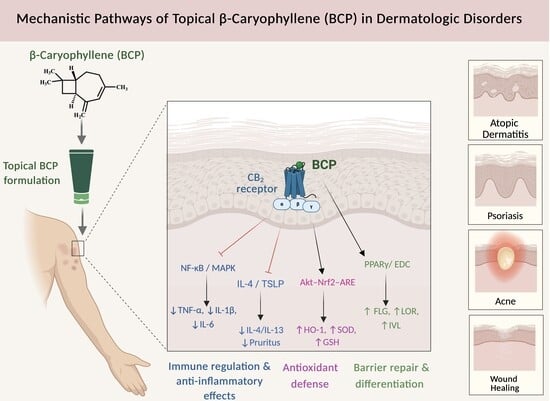
Topical β-Caryophyllene for Dermatologic Disorders: Mechanisms, Human Evidence, and Clinical Translation
Abstract
1. Introduction
2. Cannabinoid Receptors and the Endocannabinoid System in the Skin
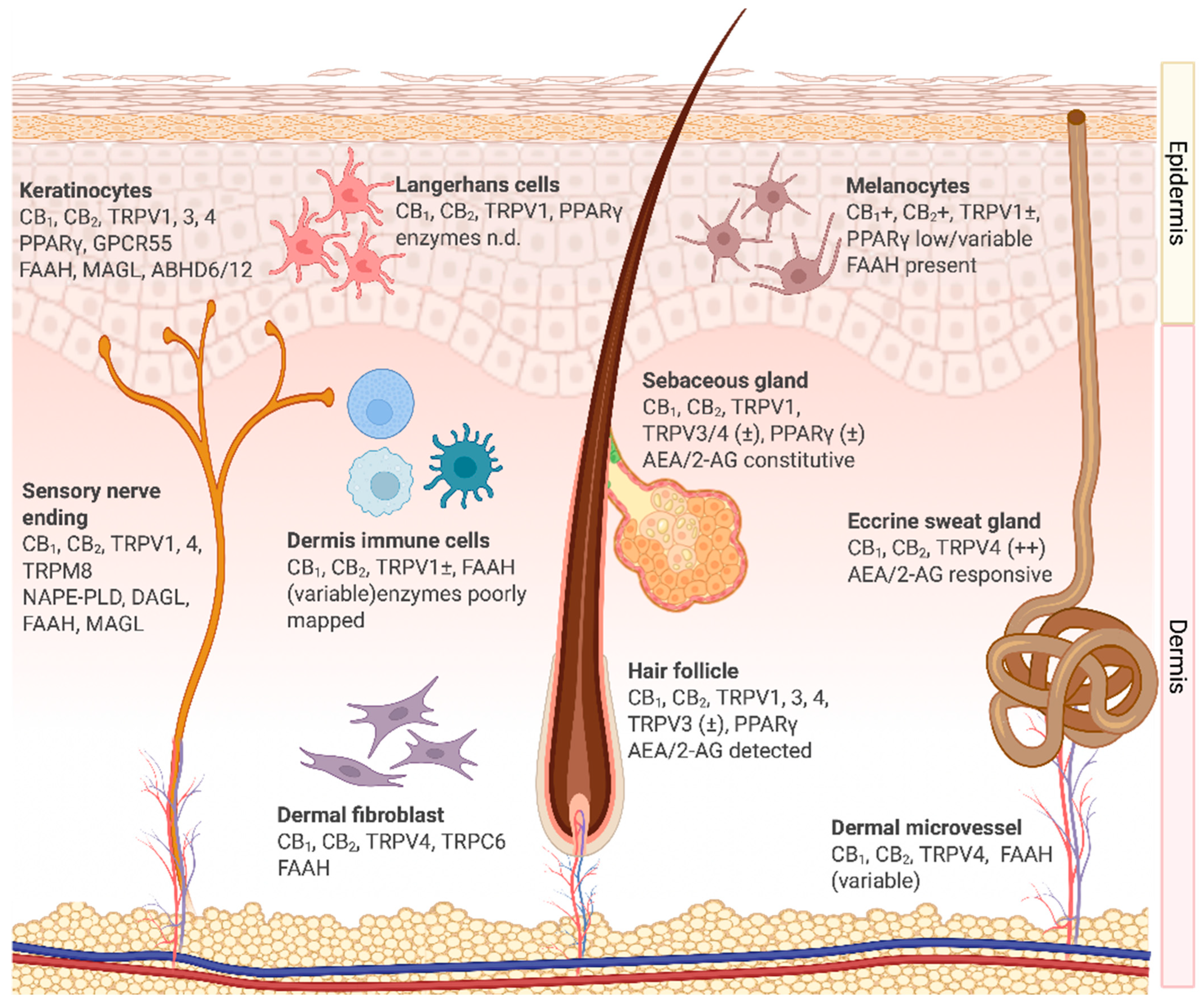
2.1. Overview of the Cutaneous Endocannabinoid System
2.2. Cellular Expression and Functions of the Endocannabinoid System in the Skin
3. Dysregulation of the Cutaneous Endocannabinoid System in Dermatological Disorders
4. Rationale for CB2 Targeting in Dermatology
5. β-Caryophyllene: Chemistry and Developability for Topical Use
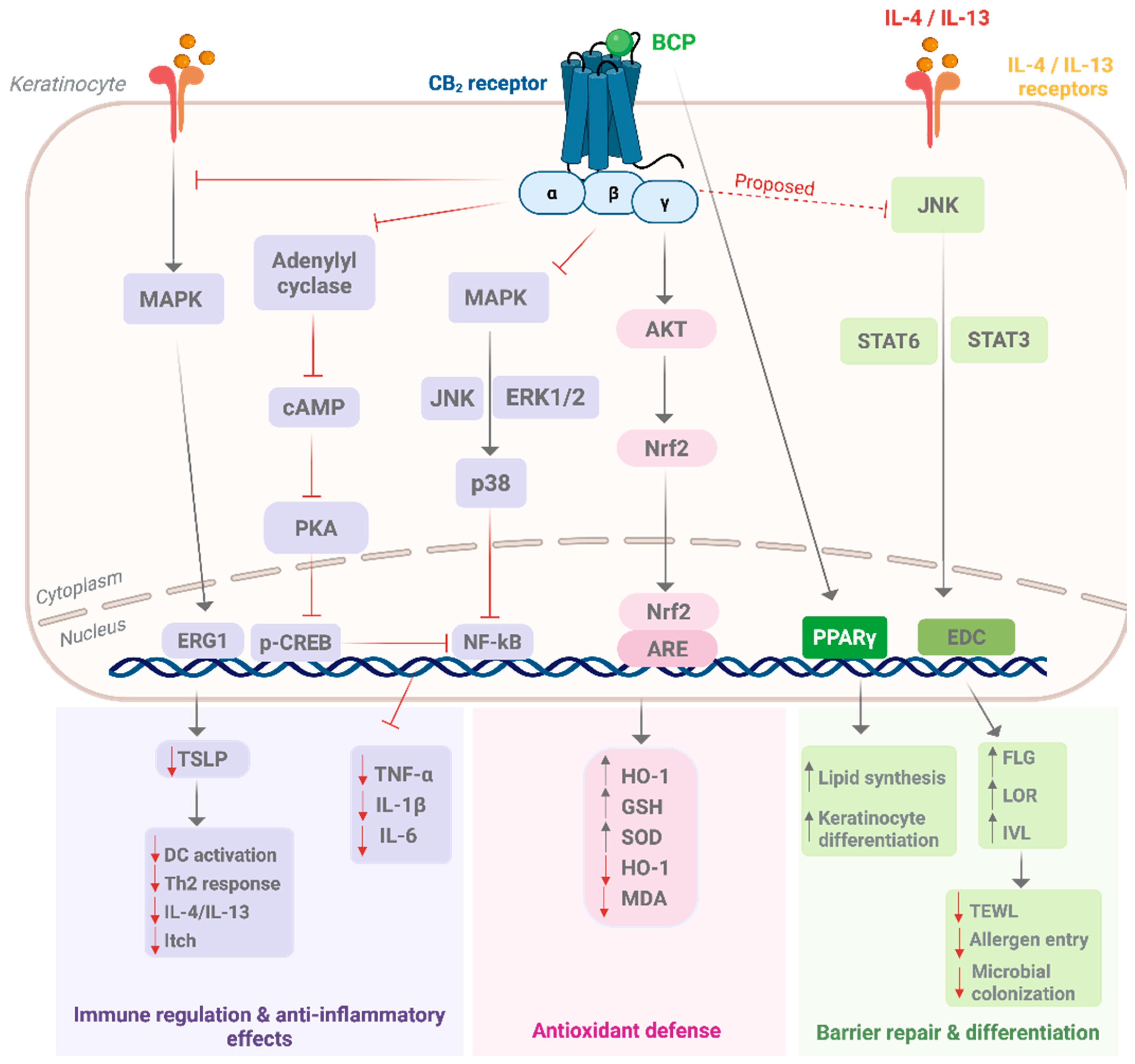
6. Molecular Pharmacology of β-Caryophyllene in Skin
7. Preclinical and Clinical Evidence of β-Caryophyllene in Skin Disorders
7.1. Mini-Methods (Search, Eligibility, and Appraisal)
7.2. Preclinical Evidence
7.2.1. In Vitro Studies
7.2.2. In Vivo Animal Models
7.3. Clinical Evidence
8. Formulation Strategies for Topical β-Caryophyllene
9. Commercial Landscape and Patent Activity
10. Safety, Tolerability, and Regulatory Considerations
11. Translational Outlook and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| ABHD6/12 | α/β-hydrolase domain–containing proteins 6 and 12 |
| AD | Atopic dermatitis |
| AEA | N-arachidonoyl ethanolamide (anandamide) |
| AKT | Protein kinase B |
| ARE | Antioxidant response element |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BCP | β-caryophyllene |
| BCPO | β-caryophyllene oxide |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| CBD | Cannabidiol |
| CREB | cAMP response element–binding protein |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| DAGLα/β | Diacylglycerol lipase α/β |
| eCBs | Endocannabinoids |
| ECS | Endocannabinoid system |
| EDC | Epidermal differentiation complex |
| EGR1 | Early growth response protein 1 |
| EMA | European Medicines Agency |
| ERK | Extracellular signal–regulated kinase |
| FAAH | Fatty acid amide hydrolase |
| FABP5/7 | Fatty acid binding proteins 5/7 |
| FDA | U.S. Food and Drug Administration |
| FEMA | Flavor and Extract Manufacturers Association |
| FLG | Filaggrin |
| GC–FID | Gas Chromatography–Flame Ionization Detection |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| GMP | Good Manufacturing Practice |
| GPCRs | G-protein-coupled receptors |
| GPR55 | G-protein-coupled receptor 55 |
| GRAS | Generally Recognized as Safe |
| GSH | Glutathione |
| HO-1 | Heme oxygenase-1 |
| HRIPT | Human repeat insult patch test |
| IL | Interleukin |
| INCI | International Nomenclature of Cosmetic Ingredients |
| IVL | Involucrin |
| IVPT | In vitro permeation testing |
| IVRT | In vitro release testing |
| JNK | c-Jun N-terminal kinase |
| LOR | Loricrin |
| LPS | Lipopolysaccharide |
| MAGL | Monoacylglycerol lipase |
| MAPKs | Mitogen-activated protein kinases |
| MDA | Malondialdehyde. |
| MHC | Major histocompatibility complex |
| MMPs | Matrix metalloproteinases |
| NAPE-PLD | N-acyl-phosphatidylethanolamine phospholipase D |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLCs | Nanostructured lipid carriers |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PD | Pharmacodynamics |
| PI3K | Phosphoinositide 3-kinase |
| PK | Pharmacokinetics |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RhoA | Ras homolog family member A |
| RIFM | Research Institute for Fragrance Materials |
| RoB 2 | Risk of Bias tool (for clinical trials) |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STAT | Signal transducer and activator of transcription |
| TEER | Transepithelial electrical resistance |
| TEWL | Transepidermal water loss |
| Th2 | T helper 2 |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TNF-α | Tumor necrosis factor-alpha |
| TRP | Transient receptor potential channels |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPC6 | Transient receptor potential canonical 6 |
| TRPM8 | Transient receptor potential melastatin 8 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TRPV3 | Transient receptor potential vanilloid 3 |
| TRPV4 | Transient receptor potential vanilloid 4 |
| TSLP | Thymic stromal lymphopoietin |
| UVB | Ultraviolet B radiation |
| Wnt | Wingless-related integration site pathway |
References
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Dreno, B.; Amici, J.M.; Demessant-Flavigny, A.L.; Wright, C.; Taieb, C.; Desai, S.R.; Alexis, A. The Impact of Acne, Atopic Dermatitis, Skin Toxicities and Scars on Quality of Life and the Importance of a Holistic Treatment Approach. Clin. Cosmet. Investig. Dermatol. 2021, 14, 623–632. [Google Scholar] [CrossRef]
- Alsohaimi, A.O.; Alghamdi, A.; Alghamdi, R.S.; Alghamdi, A.H.; Alkhathami, A.M.; Alghamdi, M.A.; Alghamdi, S.A.; Alzahrani, N.M.; Alghamdi, N.S. Prevalence of Acne Vulgaris in Adolescents and Young Adults in Al-Baha Region, Saudi Arabia. Cureus 2024, 16, e71293. [Google Scholar] [CrossRef]
- Elezbawy, B.; Fasseeh, A.N.; Fouly, E.; Esba, L.C.A.; Al Abdulkarim, H.; Al-Haddab, M.; Al-Sheikh, A.; Altawil, E.; Al Turaiki, A.; Eshmawi, M.; et al. The Humanistic and Economic Burden of Atopic Dermatitis among Adults and Adolescents in Saudi Arabia. J. Med. Econ. 2022, 25, 1231–1239. [Google Scholar] [CrossRef]
