Structure–Activity Relationship Study of 3-Alkynyl-6-aryl-isothiazolo[4,3-b]pyridines as Dual Inhibitors of the Lipid Kinases PIKfyve and PIP4K2C
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Enzymatic Assays
2.2.1. PIKfyve Inhibition
2.2.2. PIP5K2C Binding Assay
2.3. Cellular Assays
2.3.1. Antiviral Activity
2.3.2. Antitumoral Activity
3. Materials and Methods
- General
- 3-Amino-2-cyanopyridine
- This compound was prepared from 3-nitro-2-cyanopyridine (436 mg, 2.9 mmol, 1.0 eq.) and iron powder (810 mg, 14.5 mmol, 5 eq.) in acetic acid (10 mL) using a previously described protocol [25]. The title compound was isolated without further purification as a light-brown solid in 90% yield (313 mg, 2.63 mmol).
- 1H NMR (400 MHz, DMSO-d6) δ: 7.86 (dd, J = 4.3, 1.4 Hz, 1H), 7.31 (dd, J = 8.6, 4.3 Hz, 1H), 7.21 (dd, J = 8.6, 1.5 Hz, 1H), 6.25 (s, 2H).
- 13C NMR (101 MHz, DMSO-d6) δ: 148.8, 139.0, 128.1, 123.1, 117.0, 114.5.
- HRMS m/z [M+H]+ calcd for C6H5N3 120.0556, found 120.0563.
- 3-Aminopyridine-2-carbothioamide
- This compound was prepared from 3-amino-2-cyanopyridine (300 mg, 2.5 mmol, 1.0 eq.) and phosphorus pentasulfide (2.22 g, 5 mmol, 2 eq.) in ethanol (10 mL) using a previously described protocol [25]. The crude mixture was purified by silica gel chromatography (PE/EtOAc, 8:2), yielding the title compound as a light-brown solid in 65% yield (252 mg, 1.64 mmol).
- 1H NMR (400 MHz, CDCl3) δ: 9.57 (s, 1H), 7.87 (dd, J = 4.1, 1.5 Hz, 1H), 7.26 (s, 1H), 7.19 (dd, J = 8.4, 4.1 Hz, 1H), 7.07 (dd, J = 8.4, 1.4 Hz, 1H), 6.86 (s, 2H).
- 13C NMR (101 MHz, CDCl3) δ: 195.2, 146.8, 136.2, 129.9, 128.1, 126.9.
- HRMS m/z [M+H]+ calcd for C6H7N3S 154.0433, found 154.0439.
- Isothiazolo[4,3-b]pyridin-3-amine
- This compound was prepared from 3-aminopyridine-2-carbothioamide (237 mg, 1.55 mmol, 1.0 eq.) and an 30% aqueous hydrogenperoxide solution (310 μL, 3.1 mmol, 2 eq.) in methanol (5 mL) using a previously described protocol [25]. The title compound was isolated without further purification as a dark-yellow solid in 82% yield (192 mg, 1.27 mmol).
- 1H NMR (400 MHz, DMSO-d6) δ: 8.28 (dd, J = 3.8, 1.4 Hz, 1H), 7.83 (s, 2H), 7.69 (dd, J = 9.0, 1.4 Hz, 1H), 7.24 (dd, J = 9.0, 3.8 Hz, 1H).
- 13C NMR (101 MHz, DMSO-d6) δ: 172.4, 153.6, 143.4, 134.8, 127.9, 123.6.
- HRMS m/z [M+H]+ calcd for C6H5N3S 152.0277, found 152.0278.
- 3-Bromoisothiazolo[4,3-b]pyridine (1a)
- Isothiazolo[4,3-b]pyridin-3-amine (170 mg, 1.12 mmol, 1.0 eq.) was dissolved in 48% aqueous HBr (17 mL) and stirred for 10 min at room temperature. CuBr (323 mg, 2.25 mmol, 2 eq.) was added and the mixture was cooled to 0 °C. An aqueous solution (8.5 mL) of NaNO2 (233 mg, 3.37 mmol, 3 eq.) was added dropwise (0.5 mL min−1). The reaction mixture was stirred for 2 h at 0 °C then overnight at room temperature. The mixture was cooled to 0 °C, neutralized with a 2M NaOH solution and extracted with EtOAc (30 mL) three times. The combined organic layers were washed with brine (30mL) and dried over Na2SO4. The solvent was removed in vacuo and the crude was purified by silica gel chromatography (PE/EtOAc, 8:2) yielding the title compound as a yellow solid in 61% yield (147 mg, 0.68 mmol).
- 1H NMR (400 MHz, CDCl3) δ: 8.87 (dd, J = 3.9, 1.5 Hz, 1H), 8.12 (dd, J = 9.0, 1.5 Hz, 1H), 7.40 (dd, J = 9.0, 3.8 Hz, 1H).
- 13C NMR (101 MHz, CDCl3) δ: 154.9, 152.2, 146.5, 135.8, 130.0, 123.6.
- HRMS m/z [M+H]+ calcd for C6H3BrN2S 214.9274, found 214.9285.
- Sonogashira coupling at position 3 of the isothiazolo[4,3-b]pyridine scaffold
- General procedure
- A solution of 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (1 eq.) [25] and triethylamine (3 eq.) in THF, was degassed with a flow of argon for 5 min. Then, Pd(PPh3)2Cl2 (0.02 eq.) and CuI (0.01 eq.) were added and the reaction mixture was allowed to reach 30 °C. Subsequently, a solution of the appropriate acetylene in THF was added slowly over a period of 30 min. The reaction was degassed a second time, filled with argon and stirred at 30 °C overnight. After disappearance of the starting material as monitored by TLC, the volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography. Compounds 2a-b and 6a-y were made according to this procedure.
- 3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (2a)
- This compound was prepared from 3-bromoisothiazolo[4,3-b]pyridine 1a (60 mg, 0.28 mmol, 1.0 eq.) and 3-ethynylpyridine (86 mg, 0.84 mmol, 3 eq.), Pd(PPh3)2Cl2 (4 mg, 0.007 mmol, 0.025 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude mixture was purified by silica gel chromatography (PE/EtOAc, 7:3), yielding the title compound as a brown solid in 80% yield (53 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 8.91 (s, 1H), 8.89 (dd, J = 3.9, 1.5 Hz, 1H), 8.64 (dd, J = 4.9, 1.7 Hz, 1H), 8.18 (dd, J = 8.9, 1.5 Hz, 1H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H), 7.43 (dd, J = 9.0, 3.8 Hz, 1H), 7.36 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H).
- 13C NMR (75 MHz, CDCl3) δ: 154.93, 152.29, 151.92, 149.77, 148.84, 143.61, 138.71, 130.07, 123.44, 123.14, 119.24, 104.15, 80.01.
- HRMS m/z [M+H]+ calcd for C13H7N3S 238.0433, found 238.0440.
- 6-Bromo-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (2b)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light-yellow solid in 67% yield (72 mg, 0.23 mmol). Spectral data are in agreement with literature [28].
- 1H NMR (600 MHz, CDCl3) δ: 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.94–7.98 (m, 1H, arom H), 8.36 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.84 (d, J = 2.1 Hz, 1H, arom H), 8.90 (d, J = 1.5 Hz, 1H, arom H).
- HR-MS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9533.
- 6-Bromo-3-(pent-1-yn-1-yl)isothiazolo[4,3-b]pyridine (6a)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), pent-1-yne (69 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 71% yield (67.7 mg, 0.24 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 1.10 (t, J = 7.4 Hz, 3H, CH3), 1.75 (h, J = 7.2 Hz, 2H, CH2), 2.65 (t, J = 7.1 Hz, 2H, CH2), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.77 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C11H9BrN2S 280.9743, found 280.9744.
- 6-Bromo-3-(phenylethynyl)isothiazolo[4,3-b]pyridine (6b)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), ethynylbenzene (104 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 81% (87 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 8.82 (d, J = 2.0 Hz, 1H), 8.33 (d, J = 2.0 Hz, 1H), 7.67 (m, 2H), 7.43 (m, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 154.90, 152.47, 146.71, 145.93, 131.96, 131.28, 129.89, 128.54, 121.68, 120.83, 103.01 ppm.
- 6-Bromo-3-((4-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6c)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-4-fluorobenzene (122 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 89% yield (100.5 mg, 0.3 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.07–7.16 (m, 2H, arom H), 7.62–7.71 (m, 2H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.82 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrFN2S 332.9492, found 332.9483.
- 6-Bromo-3-((3-chlorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6d)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-3-chlorobenzene (139 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a light-yellow solid in 83% yield (98.6 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.31–7.44 (m, 2H, arom H) 7.52–7.58 (m, 1H, arom H), 7.65–7.68 (m, 1H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrClN2S 348.9197, found 348.9206.
- 6-Bromo-3-((2-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (6e)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 1-ethynyl-2-fluorobenzene (123 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 95:5) as mobile phase, affording the title compound as a white solid in 78% yield (88.3 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.12–7.24 (m, 2H, arom H), 7.37–7.48 (m, 1H, arom H), 7.59–7.70 (m, 1H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H6BrFN2S 332.9492, found 332.9483.
- 6-Bromo-3-((3-methoxyphenyl)ethynyl)isothiazolo[4,3-b]pyridine (6f)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylanisole (135 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general p Sonogashira coupling rocedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 95:5) as mobile phase, affording the title compound as a yellow solid in 77% yield (90.4 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.85 (s, 3H, OCH3), 6.99 (ddd, J = 8.0, 2.5, 1.4 Hz, 1H, arom H), 7.17–7.23 (m, 1H, arom H), 7.25–7.35 (m, 2H, arom H), 8.34 (d, J = 2.1 Hz, 1H, arom H), 8.83 (d, J = 2.1 Hz, 1H, arom H).
- HRMS m/z [M+H]+ calcd for C15H9BrN2OS 344.9693, found 344.9693.
- 6-Bromo-3-((6-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6g)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-methylpyridine (120 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and diethyl ether (in a ratio of 10:0.3) as mobile phase, affording the title compound as a yellow solid in 83% yield (93.2 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.61 (s, 3H, CH3), 7.22 (d, J = 8.1 Hz, 1H, arom H), 7.84 (dd, J = 8.0, 2.1 Hz, 1H, arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.78 (d, J = 1.5 Hz 1H, arom H), 8.83 (d, J = 2.0 Hz, 1H, arom H) ppm.HRMS m/z [M+H]+ calcd for C14H8BrN3S 329.9696, found 329.9696.
- 6-Bromo-3-((6-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6h)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 64% yield (75.3 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.99 (s, 3H, OCH3), 6.79 (d, J = 8.6 Hz, 1H arom H), 7.82 (dd, J = 2.3, 8.6 Hz, 1H, arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.49 (d, J = 2.0 Hz, 1H, arom H), 8.82 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3OS 345.9645, found 345.9651.
- 6-Bromo-3-((5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6i)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-methylpyridine (120 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 79% yield (88.7 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.39 (s, 3H, CH3), 7.77 (s, 1H arom H), 8.34 (d, J = 2.0 Hz, 1H, arom H), 8.47 (s, 1H, arom H), 8.71 (s, 1H, arom H), 8.83 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3S 329.9696, found 329.9694.
- 6-Bromo-3-((6-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6j)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 68% yield (77.3 mg, 0.23 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.02 (dd, J = 2.9, 8.5 Hz, 1H arom H), 8.06 (dt, J = 2.3, 8.0 Hz, 1H, arom H), 8.36 (d, J = 2.0 Hz, 1H, arom H), 8.55 (d, J = 1.5 Hz, 1H, arom H), 8.84 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9446.
