Nanotechnology for the Efficacious Delivery of Medicinal Cannabis and Pharmaceutical Medicines
Abstract
1. Introduction
2. The Gastrointestinal Tract (GIT), Cannabinoids, and Pharmaceutical Drugs
2.1. The Intestinal Microbiome/Microbiota
2.2. GIT Dysbiosis
3. Medicinal Cannabis
3.1. Medicinal Cannabis for Nausea
3.2. Medicinal Cannabis for Pain
3.3. Medicinal Cannabis for Seizures
3.4. Medicinal Cannabis for Appetite
3.5. Medicinal Cannabis for Muscle Spasticity
4. Nanotechnology for Effective Cannabinoid and Pharmaceutical Drug Delivery
4.1. Micelles
4.2. Liposomes
4.3. Dendrimers
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Bashiardes, S.; Christodoulou, C. Orally Administered Drugs and Their Complicated Relationship with Our Gastrointestinal Tract. Microorganisms 2024, 12, 242. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of Gastrointestinal Tract Variability on Oral Drug Absorption and Pharmacokinetics: An Ungap Review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An Integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Sharma, V.K.; Agrawal, M.K. A Historical Perspective of Liposomes-a Bio Nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Syama, K.; Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Development of Lipid Nanoparticles and Liposomes Reference Materials (II): Cytotoxic Profiles. Sci. Rep. 2022, 12, 18071. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to Mrna Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. Bionanoscience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Parsons, R.L. Drug Absorption in Gastrointestinal Disease with Particular Reference to Malabsorption Syndromes. Clin. Pharmacokinet. 1977, 2, 45–60. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Akhtar, S.; Zuhair, F. Advancing Nanomedicine through Electron Microscopy: Insights into Nanoparticle Cellular Interactions and Biomedical Applications. Int. J. Nanomed. 2025, 20, 2847–2878. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Rodger, M.A.; King, L. Drawing up and Administering Intramuscular Injections: A Review of the Literature. J. Adv. Nurs. 2000, 31, 574–582. [Google Scholar] [CrossRef]
- Azzouz, L.L.; Sharma, S. Physiology, Large Intestine; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Geng, H.; Ye, C.; Liu, J. Dysbiotic Alteration in the Fecal Microbiota of Patients with Polycystic Ovary Syndrome. Microbiol. Spectr. 2024, 12, e0429123. [Google Scholar] [CrossRef]
- Stecher, B. The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection. Microbiol. Spectr. 2015, 3, 297–320. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Thu, M.S.; Campbell, B.J.; Hirankarn, N.; Nopsopon, T.; Ondee, T.; Hall, S.R.; Jagota, A.; Fothergill, J.L.; Pongpirul, K. Cannabis and Cannabinoid-Microbiome Interactions in Varied Clinical Contexts: A Comprehensive Systematic Review. Biomed. Pharmacother. 2025, 182, 117764. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.K.; Zhou, J.; Chatterjee, S.; Nagarkatti, P.; Nagarkatti, M. Protective Effects of Δ(9) -Tetrahydrocannabinol against Enterotoxin-Induced Acute Respiratory Distress Syndrome Are Mediated by Modulation of Microbiota. Br. J. Pharmacol. 2020, 177, 5078–5095. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of Cannabinoids, Delta-9-Tetrahydrocannabinol (Thc) and Cannabidiol (Cbd), Mitigates Experimental Autoimmune Encephalomyelitis (Eae) by Altering the Gut Microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef]
