Abstract
Background/Objectives: Recent advances in nanotechnology have enabled the use of biosynthesized silver nanoparticles (AgNPs) in healthcare, including the management of diabetes mellitus, a metabolic disorder characterized by impaired glucose homeostasis. AgNPs have shown promising effects on enzymes, insulin signaling, gut hormones, and in vivo models. Despite the availability of oral treatments, challenges persist, prompting interest in novel therapies such as AgNPs, which are currently under investigation in various in vitro and in vivo studies. Methods: This narrative review was conducted through a PubMed search using the terms “antidiabetic + activity + AgNPs” in April 2025. Relevant articles published in English were selected and analyzed, with emphasis on studies employing biosynthesized AgNPs from plants in in vitro and in vivo models. Information was extracted regarding the experimental approaches used to evaluate antidiabetic activity, the plant sources employed, nanoparticle characteristics, concentrations tested, and corresponding outcomes. Results: The biosynthesis of AgNPs employs bioactive compounds from plants, making it an environmentally friendly green synthesis method. Plant extracts are the most common biomaterial for AgNPs biosynthesis. Most of the in vitro studies evaluated the inhibitory effect of AgNPs on α-glucosidase or α-amylase; meanwhile, in animal studies, the main parameter evaluated is blood glucose level. Conclusions: The antidiabetic potential of AgNPs is becoming increasingly evident as ongoing research continues to explore their effects through both in vitro and in vivo studies. In this review, the current state of research regarding the potential use of AgNPs for diabetes management and treatment is presented, highlighting recent findings and discussing future perspectives in the field.
1. Introduction
Diabetes mellitus (DM) is a group of metabolic disorders of carbohydrate metabolism in which glucose is both underutilized as an energy source and overproduced due to inappropriate gluconeogenesis and glycogenolysis, resulting in hyperglycemia [1,2]. DM is conventionally classified into several clinical categories: type 1 diabetes, type 2 diabetes (T2D), specific types of diabetes due to other causes, and gestational diabetes. Type 1 diabetes is due to autoimmune β-cell destruction, usually leading to absolute insulin deficiency. T2D is due to a progressive non-autoimmune loss of adequate β-cell insulin secretion, frequently with a background of insulin resistance and metabolic syndrome. Specific types of diabetes due to other causes, such as genetics or other organ-related problems, are rare. Gestational diabetes is diagnosed in the second or third trimester of pregnancy [1]. Some of the causes of DM include aging, a rapid increase in urbanization, and obesogenic environments [3].
According to the International Diabetes Federation (IDF), the current global prevalence of DM among adults aged 20–79 years is estimated at 11.1%, affecting approximately 588.7 million people in 2024. According to estimates, this figure is projected to rise to 13% by 2050, impacting around 852.5 million people. The IDF recently estimated that the total global healthcare expenditure attributable to diabetes among adults aged 20–79 years was USD 1,015 billion in 2024, with projections indicating an increase to USD 1,045 billion by 2050. Noticeably, approximately 90% of the prevalence of DM corresponds to people with T2D [3].
T2D is a disorder characterized by an impaired glucose homeostasis due to a dysregulation of carbohydrate, lipid, and protein metabolism. T2D is caused by a combination of genetic and environmental factors. However, impaired insulin secretion, insulin resistance, or a combination of both is considered the core pathophysiological defect. A T2D diagnosis is based on measurements of fasting plasma glucose, 2 h plasma glucose after an oral glucose tolerance test, and glycosylated hemoglobin (HbA1c) [4,5,6,7]. Symptoms and signs include high blood glucose levels over a prolonged period, frequent urination, increased thirst, and elevated hunger.
Prolonged and untreated DM can lead to serious complications on the cardiovascular system, kidneys and eyes, some of which are life-threatening [5,7,8]. T2D has long been identified as an incurable chronic disease. The best outcome that can be expected is the amelioration of symptoms. Approximately 50% of T2D patients will need insulin therapy within ten years of diagnosis. Although, in the past, diabetes was called chronic and irreversible, this paradigm has now changed [9]. Management for glucose control and diabetes remission includes modifications in eating and lifestyle habits [10,11]. However, sustained weight loss maintenance and complete adherence to diet and physical activity recommendations are uncommon among individuals with T2D [12].
The current therapeutics for the management of type 2 diabetes (T2D) include medications prescribed either in the presence or absence of exogenous insulin. Based on their mechanisms of action, these agents are broadly classified into four major classes: (a) insulin sensitizers, such as biguanides and thiazolidinediones; (b) insulin secretagogues, including sulfonylureas and glinides; (c) α-glucosidase inhibitors, such as acarbose and miglitol, which delay carbohydrate absorption; and (d) other agents, such as sodium–glucose co-transporter-2 (SGLT2) inhibitors and bile acid sequestrants. Some of these drugs carry the risk of side effects, including hypoglycemia, weight gain, and cardiovascular complications. However, some new drugs have been associated with weight loss [2,13,14,15].
In addition, one disadvantage of the current oral treatments of T2D is related to their low bioavailability and the immediate release of the drug into the bloodstream. In this context, nanotechnology, including different nanosystems and nanomaterials, is also applied for drug delivery, diagnostics, and tissue repair [15]. Based on the type of nanomaterial used in their composition, nanoformulations targeting T2D can be classified into polymer-based, lipid-based, and inorganic nanoformulations [16]. Inorganic nanoformulations can include the biosynthesis of silver nanoparticles (AgNPs), which can be carried out using whole plants or their parts [17].
Different parts of plants contain bioactive compounds, such as flavonoids and alkaloids, that can be used for diabetes treatment. Various techniques can be applied for their extraction. Their mechanisms of action involve improving glucose metabolism, exerting a hypolipidemic effect, enhancing the pancreatic effect, providing antioxidative benefits, managing diabetes-related complications, and exhibiting insulin-like activity [18]. According to ethnobotanical information reports, approximately 800 plants may possess antidiabetic effects [19].
Various approaches to the synthesis of metallic nanoparticles are available. These reactions have common steps: reduction, nucleation, growth, coarsening, and/or agglomeration. One widely used method involves reducing metal salts in an aqueous or organic solvent [20]. Research on AgNPs’ antidiabetic potential has focused primarily on inhibition studies of enzymes involved in carbohydrate metabolism and on animal models with drug-induced diabetes. The biosynthesis of AgNPs using parts of plants could be used for medical applications with less toxicity [21,22].
Recent studies have explored the potential application of AgNPs in the management of endocrine disorders such as DM, a prevalent metabolic disorder affecting millions worldwide. Given the global impact of DM, there is significant scientific interest in developing innovative strategies for its effective control [3,23].
An overview of existing research was conducted in PubMed in April 2025 using the search term “antidiabetic + activity + AgNPs”. Relevant articles published in English were selected and reviewed, focusing on the utilization of biosynthesized AgNPs using plants in in vitro and in vivo studies. Information regarding the methods used to evaluate the antidiabetic effects of AgNPs, the plant sources employed, the nanoparticles’ characteristics, the concentrations tested, and the corresponding results was extracted from the collected data.
In this review, the current state of research regarding the potential use of AgNPs for diabetes management and treatment is presented, highlighting recent findings and discussing future perspectives.
2. Biosynthesis of AgNPs
Nanotechnology deals with the design, fabrication, and application of small structures or small-sized materials. The size of nanoparticles spans from subnanometers to several hundred nanometers, where one nanometer is one billionth of a meter (1 × 10−9) [24]. Nanotechnology has advanced significantly in recent decades due to an improved understanding of nanostructure growth processes, as well as enhanced methods for analysis and characterization. These developments have enabled a wide range of applications across fields such as agriculture, textiles, environmental science, and medicine. Nanotechnology applied in medicine, often referred to as nanobiomedicine, has contributed to advancements in diagnostics, treatment, screening, disease prevention, and proactive healthcare [25].
The characteristics of nanoparticles, including size, shape, and the orientation of surface functional groups, are highly relevant. However, one of their most defining features is their high surface area-to-volume ratio, which facilitates their infiltration into biological tissues and fluids. This property enables processes such as endocytosis, distribution, retention, and elimination within biological systems [26].
Metallic nanoparticles exist in nature, and several historical objects, such as the pigments used by the Mayans in Chichen Itzá have been shown to contain iron and chromium nanoparticles [27]. In the 19th century, metal nanoparticles were used in photochemistry and photography and in glass molding [28]. However, current research has focused on developing different synthesis routes and evaluating their properties [29].
The synthesis of nanoparticles can be carried out using physical, chemical, and biological methods. The main disadvantage of physical and chemical methods is that they have a high cost, both in economic and environmental terms, because they involve the use of dangerous or toxic substances [30]. Therefore, recent research has focused on developing clean nanoparticle synthesis methods that do not use risky substances and are environmentally friendly; this can be achieved using biological systems with different biomaterials, such as microorganisms and plants [31,32,33,34]. Regarding plants, their seeds, leaves, fruits, flowers, peel, roots, stems, bark, and food by-products have been used; an extract is made, usually aqueous, and serves as a reducing agent for silver nitrate (silver salt) [21,22,35]. Bacteria, fungi, algae [17,36], biomolecules, and even animal parts [37,38] have also been used for the biosynthesis of AgNPs. Thus, the sources for the elaboration of AgNPs are broad (see Table 1 and Figure 1).