- Mahmoud, O.; Yosipovitch, G.; Attia, E. Burden of Disease and Unmet Needs in the Diagnosis and Management of Atopic Dermatitis in the Arabic Population of the Middle East. J. Clin. Med. 2023, 12, 4675. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse Effects of Topical Glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15, quiz 16–18. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Cordoro, K.M.; Davis, D.M.R.; Kroshinsky, D.; Paller, A.S.; Armstrong, A.W.; Connor, C.; Elewski, B.E.; Gelfand, J.M.; Gordon, K.B.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation Guidelines of Care for the Management and Treatment of Psoriasis in Pediatric Patients. J. Am. Acad. Dermatol. 2020, 82, 161–201. [Google Scholar] [CrossRef]
- Zhu, B.; Jing, M.; Yu, Q.; Ge, X.; Yuan, F.; Shi, L. Treatments in Psoriasis: From Standard Pharmacotherapy to Nanotechnology Therapy. Postepy Dermatol. Alergol. 2022, 39, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Zheng, L.; Ramanujam, V.; Gallagher, J. Novel Use of Pharmacogenetic Testing in the Identification of CYP2C9 Polymorphisms Related to NSAID-Induced Gastropathy. Pain Med. 2015, 16, 866–869. [Google Scholar] [CrossRef]
- Schick, M.A.; Schlegel, N. Clinical Implication of Phosphodiesterase-4-Inhibition. Int. J. Mol. Sci. 2022, 23, 1209. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(Ut)Annabinoid” System. Molecules 2019, 24, 918. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Haskó, G.; Paus, R.; Pacher, P. The Endocannabinoid System of the Skin in Health and Disease: Novel Perspectives and Therapeutic Opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.-K.; Zhang, M.; Li, J.-Y.; Wang, L.-L.; Guan, D.-W. Cannabinoid 2 Receptor Attenuates Inflammation during Skin Wound Healing by Inhibiting M1 Macrophages Rather than Activating M2 Macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, J.H. Cannabinoids and Their Receptors in Skin Diseases. Int. J. Mol. Sci. 2023, 24, 16523. [Google Scholar] [CrossRef]
- McCormick, E.; Han, H.; Azim, S.A.; Whiting, C.; Bhamidipati, N.; Kiss, A.; Efimova, T.; Berman, B.; Friedman, A. Topical Nanoencapsulated Cannabidiol Cream as an Innovative Strategy Combating UV-A–Induced Nuclear and Mitochondrial DNA Injury: A Pilot Randomized Clinical Study. J. Am. Acad. Dermatol. 2024, 91, 855–862. [Google Scholar] [CrossRef]
- Ferreira, B.P.; Costa, G.; Mascarenhas-Melo, F.; Pires, P.C.; Heidarizadeh, F.; Giram, P.S.; Mazzola, P.G.; Cabral, C.; Veiga, F.; Paiva-Santos, A.C. Skin Applications of Cannabidiol: Sources, Effects, Delivery Systems, Marketed Formulations and Safety. Phytochem. Rev. 2023, 22, 781–828. [Google Scholar] [CrossRef]
- Ju, T.; Labib, A.; Vander Does, A.; Yosipovitch, G. Therapeutics in Chronic Pruritus of Unknown Origin. Itch 2023, 8, e64. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Pharmacology of Cannabinoid Receptors and Their Ligands: An Overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Petrocellis, L.D.; Cascio, M.G.; Marzo, V.D. The Endocannabinoid System: A General View and Latest Additions. Br. J. Pharmacol. 2004, 141, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The Cannabinoid CB2 Receptor-Selective Phytocannabinoid Beta-Caryophyllene Exerts Analgesic Effects in Mouse Models of Inflammatory and Neuropathic Pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of Cannabinoid Receptor 1 (CB1) and 2 (CB2) on Sensory Nerve Fibers and Adnexal Structures in Human Skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Bagher, A.M. Intraplantar β-Caryophyllene Alleviates Pain and Inflammation in STZ-Induced Diabetic Peripheral Neuropathy via CB2 Receptor Activation. Int. J. Mol. Sci. 2025, 26, 4430. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Mayer, T.Z.; Simard, M.; Flamand, N.; Di Marzo, V. The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update. Molecules 2024, 29, 3381. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. Substances Added to Food (Formerly EAFUS). Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&sort=Used_for_Technical_Effect&utm_source=chatgpt.com (accessed on 14 August 2025).
- Flavor and Extract Manufacturers Association of the United States (FEMA). BETA-CARYOPHYLLENE. Available online: https://www.femaflavor.org/flavor-library/beta-caryophyllene?utm_source=chatgpt.com (accessed on 14 August 2025).
- Ahn, S.S.; Yeo, H.; Jung, E.; Ou, S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis. Int. J. Mol. Sci. 2022, 23, 14861. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, K.; Yang, J.; Wang, A.; Chen, G.; Ye, M.; Chen, Q.; Lin, D. β-Caryophyllene Promotes the Survival of Random Skin Flaps by Upregulating the PI3K/AKT Signaling Pathway. Phytomedicine 2024, 130, 155726. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; de Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K.; et al. Beta-Caryophyllene as an Antioxidant, Anti-Inflammatory and Re-Epithelialization Activities in a Rat Skin Wound Excision Model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-Caryophyllene Enhances Wound Healing through Multiple Routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef]