- 6-Bromo-3-((2-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6k)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), methyl 3-ethynyl-2-fluoropyridine (138 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a beige solid in 81% yield (92.1 mg, 0.28 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.26–7.31 (m, 1H, arom H), 8.03–8.12 (m, 1H, arom H), 8.28 (d, J = 4.4 Hz, 1H, arom H), 8.37 (d, J = 2.0 Hz, 1H, arom H), 8.85 (d, J = 1.9 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9445.
- 6-Bromo-3-((5-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6l)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 73% yield (85.9 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.91 (s, 3H, OCH3). 7.44 (bs, 1H, arom H), 8.36 (bs, 2H, arom H), 8.50 (s, 1H, arom H), 8.85 (d, J = 1.7 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H8BrN3OS 345.9645, found 345.9646.
- Methyl 5-((6-bromoisothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (6m)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), methyl 5-ethynylnicotinate (165 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a beige solid in 65% yield (82.7 mg, 0.22 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 4.00 (s, 3H, COOCH3), 8.38 (d, J = 1.9 Hz, 1H, arom H), 8.57 (t, J = 1.8 Hz, 1H, arom H), 8.86 (d, J = 1.9 Hz, 1H, arom H), 9.04 (d, J = 1.8 Hz, 1H, arom H), 9.23 (d, J = 1.8 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C15H8BrN3O2S 373.9594, found 373.9589.
- 6-Bromo-3-((6-(trifluoromethyl)pyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6n)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynyl-2-(trifluoromethyl)pyridine (175 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a light-yellow solid in 75% yield (98.0 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.76 (d, J = 8.1 Hz, 1H, arom H), 8.14 (d, J = 7.9 Hz, 1H, arom H), 8.38 (d, J = 1.8 Hz, 1H, arom H), 8.86 (d, J = 1.7 Hz, 1H, arom H), 8.98 (s, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C14H5BrF3N3S 383.9413, found 383.9413.
- 6-Bromo-3-((4-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6o)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-4-methoxypyridine (136 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as an orange solid in 71% yield (79 mg, 0.23 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.83 (d, J = 2.1 Hz, 1H), 8.75 (s, 1H), 8.55 (s, 1H), 8.34 (d, J = 2.0 Hz, 1H), 6.89 (d, J = 5.4 Hz, 1H), 4.00 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 165.7, 154.9, 154.0, 152.8, 152.1, 147.0, 145.4, 131.4, 121.0, 102.1, 82.7, 56.1 ppm.
- 6-Bromo-3-((4-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6p)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 5-ethynylpicolinonitrile (131 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 55% yield (59 mg, 0.18 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.84 (d, J = 2.1 Hz, 1H), 8.81 (s, 1H), 8.50 (d, J = 5.1 Hz, 1H), 8.35 (d, J = 2.1 Hz, 1H), 7.23 (d, J = 5.0 Hz, 1H), 2.59 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.0, 152.5, 149.9, 149.5, 147.1, 145.1, 131.4, 124.6, 121.1, 119.5, 104.1, 82.9, 20.5 ppm.
- 5-((6-Bromoisothiazolo[4,3-b]pyridin-3-yl)ethynyl)picolinonitrile (6q)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a light-brown solid in 53% yield (58 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.96 (d, J = 1.5 Hz, 1H), 8.87 (d, J = 1.9 Hz, 1H), 8.39 (d, J = 2.1 Hz, 1H), 8.09 (dd, J = 8.1, 2.1 Hz, 1H), 7.76 (dd, J = 8.1, 0.9 Hz, 1H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.5, 153.3, 147.3, 143.5, 139.6, 133.3, 131.6, 128.0, 122.6, 121.3, 116.8, 102.7, 83.4 ppm.
- 6-Bromo-3-((5-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6r)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynyl-5-fluoropyridine (124 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as an off-white solid in 56% yield (60 mg, 0.18 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.85 (d, J = 2.1 Hz, 1H), 8.72 (s, 1H), 8.53 (d, J = 2.7 Hz, 1H), 8.37 (d, J = 2.1 Hz, 1H), 7.69 (ddd, J = 8.7, 2.8, 1.6 Hz, 1H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.1, 153.2, 148.3 (d, J = 4.1 Hz), 147.2, 144.2, 139.1, 138.9, 131.6, 125.5, 125.3, 121.2, 103.2 (d, J = 2.5 Hz), 80.5 ppm.
- HRMS m/z [M+H]+ calcd for C13H5BrFN3S 333.9445, found 333.9454.
- 6 -Bromo-3-((2-ethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6s)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 2-ethoxy-3-ethynylpyridine (150 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 82% yield (101 mg, 0.28 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.82 (d, J = 2.1 Hz, 1H), 8.34 (d, J = 2.1 Hz, 1H), 8.20 (dd, J = 5.0, 2.0 Hz, 1H), 7.88 (dd, J = 7.4, 2.0 Hz, 1H), 6.92 (dd, J = 7.4, 5.0 Hz, 1H), 4.50 (q, J = 7.1 Hz, 2H), 1.48 (t, J = 7.1 Hz, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 163.5, 155.0, 152.7, 148.3, 147.0, 145.9, 142.0, 131.4, 121.0, 116.3, 106.2, 104.2, 81.4, 62.8, 14.7 ppm.
- HRMS m/z [M+H]+ calcd for C15H10BrN3OS 359.9801, found 359.9799.
- 6-Bromo-3-((5-fluoro-2-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6t)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-5-fluoro-2-methoxypyridine (290 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 78% yield (183 mg, 0.50 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 8.84 (d, J = 2.1 Hz, 1H), 8.35 (d, J = 2.1 Hz, 1H), 8.06 (d, J = 3.0 Hz, 1H), 7.66 (dd, J = 7.7, 3.0 Hz, 1H), 4.04 (s, 3H).
- 13C NMR (101 MHz, CDCl3) δ 159.9, 155.6, 154.9, 153.1, 152.9, 147.0, 144.8, 135.1, 134.8, 131.3, 129.2, 129.0, 120.9, 106.5, 106.4, 102.0, 101.9, 82.0, 54.7 ppm.
- HRMS m/z [M+H]+ calcd for C14H7BrFN3OS 363.9550, found 363.9571.
- 6-Bromo-3-((2,6-dimethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6u)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-2,6-dimethoxypyridine (313 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. This compound was obtained using 3-ethynyl-2,6-dimethoxypyridine. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as orange solid in 82% yield (200 mg, 0.53 mmol).
- 1H NMR (400 MHz, Chloroform-d) 8.80 (d, J = 2.1 Hz, 1H), 8.31 (d, J = 2.1 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 6.37 (d, J = 8.2 Hz, 1H), 4.06 (s, 3H), 3.98 (s, 3H).
- 13C NMR (101 MHz, CDCl3) 163.9, 163.4, 154.8, 152.2, 146.6, 146.4, 144.3, 131.2, 120.7, 105.1, 102.0, 96.4, 80.1, 54.2, 53.9.
- HRMS m/z [M+H]+ calcd for C15H10BrN3O2S 375.9750, found 375.9747.
- 6-Bromo-3-((2-fluoro-5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (6v)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (188 mg, 0.64 mmol, 1.0 eq.), 3-ethynyl-2-fluoro-5-methylpyridine (259 mg, 1.92 mmol, 3 eq.), Pd(PPh3)2Cl2 (10 mg, 0.014 mmol, 0.02 eq.) and CuI (1.2 mg, 0.006 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a light-brown solid in 91% yield (205 mg, 0.59 mmol).
- 1H NMR (400 MHz, Chloroform-d) 8.85 (d, J = 2.1 Hz, 1H), 8.36 (d, J = 2.1 Hz, 1H), 8.07 (dt, J = 2.1, 0.9 Hz, 1H), 7.88 (ddd, J = 8.6, 2.4, 0.8 Hz, 1H), 2.37 (s, 4H).
- 13C NMR (101 MHz, CDCl3) 162.0, 159.6, 154.9, 153.0, 148.3, 148.1, 147.1, 144.4, 144.0, 144.0, 131.4, 130.9, 130.9, 121.0, 105.5, 105.2, 99.9, 99.9, 82.1, 82.1, 17.3 ppm. HRMS m/z [M+H]+ calcd for C14H7BrFN3S 347.9601, found 347.9603.6-Bromo-3-(quinolin-3-ylethynyl)isothiazolo[4,3-b]pyridine (6w)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 3-ethynylquinoline (156 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 81% yield (100 mg, 0.27 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.62 (t, J = 7.5 Hz, 1H, arom H), 7.75–7.83 (m, 1H, arom H), 7.85 (d, J = 8.1 Hz, 1H, arom H), 8.14 (d, J = 8.5 Hz, 1H, arom H), 8.37 (d, J = 2.0 Hz, 1H, arom H), 8.49 (d, J = 1.9 Hz, 1H, arom H), 8.86 (d, J = 2.0 Hz, 1H, arom H), 9.11 (d, J = 2.0 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C17H8BrN3S 365.9696, found 365.9686.
- 6-Bromo-3-(pyridin-4-ylethynyl)isothiazolo[4,3-b]pyridine (6x)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 4-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a light-yellow solid in 59% yield (63 mg, 0.2 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.52 (dd, J = 4.5, 1.5 Hz, 2H, arom H), 8.37 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 5.9 Hz, 2H, arom H), 8.85 (d, J = 2.1 Hz, 1H, arom H) ppm. HRMS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9532.
- 6-Bromo-3-(pyridin-2-ylethynyl)isothiazolo[4,3-b]pyridine (6y)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), 2-ethynylpyridine (105 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light-yellow solid in 74% yield (79 mg, 0.25 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 7.30–7.38 (m, 1H, arom H), 7.68–7.80 (m, 2H, arom H), 8.35 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 4.8 Hz, 1H, arom H), 8.84 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C13H6BrN3S 315.9539, found 315.9535.
- tert-Butyl (3-(6-bromoisothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)carbamate (8)
- This compound was obtained from 3,6-dibromoisothiazolo[4,3-b]pyridine (101 mg, 0.34 mmol, 1.0 eq.), N-Boc-propargylamine (158 mg, 1.02 mmol, 3 eq.), Pd(PPh3)2Cl2 (5 mg, 0.007 mmol, 0.02 eq.) and CuI (0.6 mg, 0.003 mmol, 0.01 eq.) using the general Sonogashira coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a light-orange solid in 89% yield (111.2 mg, 0.3 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 1.47 (s, 9H, 3 × CH3), 4.40 (d, J = 5.1 Hz, 2H, CH2), 4.93 (bs, 1H, NH), 8.32 (d, J = 2.0 Hz, 1H, arom H), 8.79 (d, J = 2.0 Hz, 1H, arom H) ppm. HR-MS m/z [M+H]+ calcd for C14H14BrN3O2S 368.0063, found 368.0065.
- Suzuki coupling at position 6 of the isothiazolo[4,3-b]pyridine scaffold
- General procedure
- A solution of the appropriate 3-substituted-6-bromoisothiazolo[4,3-b]pyridine analogue (1 eq.) in a mixture of dioxane/water (ratio 9:1) was degassed with argon and subsequently, the corresponding boronic acid or ester (1.2 eq.), Pd(PPh3)4 (0.02 eq.) and K2CO3 (2 eq.) were added. The mixture was degassed a second time, filled with argon and stirred at 90 °C overnight. After completion of the reaction as monitored by TLC, the reaction mixture was cooled down to room temperature and the volatiles were evaporated to dryness. The resulting residue was purified by silica gel flash chromatography and precipitated with diethyl ether yielding the title compounds. Compounds 3a-p, 7a-y were synthesized according to this procedure.
