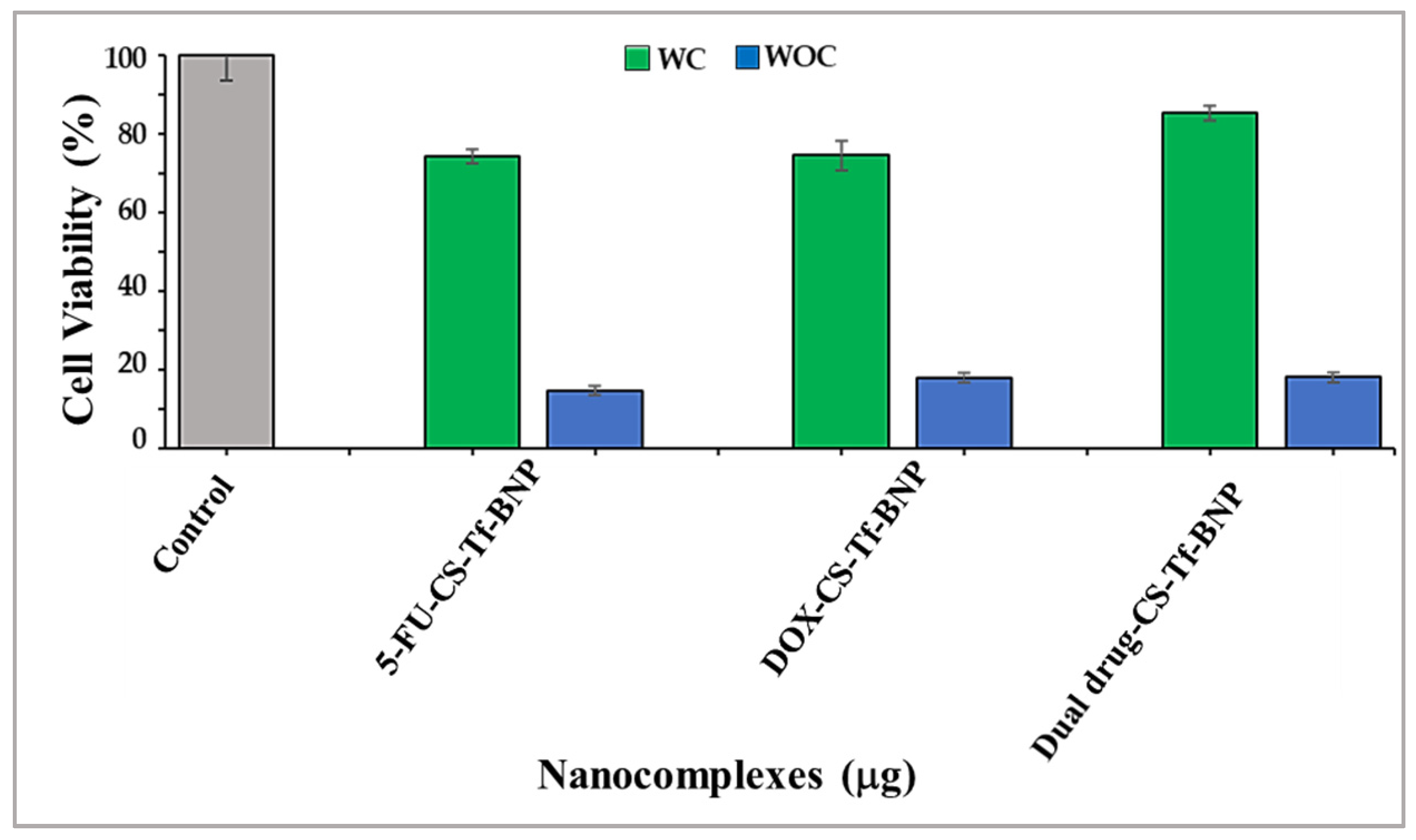
2.9. Receptor Binding Competition Study
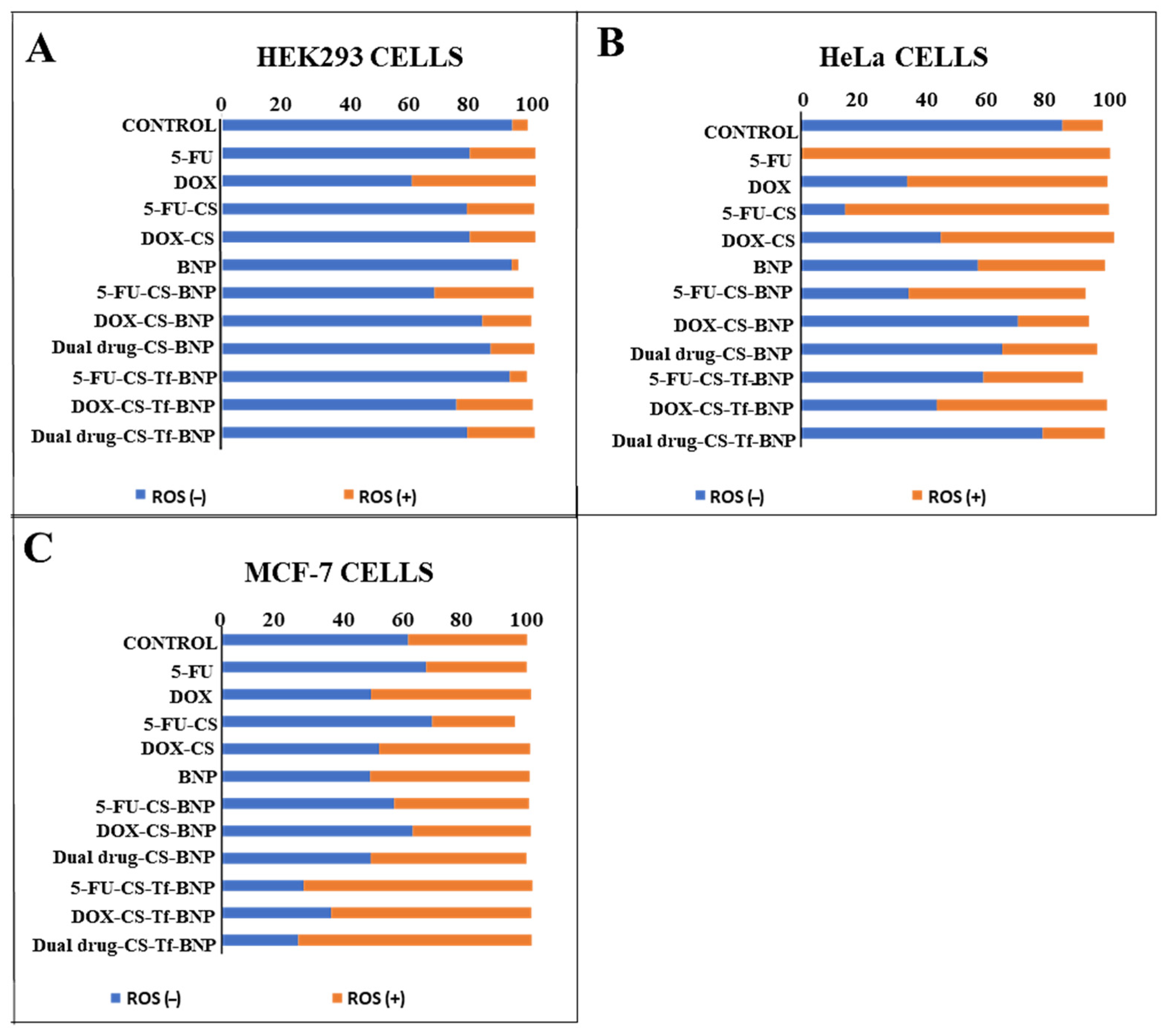
2.10. Intracellular Oxidative Stress Analysis
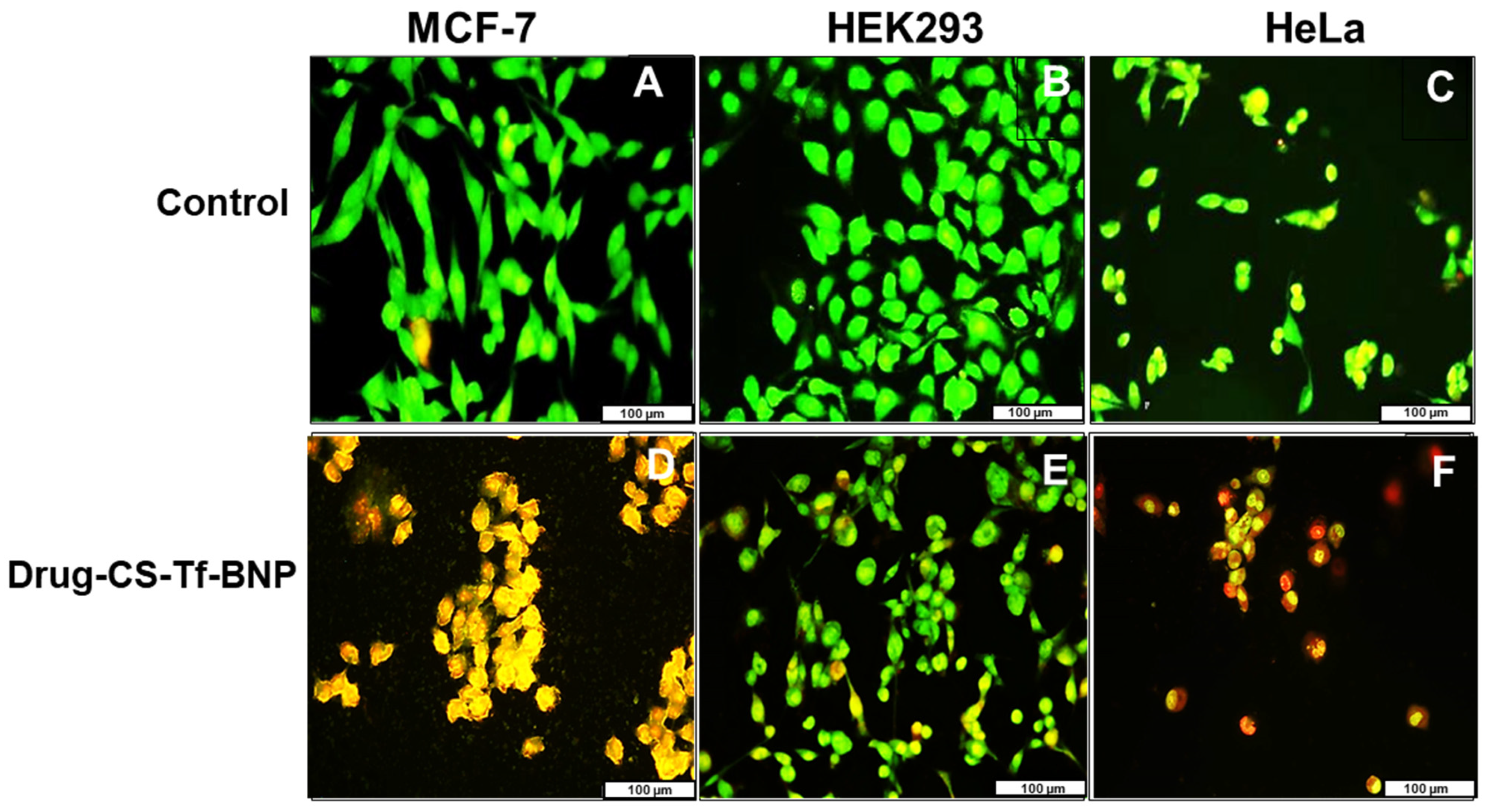
2.11. Fluorescent Apoptosis Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Dihydrogentetrachloro-Palladate (H2PdCl4)
4.2.2. Synthesis of Gold Nanoparticles (AuNPs) and the AuNP-Core
4.2.3. Synthesis of Gold-Palladium (AuPd) Bimetallic Nanoparticles (BNPs)
4.2.4. Synthesis of 5-FU and DOX-CS
4.2.5. Binding of BNP to 5-FU-CS and DOX-CS
4.2.6. Formation of Dual Drug-CS-BNP
4.2.7. Synthesis of Transferrin (Tf) Targeted BNP Nanocomplexes
4.2.8. UV-Visible (UV-Vis) Spectroscopy
4.2.9. Fourier Transform Infrared (FTIR) Spectroscopy
4.2.10. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
4.2.11. X-Ray Diffraction Analysis (XRD)
4.2.12. Transmission Electron Microscopy (TEM)
4.2.13. Nanoparticle Tracking Analysis (NTA)
4.2.14. Drug Encapsulation Efficiency
4.2.15. Drug Release Profiles and Kinetic Studies
4.2.16. In Vitro Cytotoxicity—MTT Assay
4.2.17. Receptor Binding Competition Assay
4.2.18. Intracellular Oxidative Stress Analysis
4.2.19. Apoptosis Assay
4.2.20. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| DOX | Doxorubicin |
| AuNP’s | Gold nanoparticles |
| PdNP’s | Palladium nanoparticles |
| BNP’s | Bimetallic nanoparticles |
| CS | Chitosan |
| NTA | Nanoparticle tracking analysis |
| HEK293 | Human embryonic kidney |
| HRTEM | High resolution transmission electron microscopy |
| Mw | Molecular weight |
| mM | Millimolar |
| g | Grams |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| gml | Grams per ml |
| M | Molar |
| ml | Millilitre |
| nm | Nanometre |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| FTIR | Fourier transform infra-red analysis |
| XRD | X-Ray diffraction analysis |
| ICP | Inductively coupled plasma |
| ul | Microlitre |
| mV | Millivolt |
| Tf | Transferrin |
References
- Deo, S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 report on global cancer burden: Challenges and opportunities for surgical oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Najafi, M. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef]
- Sand, F.L.; Urbute, A.; Ring, L.L.; Kjaer, A.K.; Belmonte, F.; Kjaer, S.K. The influence of overweight and obesity on participation in cervical cancer screening: A systematic review and meta-analysis. Prev. Med. 2023, 172, 107519. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Nanomedicine-mediated optimization of Immuno-therapeutic approaches in Cervical cancer. Nanomedicine 2021, 16, 1311–1328. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Cervical Cancer: A meta-analysis, Therapy and future of Nanomedicine. Ecancermedicalscience 2020, 14, 1111. [Google Scholar] [CrossRef]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef]
- Venkatas, J.; Singh, M. Curcumin-reduced gold nanoparticles facilitate IL-12 delivery to a cervical cancer in vitro cell model. Nanomedicine 2023, 18, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Wei, M.; Shang, L.; Yang, Y.W. Cucurbiturils-mediated noble metal nanoparticles for applications in sensing, sers, theranostics, and catalysis. Adv. Funct. Mater. 2021, 31, 2007277. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, J.; Wang, B.; Chen, L.; Tan, S. Recent advances in the development of noble metal NPs for cancer therapy. Bioinorg. Chem. Appl. 2022, 2022, 2444516. [Google Scholar] [CrossRef]
