Progress of ERK Pathway-Modulated Natural Products for Anti-Non-Small-Cell Lung Cancer Activity
Abstract
1. Introduction
2. ERK Pathway-Modulated Natural Compounds with Anti-Non-Small-Cell Lung Cancer Activity
2.1. Flavonoids
2.1.1. Scutellarein
2.1.2. Scutellarin
2.1.3. Morusin
2.1.4. Oroxylin A
2.1.5. Trifolium Flavonoids
2.1.6. Luteolin
2.1.7. Chrysoeriol
2.2. Terpenoids
2.2.1. Sesquiterpenoids
Parthenolide
β-Elemene
2.2.2. Diterpenes
Oridonin
Kahweol
L-Pimaric Acid
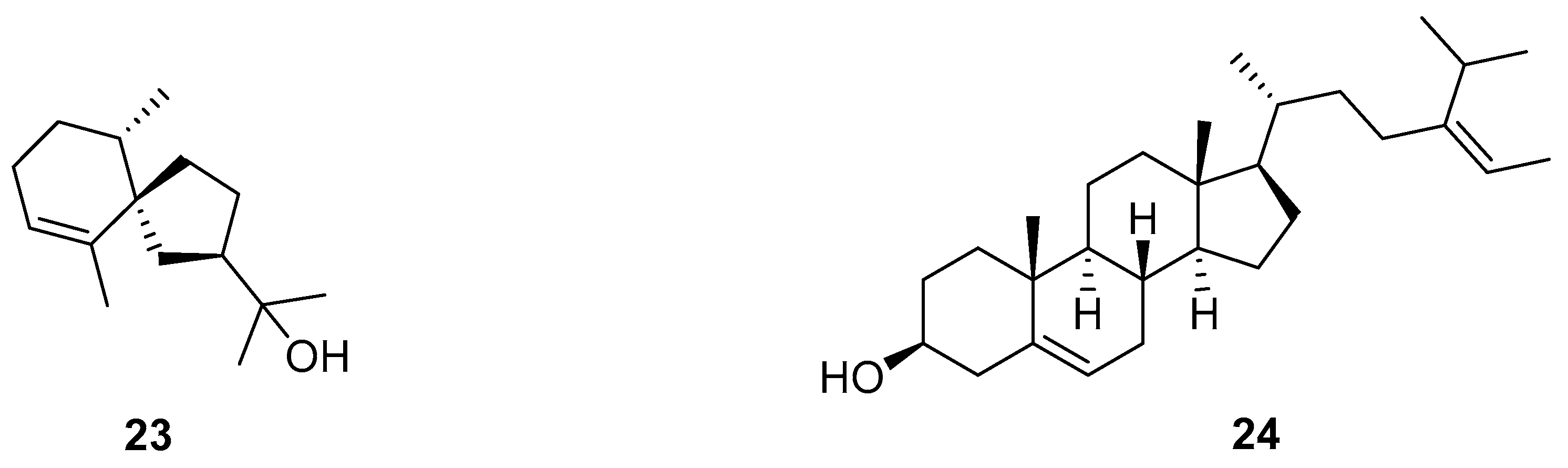
2.2.3. Triterpenes
Lupeol
Toosendanin
20(S)-Ginsenoside Rg3
| Natural Compound | Targeting Signaling Pathway Proteins | Treated Lung Cancer Cell Lines | IC50 Values (A549) | Ref |
|---|---|---|---|---|
| Parthenolide (7) | ↓ STAT3, B-Raf, c-Myc, p-MEK, p-ERK | GLC-82, A549, PC-9, H1650, H1299 | 6.07 ± 0.45 to 15.38 ± 1.13 μM | [22] |
| β-elemene (8) | ↑ Bax, PARP, p-Akt/ERK ↓ Bcl-2 | A549 | 50 µg/mL (0.24 µM) | [23] |
| Oridonin (9) | ↓ p-EGFR, p-ERK | A549, H1975 | 55.91 µM | [24] |
| Kahweol (10) | ↓ BTF3, ERK, inhibitors of MEK activation | NCI-H358, NCI-H1299 | No data | [25] |
| L-pimaric acid (11) | ↑ Bax, LC3 I, p62, p38 MAPK/JNK, ERK ↓ LC3 II, Bcl-2 | A549 | 12 μM | [26,27] |
| Lupeol (12) | ↓ pERK1/2, EMT | A549 | No data | [28,29] |
| Toosendanin (13) | ↑ ERK1/2, Snail ↓ TGF-β1 | A549, H1975 | No data | [30] |
| 20(S)-ginsenosideRg3 (14) | ↓ CDK2, CyclinA2, CyclinE1 | A549 | No data | [31] |
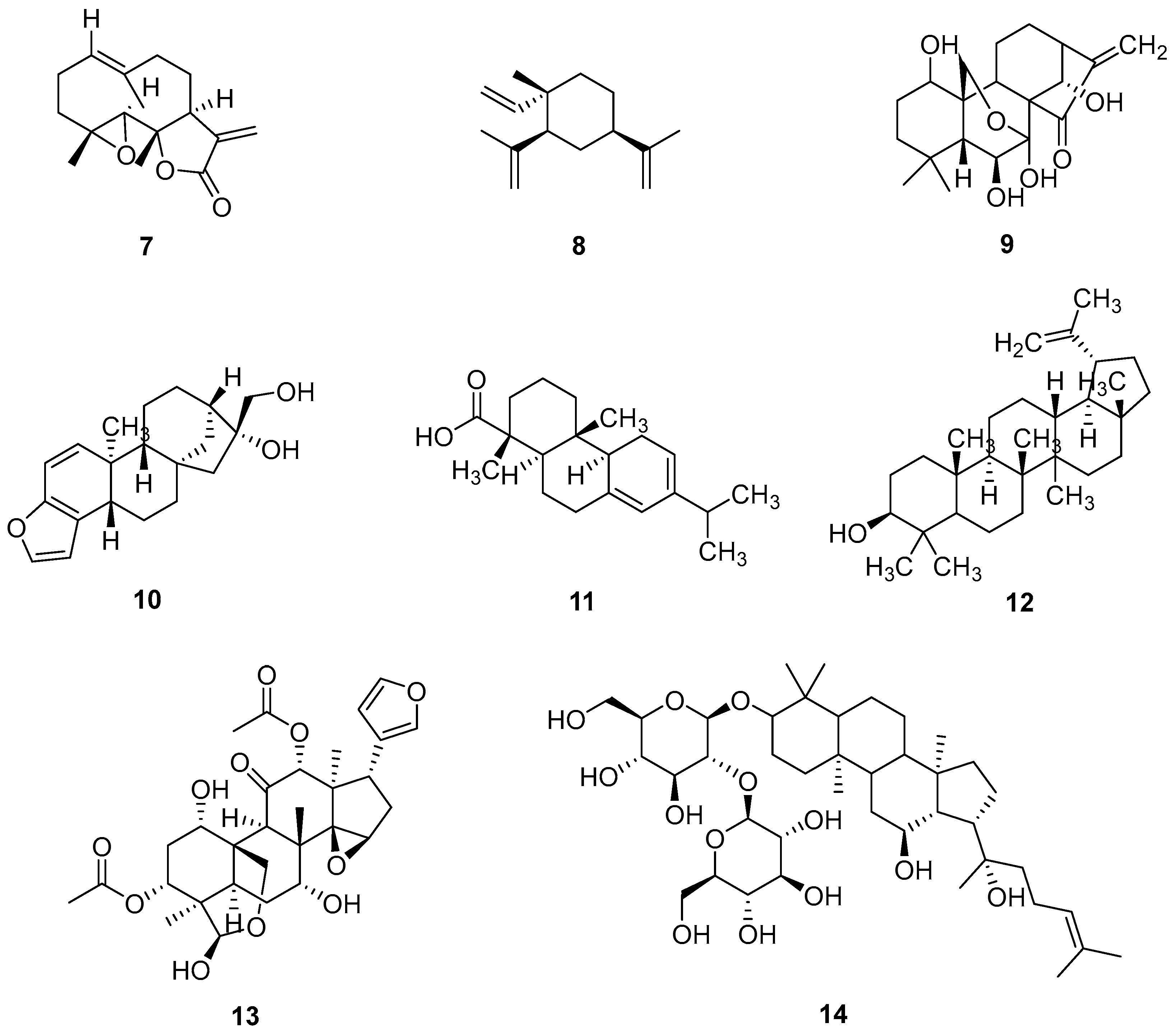
2.3. Glycosides
2.3.1. Cordycepin
2.3.2. Hydroxysafflor Yellow A
2.3.3. Periplocin
2.3.4. Salidroside
2.3.5. Echinacoside
2.3.6. Dioscin
2.3.7. Timosaponin AIII
2.3.8. Paris Saponin I
2.3.9. DT-13
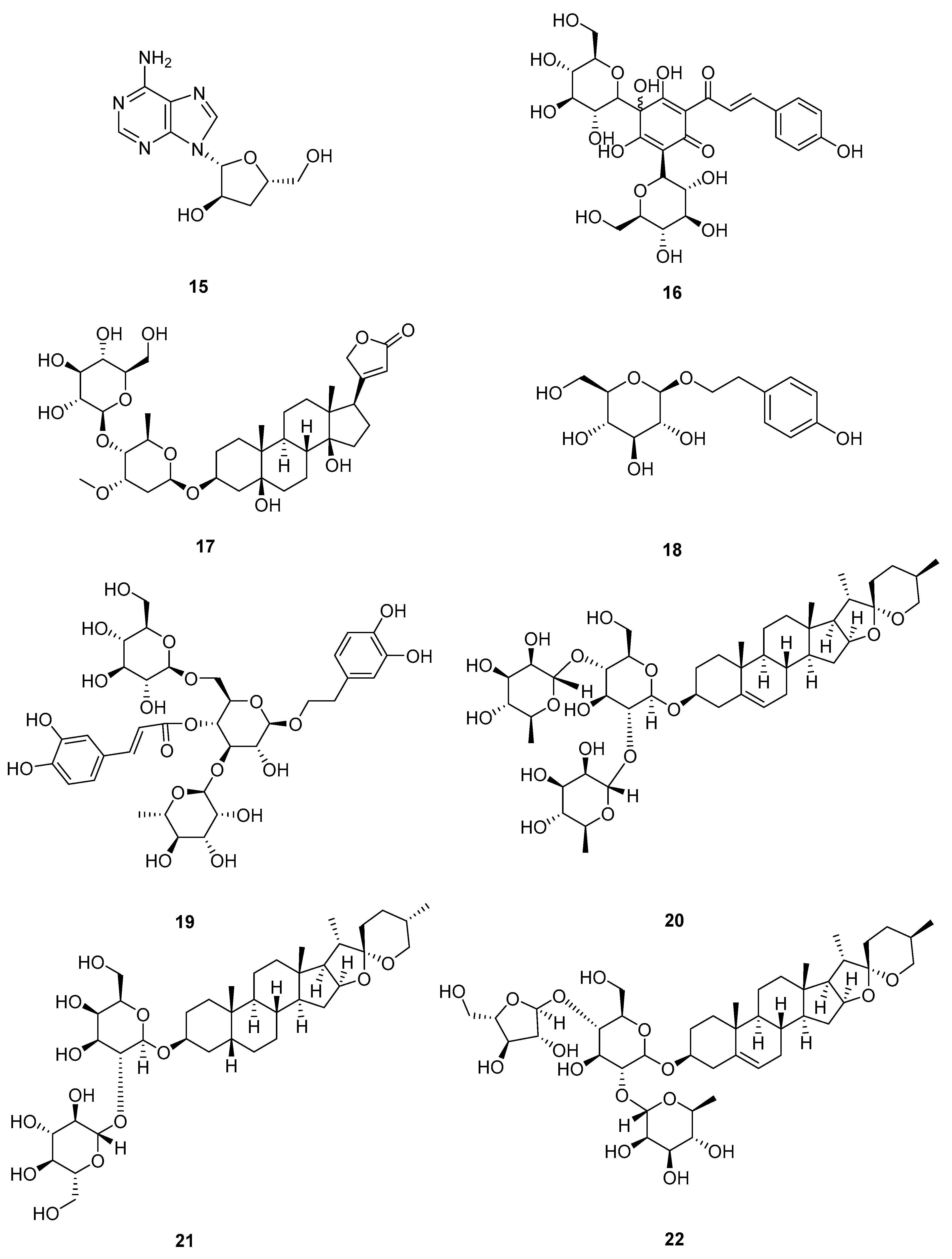
2.4. Alcohols
2.4.1. Hinesol
2.4.2. Fucosterol
2.5. Coumarins and Their Derivatives
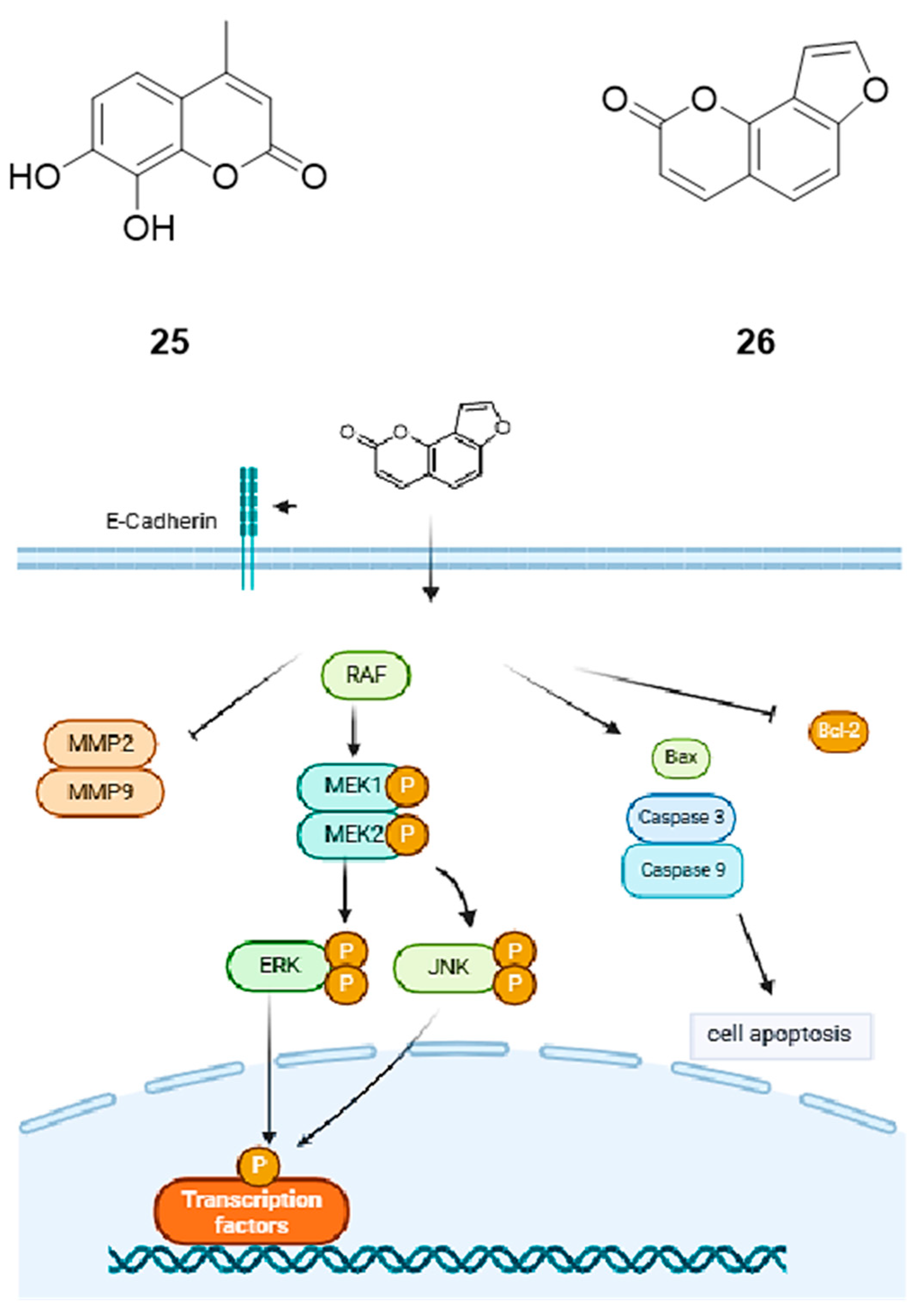
2.5.1. 7,8-Dihydroxy-4-methylcoumarin
2.5.2. Angelicin
2.5.3. Osthole Derivative NBM-T-BMX-OS01
| Natural Compound | Targeting Signaling Pathway Proteins | Treated Lung Cancer Cell Lines | IC50 Values (A549) | Ref |
|---|---|---|---|---|
| 7,8-dihydroxy-4-methylcoumarin (25) | ↑ caspase-9/-3 ↓ ERK/MAPK | A549, HeLa, Hep2, HepG2 | 160 µg/mL (0.83 µM) | [59,60,61,62,63,64,65] |
| Angelicin (26) | ↑ Bax/caspase-3/caspase-9, E-cadherin, p-ERK, p-JNK ↓ Bcl-2, MMP2, MMP9 | A549 | 50.14 µM | [66] |
2.6. Sugar/Monosaccharide
Mannose
2.7. Others
2.7.1. Ganoderan B
2.7.2. Cinnamomum Cassia Extracts
2.7.3. Atractylenolide-1
2.7.4. Solamargine
2.7.5. Thymoquinone
2.7.6. Sinomenine
2.7.7. Rosemary Extract
2.7.8. Maclurin
2.7.9. Marsdenia Tenacissima
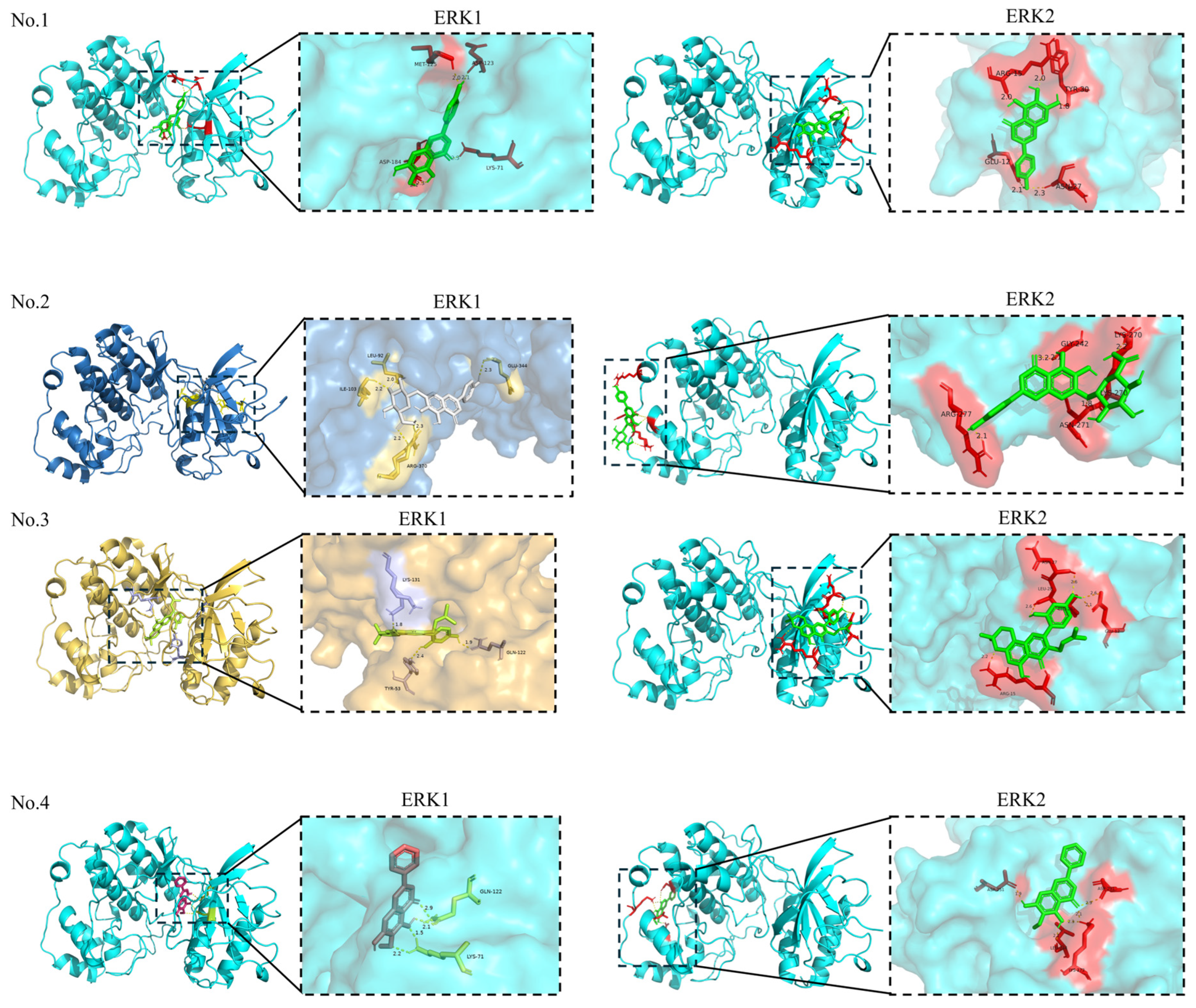
3. Molecular Docking Study Applied to the Discovery of Anti-Non-Small-Cell Lung Cancer
4. Conclusions and Perspective
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Hu, Y.; Zhang, J.; Wang, Q.; Wu, X.; Huang, W.; Wang, Q.; Cai, G.; Wang, H.; Ou, T.; et al. Wang, Therapeutic targeting RORγ with natural product N-hydroxyapiosporamide for small cell lung cancer by reprogramming neuroendocrine fate. Pharmacol. Res. 2022, 178, 106160. [Google Scholar] [CrossRef]
- Nasery, M.M.; Varzandeh, M.; Pahlavanneshan, S.; Mohamadi, N.; Sarhadi, S.; Fekri, H.S.; Mohammadinejad, R.; Ahn, K.S. Curcumin: A potential therapeutic natural product for adenocarcinomas. Phytochem. Lett. 2022, 49, 45–55. [Google Scholar] [CrossRef]
- Miri, M.R.; Zare, A.; Saberzadeh, J.; Baghban, N.; Nabipour, I.; Tamadon, A. Anti-lung Cancer Marine Compounds: A Review. Ther. Innov. Regul. Sci. 2022, 56, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 77, 1447–1457. [Google Scholar] [CrossRef]
- Bailly, C. Ready for a comeback of natural products in oncology. Biochem. Pharmacol. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Sun, X.; Xing, L.; Yuan, J.; Wang, E.; Ding, Y.; Sheng, R.; Wang, F.; Wu, W.; Yang, X.H.; Guo, R. Synthesis and biological evaluation of novel demethylzeylasteral derivatives as potential anticancer agents. Fitoterapia 2023, 167, 105504. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tian, D.; Huang, R.; Liu, X.; Luo, J.; Ma, X. Effects of cordycepin on the growth and migration of lung cancer cells. Mycosystema 2022, 41, 1088–1098. [Google Scholar]
- Kong, Y.; Shuangshuang, D.; Liang, X.; Zhou, X. RPS9 promotes the progression of NSCLC via activation Stat3 and Erk signaling pathways. J. Cancer 2022, 13, 1346–1355. [Google Scholar] [CrossRef]
