FAPI Tracer en Vogue: Evaluating [68Ga]Ga-DATA5m.SA.FAPi for Molecular Imaging of Pulmonary Fibrosis
Abstract
1. Introduction
2. Results
2.1. [68Ga]Ga-DATA5m.SA.FAPi Uptake in BLM and Control Mice
2.2. [68Ga]Ga-DATA5m.SA.FAPi Uptake in fra-2tg Mice and WT Littermates
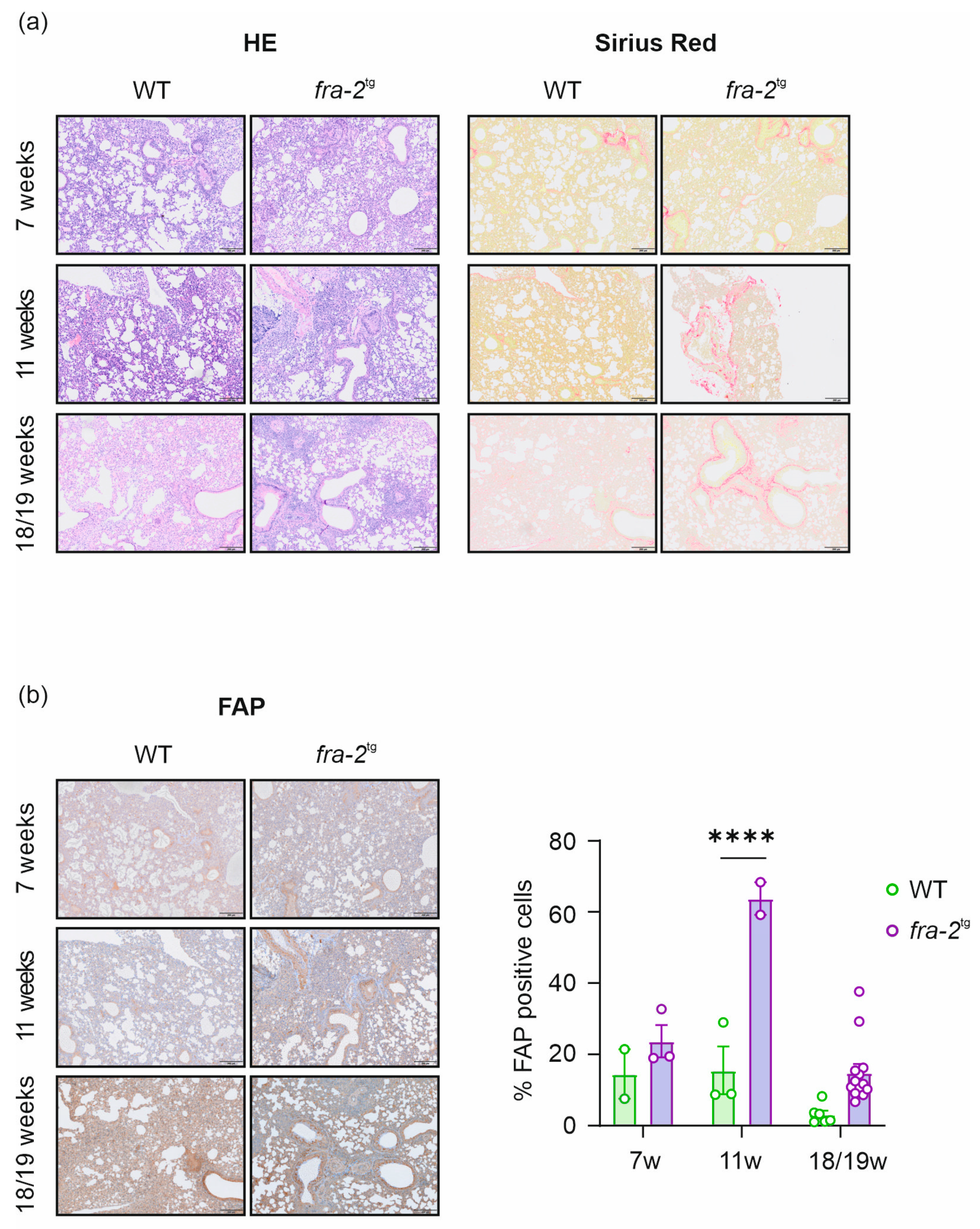
2.3. Histological and Immunohistochemical Analysis of the Lung from BLM, fra-2tg, and Control Mice
3. Discussion
4. Materials and Methods
4.1. Radiosynthesis of [68Ga]Ga-DATA5m.SA.FAPi
4.2. Mouse Models
4.2.1. Bleomycin-Treated Mice
4.2.2. fra-2tg/WT Mice
4.3. In Vivo Imaging and Data Analysis
4.4. Ex Vivo Biodistribution
4.5. Histological and Immunohistochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Amyloid light chain |
| BLM | Bleomycin |
| CT | Computed tomography |
| DATA | 2,2′-(6-((carboxymethyl)amino)-1,4-diazepane-1,4-diyl) diacetic acid |
| FAP | Fibroblast activation protein |
| FAPI | FAP inhibitor |
| fra-2 | Fos-related antigen 2 |
| Ga | Gallium |
| HCl | Hydrochloric acid |
| HE | Hematoxylin/Eosin |
| HPLC | High-performance liquid chromatography |
| HU | Hounsfield Units |
| ID | Injected dose |
| IHC | Immunohistochemistry |
| IPF | Idiopathic pulmonary fibrosis |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| MeCN | Acetonitrile |
| NSIP | Non-specific interstitial pneumonia |
| NH4OAc | Ammonium acetate |
| PET | Positron emission tomography |
| SA | Squaric acid |
| SUV | Standardized uptake value |
| Tg | Transgenic |
| TLC | Thin layer chromatography |
| WT | Wildtype |
References
- Kiani, M.; Jokar, S.; Hassanzadeh, L.; Behnammanesh, H.; Bavi, O.; Beiki, D.; Assadi, M. Recent Clinical Implications of FAPI Imaging and Therapy. Clin. Nucl. Med. 2024, 49, e538–e556. [Google Scholar] [CrossRef]
- Lartey, D.A.; Schilder, L.A.; Zwezerijnen, G.J.C.; D’Haens, G.R.A.M.; Grootjans, J.; Löwenberg, M. FAPi PET/CT Imaging to Identify Fibrosis in Immune-Mediated Inflammatory Diseases. Biomedicines 2025, 13, 775. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Atzinger, A.; Ramming, A.; Kuwert, T. FAPI PET/CT Immune-Fibrosis Imaging for New Insights into Rheumatologic Disorders. J. Nucl. Med. 2023, 64, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Q.; Zhang, Y.; Wang, J.; Wu, Y.; Yang, G.; Shi, J.; Wang, F.; Xu, Z.; Jing, H. 99mTc-Labeled FAPI SPECT Imaging in Idiopathic Pulmonary Fibrosis: Preliminary Results. Pharmaceuticals 2023, 16, 1434. [Google Scholar] [CrossRef]