- Bugwandeen, A.; Singh, K.; Daniels, A.; Singh, D.; David, L.L.; Singh, M. In Vitro Cytotoxicity profiles of some Polymers and Inorganic Nanoparticles commonly used in Nanomedicine. Curr. Topics Toxicol. 2023, 19, 1–11. [Google Scholar]
- Yaqoob, S.B.; Adnan, R.; Khan, M.; Rashid, M. Gold, silver and palladium nanoparticles as biomedical agents. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Klebowski, B.; Depciuch, J.; Parlinska Wojtan, M.; Baran, J. Gold decorated palladium and platinum nanoparticles as radiosensitisers for cancer therapy. Pharmaceutics 2021, 13, 1726. [Google Scholar] [CrossRef]
- Nagarajan, S.B.; Ramakrishnan, S.; Jayaraman, A. Theranostic aspects of palladium based bimetallic nanoparticles in biomedical field: A state-of-the-art. Health Care Sci. 2024, 3, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, E.; Gonzalez-Torres, F.; Beltrán, V.; Barrias, P.; Bonardd, S.; Ramírez, A.; Ahumada, M. Ag@Au bimetallic nanoparticles: An easy and highly reproducible synthetic approach for photocatalysis. Nanoscale Adv. 2022, 4, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 105. [Google Scholar] [CrossRef]
- Frank, L.; Onzi, G.; Morawski, A.; Pohlmann, A.; Guterres, S.; Contri, R. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and future prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Gold Nanoparticle-Based Hydrogel: Application in Anticancer Drug Delivery and Wound Healing In Vitro. Pharmaceutics 2025, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohyd. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Maney, V.; Singh, M. An in vitro assessment of Chitosan/ Bimetallic PtAu nanocomposites as delivery vehicles for Doxorubicin. Nanomedicine 2017, 12, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent advances in designing 5-fluorouracil delivery systems: A stepping stone in the safe treatment of colorectal cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Akinyelu, J.; Oladimeji, O.; Daniels, A.; Singh, M. Folate-Targeted Doxorubicin Delivery to Breast and Cervical Cancer cells using a Chitosan-Gold Nano-delivery System. J. Drug Deliv. Sci. Technol. 2022, 67, 102978. [Google Scholar] [CrossRef]
- Dongsar, T.T.; Dongsar, T.S.; Gupta, N.; Almalki, W.H.; Sahebkar, A.; Kesharwani, P. Emerging potential of 5-Fluorouracil-loaded chitosan nanoparticles in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104371. [Google Scholar] [CrossRef]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M. Enhanced cytotoxic effect of Doxorubicin conjugated gold nanoparticles on breast cancer model. BMC Chem. 2022, 16, 90. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin an agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhao, B.; Wang, Y.; Song, M.; Ye, J.; Liang, J.; Liu, B.; Wang, L. Co-delivery of 5 fluorodeoxyuridine and doxorubicin by DNA nanocarriers enhances combination chemotherapy. Int. J. Nanomed. 2020, 15, 7737–7750. [Google Scholar]
- Bilgin, S. Apoptotic effect of 5 fluorouracil doxorubicin combination on colorectal cancer cell monolayers and spheroids. Mol. Biol. Rep. 2024, 51, 603. [Google Scholar] [CrossRef]
- Yosefi, S.; Madanchi, H.; Pakdel, A.; Kokhaei, P.; Hemati, M.; Sarmadi, N.; Sirati-Sabet, M. Combinatorial Effects of Chrysin with Doxorubicin, 5-Fluorouracil, and Cyclophosphamide on Triple-Negative Breast Cancer Cell Line. Iran. J. Pharm. Res. 2025, 24, e157446. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Song, J.; Liu, X.; Liu, S.; Yang, N.; Wang, L.; Liu, Y.; Zhao, Y.; Zhou, W.; et al. Tumor cell targeting and tumor microenvironment responsive nanoplatforms for multimodal imaging guided photodynamic, photothermal and chemodynamic treatment of cervical cancer. Int. J. Nanomed. 2024, 19, 5837–5858. [Google Scholar] [CrossRef]
- Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.; Aleykutty, N.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-conjugated docetaxel–PLGA nanoparticles for tumor targeting: Influence on MCF-7 cell cycle. Polymers 2019, 11, 1905. [Google Scholar] [CrossRef]
- Singh, M.; Hawtrey, A.; Ariatti, M. Lipoplexes with biotinylated transferrin accessories: Novel, targeted, serum-tolerant gene carriers. Int. J. Pharm. 2006, 321, 124–137. [Google Scholar] [CrossRef]
- Xu, X.; Liu, T.; Wu, J.; Wang, Y.; Hong, Y.; Zhou, H. Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer. Cancer Gene Ther. 2019, 26, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Wan, C.-C.; Wang, Y.-Y. Chemical preparation of Pd nanoparticles in room temperature ethylene glycol system and its application to electroless copper deposition. J. Colloid Interface Sci. 2006, 297, 143–150. [Google Scholar] [CrossRef]
- Ho, P.-F.; Chi, K.-M. Size-controlled synthesis of Pd nanoparticles from β-diketonato complexes of palladium. Nanotechnology 2004, 15, 1059. [Google Scholar] [CrossRef]
- Akinyelu, J.; Singh, M. Folate-tagged chitosan-functionalised gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Suhail, M.; Hsieh, Y.-H.; Khan, A.; Minhas, M.U.; Wu, P.-C. Preparation and in vitro evaluation of aspartic/alginic acid-based semi-interpenetrating network hydrogels for controlled release of ibuprofen. Gels 2021, 7, 68. [Google Scholar] [CrossRef]
- Nami-Ana, S.; Nasresfahani, S.; Tashkhourian, J.; Shamsipur, M.; Zargarpour, Z.; Sheikhi, M. Nanofibers of polyaniline and Cu (II)–l-aspartic acid for a room-temperature carbon monoxide gas sensor. ACS Appl. Mater. Interfaces 2021, 13, 39791–39805. [Google Scholar] [CrossRef]
- Duca, G.; Anghel, L.; Erhan, R.V. Structural aspects of lactoferrin and serum transferrin observed by FTIR spectroscopy. Chem. J. Moldova 2018, 13, 111–116. [Google Scholar] [CrossRef]
- Samy, M.; Abd El-Alim, S.H.; Amin, A.; Ayoub, M.M. Formulation, characterisation and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 156, 783–791. [Google Scholar] [CrossRef]
- Yassin, A.E.B.; Anwer, M.K.; Mowafy, H.A.; El-Bagory, I.M.; Bayomi, M.A.; Alsarra, I.A. Optimisation of 5-flurouracil solid-lipid nanoparticles: A preliminary study to treat colon cancer. Int. J. Med. Sci. 2010, 7, 398. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Hoang, T.M.N.; Mai, T.T.T.; Nguyen, T.Q.T.; Do, H.D.; Pham, T.H.; Nguyen, T.L.; Ha, P.T. Enhanced cellular uptake and cytotoxicity of folate decorated Doxorubicin loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025005. [Google Scholar] [CrossRef]
- Mohamed, N. Synthesis of hybrid chitosan silver nanoparticles loaded with Doxorubicin with promising anti-cancer activity. BioNanoScience 2020, 10, 758–765. [Google Scholar] [CrossRef]
- Yan, X.-s.; Lin, P.; Qi, X.; Yang, L. Finnis–Sinclair potentials for fcc Au–Pd and Ag–Pt alloys. Int. J. Mater. Res. 2011, 102, 381–388. [Google Scholar] [CrossRef]
- Majimel, J.; Lamirand-Majimel, M.; Moog, I.; Féral-Martin, C.; Tréguer-Delapierre, M. Size-dependent stability of supported gold nanostructures onto ceria: An HRTEM study. J. Phys. Chem. C 2009, 113, 9275–9283. [Google Scholar] [CrossRef]