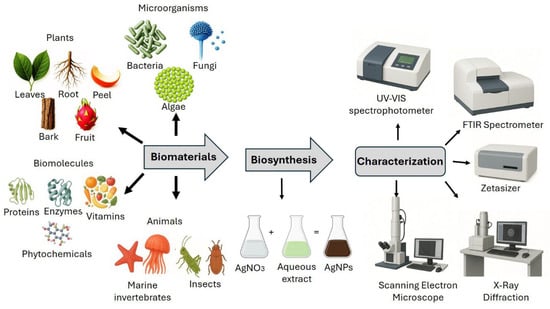
Figure 1.
Biosynthesis and characterization of AgNPs.
The synthesis of AgNPs can be influenced by different parameters, such as temperature, pH, concentration of reaction components, reducing or stabilizing agents, and the molar ratio of the surfactant/stabilizer and precursor [20]. Notably, the phytochemicals such as alkaloids, phenolics, terpenes, saponins, and steroids present in plant extracts act as reducing, capping, and stabilizing agents, facilitating the bioreduction of Ag+ ions to Ag0, and leading to the formation of AgNPs [39].
Although nanoparticles can be synthesized from different metals, AgNPs represent a clear example of the multiple applications that these materials can have, since they have been used in clothing, the food industry, electronics, biosensing, environmental remediation, medical healthcare, and consumer products [40].

Table 1.
Examples of biomaterials for the biosynthesis of silver nanoparticles.
Table 1.
Examples of biomaterials for the biosynthesis of silver nanoparticles.
| Biomaterial | Silver Nanoparticle Size | Reference | |
|---|---|---|---|
| Plants | Scientific name | ||
| Leaves | Theobroma Cacao Linneu | 10.3 nm | [41] |
| Fruit | Aegle marmelos | 159–181 nm | [42] |
| Bark | Heritiera fomes and Sonneratia apetala | 20–30 nm | [43] |
| Root | Coleus forskohlii | 5–35 nm | [44] |
| Peel | Stenocereus queretaroensis | 98.9 nm | [45] |
| Microorganisms | |||
| Bacteria | Bacillus cereus | 5–7.06 nm | [46] |
| Fungi | Aspergillus sydowii | 1–24 nm | [47] |
| Algae | Asterarcys sp. microalga | 35–52 nm | [48] |
| Biomolecules | |||
| Enzymes | Nitrate reductase from Fusarium oxysporum | 50 nm | [49] |
| Vitamins | Vitamin C | 26.5 nm | [50] |
| Proteins | Bovine serum albumin, lysozyme, among others | Non determined | [51] |
| Phytochemicals | Naringenin | 10–21 nm | [52] |
| Animals | |||
| Marine invertebrates | Marphysa moribidii | 40.19 nm average | [53] |
| Insects | Mang mao wings | 40–60 nm | [37] |
3. Characterization of AgNPs
The characterization of biosynthesized AgNPs is carried out using different methodologies, such as spectrophotometry; the surface plasmon resonance of silver ions has been detected at wavelengths of 400–460 nanometers [54,55]. X-ray diffraction analysis is used to determine the crystalline nature of AgNPs. Additionally, Fourier transform infrared spectroscopy measurements are used to determine the interactions of silver ions with the phytochemical compounds responsible for stabilizing the nanoparticles. The size and morphology of AgNPs are evaluated using scanning and transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy, whereas size distribution and stability can be determined using dynamic light scattering spectroscopy [54,56,57,58]. The chemical composition of biosynthesized AgNPs can be analyzed by utilizing scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy [57]. On the other hand, evaluating the zeta potential of a colloidal dispersion is crucial for assessing its stability and resistance to agglomeration [59].
4. Metabolism of AgNPs
Nanoparticles, ranging in size from 1 to 100 nm, have a high surface area-to-volume ratio, making them highly reactive and capable of penetrating body tissues and fluids. Their size and surface properties play a key role in endocytosis, distribution, accumulation within biological systems, and eventual elimination [26]. Due to their size, most nanoparticles do not reach their intended target and are instead sequestered by the liver and spleen, if larger than 6 nm, or eliminated through the kidneys, if smaller than 6 nm, after their administration into the body. While the route of administration influences nanoparticle processing, the liver acts as a primary biological filter, sequestering 30–99% of administered nanoparticles from the bloodstream. The development of solutions that overcome the liver will be key to enabling the use of nanoparticles for medical applications [60] when the liver is not the intended target.
The main applications of AgNPs in the biomedical area include therapeutic applications such as antioxidants, diagnosis, biomolecular detection, and controlled drug release, among others [61,62].
In vitro studies have demonstrated that the dose, size, and coating of AgNPs affect their cellular uptake; meanwhile, in vivo distribution studies have reported silver accumulation and toxicity to both local and distant organs [63].
Accumulation occurs in the liver, kidneys, spleen, brain, lungs, and testicles. Nanoparticles can persist in the body for several weeks after the end of oral exposure, particularly in the brain and testicles [64].
5. Toxicology of AgNPs
Regarding the potential toxicological risks of nanoparticles, the increasing biomedical and commercial applications of AgNPs have raised concerns about their interactions with the environment and human health [65]. The potential toxicity of silver nanoparticles is influenced by factors such as particle size, shape, concentration, and duration of exposure. However, their safety remains a matter of debate, underscoring the need for comprehensive toxicity assessments and well-designed clinical trials to establish the safety profile of AgNPs in diabetes treatment [57]. Furthermore, global harmonization of nanomaterial regulations and standa rdized toxicological testing protocols is essential to ensure the safe use of nanomaterials for manufacturers, healthcare professionals, and the general public.
Recently, an in vitro study provided a first attempt to calculate the human effect factors (HEF) value for nanoparticles, including AgNPs, which can be used to establish their impact category in a life cycle assessment. The non-carcinogenic HEF (case/kg intake) for AgNPs was 5.9 × 10−1, compared with 7.5 × 10−3 for CuO-nanoparticles, and 2.5 × 10−2 for zinc-nanoparticles [65]. Although the dose of nanoparticles in animal models varies, one study in rats estimated a no observable adverse effect level (NOAEL) of 30 mg/kg and a lowest observable adverse effect level (LOAEL) of 125 mg/kg [66]. Nevertheless, providing general warnings remains challenging, as data in the literature must take into account the type and surface charge of nanoparticles, their particle aggregation, cell lines, experimental designs, and various observational endpoints [67,68].
6. Mechanisms of Action of AgNPs in Type 2 Diabetes Mellitus
T2D risk factors include a complex combination of genetic, metabolic, and environmental factors; the main modifiable risk factors are obesity, low physical activity, and an unhealthy diet. The pathophysiological changes are characterized by β-cell dysfunction, insulin resistance, and chronic inflammation leading to hyperglycemia [4,69].
Overconsumption of food leads to obesity and is associated with gluco-lipotoxicity; it also stimulates insulin secretion and the production of reactive oxygen species, which in turn leads to an abnormal generation of inflammatory molecules. This pro-inflammatory environment causes insulin resistance in muscle, liver, and adipose tissue; as a consequence, β-cells continue the overproduction of insulin to control hyperglycemia until dysfunction of the pancreas occurs. However, other processes and behavioral risks are involved, including aging, resistance and/or deficiency of incretin hormones such as GLP-1 and gastric inhibitory polypeptide (GIP), hypersecretion of islet amyloid polypeptide, smoking, and sleep habits [4,69]. Also, gut microbiota dysbiosis has been reported to play a role in driving T2D [69,70].
On the other hand, sustained hyperglycemia induces the formation of advanced glycation end-products (AGEs), which worsen oxidative stress and non-enzymatic glycation in proteins such as hemoglobin [69]. Modulating oxidative stress holds significant therapeutic potential in the treatment of diabetes [71]. Exploring the antioxidant benefits of AgNPs along with their antidiabetic effect has also been investigated [72,73] using different in vitro methods (Table 2) and animal models.

Table 2.
In vitro methods to evaluate the antidiabetic activity of AgNPs.
The antidiabetic activity of AgNPs has been primarily evaluated in murine models, commonly induced by alloxan or streptozotocin, both of which are well known for damaging the pancreas and inducing diabetes (Table 3).

Table 3.
Animal models to evaluate the antidiabetic activity of AgNPs.