- 6-(3-Methoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3a)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-methoxyphenylboronic acid (29 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 69% yield (37.4 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.91 (s, 3H, OCH3), 7.03 (ddd, J = 8.3, 2.6, 0.8 Hz, 1H, arom H), 7.21–7.23 (m, 1H, arom H), 7.28–7.30 (m, 1H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.45–7.49 (m, 1H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.27 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.7, 1.1 Hz, 1H, arom H), 8.93 (d, J = 0.9 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.41 (OCH3), 80.02 (C triple bond), 104.15 (C triple bond), 113.39 (CH), 114.19 (CH), 119.23 (C), 119.96 (CH), 123.13 (CH), 126.44 (CH), 130.45 (CH), 136.44 (C), 138.12 (C), 138.69 (CH), 143.46 (C), 148.02 (C), 149.81 (CH), 152.33 (CH), 152.44 (CH), 155.12 (C), 160.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H13N3OS 344.0852, found 344.0835.
- 6-(2-Methoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3b)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-methoxyphenylboronic acid (29 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 74% yield (40.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.86 (s, 3H, OCH3), 7.06 (d, J = 8.2 Hz, 1H, arom H), 7.12 (td, J = 7.5, 0.9 Hz, 1H, arom H), 7.36 (ddd, J = 7.9, 5.0, 0.5 Hz, 1H, arom H), 7.41–7.48 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.64 (d, J = 3.7 Hz, 1H, arom H), 8.92 (s, 1H, arom H), 9.09 (d, J = 1.9 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.53 (OCH3), 80.16 (C triple bond), 103.74 (C triple bond), 111.37 (CH), 119.33 (C), 121.32 (CH), 123.11 (CH), 125.95 (C), 128.57 (CH), 130.46 (CH), 130.85 (CH), 134.75 (C), 138.68 (CH), 142.84 (C), 147.40 (C), 149.71 (CH), 152.32 (CH), 154.54 (CH), 155.34 (C), 156.72 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H13N3OS 344.0852, found 344.0875.
- 3-(Pyridin-3-ylethynyl)-6-(2-tolyl)isothiazolo[4,3-b]pyridine (3c)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-tolylboronic acid (26 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and diethyl ether (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 81% yield (41.8 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.35 (s, 3H, OCH3), 7.29–7.42 (m, 5H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.08 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.89 (d, J = 2.0 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H) ppm. 13C NMR (150 MHz, CDCl3) δ: 20.42 (CH3), 79.99 (C triple bond), 104.13 (C triple bond), 119.20 (C), 123.12 (CH), 126.38 (CH), 128.66 (CH), 128.79 (CH), 129.93 (CH), 130.84 (CH), 135.74 (C), 136.82 (C), 137.57 (C), 138.69 (CH), 143.44 (C), 147.60 (C), 149.78 (CH), 152.30 (CH), 153.76 (CH), 154.87 (C) ppm.
- HR-MS m/z [M+H]+ calcd for C20H13N3S 328.0903, found 328.0912.
- N,N-Dimethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)aniline (3d)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(dimethylamino)phenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase and a second time using dichloromethane and methanol (10:0.2), affording the title compound as an orange solid in 74% yield (41.7 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.07 (s, 6H, 2 ࠹NCH3), 6.84–6.91 (m, 2H, arom H), 7.38 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.62–7.68 (m, 2H, arom H), 7.95–8.03 (m, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.2 Hz, 1H, arom H), 8.94 (s, 1H, arom H), 9.19 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 40.28 (CH3), 80.26 (C triple bond), 103.64 (C triple bond), 112.74 (CH), 119.37 (C), 123.11 (CH), 123.63 (CH), 123.71 (C), 128.24 (CH), 136.49 (C), 138.68 (CH), 142.71 (C), 147.28 (C), 149.68 (CH), 150.86 (C), 152.31 (CH), 152.73 (CH), 155.72 (C) ppm.
- HR-MS m/z [M+H]+ calcd for C21H16N4S 357.1168, found 357.1167.
- 6-(4-Isopropoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3e)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-isopropoxyphenylboronic acid (34 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (47.5 mg, 0.13 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 1.40 (s, 3H, CH3), 1.42 (s, 3H, CH3), 4.62–4.70 (hept, J = 6.0 Hz, 1H, CH), 7.06 (d, J = 8.7 Hz, 2H, arom H), 7.38 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.65 (d, J = 8.7 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.7 Hz, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.66 (d, J = 4.8 Hz, 1H, arom H), 8.94 (s, 1H, arom H), 9.16 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 22.03 (CH3), 70.10 (CH), 80.15 (C triple bond), 103.99 (C triple bond), 116.57 (CH), 119.32 (C), 123.18 (CH), 125.16 (CH), 128.61 (C), 128.80 (CH), 136.24 (C), 138.76 (CH), 143.18 (C), 147.62 (C), 149.80 (CH), 152.34 (CH), 152.57 (CH), 155.40 (C), 158.84 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3OS 372.1165, found 372.1155.
- 2-Methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)aniline (3f)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-amino-3-methoxyphenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 10:0.1) as mobile phase and a second time using dichloromethane and methanol (10:0.1), affording the title compound as a light yellow solid in 77% yield (43.6 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 4.08 (bs, 2H, NH2), 6.84 (d, J = 8.0 Hz, 1H, arom H), 7.11 (d, J = 1.9 Hz, 1H, arom H), 7.18 (dd, J = 8.0, 1.9 Hz, 1H, arom H), 7.36 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.59 (OCH3), 80.18 (C triple bond), 103.77 (C triple bond), 109.25 (CH), 114.97 (CH), 119.30 (C), 120.60 (CH), 123.10 (CH), 124.28 (CH), 126.38 (C), 136.77 (C), 137.51 (C), 138.66 (CH), 142.87 (C), 147.41 (C), 147.67 (C), 149.70 (CH), 152.29 (CH), 152.69 (CH), 155.52 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H14N4OS 359.0961, found 359.0965.
- 6-(4-Fluorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3g)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-fluorophenylboronic acid (27 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 81% yield (42.4 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.22–7.28 (m, 2H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.65–7.71 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H), 9.11 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.95 (C triple bond), 104.27 (C triple bond), 116.48 (d, J = 21.8 Hz, CH), 119.20 (C), 123.24 (CH), 126.25 (CH), 129.36 (d, J = 8.3 Hz, CH), 132.85 (C), 135.57 (C), 138.70 (CH), 143.62 (C), 147.90 (C), 149.85 (CH), 152.12 (CH), 152.33 (CH), 155.00 (C), 163.36 (d, J = 249.6 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: -112.426 ppm.
- HRMS m/z [M+H]+ calcd for C19H10FN3S 332.0652, found 332.0652.
- 6-(2-Fluorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3h)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 2-fluorophenylboronic acid (27 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light-yellow solid in 72% yield (37.6 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.24–7.28 (m, 1H, arom H), 7.33 (td, J = 7.5, 1.1 Hz, 1H, arom H), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 7.44–7.49 (m, 1H, arom H), 7.56 (td, J = 7.7, 1.7 Hz, 1H, arom H), 7.96–8.00 (m, 1H, arom H), 8.29 (dd, J = 2.0, 0.9 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92–8.94 (d, J = 1.5 Hz, 1H, arom H), 9.08 (t, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.95 (C triple bond), 104.22 (C triple bond), 116.57 (d, J = 22.2 Hz, CH), 119.21 (C), 123.13 (CH), 124.64 (d, J = 13.5 Hz, C), 125.01 (d, J = 3.3 Hz, CH), 128.87 (d, J = 2.0 Hz, CH), 130.75 (d, J = 1.8 Hz, CH), 130.76 (d, J = 8.2 Hz, CH), 131.69 (C), 138.71 (CH), 143.59 (C), 147.84 (C), 149.82 (CH), 152.32 (CH), 152.98 (d, J = 3.5 Hz, CH), 154.82 (C), 159.96 (d, J = 249.6 Hz, C-F) ppm. 19F NMR (471 MHz, CDCl3) δ: −116.62 ppm.
- HRMS m/z [M+H]+ calcd for C19H10FN3S 332.06521, found 332.0646.
- 6-(3-Chlorophenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3i)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-chlorophenylboronic acid (30 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 1:1) as mobile phase, affording the title compound as a light yellow solid in 79% yield (43.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 7.36–7.39 (m, 1H, arom H), 7.44–7.51 (m, 2H, arom H), 7.58 (dt, J = 7.2, 1.6 Hz, 1H, arom H), 7.69 (t, J = 1.6 Hz, 1H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.26 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.10 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 79.90 (C triple bond), 104.39 (C triple bond), 119.16 (C), 123.15 (CH), 125.75 (CH), 126.78 (CH), 127.67 (CH), 128.98 (CH), 130.62 (CH), 135.19 (C), 135.35 (C), 138.56 (C), 138.71 (CH), 143.79 (C), 148.16 (C), 149.86 (CH), 151.80 (CH), 152.32 (CH), 154.81 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H10ClN3S 348.0357, found 348.0362.
- 6-(4-(2-Methoxyethoxy)phenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (3j)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(2-methoxyethoxy)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a light yellow solid in 79% yield (48.4 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.48 (s, 3H, OCH3), 3.76–3.83 (m, 2H, OCH2), 4.18–4.22 (m, 2H, OCH2), 7.10 (d, J = 8.7 Hz, 2H, arom H), 7.36 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.64 (d, J = 8.7 Hz, 2H, arom H), 7.97 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.19 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.4 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.13 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 59.31 (OCH3), 67.48 (OCH2), 70.95 (OCH2), 80.15 (C triple bond), 104.01 (C triple bond), 115.53 (CH), 119.29 (C), 123.17 (CH), 125.27 (CH), 128.74 (CH), 129.16 (C), 136.10 (C), 138.73 (CH), 143.20 (C), 147.64 (C), 149.80 (CH), 152.34 (CH), 152.48 (CH), 155.33 (C), 159.65 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1115.
- Methyl 2-methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzoate (3k)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-methoxy-4-methoxycarbonylphenylboronic acid (40 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure and DMF as solvent. The reaction mixture was stirred at 120 °C overnight. The residue was purified by precipitation using subsequently, methanol, dichloromethane and diethyl ether, affording the title compound as a light yellow solid in 75% yield (47.6 mg, 0.12 mmol).
- 1H NMR (300 MHz, DMSO) δ: 3.83 (s, 1H, OCH3), 3.98 (s, 1H, OCH3), 7.53–7.61 (m, 2H, arom H), 7.65 (s, 1H, arom H), 7.81 (d, J = 8.0 Hz, 1H, arom H), 8.12–8.18 (m, 1H, arom H), 8.70 (d, J = 4.8 Hz, 1H, arom H), 8.73 (d, J = 1.7 Hz, 1H, arom H), 8.91 (s, 1H, arom H), 9.34 (d, J = 1.7 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C22H15N3O3S 402.0907, found 402.0903.
- 4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenol (3l)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-hydroxyphenylboronic acid (26 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (42.1 mg, 0.13 mmol).
