Oligomeric Proanthocyanidins Reverse Lenvatinib Resistance in Hepatocellular Carcinoma Through ITGA3-Mediated Pathway
Abstract
1. Introduction
2. Results
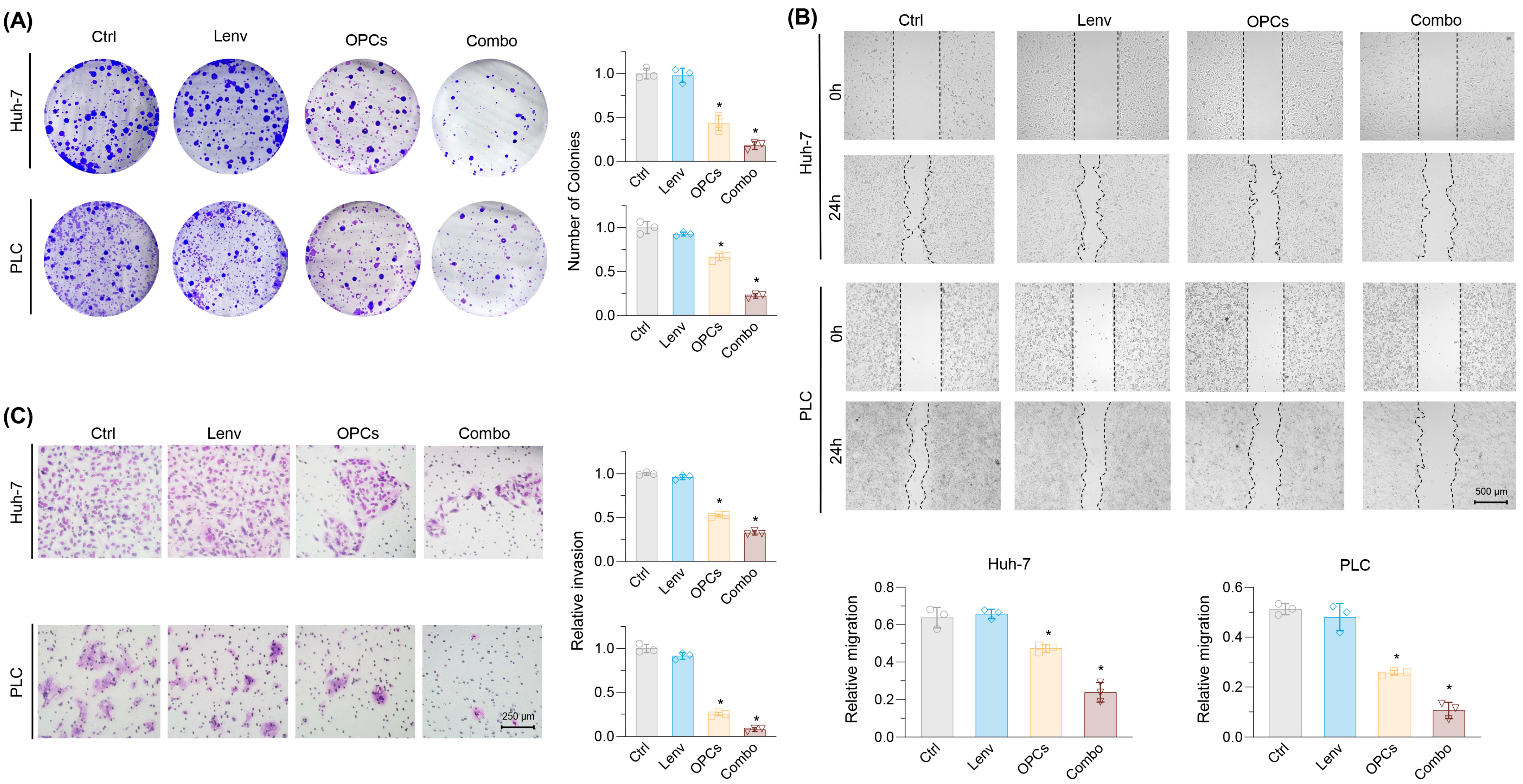
2.1. Establishment of Lenvatinib-Resistant HCC Cell Lines and Evaluation of OPCs-Mediated Synergistic Effects
2.2. Evaluation of the Effects of Lenvatinib and OPCs on Cell Migration and Invasion
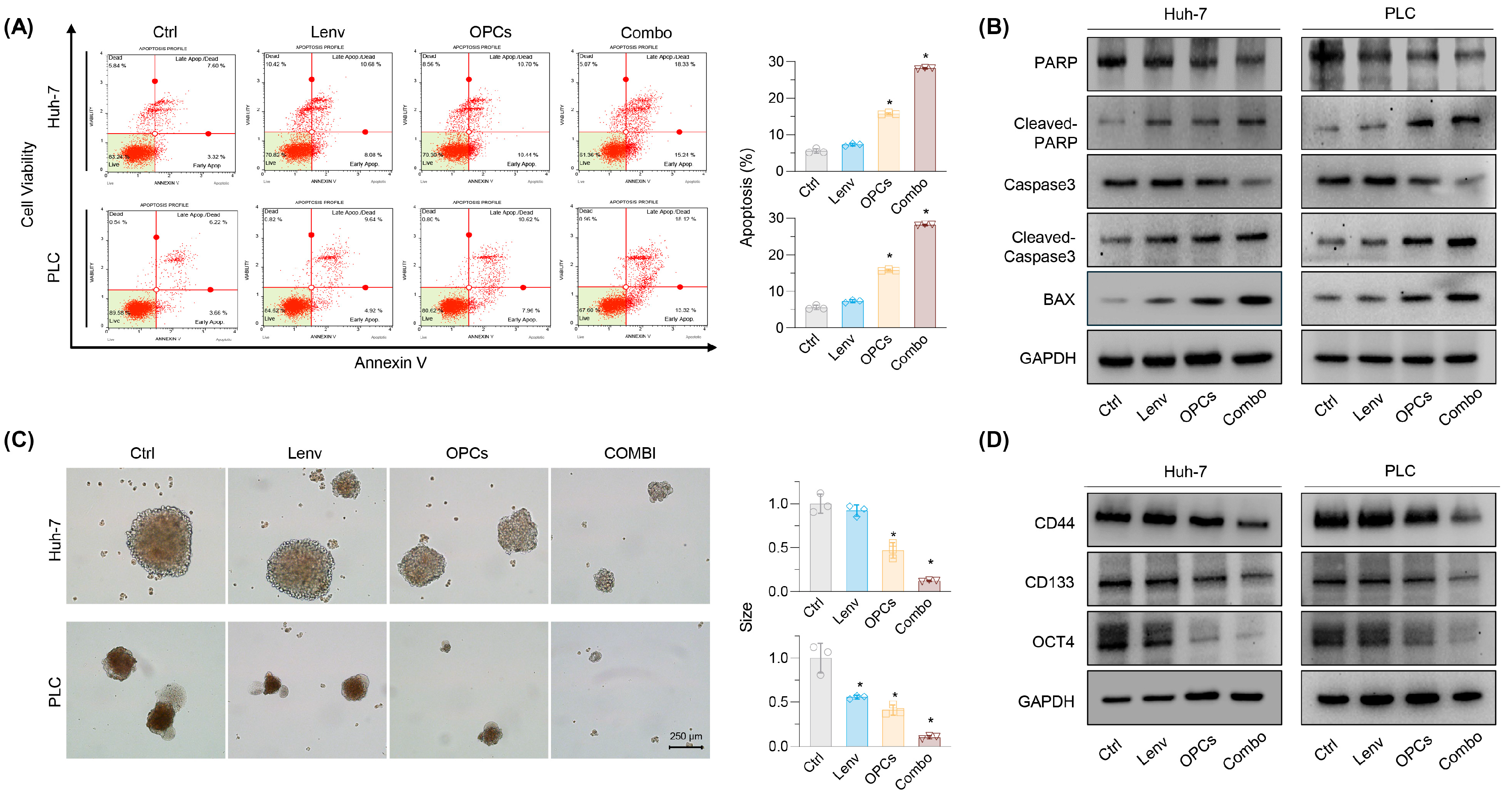
2.3. Combination of OPCs and Lenvatinib Induces Apoptosis and Suppresses Cancer Stemness in Resistance HCC Cell Lines
2.4. Transcriptome Profiling Identified Involved Pathways and Genes That Could Be Targeted to Overcome Lenvatinib Resistance
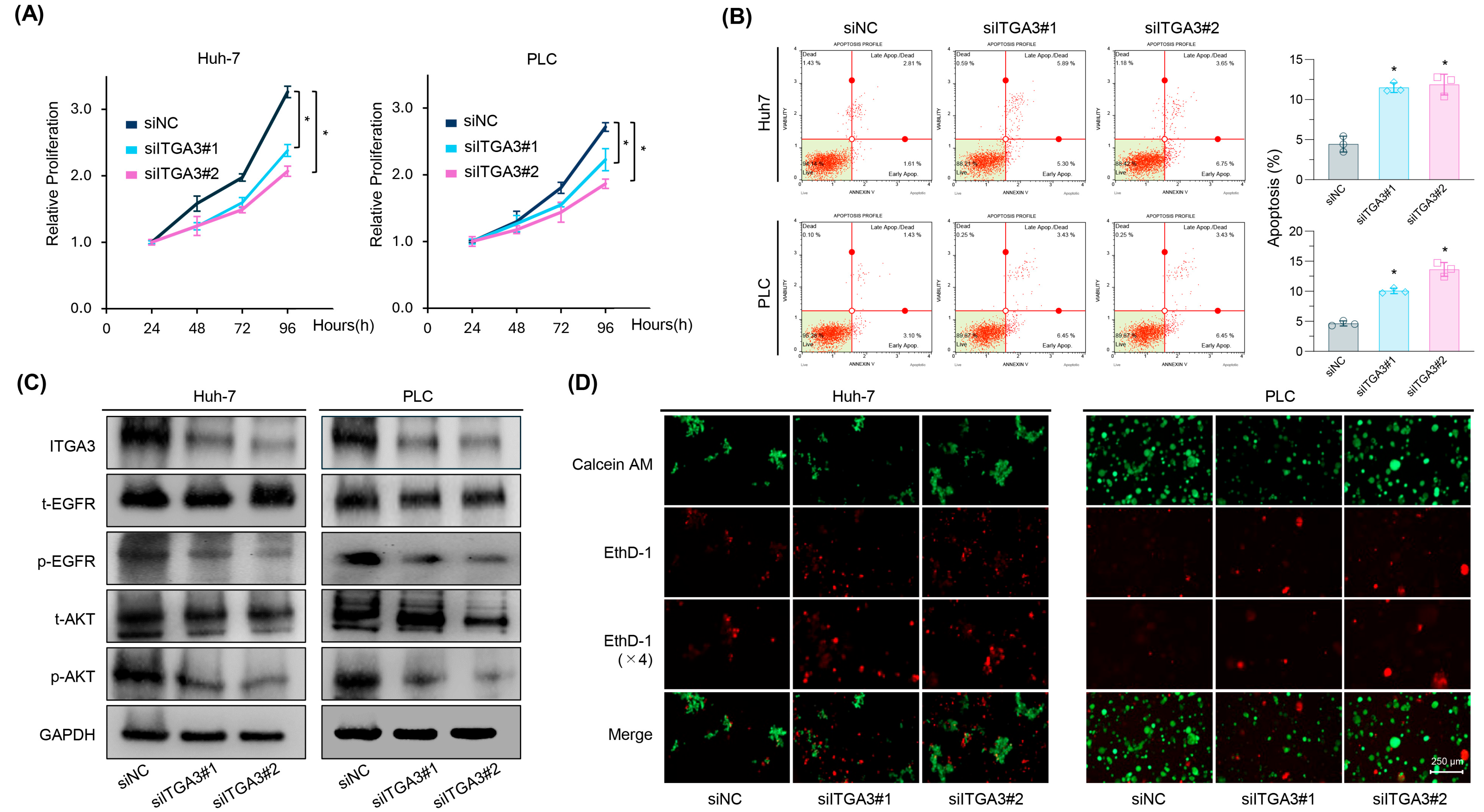
2.5. siRNA-Mediated Knockdown of ITGA3 Suppresses Tumorigenic Features and Induces Anoikis Sensitivity in HCC Cells
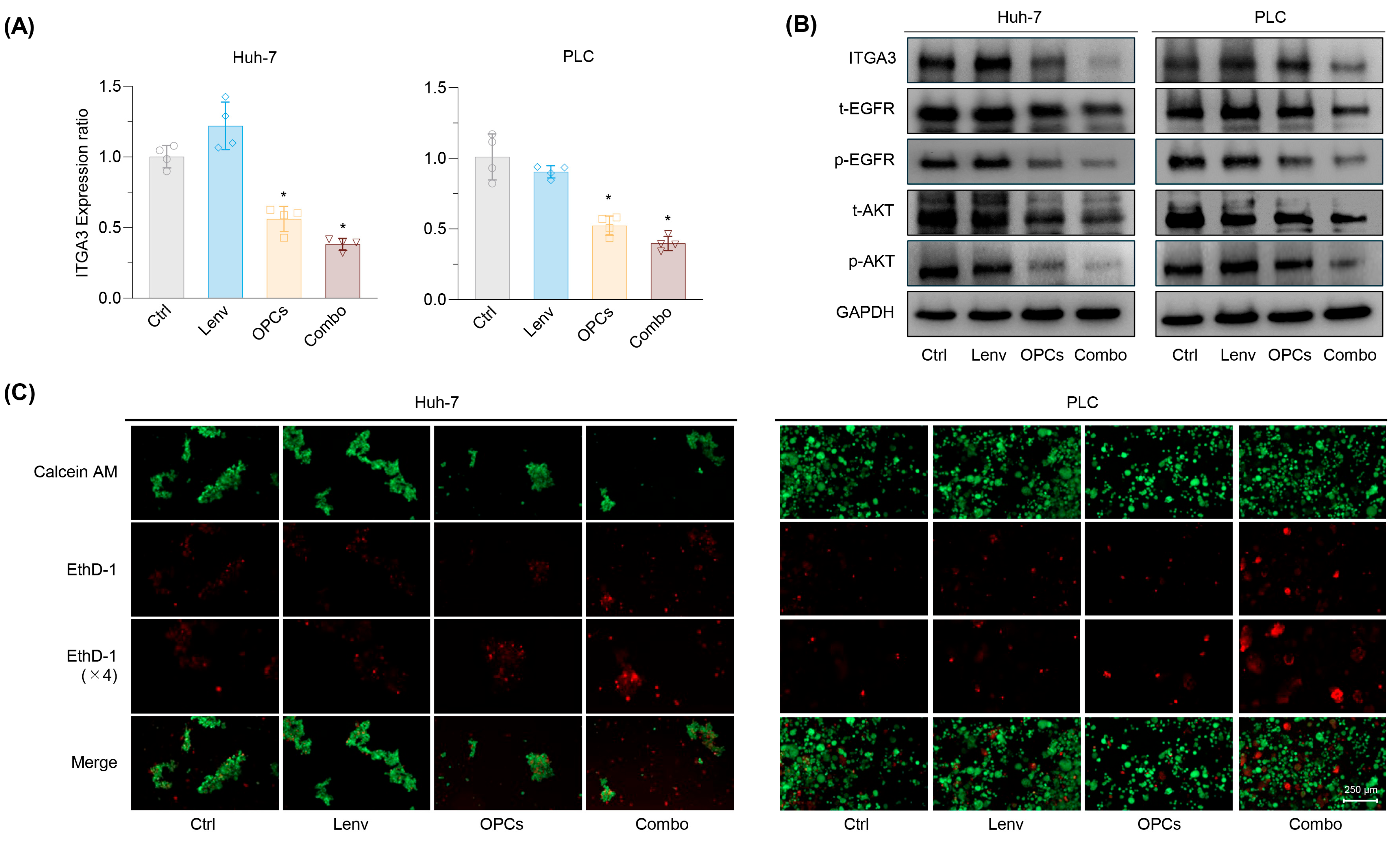
2.6. Combined OPCs and Lenvatinib Therapy Restores Anoikis Sensitivity Through ITGA3–EGFR–AKT Pathway Inhibition
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Cell Culture and Materials
4.3. Herbal Preparations and Drug Preparation
4.4. Cell Viability Assays
4.5. Establishment of Lenvatinib-Resistant HCC Cell Lines
4.6. Invasion Assay
4.7. The Wound Healing Assay
4.8. The Colony Formation Assay
4.9. Apoptosis Assay
4.10. Spheroid Formation Assays
4.11. Identification of Differentially Expressed Genes and Functional Pathway Enrichment in Lenvatinib-Resistant HCC Cells
4.12. RNA Extraction and qRT-PCR