An effective strategy for lowering elevated blood glucose levels is enzyme inhibition. α-amylase hydrolyzes starch and releases maltose and a range of branched-oligosaccharides that are further hydrolyzed by α-glucosidase into absorbable monosaccharides such as glucose. Extensive research has focused on identifying compounds that target these enzymes to regulate blood sugar levels [117]. Acarbose [118], voglibose, and miglitol are three inhibitors of these two enzymes [119]. This inhibition decreases monosaccharide cleavage from complex carbohydrates in the diet, improving hyperglycemia. The side effects of some of these drugs, such as flatulence, bloating, and diarrhea, highlight the need for the development of new inhibitors [118,119], specifically ones with stronger inhibition against α-glucosidase and a lower one against α-amylase [117]. Many studies have evaluated the inhibitory effect of AgNPs on α-glucosidase and α-amylase in vitro (Table 4). While certain studies have reported a higher inhibitory activity against α-amylase [73,74,75,80,99], others have observed a more pronounced effect on α-glucosidase [72,103]. The size of SAgNPs varies among studies, and smaller sizes appear to be associated with more efficient inhibitory activity. For example, Leucosidea sericea (2.9–7.8 nm) demonstrated α-glucosidase inhibition with IC50 values ranging from 8.75 to 21.48 μg/mL [120]. Similarly, Berberis lyceum (11.02 nm) exhibited inhibitory activity comparable to acarbose [103], while Cleome viscosa (5–50 nm) showed dual inhibitory activity against both α-amylase and α-glucosidase [85].

Table 4.
In vitro antidiabetic effects of AgNPs biosynthesized with plants.
After ingestion of a meal, insulin is released and the secretion of incretin hormones, such as glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), is increased. GLP-1 and GIP are gut-derived hormones that enhance insulin secretion, suppress glucagon release, and delay gastric emptying. These incretin hormones play a critical role in modulating glucose metabolism and reducing blood glucose levels [151]. However, the enzyme dipeptidyl peptidase-4 (DPP-4) promotes the degradation of both GLP-1 and GIP. Inhibition of DPP-4 activity results in elevated levels of these incretin hormones, thereby enhancing insulin secretion and suppressing glucagon release, ultimately contributing to improved glycemic control. DPP-4 inhibitors such as sitagliptin, vildagliptin, saxagliptin, linagliptin, and gemigliptin have been developed for clinical use [152]. AgNPs have also been evaluated as potential DPP-4 inhibitors [83]. It is worth mentioning that natural products have also been identified as DPP-4 inhibitors [151].
One approach to treating diabetic patients involves stimulating insulin secretion using thiazolidinedione drugs. Another strategy targets glucagon and related molecules, as glucagon promotes glucose production. These mechanisms have been investigated using AgNPs in in vitro models with pancreatic and liver hepatic cell lines [87].
In vitro studies have employed glucose dialysis retardation assays and glucose adsorption capacity to evaluate the potential of bioactive compounds in modulating glucose absorption. The glucose dialysis retardation assay suggests that certain compounds may delay glucose absorption in the intestine and prevent postprandial hyperglycemia [74]. Similarly, the glucose adsorption assay demonstrates that some compounds can effectively bind to glucose, reducing its availability for absorption. This mechanism has been further supported by studies in yeast models, where reduced facilitated glucose diffusion was observed [89]. Carbohydrate-rich foods are classified based on their glycemic index (GI), which represents their ability to increase blood glucose levels. Foods categorized as high GI cause a more rapid and bigger increase in blood glucose compared to medium-or low-GI foods. In addition to GI, the glycemic load (GL) is another measure used to classify foods, incorporating both the GI and the quantity of carbohydrates consumed in a meal. Foods are categorized as low-, medium-, or high-GL. Numerous studies have demonstrated the beneficial effects of dietary interventions with low-GI or low-GL foods in the management of diabetes [153]. Bioactive compounds capable of modulating GI or GL may serve as potential therapeutic agents for diabetes management.
Tyrosine protein phosphatase non-receptor type 1 (PTP1B; also known as protein tyrosine phosphatase 1B) downregulates insulin signaling through changes to insulin receptor activity. Although the physiological activity of PTP1B depends on cell type, PTP1B inhibition can improve insulin sensitivity and exert benefits on DM [58,154].
Oxidative stress has been associated with T2D and is defined as a condition characterized by elevated levels of reactive oxygen and nitrogen species, together with reduced antioxidant levels. This imbalance disrupts normal cellular processes, as the high electron reactivity of these species enables their interactions with biomolecules such as lipids, proteins, and nucleic acids. Reactive species may originate from exogenous or endogenous sources, with the mitochondrial electron transport chain—particularly under nutrient overload—being a major endogenous contributor [155]. The potential antioxidant activity of AgNPs arises from phytochemicals present in plants, such as phenolics, terpenoids, and flavonoids, which enable them to neutralize reactive species [75].
Some studies have evaluated the antidiabetic effect of AgNPs not only via amylase or glucosidase inhibition, but also by measuring their radical scavenging activity using the DPPH (1,1-diphenyl-2-picrylhydrazyl) or ABTS 2,2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) assays [75,123]. In regard to the in vitro assays among the articles found for this review, it is noted that the lowest size of AgNPs was 2.9 nm [120]; one study reported a nanoparticle size of 170 nm [125]. In one article that synthesized many types of nanoparticles from fractions of Rosa indica L., one of them was reported to be as large as 770 nm [132]. Some studies have utilized diverse doses of AgNPs to evaluate α–amylase; among the doses employed, some were as low as 0.3 μg/mL [137], utilizing 1 mL of salivary α-amylase, while other studies have used doses as high as 1.6 mg/mL [121], but employed 0.05 g/100 mL α-amylase. Other findings report using 30 mg/mL of nanoparticles to evaluate α–amylase (0.4 U/mL) inhibition [143]. This variability might be related to the experimental design, mainly the enzyme activity, but also the type of starch used, pH, and temperature [156]. Enzyme concentration must be taken into account when interpreting and comparing the effects of AgNPs treatment.
The production of AGEs is associated with the pathogenic mechanisms underlying T2D, as well as the development of diabetic complications and other chronic diseases. AGEs can be assessed through inhibition assays targeting vesperlysine-like and pentosidine-like AGEs, which are naturally fluorescent and cross-linked [95]. The anti-glycation activity of AgNPs has been previously reported [129].
Recently, one study employed in silico molecular docking approaches to evaluate interactions between silver atoms and proteins such as α-amylase, α-glucosidase, insulin, and glucagon, demonstrating that silver atoms can interact with the amino acid residues of these proteins, since the binding energy resulted in <0 kcal/mol [87].
Regarding animal models, various doses and particle sizes of AgNPs have been evaluated. Sizes as small as 9 nm [110], and up to approximately 100 nm have been tested in murine and fish models [104,115]. Most AgNPs treatments were administered intraperitoneally or orally for periods ranging from 9 to 30 days, with exceptions such as Momordica charantia, which was administered for 14 weeks [111]. Oral doses have ranged from as low as 50 μg/kg [115] to as high as 2000 mg/kg [111], while intraperitoneal administration of 10 mg/kg has also been reported [116]. As expected, blood glucose level has been the primary parameter evaluated in in vivo models, as reported by Virgen-Ortiz (2015) and more recently by Hosen E et al. (2024) [109,110]. Most diabetic models are induced using streptozotocin rather than alloxan. Several studies have demonstrated the hypoglycemic effects of biosynthesized AgNPs in diabetic animals. For example, Rumex hymenosepalus reduced blood glucose levels by 50% in diabetic rats within 9 days [110]. Benefits have been observed not only in blood glucose regulation but also in tissue morphology, including that of the liver, kidneys [80,96,102,108], and even the pancreas [80,96,108].
Consistent with in vitro findings on size, smaller AgNPs in animal models appear to exert more efficient antidiabetic effects. This was demonstrated with AgNPs biosynthesized from R. hymenosepalus (9 nm) [110], Lawsonia inermis (14.9 nm) [112], and Eysenhardtia polystachya (10–12 nm) [93], all of which lowered blood glucose and, in some cases, improved lipid profiles. Findings from animal studies suggest that AgNPs exert multitarget effects, not only reducing blood glucose but also modulating inflammatory processes and exhibiting antioxidant and regenerative activities (Table 5). The proposed mechanisms of action of AgNPs are illustrated in Figure 2.

Table 5.
Antidiabetic effects of AgNPs biosynthesized in animal models.
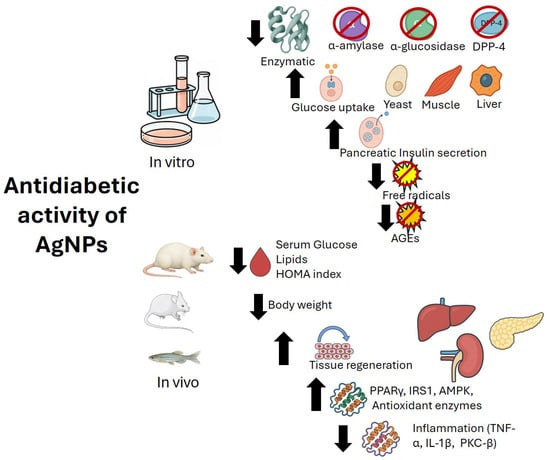
Figure 2.
Proposed mechanisms of action of AgNPs in diabetes mellitus. AGEs: Advanced glycation end products; DPP-4: Dipeptidyl peptidase 4; AMPK: AMP-activated protein kinase; IRS1: Insulin receptor substrate 1; PPARγ: Peroxisome proliferator-activated receptor gamma; TNF-α: Tumor necrosis factor-alpha; IL-1β: Interleukin-1 beta; PKC-β: Protein kinase C-beta.