- 1H NMR (500 MHz, DMSO) δ: 6.92–6.97 (m, 2H, arom H), 7.55 (dd, J = 7.9, 4.9 Hz, 1H, arom H), 7.75–7.80 (m, 2H, arom H), 8.14 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.40 (d, J = 2.1 Hz, 1H, arom H), 8.69 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.90 (d, J = 2.0 Hz, 1H, arom H), 9.25 (d, J = 2.0 Hz, 1H, arom H), 9.89 (s, 1H, OH) ppm.
- 13C NMR (126 MHz, DMSO) δ: 80.34 (C triple bond), 103.59 (C triple bond), 116.24 (CH), 118.45 (C), 123.84 (CH), 123.92 (CH), 126.45 (C), 129.04 (CH), 135.75 (C), 138.88 (CH), 142.00 (C), 147.19 (C), 150.24 (CH), 151.67 (CH), 152.61 (CH), 155.16 (C), 158.65 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H11N3OS 330.0696, found 330.0685.
- 4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3m)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-aminocarbonylphenylboronic acid (32 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and methanol (in a ratio of 10:0.3) as mobile phase, affording the title compound as a beige solid in 78% yield (44.3 mg, 0.12 mmol).
- 1H NMR (600 MHz, DMSO) δ: 7.50 (s, 1H, NH), 7.57 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H, arom H), 8.03–8.08 (m, 4H), 8.12 (s, 1H, NH), 8.15–8.18 (m, 1H, arom H), 8.66 (d, J = 2.1 Hz, 1H, arom H), 8.70 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92 (dd, J = 2.1, 0.7 Hz, 1H, arom H), 9.34 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 80.25 (C triple bond), 103.95 (C triple bond), 118.42 (C), 123.96 (CH), 126.40 (CH), 127.64 (CH), 128.42 (CH), 134.47 (C), 134.92 (C), 138.64 (C), 138.93 (CH), 142.54 (C), 147.90 (C), 150.32 (CH), 151.71 (CH), 152.38 (CH), 154.76 (C), 167.35 (C) ppm.
- HRMS m/z [M+H]+ calcd for C20H12N4OS 357.0805, found 357.0790.
- N-Ethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3n)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(N-ethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 75% yield (45.5 mg, 0.12 mmol).1H NMR (600 MHz, CDCl3) δ: 1.30 (t, J = 7.3 Hz, 3H, CH3), 3.51–3.56 (m, 2H, CH2), 6.26 (t, J = 5.1 Hz, 1H, NH), 7.38 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.75–7.79 (m, 2H, arom H), 7.93–7.97 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.30 (d, J = 2.1 Hz, 1H, arom H), 8.65 (d, J = 4.0 Hz, 1H, arom H), 8.93 (s, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 14.90 (CH3), 35.06 (CH2), 79.91 (C triple bond), 104.39 (C triple bond), 119.17 (C), 123.19 (CH), 126.82 (CH), 127.71 (CH), 127.91 (CH), 135.07 (C), 135.43 (C), 138.71 (CH), 139.55 (C), 143.77 (C), 148.17 (C), 149.85 (CH), 151.86 (CH), 152.30 (CH), 154.85 (C), 166.59 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1112.
- N,N-Dimethyl-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3o)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(N,N-dimethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 79% yield (48.0 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.06 (s, 3H, CH3), 3.16 (s, 3H, CH3), 7.37 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.61 (d, J = 8.2 Hz, 2H, arom H), 7.75 (d, J = 8.2 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.29 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.5 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.39 (CH3), 39.56 (CH3), 79.91 (C triple bond), 104.32 (C triple bond), 119.16 (C), 123.14 (CH), 126.66 (CH), 127.58 (CH), 128.15 (CH), 135.69 (C), 136.82 (C), 137.85 (C), 138.69 (CH), 143.68 (C), 148.09 (C), 149.83 (CH), 151.99 (CH), 152.29 (CH), 154.91 (C), 170.76 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1108.
- (4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenyl)(pyrrolidin-1-yl)methanone (3p)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 4-(pyrrolidine-1-carbonyl)phenylboronic acid (42 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 81% yield (52.5 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ 1.93 (p, J = 6.6 Hz, 2H, CH2), 1.96–2.05 (m, 2H, CH2), 3.51 (t, J = 6.6 Hz, 2H, CH2), 3.70 (t, J = 7.0 Hz, 2H, CH2), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.71 (d, J = 8.4 Hz, 2H, arom H), 7.75 (d, J = 8.4 Hz, 2H, arom H), 7.99 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 24.41 (CH2), 26.40 (CH2), 46.27 (CH2), 49.61 (CH2), 79.91 (C triple bond), 104.30 (C triple bond), 119.15 (C), 123.13 (CH), 126.65 (CH), 127.46 (CH), 128.19 (CH), 135.69 (C), 137.64 (C), 138.04 (C), 138.69 (CH), 143.66 (C), 148.09 (C), 149.82 (CH), 151.99 (CH), 152.28 (CH), 154.91 (C), 168.80 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H18N4OS 411.1274, found 411.1271.
- N,N-Dimethyl-3-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3q)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(N,N-dimethylaminocarbonyl)phenylboronic acid (37 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 76% yield (46.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.06 (s, 3H, CH3), 3.17 (s, 3H, CH3), 7.37 (ddd, J = 7.9, 4.9, 0.7 Hz, 1H, arom H), 7.53 (dt, J = 7.6, 1.1 Hz, 1H, arom H), 7.59 (t, J = 7.7 Hz, 1H, arom H), 7.76–7.72 (m, 1H, arom H), 7.77–7.78 (m, 1H, arom H), 7.99 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.29 (d, J = 2.1 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.41 (CH3), 39.61 (CH3), 79.93 (C triple bond), 104.30 (C triple bond), 119.17 (C), 123.13 (CH), 126.26 (CH), 126.69 (CH), 127.41 (CH), 128.62 (CH), 129.42 (CH), 135.74 (C), 137.09 (C), 137.57 (C), 138.70 (CH), 143.67 (C), 148.07 (C), 149.82 (CH), 151.99 (CH), 152.29 (CH), 154.90 (C), 170.79 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H16N4OS 385.1118, found 385.1118.
- (3-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenyl)(pyrrolidin-1-yl)methanone (3r)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(pyrrolidine-1-carbonyl)phenylboronic acid pinacol ester (61 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a yellow solid in 69% yield (44.8 mg, 0.11 mmol).1H NMR (600 MHz, CDCl3) δ: 1.89–1.96 (m, 2H, CH2), 1.98–2.04 (m, 2H, CH2), 3.51 (t, J = 6.6 Hz, 2H, CH2), 3.70 (t, J = 7.0 Hz, 2H, CH2), 7.37 (ddd, J = 7.9, 4.9, 0.8 Hz, 1H), 7.59 (t, J = 7.6 Hz, 1H, arom H), 7.62–7.65 (m, 1H, arom H), 7.74–7.77 (m, 1H, arom H), 7.88 (bs, 1H, arom H), 7.98 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.29 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.9, 1.5 Hz, 1H, arom H), 8.93 (d, J = 1.4 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.13C NMR (150 MHz, CDCl3) δ: 24.40 (CH2), 26.37 (CH2), 46.28 (CH2), 49.65 (CH2), 79.93 (C triple bond), 104.27 (C triple bond), 119.15 (C), 123.12 (CH), 126.36 (CH), 126.64 (CH), 127.43 (CH), 128.81 (CH), 129.32 (CH), 135.79 (C), 136.91 (C), 138.40 (C), 138.69 (CH), 143.62 (C), 148.03 (C), 149.79 (CH), 152.01 (CH), 152.26 (CH), 154.89 (C), 168.85 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H18N4OS 411.1274, found 411.1271.
- N-(2-Methoxyethyl)-3-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzamide (3s)
- This compound was obtained from the precursor 2b (51 mg, 0.16 mmol, 1.0 eq.), 3-(2-methoxyethylaminocarbonyl)benzeneboronic acid pinacol ester (62 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 75% yield (49.1 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.41 (s, 3H, OCH3), 3.61 (t, J = 5.0 Hz, 2H, CH2), 3.71 (dd, J = 10.3, 5.3 Hz, 2H, CH2), 6.70 (bs, 1H, NH), 7.37 (dd, J = 7.8, 4.9 Hz, 1H, arom H), 7.62 (t, J = 7.7 Hz, 1H, arom H), 7.83 (d, J = 7.8 Hz, 1H, arom H), 7.86 (d, J = 7.7 Hz, 1H, arom H), 7.96–8.02 (m, 1H, arom H), 8.16 (bs, 1H, arom H), 8.31 (d, J = 2.0 Hz, 1H, arom H), 8.65 (dd, J = 4.8, 1.3 Hz, 1H, arom H), 8.92 (d, J = 1.2 Hz, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 39.84 (CH2), 58.86 (OCH3), 71.07 (CH2), 79.94 (C triple bond), 104.33 (C triple bond), 119.18 (C), 123.14 (CH), 126.54 (CH), 126.80 (CH), 127.04 (CH), 129.58 (CH), 130.41 (CH), 135.67 (C), 135.76 (C), 137.28 (C), 138.71 (CH), 143.71 (C), 148.10 (C), 149.83 (CH), 151.98 (CH), 152.30 (CH), 154.88 (C), 166.82 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H18N4O2S 415.1223, found 415.1223.
- 6-(3,4-Dimethoxyphenyl)-3-(pent-1-yn-1-yl)isothiazolo[4,3-b]pyridine (7a)
- This compound was obtained from the precursor 2g (49 mg, 0.17 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (36 mg, 0.20 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 3:2) as mobile phase and a second purification using a mixture of hexane and dichloromethane (in a ratio of 2:3), affording the title compound as a light-yellow solid in 74% yield (44.3 mg, 0.13 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 1.11 (t, J = 7.4 Hz, 3H, CH3), 1.77 (h, J = 7.3 Hz, 2H, CH2), 2.67 (t, J = 7.1 Hz, 2H, CH2), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 7.02 (d, J = 8.3 Hz, 1H, arom H), 7.18 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.2, 2.2 Hz, 1H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 9.08 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 13.69 (CH3), 21.80 (CH2), 22.59 (CH2), 56.05 (OCH3), 68.81 (C triple bond), 110.47 (CH), 110.93 (C triple bond), 111.76 (CH), 120.19 (CH), 125.38 (CH), 129.62 (C), 136.01 (C), 145.59 (C), 147.56 (C), 149.66 (C), 149.88 (C), 151.74 (CH), 155.15 (C) ppm.
- HRMS m/z [M+H]+ calcd for C19H18N2O2S 339.1162, found 339.1162.
- 6-(3,4-Dimethoxyphenyl)-3-(phenylethynyl)isothiazolo[4,3-b]pyridine (7b)
- This compound was prepared according to a procedure described in the literature [28].
- 6-(3,4-Dimethoxyphenyl)-3-((4-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7c)
- This compound was obtained from the precursor 6c (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 83% yield (48.5 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.09–7.14 (m, 2H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.66–7.70 (m, 2H, arom H), 8.19 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.04 (OCH3), 106.85 (C triple bond), 110.43 (CH), 111.76 (CH), 115.93 (d, J = 22.2 Hz, CH), 118.10 (C), 120.20 (CH), 125.35 (CH), 129.44 (C), 133.96 (d, J = 8.5 Hz, CH) 136.17 (C), 144.15 (C), 147.52 (C), 149.68 (C), 149.94 (C), 152.11 (CH), 155.23 (C), 163.28 (d, J = 252.1 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: −108.32 ppm.
- HRMS m/z [M+H]+ calcd for C22H15FN2O2S 391.0911, found 391.0899.