- Scandiffio, R.; Bonzano, S.; Cottone, E.; Shrestha, S.; Bossi, S.; De Marchis, S.; Maffei, M.E.; Bovolin, P. Beta-Caryophyllene Modifies Intracellular Lipid Composition in a Cell Model of Hepatic Steatosis by Acting through CB2 and PPAR Receptors. Int. J. Mol. Sci. 2023, 24, 6060. [Google Scholar] [CrossRef]
- Becker, G.; Brusco, I.; Casoti, R.; Marchiori, M.C.L.; Cruz, L.; Trevisan, G.; Oliveira, S.M. Copaiba Oleoresin Has Topical Antinociceptive Activity in a UVB Radiation-Induced Skin-Burn Model in Mice. J. Ethnopharmacol. 2020, 250, 112476. [Google Scholar] [CrossRef]
- Inan, S.; Ward, S.J.; Baltazar, C.T.; Peruggia, G.A.; Javed, E.; Nayak, A.P. Epicutaneous Sensitization to the Phytocannabinoid β-Caryophyllene Induces Pruritic Inflammation. Int. J. Mol. Sci. 2023, 24, 14328. [Google Scholar] [CrossRef]
- Dobrosi, N.; Tóth, B.I.; Nagy, G.; Dózsa, A.; Géczy, T.; Nagy, L.; Zouboulis, C.C.; Paus, R.; Kovács, L.; Bíró, T. Endocannabinoids Enhance Lipid Synthesis and Apoptosis of Human Sebocytes via Cannabinoid Receptor-2-Mediated Signaling. FASEB J. 2008, 22, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM Fragrance Ingredient Safety Assessment, β-Caryophyllene, CAS Registry Number 87-44-5. Food Chem. Toxicol. 2022, 159, 112707. [Google Scholar] [CrossRef] [PubMed]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM Fragrance Ingredient Safety Assessment, Caryophyllene Oxide, CAS Registry Number 1139-30-6. Food Chem. Toxicol. 2020, 138 (Suppl. S1), 111102. [Google Scholar] [CrossRef]
- Sköld, M.; Karlberg, A.-T.; Matura, M.; Börje, A. The Fragrance Chemical Beta-Caryophyllene-Air Oxidation and Skin Sensitization. Food Chem. Toxicol. 2006, 44, 538–545. [Google Scholar] [CrossRef]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular Characterization of a Phospholipase D Generating Anandamide and Its Congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.-J.; et al. Cloning of the First Sn1-DAG Lipases Points to the Spatial and Temporal Regulation of Endocannabinoid Signaling in the Brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular Characterization of an Enzyme That Degrades Neuromodulatory Fatty-Acid Amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain Monoglyceride Lipase Participating in Endocannabinoid Inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A Comprehensive Profile of Brain Enzymes That Hydrolyze the Endocannabinoid 2-Arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. Identification of Intracellular Carriers for the Endocannabinoid Anandamide. Proc. Natl. Acad. Sci. USA 2009, 106, 6375–6380. [Google Scholar] [CrossRef]
- Marzo, V.; Petrocellis, L. Endocannabinoids as Regulators of Transient Receptor Potential (TRP)Channels: A Further Opportunity to Develop New Endocannabinoid-Based Therapeutic Drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel That Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Caterina, M.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of Skin Surface Temperature on Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kida, N.; Sokabe, T.; Kashio, M.; Haruna, K.; Mizuno, Y.; Suga, Y.; Nishikawa, K.; Kanamaru, A.; Hongo, M.; Oba, A.; et al. Importance of Transient Receptor Potential Vanilloid 4 (TRPV4) in Epidermal Barrier Function in Human Skin Keratinocytes. Pflugers Arch.-Eur. J. Physiol. 2012, 463, 715–725. [Google Scholar] [CrossRef]
- Sokabe, T.; Fukumi-Tominaga, T.; Yonemura, S.; Mizuno, A.; Tominaga, M. The TRPV4 Channel Contributes to Intercellular Junction Formation in Keratinocytes. J. Biol. Chem. 2010, 285, 18749–18758. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the Treatment of Dermatologic Conditions. JID Innov. 2022, 2, 100095. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Ramot, Y.; Sugawara, K.; Zákány, N.; Tóth, B.I.; Bíró, T.; Paus, R. A Novel Control of Human Keratin Expression: Cannabinoid Receptor 1-Mediated Signaling down-Regulates the Expression of Keratins K6 and K16 in Human Keratinocytes In Vitro and In Situ. PeerJ 2013, 1, e40. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Flores, J.M.; Quintanilla, M.; Paramio, J.M.; Guzmán, M.; Sánchez, C. The Orphan Receptor GPR55 Drives Skin Carcinogenesis and Is Upregulated in Human Squamous Cell Carcinomas. Oncogene 2013, 32, 2534–2542. [Google Scholar] [CrossRef]
- Bagood, M.D.; Isseroff, R.R. TRPV1: Role in Skin and Skin Diseases and Potential Target for Improving Wound Healing. Int. J. Mol. Sci. 2021, 22, 6135. [Google Scholar] [CrossRef]
- Pénzes, Z.; Horváth, D.; Molnár, P.; Fekete, T.; Pázmándi, K.; Bácsi, A.; Szöllősi, A.G. Anandamide Modulation of Monocyte-Derived Langerhans Cells: Implications for Immune Homeostasis and Skin Inflammation. Front. Immunol. 2024, 15, 1423776. [Google Scholar] [CrossRef]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; et al. The Endocannabinoid Anandamide Protects Neurons during CNS Inflammation by Induction of MKP-1 in Microglial Cells. Neuron 2006, 49, 67–79. [Google Scholar] [CrossRef]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, R.D. Stimulation of Cannabinoid Receptor 2 (CB2) Suppresses Microglial Activation. J. Neuroinflamm. 2005, 2, 29. [Google Scholar] [CrossRef]