- Ley, S.V.; Mitchell, C.; Pears, D.; Ramarao, C.; Yu, J.-Q.; Zhou, W. Recyclable polyurea-microencapsulated Pd (0) nanoparticles: An efficient catalyst for hydrogenolysis of epoxides. Org. Lett. 2003, 5, 4665–4668. [Google Scholar] [CrossRef]
- Biriukov, D.; Fibich, P.; Předota, M. Zeta potential determination from molecular simulations. J. Phys. Chem. C 2020, 124, 3159–3170. [Google Scholar] [CrossRef]
- Kettemann, F.; Birnbaum, A.; Witte, K.; Greiner, A.M.; Tschulik, K.; Huber, K. Reliable palladium nanoparticle syntheses in aqueous solution. CrystEngComm 2015, 17, 1865–1875. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Kiely, C.J. Strategies for the synthesis of supported gold palladium nanoparticles with controlled morphology and composition. Acc. Chem. Res. 2013, 46, 1759–1772. [Google Scholar] [CrossRef]
- Mahmudi, H.; Fathi, M.; Alavi, S.E.; Akbarzadeh, A. Tumour microenvironment penetrating chitosan nanoparticles for pH responsive drug delivery. J. Nanobiotechnol. 2022, 20, 410. [Google Scholar]
- Meng, F.; Zhong, Y.; Cheng, R.; Deng, C.; Zhong, Z. pH sensitive polymeric nanoparticles for tumor targeting doxorubicin delivery: Concept and recent advances. Nanomedicine 2014, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained release from nanocarriers a review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Javaid, S.; Javed, H.; Asim, L.; Shahid, I.; Khan, M.; Muhammad, A. Addressing artefacts of colourimetric anti-cancer assays for plant-based drug development. Med. Oncol. 2022, 39, 198. [Google Scholar] [CrossRef] [PubMed]
- Maney, V.; Singh, M. The synergism of platinum-gold bimetallic Nanoconjugates enhances 5-fluorouracil delivery in vitro. Pharmaceutics 2019, 11, 439. [Google Scholar] [CrossRef]
- Singh, P.; Jaiswal, A. Investigating the Performance of Near-Infrared Light Responsive Monometallic Gold and Bimetallic Gold-Palladium Nanorattles towards Plasmonic Photothermal Therapy. ChemistrySelect 2022, 7, e202103877. [Google Scholar] [CrossRef]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents into cancer cells. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update: The role of TfR1 in physiology and cancer. Crit. Rev. Oncol. Hematol. 2019, 132, 30–38. [Google Scholar]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.G.; Ku, S.K.; Yong, C.S.; et al. Transferrin conjugated polymeric nanoparticle for receptor mediated delivery of doxorubicin in doxorubicin resistant breast cancer cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS mediated mechanisms a radical therapeutic approach. Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5 fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Cytotoxicity, antioxidant and apoptosis studies of Quercetin-3-O-glucoside and 4-(β-D-Glucopyranosyl-1→4-α-L-Rhamnopyranosyloxy)-benzyl isothiocyanate from Moringa oleifera. Anti-Cancer Agents Med. Chem. 2016, 16, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Okitsu, K.; Maeda, Y.; Yamamoto, T.A.; Oshima, R.; Nagata, Y. Sonochemical preparation of bimetallic nanoparticles of gold/palladium in aqueous solution. J. Phys. Chem. B 1997, 101, 7033–7037. [Google Scholar] [CrossRef]