- Al-Khazaleh, A.K.; Jaye, K.; Chang, D.; Münch, G.W.; Bhuyan, D.J. Buds and Bugs: A Fascinating Tale of Gut Microbiota and Cannabis in the Fight against Cancer. Int. J. Mol. Sci. 2024, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Nation, T.; Oldfield, D.; Thomsen, M. Medicinal Cannabis and the Intestinal Microbiome. Pharmaceuticals 2024, 17, 1702. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Izzo, A.A.; Sharkey, K.A. Cannabinoids and the Gut: New Developments and Emerging Concepts. Pharmacol. Ther. 2010, 126, 21–38. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How Gut Microbes Talk to Organs: The Role of Endocrine and Nervous Routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Important Are Fatty Acids in Human Health and Can They Be Used in Treating Diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Enright, E.F.; Gahan, C.G.; Joyce, S.A.; Griffin, B.T. The Impact of the Gut Microbiota on Drug Metabolism and Clinical Outcome. Yale J. Biol. Med. 2016, 89, 375–382. [Google Scholar] [PubMed]
- Li, H.; He, J.; Jia, W. The Influence of Gut Microbiota on Drug Metabolism and Toxicity. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L.; Henson, J.D.; Hall, S. Intestinal Dysbiosis, the Tryptophan Pathway and Nonalcoholic Steatohepatitis. Int. J. Tryptophan Res. 2022, 15, 11786469211070533. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Modulation of Immunity by Tryptophan Microbial Metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Jie, S.; Linden, D.R.; Ghatak, S.; Mars, R.A.; Williams, B.B.; Pu, M.; Sonnenburg, J.L.; Fischbach, M.A.; Farrugia, G.; et al. Bacterially Derived Tryptamine Increases Mucus Release by Activating a Host Receptor in a Mouse Model of Inflammatory Bowel Disease. iScience 2020, 23, 101798. [Google Scholar] [CrossRef]
- Bortolotti, P.; Hennart, B.; Thieffry, C.; Jausions, G.; Faure, E.; Grandjean, T.; Thepaut, M.; Dessein, R.; Allorge, D.; Guery, B.P.; et al. Tryptophan Catabolism in Pseudomonas aeruginosa and Potential for Inter-Kingdom Relationship. BMC Microbiol. 2016, 16, 137. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Malik, Z.; Baik, D.; Schey, R. The Role of Cannabinoids in Regulation of Nausea and Vomiting, and Visceral Pain. Curr. Gastroenterol. Rep. 2015, 17, 429. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Fu, Y.; Lyu, J.; Wang, S. The Role of Intestinal Microbes on Intestinal Barrier Function and Host Immunity from a Metabolite Perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Association between Gut Dysbiosis and the Occurrence of Sibo, Libo, Sifo and Imo. Microorganisms 2023, 11, 573. [Google Scholar] [CrossRef]
- Castonguay-Paradis, S.; Lacroix, S.; Rochefort, G.; Parent, L.; Perron, J.; Martin, C.; Lamarche, B.; Raymond, F.; Flamand, N.; Di Marzo, V.; et al. Dietary Fatty Acid Intake and Gut Microbiota Determine Circulating Endocannabinoidome Signaling Beyond the Effect of Body Fat. Sci. Rep. 2020, 10, 15975. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing Human Gut Microbiota Research by Considering Gut Transit Time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef]