- Ji, H.; Song, X.; Lv, X.; Shao, F.; Long, Y.; Song, Y.; Song, W.; Qiao, P.; Gai, Y.; Jiang, D.; et al. [68Ga]FAPI PET for Imaging and Treatment Monitoring in a Preclinical Model of Pulmonary Fibrosis: Comparison to [18F]FDG PET and CT. Pharmaceuticals 2024, 17, 726. [Google Scholar] [CrossRef]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-Art of FAPI-PET Imaging: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef]
- Song, Y.; Qin, C.; Chen, Y.; Ruan, W.; Gai, Y.; Song, W.; Gao, Y.; Hu, W.; Qiao, P.; Song, X.; et al. Non-Invasive Visualization of Liver Fibrosis with [68Ga]Ga-DOTA-FAPI-04 PET from Preclinical Insights to Clinical Translation. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3572–3584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, X.; Liu, H.; Luo, W.; Liu, H.; Lv, T.; Wang, J.; Qin, J.; Ou, S.; Chen, Y. Value of [68Ga]Ga-FAPI-04 Imaging in the Diagnosis of Renal Fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Johnsen, R.H.; Mortensen, J.; Shaker, S.B.; Nielsen, C.T.H. Radio-Labelled Fibroblast Activation Protein Inhibitors in Interstitial Lung Diseases—A Systematic Review. Autoimmun. Rev. 2025, 24, 103856. [Google Scholar] [CrossRef]
- Hou, P.; Chen, H.; Liang, S.; Guo, W.; Zhao, R.; Pan, H.; Liu, H.; Li, Y.; Lv, J.; Zhong, K.; et al. Targeting Fibroblast Activation Protein for Molecular Imaging of Fibrotic Remodeling in Pulmonary Arterial Hypertension. J. Nucl. Med. 2025, 66, 98–103. [Google Scholar] [CrossRef]
- Sun, R.; Huang, Y.; Deng, H.; Wang, Q.; Xiao, C.; Guo, C.; Wang, T.; Liu, L.; Hua, J.; Chen, X. 68Ga-FAPI PET/CT for Diagnostic Accuracy and Therapeutic Response Assessment in Bleomycin-Induced Pulmonary Fibrosis: An Integrated Preclinical Study. Front. Med. 2025, 12, 1613010. [Google Scholar] [CrossRef]
- Mori, Y.; Dendl, K.; Cardinale, J.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U. FAPI PET: Fibroblast Activation Protein Inhibitor Use in Oncologic and Nononcologic Disease. Radiology 2023, 306, e220749. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Distler, J.H.W.; Treutlein, C.; Tascilar, K.; Müller, A.T.; Atzinger, A.; Matei, A.E.; Knitza, J.; Györfi, A.H.; Lück, A.; et al. 68Ga-FAPI-04 PET-CT for Molecular Assessment of Fibroblast Activation and Risk Evaluation in Systemic Sclerosis-Associated Interstitial Lung Disease: A Single-Centre, Pilot Study. Lancet Rheumatol. 2021, 3, e185–e194. [Google Scholar] [CrossRef]