- Sugawara, K.; Bíró, T.; Tsuruta, D.; Tóth, B.I.; Kromminga, A.; Zákány, N.; Zimmer, A.; Funk, W.; Gibbs, B.F.; Zimmer, A.; et al. Endocannabinoids Limit Excessive Mast Cell Maturation and Activation in Human Skin. J. Allergy Clin. Immunol. 2012, 129, 726–738.e8. [Google Scholar] [CrossRef]
- Telek, A.; Bíró, T.; Bodó, E.; Tóth, B.I.; Borbíró, I.; Kunos, G.; Paus, R. Inhibition of Human Hair Follicle Growth by Endo- and Exocannabinoids. FASEB J. 2007, 21, 3534–3541. [Google Scholar] [CrossRef]
- Dvorak, M.; Watkinson, A.; McGlone, F.; Rukwied, R. Histamine Induced Responses Are Attenuated by a Cannabinoid Receptor Agonist in Human Skin. Inflamm. Res. 2003, 52, 238–245. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Harding-Rose, C.; Simone, D.A.; Seybold, V.S. Differential Effects of CB1 and Opioid Agonists on Two Populations of Adult Rat Dorsal Root Ganglion Neurons. J. Neurosci. 2004, 24, 1744–1753. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Suplita, R.L. Endocannabinoid Mechanisms of Pain Modulation. AAPS J. 2006, 8, E693–E708. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Szöllősi, A.G.; Tóth, B.I.; Demaude, J.; Bouez, C.; Breton, L.; Bíró, T. Endocannabinoids Regulate Growth and Survival of Human Eccrine Sweat Gland-Derived Epithelial Cells. J. Investig. Dermatol. 2012, 132, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Bíró, T. TRP Channels in the Skin. Br. J. Pharmacol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.; Huang, S.M.; et al. TRP Channel Regulates EGFR Signaling in Hair Morphogenesis and Skin Barrier Formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef]
- Zákány, N.; Oláh, A.; Markovics, A.; Takács, E.; Aranyász, A.; Nicolussi, S.; Piscitelli, F.; Allarà, M.; Pór, Á.; Kovács, I.; et al. Endocannabinoid Tone Regulates Human Sebocyte Biology. J. Investig. Dermatol. 2018, 138, 1699–1706. [Google Scholar] [CrossRef]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic Control of Skin Differentiation Genes by Phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Kovalchuk, O.; Kovalchuk, I. Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts. Cells 2022, 11, 3939. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The Putative Cannabinoid Receptor GPR55 Affects Osteoclast Function in Vitro and Bone Mass in Vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; Kunos, G. Functional CB1 Cannabinoid Receptors in Human Vascular Endothelial Cells. Biochem. J. 2000, 346 Pt 3, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Hartmannsgruber, V.; Heyken, W.-T.; Kacik, M.; Kaistha, A.; Grgic, I.; Harteneck, C.; Liedtke, W.; Hoyer, J.; Köhler, R. Arterial Response to Shear Stress Critically Depends on Endothelial TRPV4 Expression. PLoS ONE 2007, 2, e827. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic Immune Mechanisms in Atopic Dermatitis and Psoriasis with Implications for Treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of Allergic Contact Dermatitis through the Endocannabinoid System. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Wakui, J.; Ikeda, S.; Yanagimoto, S.; Kishimoto, S.; Gokoh, M.; Nasui, M.; Sugiura, T. Involvement of the Cannabinoid CB2 Receptor and Its Endogenous Ligand 2-Arachidonoylglycerol in Oxazolone-Induced Contact Dermatitis in Mice. J. Immunol. 2006, 177, 8796–8805. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Ge, W.; Chen, C.; Huang, Y.; Jin, Z.; Zhan, M.; Duan, X.; Liu, X.; Kong, Y.; et al. CB2R Deficiency Exacerbates Imiquimod-Induced Psoriasiform Dermatitis and Itch Through the Neuro-Immune Pathway. Front. Pharmacol. 2022, 13, 790712. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The Endocannabinoid System in Human Keratinocytes. Evidence That Anandamide Inhibits Epidermal Differentiation through CB1 Receptor-Dependent Inhibition of Protein Kinase C, Activation Protein-1, and Transglutaminase. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium acnes) and Acne Vulgaris: A Brief Look at the Latest Updates. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New Developments in Our Understanding of Acne Pathogenesis and Treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Filipiuc, S.-I.; Neagu, A.-N.; Uritu, C.M.; Tamba, B.-I.; Filipiuc, L.-E.; Tudorancea, I.M.; Boca, A.N.; Hâncu, M.F.; Porumb, V.; Bild, W. The Skin and Natural Cannabinoids-Topical and Transdermal Applications. Pharmaceuticals 2023, 16, 1049. [Google Scholar] [CrossRef]
- Makhakhe, L. Topical Cannabidiol (CBD) in Skin Pathology—A Comprehensive Review and Prospects for New Therapeutic Opportunities. S. Afr. Fam. Pract. 2022, 64, e1–e4. [Google Scholar] [CrossRef]
- Bizarro, A.F.; Schmidt, V.M.; Fernandes, B.; Pinto, M.; Pereira, H.; Marto, J.; Lourenço, A.M. The Potential of Cannabidiol for Treating Canine Atopic Dermatitis. Vet. Sci. 2025, 12, 159. [Google Scholar] [CrossRef]
