Promising Norlabdane-Heterocyclic Hybrids: Synthesis, Structural Characterization and Antimicrobial Activity Evaluation
Abstract
1. Introduction
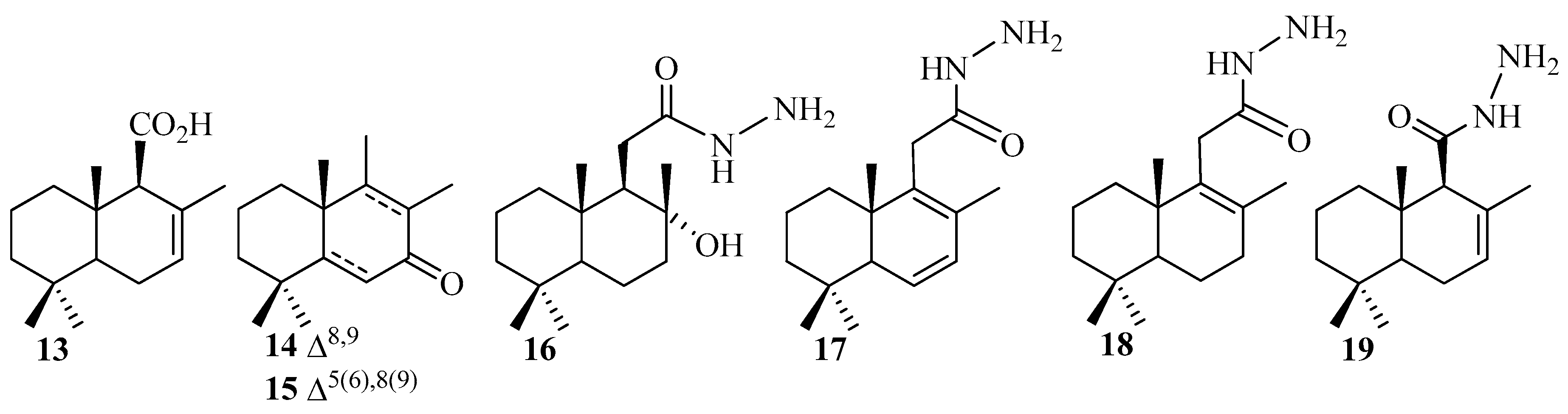
2. Results
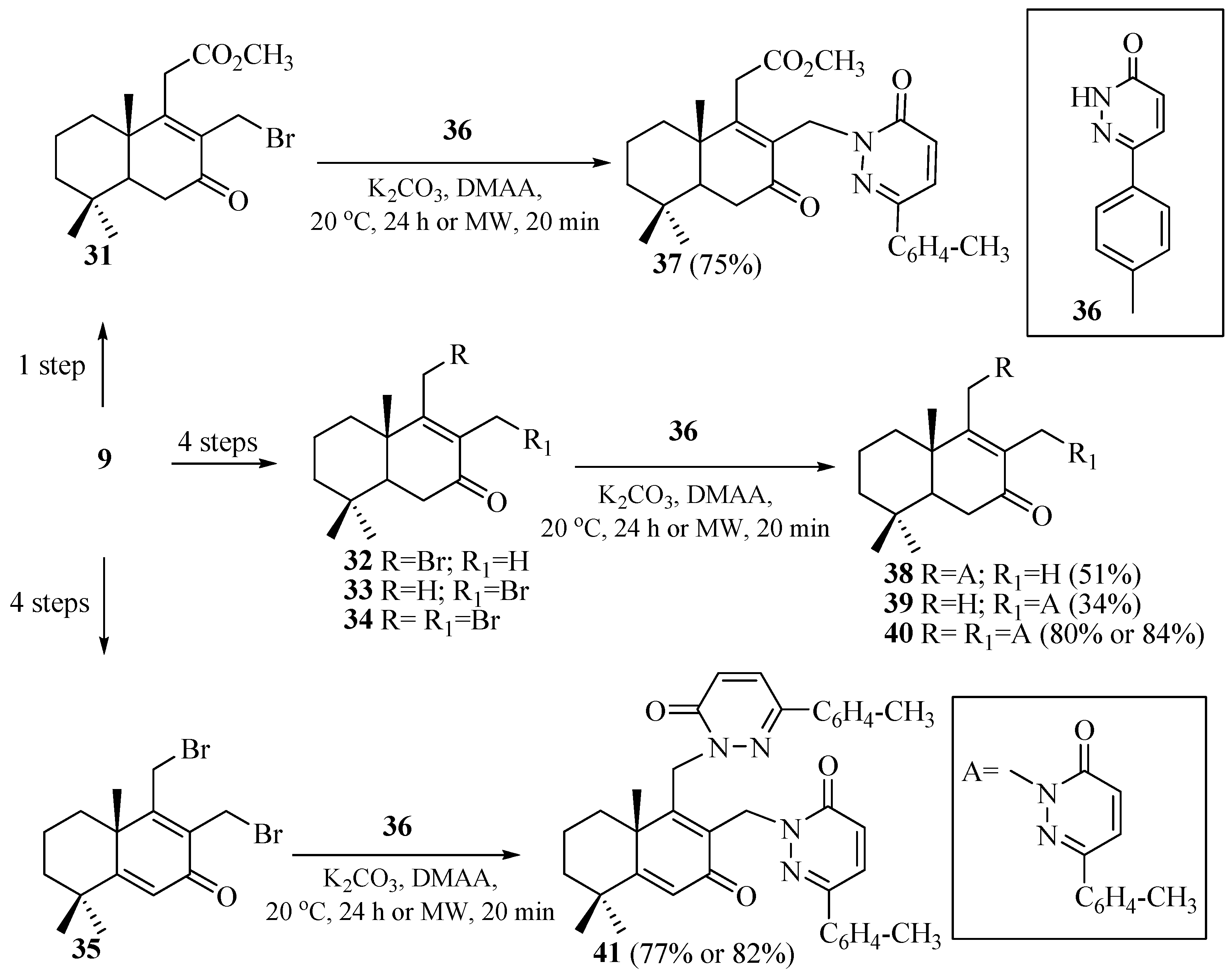
2.1. Synthesis of Norlabdane–Diazine Hybrids
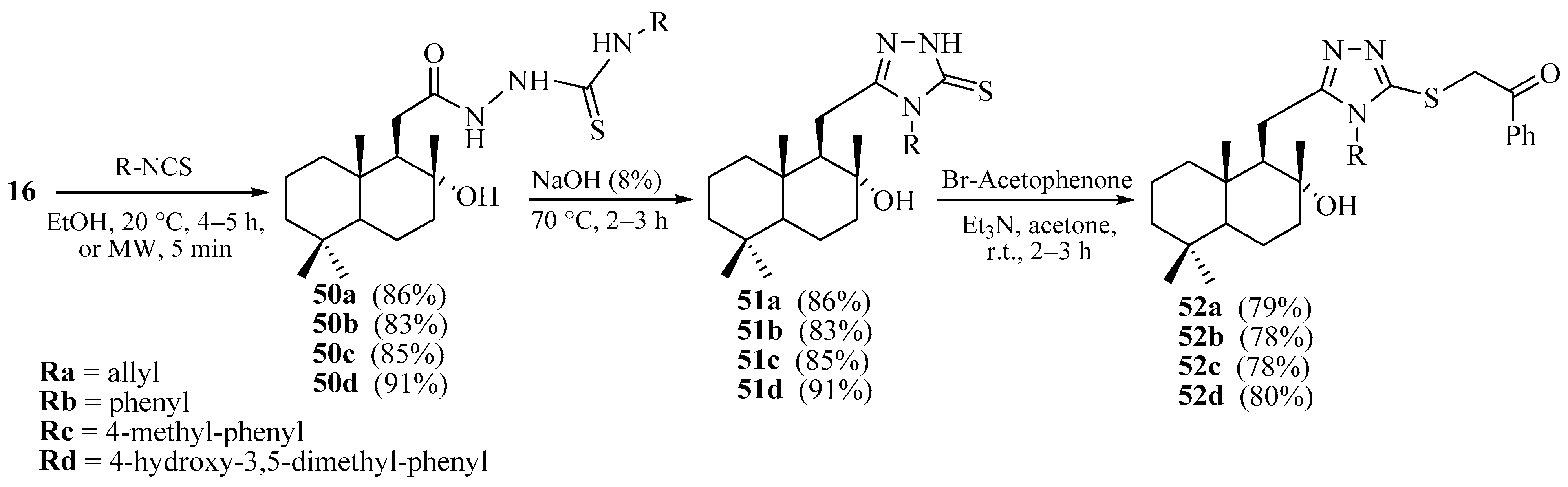
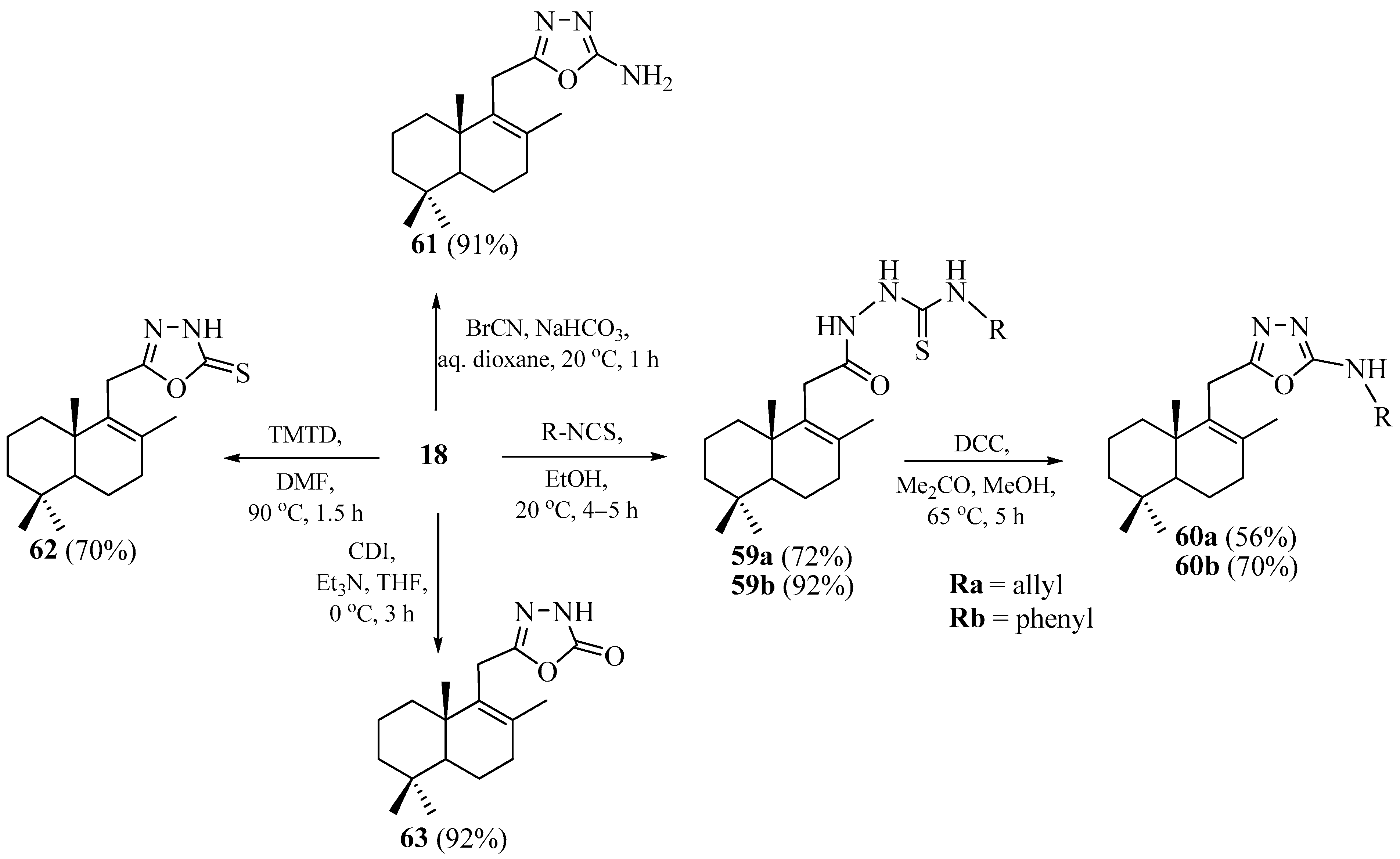
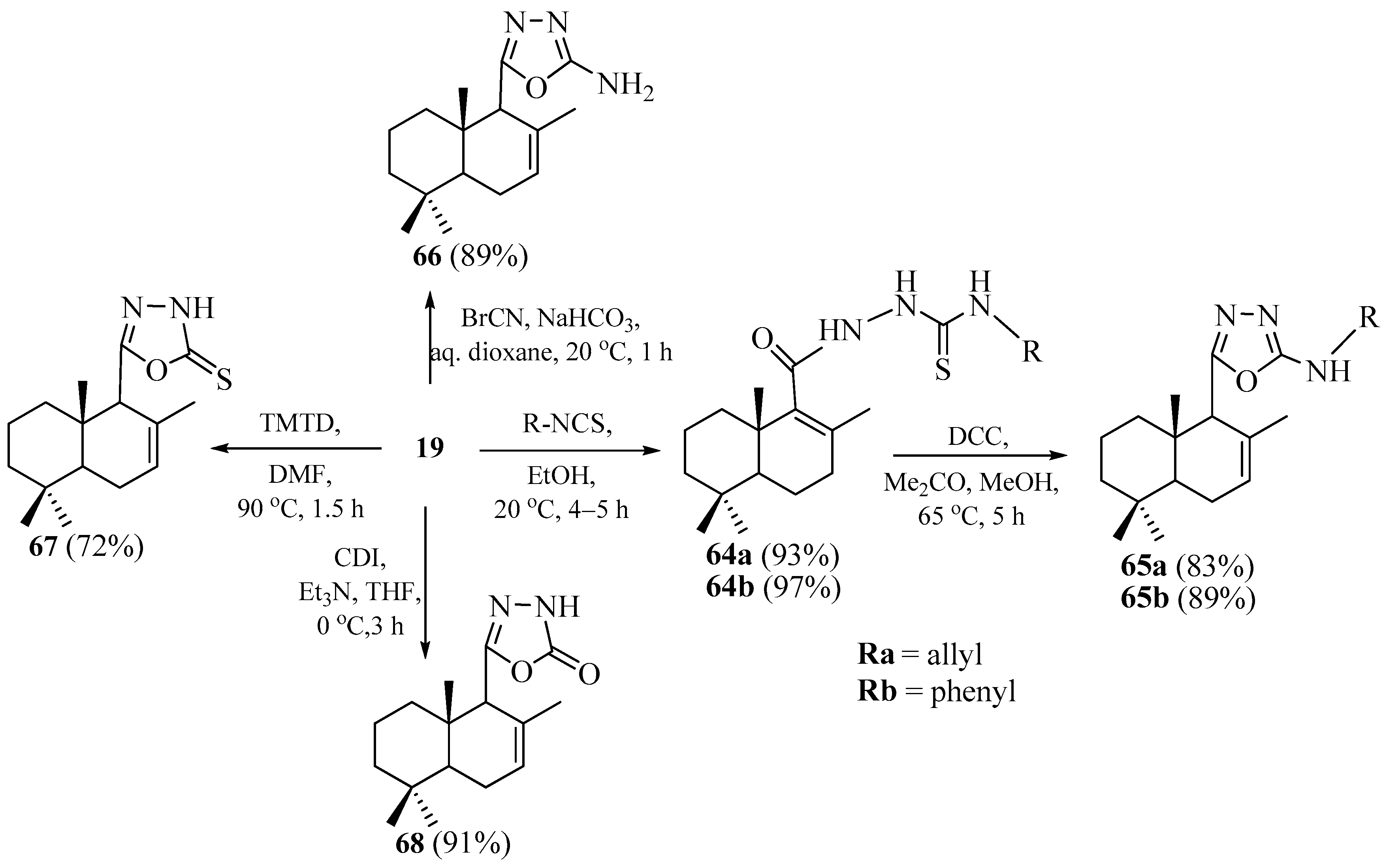
2.2. Synthesis of Norlabdane-1,2,4-triazole and Carbazole Hybrids
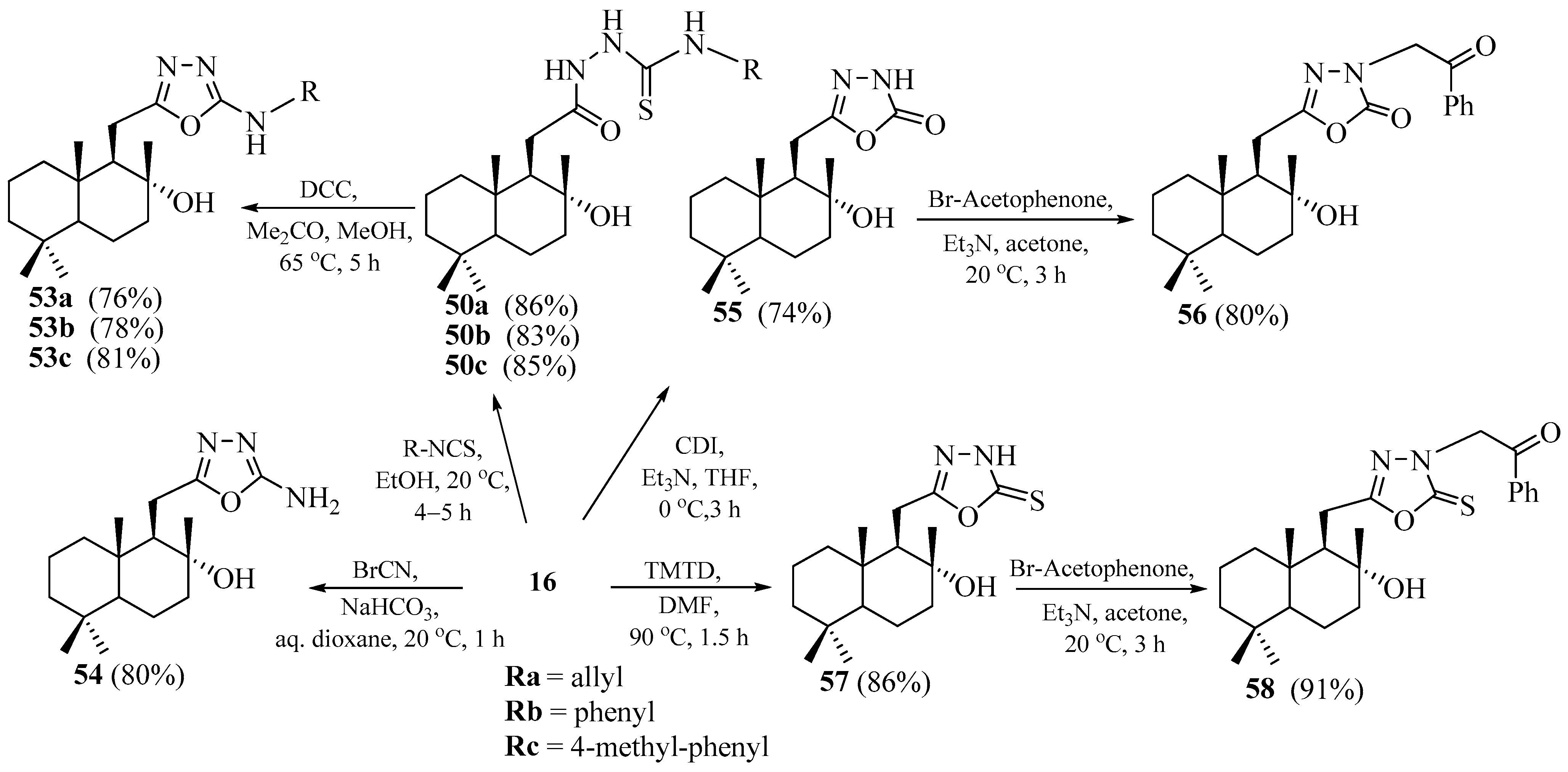
2.3. Synthesis of Norlabdane-1,3,4-oxadiazole Hybrids
2.4. Synthesis of Norlabdane-1,3,4-thiadiazole Hybrids
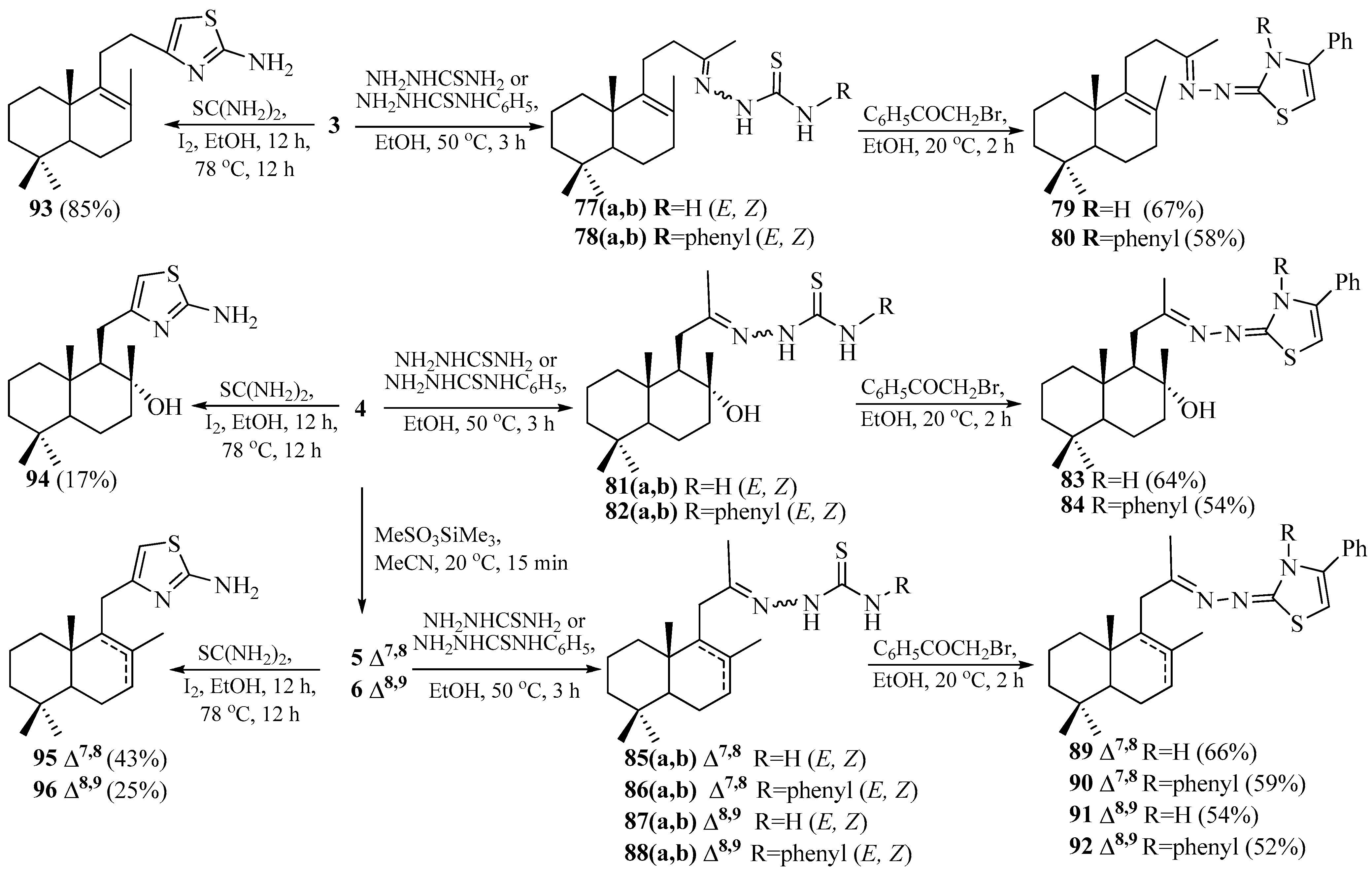
2.5. Synthesis of Norlabdane-1,3-thiazole Hybrids
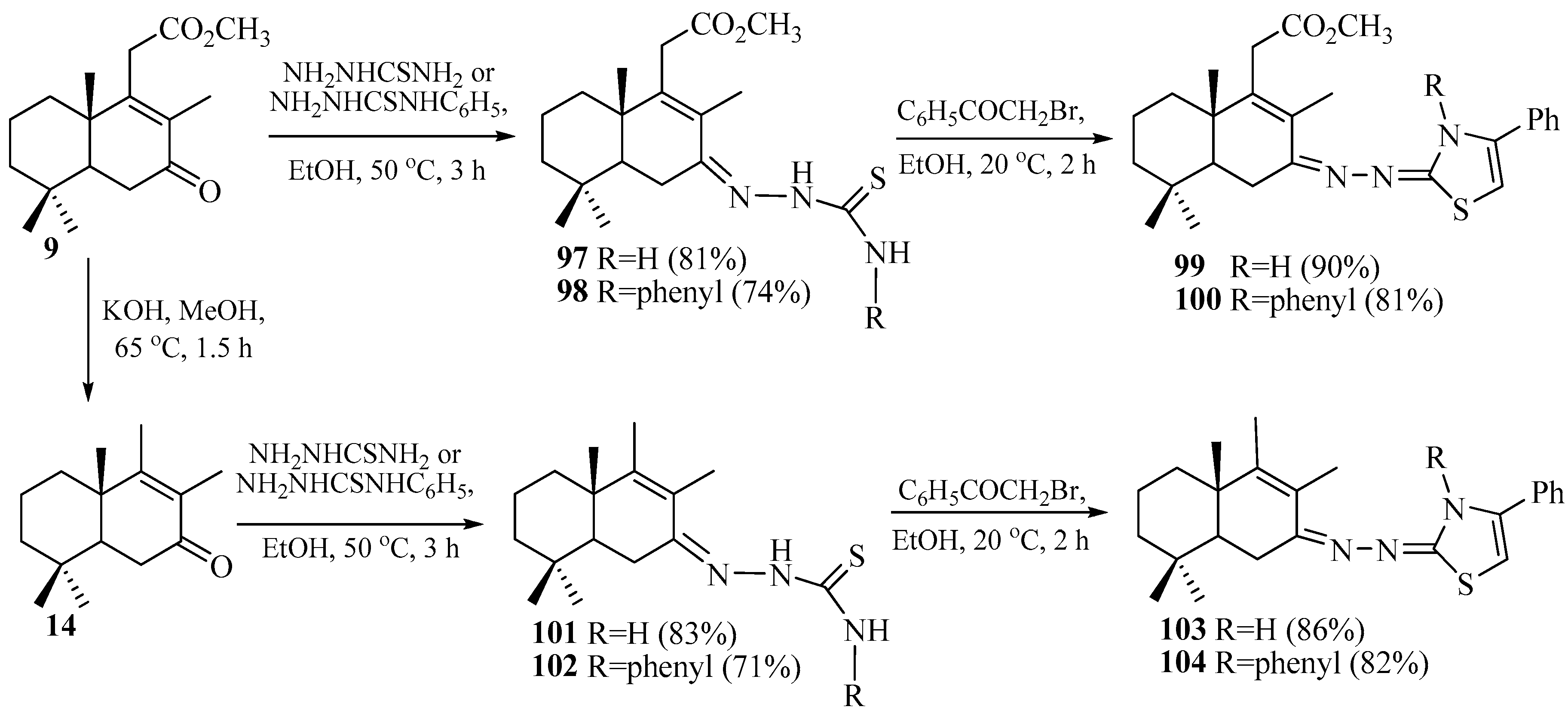
2.6. Synthesis of Norlabdane-1,3-benzothiazole Hybrids

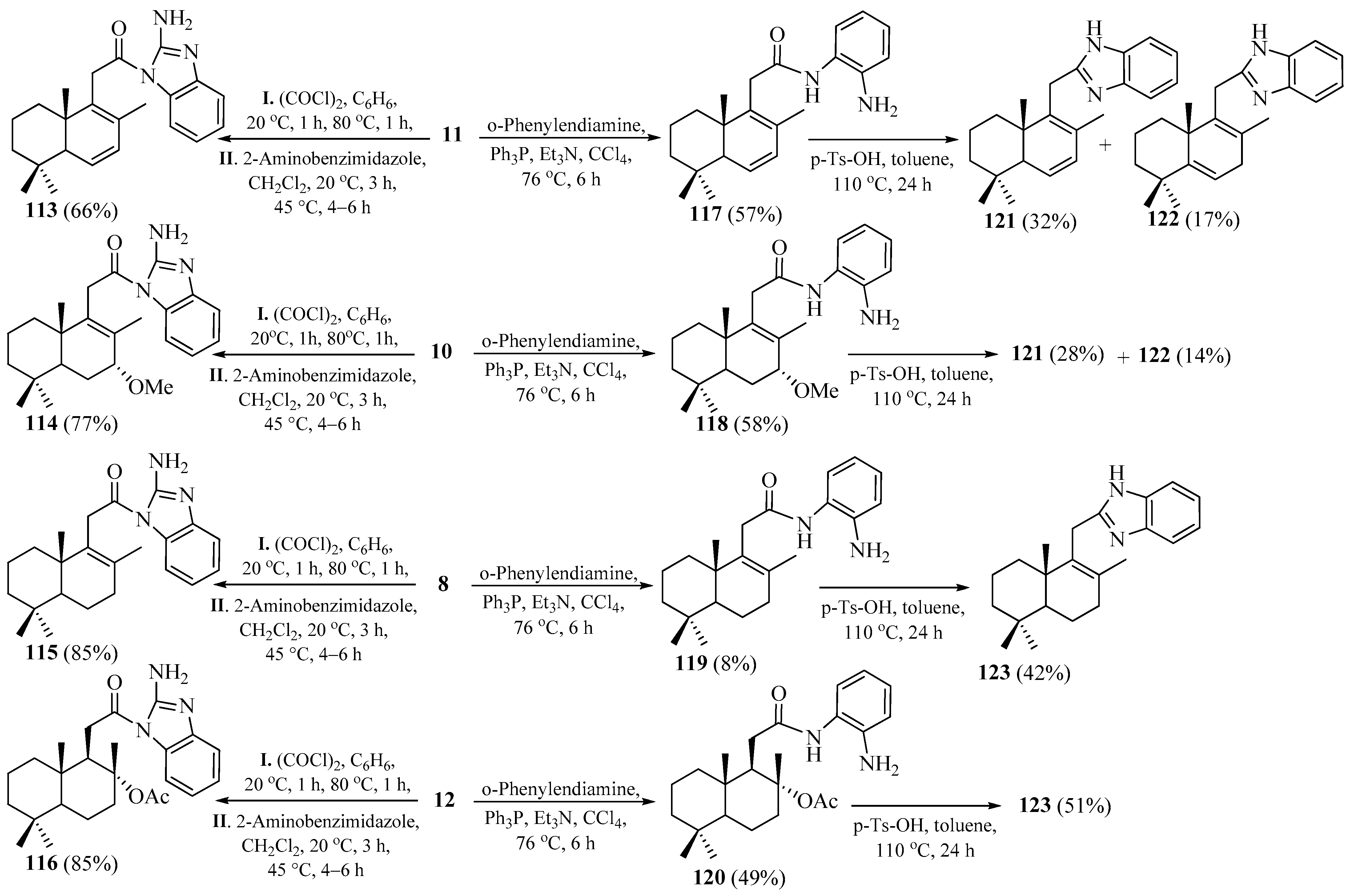
2.7. Synthesis of Norlabdane-1,3-benzimidazole Hybrids
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDI | 1,1′-Carbonyl diimidazole |
| (COCl)2 | Oxalyl chloride |
| CH2Cl2 | Dichloromethane |
| CCl4 | Tetrachloromethane |
| CNBr | Cyanogen bromide |
| C6H5COCH2Br | 2-Bromoacetophenone |
| C6H6 | Benzene |
| 13C NMR | Carbon-13 nuclear magnetic resonance |
| 1H NMR | Proton-1 nuclear magnetic resonance |
| 15N NMR | Nitrogen-15 nuclear magnetic resonance |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAA | N,N-Dimethylacetamide |
| DMAP | Dimethylaminopyridine |
| DMF | Dimethylformamide |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| Et3N | Triethyl amine |
| EtOAc | Ethyl acetate |
| EtOH | Ethyl alcohol |
| Et2O | Diethyl ether |
| HeLa | Human cervical cells |
| HepG2 | Human liver cancer cells |
| HCT116 | Human colon cancer cells |
| HRMS(EI) | High-resolution mass spectrometry |
| K2CO3 | Potassium carbonate |
| IR | Infrared spectroscopy |
| MeOH | Methanol |
| Me2CO | Acetone |
| MW | Microwave irradiation |
| MeSO3SiMe3 | Trimethylsilyl methanesulfonate |
| MeCN | Acetonitrile |
| MIC | Minimum inhibitory concentration |
| KOH | Potassium hydroxide |
| NaHCO3 | Sodium bicarbonate |
| NH2-NH2∙H2O | Hydrazine monohydrate |
| NH2NHCSNH2 | Thiosemicarbazide |