4.13. Protein Isolation and Western Blotting
4.14. Transfection of Small Interfering RNA
4.15. Anoikis Assay
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| ICIs | Immune checkpoint inhibitors |
| TKIs | Multi-tyrosine kinase inhibitors |
| OS | Overall survival |
| IC50 | Half-maximal inhibitory concentration |
| OPCs | Oligomeric proanthocyanidins |
| PLC | PLC-PRF-5 |
| FC | Fold change |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CCK-8 | Cell Counting Kit-8 |
| PBS | Phosphate-buffered saline |
| SD | Standard deviation |
| HR | Hazard ratios |
| CI | Confidence intervals |
| cDNA | Complementary DNA |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Johnson, P.J.; Qin, S.; Park, J.W.; Poon, R.T.; Raoul, J.L.; Philip, P.A.; Hsu, C.H.; Hu, T.H.; Heo, J.; Xu, J.; et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur. J. Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Rimini, M.; Rimassa, L.; Ueshima, K.; Burgio, V.; Shigeo, S.; Tada, T.; Suda, G.; Yoo, C.; Cheon, J.; Pinato, D.J.; et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open 2022, 7, 100591. [Google Scholar] [CrossRef]
- He, X.; Hikiba, Y.; Suzuki, Y.; Nakamori, Y.; Kanemaru, Y.; Sugimori, M.; Sato, T.; Nozaki, A.; Chuma, M.; Maeda, S. EGFR inhibition reverses resistance to lenvatinib in hepatocellular carcinoma cells. Sci. Rep. 2022, 12, 8007. [Google Scholar] [CrossRef]