7. Current Challenges in the Treatment of Type 2 Diabetes Mellitus
Some studies have shown that weight loss can produce a remission of T2D, defined as no longer meeting the diagnostic criteria for T2D. Both diet and a physically active lifestyle are the cornerstones of remission, and it is worth mentioning that long-term maintenance of weight loss is challenging [157].
Dietary restrictions are commonly prescribed to improve metabolic control in T2D. National and international guidelines for nutritional and lifestyle recommendations are available. Some recommendations focus on the consumption of low glycemic index foods to improve hyperglycemia [153,158]. Other dietetic approaches have even had an impact on the low-grade inflammation present in T2D [159]. Regular exercise is essential for the management of T2D. However, exercise training alone, without any sort of dietary intervention, may not lead to diabetes remission [157].
DM is a major public health epidemic despite recent advances in both pharmaceutical and technological treatment options [9]. The pharmaceutical industry encounters challenges in innovation, including the complexity of drug discovery, high research and development costs, risk of failure, and prolonged regulatory approval processes, among other factors [160,161]. The interest in natural products in drug development seems undeniable [161].
8. Study Limitations
Doses of AgNPs are reported using various units, and not all studies provide IC50 values for α-amylase and α-glucosidase inhibition. Many investigations rely solely on enzymatic activity to support antidiabetic effects, with relatively few animal studies compared to in vitro studies. Another limitation of studies with AgNPs is the lack of safety evaluations using appropriate methodologies to establish toxicological risk and its possible effects on the environment.
9. Conclusions
This review provides an extensive overview of the antidiabetic effects of AgNPs, with particular emphasis on differentiating findings from in vitro and animal studies. Such distinctions are essential for interpreting current evidence and guiding future research toward clinical translation. Most studies have focused on the enzyme inhibitory activity of AgNPs; however, a smaller number have examined glucose uptake in cells, the expression of relevant genes, and the non-enzymatic glycosylation of proteins. In addition, a few other types of studies have also been reported, although they remain limited.
The biosynthesis of AgNPs employs bioactive compounds from plants instead of hazardous chemicals, making it an environmentally friendly green synthesis method. Plant extracts are the most common biomaterial for AgNP biosynthesis. Optimization of the biosynthesis conditions is highly recommended to enhance the efficiency and reproducibility of AgNP production. However, safety concerns must still be addressed given the increasing utilization of AgNPs; this, in turn, requires further investigation into their toxicological effects. Most of the antidiabetic screening found in this review was performed in vitro using cellular and enzymatic assays and a few animal models. In addition, the shape and size of AgNPs may influence their antidiabetic efficacy and should therefore be considered in future investigations.
T2D remains a major public health challenge despite recent advances in nanobiomedicine. The antidiabetic potential of AgNPs is increasingly supported by evidence from both in vitro and in vivo studies, suggesting their promise in the development of nanoparticle-based therapies. Nevertheless, further investigations are required, particularly regarding pharmacological properties, biocompatibility, and cytotoxicity.
Additionally, although several mechanisms of action have been proposed, the precise mode of action in the altered processes in T2D remains unclear. Such studies are essential to optimize dosage, stability, and safety, thereby enabling the design of clinical trials and advancing the potential commercialization (i.e., scaling up the biosynthesis process, regulatory alignment) of AgNP-based antidiabetic agents.
Author Contributions
Conceptualization, E.P.-C. and A.S.G.-G.; Literature review, A.S.G.-G., I.M.S.-H., O.R.T.-G. and E.P.-C.; Drafting the article, A.S.G.-G.; Review and editing: I.M.S.-H. and O.R.T.-G.; Critical revision for relevant intellectual content, E.P.-C.; Final approval of the version to be published, A.S.G.-G., O.R.T.-G., I.M.S.-H. and E.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was also supported by a postdoctoral fellowship No. 3097257 from Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT/SECIHTI), to Angélica Sofía González Garibay (CVU 298099). This study received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Singh, A.; Shadangi, S.; Gupta, P.K.; Rana, S. Type 2 Diabetes Mellitus: A Comprehensive Review of Pathophysiology, Comorbidities, and Emerging Therapies. Compr. Physiol. 2025, 15, e70003. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes Mellitus Statistics on Prevalence and Mortality: Facts and Fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of Methods for Detecting Glycemic Disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Hou, Y.; Xiang, J.; Wang, B.; Duan, S.; Song, R.; Zhou, W.; Tan, S.; He, B. Pathogenesis and comprehensive treatment strategies of sarcopenia in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2024, 8, 1263650. [Google Scholar] [CrossRef]
- Hallberg, S.J.; Gershuni, V.M.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Huang, Q.; Zhang, Q.; Li, M.; Wu, Y. The Effectiveness of Lifestyle Interventions for Diabetes Remission on Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Worldviews Evid. Based Nurs. 2023, 20, 64–78. [Google Scholar] [CrossRef]
- Tripathi, P.; Kathrikolly, T.R. Lifestyle Intervention for Diabetes Remission: A Paradigm Shift in Diabetes Care and Management. Curr. Diabetes Rev. 2023, 20, 45–49. [Google Scholar] [CrossRef]
- Cartagena, M.V.; Tort-Nasarre, G.; Arnaldo, E.R. Barriers and Facilitators for Physical Activity in Adults with Type 2 Diabetes Mellitus: A Scoping Review. Int. J. Environ. Res. Public. Health 2021, 18, 5359. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.O.; Ali, I.I.; Adeghate, J.O.; Tekes, K.; Kalász, H.; Adeghate, E.A. An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 9328. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of Nanotechnology in Type 2 Diabetes Mellitus Treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Nanoformulation of Plant-Based Natural Products for Type 2 Diabetes Mellitus: From Formulation Design to Therapeutic Applications. Curr. Ther. Res. Clin. Exp. 2022, 96, 100672. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Alqahtani, A.A.; Ahmed, M.M. Application of Green Synthesized Silver Nanoparticles in Burn Therapy: A Review. Pharmazie 2024, 79, 42–48. [Google Scholar] [CrossRef]
- Chan, C.H.; Ngoh, G.C.; Yusoff, R. A Brief Review on Anti Diabetic Plants: Global Distribution, Active Ingredients, Extraction Techniques and Acting Mechanisms. Pharmacogn. Rev. 2012, 6, 22–28. [Google Scholar] [CrossRef]
- Arumugam, G.; Manjula, P.; Paari, N. A Review: Anti Diabetic Medicinal Plants Used for Diabetes Mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Addissouky, T.; Ali, M.; El Sayed, I.E.T.; Wang, Y. Revolutionary Innovations in Diabetes Research: From Biomarkers to Genomic Medicine. Iran. J. Diabetes Obes. 2023, 15, 228–242. [Google Scholar] [CrossRef]
- Cao, G.; Ying, W. Nanostructures and Nanomaterials. Synthesis, Properties, and Applications, 2nd ed.; Imperial College Press: Singapore, 2011; ISBN 978-981-4340-57-1. [Google Scholar]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Cai, H.; Yang, M. The Application of Nanotechnology for the Diagnosis and Treatment of Endocrine Disorders: A Review of Current Trends, Toxicology and Future Perspective. Int. J. Nanomed. 2024, 19, 9921–9942. [Google Scholar] [CrossRef]
- José-Yacamán, M.; Rendón, L.; Arenas, J.; Carmen, M.; Puche, S. Maya Blue Paint: An Ancient Nanostructured Material. Science 1996, 273, 223–225. [Google Scholar] [CrossRef]