| NH2NHCSNHC6H5 | 4-Phenylthiosemicarbazide |
| NMR | Nuclear magnetic resonance |
| PPh3 | Triphenylphosphine |
| SC(NH2)2 | Thiourea |
| R-NCS | Substituted aryl isothiocyanates |
| THF | Tetrahydrofuran |
| TMTD | Tetramethylthiuram disulfide |
References
- Grahan, P.L. An Introduction to Medicinal Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2001; p. 646. [Google Scholar]
- Greenwood, D.; Finch, R.; Davey, P.; Wilcox, M. Antimicrobial Chemotherapy, 5th ed.; Oxford University Press: Oxford, UK, 2007; p. 504. [Google Scholar]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.M.; De Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Allouche, N.; Apel, C.; Martin, M.-T.; Dumontet, V.; Guéritte, F.; Litaudon, M. Cytotoxic sesquiterpenoids from Winteraceae of Caledonian rainforest. Phytochemistry 2009, 70, 546–553. [Google Scholar] [CrossRef]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic, radical scavenging and antimicrobial activities of sesquiterpenoids from the Tahitian liverwort Mastigophora diclados (Brid.) Nees (Mastigophoraceae). J. Nat. Med. 2010, 64, 417–422. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, T.; Cai, S.; Gu, Q.; Li, D. Drimane sesquiterpenoids from the mangrove-derived fungus Aspergillus ustus. Chem. Pharm. Bull. 2011, 59, 762–766. [Google Scholar] [CrossRef]
- Lhinhatrakool, T.; Prabpai, S.; Kongsaeree, P.; Sutthivaiyakit, S. Antiplasmodial sesquiterpene alkaloids from the roots of Maytenus mekongensis. J. Nat. Prod. 2011, 74, 1386–1391. [Google Scholar] [CrossRef]
- Zhao, G.; Li, X.; Chen, W.; Xi, Z.; Sun, L. Three new sesquiterpenes from Tithonia diversifolia and their anti-hyperglycemic activity. Fitoterapia 2012, 83, 1590–1597. [Google Scholar] [CrossRef]
- Lelyukh, M.I.; Komarenska, Z.M.; Chaban, T.I.; Chaban, I.H. An overview of the synthetic routes toward [1,2,4]triazolo[3,4b][1,3,4]thiadiazoles. Chem. Heterocycl. Compd. 2024, 60, 342–344. [Google Scholar] [CrossRef]