- Xiang, M.; Jiang, H.; Shu, Y.; Chen, Y.; Jin, J.; Zhu, Y.; Li, M.; Wu, J.; Li, J. Bisdemethoxycurcumin enhances the sensitivity of non-small cell Lung cancer cells to Icotinib via dual induction of autophagy and apoptosis. Int. J. Biol. Sci. 2020, 16, 1536–1550. [Google Scholar] [CrossRef]
- Yu, X.; Luo, X.; Cao, Y. The mechanism of antitumor natural products targeting on MAPK signal pathway. Chem. Life 2010, 30, 871–876. [Google Scholar]
- Sankaranarayanan, R.; Kumar, D.R.; Patel, J.; Bhat, G.J. Do Aspirin and Flavonoids Prevent Cancer through a Common Mechanism Involving Hydroxybenzoic Acids?—The Metabolite Hypothesis. Molecules 2020, 25, 2243. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. [Google Scholar] [CrossRef]
- Wan, P.; Li, X.; Guo, S.; Zhao, X. Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway. Open Life Sci. 2024, 19, 20220977. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Hu, C.-C.; Yang, H.-J.; Lee, M.-C.; Kao, S.-E. Inhibitory Effects of Scutellarein on Proliferation of Human Lung Cancer A549 Cells through ERK and NFκB Mediated by the EGFR Pathway. Chin. J. Physiol. 2014, 57, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhu, Y.; Li, X.; Wang, X.; Tang, L.; Su, Z.; Li, C.; Zheng, G.; Feng, B. Scutellarin Increases Cisplatin-Induced Apoptosis and Autophagy to Overcome Cisplatin Resistance in Non-small Cell Lung Cancer via ERK/p53 and c-met/AKT Signaling Pathways. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zheng, H.; Liu, Q.; Zhang, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Morusin induces apoptosis and autophagy via JNK, ERK and PI3K/Akt signaling in human lung carcinoma cells. Chem. Biol. Interact. 2020, 331, 109279. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yao, Y.; Zhao, K.; Huang, Y.; Zhou, Y.; Zhao, L.; Guo, Q.; Lu, N. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells. Mol. Carcinog. 2016, 55, 2121–2134. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, B.; Yu, Z.; He, Q.; Hu, Z.; Zhou, S.; Chen, M.; Zhu, L.; Galani, V. Trifolium Flavonoids Overcome Gefitinib Resistance of Non-Small-Cell Lung Cancer Cell by Suppressing ERK and STAT3 Signaling Pathways. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef]
- Wei, W.; He, J.; Ruan, H.; Wang, Y. In vitro and in vivo cytotoxic effects of chrysoeriol in human lung carcinoma are facilitated through activation of autophagy, sub-G1 cell cycle arrest, cell migration and invasion inhibition and modulation of MAPK/ERK signaling pathway. J. BUON 2019, 24, 936–942. [Google Scholar]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J.; et al. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436–23447. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Qu, X.; Zhao, M.; Yu, P.; Kang, J.; Liu, Y.; Hu, X. Cbl-regulated Akt and ERK signals are involved in β-elemene-induced cell apoptosis in lung cancer cells. Mol. Med. Rep. 2011, 4, 1243–1246. [Google Scholar] [CrossRef]
- Xiao, X.; He, Z.; Cao, W.; Cai, F.; Zhang, L.; Huang, Q.; Fan, C.; Duan, C.; Wang, X.; Wang, J.; et al. Oridonin inhibits gefitinib-resistant lung cancer cells by suppressing EGFR/ERK/MMP-12 and CIP2A/Akt signaling pathways. Int. J. Oncol. 2016, 48, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Bang, W.; Cho, J.H.; Lee, R.H.; Kim, S.; Kim, M.S.; Park, S.; Shin, J.; Chung, H.; Oh, K.B.; et al. Kahweol induces apoptosis by suppressing BTF3 expression through the ERK signaling pathway in non-small cell lung cancer cells. Int. J. Oncol. 2016, 49, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, J. In vitro anticancer effects of levopimaric acid in cisplatin resistant human lung carcinoma are mediated via autophagy, ROS-mediated mitochondrial dysfunction, cell apoptosis and modulation of ERK/MAPK/JNK signalling pathway. J. BUON 2020, 25, 248–254. [Google Scholar]
- Kersten, P.J.; Kopper, B.J.; Raffa, K.F.; Illman, B.L. Rapid Analysis of Abietanes in Conifers. J. Chem. Ecol. 2006, 32, 2679–2685. [Google Scholar] [CrossRef]
- Bhatt, M.; Patel, M.; Adnan, M.; Reddy, M.N. Anti-Metastatic Effects of Lupeol via the Inhibition of MAPK/ERK Pathway in Lung Cancer. Anti-Cancer Agents Med. Chem. 2020, 21, 201–206. [Google Scholar] [CrossRef]