- Turner, P. History of Photography. Metal Nano Particles Synthesis Characterization and Applications, Colloidal Gold. In History of photography; Hamlyn: London, UK, 1987. [Google Scholar]
- Kiranmai, M. Biological and Non-Biological Synthesis of Metallic Nanoparticles: Scope for Current Pharmaceutical Research. Indian J. Pharm. Sci. 2017, 79, 501–512. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological Properties of “Naked” Metal Nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Ali, Z.A.; Yahya, R.; Sekaran, S.D.; Puteh, R. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties. Adv. Mater. Sci. Eng. 2016, 2016, 4102196. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Coriander Leaf Mediated Biosynthesis of Gold Nanoparticles. Mater. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. J. Chem. 2009, 6, 61–70. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Zakariya, N.A.; Hashim, R.; Ansari, M.T.; Alkahtani, S.; Hasnain, M.S. In Vitro Evaluation of Antibacterial, Antioxidant, and Antidiabetic Activities and Glucose Uptake through 2-NBDG by Hep-2 Liver Cancer Cells Treated with Green Synthesized Silver Nanoparticles. Oxid. Med. Cell Longev. 2022, 2022, 1646687. [Google Scholar] [CrossRef] [PubMed]
- Jakinala, P.; Lingampally, N.; Hameeda, B.; Sayyed, R.Z.; Yahya Khan, M.; Elsayed, E.A.; Enshasy, H. El Silver Nanoparticles from Insect Wing Extract: Biosynthesis and Evaluation for Antioxidant and Antimicrobial Potential. PLoS ONE 2021, 16, e0241729. [Google Scholar] [CrossRef]
- George, I.E.; Cherian, T.; Ragavendran, C.; Mohanraju, R.; Dailah, H.G.; Hassani, R.; Alhazmi, H.A.; Khalid, A.; Mohan, S. One-Pot Green Synthesis of Silver Nanoparticles Using Brittle Star Ophiocoma scolopendrina: Assessing Biological Potentialities of Antibacterial, Antioxidant, Anti-Diabetic and Catalytic Degradation of Organic Dyes. Heliyon 2023, 9, e14538. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.N.; Matussin, S.N.; Fariduddin, Q.; Khan, M.M. Potentials of Roots, Stems, Leaves, Flowers, Fruits, and Seeds Extract for the Synthesis of Silver Nanoparticles. Bioprocess. Biosyst. Eng. 2024, 47, 1119–1137. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver Nanoparticle Applications and Human Health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Herrera-Marín, P.; Fernández, L.; Pilaquinga, F.F.; Debut, A.; Rodríguez, A.; Espinoza-Montero, P. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of the Leaves of Fine Aroma Cocoa Theobroma cacao linneu (Malvaceae): Optimization by Electrochemical Techniques. Electrochim. Acta 2023, 447, 142122. [Google Scholar] [CrossRef]
- Devi, M.; Devi, S.; Sharma, V.; Rana, N.; Bhatia, R.K.; Bhatt, A.K. Green Synthesis of Silver Nanoparticles Using Methanolic Fruit Extract of Aegle marmelos and Their Antimicrobial Potential against Human Bacterial Pathogens. J. Tradit. Complement. Med. 2020, 10, 158–165. [Google Scholar] [CrossRef]
- Thatoi, P.; Kerry, R.G.; Gouda, S.; Das, G.; Pramanik, K.; Thatoi, H.; Patra, J.K. Photo-Mediated Green Synthesis of Silver and Zinc Oxide Nanoparticles Using Aqueous Extracts of Two Mangrove Plant Species, Heritiera fomes and Sonneratia apetala and Investigation of Their Biomedical Applications. J. Photochem. Photobiol. B 2016, 163, 311–318. [Google Scholar] [CrossRef]
- Dhayalan, M.; Denison, M.I.J.; Ayyar, M.; Gandhi, N.N.; Krishnan, K.; Abdulhadi, B. Biogenic Synthesis, Characterization of Gold and Silver Nanoparticles from Coleus forskohlii and Their Clinical Importance. J. Photochem. Photobiol. B 2018, 183, 251–257. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Ramirez-Rodriguez, P.; Diaz, E.; Wille, H.; Flores-Fernandez, J.M. Biosynthesis of Silver Nanoparticles Using Stenocereus queretaroensis Fruit Peel Extract: Study of Antimicrobial Activity. Materials 2021, 14, 4543. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for Biogenic Microbial Synthesis of Silver Nanoparticles through Response Surface Methodology, Characterization, Their Antimicrobial, Antioxidant, and Catalytic Potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-Mediated Green Synthesis of Nano-Silver Using Aspergillus sydowii and Its Antifungal/Antiproliferative Activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kumawat, G.; Khandelwal, M.; Khangarot, R.K.; Saharan, V.; Nigam, S. Harish Phyco-Synthesis of Silver Nanoparticles by Environmentally Safe Approach and Their Applications. Sci. Rep. 2024, 14, 9568. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Akbarzadeh, A.; Norouzian, D.; Amini, A.; Gholami-Shabani, Z.; Imani, A.; Chiani, M.; Riazi, G.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Antimicrobial Activity and Physical Characterization of Silver Nanoparticles Green Synthesized Using Nitrate Reductase from Fusarium oxysporum. Appl. Biochem. Biotechnol. 2014, 172, 4084–4098. [Google Scholar] [CrossRef]
- Hassan (El-Moushy), R.M. Novel Green Synthesis of Cluster AgNPs by Reduction of Silver (I) by Vitamin C in Presence of Alginate Surfactant Powder in Aqueous Media: Characteristics and Applications. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100971. [Google Scholar] [CrossRef]
- Chakraborty, I.; Feliu, N.; Roy, S.; Dawson, K.; Parak, W.J. Protein-Mediated Shape Control of Silver Nanoparticles. Bioconjug Chem. 2018, 29, 1261–1265. [Google Scholar] [CrossRef]
- Sathianathan, N.; Thazhenandayipurath, V.V.; Mukundan, A.V.; Raj, A.; Latha, V.; Krishnankutty, R.E.; Chellappanpillai, S. One-Pot Synthesis and Characterization of Naringenin-Capped Silver Nanoparticles with Enhanced Biological Activities. Appl. Biochem. Biotechnol. 2025, 197, 3780–3799. [Google Scholar] [CrossRef]
- Rosman, N.S.R.; Harun, N.A.; Idris, I.; Ismail, W.I.W. Eco-Friendly Silver Nanoparticles (AgNPs) Fabricated by Green Synthesis Using the Crude Extract of Marine Polychaete, Marphysa moribidii: Biosynthesis, Characterisation, and Antibacterial Applications. Heliyon 2020, 6, e05462. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile Green Synthesis of Silver Nanoparticles Using Berberis vulgaris Leaf and Root Aqueous Extract and Its Antibacterial Activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Mikhailova, E.O. Elemental Silver Nanoparticles: Biosynthesis and Bio Applications. Materials 2019, 12, 3177. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016, 6, 4. [Google Scholar] [CrossRef]
- Paul, S.; Sarkar, I.; Sarkar, N.; Bose, A.; Chakraborty, M.; Chakraborty, A.; Mukherjee, S. Silver Nanoparticles in Diabetes Mellitus: Therapeutic Potential and Mechanistic Insights. Bull. Natl. Res. Cent. 2024, 48, 33. [Google Scholar] [CrossRef]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A Systematic Review on Green Synthesis of Silver Nanoparticles Using Plants Extract and Their Bio-Medical Applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of Cosurfactant Effect on Nanoemulsifying Area and Development of Lercanidipine Loaded (SNEDDS) Self Nanoemulsifying Drug Delivery System. Colloids Surf. B Biointerfaces 2011, 86, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Mashwani, Z.U.R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in Plants and Plant Extracts of Silver Nanoparticles with Potent Antimicrobial Properties: Current Status and Future Prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of Silver Nanoparticles Using Alternanthera sessilis (Linn.) Extract and Their Antimicrobial, Antioxidant Activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Houdeau, E.; Thomas, M. Mucus and Microbiota as Emerging Players in Gut Nanotoxicology: The Example of Dietary Silver and Titanium Dioxide Nanoparticles. Crit. Rev. Food Sci. Nutr. 2018, 58, 1023–1032. [Google Scholar] [CrossRef]
- Salieri, B.; Kaiser, J.P.; Rösslein, M.; Nowack, B.; Hischier, R.; Wick, P. Relative Potency Factor Approach Enables the Use of in Vitro Information for Estimation of Human Effect Factors for Nanoparticle Toxicity in Life-Cycle Impact Assessment. Nanotoxicology 2020, 14, 275–286. [Google Scholar] [CrossRef]