- 6-(3,4-Dimethoxyphenyl)-3-((3-chlorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7d)
- This compound was obtained from the precursor 6d (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 84% yield (49.2 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.33–7.37 (m, 1H, arom H), 7.39–7.42 (m, 1H, arom H), 7.57 (dt, J = 7.5, 1.3 Hz, 1H, arom H), 7.69 (t, J = 1.6 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.04 (OCH3), 78.03 (C triple bond), 106.09 (C triple bond), 110.42 (CH), 111.76 (CH), 120.21 (CH), 123.60 (C), 125.33 (CH), 129.37 (C), 129.72 (CH), 129.83 (CH), 129.93 (CH), 131.63 (CH), 134.40 (C), 136.22 (C), 143.62 (C), 147.64 (C), 149.67 (C), 149.96 (C), 152.28 (CH), 155.23 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H15ClN2O2S 407.0615, found 407.0581.
- 6-(3,4-Dimethoxyphenyl)-3-((2-fluorophenyl)ethynyl)isothiazolo[4,3-b]pyridine (7e)
- This compound was obtained from the precursor 6e (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 81% yield (47.4 mg, 0.12 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.4 Hz, 1H, arom H), 7.14–7.22 (m, 1H), 7.28 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.39–7.45 (m, 1H, arom H), 7.68 (td, J = 7.3, 1.7 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.05 (OCH3), 81.67 (C triple bond), 101.02 (C triple bond), 110.46 (CH), 110.83 (d, J = 15.5 Hz, C), 111.79 (CH), 115.76 (d, J = 20.5 Hz, CH), 120.22 (CH), 124.12 (d, J = 3.3 Hz, CH), 125.30 (CH), 129.46 (C), 131.52 (d, J = 7.9 Hz, CH), 133.53 (CH), 136.18 (C), 143.73 (C), 147.69 (C), 149.68 (C), 149.95 (C), 152.30 (CH), 155.20 (C), 162.52 (d, J = 253.9 Hz, C-F) ppm.
- 19F NMR (471 MHz, CDCl3) δ: −107.77 ppm.
- HRMS m/z [M+H]+ calcd for C22H15FN2O2S 391.0911, found 391.0899.
- 6-(3,4-Dimethoxyphenyl)-3-((3-methoxyphenyl)ethynyl)isothiazolo[4,3-b]pyridine (7f)
- This compound was obtained from the precursor 6e (50 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. This compound was obtained using the precursor 6f and 3,4-dimethoxyphenylboronic acid. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 72% yield (41.9 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.85 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 6.99 (ddd, J = 8.1, 2.6, 1.3 Hz, 1H, arom H), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.21 (dd, J = 2.6, 1.3 Hz, 1H, arom H), 7.27–7.30 (m, 2H, arom H), 7.32 (t, J = 7.8 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 9.13 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 55.40 (OCH3), 56.04 (OCH3), 108.04 (C), 110.43 (CH), 111.77 (CH), 116.29 (CH), 116.57 (CH), 120.21 (CH), 122.87 (C), 124.52 (CH), 125.37 (CH), 129.47 (C), 129.57 (CH), 136.15 (C), 144.33 (C), 147.49 (C), 149.67 (C), 149.93 (C), 152.08 (CH), 155.23 (C), 159.36 (C) ppm.HR-MS m/z [M+H]+ calcd for C23H18N2O3S 403.1111, found 403.1093.
- 6-(3,4-Dimethoxyphenyl)-3-((6-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7g)
- This compound was obtained from the precursor 6g (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 79% yield (46.3 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.62 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.23 (d, J = 8.0 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.86 (dd, J = 8.0, 2.2 Hz, 1H, arom H), 8.21 (d, J = 2.1 Hz, 1H, arom H), 8.81 (d, J = 1.6 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 24.67 (CH3), 56.05 (OCH3), 79.43 (C), 104.66 (C), 110.42 (CH), 111.71 (CH), 116.14 (C), 120.22 (CH), 122.85 (CH), 125.38 (CH), 129.37 (C), 136.25 (C), 138.90 (CH), 143.65 (C), 147.57 (C), 149.66 (C), 149.97 (C), 151.71 (CH), 152.30 (CH), 155.23 (C), 159.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1111.
- 6-(3,4-Dimethoxyphenyl)-3-((6-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7h)
- This compound was obtained from the precursor 6h (48 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 8:2) as mobile phase and a second time using dichloromethane and methanol (10:0.1), affording the title compound as a yellow solid in 71% yield (41.3 mg, 0.10 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 6.80 (d, J = 8.6 Hz, 1H, arom H), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 1.9 Hz, 1H, arom H), 7.26–7.32 (m, 1H, arom H), 7.84 (dd, J = 2.3, 8.6 Hz, 1H, arom H), 8.20 (d, J = 1.9 Hz, 1H, arom H), 8.51 (d, J = 2.0 Hz, 1H, arom H), 9.12 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 53.90 (OCH3), 56.08 (OCH3), 78.57 (C), 105.16 (C), 110.47 (CH), 111.01 (CH), 111.81 (CH), 111.92 (C), 120.25 (CH), 125.41 (CH), 129.46 (C), 136.23 (C), 141.27 (CH), 144.16 (C), 147.47 (C), 149.71 (C), 149.98 (C), 150.71 (CH), 152.13 (CH), 155.24 (C), 164.24 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1065.
- 6-(3,4-Dimethoxyphenyl)-3-((5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7i)
- This compound was obtained from the precursor 6i (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a yellow solid in 81% yield (47.5 mg, 0.12 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.39 (s, 3H, CH3), 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.20 (d, J = 1.7 Hz, 1 H, arom H), 7.26–7.31 (m, 1H, arom H), 7.80 (s, 1H, arom H), 8.21 (d, J = 1.8 Hz, 1H), 8.47 (s, 1H, arom H), 8.73 (s, 1H, arom H), 9.14 (d, J = 1.8 Hz, 1H, arom H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 18.25 (CH3), 56.08 (OCH3), 79.79 (C), 104.42 (C), 110.47 (CH), 111.82 (CH), 118.68 (C), 120.27 (CH), 125.40 (CH), 129.38 (C), 132.88 (C), 136.32 (C), 139.09 (CH), 143.48 (C), 147.70 (C), 149.48 (CH), 149.73 (C), 150.02 (C), 150.50 (CH), 152.41 (CH), 155.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1115.
- 6-(3,4-Dimethoxyphenyl)-3-((6-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7j)
- This compound was obtained from the precursor 6j (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 75% yield (43.9 mg, 0.11 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.00–7.05 (m, 2H, arom H), 7.19 (d, J = 1.8 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.0 Hz, 1H, arom H), 8.04–8.10 (m, 1H, arom H), 8.22 (d, J = 2.0 Hz, 1H, arom H), 8.56 (s, 1H, arom H), 9.15 (d, J = 2.0 Hz, 1H, arom H) ppm. 19F NMR (282 MHz, CDCl3) δ: −63.82 ppm.
- 13C NMR (126 MHz, CDCl3) δ: 56.03 (OCH3), 79.94 (C), 102.56 (C), 109.73 (CH, d, J = 37.9 Hz), 110.34 (CH), 111.72 (CH), 117.13 (C), 120.22 (CH), 125.38 (CH), 129.23 (C), 136.28 (C), 142.98 (C), 143.85 (CH, d, J = 8.3 Hz), 147.67 (C), 149.66 (C), 149.97 (C), 151.03 (CH, d, J = 15.6 Hz), 152.51 (CH), 155.23 (C), 163.22 (CF, d, J = 244.3 Hz) ppm.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0870.
- Methyl 5-((6-(3,4-dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (7k)
- This compound was obtained from the precursor 6k (65 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (42 mg, 0.3 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a light yellow solid in 76% yield (44.5 mg, 0.11 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 1.9 Hz, 1H, arom H), 7.24–7.32 (m, 2H, arom H), 8.04–8.13 (m, 1H, arom H), 8.21 (d, J = 2.0 Hz, 1H, arom H), 8.28 (d, J = 4.3 Hz, 1H, arom H), 9.15 (d, J = 1.9 Hz, 1H, arom H) ppm.
- 19F NMR (282 MHz, CDCl3) δ -61.97 ppm.
- 13C NMR (75 MHz, CDCl3) δ: 56.08 (OCH3), 83.12 (C), 98.68 (d, J = 5.1 Hz, C), 106.54 (d, J = 31.0 Hz, C), 110.45 (CH), 111.81 (CH), 120.27 (CH), 121.2 (d, J = 4.4 Hz, CH), 125.37 (CH), 129.31 (C), 136.36 (C), 142.72 (C), 143.58 (CH), 147.90 (C), 148.15 (d, J = 14.3 Hz, CH), 149.88 (d, J = 23.5 Hz, C), 152.68 (CH), 155.24 (C), 160.34 (d, J = 244.4 Hz, C) ppm.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0859.
- 6-(3,4-Dimethoxyphenyl)-3-((5-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7l)
- This compound was obtained from the precursor 6l (48.5 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 71% yield (41.3 mg, 0.10 mmol).
- 1H NMR (300 MHz, DMSO) δ: 3.84 (s, 3H, OCH3). 3.91 (s, 6H, 2 x OCH3), 7.12 (d, J = 8.8 Hz, 1H, arom H), 7.50 (bs, 2H, arom H), 7.73 (s, 1H, arom H), 8.41 (d, J = 2.4 Hz, 1H, arom H), 8.48 (s, 1H, arom H), 8.54 (s, 1H, arom H), 9.31 (s, 1H, arom H) ppm.
- 13C NMR (75 MHz, DMSO) δ: 56.11 (OCH3), 56.21 (OCH3), 56.34 (OCH3), 80.49 (C), 103.98 (C), 111.51 (CH), 112.68 (CH), 119.08 (C), 120.63 (CH), 122.39 (CH), 125.00 (CH), 128.74 (C), 136.02 (C), 139.67 (CH), 142.41 (C), 144.09 (CH), 147.73 (C), 149.85 (C), 150.25 (C), 153.10 (CH), 155.47 (C), 155.52 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1067.
- Methyl 5-((6-(3,4-dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)nicotinate (7m)
- This compound was obtained from the precursor 6m (49 mg, 0.13 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.3 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2.2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 4:1) as mobile phase, affording the title compound as an orange solid in 69% yield (39.8 mg, 0.09 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 6H, 2 x OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.20 (d, J = 1.8 Hz, 1H, arom H), 7.30 (dd, J = 8.3, 1.9 Hz, 1H, arom H), 8.23 (d, J = 1.9 Hz, 1H, arom H), 8.59 (t, J = 1.8 Hz, 1H, arom H), 9.06 (d, J = 1.8 Hz, 1H, arom H), 9.16 (d, J = 1.9 Hz, 1H, arom H), 9.22 (d, J = 1.8 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 52.70 (OCH3), 56.05 (OCH3), 81.01 (C), 102.68 (C), 110.41 (CH), 111.78 (CH), 119.29 (C), 120.26 (CH), 125.40 (CH), 125.73 (C), 129.25 (C), 136.40 (C), 139.62 (CH), 142.69 (C), 147.82 (C), 149.71 (C), 150.03 (C), 150.51 (CH), 152.66 (CH), 155.27 (C), 155.36 (CH),164.84 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H17N3O4S 432.1012, found 432.1021.
- 6-(3,4-Dimethoxyphenyl)-3-((6-(trifluoromethyl)pyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7n)
- This compound was obtained from the precursor 6n (50 mg, 0.13 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.3 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2.2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 74% yield (42.5 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.76 (dd, J = 8.1, 0.6 Hz, 1H, arom H), 8.16 (dd, J = 8.0, 1.6 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 9.00 (d, J = 1.6 Hz, 1H, arom H), 9.17 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 19F NMR (282 MHz, CDCl3) δ: −68.07 ppm.