- Tsai, F.; Lin, L.; Wu, C. Lupeol and Its Role in Chronic Diseases. Drug Discov. Mother Nat. 2016, 7, 145–175. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Sun, W.; Lu, J.J.; Wang, Y.; Chen, X. Toosendanin; a natural product, inhibited TGF-β1-induced epithelial-mesenchymal transition through ERK/Snail pathway. Phytother. Res. 2018, 32, 2009–2020. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, T.; Jing, S.; Zuo, P.; Li, T.; Wang, Y.; Xing, S.; Zhang, J.; Wei, Z. 20(S)-Ginsenoside Rg3 Inhibits Lung Cancer Cell Proliferation by Targeting EGFR-Mediated Ras/Raf/MEK/ERK Pathway. Am. J. Chin. Med. 2021, 49, 753–765. [Google Scholar] [CrossRef]
- Cho, S.H.; Kang, I. The inhibitory effect of Cordycepin on the proliferation of cisplatin-resistant A549 lung cancer cells. Biochem. Biophys. Res. Commun. 2018, 498, 431–436. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, L.Y.; Xu, N.; An, Q. Hydroxysafflor yellow A inhibited lipopolysaccharide induced non-small cell lung cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT/mTOR and ERK/MAPK signaling pathways. Thorac. Cancer 2019, 10, 1319–1333. [Google Scholar] [CrossRef]
- Xu, R.; Xu, Z.; Ge, R. Effects of hydroxysafflor yellow A on the activity and mRNA expression of four CYP isozymes in rats. J. Ethnopharmacol. 2014, 151, 1141–1146. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Y.; Song, Q.; Qin, Z.; Zhang, H.; Zhou, Y.; Gou, L.; Yang, J.; Luo, F. Periplocin Inhibits Growth of Lung Cancer in vitro and in vivo by Blocking AKT/ERK Signaling Pathways. Cell. Physiol. Biochem. 2010, 26, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.I.; Kwon, J.H.; Park, K.H.; Oh, M.H.; Kim, M.H.; Kim, H.H.; Cho, S.H.; Chung, E.K.; Ha, S.Y.; Lee, M.W. The Antioxidant and Anti-inflammatory Effects of Phenolic Compounds Isolated from the Root of Rhodiola sachalinensis A. BOR. Molecules 2012, 17, 11484–11494. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Li, F.; Liang, D.; Liu, Z.; Zhang, X.; et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-jB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm. Res. 2012, 62, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Perfumi, M.; Mattioli, L. Adaptogenic and Central Nervous System Effects of Single Doses of 3% Rosavin and 1% Salidroside Rhodiola rosea L. Extract in Mice. Phytother. Res. 2006, 21, 37–43. [Google Scholar] [CrossRef]
- Qian, E.W.; Ge, D.T.; Kong, S. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J. Ethnopharmacol. 2011, 133, 308–314. [Google Scholar] [CrossRef]
- Ren, M.; Xu, W.; Xu, T. Salidroside represses proliferation, migration and invasion of human lung cancer cells through AKT and MEK/ERK signal pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1014–1021. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhou, J.; Sun, X.; Wang, S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine 2009, 16, 146–155. [Google Scholar] [CrossRef]
- Xu, M.; Shi, H.; Wang, H.; Gao, X. Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation. Mol. Med. Rep. 2013, 8, 147–153. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, H.; Liu, Z.; Xi, L.; Dong, C.; Wang, F. Echinacoside Induces Mitochondria-Mediated Pyroptosis through Raf/MEK/ERK Signaling in Non-Small Cell Lung Cancer Cells. J. Immunol. Res. 2022, 2022, 3351268. [Google Scholar] [CrossRef]
- Wang, Y.; Che, C.; Chiu, J.; He, Q. Dioscin (Saponin)-Induced Generation of Reactive Oxygen Species through Mitochondria Dysfunction: A Proteomic-Based Study. J. Proteome Res. 2007, 6, 4703–4710. [Google Scholar] [CrossRef]
- Sautour, M.; Mitaine, A.; Miyamoto, T.; Dongmo, A.; Lacaille, M. A New Steroidal Saponin from Dioscorea cayenensis. Chem. Pharm. Bull. 2004, 52, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Tsai, T.; Hsieh, Y.; Wang, C.; Chiou, H. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch. Toxicol. 2013, 87, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Kaskiw, M.J.; Tassotto, M.L.; Mok, M.; Tokar, S.L.; Pycko, R.; Th’ng, J.; Jiang, Z.-H. Structural analogues of diosgenyl saponins: Synthesis and anticancer activity. Bioorg. Med. Chem. 2009, 17, 7670–7679. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yin, L.; Xu, L.; Peng, J. Application of Proteomic and Bioinformatic Techniques for Studying the Hepatoprotective Effect of Dioscin against CCl4-induced Liver Damage in Mice. Planta Medica 2010, 77, 407–415. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Li, J.; Zhong, M.; Li, J.; Chen, X.; Bi, K. Determination of protodioscin in rat plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2007, 848, 363–368. [Google Scholar] [CrossRef]