- Soon Kim, Y.; Yong Song, M.; Duck Park, J.; Seuk Song, K.; Ryol Ryu, H.; Hyun Chung, Y.; Kyung Chang, H.; Hyun Lee, J.; Hui Oh, K.; Kelman, B.J.; et al. Subchronic Oral Toxicity of Silver Nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering Gut Microbiota in Treatment of Type 2 Diabetes Mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Synthesis of Bio-Mediated Silver Nanoparticles from Silybum marianum and Their Biological and Clinical Activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef]
- Kurra, H.; Velidandi, A.; Sarvepalli, M.; Pabbathi, N.P.P.; Godishala, V. Aqueous Cymbopogon citratus Extract Mediated Silver Nanoparticles: Part I. Influence of Synthesis Parameters, Characterization, and Biomedical Studies. Nanomaterials 2025, 15, 328. [Google Scholar] [CrossRef]
- Abideen, S.; Vijaya Sankar, M. In-Vitro Screening of Antidiabetic and Antimicrobial Activity against Green Synthesized AgNO3 Using Seaweeds. J. Nanomed. Nanotechnol. 2015, 10, 2157–7439. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of Silver Nanoparticles from Melia azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of Antioxidant, Antibacterial, Antidiabetic, and Cytotoxicity Potential of Silver Nanoparticles Synthesized Using the Outer Peel Extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Benelli, G.; Kumar, G.; Pugazhendhi, A.; Kim, D.S.; Shin, H.S. Anti-Diabetic Potential of Silver Nanoparticles Synthesized with Argyreia nervosa Leaf Extract High Synergistic Antibacterial Activity with Standard Antibiotics Against Foodborne Bacteria. J. Clust. Sci. 2017, 28, 1709–1727. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ahn, S.; Shin, H.S. Grape Pomace Extracted Tannin for Green Synthesis of Silver Nanoparticles: Assessment of Their Antidiabetic, Antioxidant Potential and Antimicrobial Activity. Polymers 2021, 13, 4355. [Google Scholar] [CrossRef]
- Anandachockalingam, A.; Shanmugam, R.; Ryntathiang, I.; Dharmalingam Jothinathan, M.K. Green Synthesis of Silver Nanoparticles Using Zingiber officinale and Ocimum gratissimum Formulation for Its Anti-Inflammatory and Antidiabetic Activity: An In Vitro Study. Cureus 2024, 16, e58098. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Ghufran, M.; Shams, S.; Jamal, A.; Khan, A.; Abdullah; Awan, Z.A.; Khan, M.I. Green Synthesis of Silver Nanoparticles from Plant Fagonia cretica and Evaluating Its Anti-Diabetic Activity through Indepth in-Vitro and in-Vivo Analysis. Front. Pharmacol. 2023, 14, 1194809. [Google Scholar] [CrossRef]
- Ahmed, F.; Sairam, S.; Urooj, A. In Vitro Hypoglycemic Effects of Selected Dietary Fiber Sources. J. Food Sci. Technol. 2011, 48, 285–289. [Google Scholar] [CrossRef]
- Khaleel Basha, S.; Govindaraju, K.; Manikandan, R.; Ahn, J.S.; Bae, E.Y.; Singaravelu, G. Phytochemical Mediated Gold Nanoparticles and Their PTP 1B Inhibitory Activity. Colloids Surf. B Biointerfaces 2010, 75, 405–409. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, Antibacterial, Antioxidant, Antidiabetic, Anti-Inflammatory and Antityrosinase Activity of Green Synthesized Silver Nanoparticles Using Calophyllum tomentosum Leaves Extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Seneviratne, C.; Narayanan, R.; Liu, W.; Dain, J.A. The in Vitro Inhibition Effect of 2 nm Gold Nanoparticles on Non-Enzymatic Glycation of Human Serum Albumin. Biochem. Biophys. Res. Commun. 2012, 422, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yarrappagaari, S.; Gutha, R.; Narayanaswamy, L.; Thopireddy, L.; Benne, L.; Mohiyuddin, S.S.; Vijayakumar, V.; Saddala, R.R. Eco-Friendly Synthesis of Silver Nanoparticles from the Whole Plant of Cleome viscosa and Evaluation of Their Characterization, Antibacterial, Antioxidant and Antidiabetic Properties. Saudi J. Biol. Sci. 2020, 27, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Ansari, M.A.; Fatma, S.; Abdullah, S.M.S.; Iqbal, J.; Madkhali, A.; Hamali, A.H.; Ahmad, S.; Jerah, A.; Echeverria, V.; et al. Inhibiting Effect of Zinc Oxide Nanoparticles on Advanced Glycation Products and Oxidative Modifications: A Potential Tool to Counteract Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2018, 55, 7438–7452. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In Vitro and in Silico Studies of Silver Nanoparticles (AgNPs) from Allium sativum against Diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef] [PubMed]
- Rehman, G.; Umar, M.; Shah, N.; Hamayun, M.; Ali, A.; Khan, W.; Khan, A.; Ahmad, S.; Alrefaei, A.F.; Almutairi, M.H.; et al. Green Synthesis and Characterization of Silver Nanoparticles Using Azadirachta indica Seeds Extract: In Vitro and In Vivo Evaluation of Anti-Diabetic Activity. Pharmaceuticals 2023, 16, 1677. [Google Scholar] [CrossRef]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Hasabe, T.S. In Vitro Hypoglycemic Effects of Unripe and Ripe Fruits of Musa sapientum. Braz. J. Pharm. Sci. 2017, 53, e00159. [Google Scholar] [CrossRef]
- Johnson, E.C.; Ebong, S.S.; Ejinwa, G.; Eseyin, O.A. Comparative Assessment of Antidiabetic Properties of Aqueous Extract and Its Silver Nanoparticles from Vernonia amygdalina (ASTERACEAE). Niger. J. Pharm. Appl. Sci. Res. 2024, 13, 2971–7388. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Yadav, M.; Chahal, J.; Dalal, S.; Kataria, S.K. Phytochemical Screening and Antidiabetic Efficacy of Balanites aegyptiaca Seed Extract and Their Silver Nanoparticles on Muscle and Pancreatic Cell Lines. ACS Omega 2024, 9, 22660–22676. [Google Scholar] [CrossRef]
- Sengani, M.; Bavithra, V.; Banerjee, M.; Choudhury, A.A.; Chakraborty, S.; Ramasubbu, K.; Rajeswari, V.D.; Al Obaid, S.; Alharbi, S.A.; Subramani, B.; et al. Evaluation of the Anti-Diabetic Effect of Biogenic Silver Nanoparticles and Intervention in PPARγ Gene Regulation. Environ. Res. 2022, 215, 114408. [Google Scholar] [CrossRef]
- Garcia-Campoy, A.H.; Perez-Gutierrez, R.M.; Manriquez-Alvirde, G.; Muñiz-Ramirez, A. Protection of Silver Nanoparticles Using Eysenhardtia polystachya in Peroxide-Induced Pancreatic β-Cell Damage and Their Antidiabetic Properties in Zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef]
- Chaudhari, M.G.; Joshi, B.B.; Mistry, K.N. In Vitro Anti-diabetic and Anti-inflammatory Activity of Stem Bark of Bauhinia purpurea. Bull. Pharm. Med. Sci. (BOPAMS) 2013, 1, 139–150. [Google Scholar]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.; Rashid, F.; Ali, S.; Summer, M.; Afzal, M. Synthesis, Characterization, Phytochemistry, and Therapeutic Potential of Azadirachta indica Conjugated Silver Nanoparticles: A Comprehensive Study on Antidiabetic and Antioxidant Properties. Biol. Trace Elem. Res. 2024, 203, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.; Bhatti, A.; John, P. Citation: Evaluation of Antidiabetic Activity of Biogenic Silver Nanoparticles Using Thymus serpyllum on Streptozotocin-Induced Diabetic BALB/c Mice. Polymers 2022, 14, 3138. [Google Scholar] [CrossRef]
- Shah, A.H.; Khan, A.; Khan, N.; Jannat, S.; Alarjan, K.M.; Elshikh, M.S.; Afareen, A.; Hameed, H. Synergistic Phytochemical and Nanotechnological Exploration of Melia azedarach With Silver Nitrate: Elucidating Multifaceted Antimicrobial, Antioxidant, Antidiabetic, and Insecticidal Potentials. Microsc. Res. Tech. 2025, 88, 542–554. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting Antidiabetic Activity of Silver Nanoparticles Synthesized Using Punica granatum Leaves and Anticancer Potential against Human Liver Cancer Cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Basavegowda, N.; Vishnuprasad, C.N.; Shin, H.S. Comparative Study on Antidiabetic, Cytotoxicity, Antioxidant and Antibacterial Properties of Biosynthesized Silver Nanoparticles Using Outer Peels of Two Varieties of Ipomoea batatas (L.) Lam. Int. J. Nanomed. 2019, 14, 4741–4754. [Google Scholar] [CrossRef]
- Dugganaboyana, G.K.; Kumar Mukunda, C.; Jain, A.; Kantharaju, R.M.; Nithya, R.R.; Ninganna, D.; Ahalliya, R.M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; et al. Environmentally Benign Silver Bio-Nanomaterials as Potent Antioxidant, Antibacterial, and Antidiabetic Agents: Green Synthesis Using Salacia oblonga Root Extract. Front. Chem. 2023, 11, 1114109. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.W.A.; Qureshi, M.T.; Hussain, Z.; Ullah, I.; Kalsoom, U.E.; Rahim, F.; Rahman, S.S.U.; Sultana, N.; Khan, M.K. Antidiabetic and Hypolipidemic Potential of Green AgNPs against Diabetic Mice. ACS Appl. Bio Mater. 2021, 4, 3433–3442. [Google Scholar] [CrossRef]