- Mosleh, I.; Abbaspourrad, A. Peptide-directed Pd-decorated Au and PdAu nanocatalysts for degradation of nitrite in water. RSC Adv. 2021, 11, 32615–32621. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Karmakar, S. Particle size distribution and zeta potential based on dynamic light scattering: Techniques to characterise stability and surface charge distribution of charged colloids. Recent Trends Mater. Phys. Chem. 2019, 28, 117–159. [Google Scholar]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical characterisation of nanoparticle size and surface modification using particle scattering diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Salem, D.S.; Hegazy, S.F.; Obayya, S.S. Nanogold-loaded chitosan nanocomposites for pH/light-responsive drug release and synergistic chemo-photothermal cancer therapy. Colloid Interface Sci. Commun. 2021, 41, 100361. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Preparation and characterisation of alginate-PVA-based semi-IPN: Controlled release pH-responsive composites. Polym. Bull. 2018, 75, 1075–1099. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.-K. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Abdouss, H.; Pourmadadi, M.; Zahedi, P.; Abdouss, M.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Green synthesis of chitosan/polyacrylic acid/graphitic carbon nitride nanocarrier as a potential pH-sensitive system for curcumin delivery to MCF-7 breast cancer cells. Int. J. Biol. Macromol. 2023, 242, 125134. [Google Scholar] [CrossRef]
- Sukhodub, L.; Kumeda, M.; Sukhodub, L.; Bielai, V.; Lyndin, M. Metal ions doping effect on the physicochemical, antimicrobial, and wound healing profiles of alginate-based composite. Carbohyd. Polym. 2023, 304, 120486. [Google Scholar] [CrossRef]
- Tian, X.-P.; Wang, C.-Y.; Jin, X.-H.; Li, M.; Wang, F.-W.; Huang, W.-J.; Yun, J.-P.; Xu, R.-H.; Cai, Q.-Q.; Xie, D. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics 2019, 9, 1965–1979. [Google Scholar] [CrossRef]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of magnetic and supermagnetic nanoparticles in new approach of targeting drug delivery and cancer therapy application. Drug Metab. Rev. 2020, 52, 157–184. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- David, L.L.; Daniels, A.; Habib, S.; Singh, M. Gold Nanoparticles in Transferrin-targeted dual-drug delivery in vitro. J. Drug Deliv. Sci. Technol. 2023, 90, 105168. [Google Scholar] [CrossRef]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Potential roles of the glass transition temperature of PLGA microparticles in drug release kinetics. Mol. Pharm. 2020, 18, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Stiepel, R.T.; Pena, E.S.; Ehrenzeller, S.A.; Gallovic, M.D.; Lifshits, L.M.; Genito, C.J.; Bachelder, E.M.; Ainslie, K.M. A predictive mechanistic model of drug release from surface eroding polymeric nanoparticles. J. Control. Release 2022, 351, 883–895. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Ettlinger, R.; Lächelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.E.; Wuttke, S. Toxicity of metal–organic framework nanoparticles: From essential analyses to potential applications. Chem. Soc. Rev. 2022, 51, 464–484. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2021, 8, e08674. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [PubMed]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Weng, M.-F.; Chiang, S.-Y.; Wang, N.-S.; Niu, H. Fluorescent nanodiamonds for specifically targeted bioimaging: Application to the interaction of transferrin with transferrin receptor. Diam. Relat. Mater. 2009, 18, 587–591. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Tan, X.; Li, J.; Song, C.; Zhang, R.; Cui, Y. A straightforward route to the synthesis of a surface-enhanced Raman scattering probe for targeting transferrin receptor-overexpressed cells. Nanotechnology 2010, 21, 345101. [Google Scholar] [CrossRef] [PubMed]