- Sugihara, M.; Takeuchi, S.; Sugita, M.; Higaki, K.; Kataoka, M.; Yamashita, S. Analysis of Intra- and Intersubject Variability in Oral Drug Absorption in Human Bioequivalence Studies of 113 Generic Products. Mol. Pharm. 2015, 12, 4405–4413. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral Drug Delivery with Polymeric Nanoparticles: The Gastrointestinal Mucus Barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of Cancer Metabolism: The Therapeutic Potential of Cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, D.; Prasanna, V.; Firdous, A.; Thakur, S. A Systemic Review on Chemotherapy Induced Nausea and Vomiting- Risk and Clinical Management with Alternative Therapies. Cancer Treat. Res. Commun. 2025, 44, 100938. [Google Scholar] [CrossRef] [PubMed]
- Braun, I.M.; Bohlke, K.; Abrams, D.I.; Anderson, H.; Balneaves, L.G.; Bar-Sela, G.; Bowles, D.W.; Chai, P.R.; Damani, A.; Gupta, A.; et al. Cannabis and Cannabinoids in Adults with Cancer: Asco Guideline. J. Clin. Oncol. 2024, 42, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.M.; Stéfano, S.; Haiek, R.D.C.; Oliveira, L.R.; Da Silveira, D. Therapeutic Use of Cannabis Sativa on Chemotherapy-Induced Nausea and Vomiting among Cancer Patients: Systematic Review and Meta-Analysis. Eur. J. Cancer Care (Engl.) 2008, 17, 431–443. [Google Scholar]
- Duran, M.; Pérez, E.; Abanades, S.; Vidal, X.; Saura, C.; Majem, M.; Arriola, E.; Rabanal, M.; Pastor, A.; Farré, M.; et al. Preliminary Efficacy and Safety of an Oromucosal Standardized Cannabis Extract in Chemotherapy-Induced Nausea and Vomiting. Br. J. Clin. Pharmacol. 2010, 70, 656–663. [Google Scholar] [CrossRef]
- Venkatesan, T.; Levinthal, D.J.; Li, B.U.K.; Tarbell, S.E.; Adams, K.A.; Issenman, R.M.; Sarosiek, I.; Jaradeh, S.S.; Sharaf, R.N.; Sultan, S.; et al. Role of Chronic Cannabis Use: Cyclic Vomiting Syndrome Vs Cannabinoid Hyperemesis Syndrome. Neurogastroenterol. Motil. 2019, 31 (Suppl. S2), e13606. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Gangopadhyay, S.; Clauw, D.J.; Haffajee, R.L. Qualifying Conditions of Medical Cannabis License Holders in the United States. Health Aff. 2019, 38, 295–302. [Google Scholar] [CrossRef]
- Mucke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Hauser, W. Cannabis-Based Medicines for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2018, 2020, Cd012182. [Google Scholar] [CrossRef]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of Cannabis-Based Medicines for Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [CrossRef]
- Yanes, J.A.; McKinnell, Z.E.; Reid, M.A.; Busler, J.N.; Michel, J.S.; Pangelinan, M.M.; Sutherland, M.T.; Younger, J.W.; Gonzalez, R.; Robinson, J.L. Effects of Cannabinoid Administration for Pain: A Meta-Analysis and Meta-Regression. Exp. Clin. Psychopharmacol. 2019, 27, 370–382. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low-Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. J. Pain 2013, 14, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Wang, T.; Shapiro, S.; Robinson, A.; Ducruet, T.; Huynh, T.; Gamsa, A.; Bennett, G.J.; Collet, J.P. Smoked Cannabis for Chronic Neuropathic Pain: A Randomized Controlled Trial. CMAJ 2010, 182, E694–E701. [Google Scholar] [PubMed]