- Mughal, T.A.; Ali, S.; Mumtaz, S.; Summer, M.; Saleem, M.Z.; Hassan, A.; Hameed, M.U. Evaluating the Biological (Antidiabetic) Potential of TEM, FTIR, XRD, and UV-Spectra Observed Berberis lyceum Conjugated Silver Nanoparticles. Microsc. Res. Tech. 2024, 87, 1286–1305. [Google Scholar] [CrossRef]
- Iqbal, R.; Asghar, A.; Habib, A.; Ali, S.; Zahra, S.; Hussain, M.I.; Ahsan, A.B.; Liang, Y. Therapeutic Potential of Green Synthesized Silver Nanoparticles for Promoting Wound-Healing Process in Diabetic Mice. Biol. Trace Elem. Res. 2024, 202, 5545–5555. [Google Scholar] [CrossRef] [PubMed]
- Sengottaiyan, A.; Aravinthan, A.; Sudhakar, C.; Selvam, K.; Srinivasan, P.; Govarthanan, M.; Manoharan, K.; Selvankumar, T. Synthesis and Characterization of Solanum nigrum-Mediated Silver Nanoparticles and Its Protective Effect on Alloxan-Induced Diabetic Rats. J. Nanostructure Chem. 2016, 6, 41–48. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Mahmoudi, F.; Gollo, K.H.; Amini, M.M. Biosynthesis of Novel Silver Nanoparticles Using Eryngium thyrsoideum boiss Extract and Comparison of Their Antidiabetic Activity with Chemical Synthesized Silver Nanoparticles in Diabetic Rats. Biol. Trace Elem. Res. 2021, 199, 1967–1978. [Google Scholar] [CrossRef]
- Ul Haq, M.N.; Shah, G.M.; Gul, A.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S.; Hussain, M.; Alkreathy, H.M.; Ullah, I.; Khan, A.M.; et al. Biogenic Synthesis of Silver Nanoparticles Using Phagnalon niveum and Its In Vivo Anti-Diabetic Effect against Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 830. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Rehman, G.; Shah, N.; Hamayun, M.; Ali, S.; Ali, A.; Shah, S.k.; Khan, W.; Shah, M.I.A.; Alrefaei, A.F. Biosynthesis and Characterization of Silver Nanoparticles Using Tribulus terrestris Seeds: Revealed Promising Antidiabetic Potentials. Molecules 2023, 28, 4203. [Google Scholar] [CrossRef]
- Hosen, E.; Rahman, A.; Rahman, S.; Akash, S.; Khalekuzzaman; Alsahli, A.A.; Bourhia, M.; Nafidi, H.A.; Islam, A.; Zaman, R. Synthesis of Silver Nanoparticles Using Camellia sinensis Leaf Extract: Promising Particles for the Treatment of Cancer and Diabetes. Chem. Biodivers. 2024, 21, e202301661. [Google Scholar] [CrossRef]
- Virgen-Ortiz, A.; Limón-Miranda, S.; Soto-Covarrubias, M.A.; Apolinar-Iribe, A.; Rodríguez-León, E.; Iñiguez-Palomares, R. Biocompatible silver nanoparticles synthesized using Rumex hymenosepalus extract decreases fasting glucose levels in diabetic rats. Dig. J. Nanomater. Biostruct 2015, 10, 927–933. [Google Scholar]
- Shanker, K.; Naradala, J.; Mohan, G.K.; Kumar, G.S.; Pravallika, P.L. A Sub-Acute Oral Toxicity Analysis and Comparative: In Vivo Anti-Diabetic Activity of Zinc Oxide, Cerium Oxide, Silver Nanoparticles, and Momordica charantia in Streptozotocin-Induced Diabetic Wistar Rats. RSC Adv. 2017, 7, 37158–37167. [Google Scholar] [CrossRef]
- Kalakotla, S.; Jayarambabu, N.; Mohan, G.K.; Mydin, R.B.S.M.N.; Gupta, V.R. A Novel Pharmacological Approach of Herbal Mediated Cerium Oxide and Silver Nanoparticles with Improved Biomedical Activity in Comparison with Lawsonia inermis. Colloids Surf. B Biointerfaces 2019, 174, 199–206. [Google Scholar] [CrossRef]
- Nagaraja, S.; Ahmed, S.S.; Bharathi, D.R.; Goudanavar, P.; Rupesh, K.M.; Fattepur, S.; Meravanige, G.; Shariff, A.; Shiroorkar, P.N.; Habeebuddin, M.; et al. Green Synthesis and Characterization of Silver Nanoparticles of Psidium guajava Leaf Extract and Evaluation for Its Antidiabetic Activity. Molecules 2022, 27, 4336. [Google Scholar] [CrossRef]
- Khodeer, D.M.; Nasr, A.M.; Swidan, S.A.; Shabayek, S.; Khinkar, R.M.; Aldurdunji, M.M.; Ramadan, M.A.; Badr, J.M. Characterization, Antibacterial, Antioxidant, Antidiabetic, and Anti-Inflammatory Activities of Green Synthesized Silver Nanoparticles Using Phragmanthera austroarabica A. G. Mill and J. A. Nyberg Extract. Front. Microbiol. 2023, 13, 1078061. [Google Scholar] [CrossRef] [PubMed]
- Pirabbasi, E.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R.; Kalantar, M. Chemical Characterization and Effect of Ziziphora clinopodioides Green-Synthesized Silver Nanoparticles on Cytotoxicity, Antioxidant, and Antidiabetic Activities in Streptozotocin-Induced Hepatotoxicity in Wistar Diabetic Male Rats. Food Sci. Nutr. 2024, 12, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bao, M.; Peng, Y.; Gao, J.; Bao, J. Eco-Friendly Nanoparticles Synthesized from Salvia sclarea Ethanol Extract Protect against STZ-Induced Diabetic Nephropathy in Rats via Antioxidant, Anti-Inflammatory, and Apoptosis Mechanisms. J. Oleo Sci. 2024, 73, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-Derived Non-Phenolic α-Amylase and α-Glucosidase Inhibitors for Controlling Starch Digestion Rate and Guiding Diabetes-Friendly Recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Smith, D.L.; Orlandella, R.M.; Allison, D.B.; Norian, L.A. Diabetes Medications as Potential Calorie Restriction Mimetics—A Focus on the Alpha-Glucosidase Inhibitor Acarbose. Geroscience 2021, 43, 1123–1133. [Google Scholar] [CrossRef]
- Khan, F.; Shah, A.A.; Kumar, A.; Akhtar, S. In Silico Investigation against Inhibitors of Alpha-Amylase Using Structure-Based Screening, Molecular Docking, and Molecular Simulations Studies. Cell Biochem. Biophys. 2024, 82, 2873–2888. [Google Scholar] [CrossRef]
- Badeggi, U.M.; Badmus, J.A.; Botha, S.S.; Ismail, E.; Marnewick, J.L.; Africa, C.W.J.; Hussein, A.A. Biosynthesis, Characterization, and Biological Activities of Procyanidin Capped Silver Nanoparticles. J. Funct. Biomater. 2020, 11, 66. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing Antidiabetic and Antimicrobial Performance of Ocimum basilicum, and Ocimum sanctum (L.) Using Silver Nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef]
- Patra, J.K.; Shin, H.S.; Das, G. Characterization and Evaluation of Multiple Biological Activities of Silver Nanoparticles Fabricated from Dragon Tongue Bean Outer Peel Extract. Int. J. Nanomed. 2021, 16, 977–987. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Shin, H.S. Facile Green Biosynthesis of Silver Nanoparticles Using Pisum sativum L. Outer Peel Aqueous Extract and Its Antidiabetic, Cytotoxicity, Antioxidant, and Antibacterial Activity. Int. J. Nanomed. 2019, 14, 6679–6690. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.S.; Patra, J.K. Comparative Assessment of Antioxidant, Anti-Diabetic and Cytotoxic Effects of Three Peel/ Shelle Food Waste Extract-Mediated Silver Nanoparticles. Int. J. Nanomed. 2020, 15, 9075–9088. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.K.; Shin, H.S. Biosynthesis, and Potential Effect of Fern Mediated Biocompatible Silver Nanoparticles by Cytotoxicity, Antidiabetic, Antioxidant and Antibacterial, Studies. Mater. Sci. Eng. C 2020, 114, 111011. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Kour, G.; Bartwal, A.S.; Sati, M.D. Biosynthesis of Metal Nanoparticles from Leaves of Ficus Palmata and Evaluation of Their Anti-Inflammatory and Anti-Diabetic Activities. Biochemistry 2020, 59, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Badmus, J.A.; Oyemomi, S.A.; Adedosu, O.T.; Yekeen, T.A.; Azeez, M.A.; Adebayo, E.A.; Lateef, A.; Badeggi, U.M.; Botha, S.; Hussein, A.A.; et al. Photo-Assisted Bio-Fabrication of Silver Nanoparticles Using Annona muricata Leaf Extract: Exploring the Antioxidant, Anti-Diabetic, Antimicrobial, and Cytotoxic Activities. Heliyon 2020, 6, e05413. [Google Scholar] [CrossRef]
- Antonysamy Johnson, M.A.; Shibila, T.; Amutha, S.; Menezes, I.R.A.; Da Costa, J.G.M.; Sampaio, N.F.L.; Coutinho, H.D.M. Synthesis of Silver Nanoparticles Using Odontosoria chinensis (L.) J. Sm. and Evaluation of Their Biological Potentials. Pharmaceuticals 2020, 13, 66. [Google Scholar] [CrossRef]