- 13C NMR (151 MHz, CDCl3) δ: 56.03 (OCH3), 56.05 (OCH3), 82.19 (C), 102.13 (C), 110.38 (CH), 111.77 (CH), 120.02 (CH), 120.25 (CH), 122.23 (C), 125.39 (CH), 129.14 (C), 136.44 (C), 139.97 (CH), 142.23 (C), 147.61 (q, J = 35.3 Hz, C). 147.85 (C), 149.71 (C), 150.06 (C), 152.16 (CH), 152.80 (CH), 155.27 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H14F3N3O2S 442.0831, found 442.0828.
- 6-(3,4-Dimethoxyphenyl)-3-((4-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7o)
- This compound was obtained from the precursor 6o (62 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as an orange solid in 71% yield (51 mg, 0.13 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.14 (d, J = 2.1 Hz, 1H), 8.76 (s, 1H), 8.52 (d, J = 5.8 Hz, 1H), 8.20 (d, J = 2.1 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.4 Hz, 1H), 6.89 (d, J = 5.8 Hz, 1H), 4.02 (s, 3H), 3.99 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 165.72, 155.35, 154.06, 152.45, 151.98, 150.11, 149.84, 147.84, 143.96, 136.37, 129.62, 125.48, 120.39, 111.94, 110.61, 106.36, 101.14, 83.25, 56.2, 56.1 ppm. HRMS m/z [M+H]+ calcd for C22H17N3O3S 404.1063, found 404.1058.
- 6-(3,4-Dimethoxyphenyl)-3-((4-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7p)
- This compound was obtained from the precursor 6p (46 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as a brown solid in 95% yield (67 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.15 (d, J = 2.1 Hz, 1H), 8.83 (s, 1H), 8.49 (d, J = 5.1 Hz, 1H), 8.21 (d, J = 2.1 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.24 (d, J = 5.0 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.97 (s, 3H), 2.61 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.42, 152.56, 152.50, 150.12, 149.83, 149.70, 149.38, 147.90, 143.63, 136.40, 129.52, 125.43, 124.54, 120.37, 119.72, 111.93, 110.56, 103.23, 83.51, 56.20, 56.21, 20.57 ppm.
- HRMS m/z [M+H]+ calcd for C22H17N3O2S 388.1114, found 388.1114.
- 5-((6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)ethynyl)picolinonitrile (7q)
- This compound was obtained from the precursor 6q (48 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 7:3) as mobile phase, affording the title compound as an orange solid in 70% yield (40 mg, 0.10 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.17 (d, J = 2.1 Hz, 1H), 8.98 (dd, J = 2.1, 0.9 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.10 (dd, J = 8.0, 2.1 Hz, 1H), 7.76 (dd, J = 8.1, 0.9 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 155.46, 153.29, 153.13, 150.27, 149.90, 148.10, 141.98, 139.47, 136.70, 133.12, 129.23, 127.95, 125.57, 122.91, 120.44, 116.90, 119.97, 110.57, 101.93, 84.10, 56.24, 56.22 ppm.
- HRMS m/z [M+H]+ calcd for C22H14N4O2S 399.0910, found 399.0912.
- 6-(3,4-Dimethoxyphenyl)-3-((5-fluoropyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7r)
- This compound was obtained from the precursor 6r (50 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 4:1) as mobile phase, affording the title compound as a yellow solid in 75% yield (44 mg, 0.11 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.16 (d, J = 2.1 Hz, 1H), 8.74 (s, 1H), 8.52 (d, J = 2.8 Hz, 1H), 8.22 (d, J = 2.0 Hz, 1H), 7.70 (ddd, J = 8.7, 2.8, 1.7 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.20 (d, J = 2.2 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 154.14 (d, J = 259 Hz), 152.84, 150.20, 149.88, 148.34 (d, J = 4.1 Hz), 147.99, 142.72, 138.89, 138.77(d, J = 23 Hz), 136.56, 129.39, 125.54, 125.34 (d, J= 20 Hz), 120.41, 111.95, 110.58, 102.34 (d, J = 2.6 Hz), 81.11, 56.23, 56.21.
- HRMS m/z [M+H]+ calcd for C21H14FN3O2S 392.0863, found 392.0863.
- 6-(3,4-Dimethoxyphenyl)-3-((2-ethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7s)
- This compound was obtained from the precursor 6s (90 mg, 0.25 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (55 mg, 0.3 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (69 mg, 0.5 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 67% yield (70 mg, 0.17 mmol).
- 1H NMR (400 MHz, Chloroform-d) δ 9.13 (d, J = 2.0 Hz, 1H), 8.20 (s, 1H), 7.90 (dd, J = 7.4, 1.9 Hz, 1H), 7.29 (dd, J = 8.3, 2.1 Hz, 1H), 7.20 (d, J = 2.1 Hz, 1H), 7.03 (d, J = 8.3 Hz, 1H), 6.92 (dd, J = 7.4, 5.0 Hz, 1H), 4.52 (q, J = 7.0 Hz, 2H), 3.99 (s, 3H), 3.97 (s, 3H), 1.49 (t, J = 7.0 Hz, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) δ 163.47, 155.34, 152.27, 150.08, 149.83, 148.02, 147.78, 144.37, 142.00, 136.32, 129.66, 125.49, 120.38, 116.33, 111.93, 110.61, 106.47, 103.22, 81.96, 62.79, 56.23, 56.21, 14.72 ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O3S 418.1220, found 418.1208.
- 6-(3,4-Dimethoxyphenyl)-3-((5-fluoro-2-methoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7t)
- This compound was obtained from the precursor 6s (98 mg, 0.27 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (60 mg, 0.32 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (75 mg, 0.54 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 85:15) as mobile phase, affording the title compound as a yellow solid in 78% yield (90 mg, 0.21 mmol).
- 1H NMR (400 MHz, CDCl3) 9.14 (d, J = 2.1 Hz, 1H), 8.20 (d, J = 2.1 Hz, 1H), 8.06 (d, J = 3.0 Hz, 1H), 7.67 (dd, J = 7.8, 3.0 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 4.05 (s, 3H), 3.99 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 159.88, 155.6, 155.22, 154.36 (d, J = 246 Hz), 152.45, 150.02, 149.73, 147.84, 143.31, 136.32, 134.66 (d, J = 26 Hz), 129.40, 129.05 (d, J = 23 Hz), 125.39, 120.28, 111.81, 110.47, 106.75 (d, J = 6 Hz), 101.13, 82.62, 56.10, 56.08, 54.67 ppm.
- HRMS m/z [M+H]+ calcd for C22H16FN3O3S 422.0969, found 422.0958.
- 6-(3,4-Dimethoxyphenyl)-3-((2,6-dimethoxypyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7u)
- This compound was obtained from the precursor 6u (100 mg, 0.27 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (60 mg, 0.32 mmol, 1.2 eq.), Pd(PPh3)4 (6 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (75 mg, 0.54 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as a yellow solid in 61% yield (70 mg, 0.16 mmol).
- 1H NMR (400 MHz, CDCl3) 9.11 (d, J = 2.1 Hz, 1H), 8.18 (d, J = 2.1 Hz, 1H), 7.80 (d, J = 8.2 Hz, 1H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H), 7.19 (d, J = 2.1 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 6.38 (d, J = 8.2 Hz, 1H), 4.07 (s, 3H), 3.99 (s, 3H), 3.98 (s, 3H), 3.96 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 163.75, 163.35, 155.16, 151.80, 149.91, 149.69, 147.46, 144.94, 144.33, 136.09, 129.65, 125.35, 120.23, 111.79, 110.50, 104.02, 101.92, 96.76, 80.55, 56.08, 54.15, 53.86 ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O4S 434.1169, found 434.1162.
- 6-(3,4-Dimethoxyphenyl)-3-((2-fluoro-5-methylpyridin-3-yl)ethynyl)isothiazolo[4,3-b]pyridine (7v)
- This compound was obtained from the precursor 6v (101 mg, 0.29 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (63 mg, 0.35 mmol, 1.2 eq.), Pd(PPh3)4 (6.7 mg, 0.0058 mmol, 0.02 eq.) and K2CO3 (80 mg, 0.58 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as an orange solid in 92% yield (107 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3). 9.15 (d, J = 2.0 Hz, 1H), 8.21 (d, J = 2.1 Hz, 1H), 8.06 (s, 1H), 7.90 (d, J = 8.7 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.19 (d, J = 2.2 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.97 (s, 3H), 2.37 (s, 3H) ppm.
- 13C NMR (101 MHz, CDCl3) 160.81 (d, J = 240 Hz), 155.22, 152.59, 150.02, 149.72, 147.94 (d, J = 14 Hz), 147.85, 143.98, 142.87, 136.33, 130.88 (d, J = 5 Hz), 129.33, 125.36, 120.27, 111.81, 110.44, 105.62 (d, J = 32 Hz), 99.06 (d, J = 6 Hz), 82.74, 56.07, 17.30 ppm
- HRMS m/z [M+H]+ calcd for C22H16FN3O2S 406.1020, found 406.1014.
- 6-(3,4-Dimethoxyphenyl)-3-(quinolin-3-ylethynyl)isothiazolo[4,3-b]pyridine (7w)
- This compound was obtained from the precursor 6w (51 mg, 0.14 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (32 mg, 0.17 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (39 mg, 0.28 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase and a second time using dichloromethane and methanol (in a ratio of 10:0.2), affording the title compound as a yellow solid in 75% yield (43.3 mg, 0.10 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.3 Hz, 1H, arom H), 7.21 (d, J = 2.1 Hz, 1H, arom H), 7.30 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.60–7.65 (m, 1H, arom H), 7.77–7.83 (m, 1H, arom H), 7.86 (d, J = 7.9 Hz, 1H, arom H), 8.15 (d, J = 8.5 Hz, 1H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.51 (d, J = 1.8 Hz, 1H, arom H), 9.14 (d, J = 1.9 Hz, 1H, arom H), 9.17 (d, J = 2.0 Hz, 1H, arom H) ppm. 13C NMR (150 MHz, CDCl3) δ: 56.1 (OCH3), 56.06 (OCH3), 80.05 (C), 104.87 (C), 110.44 (CH), 111.79 (CH), 116.14 (C), 120.25 (CH), 125.41 (CH), 127.01 (C), 127.61 (CH), 127.92 (CH), 129.36 (C), 129.54 (CH), 130.89 (CH), 136.33 (C), 139.24 (CH), 143.42 (C), 147.36 (C), 147.75 (C), 149.74 (C), 149.71 (C), 151.51 (CH), 152.44 (CH), 155.28 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H17N3O2S 424.1114, found 424.1113.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-4-ylethynyl)isothiazolo[4,3-b]pyridine (7x)
- This compound was obtained from the precursor 6w (47 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (39 mg, 0.22 mmol, 1.2 eq.), Pd(PPh3)4 (5.5 mg, 0.005 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 71% yield (41.8 mg, 0.11 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 7.04 (d, J = 8.3, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.53–7.55 (m, 2H, arom H), 8.23 (d, J = 2.1 Hz, 1H, arom H), 8.70 (d, J = 5.8 Hz, 2H, arom H), 9.16 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.08 (OCH3), 80.92 (C triple bond), 104.17 (C triple bond), 110.44 (CH), 111.81 (CH), 120.27 (CH), 125.38 (CH), 125.40 (CH), 129.26 (C), 129.97 (C), 136.42 (C), 142.67 (C), 147.84 (C), 149.74 (C), 149.98 (CH), 150.06 (C), 152.73 (CH), 155.31 (C) ppm. HRMS m/z [M+H]+ calcd for C21H15N3O2S 374.0958, found 374.0948.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-2-ylethynyl)isothiazolo[4,3-b]pyridine (7y)
- This compound was obtained from the precursor 6y (50 mg, 0.16 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (35 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.025 eq.) and K2CO3 (44 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase, affording the title compound as a light yellow solid in 59% yield (34.6 mg, 0.09 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.04 (d, J = 8.4 Hz, 1H, arom H), 7.20 (d, J = 2.1 Hz, 1H, arom H), 7.29 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 7.32–7.35 (m, 1H, arom H), 7.72–7.78 (m, 2H, arom H), 8.22 (d, J = 2.1 Hz, 1H, arom H), 8.70–8.72 (m, 1H, arom H), 9.15 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 56.06 (OCH3), 56.07 (OCH3), 76.45 (C triple bond), 106.11 (C triple bond), 110.45 (CH), 111.78 (CH), 120.25 (CH), 123.79 (CH), 125.38 (CH), 127.69 (CH), 129.42 (C), 136.30 (C), 136.30 (CH), 142.37 (C), 143.10 (C), 148.04 (C), 149.70 (C), 149.97 (C), 150.39 (CH), 152.46 (CH), 155.19 (C) ppm.