- Ellis, R.J.; Toperoff, W.; Vaida, F.; van den Brande, G.; Gonzales, J.; Gouaux, B.; Bentley, H.; Atkinson, J.H. Smoked Medicinal Cannabis for Neuropathic Pain in Hiv: A Randomized, Crossover Clinical Trial. Neuropsychopharmacology 2009, 34, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Sohler, N.L.; Starrels, J.L.; Khalid, L.; Bachhuber, M.A.; Arnsten, J.H.; Nahvi, S.; Jost, J.; Cunningham, C.O. Cannabis Use Is Associated with Lower Odds of Prescription Opioid Analgesic Use among Hiv-Infected Individuals with Chronic Pain. Subst. Use Misuse 2018, 53, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in Painful Hiv-Associated Sensory Neuropathy: A Randomized Placebo-Controlled Trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The National Academies Collection: Reports Funded by National Institutes of Health. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press (US): Washington, DC, USA, 2017. [Google Scholar]
- Boland, E.G.; Bennett, M.I.; Allgar, V.; Boland, J.W. Cannabinoids for Adult Cancer-Related Pain: Systematic Review and Meta-Analysis. BMJ Support. Palliat. Care 2020, 10, 14–24. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabis and Pain. Pain Med. 2019, 20, 2083–2085. [Google Scholar] [CrossRef]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood Cannabinoids. I. Absorption of Thc and Formation of 11-Oh-Thc and Thccooh during and after Smoking Marijuana. J. Anal. Toxicol. 1992, 16, 276–282. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (Cbd) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of Cannabinoids in the Treatment of Epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- Arzimanoglou, A.; Brandi, U.; Cross, J.H.; Gil-Nagel, A.; Lagae, L.; Landmark, C.J.; Specchio, N.; Nabbout, R.; Thiele, E.A.; Gubbay, O.; et al. Epilepsy and Cannabidiol: A Guide to Treatment. Epileptic Disord. 2020, 22, 1–14. [Google Scholar] [PubMed]
- Borowicz-Reutt, K.; Czernia, J.; Krawczyk, M. Cbd in the Treatment of Epilepsy. Molecules 2024, 29, 1981. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in Patients with Treatment-Resistant Epilepsy: An Open-Label Interventional Trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.J.; Moody, K.A.; Geffrey, A.L.; Pollack, S.F.; Skirvin, L.A.; Bruno, P.L.; Paolini, J.L.; Thiele, E.A. Cannabidiol as a New Treatment for Drug-Resistant Epilepsy in Tuberous Sclerosis Complex. Epilepsia 2016, 57, 1617–1624. [Google Scholar] [CrossRef]
- Razmovski-Naumovski, V.; Luckett, T.; Amgarth-Duff, I.; Agar, M.R. Efficacy of Medicinal Cannabis for Appetite-Related Symptoms in People with Cancer: A Systematic Review. Palliat. Med. 2022, 36, 912–927. [Google Scholar] [CrossRef]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-Tetrahydrocannabinol May Palliate Altered Chemosensory Perception in Cancer Patients: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Ann. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.-D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of Orally Administered Cannabis Extract and Delta-9-Tetrahydrocannabinol in Treating Patients with Cancer-Related Anorexia-Cachexia Syndrome: A Multicenter, Phase Iii, Randomized, Double-Blind, Placebo-Controlled Clinical Trial from the Cannabis-in-Cachexia-Study-Group. J. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar]
- Côté, M.; Trudel, M.; Wang, C.; Fortin, A. Improving Quality of Life with Nabilone during Radiotherapy Treatments for Head and Neck Cancers: A Randomized Double-Blind Placebo-Controlled Trial. Ann. Otol. Rhinol. Laryngol. 2016, 125, 317–324. [Google Scholar] [CrossRef]