- Anjum, S.; Khan, A.K.; Qamar, A.; Fatima, N.; Drouet, S.; Renouard, S.; Blondeau, J.P.; Abbasi, B.H.; Hano, C. Light Tailoring: Impact of UV-C Irradiation on Biosynthesis, Physiognomies, and Clinical Activities of Morus macroura-Mediated Monometallic (Ag and ZnO) and Bimetallic (Ag and ZnO) Nanoparticles. Int. J. Mol. Sci. 2021, 22, 11294. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.S.; Patra, J.K. Multitherapeutic Efficacy of Curly Kale Extract Fabricated Biogenic Silver Nanoparticles. Int. J. Nanomed. 2022, 17, 1125–1137. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.S.; Patra, J.K. Key Health Benefits of Korean ueong Dry Root Extract Combined Silver Nanoparticles. Int. J. Nanomed. 2022, 17, 4261–4275. [Google Scholar] [CrossRef]
- Balu, S.K.; Andra, S.; Damiri, F.; Sivaramalingam, A.; Sudandaradoss, M.V.; Kumarasamy, K.; Bhakthavachalam, K.; Ali, F.; Kundu, M.K.; Rahman, M.H.; et al. Size-Dependent Antibacterial, Antidiabetic, and Toxicity of Silver Nanoparticles Synthesized Using Solvent Extraction of Rosa indica L. Petals. Pharmaceuticals 2022, 15, 689. [Google Scholar] [CrossRef]
- Zubair, M.; Azeem, M.; Mumtaz, R.; Younas, M.; Adrees, M.; Zubair, E.; Khalid, A.; Hafeez, F.; Rizwan, M.; Ali, S. Green Synthesis and Characterization of Silver Nanoparticles from Acacia nilotica and Their Anticancer, Antidiabetic and Antioxidant Efficacy. Environ. Pollut. 2022, 304, 119249. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Dharavath, S.B.; Taduri, S. Eco-Friendly Green Synthesis of Silver Nanoparticles from Leaf Extract of Solanum khasianum: Optical Properties and Biological Applications. Appl. Biochem. Biotechnol. 2023, 195, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Hannachi, H.; Nouir, R.; Mottola, F.; Rocco, L. Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima Subsp. Maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens. Nanomaterials 2023, 13, 1964. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Dridi, R.; Mottola, F.; Rocco, L.; Zid, M.F.; Hannachi, H. Biosynthesis and Characterization of Silver Nanoparticles from the Extremophile Plant Aeonium haworthii and Their Antioxidant, Antimicrobial and Anti-Diabetic Capacities. Nanomaterials 2023, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Sumra, A.A.; Zain, M.; Saleem, T.; Yasin, G.; Azhar, M.F.; Zaman, Q.U.; Budhram-Mahadeo, V.; Ali, H.M. Biogenic Synthesis, Characterization, and In Vitro Biological Evaluation of Silver Nanoparticles Using Cleome brachycarpa. Plants 2023, 12, 1578. [Google Scholar] [CrossRef]
- Yousefzadeh-Valendeh, S.; Fattahi, M.; Asghari, B.; Alizadeh, Z. Dandelion Flower-Fabricated Ag Nanoparticles versus Synthetic Ones with Characterization and Determination of Photocatalytic, Antioxidant, Antibacterial, and α-Glucosidase Inhibitory Activities. Sci. Rep. 2023, 13, 15444. [Google Scholar] [CrossRef]
- Dilipan, E.; Sivaperumal, P.; Kamala, K.; Ramachandran, M.; Vivekanandhan, P. Green Synthesis of Silver Nanoparticles Using Seagrass Cymodocea serrulata (R.Br.) Asch. & Magnus, Characterization, and Evaluation of Anticancer, Antioxidant, and Antiglycemic Index. Biotechnol. Appl. Biochem. 2023, 70, 1346–1356. [Google Scholar] [CrossRef]
- Paramasivam, D.; Balasubramanian, B.; Suresh, R.; Kumaravelu, J.; Vellingiri, M.M.; Liu, W.C.; Meyyazhagan, A.; Alanazi, A.M.; Rengasamy, K.R.R.; Arumugam, V.A. One-Pot Synthesis of Silver Nanoparticles Derived from Aqueous Leaf Extract of Ageratum conyzoides and Their Biological Efficacy. Antibiotics 2023, 12, 688. [Google Scholar] [CrossRef]
- Kiani, B.H.; Arshad, I.; Najeeb, S.; Okla, M.K.; Almanaa, T.N.; Al-Qahtani, W.H.; Abdel-Maksoud, M.A. Evaluation of Biogenic Silver Nanoparticles Synthesized from Vegetable Waste. Int. J. Nanomed. 2023, 18, 6527–6544. [Google Scholar] [CrossRef]
- Nagime, P.V.; Singh, S.; Shaikh, N.M.; Gomare, K.S.; Chitme, H.; Abdel-Wahab, B.A.; Alqahtany, Y.S.; Khateeb, M.M.; Habeeb, M.S.; Bakir, M.B. Biogenic Fabrication of Silver Nanoparticles Using Calotropis procera Flower Extract with Enhanced Biomimetics Attributes. Materials 2023, 16, 4058. [Google Scholar] [CrossRef]
- Khan, Z.U.R.; Assad, N.; Naeem-ul-Hassan, M.; Sher, M.; Alatawi, S.; Alatawi, M.S.; Omran, A.M.E.; Jame, R.M.A.; Adnan, M.; Khan, M.N.; et al. Aconitum lycoctonum L. (Ranunculaceae) Mediated Biogenic Synthesis of Silver Nanoparticles as Potential Antioxidant, Anti-Inflammatory, Antimicrobial and Antidiabetic Agents. BMC Chem. 2023, 17, 128. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, N.; Perinmbam, K.; Devanesan, S.; AlSalhi, M.S.; Asemi, N.; Nicoletti, M. Synthesis of Silver Nanoparticles by Leaf Extract of Cucumis melo L. and Their In Vitro Antidiabetic and Anticoccidial Activities. Molecules 2023, 28, 4995. [Google Scholar] [CrossRef]
- Ejaz, U.; Afzal, M.; Mazhar, M.; Riaz, M.; Ahmed, N.; Rizg, W.Y.; Alahmadi, A.A.; Badr, M.Y.; Mushtaq, R.Y.; Yean, C.Y. Characterization, Synthesis, and Biological Activities of Silver Nanoparticles Produced via Green Synthesis Method Using Thymus vulgaris Aqueous Extract. Int. J. Nanomed. 2024, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ashraf, G.J.; Baishya, T.; Dua, T.K.; Paul, P.; Nandi, G.; Dutta, A.; Limbu, D.; Kumar, A.; Adhikari, M.D.; et al. Formulation of Silver Nanoparticles Using Duabanga grandiflora Leaf Extract and Evaluation of Their Versatile Therapeutic Applications. Bioprocess. Biosyst. Eng. 2024, 47, 1139–1150. [Google Scholar] [CrossRef]
- Geremew, A.; Gonzalles, J.; Peace, E.; Woldesenbet, S.; Reeves, S.; Brooks, N.; Carson, L. Green Synthesis of Novel Silver Nanoparticles Using Salvia blepharophylla and Salvia greggii: Antioxidant and Antidiabetic Potential and Effect on Foodborne Bacterial Pathogens. Int. J. Mol. Sci. 2024, 25, 904. [Google Scholar] [CrossRef]
- Arya, A.; Chahar, D.; Bhakuni, K.; Vandana; Kumar, S.; Venkatesu, P. Green Synthesis of Silver Nanoparticles Using Drymaria cordata and Their Biocompatibility with Hemoglobin: A Therapeutic Potential Approach. ACS Appl. Bio Mater. 2024, 7, 977–989. [Google Scholar] [CrossRef]
- Naveed, M.; Mahmood, S.; Aziz, T.; Azeem, A.; Rajpoot, Z.; Rehman, S.U.; Al-Asmari, F.; Alahmari, A.S.; Saleh, O.; Sameeh, M.Y.; et al. Green-Synthesis of Silver Nanoparticles AgNPs from Podocarpus macrophyllus for Targeting GBM and LGG Brain Cancers via NOTCH2 Gene Interactions. Sci. Rep. 2024, 14, 25489. [Google Scholar] [CrossRef]
- Sasikala, M.; Mohan, S.; Karuppaiah, A.; Karthick, V.; Ragul, P.A.; Nagarajan, A. NanoFlora: Unveiling the Therapeutic Potential of Ipomoea aquatica Nanoparticles. J. Genet. Eng. Biotechnol. 2025, 23, 100470. [Google Scholar] [CrossRef]
- Hussain, H.; Abbas, G.; Green, I.R.; Ali, I. Dipeptidyl Peptidase IV Inhibitors as a Potential Target for Diabetes: Patent Review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 535–553. [Google Scholar] [CrossRef]
- Shao, D.W.; Zhao, L.J.; Sun, J.F. Synthesis and Clinical Application of Representative Small-Molecule Dipeptidyl Peptidase-4 (DPP-4) Inhibitors for the Treatment of Type 2 Diabetes Mellitus (T2DM). Eur. J. Med. Chem. 2024, 272, 116464. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.; Costa, H.S.; Silva, M.A.; Albuquerque, T.G. The Health Effects of Low Glycemic Index and Low Glycemic Load Interventions on Prediabetes and Type 2 Diabetes Mellitus: A Literature Review of RCTs. Nutrients 2023, 15, 5060. [Google Scholar] [CrossRef] [PubMed]
- Coronell-Tovar, A.; Pardo, J.P.; Rodríguez-Romero, A.; Sosa-Peinado, A.; Vásquez-Bochm, L.; Cano-Sánchez, P.; Álvarez-Añorve, L.I.; González-Andrade, M. Protein Tyrosine Phosphatase 1B (PTP1B) Function, Structure, and Inhibition Strategies to Develop Antidiabetic Drugs. FEBS Lett. 2024, 598, 1811–1838. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The Interplay of α-Amylase and Amyloglucosidase Activities on the Digestion of Starch in in Vitro Enzymic Systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and Exercise in the Prevention and Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Marchignoli, F.; Sasdelli, A.S.; Caraceni, P.; Marchesini, G.; Ravaioli, F. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- Khanna, I. Drug Discovery in Pharmaceutical Industry: Productivity Challenges and Trends. Drug Discov. Today 2012, 17, 1088–1102. [Google Scholar] [CrossRef]
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).