- Patel, Z.M.; Shahi, P.J.; Chavda, S.S.; Patel, U.P.; Dabhi, R.C.; Shrivastav, P.S.; Maru, J.J. Synthetic pathway to 5-fluoroindolin-2-one: Key intermediate for sunitinib. Chem. Heterocycl. Compd. 2024, 60, 422–429. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; Da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Schmiderer, C.; Grassi, P.; Novak, J.; Weber, M.; Franz, C. Diversity of essential oil glands of clary sage (Salvia sclarea L., Lamiaceae). Plant Biol. 2008, 10, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.-L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol. 2012, 12, 119. [Google Scholar] [CrossRef]
- Kaneko, H. The Aroma of cigar tobacco. Agric. Biol. Chem. 1971, 35, 1461–1462. [Google Scholar] [CrossRef]
- Guo, Z.; Wagner, G.J. Biosynthesis of labdenediol and sclareol in cell-free extracts from trichomes of Nicotiana glutinosa. Planta 1995, 197, 627–632. [Google Scholar] [CrossRef]
- Bakir, D.; Akdeniz, M.; Ertas, A.; Yilmaz, M.A.; Yener, I.; Firat, M.; Kolak, U. A GC-MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & C.A. Mey. of Turkey: Enzyme inhibitory potential of ferruginol. J. Food Biochem. 2020, 44, e13350. [Google Scholar] [CrossRef]
- Ruzicka, L.; Seidel, C.F.; Engel, L.L. Zur Kenntnis der Diterpene. (53. Mitteilung). Oxydation des Sclareols mit Kaliumpermanganat. Helv. Chim. Acta 1942, 25, 621. [Google Scholar] [CrossRef]
- Ohloff, G. Fragrance chemistry. In The Science of the Sense of Smell; Theimer, E.T., Ed.; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Amariucai-Mantu, D.; Mangalagiu, V.; Bejan, I.; Aricu, A.; Mangalagiu, I.I. Hybrid azine derivatives: A useful approach for antimicrobial therapy. Pharmaceutics 2022, 14, 2026. [Google Scholar] [CrossRef]
- Kuchkova, K.; Aricu, A.; Barba, A.; Vlad, P.; Shova, S.; Secara, E.; Ungur, N.; Zbancioc, G.; Mangalagiu, I.I. An efficient and straightforward method to new organic compounds: Homodrimane sesquiterpenoids with diazine units. Synlett 2013, 24, 697–700. [Google Scholar] [CrossRef]
- Kuckova, K.; Aricu, A.; Secara, E.; Barba, A.; Vlad, P.; Ungur, N.; Mangalagiu, I.I. Design, synthesis, and antimicrobial activity of some novel homodrimane sesquiterpenoids with diazine skeleton. Med. Chem. Res. 2014, 23, 1559–1568. [Google Scholar] [CrossRef]