- Mori, Y.; Kramer, V.; Novruzov, E.; Mamlins, E.; Röhrich, M.; Fernández, R.; Amaral, H.; Soza-Ried, C.; Monje, B.; Sabbagh, E.; et al. Initial Results with [18F]FAPI-74 PET/CT in Idiopathic Pulmonary Fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1605–1611. [Google Scholar] [CrossRef]
- Rosenkrans, Z.T.; Massey, C.F.; Bernau, K.; Ferreira, C.A.; Jeffery, J.J.; Schulte, J.J.; Moore, M.; Valla, F.; Batterton, J.M.; Drake, C.R.; et al. [68Ga]Ga-FAPI-46 PET for Non-Invasive Detection of Pulmonary Fibrosis Disease Activity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3705–3716. [Google Scholar] [CrossRef] [PubMed]
- Sewald, K.; Kafert-Kasting, S. Fraunhofer Projekt FibroPaths. Available online: http://www.item.fraunhofer.de (accessed on 29 June 2022).
- Prasse, A.; Behr, J.; Hemholtz Zentrum München; Deutsches Zentrum für Lungenforschung. Lungenfibrose: Verbreitung. Available online: https://www.lungeninformationsdienst.de/krankheiten/lungenfibrose/verbreitung (accessed on 28 November 2022).
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting Fibrosis, Mechanisms and Clinical Trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Götz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Disentangling Inflammatory from Fibrotic Disease Activity by Fibroblast Activation Protein Imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491. [Google Scholar] [CrossRef]
- Durant, F.; Whited, J.L. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv. Sci. 2021, 8, 2100407. [Google Scholar] [CrossRef]
- Rodriguez, L.; Hecht, S. Iron(II)-Bleomycin. Biochemical and Spectral Properties in the Presence of Radical Scavengers. Biochem. Biophys. Res. Commun. 1982, 104, 1470–1476. [Google Scholar] [CrossRef]
- Williamson, J.D.; Sadofsky, L.R.; Hart, S.P. The Pathogenesis of Bleomycin-Induced Lung Injury in Animals and Its Applicability to Human Idiopathic Pulmonary Fibrosis. Exp. Lung Res. 2015, 41, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.; Chow, M.S.; Kemsley, J.N.; Lehnert, N.; Solomon, E.I. Direct Hydrogen-Atom Abstraction by Activated Bleomycin: An Experimental and Computational Study. J. Am. Chem. Soc. 2006, 128, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.V.; Iii, C.B.B.; Wong, S.D.; Wilson, S.A.; Kwak, Y.; Chow, M.S.; Zhao, J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I.; et al. Definition of the Intermediates and Mechanism of the Anticancer Drug Bleomycin Using Nuclear Resonance Vibrational Spectroscopy and Related Methods. Proc. Natl. Acad. Sci. USA 2010, 107, 22419–22424. [Google Scholar] [CrossRef]