- Stoco, A.A.G.; Mazzola, P.G. Therapeutic Potential of Cannabinoids for Treating Atopic Dermatitis. J. Cannabis Res. 2025, 7, 57. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.-P.; Kim, B.; Kim, B.-W. Selective Cannabinoid Receptor-1 Agonists Regulate Mast Cell Activation in an Oxazolone-Induced Atopic Dermatitis Model. Ann. Dermatol. 2016, 28, 22–29. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Katerina, V.; Klara, U.; Samnang, N.; Ladislav, K. Chemical Composition of Essential Oils and Supercritical Carbon Dioxide Extracts from Amomum Kravanh, Citrus Hystrix and Piper Nigrum “Kampot”. Molecules 2023, 28, 7748. [Google Scholar] [CrossRef]
- PubChem. (+)-Beta-Caryophyllene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/20831623 (accessed on 9 August 2025).
- Chicca, A.; Marazzi, J.; Gertsch, J. The Antinociceptive Triterpene Β-amyrin Inhibits 2-arachidonoylglycerol (2-AG) Hydrolysis without Directly Targeting Cannabinoid Receptors. Br. J. Pharmacol. 2012, 167, 1596–1608. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating Effect of β-Caryophyllene on Anticancer Activity of α-Humulene, Isocaryophyllene and Paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Goindi, S.; Narula, M.; Kalra, A. Microemulsion-Based Topical Hydrogels of Tenoxicam for Treatment of Arthritis. AAPS PharmSciTech 2016, 17, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Santos Porto, D.; Da Costa Bernardo Port, B.; Conte, J.; Fretes Argenta, D.; Pereira Balleste, M.; Amadeu Micke, G.; Machado Campos, Â.; Silva Caumo, K.; Caon, T. Development of Ophthalmic Nanoemulsions of β-Caryophyllene for the Treatment of Acanthamoeba Keratitis. Int. J. Pharm. 2024, 659, 124252. [Google Scholar] [CrossRef]
- Weimer, P.; Kreutz, T.; Limberger, R.P.; Rossi, R.C.; De Lima, Á.A.N.; Veiga, V.F.; De Araújo, B.V.; Koester, L.S. Correlation between the Skin Permeation Profile of the Synthetic Sesquiterpene Compounds, Beta-Caryophyllene and Caryophyllene Oxide, and the Antiedematogenic Activity by Topical Application of Nanoemulgels. Biomolecules 2022, 12, 1102. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 67–134. ISBN 978-0-12-811232-8. [Google Scholar]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells: An in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Myslivečková, Z.; Szotáková, B.; Špičáková, A.; Lněničková, K.; Ambrož, M.; Kubíček, V.; Krasulová, K.; Anzenbacher, P.; Skálová, L. The Inhibitory Effects of β-Caryophyllene, β-Caryophyllene Oxide and α-Humulene on the Activities of the Main Drug-Metabolizing Enzymes in Rat and Human Liver in Vitro. Chem. Biol. Interact. 2017, 278, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Sertznig, P.; Reichrath, J. Peroxisome Proliferator-Activated Receptors (PPARs) in Dermatology: Challenge and Promise. Dermato-Endocrinology 2011, 3, 130–135. [Google Scholar] [CrossRef]
- Zhang, H.; Boyette-Davis, J.A.; Kosturakis, A.K.; Li, Y.; Yoon, S.-Y.; Walters, E.T.; Dougherty, P.M. Induction of Monocyte Chemoattractant Protein-1 (MCP-1) and Its Receptor CCR2 in Primary Sensory Neurons Contributes to Paclitaxel-Induced Peripheral Neuropathy. J. Pain 2013, 14, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhu, Z.; Zhai, Y.; Zeng, J.; Li, L.; Wang, D.; Deng, F.; Chang, B.; Zhou, J.; Sun, L. The Role of TSLP in Atopic Dermatitis: From Pathogenetic Molecule to Therapeutical Target. Mediators Inflamm. 2023, 2023, 7697699. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human Epithelial Cells Trigger Dendritic Cell–Mediated Allergic Inflammation by Producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Mamdouh Hashiesh, H.; Sheikh, A.; Meeran, M.F.N.; Saraswathiamma, D.; Jha, N.K.; Sadek, B.; Adeghate, E.; Tariq, S.; Al Marzooqi, S.; Ojha, S. β-Caryophyllene, a Dietary Phytocannabinoid, Alleviates Diabetic Cardiomyopathy in Mice by Inhibiting Oxidative Stress and Inflammation Activating Cannabinoid Type-2 Receptors. ACS Pharmacol. Transl. Sci. 2023, 6, 1129–1142. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Che-Wen, Y.; Lin, F.-L.; Chen, K.-H.; Cheng, Y.-P.; Cheng, Y.-C.; Guo, J.-W. Transcriptional Dysregulation of Skin Barrier Genes in Atopic Dermatitis and Psoriasis: Mechanistic Insights and Emerging Therapeutic Strategies. Biomed. Pharmacother. 2025, 191, 118508. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.-S.; Chun, H.S.; Kwon, S.-H.; et al. β-Caryophyllene Potently Inhibits Solid Tumor Growth and Lymph Node Metastasis of B16F10 Melanoma Cells in High-Fat Diet–Induced Obese C57BL/6N Mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef]
- Del Río, C.; Navarrete, C.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; Calzado, M.A.; et al. The Cannabinoid Quinol VCE-004.8 Alleviates Bleomycin-Induced Scleroderma and Exerts Potent Antifibrotic Effects through Peroxisome Proliferator-Activated Receptor-γ and CB2 Pathways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef] [PubMed]
- Seminotti, B.; Grings, M.; Glänzel, N.M.; Vockley, J.; Leipnitz, G. Peroxisome Proliferator-Activated Receptor (PPAR) Agonists as a Potential Therapy for Inherited Metabolic Disorders. Biochem. Pharmacol. 2023, 209, 115433. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Porreca, F.; Lai, J.; Albrecht, P.J.; Rice, F.L.; Khodorova, A.; Davar, G.; Makriyannis, A.; Vanderah, T.W.; Mata, H.P.; et al. CB2 Cannabinoid Receptor Activation Produces Antinociception by Stimulating Peripheral Release of Endogenous Opioids. Proc. Natl. Acad. Sci. USA 2005, 102, 3093–3098. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Xu, L.; Xu, J. Emerging Roles of Keratinocytes in Nociceptive Transduction and Regulation. Front. Mol. Neurosci. 2022, 15, 982202. [Google Scholar] [CrossRef]