- Turcott, J.G.; Del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The Effect of Nabilone on Appetite, Nutritional Status, and Quality of Life in Lung Cancer Patients: A Randomized, Double-Blind Clinical Trial. Support. Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Jatoi, A.; Windschitl, H.E.; Loprinzi, C.L.; Sloan, J.A.; Dakhil, S.R.; Mailliard, J.A.; Pundaleeka, S.; Kardinal, C.G.; Fitch, T.R.; Krook, J.E.; et al. Dronabinol Versus Megestrol Acetate Versus Combination Therapy for Cancer-Associated Anorexia: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2002, 20, 567–573. [Google Scholar] [CrossRef][Green Version]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Pryce, G.; Baker, D. Endocannabinoids in Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Handb. Exp. Pharmacol. 2015, 231, 213–231. [Google Scholar]
- Ball, S.; Vickery, J.; Hobart, J.; Wright, D.; Green, C.; Shearer, J.; Nunn, A.; Cano, M.G.; MacManus, D.; Miller, D.; et al. The Cannabinoid Use in Progressive Inflammatory Brain Disease (Cupid) Trial: A Randomised Double-Blind Placebo-Controlled Parallel-Group Multicentre Trial and Economic Evaluation of Cannabinoids to Slow Progression in Multiple Sclerosis. Health Technol. Assess. 2015, 19, 1–187, 7–8, 25–31. [Google Scholar]
- Iskedjian, M.; Bereza, B.; Gordon, A.; Piwko, C.; Einarson, T.R. Meta-Analysis of Cannabis Based Treatments for Neuropathic and Multiple Sclerosis-Related Pain. Curr. Med. Res. Opin. 2007, 23, 17–24. [Google Scholar]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of Thc/Cbd Oromucosal Spray in Combination with the Existing Treatment Regimen, in the Relief of Central Neuropathic Pain in Patients with Multiple Sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 12, 1–26. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, H. Emerging Applications of Nanotechnology in Drug Delivery and Medical Imaging: Review. Curr. Radiopharm. 2023, 16, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, X.; Liu, S.; Liu, X.; Pei, X.; Sun, K.; Li, M.; Wang, P.; Chang, Y.; Wang, T.; et al. Recent Advances in Self-Targeting Natural Product-Based Nanomedicines. J. Nanobiotechnol. 2025, 23, 31. [Google Scholar] [CrossRef]
- Singh, V.; Vihal, S.; Rana, R.; Rathore, C. Nanocarriers for Cannabinoid Delivery: Enhancing Therapeutic Potential. Recent Adv. Drug Deliv. Formul. 2024, 18, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Bramlett, K.; Onel, E.; Viscusi, E.R.; Jones, K. A Randomized, Double-Blind, Dose-Ranging Study Comparing Wound Infiltration of Depofoam Bupivacaine, an Extended-Release Liposomal Bupivacaine, to Bupivacaine Hcl for Postsurgical Analgesia in Total Knee Arthroplasty. Knee 2012, 19, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, Q.; Lu, M.; Chen, S.-T.; Tseng, H.-W.; Lo, Y.-C.; Ho, C. A New Approach to Deliver Anti-Cancer Nanodrugs with Reduced Off-Target Toxicities and Improved Efficiency by Temporarily Blunting the Reticuloendothelial System with Intralipid. Sci. Rep. 2017, 7, 16106. [Google Scholar] [CrossRef] [PubMed]
- Passero, F.C., Jr.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The Safety and Efficacy of Onivyde (Irinotecan Liposome Injection) for the Treatment of Metastatic Pancreatic Cancer Following Gemcitabine-Based Therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [PubMed]