- Umezawa, H.; Ishizuka, M.; Maeda, K.; Takeuchi, T. Studies on Bleomycin. Cancer 1967, 20, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; De Lena, M.; Monfardini, S.; Bartoli, C.; Bajetta, E.; Beretta, G.; Fossati-Bellani, F. Clinical Trials with Bleomycin in Lymphomas and in Solid Tumors; Pergamon Press: New York, NY, USA, 1972; Volume 8. [Google Scholar]
- Degryse, A.L.; Tanjore, H.; Xu, X.C.; Polosukhin, V.V.; Jones, B.R.; McMahon, F.B.; Gleaves, L.A.; Blackwell, T.S.; Lawson, W.E. Repetitive Intratracheal Bleomycin Models Several Features of Idiopathic Pulmonary Fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L442–L452. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, E.J.; Lee, S.Y.; Kim, J.H.; Shim, J.J.; Shin, C.; In, K.H.; Kang, K.H.; Uhm, C.S.; Kim, H.K.; et al. The Effect of Adipose Stem Cell Therapy on Pulmonary Fibrosis Induced by Repetitive Intratracheal Bleomycin in Mice. Exp. Lung Res. 2014, 40, 117–125. [Google Scholar] [CrossRef]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front. Med. 2017, 4, 118. [Google Scholar] [CrossRef]
- Kimura, T.; Monslow, J.; Klampatsa, A.; Leibowitz, M.; Sun, J.; Woodruff, P.; Moon, E.; Todd, L.; Puré, E.; Albelda, S.M. Loss of Cells Expressing Fibroblast Activation Protein (FAP) Has Variable Effects in Models of TGF-β and Chronic Bleomycin-Induced Fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L271–L282. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.J.; Chen, Z.; Xu, A.L.; Zhou, H.; Bi, X.L.; Yan, P.Y.; Xie, Y. A Novel Quantification Method of Lung Fibrosis Based on Micro-CT Images Developed with the Optimized Pulmonary Fibrosis Mice Model Induced by Bleomycin. Heliyon 2023, 9, e13598. [Google Scholar] [CrossRef]
- Lawson, W.E.; Polosukhin, V.V.; Stathopoulos, G.T.; Zoia, O.; Han, W.; Lane, K.B.; Li, B.; Donnelly, E.F.; Holburn, G.E.; Lewis, K.G.; et al. Epithelial and Mesenchymal Cell Biology Increased and Prolonged Pulmonary Fibrosis in Surfactant Protein C-Deficient Mice Following Intratracheal Bleomycin. Am. J. Pathol. 2005, 167, 1267–1277. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Al-Saedi, H.F.S.; Mohammed, A.Q.; Amir, A.A.; Radi, U.K.; Sattar, R.; Ahmad, I.; Ramadan, M.F.; Alshahrani, M.Y.; Balasim, H.M.; et al. Mechanisms of Bleomycin-Induced Lung Fibrosis: A Review of Therapeutic Targets and Approaches. Cell Biochem. Biophys. 2024, 82, 1845–1870. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kuninaka, Y.; Mukaida, N.; Kondo, T. Immune Mechanisms of Pulmonary Fibrosis with Bleomycin. Int. J. Mol. Sci. 2023, 24, 3149. [Google Scholar] [CrossRef]