- Jung, O.; Lee, J.; Lee, Y.; Yun, J.; Son, Y.; Cho, J.; Ryou, C.; Lee, S. Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and b-catenin signaling pathways. Bioorg. Med. Chem. Lett. 2016, 26, 3963–3967. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Q.; Chen, W.; Wu, M.; Pan, G.; Yang, K.; Li, X.; Man, S.; Teng, Y.; Yu, P.; et al. Chemosensitizing effect of Paris Saponin I on Camptothecin and 10 hydroxycamptothecin in lung cancer cells via p38 MAPK, ERK, and Akt signaling pathways. Eur. J. Med. Chem. 2017, 125, 760–769. [Google Scholar] [CrossRef]
- Kou, J.; Sun, Y.; Lin, Y.; Cheng, Z.; Zheng, W.; Yu, B.; Xu, Q. Anti-inflammatory Activities of Aqueous Extract from Radix Ophiopogon japonicus and Its Two Constituents. Biol. Pharm. Bull. 2005, 28, 1234–1238. [Google Scholar] [CrossRef]
- Kou, J.; Tian, Y.; Tang, Y.; Yan, J.; Yu, B. Antithrombotic Activities of Aqueous Extract from Radix Ophiopogon japonicus and Its Two Constituents. Biol. Pharm. Bull. 2006, 29, 1267–1270. [Google Scholar] [CrossRef]
- Sun, L.; Lin, S.; Zhao, R.; Yu, B.; Yuan, S.; Zhang, L. The Saponin Monomer of Dwarf Lilyturf Tuber, DT-13, Reduces Human Breast Cancer Cell Adhesion and Migration during Hypoxia via Regulation of Tissue Factor. Biol. Pharm. Bull. 2010, 33, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, L.; Li, H.; Lv, X.; Semukunzi, H.; Li, R.; Yu, J.; Yuan, S.; Lin, S. DT-13, a saponin monomer 13 of the Dwarf lilyturf tuber, synergized with vinorelbine to induce mitotic arrest via activation of ERK signaling pathway in NCI-H1299 cells. Biomed. Pharmacother. 2017, 89, 1277–1285. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Kou, J.; Yu, J.; Yu, B. DT-13 suppresses MDA-MB-435 cell adhesion and invasion by inhibiting MMP-2/9 via the p38 MAPK pathway. Mol. Med. Rep. 2012, 6, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Ju, X.; Du, J.; Xu, B.; Yuan, H.; Qin, F.; Li, L. Theantitumoreffect of hinesol, extract from Atractylodes lancea (Thunb.) DC. byproliferation, inhibition, and apoptosis induction via MEK/ERKandNF-κBpathwayin non–small cell lung cancer cell lines A549 and NCI-H1299. J. Cell. Biochem. 2019, 120, 18600–18607. [Google Scholar] [CrossRef]
- Mao, Z.; Shen, X.; Dong, P.; Liu, G.; Pan, S.; Sun, X.; Hu, H.; Pan, L.; Huang, J. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cell cycle arrest and targeting of Raf/MEK/ERK signalling pathway. Phytomedicine 2019, 61, 152809. [Google Scholar] [CrossRef]
- Hoult, J.R.S.; Payá, M. Pharmacological and Biochemical Actions of Simple Coumarins: Natural Products with Therapeutic Potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Laurin, P.; Ferroud, D.; Schio, L.; Klich, M.; Dupuis-Hamelin, C.; Mauvais, P.; Lassaigne, P.; Bonnefoy, A.; Musicki, B. Structure-activity Relationship in two series of Aminoalkyl Substituted Coumarin Inhibitors of Gyrase B. Bioorg. Med. Chem. Lett. 1999, 9, 2875–2880. [Google Scholar] [CrossRef]
- Okamoto, T.; Kobayashi, T.; Yoshida, S. Chemical Aspects of Coumarin Compounds for the Prevention of Hepatocellular Carcinomas. Anti-Cancer Agents Med. Chem. 2005, 5, 47–51. [Google Scholar] [CrossRef]
- Elinos-Báez, C.M.; León, F.; Santos, E. Effects of coumarin and 7OH-coumarin on bcl-2 and Bax expression in two human lung cancer cell lines in vitro. Cell Biol. Int. 2005, 29, 703–708. [Google Scholar] [CrossRef]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. 7,8-Dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007, 581, 2447–2454. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 2004, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Zhu, H.; Liu, W.; Zhang, P.; Yu, S.; Gao, Q.; Shen, G.; Li, B.; Wang, G. Scoparone suppresses proliferation and cell cycle of hepatocellular carcinoma cells via inhibiting AKT/GSK-3β/cyclin D1 signaling pathway. Transl. Cancer Res. 2025, 14, 1638–1650. [Google Scholar] [CrossRef]