- Alfayez, M.; Kantarjian, H.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. Cpx-351 (Vyxeos) in Aml. Leuk. Lymphoma 2020, 61, 288–297. [Google Scholar] [CrossRef]
- Ohlmann, C.-H.; Gross-Langenhoff, M. Efficacy and Tolerability of Leuprorelin Acetate (Eligard®) in Daily Practice in Germany: Pooled Data from 2 Prospective, Non-Interventional Studies with 3- or 6-Month Depot Formulations in Patients with Advanced Prostate Cancer. Urol. Int. 2018, 100, 66–71. [Google Scholar]
- Fishburn, C.S. The Pharmacology of Pegylation: Balancing Pd with Pk to Generate Novel Therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef]
- Goel, N.; Stephens, S. Certolizumab Pegol. MAbs 2010, 2, 137–147. [Google Scholar]
- Freeman, T.P.; Hindocha, C.; Green, S.F.; Bloomfield, M.A.P. Medicinal Use of Cannabis Based Products and Cannabinoids. Bmj 2019, 365, l1141. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond Thc and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef]
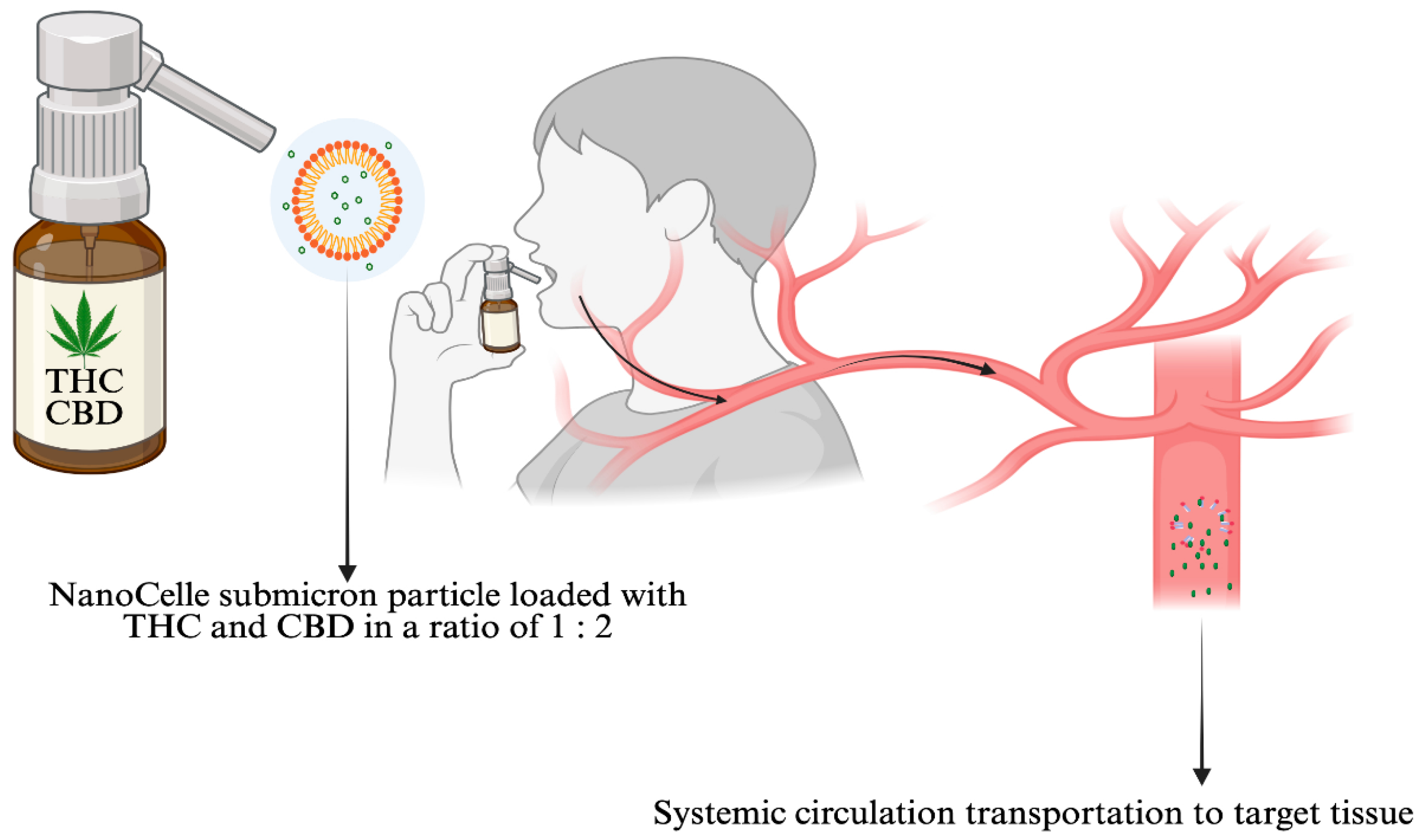
- Reuter, S.E.; Schultz, H.B.; McLachlan, A.J.; Henson, J.D.; Vitetta, L. Pharmacokinetics and Bioavailability of Cannabinoids Administered Via a Novel Orobuccal Nanoparticle Formulation (Nanocelle™) in Patients with Advanced Cancer. Cannabis Cannabinoid Res. 2025. Ahead of print. [Google Scholar] [CrossRef]
- Vitetta, L.; Zhou, J.; Manuel, R.; Dal Forno, S.; Hall, S.; Rutolo, D. Route and Type of Formulation Administered Influences the Absorption and Disposition of Vitamin B(12) Levels in Serum. J. Funct. Biomater. 2018, 9, 12. [Google Scholar]
- Clarke, S.; Butcher, B.E.; McLachlan, A.J.; Henson, J.D.; Rutolo, D.; Hall, S.; Vitetta, L. Pilot Clinical and Pharmacokinetic Study of Δ9-Tetrahydrocannabinol (Thc)/Cannabidiol (Cbd) Nanoparticle Oro-Buccal Spray in Patients with Advanced Cancer Experiencing Uncontrolled Pain. PLoS ONE 2022, 17, e0270543. [Google Scholar]
- Vitetta, L.; Butcher, B.; Henson, J.D.; Rutolo, D.; Hall, S. A Pilot Safety, Tolerability and Pharmacokinetic Study of an Oro-Buccal Administered Cannabidiol-Dominant Anti-Inflammatory Formulation in Healthy Individuals: A Randomized Placebo-Controlled Single-Blinded Study. Inflammopharmacology 2021, 29, 1361–1370. [Google Scholar] [CrossRef]
- Janani, G.; Girigoswami, A.; Deepika, B.; Udayakumar, S.; Girigoswami, K. Unveiling the Role of Nano-Formulated Red Algae Extract in Cancer Management. Molecules 2024, 29, 2077. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Wu, D.; Zhu, H. Nanoparticles as a Novel Platform for Cardiovascular Disease Diagnosis and Therapy. Int. J. Nanomed. 2024, 19, 8831–8846. [Google Scholar] [CrossRef]
- Hari Priya, V.M.; Ganapathy, A.A.; Veeran, M.G.; Raphael, M.S.; Kumaran, A. Nanotechnology-Based Drug Delivery Platforms for Erectile Dysfunction: Addressing Efficacy, Safety, and Bioavailability Concerns. Pharm. Dev. Technol. 2024, 29, 996–1015. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Bedocs, P.; Rozsnyay, Z.; Weiszhár, Z.; Urbanics, R.; Rosivall, L.; Cohen, R.; Garbuzenko, O.; Báthori, G.; Tóth, M.; et al. Liposome-Induced Complement Activation and Related Cardiopulmonary Distress in Pigs: Factors Promoting Reactogenicity of Doxil and Ambisome. Nanomedicine 2012, 8, 176–184. [Google Scholar] [CrossRef]