- Shi, K.; Jiang, J.; Ma, T.; Xie, J.; Duan, L.; Chen, R.; Song, P.; Yu, Z.; Liu, C.; Zhu, Q.; et al. Pathogenesis Pathways of Idiopathic Pulmonary Fibrosis in Bleomycin-Induced Lung Injury Model in Mice. Respir. Physiol. Neurobiol. 2014, 190, 113–117. [Google Scholar] [CrossRef]
- Della Latta, V.; Cecchettini, A.; Del Ry, S.; Morales, M.A. Bleomycin in the Setting of Lung Fibrosis Induction: From Biological Mechanisms to Counteractions. Pharmacol. Res. 2015, 97, 122–130. [Google Scholar] [CrossRef]
- Taooka, Y.; Maeda, A.; Hiyama, K.; Ishioka, S.; Yamakido, M. Effects of Neutrophil Elastase Inhibitor on Bleomycin-Induced Pulmonary Fibrosis in Mice. Am. J. Respir. Crit. Care Med. 1997, 156, 260–265. [Google Scholar]
- Maeda, A.; Ishioka, S.; Taooka, Y.; Hiyama, K.; Yamakido, M. Expression of Transforming Growth Factor-β1 and Tumour Necrosis Factor-α in Bronchoalveolar Lavage Cells in Murine Pulmonary Fibrosis after Intraperitoneal Administration of Bleomycin. Respirology 1999, 4, 359–363. [Google Scholar] [CrossRef]
- Tabata, C.; Kadokawa, Y.; Tabata, R.; Takahashi, M.; Okoshi, K.; Sakai, Y.; Mishima, M.; Kubo, H. All-Trans-Retinoic Acid Prevents Radiation- or Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1352–1360. [Google Scholar] [CrossRef]
- Yasui, H.; Gabazza, E.C.; Tamaki, S.; Kobayashi, T.; Hataji, O.; Yuda, H.; Shimizu, S.; Suzuki, K.; Adachi, Y.; Taguchi, O. Intratracheal Administration of Activated Protein C Inhibits Bleomycin-Induced Lung Fibrosis in the Mouse. Am. J. Respir. Crit. Care Med. 2001, 163, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Takaki, H.; Hirata, Y.; Ueshima, E.; Kimura, Y.; Wada, R.; Osuga, K.; Yamakado, K. Unilateral Bleomycin-Induced Interstitial Pneumonitis Mouse Model With Both a Healthy and a Diseased Lung. In Vivo 2025, 39, 251–256. [Google Scholar] [CrossRef]
- Abbaszadeh, M.E.; Khezri, M.R.; Ghasemnejad-Berenji, M. The Protective Effects of Metformin and Vitamin C and Their Co-Administration in Bleomycin-Induced Pulmonary Fibrosis in Mice. Adv. Pharmacol. Pharm. Sci. 2025, 2025, 5227142. [Google Scholar] [CrossRef]