- Desroches, J.; Bouchard, J.-F.; Gendron, L.; Beaulieu, P. Involvement of Cannabinoid Receptors in Peripheral and Spinal Morphine Analgesia. Neuroscience 2014, 261, 23–42. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Mazzantini, C.; El Bourji, Z.; Parisio, C.; Davolio, P.L.; Cocchi, A.; Pellegrini-Giampietro, D.E.; Landucci, E. Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals 2024, 17, 467. [Google Scholar] [CrossRef]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Gullì, M.; Percaccio, E.; Di Giacomo, S.; Di Sotto, A. Novel Insights into the Immunomodulatory Effects of Caryophyllane Sesquiterpenes: A Systematic Review of Preclinical Studies. Appl. Sci. 2022, 12, 2292. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol Exerts Sebostatic and Antiinflammatory Effects on Human Sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Reck, A.M.; Siderovski, D.P.; Kinsey, S.G. The Synthetic Cannabinoid Agonist WIN 55,212-2 Reduces Experimental Pruritus via CB2 Receptor Activation. Neuropharmacology 2025, 264, 110216. [Google Scholar] [CrossRef] [PubMed]
- da Trindade, R.; da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef]
- Waibel, J.; Patel, H.; Cull, E.; Sidhu, R.; Lupatini, R. Prospective, Randomized, Double-Blind, Placebo-Controlled Study on Efficacy of Copaiba Oil in Silicone-Based Gel to Reduce Scar Formation. Dermatol. Ther. 2021, 11, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Cardinelli, C.C.; Passos, J.T.G.; Veiga-Junior, V.F.; de Oliveira, B.G.R.B.; Santos, E.P.d.; Neto, G.G.; Di Piero, K.C.; de Freitas, Z.M.F. Skin Tear Treatment with Copaifera Multijuga Hayne in Polymeric Hydrogel: A Randomized Clinical Trial. Pharmaceuticals 2024, 17, 1691. [Google Scholar] [CrossRef]
- Leite, V.; Januário, L.H.; Borges, E.; Ruas, C. Cicatrização de feridas crónicas tratadas com oleorresina de copaíba. Rev. Enf. Ref. 2023, 6, e22045. [Google Scholar] [CrossRef]
- da Silva, J.T.; Evangelista, B.G.; Venega, R.A.G.; Seminowicz, D.A.; Chacur, M. Anti-NGF Treatment Can Reduce Chronic Neuropathic Pain by Changing Peripheral Mediators and Brain Activity in Rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Louw, S. Recent Trends in the Chromatographic Analysis of Volatile Flavor and Fragrance Compounds: Annual Review 2020. Anal. Sci. Adv. 2021, 2, 157–170. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Product-Specific Guidances for Generic Drug Development; FDA: Silver Spring, MD, USA, 2025.
- Abraham, J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill|Nijhoff: Leiden, The Netherlands, 2010; pp. 1041–1053. ISBN 978-90-04-18156-4. [Google Scholar]
- Alharthi, S.; Ziora, Z.M.; Mustafa, G.; Chaubey, P.; El Kirdasy, A.F.; Alotaibi, G. β-Caryophyllene-Loaded Microemulsion-Based Topical Hydrogel: A Promising Carrier to Enhance the Analgesic and Anti-Inflammatory Outcomes. Gels 2023, 9, 634. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hani, U.; Alqarni, M.H.; Alam, A. Beta Caryophyllene-Loaded Nanostructured Lipid Carriers for Topical Management of Skin Disorders: Statistical Optimization, In Vitro and Dermatokinetic Evaluation. Gels 2023, 9, 550. [Google Scholar] [CrossRef]
- Amalraj, A.; Jacob, J.; Varma, K.; Gopi, S. Preparation and Characterization of Liposomal β-Caryophyllene (Rephyll) by Nanofiber Weaving Technology and Its Effects on Delayed Onset Muscle Soreness (DOMS) in Humans: A Randomized, Double-Blinded, Crossover-Designed, and Placebo-Controlled Study. ACS Omega 2020, 5, 24045–24056. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, F.; Wei, X.; Gu, J.; Qiao, P.; Zhu, X.; Yin, S.; Ouyang, D.; Dong, J.; Yao, J.; et al. Investigation of β-Caryophyllene as Terpene Penetration Enhancer: Role of Stratum Corneum Retention. Eur. J. Pharm. Sci. 2023, 183, 106401. [Google Scholar] [CrossRef]
- Barry, B.W. Novel Mechanisms and Devices to Enable Successful Transdermal Drug Delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R.; et al. Nanoparticles--an Efficient Carrier for Drug Delivery into the Hair Follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Weimer, P.; de Araújo Lock, G.; Amaral Antunes Nunes, K.; Rossi, R.C.; Koester, L.S. Association Effect of the Phytocannabinoid Beta-Caryophyllene and Indomethacin Carried in Topical Nanoemulgels: An Evaluation by in Vivo Anti-Inflammatory Model. Nat. Prod. Res. 2025, 39, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Abdesselam, Z.; Dominique, B.; Caroline, S.; Florence, S. Antibacterial Activities of Homemade Matrices Miming Essential Oils Compared to Commercial Ones. Antibiotics 2021, 10, 584. [Google Scholar] [CrossRef]
- European Commission. Cosmetic Ingredient Database. Available online: https://single-market-economy.ec.europa.eu/sectors/cosmetics/cosmetic-ingredient-database_en (accessed on 25 September 2025).