- David, L.L.; Singh, M. Palladium nanoparticles: Potential for receptor-mediated drug delivery in vitro. Nano-Struct. Nano-Objects 2025, 41, 101428. [Google Scholar] [CrossRef]
- Zenze, M.; Singh, M. Receptor targeting using copolymer-modified gold nanoparticles for pCMV-Luc gene delivery to liver cancer cells in vitro. Int. J. Mol. Sci. 2024, 25, 5016. [Google Scholar] [CrossRef]
- Arslanbaeva, L.R.; Santoro, M.M. Adaptive Redox homeostasis in cutaneous melanoma. Redox Biol. 2020, 37, 101753. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615. [Google Scholar] [CrossRef]
- Liu, C.-L.; Hsu, Y.-C.; Lee, J.-J.; Chen, M.-J.; Lin, C.-H.; Huang, S.-Y.; Cheng, S.-P. Targeting the pentose phosphate pathway increases reactive oxygen species and induces apoptosis in thyroid cancer cells. Mol. Cell. Endocrinol. 2020, 499, 110595. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Ham, Y.-H.; Jason Chan, K.; Chan, W. Thioproline serves as an efficient antioxidant protecting human cells from oxidative stress and improves cell viability. Chem. Res. Toxicol. 2020, 33, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Hah, Y.-S.; Kim, W.Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef]
- Byvaltsev, V.A.; Bardonova, L.A.; Onaka, N.R.; Polkin, R.A.; Ochkal, S.V.; Shepelev, V.V.; Aliyev, M.A.; Potapov, A.A. Acridine orange: A review of novel applications for surgical cancer imaging and therapy. Front. Oncol. 2019, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Vikas, B.; Kunjiraman, S.; Rajam, S.S.N.; Anil, S. The Apoptotic Properties of Leaf Extracts of Simarouba glauca against Human Leukemic Cancer Cells. Asian Pac. J. Cancer Prev. 2021, 22, 1305. [Google Scholar] [CrossRef] [PubMed]
- Saranya, J.; Saminathan, P.; Ankireddy, S.R.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Cerium Oxide/Graphene Oxide Hybrid: Synthesis, Characterisation, and Evaluation of Anti-cancer Activity in a Breast Cancer Cell Line (MCF-7). Biomedicines 2023, 11, 531. [Google Scholar] [CrossRef]
- Vikas, B.; Sujathan, K.; Rajam, S.S.N.; Anil, S. Caspase-Dependent Apoptosis Induced by Simarouba Glauca on Human Non-Small-Cell Lung Cancer, A549 Cells. Asian Pac. J. Cancer Prev. 2022, 23, 1867. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Cui, P.; Liu, H.; Yang, J. Gold-catalysed formation of core–shell gold–palladium nanoparticles with palladium shells up to three atomic layers. J. Mater. Chem. A 2016, 4, 3813–3821. [Google Scholar] [CrossRef]
- Shanthi, K.; Vimala, K.; Gopi, D.; Kannan, S. Fabrication of a pH responsive DOX conjugated PEGylated palladium nanoparticle mediated drug delivery system: An in vitro and in vivo evaluation. RSC Adv. 2015, 5, 44998–45014. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Cerea, M.; Maroni, A.; Palugan, L.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Oral hydrophilic matrices having non uniform drug distribution for zero-order release: A literature review. J. Control. Release 2020, 325, 72–83. [Google Scholar] [CrossRef]
- Zeng, L.; An, L.; Wu, X. Modeling drug-carrier interaction in the drug release from nanocarriers. J. Drug Deliv. 2011, 2011, 370308. [Google Scholar] [CrossRef] [PubMed]