- Jin, H.; Shi, Y.; Lv, Y.; Yuan, S.; Ramirez, C.F.A.; Lieftink, C.; Wang, L.; Wang, S.; Wang, C.; Dias, M.H.; et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 2021, 595, 730–734. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Dong, W.; Zhang, C.; Hu, M.; Ma, W.; Jiang, X.; Li, H.; Yang, P.; Xiang, D. N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells’ Properties and Lenvatinib Resistance Through WNT/beta-Catenin and Hippo Signaling Pathways. Gastroenterology 2023, 164, 990–1005. [Google Scholar] [CrossRef]
- Yu, T.; Yu, J.; Lu, L.; Zhang, Y.; Zhou, Y.; Zhou, Y.; Huang, F.; Sun, L.; Guo, Z.; Hou, G.; et al. MT1JP-mediated miR-24-3p/BCL2L2 axis promotes Lenvatinib resistance in hepatocellular carcinoma cells by inhibiting apoptosis. Cell Oncol. 2021, 44, 821–834. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiong, P.; Nie, M.; Pan, Y.; Wang, J.; Hu, D.; Zhou, Z.; Zhang, Y.; Chen, M.; Xu, L. The combination treatment strategy of lenvatinib for hepatocellular carcinoma: A real-world study. J. Cancer Res. Clin. Oncol. 2023, 149, 2491–2500. [Google Scholar] [CrossRef]
- Bermudez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espin, J.C.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef]
- Huai, Z.; Li, Z.; Xue, W.; Li, S.; Huang, Y.; Cao, X.; Wei, Q.; Wang, Y. Novel curcumin derivatives N17 exert anti-cancer effects through the CSNK1G3/AKT axis in triple-negative breast cancer. Biochem. Pharmacol. 2024, 229, 116472. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Potentiating therapeutic effects by enhancing synergism based on active constituents from traditional medicine. Phytother. Res. 2014, 28, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Sharma, P.; Sharma, G.G.; Banwait, J.K.; Goel, A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci. Rep. 2021, 11, 7548. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis 2018, 39, 767–777. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Goel, A. Oligomeric proanthocyanidins (OPCs) from grape seed extract suppress the activity of ABC transporters in overcoming chemoresistance in colorectal cancer cells. Carcinogenesis 2019, 40, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Ravindranathan, P.; Gu, J.; Cardenas, J.; Yuchang, M.; Goel, A. Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Sci. Rep. 2018, 8, 3335. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Kariya, Y.; Nishita, M. Integrins in Cancer Drug Resistance: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 3143. [Google Scholar] [CrossRef]
- Nasimi Shad, A.; Moghbeli, M. Integrins as the pivotal regulators of cisplatin response in tumor cells. Cell Commun. Signal 2024, 22, 265. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef]
- Song, W.; Hu, H.; Yuan, Z.; Yao, H. A prognostic model for anoikis-related genes in pancreatic cancer. Sci. Rep. 2024, 14, 15200. [Google Scholar] [CrossRef] [PubMed]
- Sa, K.D.; Zhang, X.; Li, X.F.; Gu, Z.P.; Yang, A.G.; Zhang, R.; Li, J.P.; Sun, J.Y. A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem. Biophys. Res. Commun. 2018, 501, 758–764. [Google Scholar] [CrossRef]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Ou, Y.; Zou, L.; Zhang, D.; Yang, Q.; Qin, Y.; Du, X.; Li, W.; Yuan, Z.; et al. Anoikis resistance--protagonists of breast cancer cells survive and metastasize after ECM detachment. Cell Commun. Signal 2023, 21, 190. [Google Scholar] [CrossRef]
- Gonzalez-Llorente, L.; Santacatterina, F.; Garcia-Aguilar, A.; Nuevo-Tapioles, C.; Gonzalez-Garcia, S.; Tirpakova, Z.; Toribio, M.L.; Cuezva, J.M. Overexpression of Mitochondrial IF1 Prevents Metastatic Disease of Colorectal Cancer by Enhancing Anoikis and Tumor Infiltration of NK Cells. Cancers 2019, 12, 22. [Google Scholar] [CrossRef]
- Wade, C.A.; Kyprianou, N. Profiling Prostate Cancer Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 904. [Google Scholar] [CrossRef]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef]
- Liu, G.; Shi, A.; Wang, N.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Tao, Y.; Wang, Q.; et al. Polyphenolic Proanthocyanidin-B2 suppresses proliferation of liver cancer cells and hepatocellular carcinogenesis through directly binding and inhibiting AKT activity. Redox Biol. 2020, 37, 101701. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Busselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, S.Y.; Sun, Q.Y.; Ren, J.; Liu, H.X.; Li, H. Proanthocyanidin B2 attenuates high-glucose-induced neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt signaling pathway. Neural Regen. Res. 2018, 13, 1628–1636. [Google Scholar]