- Adamson, I.Y.R.; Bowden, D.H. The Pathogenesis of Bleomycin-Induced Pulmonary Fibrosis in Mice. Am. J. Pathol. 1974, 77, 185. [Google Scholar] [PubMed]
- Gul, A.; Yang, F.; Xie, C.; Du, W.; Mohammadtursun, N.; Wang, B.; Le, J.; Dong, J. Pulmonary Fibrosis Model of Mice Induced by Different Administration Methods of Bleomycin. BMC Pulm. Med. 2023, 23, 91. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Lu, R.; Zhao, P.; Tian, Y.; Li, J. Bleomycin Pollution and Lung Health: The Therapeutic Potential of Peimine in Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Glycolysis. Ecotoxicol. Environ. Saf. 2024, 289, 117451. [Google Scholar] [CrossRef] [PubMed]
- Ayilya, B.L.; Balde, A.; Ramya, M.; Benjakul, S.; Kim, S.K.; Nazeer, R.A. Insights on the Mechanism of Bleomycin to Induce Lung Injury and Associated in Vivo Models: A Review. Int. Immunopharmacol. 2023, 121, 110493. [Google Scholar] [CrossRef]
- Eferl, R.; Hasselblatt, P.; Rath, M.; Popper, H.; Zenz, R.; Komnenovic, V.; Idarraga, M.H.; Kenner, L.; Wagner, E.F. Development of Pulmonary Fibrosis through a Pathway Involving the Transcription Factor Fra-2/AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 10525–10530. [Google Scholar] [CrossRef]
- Grigoriadis, A.E.; Schellander, K.; Wang, Z.-Q.; Wagner, E.E. Osteoblasts Are Target Cells for Transformation in C-Fos Transgenic Mice. J. Cell. Biol. 1993, 122, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Jochum, W.; Jean-Pierre, D.; Elliott, C.C.E.; Wutz, A.; Plenk, H., Jr.; Matsuo, K.; Wagner, E.F. Increased Bone Formation and Osteosclerosis in Mice Overexpressing the Transcription Factor Fra-1. Nat. Med. 2000, 6, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Sabatakos, G.; Sims, N.A.; Chen, J.; Aoki, K.; Kelz, M.B.; Amling, M.; Bouali, Y.; Mukhopadhyay, K.; Ford, K.; Nestler, E.J.; et al. Overexpression of ΔFosB Transcription Factor (s) Increases Bone Formation and Inhibits Adipogenesis. Nat. Med. 2000, 6, 985–990. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A Double-Edged Sword in Tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Tsujino, K.; Reed, N.I.; Atakilit, A.; Ren, X.; Sheppard, D. Transforming Growth Factor-Plays Divergent Roles in Modulating Vascular Remodeling, Inflammation, and Pulmonary Fibrosis in a Murine Model of Scleroderma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, 22–31. [Google Scholar] [CrossRef]