- Li, G.; He, Y.; Yao, J.; Huang, C.; Song, X.; Deng, Y.; Xie, S.; Ren, J.; Jin, M.; Liu, H. Angelicin inhibits human lung carcinoma A549 cell growth and migration through regulating JNK and ERK pathways. Oncol. Rep. 2016, 36, 3504–3512. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Y.; Ning, J.; Yang, T.; Ren, H.; Li, Y.; Zhang, S.; Chen, M. NBM-T-BMX-OS01, an Osthole Derivative, Sensitizes Human Lung Cancer A549 Cells to Cisplatin through AMPK-Dependent Inhibition of ERK and Akt Pathway. Cell. Physiol. Biochem. 2015, 36, 893–906. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; He, B. Mannose shows antitumour properties against lung cancer via inhibiting proliferation, promoting cisplatin mediated apoptosis and reducing metastasis. Mol. Med. Rep. 2020, 22, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gou, X.; Xue, H.; Liu, K.; Growth, R.T. Motility and Apoptosis of Non-Small Cell Lung Cancer Cells Through ERK Signaling Pathway in Vitro and in Vivo. OncoTargets Ther. 2019, 12, 8821–8832. [Google Scholar] [CrossRef]
- Chang, W.L.; Cheng, F.C.; Wang, S.P.; Chou, S.T.; Shih, Y. Cinnamomum cassia Essential Oil and Its Major Constituent Cinnamaldehyde Induced Cell Cycle Arrest and Apoptosis in Human Oral Squamous Cell Carcinoma HSC-3 Cells. Environ. Toxicol. 2016, 32, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wu, S.J.; Chang, C.H.; Ng, L.T. Antioxidant Activity of Cinnamomum cassia. Phytother. Res. 2003, 17, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Zaidi, N.; Amraiz, D.; Afzal, F. In Vitro Antiviral Activity of Cinnamomum cassia and Its Nanoparticles Against H7N3 Influenza A Virus. J. Microbiol. Biotechnol. 2016, 26, 151–159. [Google Scholar] [CrossRef]
- Wu, H.; Horng, C.; Lee, Y.; Chen, P.; Lin, C.; Liao, C.; Hsieh, Y.; Chu, S. Cinnamomum cassia Extracts Suppress Human Lung Cancer Cells Invasion by Reducing u-PA/MMP Expression through the FAK to ERK Pathways. Int. J. Med. Sci. 2018, 15, 115–123. [Google Scholar] [CrossRef]
- Huang, H.L.; Lin, T.W.; Huang, Y.L.; Huang, R.L. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 1905–1909. [Google Scholar] [CrossRef]
- Ji, G.; Chen, R.; Zheng, J. Atractylenolide I inhibits lipopolysaccharide-induced inflammatory responses via mitogen-activated protein kinase pathways in RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2014, 36, 420–425. [Google Scholar] [CrossRef]
- Li, W.; Zhi, W.; Liu, F.; He, Z.; Wang, X.; Niu, X. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp. Cell Res. 2017, 353, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Y.; Zhang, T.; Zhao, Z.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X. Anti-Tumor Effects of Atractylenolide I Isolated from Atractylodes macrocephala in Human Lung Carcinoma Cell Lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zheng, F.; Wu, J.; Tang, Q.; Wang, W.; Hann, S.S. Activation of ERK and Mutual Regulation of Stat3 and SP1 Contribute to Inhibition of PDK1 Expression by Atractylenolide-1 in Human Lung Cancer Cells. Cell. Physiol. Biochem. 2017, 43, 2353–2366. [Google Scholar] [CrossRef]
- Yu, R.; Yu, B.X.; Chen, J.F.; Lv, X.Y.; Yan, Z.J.; Cheng, Y.; Ma, Q. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 40. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Q.; Xiao, Q.; Yang, L.; Hann, S.S. Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine inhibited growth of lung cancer cells. J. Cell. Mol. Med. 2016, 21, 222–233. [Google Scholar] [CrossRef]
- Chou, S.C.; Huang, T.J.; Lin, E.H.; Huang, C.H.; Chou, C.H. Antihepatitis B Virus Constituents of Solanum erianthum. Nat. Prod. Commun. 2012, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.Z.; Moreira, R.D.; Planeta, C.; Almeida, A.; Bastos, J.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.D.C.; Martins, G. Effects of the extract and glycoalkaloids of Solanum lycocarpum St. Hill on Giardia lamblia trophozoites. Pharmacogn. Mag. 2015, 11, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.C.; de Oliveira, P.F.; Campos, J.C.L.; Martins, S.D.P.L.; Da Costa, J.C.; Bastos, J.K.; Tavares, D.C. Antiproliferative activity of Solanum lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J. Nat. Med. 2013, 68, 236–241. [Google Scholar] [CrossRef]
- Sani, I.K.; Marashi, S.H.; Kalalinia, F. Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. Toxicol. Vitr. 2015, 29, 893–900. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, Q.; Zhao, S.; Zhang, F.; Li, L.; Wu, W.; Wang, Z.; Hann, S. Targeting signal transducer and activator of transcription 3 contributes to the solamargine-inhibited growth and-induced apoptosis of human lung cancer cells. Tumor Biol. 2014, 35, 8169–8178. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine 2004, 11, 56–64. [Google Scholar] [CrossRef]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2014, 36, 259–269. [Google Scholar] [CrossRef]