- Duca, G.; Aricu, A.; Lungu, L.; Tenu, N.; Ciocarlan, A.; Gutu, Y.; Dragalin, I.; Barba, A. Synthesis of new homodrimane sesquiterpenoids containing diazine, 1,2,4-triazole and carbazole rings. Chem. J. Mold. 2018, 13, 69–73. [Google Scholar] [CrossRef]
- Aricu, A.N.; Kuchkova, K.I.; Sekara, E.S.; Barba, A.N.; Dragalin, I.P.; Ungur, N.D.; Melnic, E.; Kravtsov, V.K. Synthesis and structure of drimane sesquiterpenoids containing diazine, 1,2,4-triazole and carbazole rings. Chem. Nat. Compd. 2018, 54, 455–460. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Aricu, A.; Lungu, L.; Edu, C.; Barba, A.; Shova, S.; Mangalagiu, I.I.; D’Ambrosio, M.; Nicolescu, A.; Deleanu, C.; et al. Synthesis of novel tetranorlabdane derivatives with unprecedented carbon skeleton. Synlett 2017, 28, 565–571. [Google Scholar] [CrossRef]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I.; D’Ambrosio, M.; Vornicu, N. Synthesis, antibacterial, and antifungal activities of new drimane sesquiterpenoids with azaheterocyclic units. Med. Chem. Res. 2016, 25, 2316–2323. [Google Scholar] [CrossRef]
- Arîcu, A.; Mangalagiu, I.I.; Ciocârlan, A.; Lungu, L.; Zbâncioc, G.; Vornicu, N. 11,12-Bis-p-tolyl-Pyridazonyl-drim-5(6),8(9)-dien-7-one Exhibiting Antifungal and Antibacterial Properties. MD Patent 4370, 30 September 2015. [Google Scholar]
- Kharb, R.; Sharma, P.S.; Yar, M.S.J. Pharmacological significance of triazole scaffold. J. Enzym. Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K.J. Synthesis, antifungal and antibacterial activity of novel 1,2,4-triazole derivatives. J. Adv. Pharm. Technol. Res. 2015, 6, 141–146. [Google Scholar] [CrossRef]
- Shen, Q.-K.; Liu, C.-F.; Zhang, H.-J.; Tian, Y.-S.; Quan, Z.-S. Design and synthesis of new triazoles linked to xanthotoxin for potent and highly selective anti-gastric cancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 4871–4875. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ortiz, A.V.; Bratoeff, E.; Ramírez-Apan, T.; Heuze, Y.; Sánchez, A.; Soriano, J.; Cabeza, M. Synthesis and activity of novel 16-dehydropregnenolone acetate derivatives as inhibitors of type 1 5α-reductase and on cancer cell line SK-LU-1. Bioorg. Med. Chem. 2015, 23, 7535–7542. [Google Scholar] [CrossRef] [PubMed]