- Ucero, A.C.; Bakiri, L.; Roediger, B.; Suzuki, M.; Jimenez, M.; Mandal, P.; Braghetta, P.; Bonaldo, P.; Paz-Ares, L.; Fustero-Torre, C.; et al. Fra-2–Expressing Macrophages Promote Lung Fibrosis. J. Clin. Investig. 2019, 129, 3293–3309. [Google Scholar] [CrossRef]
- Maurer, B.; Distler, J.H.W.; Distler, O. The Fra-2 Transgenic Mouse Model of Systemic Sclerosis. Vascul. Pharmacol. 2013, 58, 194–201. [Google Scholar] [CrossRef]
- Maurer, B.; Reich, N.; Juengel, A.; Kriegsmann, J.; Gay, R.E.; Schett, G.; Michel, B.A.; Gay, S.; Distler, J.H.W.; Distler, O. Fra-2 Transgenic Mice as a Novel Model of Pulmonary Hypertension Associated with Systemic Sclerosis. Ann. Rheum. Dis. 2012, 71, 1382–1387. [Google Scholar] [CrossRef]
- Reich, N.; Maurer, B.; Akhmetshina, A.; Venalis, P.; Dees, C.; Zerr, P.; Palumbo, K.; Zwerina, J.; Nevskaya, T.; Gay, S.; et al. The Transcription Factor Fra-2 Regulates the Production of Extracellular Matrix in Systemic Sclerosis. Arthritis Rheum. 2010, 62, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, V.; Weber, L.; Schlederer, M.; Silva Sousa, L.; Stampfer, A.; Baydar, S.; Nakuz, T.; Calabretta, R.; Antunes Goncalves, A.I.; Li, X.; et al. Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals 2025, 18, 658. [Google Scholar] [CrossRef]
- Orstavik, H. Why Are Autoimmune Diseases More Prevalent in Women? Tidsskr. Den Nor. legeforening 2017, 137, 866–868. [Google Scholar] [CrossRef]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting Fibroblast Activation Protein (FAP): Next Generation PET Radiotracers Using Squaramide Coupled Bifunctional DOTA and DATA5m Chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef]
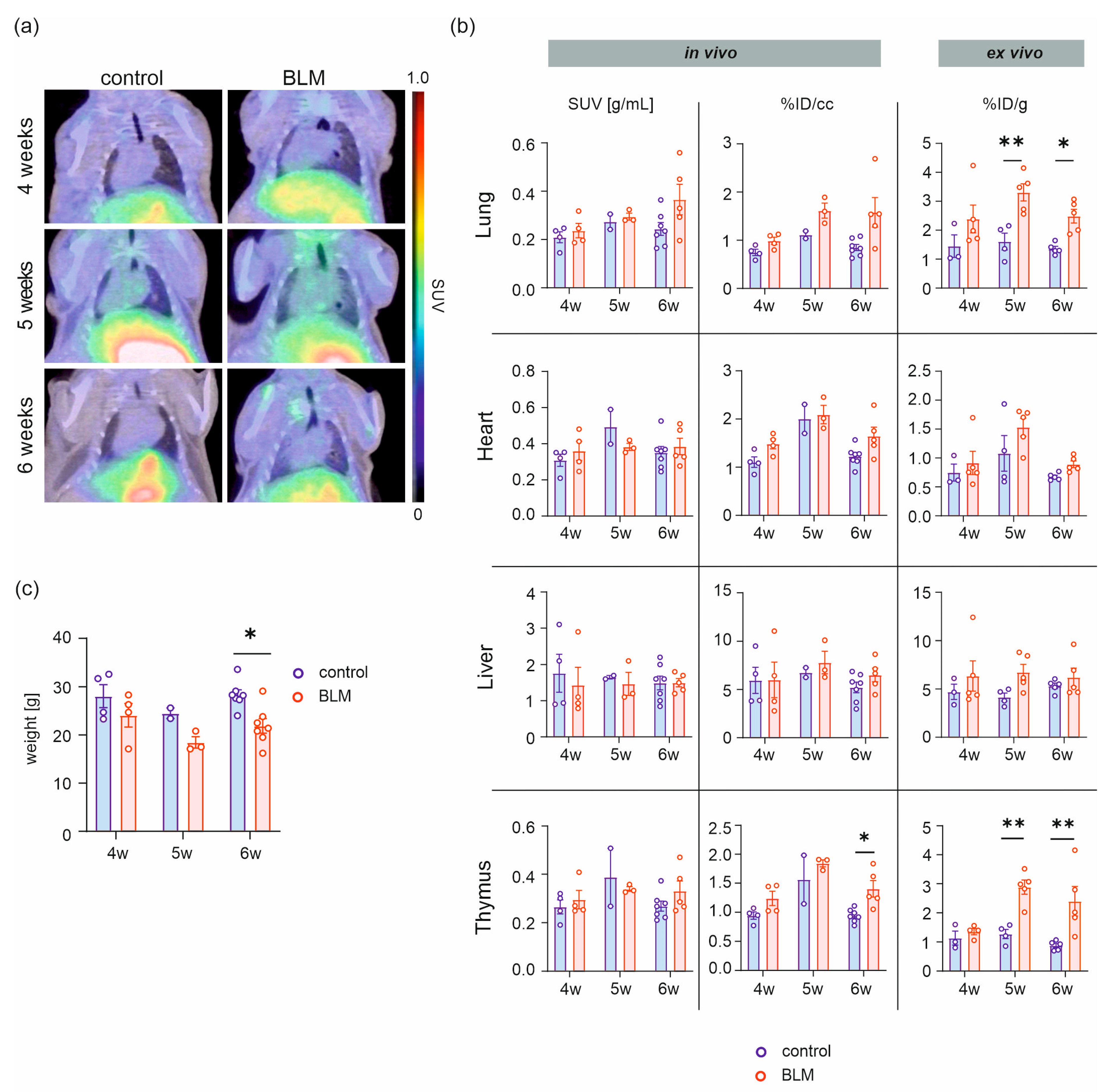
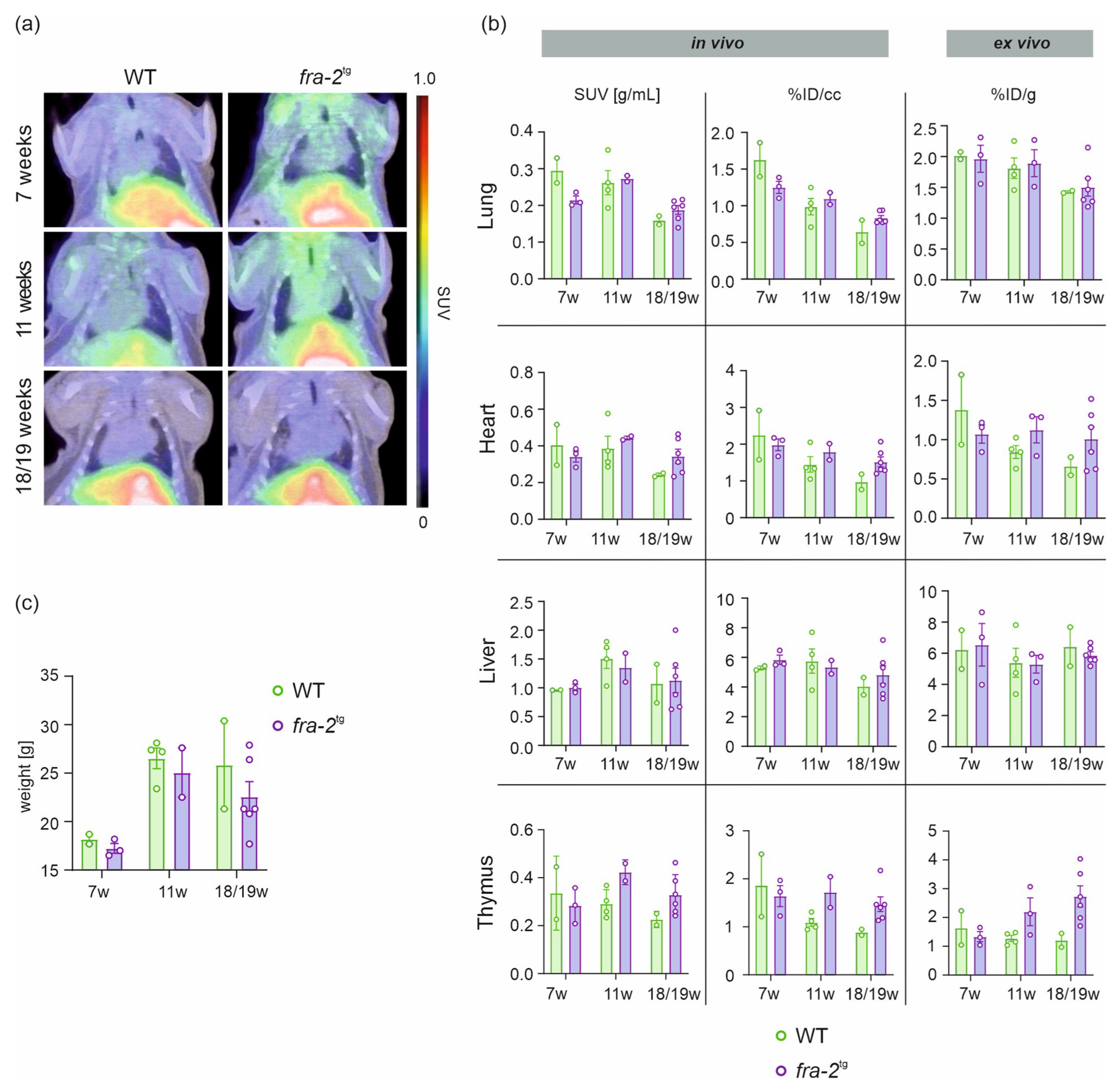

| Timepoint | Mouse Model | In Vivo | Ex Vivo | |||
|---|---|---|---|---|---|---|
| %ID/cc | SUV [g/mL] | n | %ID/g | n | ||
| 4 weeks | Control | 0.75 ± 0.07 | 0.21 ± 0.02 | 4 | 1.45 ± 0.39 | 3 |
| BLM | 0.99 ± 0.08 | 0.24 ± 0.03 | 4 | 2.39 ± 0.48 | 5 | |
| 5 weeks | Control | 1.11 ± 0.08 | 0.27 ± 0.03 | 2 | 1.61 ± 0.29 | 4 |
| BLM | 1.62 ± 0.16 | 0.30 ± 0.01 | 3 | 3.31 ± 0.29 ** | 5 | |
| 6 weeks | Control | 0.85 ± 0.07 | 0.24 ± 0.03 | 7 | 1.36 ± 0.09 | 5 |
| BLM | 1.58 ± 0.31 | 0.37 ± 0.06 | 5 | 2.49 ± 0.24 * | 5 | |
| 7 weeks | WT | 1.63 ± 0.23 | 0.30 ± 0.03 | 2 | 2.02 ± 0.06 | 2 |
| fra-2tg | 1.25 ± 0.08 | 0.22 ± 0.01 | 3 | 1.97 ± 0.22 | 3 | |
| 11 weeks | WT | 0.99 ± 0.11 | 0.26 ± 0.03 | 4 | 1.81 ± 0.17 | 4 |
| fra-2tg | 1.10 ± 0.08 | 0.27 ± 0.01 | 2 | 1.89 ± 0.22 | 3 | |