- Vieregge, B.; Resch, K.; Kaeve, V. Synergistic Effects of the Alkaloid Sinomenine in Combination with the Immunosuppressive Drugs Tacrolimus and Mycophenolic Acid. Planta Medica 1999, 65, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, L.; Qi, C.; Deng, B.; Cai, X. Sinomenine Versus NSAIDs for the Treatment of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Planta Medica 2008, 74, 1423–1429. [Google Scholar] [CrossRef]
- Zhou, L.; Luan, H.; Liu, Q.; Jiang, T.; Liang, H.; Dong, X.; Shang, H. Activation of PI3K/Akt and ERK signaling pathways antagonized sinomenine-induced lung cancer cell apoptosis. Mol. Med. Rep. 2012, 5, 1256–1260. [Google Scholar] [CrossRef]
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERKandAMPK. Life 2021, 12, 52. [Google Scholar] [CrossRef]
- Ku, M.J.; Kim, J.H.; Lee, J.; Cho, J.Y.; Chun, T.; Lee, S.Y. Maclurin suppresses migration and invasion of human non-small cell lung cancer cells via anti-oxidative activity and inhibition of the Src/FAK–ERK–b-catenin pathway. Mol. Cell. Biochem. 2015, 402, 243–252. [Google Scholar] [CrossRef]
- Jiao, Y.N.; Wu, L.N.; Xue, D.; Liu, X.J.; Tian, Z.H.; Jiang, S.T.; Han, S.Y.; Li, P.P. Marsdenia tenacissima extract induces apoptosis and suppresses autophagy through ERK activation in lung cancer cells. Cancer Cell Int. 2018, 18, 149. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, S.; Wan, Z.; Dong, H.; Liu, L.; Fan, Z.; Li, T.; Peng, F.; Xiong, Y.; Han, Y. TCF19 promotes cell proliferation and tumor formation in lung cancer by activating the Raf/MEK/ERK signaling pathway. Transl. Oncol. 2024, 45, 101978. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.T.; Sabnis, A.J.; Williams, P.M.; Chowdhuri, S.R.; Patton, D.R.; Coffey, B.; Reid, J.M.; Piao, J.; Saguilig, L.; Alonzo, T.A.; et al. Phase II study of Ulixertinib in children and young adults with tumors harboring activating mitogen-activated protein kinase pathway alterations: APEC1621J of the national cancer institute-children’s oncology group pediatric MATCH trial. JCO Precis. Oncol. 2024, 8, e2400103. [Google Scholar] [CrossRef] [PubMed]
- Chaikuad, A.; Tacconi, E.M.C.; Zimmer, J.; Liang, Y.; Gray, N.S.; Tarsounas, M.; Knapp, S. A unique inhibitor binding site in ERK1/2 is associated with slow binding kinetics. Nat. Chem. Biol. 2014, 10, 853–860. [Google Scholar] [CrossRef]
- Denis, J.D.S.; Chessari, G.; Cleasby, A.; Cons, B.D.; Cowan, S.; Dalton, S.E.; East, C.; Murray, C.W.; O’Reilly, M.; Peakman, T.; et al. X-ray screening of an electrophilic fragment library and application toward the development of a novel ERK 1/2 covalent inhibitor. J. Med. Chem. 2022, 65, 12319–12333. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, Q.; Chen, J.; He, J.; Yuan, A.; Cao, P.; Zhang, L. Exploring the mechanisms of artemisinin and its derivatives in the treatment of atopic dermatitis based on network pharmacology and molecular docking. Medicine 2025, 104, e42287. [Google Scholar] [CrossRef]
- Wu, X.R.; Wang, L.Z.; Gao, Y.L.; Yang, M.; Liu, Y.H.; Xu, X.Y.; Liu, K. Bioassay Screening and Binding Mode Study of Inhibitors Targeting at ERK2. J. Guangxi Acad. Sci. 2024, 40, 189–196. (In Chinese) [Google Scholar]
- Zhou, Y.; Xiang, S.; Yang, F.; Lu, X.Y. Targeting gatekeeper mutations for kinase drug discovery. J. Med. Chem. 2022, 65, 15540–15558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Jang, H.; Nussinov, R. Strategy toward kinase-selective drug discovery. J. Chem. Theory Comput. 2023, 19, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
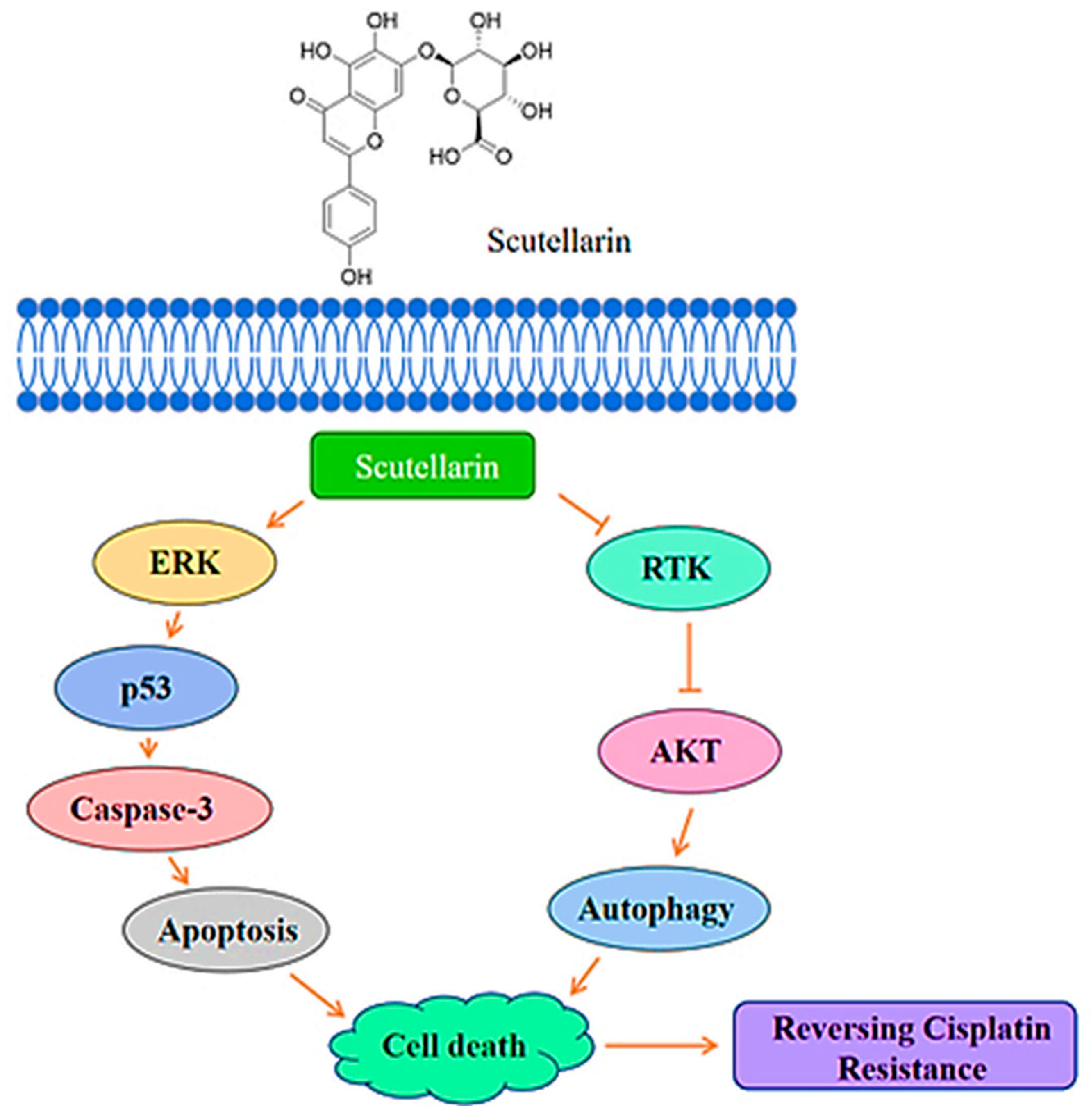

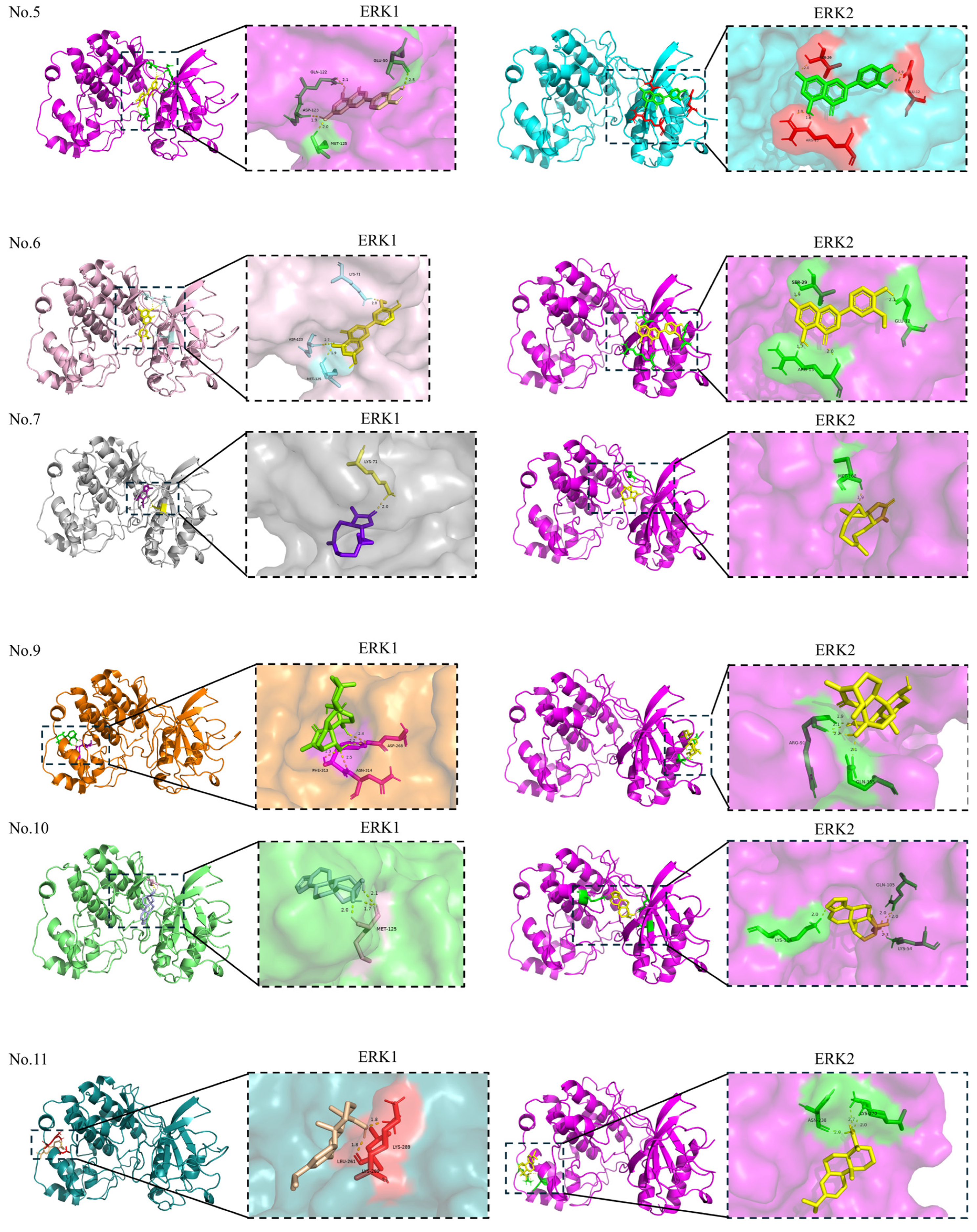
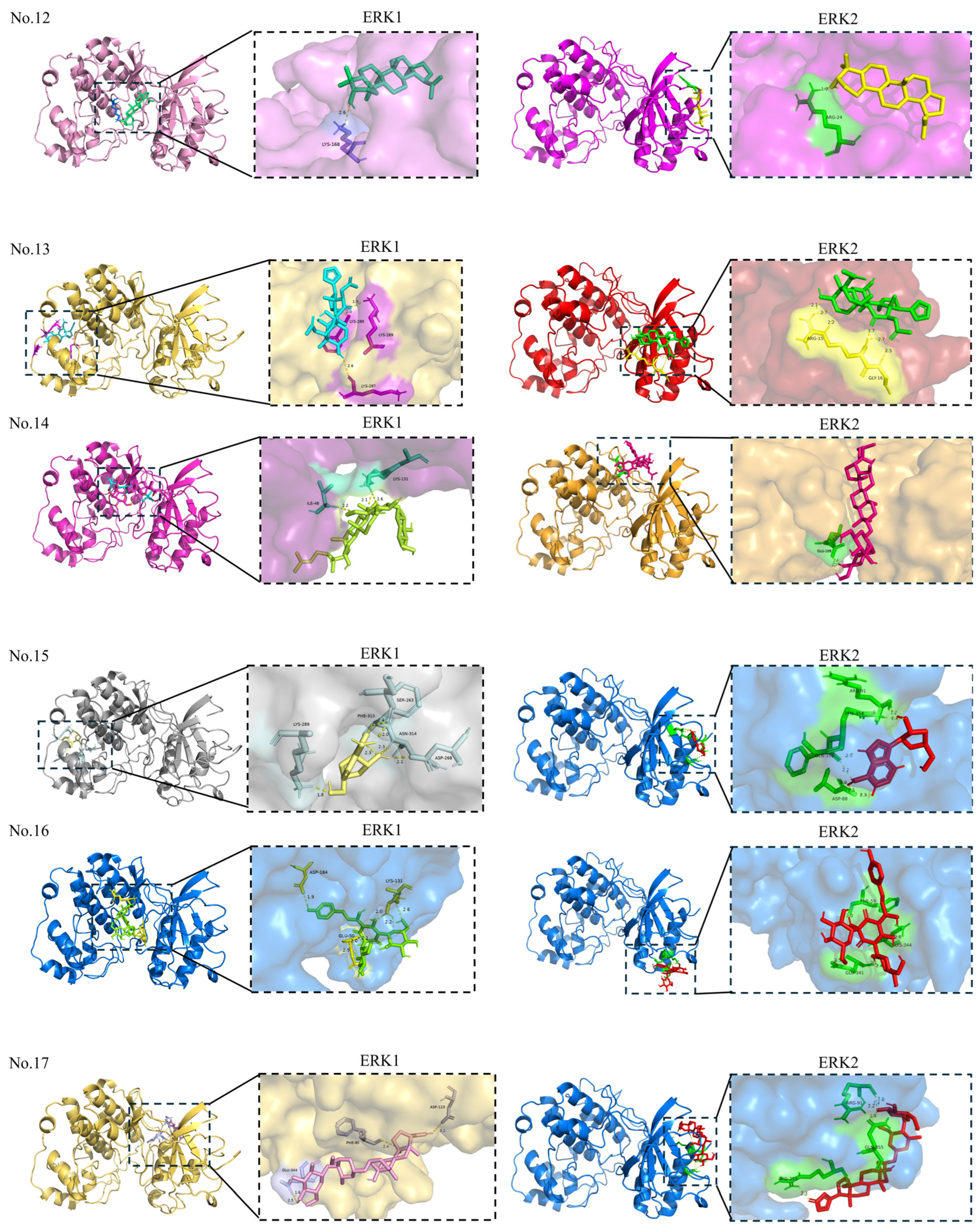
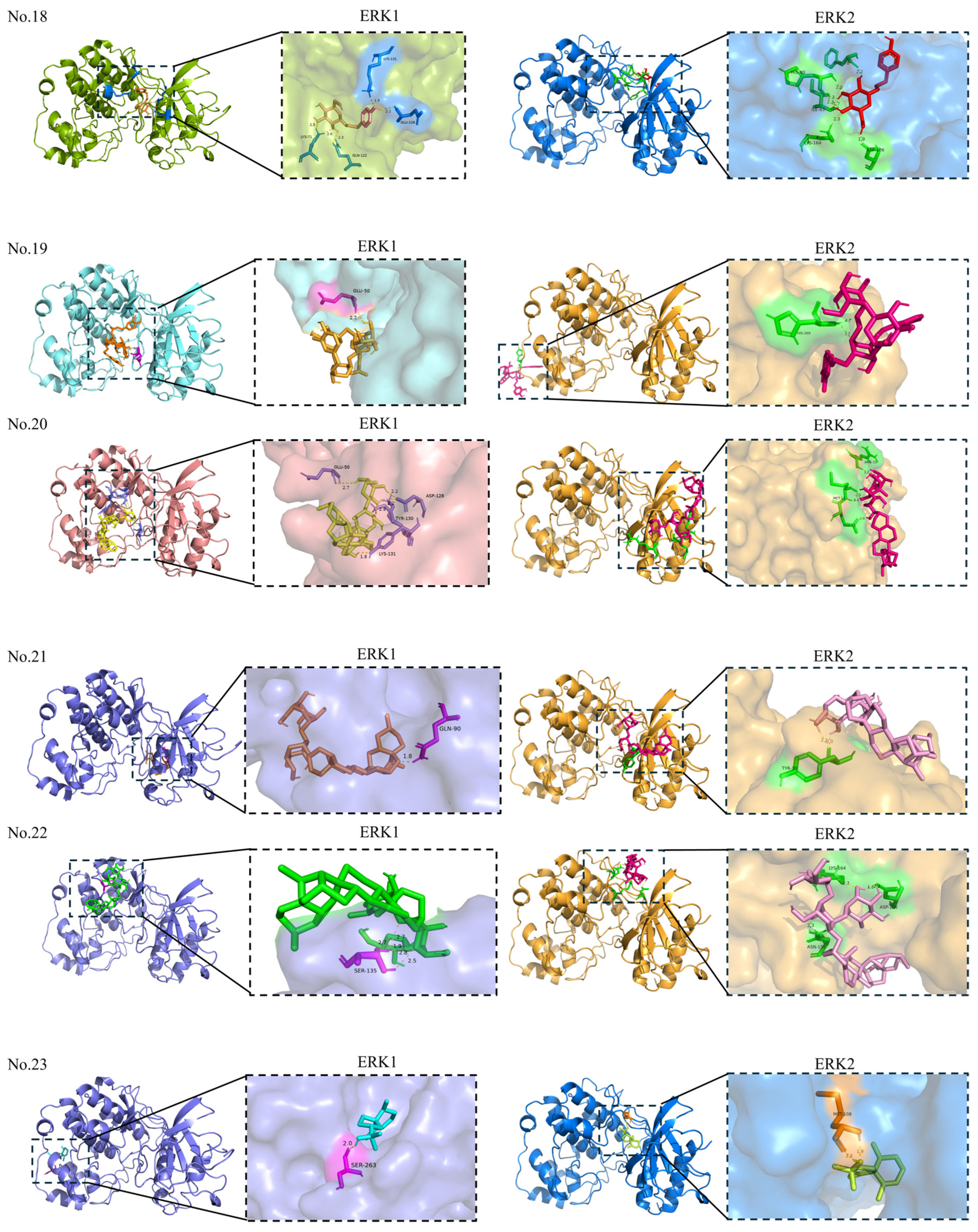
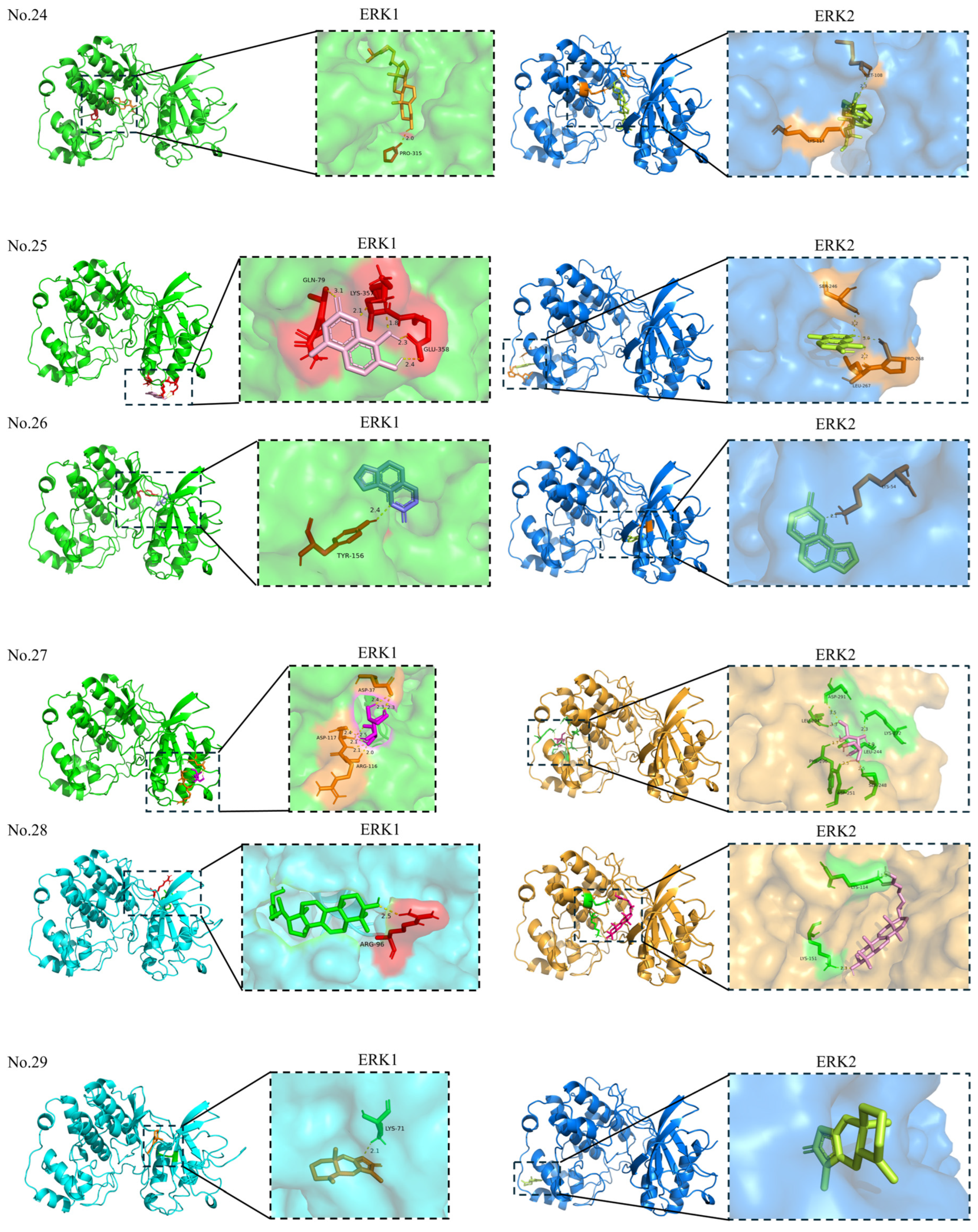
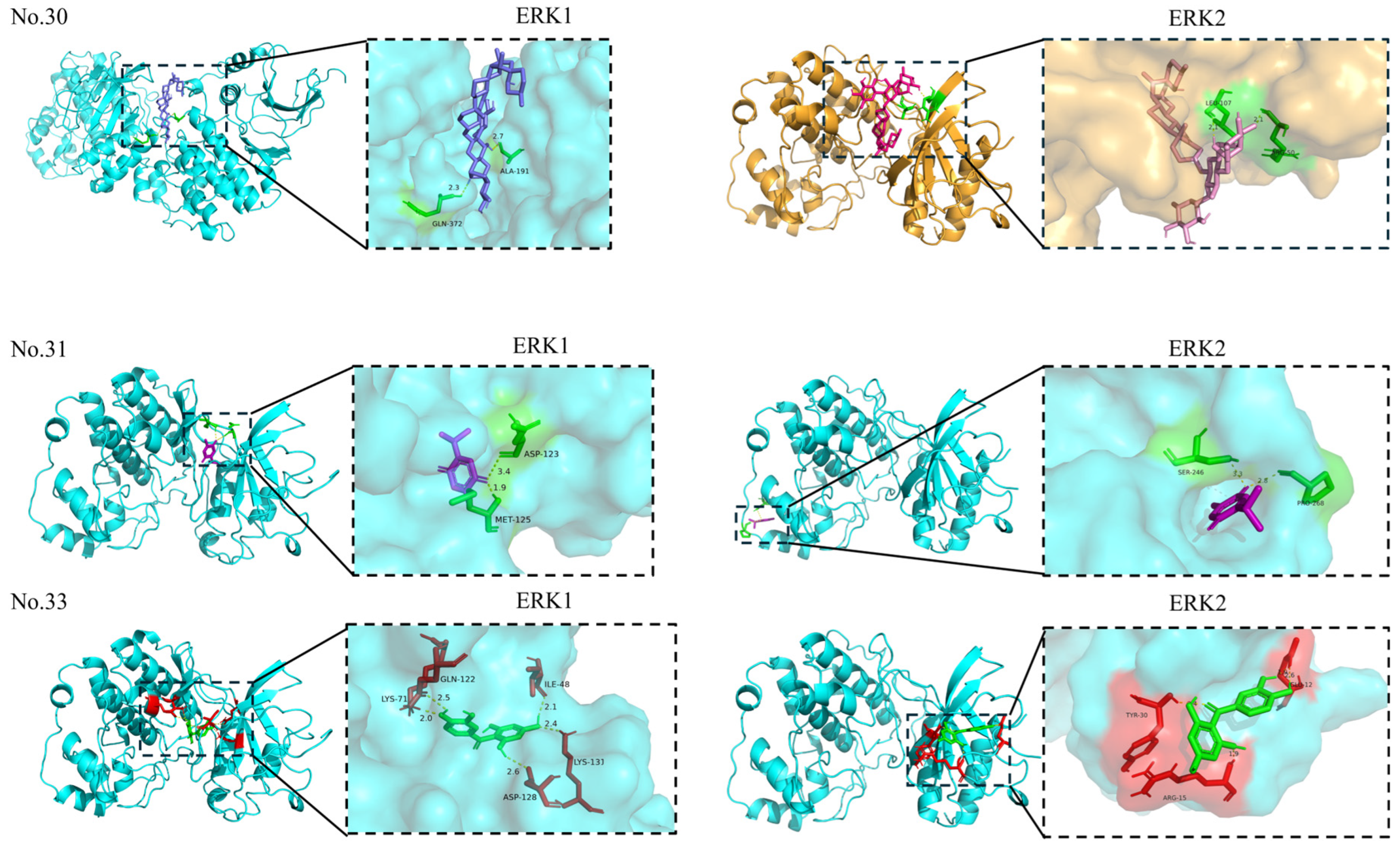
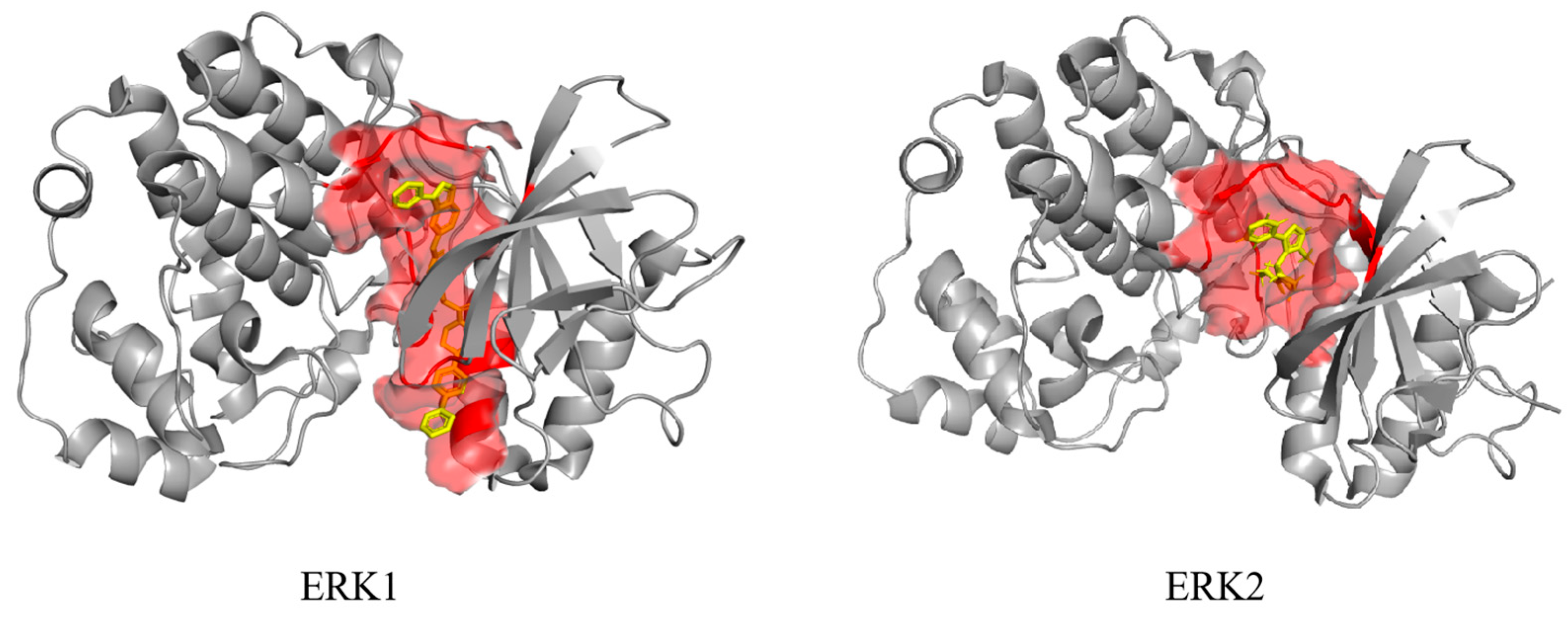
| Diseases | Mechanisms and Pathways | In Vitro Experiment | In Vivo Experiment | Ref |
|---|---|---|---|---|
| Colorectal cancer | Akt/mTOR pathway, NF-κB pathway, Wnt signaling pathway, AMPK | HCT-116, HT-29 cells | Rat model. The absorption of flavonoids is 1–15% in the intestine. | [11] |