- Kuchkova, K.; Arycu, A.; Sekara, E.; Barba, A.; Vlad, P.; Makaev, F.; Mel’nik, E.; Kravtsov, V. Synthesis and structure of homodrimane sesquiterpenoids containing 1,2,4-triazole and carbazole rings. Chem. Nat. Compd. 2015, 51, 684–688. [Google Scholar] [CrossRef]
- Cucicova, C.; Rudic, V.; Aricu, A.; Cepoi, L.; Rudi, L.; Secara, E.; Valuta, A.; Barba, A.; Miscu, V.; Vlad, P.; et al. N-(Δ8,13-Bicyclohomofarnesenoyl)-3-Amino-1,2,4-Triazole Compound and Process for the Cultivation of Nostoc linckia Cyanobacterium with Its Use. MD Patent 4327, 28 February 2015. [Google Scholar]
- Lungu, L.; Ciocarlan, A.; Barba, A.; Shova, S.; Pogrebnoi, S.; Mangalagiu, I.; Moldoveanu, C.; Vornicu, N.; Ambrosio, M.; Babak, M.V.; et al. Synthesis and evaluation of biological activity of homodrimane sesquiterpenoids bearing hydrazinecarbothioamide or 1,2,4-triazole unit. Chem. Heterocycl. Compd. 2019, 55, 716–724. [Google Scholar] [CrossRef]
- Ahsan, M.J. 1,3,4-Oxadiazole containing compounds as therapeutic targets for cancer therapy. Mini Rev. Med. Chem. 2022, 22, 164–197. [Google Scholar] [CrossRef]
- Patel, N.; Patel, J. Synthesis and antimicrobial activity of 3-(1,3,4-oxadiazol-2-yl)quinazolin-4(3H)-ones. Sci. Pharm. 2010, 78, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, D.-L.; Imafuku, K. Microwave-assisted synthesis and antifungal activity of 2,5-disubstituted-1,3,4-oxadiazoles containing azulene moiety. Synth. Commun. 2009, 39, 2196–2204. [Google Scholar] [CrossRef]
- Kashaw, S.; Gupta, V.; Kashaw, V.; Mishra, P.; Stables, J.; Jain, N. Anticonvulsant and sedative-hypnotic activity of some novel 3-[5-(4-substituted)phenyl-1,3,4-oxadiazole-2-yl]-2-styrylquinazoline-4(3H)-ones. Med. Chem. Res. 2010, 19, 250–261. [Google Scholar] [CrossRef]
- Bondock, S.; Adel, S.; Etman, H.; Badria, F. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012, 48, 192–199. [Google Scholar] [CrossRef]