- European Commission. CosIng—Cosmetics Ingredients: Function—Skin Conditioning. Available online: https://ec.europa.eu/growth/tools-databases/cosing/reference/functions/list/SKIN%20CONDITIONING?utm_source=chatgpt.com (accessed on 15 August 2025).
- U.S. Food and Drug Administration (FDA). FDA Authority Over Cosmetics: How Cosmetics Are Not FDA-Approved, but Are FDA-Regulated. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated (accessed on 15 August 2025).
- Panag Pharma Inc. Randomized, Double Blind, Placebo Controlled Crossover Trial with Open Label Extension of Topical 20% Beta Caryophyllene Alone and in Combination with 0.025% Capsaicin in The Treatment of Pain Caused by Osteoarthritis of The Knee. 2020. Available online: https://clinicaltrials.gov/study/NCT03152578 (accessed on 17 September 2025).
- Sinai, A.; Turner, Z.; Baruch, Y. Cannabis-Based Extracts and Topical Formulations for Use in Skin Disorders. U.S. Patent 2018/0042890 A1, 15 February 2018. [Google Scholar]
- Ofir, R.; Rachmilevich, S.; Amiel, E.; Dudai, N.; Rabinski, T. Compositions Comprising Beta-Caryophyllene and Methods of Utilizing the Same. WO2012104845A1, 9 August 2012. [Google Scholar]
- Maida, V.; Corban, J. Topical Medical Cannabis: A New Treatment for Wound Pain—Three Cases of Pyoderma Gangrenosum. J. Pain Symptom Manag. 2017, 54, 732–736. [Google Scholar] [CrossRef]
- Mata, J.E. Multifunctional Topical Cream Comprising Beta-Caryophyllene, Essential Oils, in a Phospholipid and Triglyceride Base. U.S. Patent 2020/0030252 A1, 21 December 2021. [Google Scholar]
- Niyangoda, D.; Muayad, M.; Tesfaye, W.; Bushell, M.; Ahmad, D.; Samarawickrema, I.; Sinclair, J.; Kebriti, S.; Maida, V.; Thomas, J. Cannabinoids in Integumentary Wound Care: A Systematic Review of Emerging Preclinical and Clinical Evidence. Pharmaceutics 2024, 16, 1081. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A. The Human Repeated Insult Patch Test in the 21st Century: A Commentary. Cutan. Ocul. Toxicol. 2009, 28, 49–53. [Google Scholar] [CrossRef]
- Na, M.; Ritacco, G.; O’Brien, D.; Lavelle, M.; Api, A.M.; Basketter, D. Fragrance Skin Sensitization Evaluation and Human Testing: 30-Year Experience. Dermatitis 2021, 32, 339–352. [Google Scholar] [CrossRef]
- Matura, M.; Sköld, M.; Börje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Selected Oxidized Fragrance Terpenes Are Common Contact Allergens. Contact Dermat. 2005, 52, 320–328. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast); European Commission: Brussels, Belgium, 2009; Volume 342. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Modernization of Cosmetics Regulation Act of 2022 (MoCRA). Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra (accessed on 15 August 2025).
- Scientific Committee on Consumer Safety (SCCS). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation—12th Revision. 2023. Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 18 September 2025).
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- Reich, A.; Riepe, C.; Anastasiadou, Z.; MMędrek, K.; Augustin, M.; Szepietowski, J.C.; Ständer, S. Itch Assessment with Visual Analogue Scale and Numerical Rating Scale: Determination of Minimal Clinically Important Difference in Chronic Itch. Acta Dermato-Venereol. 2016, 96, 978–980. [Google Scholar] [CrossRef]
- Schram, M.E.; Spuls, P.I.; Leeflang, M.M.G.; Lindeboom, R.; Bos, J.D.; Schmitt, J. EASI, (Objective) SCORAD and POEM for Atopic Eczema: Responsiveness and Minimal Clinically Important Difference. Allergy 2012, 67, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, K.A.; Johansen, J.D.; Kezic, S.; Linneberg, A.; Thyssen, J.P. The Effect of Environmental Humidity and Temperature on Skin Barrier Function and Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Heath, C.R.; Alexis, A.; Silverberg, J.I. Racial Disparities in Dermatology. Arch. Dermatol. Res. 2023, 315, 1215–1223. [Google Scholar] [CrossRef] [PubMed]

| Study Type/Model | Dose/Exposure | Main Outcomes | Limitations/Notes | Ref. |
|---|---|---|---|---|
| In vitro—Inflammation (HaCaT keratinocytes) † | 1–100 µM for 24 h | ↓ p65, COX-2, IL-1β at low-mid µM; no cytotoxicity ≤ 50 µM | Short exposure; no antagonist control | [129] |
| In vitro—Th2/TSLP axis (HaCaT keratinocytes, IL-4 challenge) | 0.1–0.2 µg/mL (≈0.5–1 µM) | ↓ TSLP; ↓ MAPKs; ↓ EGR1; reduced promoter activity at −369/+18 | In vitro only; no animal validation | [30] |
| In vitro—Migration/Repair (fibroblasts, keratinocytes) * | 5–10 µM for 6–24 h | ↑ Migration/chemotaxis (~2× vs. control) | No mechanistic receptor analysis | [33] |
| In vivo—Full-thickness excisional wound (mouse; ♀ > ♂) | Topical 50 mg/kg in olive oil daily | ↑ Closure; ↑ proliferation/migration markers; CB2 involvement | Sex difference not explained; no oxidative biomarkers | [33] |
| In vivo—Cutaneous wound healing (rat) | 1% w/w daily | ↑ Contraction/remodeling; ↓ IL-1β/IL-6/TNF-α; ↑ IL-10; no systemic toxicity | Single dose level; short follow-up | [32] |
| In vivo—atopic dermatitis-like model (DNCB, BALB/c mouse) | 0.001–100 µg/mL; 1–2 weeks | ↓ Thickening; ↓ infiltration; ↓ EGR1/TSLP | No CB2 blocker confirmation | [30] |
| In vivo (safety)—Epicutaneous sensitization (mouse) | 0.1–10 mg/mL × 4 wk | Dose-dependent ↑ scratching/dermatitis; ↑ IgE; mast-cell recruitment; ↓ FLG | Irritant risk at high dose | [36] |
| In vivo—UVB-induced skin burn (mouse) ‡ | Topical 3% copaiba cream; UVB 0.75 J·cm−2 | ↓ Mechanical allodynia; ↓ thermal hyperalgesia ↓ leukocyte infiltration; no change in dermal thickness; formulation stable ~2 mo | Mixture (copaiba); BCP not isolated | [35] |
| Study (Year) | Design/Population | Intervention | Comparator | Duration | Primary Outcomes | Main Outcomes | Limitations/Notes |
|---|---|---|---|---|---|---|---|
| Waibel et al., 2021 [135] | RCT; adults with abnormal scars | Silicone gel + copaiba oil | Placebo gel | 84 days | Manchester Scar Scale (MSS) | Significant improvement in MSS vs. placebo | Multicomponent gel; attribution to BCP not definitive |
| Cardinelli et al., 2024 [136] | 3-arm RCT; hospital patients with skin tears | Polymeric hydrogels (2% or 10% Copaifera multijuga oil) | Vehicle hydrogel | Until closure | Wound healing time, safety | All wounds healed; 2% hydrogel accelerated closure; no adverse events | Multicomponent oleoresin; no BCP-specific PK data |