- HRMS m/z [M+H]+ calcd for C21H15N3O2S 374.0958, found 374.0953.
- tert-Butyl (3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)carbamate (9)
- This compound was obtained from the precursor 8 (59 mg, 0.16 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (35 mg, 0.19 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.025 eq.) and K2CO3 (44 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 3:2) as mobile phase, affording the title compound as a light yellow solid in 87% yield (57.7 mg, 0.13 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 1.48 (s, 9H, 3× CH3), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.42 (d, J = 4.2 Hz, 2H, CH2), 4.98 (bs, 1H, NH), 7.02 (d, J = 8.4 Hz, 1H, arom H), 7.17 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 28.3 (CH3 tBuO), 31.8 (CH2), 56.0 (OCH3), 71.0 (C triple bond), 80.4 (C), 104.8 (C triple bond), 110.5 (CH), 111.8 (CH), 120.2 (CH), 125.4 (CH), 129.4 (C), 136.2 (C), 143.7 (C), 147.8 (C), 149.7 (C), 150.0 (C), 152.3 (CH), 155.1 (C) ppm.
- HRMS m/z [M+H]+ calcd for C22H23N3O4S 426.1482, found 426.1489.
- 3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-aminium chloride (10)
- To a solution of the Boc-amino precursor 9 (100 mg, 0.235 mmol, 1.0 eq.) in 1,4-dioxane (5 mL) at 0 °C, hydrogen chloride solution 4.0 M in 1,4-dioxane (10 mL) was added carefully. The resulting mixture was stirred at room temperature for 4–5 h. After completion of the reaction as monitored by TLC, the volatiles were evaporated to dryness and the resulting residue was co-evaporated with dichloromethane and precipitate with a mixture of dichloromethane and diethyl ether (in a ratio 1:4), yielding the title compound as hydrochloric salt (orange solid) in 98% yield (83.3 mg, 0.23 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.90 (s, 2H, CH2), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.18 (d, J = 2.1 Hz, 1H, arom H), 7.27 (dd, J = 8.3, 2.2 Hz, 1H, arom H), 8.18 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C17H15N3O2S 326.0958, found 326.0962.
- Derivatisation of the propargylamino moiety at position 3 of the isothiazolo[4,3-b]pyridine scaffold (11a-c)
- General procedure
- To a solution of the amino precursor 10 (1 eq.) in dry dichloromethane (15 mL), dry triethylamine (2 eq.) was added. The reaction mixture was cooled at 0 °C and the appropriated acid chloride, sulfonyl chloride or isocyanate (1.2 eq.) was added. The resulting mixture was stirred at room temperature overnight. After completion of the reaction as monitored by TLC, the volatiles were evaporated to dryness and the resulting residue was purified by silica gel and precipitated with diethyl ether, yielding the corresponding 3-modified isothiazolo[4,3-b]pyridine. Compounds 11a-c were made according to this procedure.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)benzamide (11a)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0 eq.), benzoyl chloride (24 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 2:3) as mobile phase and a second purification using a mixture of dichloromethane and acetone (in a ratio of 9:1), affording the title compound as a yellow solid in 68% yield (40.3 mg, 0.09 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.75 (d, J = 5.2 Hz, 2H, CH2), 6.80 (bs, 1H, NH), 7.02 (d, J = 8.4 Hz, 1H, arom H), 7.17 (d, J = 2.1 Hz, 1H, arom H), 7.23–7.29 (m, 1H, arom H), 7.44–7.50 (m, 2H, arom H), 7.50–7.56 (m, 1H, arom H), 7.83–7.89 (m, 2H, arom H), 8.19 (d, J = 2.1 Hz, 1H, arom H), 9.08 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (126 MHz, CDCl3) δ: 31.16 (CH2), 56.03 (OCH3), 56.06 (OCH3), 71.49 (C), 104.07 (C), 110.43 (CH), 111.77 (CH), 120.22 (CH), 125.50 (CH), 127.10 (CH), 128.63 (CH), 129.27 (C), 131.87 (CH); 136.25 (C), 133.59 (C), 143.38 (C), 147.86 (C), 149.70 (C), 150.00 (C), 152.25 (CH), 155.08 (C), 167.09 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H19N3O3S 430.1220, found 430.1218.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)benzenesulfonamide (11b)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0eq.), benzenesulfonyl chloride (30 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 98:2) as mobile phase, affording the title compound as a yellow solid in 61% yield (39.2 mg, 0.08 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.35 (d, J = 6.1 Hz, 2H, CH2), 5.30 (t, J = 6.0 Hz, 1H, NH), 7.01 (d, J = 8.3 Hz, 1H, arom H), 7.15 (d, J = 2.0 Hz, 1H, arom H), 7.24 (dd, J = 8.3, 2.1 Hz, 1H, arom H), 7.47–7.58 (m, 3H, arom H), 7.95–7.99 (m, 2H, arom H), 8.16 (d, J = 2.0 Hz, 1H, arom H), 9.05 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 34.33 (CH2), 56.04 (OCH3), 56.07 (OCH3), 72.54 (C triple bond), 102.13 (C triple bond), 110.38 (CH), 111.75 (CH), 120.21 (CH), 125.38 (CH), 127.43 (CH), 129.16 (CH), 133.08 (CH), 136.31 (C), 139.70 (C), 142.55 (C), 147.71 (C), 149.70 (C), 150.04 (C), 152.40 (CH), 155.03 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O4S2 466.0890, found 466.0888.
- 1-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)-3-phenylurea (11c)
- This compound was obtained from amino precursor 10 (50 mg, 0.14 mmol, 1.0 eq.), phenyl isocyanate (20 mg, 0.17 mmol, 1.2 eq.) and triethylamine (28 mg, 0.28 mmol, 2 eq.) using above mentioned procedure. The crude residue was purified by flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 95:5) as mobile phase, affording the title compound as a light yellow solid in 54% yield (33.1 mg, 0.07 mmol).
- 1H NMR (600 MHz, DMSO) δ: 3.83 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 4.41 (d, J = 5.8 Hz, 2H, CH2), 6.74 (t, J = 5.8 Hz, 1H, NH), 6.89–6.94 (m, 1H, arom H), 7.12 (d, J = 9.0 Hz, 1H, arom H), 7.22–7.26 (m, 2H, arom H), 7.43 (dd, J = 8.6, 1.0 Hz, 2H, arom H), 7.47 (dd, J = 6.9, 2.0 Hz, 2H, arom H), 8.50 (d, J = 2.1 Hz, 1H, arom H), 8.74 (s, 1H, NH), 9.24 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 30.31 (CH2), 55.72 (OCH3), 55.82 (OCH3), 69.73 (C triple bond), 107.47 (C triple bond), 111.13 (CH), 112.30 (CH), 117.96 (CH), 120.20 (CH), 121.51 (CH), 124.61 (CH), 128.43 (C), 128.77 (CH), 135.48 (C), 140.22 (C), 143.19 (C), 147.30 (C), 149.45 (C), 149.82 (C), 152.31 (CH), 154.89 (C), 155.14 (C) ppm.
- HRMS m/z [M+H]+ calcd for C24H20N4O3S 445.1329, found 445.1322.
- 2-Methoxy-4-(3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)benzoic acid (4)
- To a solution of the precursor 3j (50 mg, 0.13 mmol, 1.0 eq.) in a mixture of THF/H2O (in a ratio of 4:2) (6 mL), LiOH (6.2 mg, 0.26 mmol, 2 eq.) was added and the reaction mixture was stirred at 50 °C overnight. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and a solution of HCl (1 M) was added slowly until pH acid was reached (pH = 2–4). The volatiles were evaporated to dryness and the crude residue was precipitated with methanol and subsequently, with diethyl ether, affording the title compound as a light yellow solid in 94% yield (45.4 mg, 0.12 mmol).
- 1H NMR (600 MHz, DMSO) δ: 3.98 (s, 3H, OCH3), 7.55 (dd, J = 7.9, 1.3 Hz, 1H), 7.60–7.63 (m, 2H, arom H), 7.80 (d, J = 7.9 Hz, 1H, arom H), 8.19–8.23 (m, 1H, arom H), 8.70–8.74 (m, 2H, arom H), 8.94 (d, J = 1.4 Hz, 1H, arom H), 9.34 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, DMSO) δ: 56.18 (OCH3), 80.47 (C), 103.69 (C), 111.91 (CH), 118.64 (C), 119.37 (CH), 121.54 (C), 124.24 (CH), 126.74 (CH), 131.53 (CH), 134.96 (C), 139.61 (CH), 140.60 (C), 142.43 (C), 148.01 (C), 149.70 (CH), 151.15 (CH), 152.46 (CH), 154.74 (C), 158.75 (C), 167.05 (C) ppm.
- HRMS m/z [M+H]+ calcd for C21H13N3O3S 388.0750, found 388.0746.
- 4-(2-(4-(3-(Pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridin-6-yl)phenoxy)ethyl)morpholine (5a)
- To a solution of the precursor 3k (99 mg, 0.30 mmol, 1.0 eq.) in acetone, K2CO3 (62 mg, 0.45 mmol, 1.5 eq.) was added and the mixture was stirred at 50 °C for 20 min. Separately, to a second solution of 4-(2-chloroethyl)morpholine hydrochloride (67 mg, 0.36 mmol, 1.2 eq.) in acetone, K2CO3 (62 mg, 0.45 mmol, 1.5 eq.) and KI (100 mg, 0.6 mmol, 2 eq.) was added and the mixture was stirred at 50 °C for 20 min. Then, the second solution was added to the one contained the precursor 3k and the final mixture was stirred at reflux overnight. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase (adding 6 drops of ammonia 25% in 100 mL of solvent), affording the title compound as a light yellow solid in 48% yield (64.5 mg, 0.14 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 2.58–2.64 (m, 4H, 2 x CH2), 2.86 (t, J = 5.7 Hz, 2H, CH2), 3.74–3.79 (m, 4H, 2 x CH2), 4.20 (t, J = 5.7 Hz, 2H, CH2), 7.06–7.10 (m, 2H, arom H), 7.37 (ddd, J = 7.8, 4.9, 0.8 Hz, 1H, arom H), 7.62–7.67 (m, 2H, arom H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H, arom H), 8.20 (d, J = 2.1 Hz, 1H, arom H), 8.64 (dd, J = 4.9, 1.6 Hz, 1H, arom H), 8.92 (dd, J = 2.0, 0.7 Hz, 1H, arom H), 9.14 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 54.10 (CH2), 57.54 (CH2), 66.01 (CH2), 66.91 (CH2), 80.07 (C), 104.00 (C) 115.49 (CH), 119.25 (C), 123.12 (CH), 125.27 (CH), 128.76 (CH), 129.16 (C), 136.07 (C), 138.68 (CH), 143.23 (C), 147.63 (C), 149.78 (CH), 152.32 (CH), 152.43 (CH), 155.30 (C), 159.53 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H22N4O2S 443.1536, found 443.1531.