- Soares De Oliveira, C.; Lira, B.; Barbosa-Filho, J.; Lorenzo, J.; Filgueiras De Athayde-Filho, P. Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: A review of the literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Smigon, C.; Ozer, I.; Shova, S.; Gutu, I.; Vornicu, N.; Mangalagiu, I.; D’Ambrosio, M.; Aricu, A. Synthesis and evaluation of biological activity of homodrimane sesquiterpenoids bearing 1,3,4-oxadiazole or 1,3,4-thiadiazole units Chem. Heterocycl. Compd. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Omar, F.; Mahfouz, N.; Rahman, M. Design, synthesis and anti-inflammatory activity of some 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem. 1996, 31, 819–825. [Google Scholar] [CrossRef]
- Boschelli, D.; Connor, D.; Bornemeier, D.; Dyer, R.; Kennedy, J.; Kuipers, P.; Okonkwo, G.; Schrier, D.; Wright, C. 1,3,4-Oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazole analogs of the fenamates: In vitro inhibition of cyclooxygenase and 5-lipoxygenase activities. J. Med. Chem. 1993, 36, 802–1810. [Google Scholar] [CrossRef]
- Rusu, G.; Gutu, E.; Barba, N. Reaction of aminobenzoic acid hydrazides with tetramethylthiuram disulfide. Russ. J. Org. Chem. 1995, 31, 1721. [Google Scholar]
- Macaev, F.; Rusu, G.; Pogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure–antimycobacterial activities. Bioorg. Med. Chem. 2005, 13, 4842–4850. [Google Scholar] [CrossRef]
- Aricu, A.N.; Kuchkova, K.I.; Secara-Cushnir, E.S.; Barba, A.N.; Ungur, N.D. Synthesis and antimicrobial activity of new drimane and homodrimane sesquiterpenoids with oxadiazole and thiadiazole fragments. Chem. Nat. Compd. 2020, 56, 656–662. [Google Scholar] [CrossRef]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its derivatives: A review on recent progress in biological activities. Chem. Biol. Drug. Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Blaja, S.; Lungu, L.; Ciocarlan, A.; Vornicu, N.; Aricu, A. Synthesis and evaluation of antimicrobial activity of tetranorlabdane compounds bearing 1,3,4-thiadiazole units. Chem. J. Mold. 2023, 18, 86–91. [Google Scholar] [CrossRef]
- Antimicrobial Susceptibility Testing (AST). Standards M07 and M100, National Committee on Clinical Laboratory Standards: Wayne. 2003. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 19 March 2024).