- Crisafulli, S.; Cutroneo, P.M.; Luxi, N.; Fontana, A.; Ferrajolo, C.; Marchione, P.; Sottosanti, L.; Zanoni, G.; Moretti, U.; Franzè, S.; et al. Is Pegylation of Drugs Associated with Hypersensitivity Reactions? An Analysis of the Italian National Spontaneous Adverse Drug Reaction Reporting System. Drug Saf. 2023, 46, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.D. U.S. Food and Drug Administration Approval of Ambisome (Liposomal Amphotericin B) for Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 1999, 28, 49–51. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Alnuhait, M.A.; Alqahtani, T. The Clinical Safety and Efficacy of Cytarabine and Daunorubicin Liposome (Cpx-351) in Acute Myeloid Leukemia Patients: A Systematic Review. Cancer Rep. 2025, 8, e70199. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Kitchens, K.M.; Nevels, K.J.; Ghandehari, H.; Butko, P. Kinetic Analysis of the Interaction between Poly(Amidoamine) Dendrimers and Model Lipid Membranes. Biochim. Biophys. Acta 2011, 1808, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Henson, J.D.; Vitetta, L.; Quezada, M.; Hall, S. Enhancing Endocannabinoid Control of Stress with Cannabidiol. J. Clin. Med. 2021, 10, 5852. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Phillips, S.; ElSohly, M.A.; Walker, L.A. Current Status and Prospects for Cannabidiol Preparations as New Therapeutic Agents. Pharmacotherapy 2016, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.W.; Flint, M.E. A Single Centre, Placebo-Controlled, Four Period, Crossover, Tolerability Study Assessing, Pharmacodynamic Effects, Pharmacokinetic Characteristics and Cognitive Profiles of a Single Dose of Three Formulations of Cannabis Based Medicine Extracts (Cbmes) (Gwpd9901), Plus a Two Period Tolerability Study Comparing Pharmacodynamic Effects and Pharmacokinetic Characteristics of a Single Dose of a Cannabis Based Medicine Extract Given Via Two Administration Routes (Gwpd9901 Ext). J. Cannabis Ther. 2004, 3, 35–77. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lindgren, J.-E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-Dose Kinetics of Deuterium-Labelled Cannabidiol in Man after Smoking and Intravenous Administration. Biomed. Environ. Mass. Spectrom. 1986, 13, 77–83. [Google Scholar]

| Drug Manufacturer Brand/Trade Name [Ref] | Pharmaceutical | Method of Delivery | Indication | Advantages and Disadvantages |
|---|---|---|---|---|
| MICELLES Usual size range 5 to 100 nm|surface charge can be anionic|cationic|zwitterionic | ||||
| Celgene, Summit, NJ, USA Abraxane [92] | Paclitaxel | Intravenous Administration | -Breast cancer -Non-Small Lung cancer -Pancreatic cancer -Ovarian cancer | Advantages ↑drug solubility| Nanosize prolonged circulation|Hydrophilic shells| Improved bioavailability of hydrophobic drugs| Disadvantages Drug loading efficiency|Difficulty controlling particle uniformity| Premature drug release|Instability in physiological environments| Potential immunogenicity or toxicity issues| |
| Medlab Group, Paradise Point, QLD, Australia MDCNB-01 (THC + CBD) [93] | MDCNB-01 (NanaBis) | Orobuccal Administration | -Cancer Pain -Cancer Nausea | |
| Zentopia, Boise, ID, USA Pink Lemon Smash THC + CBD [www.Zentopia.com] | Pink Lemon Smash | Oral liquid | -General consumption | |