| 18/19 weeks | WT | 0.65 ± 0.16 | 0.16 ± 0.01 | 2 | 1.43 ± 0.02 | 2 |
| fra-2tg | 0.84 ± 0.03 | 0.19 ± 0.01 | 6 | 1.50 ± 0.15 | 6 | |
| Product | Product Number | Company |
|---|---|---|
| DATA5m.SA.FAPi Precursor | Johannes Gutenberg University Mainz, Mainz, Germany | |
| DMF | 047390.AE | Thermo Fisher Scientific, Waltham, MA, USA |
| 0.9% NaCl | 3505731 | Braun, Melsungen, Germany |
| HCl Reag.Ph.Eur. 0.1N | 6789 | Carl Roth GmbH, Karlsruhe, Germany |
| Cartridge SEP-PAK C18 light | WAT023501 | Waters Corporation, Milford, MA, USA |
| iTLC-SG plates | SGI0001 | Agilent Technologies, Santa Clara, CA, USA |
| 68Ge/68Ga Generator | 438495 | IRE Galli Eo®, Fleurus, Belgium |
| Dry block heater | 0004025100 | IKA®, Staufen, Germany |
| HPLC VWR Hitachi Chromaster | 5410 UV Detector, 5310 Column Oven, 5160 Pump | VWR, Tokyo, Japan |
| Chromolith® Performance RP-18e (100–4.6 mm) column | 102129 | Merck KGaA, Darmstadt, Germany |
| TLC scanner Elysia Raytest GITA* | B00002881 | Elysia-Raytest, Straubenhardt, Germany |
| CLARITY 7.3 software | C50 | Data Apex, Prague, Czech Republic |
| Gina Star TLC 6.3 | Elysia-Raytest, Straubenhardt, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Weissenböck, V.; Schlederer, M.; Bakiri, L.; Schaffenrath, J.; Wagner, E.F.; Rösch, F.; Hacker, M.; Kenner, L.; Philippe, C. FAPI Tracer en Vogue: Evaluating [68Ga]Ga-DATA5m.SA.FAPi for Molecular Imaging of Pulmonary Fibrosis. Pharmaceuticals 2026, 19, 34. https://doi.org/10.3390/ph19010034
Weissenböck V, Schlederer M, Bakiri L, Schaffenrath J, Wagner EF, Rösch F, Hacker M, Kenner L, Philippe C. FAPI Tracer en Vogue: Evaluating [68Ga]Ga-DATA5m.SA.FAPi for Molecular Imaging of Pulmonary Fibrosis. Pharmaceuticals. 2026; 19(1):34. https://doi.org/10.3390/ph19010034
Chicago/Turabian StyleWeissenböck, Victoria, Michaela Schlederer, Latifa Bakiri, Johanna Schaffenrath, Erwin F. Wagner, Frank Rösch, Marcus Hacker, Lukas Kenner, and Cécile Philippe. 2026. "FAPI Tracer en Vogue: Evaluating [68Ga]Ga-DATA5m.SA.FAPi for Molecular Imaging of Pulmonary Fibrosis" Pharmaceuticals 19, no. 1: 34. https://doi.org/10.3390/ph19010034
APA StyleWeissenböck, V., Schlederer, M., Bakiri, L., Schaffenrath, J., Wagner, E. F., Rösch, F., Hacker, M., Kenner, L., & Philippe, C. (2026). FAPI Tracer en Vogue: Evaluating [68Ga]Ga-DATA5m.SA.FAPi for Molecular Imaging of Pulmonary Fibrosis. Pharmaceuticals, 19(1), 34. https://doi.org/10.3390/ph19010034