- Aricu, A.; Lungu, L.; Ciocarlan, A.; Vornicu, N. Compound(1R,2R,8aS)-1-((5-Mercapto-1,3,4-Thiadiazol-2-yl)Methyl)-2,5,5,8α-Tetramethyldecahydronaphthalen-2-ol with Antifungal and Antibacterial Properties. MD Patent 4580, 31 July 2018. [Google Scholar]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Blaja, S.; Vornicu, N. 5-(Homodrim-6,8-Dien-11-yl)-1,3,4-Thiadiazol-2(3H)-Imine with Antifungal Properties. MD Patent 4765, 31 August 2021. [Google Scholar]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amides, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis, characterization and antimicrobial activities of some thiazole derivatives. Am. J. Org. Chem. 2012, 2, 69–73. [Google Scholar] [CrossRef]
- El-Subbagh, H.I.; Abadi, A.H.; Lehmann, J. Synthesis and antitumor activity of ethyl 2-substituted-aminothiazole-4-carboxylate analogs. Arch. Pharm. 1999, 332, 137–142. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Chawla, A.; Baghel, U.S.; Dhawan, R.K. A review: Synthesis schemes of antimicrobial and anticancer thiazole derivatives. JGTPS 2014, 5, 1684–1691. [Google Scholar]
- Rajasekaran, A.; Murugesan, S. Synthesis and antimicrobial evaluation of thiosemicarbazones (I). ChemInform 2003, 34, 544–545. [Google Scholar] [CrossRef]
- Beraldo, H.; Gambino, D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [CrossRef]
- Matesanz, A.; Leitao, I.; Souza, P. Palladium(II) and platinum(II) bis(thiosemicarbazone) complexes of the 2,6-diacetylpyridine series with high cytotoxic activity in Cisplatin resistant A2780cisR tumor cells and reduced toxicity. J. Inorg. Biochem. 2013, 125, 26–31. [Google Scholar] [CrossRef]
- Vandresen, F.; Falzirolli, H.; Almeida Batista, S.; Da Silva-Giardini, A.; De Oliveira, D.; Catharino, R.; Ruiz, A.; De Carvalho, J.; Foglio, M.; Da Silva, C. Novel R-(+)-limonene-based thiosemicarbazones and their antitumor activity against human tumor cell lines. Eur. J. Med. Chem. 2014, 79, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kirin, V.; Maksakov, V.; Virovets, A.; Popov, S.; Tkachev, A. Optically active triosmium cluster with bridging terpenic thioureato-like ligand—Synthesis, structure, and absolute configuration. Inorg. Chem. Commun. 2002, 5, 32–34. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Zhao, Z.; Xu, S.; Bi, W. Synthesis and cytotoxic activity of amides from isopimaric acid. Chem. Nat. Compd. 2018, 54, 695–700. [Google Scholar] [CrossRef]
- Blaja, S.; Lungu, L.; Kuchkova, K.; Ciocarlan, A.; Barba, A.; Vornicu, N.; Aricu, A. Norlabdane compounds containing thiosemicarbazone or 1,3-thiazole fragments: Synthesis and antimicrobial activity. Chem. Nat. Compd. 2021, 57, 101–110. [Google Scholar] [CrossRef]
- Kuchkova, K.; Chiumakov, Y.; Simonov, Y.; Bocelli, G.; Panasenko, A.; Vlad, P. A short efficient synthesis of 11-monoacetate of drimane-8α-11-diol from norambreinolide. Synthesis 1997, 1997, 1045–1048. [Google Scholar] [CrossRef]
- Cucicova, C.; Aricu, A.; Secara, E.; Vlad, P.; Ungur, N. Process for Producing 14,15-Bisnorlabdane-8(9)-En-13-One. MD Patent 4248, 31 August 2013. [Google Scholar]
- Blaja, S.P. Synthesis of new di- and tri-norlabdane compounds with 2-amino-1,3-thiazole units. Chem. J. Mold. 2019, 14, 72–78. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lungu, L.; Blaja, S.; Dragalin, I.; Aricu, A. The use of some non-conventional methods in chemistry of bicyclohomofarnesenic methyl esters. Chem. J. Mold. 2020, 15, 69–77. [Google Scholar] [CrossRef]
- Mohareb, R.; Wardakhan, W.; Elmegeed, G.; Ashour, R. Heterocyclizations of pregnenolone: Novel synthesis of thiosemicarbazone, thiophene, thiazole, thieno[2,3-b]pyridine derivatives and their cytotoxicity evaluations. Steroids 2012, 77, 1560–1569. [Google Scholar] [CrossRef]
- Arîcu, A.; Ciocârlan, A.; Lungu, L.; Blaja, S.; Vornicu, N. (Z/E)-2-(1-((1R,2R,8aS)-2-Hydroxy-2,5,5,8a-Tetramethyldecahydronaphthalen-1-yl)Propan-2-Ylidene)Hydrazinecarbothioamide for Use as an Antifungal Agent. MD Patent 4769, 31 October 2021. [Google Scholar]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Kumar, S.A.; Mishra, A.K. Advancement in pharmacological activities of benzothiazole and its derivatives: An up to date review. Mini Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar] [CrossRef]
- Banerjee, S.; Payra, S.; Saha, A. A review on synthesis of benzothiazole derivatives. Curr. Organocatal. 2018, 4, 164–181. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Z.-B. A Review on domino condensation/cyclization reactions for the synthesis of 2-substituted 1,3-benzothiazole derivatives. Eur. J. Org. Chem. 2020, 2020, 408–419. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Zuo, X.; Feng, X.; Gao, Y. Recent advances in synthesis of benzothiazole compounds related to green chemistry. Molecules 2020, 25, 1675. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Zahra Naqvi, S.A.; Islam, A.; Osman, S.M.; Nocentini, A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2019, 35, 265–279. [Google Scholar] [CrossRef]
- Kumari, B.; Chauhan, K.; Trivedi, J.; Jaiswal, V.; Kanwar, S.S.; Pokharel, Y.R. Benzothiazole-based-bioconjugates with improved antimicrobial, anticancer and antioxidant potential. ChemistrySelect 2018, 3, 11326–11332. [Google Scholar] [CrossRef]
- Azzam, R.A.; Osman, R.R.; Elgemeie, G.H. Efficient synthesis and docking studies of novel benzothiazole-based pyrimidinesulfonamide scaffolds as new antiviral agents and Hsp90α inhibitors. ACS Omega 2020, 5, 1640–1655. [Google Scholar] [CrossRef]