- Santadkha, T.; Skolpap, W.; Thitapakorn, V. Diffusion Modelling and In Vitro Release Kinetics Studies of Curcumin—Loaded Superparamagnetic Nanomicelles in Cancer Drug Delivery System. J. Pharm. Sci. 2022, 111, 1690–1699. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Hixson, A.; Crowell, J. Dependence of reaction velocity upon surface and agitation. Indust. Eng. Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: Effect of entrapped air. J. Pharm. Sci. 1983, 72, 1189–1191. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anti-cancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilising Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2020, 7, 779–786. [Google Scholar] [CrossRef]
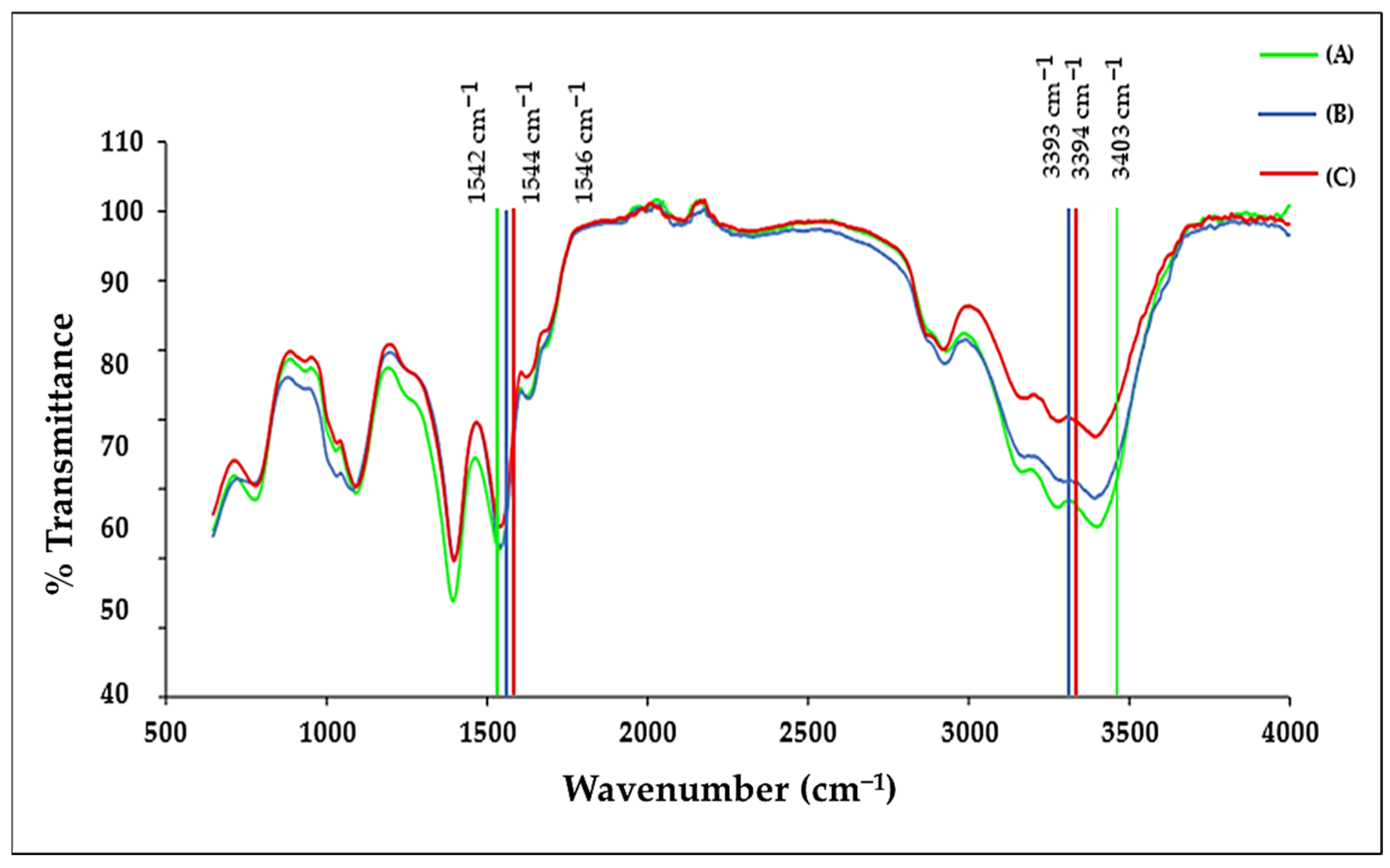
| Molecule | Characteristic Peak (cm−1) | Assignment |
|---|---|---|
| Chitosan (CS) | 3000–3600 (broad) | O–H and N–H stretching |
| 1648 | Amide I (C=O stretch) | |
| Transferrin (Tf) | 1635 | Amide I/histidine stretching |
| 1402 | Aspartic acid—COO− symmetric stretch | |
| 5-FU (5-FU) | 2800–3100 | C–H (imide) stretching |
| 1431–1658 | C=C and C=N stretching | |
| ~1398 | Aromatic ring vibration | |
| Doxorubicin (DOX) | 3382 | O–H stretch |
| 1618 | C=O/aromatic stretching |
| No. | h | k | l | d [Å] | 2θ [°] | I [%] |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 2.33249 | 45.101 | 100.0 |
| 2 | 0 | 0 | 2 | 2.02000 | 52.568 | 47.9 |
| 3 | 0 | 2 | 2 | 1.42836 | 77.548 | 28.8 |
| 4 | 1 | 1 | 3 | 1.21811 | 94.503 | 32.7 |
| 5 | 2 | 2 | 2 | 1.16625 | 100.169 | 9.4 |
| Sample | Size (nm) | Zeta Potential (mV) | PDI (σ/D)2 |
|---|---|---|---|
| AuNP | 73.4 ± 9.8 | −16.3 ± 2.3 | 0.101239669 |
| 5-FU-CS | 130.3 ± 13.6 | 21.4 ± 0.2 | 0.16599859 |
| DOX-CS | 128.7 ± 3.5 | 18.3 ± 0.8 | 0.054837146 |
| 5-FU-CS-BNP | 85.9 ± 8.4 | 16.1 ± 0.3 | 0.108083068 |
| DOX-CS-BNP | 96.5 ± 48 | 5.4 ± 6.5 | 0.164671288 |
| Dual-Drug-CS-BNP | 91.8 ± 47.2 | 2.8 ± 0.4 | 0.194212819 |
| 5-FU-CS-Tf-BNP | 105.6 ± 10.7 | 16.9 ± 0.3 | 0.138109753 |
| DOX-CS-Tf-BNP | 107.1 ± 18.1 | 14.4 ± 1.8 | 0.098792686 |
| Dual-Drug-CS-Tf-BNP | 112.8 ± 0.9 | 9.4 ± 1.9 | 0.081632653 |
| Day | Bimetallic Nanoparticle Size (nm) | Zeta Potential (mV) |
|---|---|---|
| 0 | 73.4 ± 9.8 nm | −28.7 ± 0.2 mV |
| 7 | 52.1 ± 2.9 nm | −36.2 ± −7.0 mV |
| 14 | 83.8 ± 21.9 nm | −31.5 ± 0.4 mV |
| 21 | 94.0 ± 6.4 nm | −25.7 ± 0.4 mV |
| 28 | 61.5 ± 4.3 nm | −27.3 ± 6.0 mV |
| 216 | 76.01 ± 5.9 nm | −69.27 ± 2.0 mV |
| pH | Zero-Order | First-Order | Higuchi’s | Hixon-Crowell’s | Korsmeyer-Peppa’s | Kopcha’s |
|---|---|---|---|---|---|---|
| 4.2 | 0.8756 | 0.9143 | 0.963 | 0.9029 | 0.5008 n = 0.52 | 0.9173 |
| 5.2 | 0.792 | 0.08674 | 0.9602 | 0.845 | 0.604 n= 0.43 | 0.9879 |
| 7.4 | 0.8201 | 0.873 | 0.9375 | 0.8569 | 0.6338 n= 0.56 | 0.9884 |
| pH | Kopcha Model | ||
|---|---|---|---|
| A | B | A/B | |
| 4.2 | 210.12 | 99.098 | 2.120325 |
| 5.2 | 16.132 | 0.266 | 60.64662 |
| 7.4 | 10.71 | 0.7688 | 13.9308 |
| pH | Zero-Order | First-Order | Higuchi’s | Hixon-Crowell’s | Korsmeyer-Peppa’s | Kopcha’s |
|---|---|---|---|---|---|---|
| 4.2 | 0.9522 | 0.9694 | 0.9559 | 0.9679 | 0.5972 n = 0.65 | 0.9223 |
| 5.2 | 0.8428 | 0.9087 | 0.9782 | 0.889 | 0.644 n = 0.43 | 0.9728 |
| 7.4 | 0.7659 | 0.8069 | 0.926 | 0.7938 | 0.6903 n = 0.30 | 0.9989 |
| pH | Kopcha Model | ||
|---|---|---|---|
| A | B | A/B | |
| 4.2 | 17.585 | 0.8439 | 20.83778 |
| 5.2 | 13.64 | 0.8158 | 16.71978 |
| 7.4 | 8.3373 | 0.5348 | 15.58957 |
| pH | Zero-Order | First-Order | Higuchi’s | Hixon-Crowell’s | Korsmeyer-Peppa’s | Kopcha’s |
|---|---|---|---|---|---|---|
| 5-FU/DOX | 5-FU/DOX | 5-FU/DOX | 5-FU/DOX | 5-FU/DOX | 5-FU/DOX | |