| Leite et al., 2023 [137] | Case series; chronic wounds | Topical copaiba oleoresin | None | Variable | Wound healing | Progressive wound closure | Small, uncontrolled; multicomponent extract |
| da Silva et al., 2019 [138] | RCT; acne vulgaris patients | Copaifera langsdorffii essential oil (topical) | Placebo | Several weeks | Clinical acne severity | Clinical improvement vs. placebo | Multicomponent essential oil; no BCP isolation |
| Domain | Required Reporting Items | Typical Methods/Notes |
|---|---|---|
| Assay/ Degradation | Quantify BCP and BCPO; report peroxide value; perform forced-degradation and photostability studies with preset acceptance limits | GC–MS or GC–FID for BCP/oxide; ICH-style stress protocols |
| Volatility | Open-system mass-loss testing (~32 °C); headspace GC; include dose-to-skin mass balance | Specify test duration, ambient conditions, and occlusion status |
| Performance | Finite-dose IVRT/IVPT with defined dose/area, occlusion status, receptor phase (composition), skin source, and temperature | Use human or porcine skin; report membrane integrity checks |
| Skin Deposition | Depth-resolved tape-stripping or follicular biopsy; specify intact vs. compromised skin | Report number of strips/depth, anatomical site, and recovery efficiency |
| Packaging and Safety | Oxygen- and light-barrier (airless) packaging; in-use stability under repeated openings; sorption to container parts; TEWL/erythema; HRIPT (where appropriate) | Include container–closure compatibility data; state opening regimen and timepoints |
| Study (Year) | Study Type/Model | Delivery System/Formulation Type | Core Components/Vehicle | Main Outcomes | Limitations/Notes/Safety |
|---|---|---|---|---|---|
| Gushiken et al., 2022 [32] | In vivo rat excision-wound | Conventional emulgel (1% BCP) | Medium-chain triglyceride oil; propylene glycol; polymeric emulsifier | ↑ Wound closure; improved inflammatory and antioxidant markers | Simple semisolid system; oxidation monitoring not reported |
| Alharthi et al., 2023 [142] | Ex vivo permeation; In vivo anti-inflammatory/analgesic | Microemulsion hydrogel (1% BCP) | Isopropyl myristate; propylene glycol; non-ionic surfactants | ↑ Dermal permeation (~2×); ↑ retention; ↑ anti-inflammatory and analgesic effects | Direct enhancer evidence; no oxidative stability assessment |
| Weimer et al., 2022 [103] | Ex vivo permeation; in vivo antiedematogenic | Nanoemulgel | Medium-chain triglyceride oil; non-ionic surfactants; hydrogel base | ↑ Dermal permeation; ↑ anti-swelling effect; distinct BCP/BCPO behavior | PK–PD link shown; antioxidant controls not detailed |
| Ghazwani et al., 2023 [143] | In vitro; ex vivo dermatokinetics | Nanostructured lipid carriers (NLCs) | Solid-lipid matrix with BCP | Controlled release (24 h); ↑ retention (~1.9-fold vs. gel) | In vivo PD and HRIPT not yet performed; oxidation monitoring not reported |
| Amalraj et al., 2020 [144] | Physicochemical characterization; manufacturability/scale-up | Liposomal BCP (powder, re-dispersible) | Phospholipid vesicles | Improved BCP stability within liposomes; scalable manufacturing validated | Dermal PK/PD not established; efficacy data from oral use only |
| Tang et al., 2023 [145] | In vitro/ex vivo permeation; in vivo PDT | BCP as penetration enhancer (co-treatment) | Co-administered with 5-aminolevulinic acid in photodynamic therapy | ↑ Stratum corneum fluidization; ↑ adjunct active retention; ↑ PDT efficacy | Supports ‘BCP as co-enhancer’ concept; requires finite-dose and oxidation validation |
| Segment | Brand and Product (Region) | Declared Active(s)/BCP Role | Claims Posture and Regulatory Status * |
|---|---|---|---|
| Acne cleanser (rinse-off) | ISA BEAUTY, Petal’s Flower Acne Facial Wash | No U.S. OTC monograph active declared; BCP present in INCI (fragrance/terpene stack) | “Acne wash” cosmetic positioning; Cosmetic, regulated, not FDA-approved |
| Acne/rosacea serum | Hemptouch, Skin Perfection Azelaic Serum (EU) | Azelaic acid listed; BCP present as terpene component | Anti-acne/anti-redness; Cosmetic in EU (≤10% azelaic acid); not a U.S. prescription product |
| Rosacea serum | Rosacea Care, Willowherb Serum with Vitamin K | No monograph drug active; BCP listed as perfuming component | Redness/soothing for rosacea; Cosmetic, regulated, not FDA-approved |
| Scalp serum (dandruff/itch) | Australian Bodycare, Tea Tree Oil Scalp Serum (EU/UK) | No OTC drug active declared; BCP present with tea tree oil | Dandruff/itchy scalp positioning; Cosmetic, regulated, not FDA-approved |
| Natural acne/eczema serum | Myrto Natural Cosmetics, Copaiba Blemish Serum (EU) | Copaiba balsam (natural resin rich in BCP) | Acne, psoriasis, skin irritation; marketed as natural cosmetic, not drug |
| Soothing balm | BareFut, Beta-Caryophyllene Balm (US wellness market) | BCP listed as key active with coconut oil and beeswax | General soothing, relief of irritated/dry skin; Cosmetic, no FDA drug approval |
| Premium cleansing balm | Eve Lom, Cleansing Balm (EU/US) | Contains clove oil (Eugenia caryophyllus; BCP source) among essential oils | Skin cleansing, texture renewal; Cosmetic, regulated, not FDA-approved |
| Rosacea/anti-redness care | Rosacea Care, Serum with Willowherb and Vitamin K | BCP present as fragrance/terpene excipient | Anti-redness, soothing; Cosmetic positioning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagher, A.M. Topical β-Caryophyllene for Dermatologic Disorders: Mechanisms, Human Evidence, and Clinical Translation. Pharmaceuticals 2025, 18, 1605. https://doi.org/10.3390/ph18111605
Bagher AM. Topical β-Caryophyllene for Dermatologic Disorders: Mechanisms, Human Evidence, and Clinical Translation. Pharmaceuticals. 2025; 18(11):1605. https://doi.org/10.3390/ph18111605
Chicago/Turabian StyleBagher, Amina M. 2025. "Topical β-Caryophyllene for Dermatologic Disorders: Mechanisms, Human Evidence, and Clinical Translation" Pharmaceuticals 18, no. 11: 1605. https://doi.org/10.3390/ph18111605
APA StyleBagher, A. M. (2025). Topical β-Caryophyllene for Dermatologic Disorders: Mechanisms, Human Evidence, and Clinical Translation. Pharmaceuticals, 18(11), 1605. https://doi.org/10.3390/ph18111605