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzym. Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; Omar, H.A. Design, synthesis and biological evaluation of some novel benzothiazole/benzoxazole and/or benzimidazole derivatives incorporating a pyrazole scaffold as antiproliferative agents. Bioorg. Chem. 2017, 74, 82–90. [Google Scholar] [CrossRef]
- Baba, H.; Yaoita, Y.; Kikuchi, M. Sesquiterpenoids and lactone derivatives from Ligularia dentata. Helv. Chim. Acta 2007, 90, 1028–1037. [Google Scholar] [CrossRef]
- Sandargo, B.; Thongbai, B.; Stadler, M.; Surup, F. Cysteine-Derived pleurotin congeners from the nematode-trapping basidiomycete Hohenbuehelia grisea. J. Nat. Prod. 2018, 81, 286–291. [Google Scholar] [CrossRef]
- Hu, Y.; MacMillan, J.B. Erythrazoles A–B, cytotoxic benzothiazoles from a marine-derived Erythrobacter sp. Org. Lett. 2011, 13, 6580–6583. [Google Scholar] [CrossRef]
- Coelho, F.L.; Campo, L.F. Synthesis of 2-arylbenzothiazoles via direct condensation between in situ generated 2-aminothiophenol from disulfide cleavage and carboxylic acids. Tetrahedron Lett. 2017, 58, 2330–2333. [Google Scholar] [CrossRef]
- Jesberger, M.; Davis, T.P.; Barner, L. Applications of Lawesson’s reagent in organic and organometallic syntheses. Synthesis 2003, 13, 1929–1958. [Google Scholar] [CrossRef]
- Lungu, L.; Cucicova, C.; Blaja, S.; Ciocarlan, A.; Dragalin, I.; Vornicu, N.; Geana, E.; Mangalagiu, I.I.; Aricu, A. Synthesis of homodrimane sesquiterpenoids bearing 1,3-benzothiazole unit and their antimicrobial activity evaluation. Molecules 2022, 27, 5082. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Wang, Z.; Wan, W.; Lua, W.; Hao, J. One-pot synthesis of 2-trifluoromethyl and 2-difluoromethyl substituted benzo-1,3-diazoles. Tetrahedron Lett. 2007, 48, 3251–3254. [Google Scholar] [CrossRef]
- Pathare, B.; Bansode, T. Review—Biological active benzimidazole derivatives. Results Chem. 2021, 3, 100200. [Google Scholar] [CrossRef]
- Ebenezer, O.; Oyetunde-Joshua, F.; Omotoso, O.D.; Micheal Shapi, M. Benzimidazole and its derivatives: Recent advances (2020–2022). Results Chem. 2023, 5, 100925. [Google Scholar] [CrossRef]
- Soliman, M.A.; Eltamany, E.H.; Boraei, A.T.A.; Aouad, M.R.; Aljuhani, A.; Almohaywi, B.; Awaji, A.A.; Alghamdi, R.; Aljohani, A.K.B.; Ahmed, H.E.A. Novel benzimidazole-1,2,3-triazole hybrids: Synthesis, dual antimicrobial, anticancer activity, mechanistic insights, and computational studies. Chem. Sel. 2025, 10, e202500813. [Google Scholar] [CrossRef]
- Ouahrouch, A.; Ighachane, H.; Taourirte, M.; Engels, J.W.; Sedra, M.H.; Lazrek, H.B. Benzimidazole-1,2,3-triazole hybrid molecules: Synthesis and evaluation for antibacterial/antifungal activity. Arch. Pharm. 2014, 347, 748–755. [Google Scholar] [CrossRef]
- Lungu, L.; Cucicova, C.; Blaja, S.; Ciocarlan, A.; Dragalin, I.; Barba, A.; Kulcițki, V.; Vornicu, V.; Geana, E.; Mangalagiu, I.I.; et al. Synthesis and antimicrobial activity evaluation of homodrimane sesquiterpenoids with a benzimidazole unit. Molecules 2023, 28, 933. [Google Scholar] [CrossRef]
- Mariappan, G.; Hazarika, R.; Alam, F.; Karki, R.; Patangia, U.; Nath, S. Synthesis and biological evaluation of 2-substituted benzimidazole derivatives. Arab. J. Chem. 2015, 8, 715–719. [Google Scholar] [CrossRef]
- Leonard, J.T.; Jeyaseeli, L.; Shivakumar, R.; Gunasekaran, V. Synthesis, anti-imflamatory and antibacterial activities of 2-hydroxyphenyl benzimidazoles. Asian J. Chem. 2005, 17, 2674–2678. [Google Scholar]
- Sharma, M.C.; Kohli, D.V.; Sharma, S.; Sharma, A.D. Synthesis and antihypertensive activity of some new benzimidazole derivatives of 4′-(6-methoxy-2-substituted-benzimidazole-1-ylmethyl)-biphenyl-2-carboxylic acid in the presence of BF3-Et2O. Pharm. Sin. 2010, 1, 104–115. [Google Scholar]
- Charton, J.; Girault-Mizzi, S.; Sergheraert, C. Conversion of sterically hindered diacylated 1,2-phenylenediamines into 2-substituted benzimidazoles. Chem. Pharm. Bull. 2005, 53, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lungu, L.; Ciocarlan, A.; Cucicova, C.; Blaja, S.; Aricu, A.; Vornicu, N. N-(2-Aminophenyl)-8α-Acetoxy-Homotriacetamide with Antifungal and Antibacterial Properties. MD Patent 4891, 30 April 2024. [Google Scholar]
- Lungu, L.; Ciocarlan, A.; Cucicova, C.; Blaja, S.; Aricu, A.; Vornicu, N. 2-Amino-1-(Δ8,9-Bicyclo Homofarnesenoyl)-Benzimidazole with Antifungal and Antibacterial Properties. MD Patent 4878, 31 September 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, L.; Ciocarlan, A.; Mangalagiu, I.I.; Aricu, A. Promising Norlabdane-Heterocyclic Hybrids: Synthesis, Structural Characterization and Antimicrobial Activity Evaluation. Pharmaceuticals 2025, 18, 1411. https://doi.org/10.3390/ph18091411
Lungu L, Ciocarlan A, Mangalagiu II, Aricu A. Promising Norlabdane-Heterocyclic Hybrids: Synthesis, Structural Characterization and Antimicrobial Activity Evaluation. Pharmaceuticals. 2025; 18(9):1411. https://doi.org/10.3390/ph18091411
Chicago/Turabian StyleLungu, Lidia, Alexandru Ciocarlan, Ionel I. Mangalagiu, and Aculina Aricu. 2025. "Promising Norlabdane-Heterocyclic Hybrids: Synthesis, Structural Characterization and Antimicrobial Activity Evaluation" Pharmaceuticals 18, no. 9: 1411. https://doi.org/10.3390/ph18091411
APA StyleLungu, L., Ciocarlan, A., Mangalagiu, I. I., & Aricu, A. (2025). Promising Norlabdane-Heterocyclic Hybrids: Synthesis, Structural Characterization and Antimicrobial Activity Evaluation. Pharmaceuticals, 18(9), 1411. https://doi.org/10.3390/ph18091411