| Breast cancer | DNA methyltransferases (DNMTs), Histone acetyltransferases (HATs), Histone deacetylases (HDACs), Histone methyltransferases (HMTs), Histone demethylases (HDMs) | MCF-7, MDA-MB-157, MDA-MB-231 cells | Genistein did not show significant cancer-reducing activity in animal models | [12] |
| Prostate cancer | Fyn or Src proteins, Ras/Raf/MEK/MAPK pathways | PC3, LNCaP cells | — | [13] |
| Lung cancer | STAT3 and FAK signaling pathways | A549 cells | — | [14] |
| Natural Compound | Targeting Signaling Pathway Proteins | Treated Lung Cancer Cell Lines | IC50 Values (A549) | Ref |
|---|---|---|---|---|
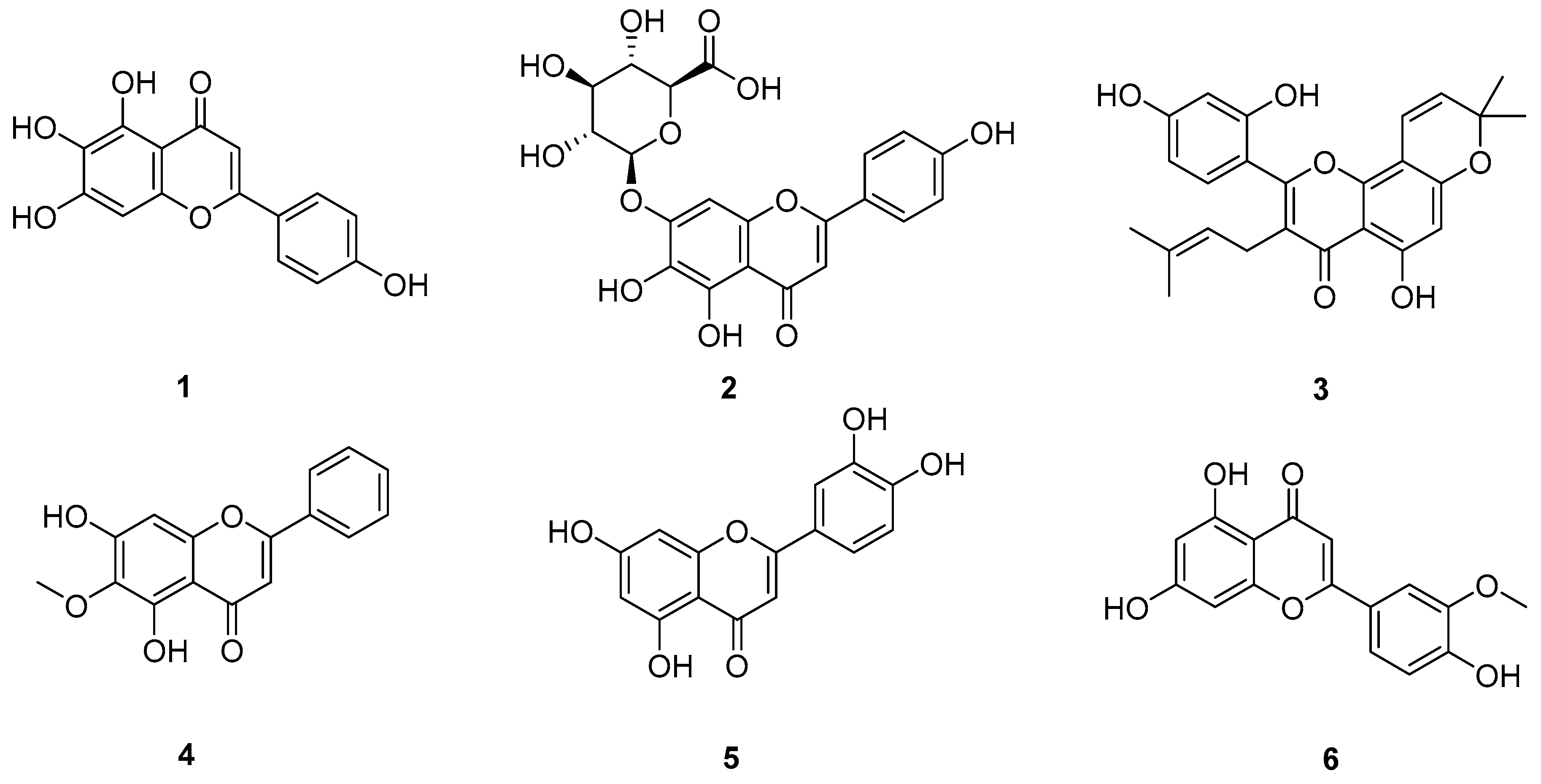
| Scutellarein (1) | ↓ p-EGFR/p-ERK/p-NFκB, COX-2 | A549 | 50 μM | [15] |
| Scutellarin (2) | ↑ ERK | A549, A549/DDP | 0.43 µg/mL (0.0009 μM) | [16] |
| Morusin (3) | ↑ Bax, JNK, ERK ↓ Bcl-2, PI3K/Akt | A549, NCI-H292 | 12.32 μM | [17] |
| Oroxylin A (4) | ↓ MMP-2, MMP-9, ERK/GSK-3β | A549, human lung giant-cell carcinoma 95-D | No data | [18] |
| Luteolin (5) | ↑ caspases-3/-9, Bax, p-MEK/Akt ↓ Bcl-2 | A549 | 63.3 μM | [20] |
| Chrysoeriol (6) | ↓ p-p38, p-ERK1/2 | A549 | 15 μM | [21] |
| Natural Compound | Targeting Signaling Pathway Proteins | Treated Lung Cancer Cell Lines | IC50 Values (A549) | Ref |
|---|---|---|---|---|
| Hydroxysafflor yellow A (16) | ↑ Bcl-2 ↓ Bax, caspase-3, caspase-9, ERK/MAPK, PI3K/Akt/mTOR, COX-2/MMP-2/MMP-9 | A549, H1299 | No data | [33] |
| Salidroside (18) | ↑ Bax, caspase-3, caspase-9 ↓ Bcl-2, MP2/RhoA/ROCK1, MEK/ERK, AKT | A549 | 6.2 μM | [36] |
| Echinacoside (19) | ↓ p-Raf, p-MEK1/2, p-ERK1/2, c-Myc, c-Fos | A549, H1299 | 45.35 μM | [43] |
| No. | Compounds | PubChem ID | Score (kcal/mol) | Residues | Combined Site Type | Number of Hydrogen Bonds |
|---|---|---|---|---|---|---|
| 1 | Scutellarein | 5281697 | −6.81 | MET-125; ASP-123; LYS-71; ASP-184 | Orthosteric site | 5 |
| 2 | Scutellarin | 185617 | −5.59 | ARG-370; ILE-103; LEU-92; GLU-344 | Allosteric site | 5 |
| 3 | Morusin | 5281671 | −8.08 | LYS-131; TYR-53; LYS-71; GLN-122 | Orthosteric site | 4 |
| 4 | Oroxylin A | 5320315 | −6.97 | LYS-71; GLN-122 | Orthosteric site | 4 |
| 5 | Luteolin | 5280445 | −6.46 | MET-125; ASP-123; GLN-122; GLU-50 | Orthosteric site: MET-125; ASP-123; GLN-122 Allosteric site: GLU-50 | 4 |
| 6 | Chrysoeriol | 5280666 | −6.62 | MET-125; ASP-123; LYS-71 | Orthosteric site | 3 |
| 7 | Parthenolide | 7251185 | −7.05 | LYS-71 | Orthosteric site | 1 |
| 8 | β-elemene | — | −6.03 | — | — | — |
| 9 | Oridonin | 5321010 | −6.33 | ASP-268; ASN-314; PHE-313 | Allosteric site | 4 |
| 10 | Kahweol | 114778 | −7.93 | MET-125 | Orthosteric site | 3 |
| 11 | L-Pimaric acid | 221062 | −8.00 | LEU-261; LYS-289 | Allosteric site | 2 |
| 12 | Lupeol | 259846 | −8.18 | LYS-168 | Allosteric site | 1 |
| 13 | Toosendanin | 9851101 | −5.83 | LYS-287; PHE-313; LYS-289 | Allosteric site | 3 |
| 14 | 20(S)-Ginsenoside Rg3 | — | −1.47 | ILE-48; LYS-131 | Orthosteric site | 3 |
| 15 | Cordycepin | 6303 | −4.33 | LYS-289; SER-263; ASP-268; ASN-314; PHE-313 | Allosteric site | 6 |
| 16 | Hydroxysafflor yellow A | 6443665 | −2.43 | ASP-184; GLU-50; LYS-131 | Orthosteric site: ASP-184; LYS-131 Allosteric site: GLU-50 | 7 |
| 17 | Periplocin | 14463159 | −5.29 | GLU-344; PHE-95; ASP-123 | Orthosteric site: ASP-123 Allosteric site: GLU-344; PHE-95 | 5 |
| 18 | Salidroside | 159278 | −4.96 | LYS-71; GLN-122; GLU-126; LYS-131 | Orthosteric site | 5 |
| 19 | Echinacoside | — | 3.93 | GLU-50 | Allosteric site | 1 |
| 20 | Dioscin | — | −4.18 | GLU-50; LYS-131; TYR-130; ASP-128 | Orthosteric site: LYS-131; ASP-128 Allosteric site: GLU-50; TYR-130 | 5 |
| 21 | Timosaponin AIII | — | −2.67 | GLN-90 | Allosteric site | 1 |
| 22 | Paris saponin I | — | −2.83 | SER-135 | Allosteric site | 5 |
| 23 | Hinesol | 10878761 | −6.56 | SER-263 | Allosteric site | 1 |
| 24 | Fucosterol | 5281328 | −8.30 | PRO-315 | Allosteric site | 1 |
| 25 | 7,8-Dihydroxy-4-methylcoumarin | 5355836 | −6.12 | GLU-358; LYS-357; GLN-79 | Allosteric site | 5 |
| 26 | Angelicin | 10658 | −6.63 | TYR-156 | Allosteric site | 1 |
| 27 | Mannose | — | −2.73 | ARG-116; ASP-117; ASP-37 | Allosteric site | 8 |
| 28 | Ganoderan B | — | −7.30 | ARG-96 | Allosteric site | 1 |
| 29 | Atractylenolide-1 | 5321018 | −7.61 | LYS-71 | Orthosteric site | 1 |