| Vacay, Valens Agritech Ltd., Kelowna, BC, Canada Vacay’s Island Punch [https://www.vacayedibles.com] | Vacay’s Island Punch | Oral liquid | -General consumption | |
| LIPOSOMES Usual size range 25 nm to 2.5 μm|surface charges can be neutral, negative, or positive | ||||
| Pacira Pharmaceuticals, San Diego, CA, USA DepoDur [94] | Morphine injectable (slow release) | Epidural Administration | -Postoperative pain | Advantages ↑ Drug stability| ↑ Targeted delivery| ↑ Bioavailability| ↓Toxicity| Biocompatible|Biodegradable nature ideal for delivering drugs and genes to specific tissues Disadvantages High production costs| Poor physical stability| Short circulation times Potential leakage or fusion of encapsulated substance|Chemical degradation of phospholipids|Instability in physiological environments| Potential immunogenicity or toxicity issues| |
| Marqibo (Spectrum Pharmaceuticals), Boston, MA, USA Oncovin|Vincasar PFS|Vincrex [95] | Vincristine | Intravenous Infusion | -Acute Lymphoblastic Leukemia | |
| Ipsen Biopharmaceuticals, Paris, France Onivyde [96] | Irinotecan | Intravenous Injection | -Metastatic Adenocarcinoma of the Pancreas | |
| Jazz Pharmaceuticals, Dublin, Ireland Vyxeos [97] | Daunorubicin and Cytarabine | -Intramuscular -Intrathecal -Subcutaneous Injection | -Acute Myeloid Leukemia | |
| Creative Biostructure, Shirley, NY, USA Lipo-308C [CBD] Cannabis sativa extract | Liposome for cosmetics| Liposomes for food | Sprays Oils | -General consumption | |
| DENDRIMERS (polymer nanoparticles) Usual size range 1 to 100 nm|positive surface charge for cell entry acquisition | ||||
| Tolmar, Chicago, IL, USA Eligard [98] | Leuprolide acetate and polymer | Subcutaneous Injections | -Prostate cancer | Advantages Precise drug delivery| ↑ Solubility| ↑ Targeting due unique branched monodisperse structure and customizable surface| Disadvantages High manufacturing costs|Potential toxicity issues [e.g., cationic dendrimers] Challenges in achieving pure and large-scale synthesis| |
| Pfizer, New York, NY, USA Somavert [99] | Pegvisomant | Subcutaneous Injections | -Acromegaly | |
| UCB, Brussels, Belgium Cimzia [100] | Certolizumab | Tablets or Intravenous Injections | -Rheumatoid Arthritis -Crohn’s Disease -Psoriatic Arthritis, -Ankylosing Spondylitis | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitetta, L.; Henson, J.D.; Hayes, E.; Rutolo, D.; Hall, S. Nanotechnology for the Efficacious Delivery of Medicinal Cannabis and Pharmaceutical Medicines. Pharmaceuticals 2025, 18, 1385. https://doi.org/10.3390/ph18091385
Vitetta L, Henson JD, Hayes E, Rutolo D, Hall S. Nanotechnology for the Efficacious Delivery of Medicinal Cannabis and Pharmaceutical Medicines. Pharmaceuticals. 2025; 18(9):1385. https://doi.org/10.3390/ph18091385
Chicago/Turabian StyleVitetta, Luis, Jeremy David Henson, Evan Hayes, David Rutolo, and Sean Hall. 2025. "Nanotechnology for the Efficacious Delivery of Medicinal Cannabis and Pharmaceutical Medicines" Pharmaceuticals 18, no. 9: 1385. https://doi.org/10.3390/ph18091385
APA StyleVitetta, L., Henson, J. D., Hayes, E., Rutolo, D., & Hall, S. (2025). Nanotechnology for the Efficacious Delivery of Medicinal Cannabis and Pharmaceutical Medicines. Pharmaceuticals, 18(9), 1385. https://doi.org/10.3390/ph18091385