| 4.2 | 0.8535/0.7818 | 0.9232/0.8575 | 0.9734/0.9604 | 0.902/0.902 | 0.7554; n = 0.58/ 0.4422; n = 0.77 | 0.923/0.9785 |
| 5.2 | 0.8089/0.7172 | 0.9022/0.8243 | 0.9739/0.8996 | 0.8741/0.79 | 0.5029; n = 0.73/ 0.7209; n = 0.31 | 0.2472/0.9826 |
| 7.4 | 0.695/0.7216 | 0.7453/0.7807 | 0.8758/0.9186 | 0.727/0.7618 | 0.6833; n = 0.56/ 0.9857; n = 1 | 0.9859/0.9822 |
| pH | Kopcha Model | |||||
|---|---|---|---|---|---|---|
| A | B | A/B | ||||
| 5-FU | DOX | 5-FU | DOX | 5-FU | DOX | |
| 4.2 | 7.166 | 31.687 | 1.2963 | 1.4891 | 5.528041348 | 21.27929622 |
| 5.2 | 24.082 | 4.6556 | 2.0621 | 2.6393 | 11.67838611 | 1.763952563 |
| 7.4 | 9.1559 | 10.451 | 0.3707 | 0.5873 | 24.69894794 | 17.79499404 |
| Nanoparticle/Nanocomplex | Ratio | Nanocomplex Ratio Drug Conc | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| BNP | 1:0 | 0.134 µg | 0.268 µg | 0.402 µg |
| Free DOX | 1:0 | 0.1701 µg | 0.3401 µg | 0.5103 µg |
| Free 5-FU | 1:0 | 0.1701 µg | 0.3401 µg | 0.5105 µg |
| 5-FU-CS | 1:4 = 1 * | 0.1702 µg | 0.3404 µg | 0.5106 µg |
| DOX-CS | 1:5 = 1 * | 0.1701 µg | 0.3402 µg | 0.5103 µg |
| 5-FU-CS-BNP | 1:2 = 1 * | 0.1701 µg | 0.3403 µg | 0.5105 µg |
| DOX-CS-BNP | 1:2 = 1 * | 0.1701 µg | 0.3402 µg | 0.5103 µg |
| Dual Drug-CS-BNP | 1:1:1 = 1 * | 0.1701 µg | 0.3402 µg | 0.5104 µg |
| 5-FU-CS-Tf-BNP | 20:1 | 0.1701 µg | 0.3403 µg | 0.5105 µg |
| DOX-CS-Tf-BNP | 20:1 | 0.1701 µg | 0.3402 µg | 0.5103 µg |
| Dual Drug-CS-Tf-BNP | 20:1 | 0.1701 µg | 0.3402 µg | 0.5104 µg |
| Nanoparticle/Nanocomplex | IC50 Concentrations (µg) | ||
|---|---|---|---|
| HEK293 | HeLa | MCF-7 | |
| BNP | 4.90 | 3.12 | 5.56 |
| Free 5-FU | 0.062 | 0.463 | 0.062 |
| Free DOX | 0.103 | 0.351 | 0.038 |
| 5-FU-CS | 0.160 | 0.670 | 0.502 |
| DOX-CS | 0.133 | 0.244 | 0.136 |
| 5-FU-CS-BNP | 1.286 | 0.968 | 1.997 |
| DOX-CS-BNP | 0.650 | 0.183 | 0.972 |
| Dual Drug-CS-BNP | 3.183 | 0.491 | 0.763 |
| 5-FU-CS-Tf-BNP | 3.998 | 0.461 | 2.221 |
| DOX-CS-Tf-BNP | 3.741 | 0.221 | 1.324 |
| Dual Drug-CS-Tf-BNP | 3.104 | 0.0265 | 1.241 |
| Sample | HEK293 | HeLa | MCF-7 |
|---|---|---|---|
| BNP | 0.07 | 0.14 | 0.12 |
| Free 5-FU | 0.35 | 0.81 | 0.35 |
| Free DOX | 0.41 | 0.86 | 0.61 |
| 5-FU-CS | 0.31 | 0.72 | 0.29 |
| DOX-CS | 0.38 | 0.71 | 0.49 |
| 5-FU-CS-BNP | 0.29 | 0.77 | 0.47 |
| DOX-CS-BNP | 0.25 | 0.84 | 0.43 |
| Dual Drug-CS-BNP | 0.22 | 0.86 | 0.42 |
| 5-FU-CS-Tf-BNP | 0.19 | 0.85 | 0.28 |
| DOX-CS-Tf-BNP | 0.22 | 0.89 | 0.31 |
| Dual Drug-CS-Tf-BNP | 0.21 | 0.96 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
David, L.L.; Singh, M. Functionalized Core/Shell Gold-Palladium Bimetallic Nanoparticles in Transferrin-Targeted Dual-Drug Delivery in a Cervical Cancer Cell Model. Pharmaceuticals 2026, 19, 74. https://doi.org/10.3390/ph19010074
David LL, Singh M. Functionalized Core/Shell Gold-Palladium Bimetallic Nanoparticles in Transferrin-Targeted Dual-Drug Delivery in a Cervical Cancer Cell Model. Pharmaceuticals. 2026; 19(1):74. https://doi.org/10.3390/ph19010074
Chicago/Turabian StyleDavid, Lorenzo Lance, and Moganavelli Singh. 2026. "Functionalized Core/Shell Gold-Palladium Bimetallic Nanoparticles in Transferrin-Targeted Dual-Drug Delivery in a Cervical Cancer Cell Model" Pharmaceuticals 19, no. 1: 74. https://doi.org/10.3390/ph19010074
APA StyleDavid, L. L., & Singh, M. (2026). Functionalized Core/Shell Gold-Palladium Bimetallic Nanoparticles in Transferrin-Targeted Dual-Drug Delivery in a Cervical Cancer Cell Model. Pharmaceuticals, 19(1), 74. https://doi.org/10.3390/ph19010074