| 30 | Solamargine | — | −6.40 | ALA-191; GLN-372 | Allosteric site | 2 |
| 31 | Thymoquinone | 10281 | −5.35 | MET-125; ASP-123 | Orthosteric site | 2 |
| 32 | Sinomenine | 5459308 | −6.16 | — | — | — |
| 33 | Maclurin | 68213 | −4.67 | ASP-128; LYS-131; LYS-71; GLN-122; ILE-48 | Orthosteric site | 5 |
| No. | Compounds | PubChem ID | Score (kcal/mol) | Residues | Combined Site Type | Number of Hydrogen Bonds |
|---|---|---|---|---|---|---|
| 1 | Scutellarein | 5281697 | −6.72 | ARG-15; TYR-30; GLU-12; ASN-27 | Allosteric site | 5 |
| 2 | Scutellarin | 185617 | −5.44 | ARG-277; GLY-242; ASN-271; LYS-270 | Allosteric site | 5 |
| 3 | Morusin | 5281671 | −7.54 | ARG-15; LEU-28; GLU-12; ASN-27 | Allosteric site | 5 |
| 4 | Oroxylin A | 5320315 | −6.49 | ASP-251; LEU-244; ASP-291; LYS-272 | Allosteric site | 5 |
| 5 | Luteolin | 5280445 | −6.65 | SER-29; GLU-12; ARG-15 | Allosteric site | 5 |
| 6 | Chrysoeriol | 5280666 | −6.18 | SER-29; GLU-12; ARG-15 | Allosteric site | 4 |
| 7 | Parthenolide | 7251185 | −6.44 | MET-108 | Orthosteric site | 1 |
| 8 | β-elemene | — | −5.72 | — | — | — |
| 9 | Oridonin | 5321010 | −7.53 | ARG-91; GLN-355 | Allosteric site | 4 |
| 10 | Kahweol | 114778 | −8.14 | LYS-114; GLN-105; LYS-54 | Orthosteric site | 4 |
| 11 | L-Pimaric acid | 221062 | −7.62 | ASN-238; LYS-270 | Allosteric site | 3 |
| 12 | Lupeol | 259846 | −8.04 | ARG-24 | Allosteric site | 1 |
| 13 | Toosendanin | 9851101 | −6.79 | ARG-15; GLY-16 | Allosteric site | 6 |
| 14 | 20(S)-Ginsenoside Rg3 | — | −3.97 | GLU-109 | Orthosteric site | 2 |
| 15 | Cordycepin | 6303 | −4.26 | ARG-91; PHE-354; GLN-355; ASP-88 | Allosteric site | 7 |
| 16 | Hydroxysafflor yellow A | 6443665 | −3.13 | PHE-59; LYS-344; GLU-341 | Allosteric site | 5 |
| 17 | Periplocin | 14463159 | −5.05 | ARG-91; GLN-355; ARG-353 | Allosteric site | 6 |
| 18 | Salidroside | 159278 | −4.58 | PHE-78; HIS-80; ILE-83; LYS-164; ASP-106 | Orthosteric site: ASP-106 Allosteric site: PHE-78; HIS-80; ILE-83; LYS-164 | 8 |
| 19 | Echinacoside | — | −1.90 | HIS-269 | Allosteric site | 2 |
| 20 | Dioscin | — | −5.18 | ARG-15; MET-13; GLU-12 | Allosteric site | 4 |
| 21 | Timosaponin AIII | — | −5.00 | TYR-30 | Allosteric site | 2 |
| 22 | Paris saponin I | — | −5.12 | LYS-164; ASP-106; ASN-158 | Allosteric site | 4 |
| 23 | Hinesol | 10878761 | −6.49 | MET-108 | Orthosteric site | 2 |
| 24 | Fucosterol | 5281328 | −7.84 | MET-108; LYS-114 | Orthosteric site | 2 |
| 25 | 7,8-Dihydroxy-4-methylcoumarin | 5355836 | −5.07 | SER-246; PRO-268; LEU-267 | Allosteric site | 3 |
| 26 | Angelicin | 10658 | −6.42 | LYS-54 | Orthosteric site | 1 |
| 27 | Mannose | — | −1.92 | ASP-291; LEU-294; LYS-272; LEU-244; PHE-296; SER-248; ASP-251 | Allosteric site | 7 |
| 28 | Ganoderan B | — | −7.68 | LYS-114; LYS-151 | Orthosteric site: LYS-114 Allosteric site: LYS-151 | 2 |
| 29 | Atractylenolide-1 | 5321018 | −7.27 | — | — | — |
| 30 | Solamargine | — | −9.30 | LEU-107; ARG-50 | Orthosteric site: LEU-107 Allosteric site: ARG-50 | 2 |
| 31 | Thymoquinone | 10281 | −5.64 | SER-246; PRO-268 | Allosteric site | 2 |
| 32 | Sinomenine | 5459308 | −6.90 | LEU-107; ARG-50 | Allosteric site | 2 |
| 33 | Maclurin | 68213 | −4.68 | GLU-12; TYR-30; ARG-15 | Allosteric site | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Zhang, C.; Yuan, J.; Zhu, K.; Tomás, H.; Sheng, R.; Yang, X.H.; Tu, Q.; Guo, R. Progress of ERK Pathway-Modulated Natural Products for Anti-Non-Small-Cell Lung Cancer Activity. Pharmaceuticals 2025, 18, 1371. https://doi.org/10.3390/ph18091371
Xing L, Zhang C, Yuan J, Zhu K, Tomás H, Sheng R, Yang XH, Tu Q, Guo R. Progress of ERK Pathway-Modulated Natural Products for Anti-Non-Small-Cell Lung Cancer Activity. Pharmaceuticals. 2025; 18(9):1371. https://doi.org/10.3390/ph18091371
Chicago/Turabian StyleXing, Lin, Chi Zhang, Jieying Yuan, Kai Zhu, Helena Tomás, Ruilong Sheng, Xiuwei H. Yang, Qidong Tu, and Ruihua Guo. 2025. "Progress of ERK Pathway-Modulated Natural Products for Anti-Non-Small-Cell Lung Cancer Activity" Pharmaceuticals 18, no. 9: 1371. https://doi.org/10.3390/ph18091371
APA StyleXing, L., Zhang, C., Yuan, J., Zhu, K., Tomás, H., Sheng, R., Yang, X. H., Tu, Q., & Guo, R. (2025). Progress of ERK Pathway-Modulated Natural Products for Anti-Non-Small-Cell Lung Cancer Activity. Pharmaceuticals, 18(9), 1371. https://doi.org/10.3390/ph18091371