- Vogel, A.; Qin, S.; Kudo, M.; Su, Y.; Hudgens, S.; Yamashita, T.; Yoon, J.H.; Fartoux, L.; Simon, K.; Lopez, C.; et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: Patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 649–658. [Google Scholar] [CrossRef]
- Capozzi, M.; De Divitiis, C.; Ottaiano, A.; von Arx, C.; Scala, S.; Tatangelo, F.; Delrio, P.; Tafuto, S. Lenvatinib, a molecule with versatile application: From preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res. 2019, 11, 3847–3860. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, H.; Miao, S.; Zheng, J.; Xie, Z.; Zhao, H. Anti-tumor effect of cisplatin in human oral squamous cell carcinoma was enhanced by andrographolide via upregulation of phospho-p53 in vitro and in vivo. Tumour. Biol. 2017, 39, 1010428317705330. [Google Scholar] [CrossRef]
- Sun, C.; Jin, W.; Shi, H. Oligomeric proanthocyanidins protects A549 cells against H2O2-induced oxidative stress via the Nrf2-ARE pathway. Int. J. Mol. Med. 2017, 39, 1548–1554. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef]
- Roy, S.; Zhao, Y.; Yuan, Y.C.; Goel, A. Metformin and ICG-001 Act Synergistically to Abrogate Cancer Stem Cells-Mediated Chemoresistance in Colorectal Cancer by Promoting Apoptosis and Autophagy. Cancers 2022, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Goel, A. Andrographis overcomes 5-fluorouracil-associated chemoresistance through inhibition of DKK1 in colorectal cancer. Carcinogenesis 2021, 42, 814–825. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noma, T.; Li, Y.; Wada, Y.; Morine, Y.; Ikemoto, T.; Saito, Y.; Yamada, S.; Teraoku, H.; Shimada, M.; Goel, A. Oligomeric Proanthocyanidins Reverse Lenvatinib Resistance in Hepatocellular Carcinoma Through ITGA3-Mediated Pathway. Pharmaceuticals 2025, 18, 1361. https://doi.org/10.3390/ph18091361
Noma T, Li Y, Wada Y, Morine Y, Ikemoto T, Saito Y, Yamada S, Teraoku H, Shimada M, Goel A. Oligomeric Proanthocyanidins Reverse Lenvatinib Resistance in Hepatocellular Carcinoma Through ITGA3-Mediated Pathway. Pharmaceuticals. 2025; 18(9):1361. https://doi.org/10.3390/ph18091361
Chicago/Turabian StyleNoma, Takayuki, Yuan Li, Yuma Wada, Yuji Morine, Tetsuya Ikemoto, Yu Saito, Shinichiro Yamada, Hiroki Teraoku, Mitsuo Shimada, and Ajay Goel. 2025. "Oligomeric Proanthocyanidins Reverse Lenvatinib Resistance in Hepatocellular Carcinoma Through ITGA3-Mediated Pathway" Pharmaceuticals 18, no. 9: 1361. https://doi.org/10.3390/ph18091361
APA StyleNoma, T., Li, Y., Wada, Y., Morine, Y., Ikemoto, T., Saito, Y., Yamada, S., Teraoku, H., Shimada, M., & Goel, A. (2025). Oligomeric Proanthocyanidins Reverse Lenvatinib Resistance in Hepatocellular Carcinoma Through ITGA3-Mediated Pathway. Pharmaceuticals, 18(9), 1361. https://doi.org/10.3390/ph18091361