- 3-(Pyridin-3-ylethynyl)-6-(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)isothiazolo[4,3-b]pyridine (5b)
- To a solution of the precursor 3k (250 mg, 0.76 mmol, 1.0 eq.) in DMF (10 mL), K2CO3 (157 mg, 1.14 mmol, 1.5 eq.) was added and the mixture was stirred at 50 °C for 20 min. Separately, to a second solution of 1-(2-chloroethyl)pyrrolidine hydrochloride (170 mg, 0.91 mmol, 1.2 eq.) in DMF (10 mL), K2CO3 (157 mg, 1.14 mmol, 1.5 eq.) and KI (252 mg, 1.52 mmol, 2 eq.) was added and the mixture was stirred at 50 °C for 20 min. After this time, the second solution was added to the one contained the precursor 3k and the final mixture was stirred at 100 °C for 5 h. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of hexane and acetone (in a ratio of 7:3) as mobile phase (adding 6 drops of ammonia 25% in 100 mL of solvent), affording the title compound as a light yellow solid in 25% yield (80.9 mg, 0.19 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 1.82–1.87 (m, 4H, 2 x CH2), 2.64–2.69 (m, 4H, 2 × CH2), 2.96 (t, J = 5.9 Hz, 2H, CH2), 4.20 (t, J = 5.9 Hz, 2H, CH2), 7.09 (d, J = 8.7 Hz, 2H, arom H), 7.37 (dd, J = 7.6, 5.0 Hz, 1H, arom H), 7.65 (d, J = 8.7 Hz, 2H, arom H), 7.98 (dt, J = 7.9, 1.8 Hz, 1H, arom H), 8.20 (d, J = 2.0 Hz, 1H, arom H), 8.64 (dd, J = 4.8, 1.5 Hz, 1H, arom H), 8.92 (d, J = 1.4 Hz, 1H, arom H), 9.14 (d, J = 2.0 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 23.48 (CH2), 54.74 (CH2), 54.98 (CH2), 67.28 (CH2), 80.09 (C), 103.96 (C), 115.49 (CH), 119.26 (C), 123.11 (CH), 125.20 (CH), 128.71 (CH), 128.96 (C), 136.13 (C), 138.68 (CH), 143.17 (C), 147.60 (C), 149.76 (CH), 152.31 (CH), 152.49 (CH), 155.33 (C), 159.71 (C) ppm.
- HRMS m/z [M+H]+ calcd for C25H22N4OS 427.1587, found 427.1582.
- N-(Propargyl)aniline
- To a suspension of aniline (94 mg, 1.0 mmol, 1.2 eq.) and K2CO3 (174 mg, 1.26mmol, 1.5 eq.) in DMF at 0 °C, propargyl bromide 80% w/w in toluene (125 mg, 0.84 mmol, 1.0 eq.) was added. The reaction mixture was stirred at room temperature overnight. The volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 9:1) as mobile phase, affording the title compound as colourless oil in 87% yield (95.9 mg, 0.73 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 2.21 (t, J = 2.4 Hz, 1H, CH), 3.92 (d, J = 2.4 Hz, 2H, CH2), 6.65–6.72 (m, 2H, arom H), 6.79 (t, J = 7.3 Hz, 1H, arom H), 7.15–7.29 (m, 2H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C9H9N 132.0808, found 132.0802.
- N-(3-(6-Bromoisothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)aniline (12)
- A solution of 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (100 mg, 0.34 mmol, 1 eq.) in dry THF was degassed with a flow of argon for 5 min. Subsequently, Pd(PPh3)2Cl2 (4.8 mg, 0.0068 mmol, 0.02 eq.), CuI (0.65 mg, 0.0034 mmol, 0.01 eq.) and N-(propargyl)aniline (45 mg, 0.34 mmol, 1.0 eq.) were added and the reaction mixture was filled with argon and stirred at room temperature for 5 h. After disappearance of the starting material as monitored by TLC, the volatiles were evaporated in vacuo and the crude residue was purified by silica gel flash chromatography using a mixture of hexane and ethyl acetate (in a ratio of 8:2) as mobile phase, affording the title compound as dark oil in 78% yield (91.3 mg, 0.26 mmol).
- 1H NMR (300 MHz, CDCl3) δ: 4.11 (bs, 1H, NH), 4.40 (s, 2H, CH2), 6.72–6.87 (m, 3H, arom H), 7.19–7.31 (m, 2H, arom H), 8.31 (d, J = 2.1 Hz, 1H, arom H), 8.79 (d, J = 2.1 Hz, 1H, arom H) ppm.
- HRMS m/z [M+H]+ calcd for C15H10BrN3S 343.9852, found 343.9842.
- N-(3-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)prop-2-yn-1-yl)aniline (13)
- This compound was obtained from the precursor 12 (52 mg, 0.15 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (33 mg, 0.18 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.03 eq.) and K2CO3 (50 mg, 0.36 mmol, 2 eq.) using general Suzuki coupling procedure.
- The crude residue was purified by flash chromatography using a mixture of hexane and acetone (in a ratio of 8:2) as mobile phase., affording the title compound as a yellow solid in 81% yield (47.2 mg, 0.12 mmol).
- 1H NMR (600 MHz, CDCl3) δ: 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.14 (bs, 1H, NH), 4.42 (s, 2H, CH2), 6.76–6.80 (m, 2H, arom H), 6.82 (tt, J = 7.4, 1.0 Hz, 1H, arom H), 7.02 (d, J = 8.3 Hz, 1H, arom H), 7.16 (d, J = 2.1 Hz, 1H, arom H), 7.23–7.28 (m, 3H, arom H), 8.16 (d, J = 2.1 Hz, 1H, arom H), 9.09 (d, J = 2.1 Hz, 1H, arom H) ppm.
- 13C NMR (150 MHz, CDCl3) δ: 35.18 (CH2), 56.03 (OCH3), 56.05 (OCH3), 71.05 (C triple bond), 105.98 (C triple bond), 110.42 (CH), 111.74 (CH), 113.59 (CH), 118.80 (CH), 120.20 (CH), 125.38 (CH), 129.32 (CH), 129.32 (C), 136.15 (C), 143.85 (C), 146.66 (C), 147.76 (C), 149.66 (C), 149.93 (C), 152.16 (CH), 155.07 (C) ppm.
- HRMS m/z [M+H]+ calcd for C23H19N3O2S 402.1271, found 402.1263.
- 6-Bromo-N-(pyridin-3-ylmethyl)isothiazolo[4,3-b]pyridin-3-amine (14)
- The mixture of 3,6-dibromoisothiazolo[4,3-b]pyridine (147 mg, 0.5 mmol, 1.0 eq.), pyridin-3-ylmethanamine (216 mg, 2.0 mmol, 4.0 eq.) and K2CO3 (276 mg, 2.0 mmol, 4 eq.) in acetonitrile (5 mL) was heated under reflux till the starting material was almost disappeared on TLC. After cooling down to room temoerature, the mixture was concentrated under reduced pressure. The residue was purified with silica gel column (acetone in dichloromethane from 0 to 20% in 15 min.) to yield the title compounds 109 mg (0.34 mmol, 68%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 8.70 (s, 1H), 8.62 (d, J = 4.6 Hz, 1H), 8.31 (s, 1H), 7.98 (s, 1H), 7.75 (d, J = 7.7 Hz, 1H), 7.32 (dd, J = 7.7 & 5.0 Hz, 1H), 6.69 (br., 1H), 4.59 (d, J = 5.5 Hz, 2H), ppm.
- 13C NMR (75 MHz, CDCl3) δ: 154.2, 150.0, 149.5, 145.8, 135.6, 133.1, 131.1, 130.1, 123.8, 121.4, 49.3 ppm.
- 6-(3,4-Dimethoxyphenyl)-N-(pyridin-3-ylmethyl)isothiazolo[4,3-b]pyridin-3-amine (15)
- This compound was obtained from the precursor 14 (55 mg, 0.17 mmol, 1.0 eq.), 3,4-dimethoxyphenylboronic acid (36 mg, 0.20 mmol, 1.2 eq.), Pd(PPh3)4 (5 mg, 0.004 mmol, 0.027 eq.) and K2CO3 (45 mg, 0.32 mmol, 2 eq.) using general Suzuki coupling procedure. The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 50 mg (0.12 mmol, 70%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 8.72 (s, 1H), 8.59 (m, 2H), 7.88 (s, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.30 (m, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.14 (s, 1H), 6.98 (d, J = 8.2 Hz, 1H), 6.86 (t, J = 5.5 Hz, 1H), 4.61 (d, J = 5.5 Hz, 2H), 3.96 (s, 3H), 3.94 (s, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 171.01, 154.50, 149.88, 149.61, 149.51, 145.21, 136.82, 135.52, 133.77, 131.49, 130.35, 124.67, 123.75, 119.99, 111.68, 110.48, 56.04, 49.35 ppm.
- HRMS m/z [M+H]+ calcd for C20H18N4O2S 379.1223, found 379.1224.
- 6-Bromo-3-(pyridin-3-yl)isothiazolo[4,3-b]pyridine (16)
- The title compound was synthesized from 3,6-dibromoisothiazolo[4,3-b]pyridine 1b (294mg, 1.0 mmol, 1.0 eq.) and pyridin-3-ylboronic acid (148 mg, 1.2 mmol, 1.2 eq.) Pd(PPh3)4 (14 mg, 0.02 mmol, 0.02 eq.) and K2CO3 (414 mg, 3.0 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 3 h). The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 248 mg (0.85 mmol, 85%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.28 (s, 1H), 8.82 (s, 1H), 8.72 (d, J = 4.4 Hz, 1H), 8.55 (d, J = 7.6 Hz, 1H), 8.34 (s, 1H), 7.50 (dd, J = 7.6 & 5.2 Hz, 1H) ppm.
- 6-(3,4-Dimethoxyphenyl)-3-(pyridin-3-yl)isothiazolo[4,3-b]pyridine (17)
- The title compound was synthesized from intemediate 16 (246 mg, 0.5 mmol, 1.0 eq.) and 3,4-dimethoxyphenylboronic acid (109 mg, 0.6 mmol, 1.2 eq.), Pd(PPh3)4 (7 mg, 0.01 mmol, 0.02 eq.) and K2CO3 (212 mg, 1.5 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 4 h). The crude residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 140 mg (0.40 mmol, 80%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.33 (s, 1H), 9.13 (s, 1H), 8.71 (d, J = 3.2 Hz, 1H), 8.62 (d, J = 7.6 Hz, 1H), 8.20 (s, 1H), 7.51 (t, J = 5.3 Hz, 1H), 7.30 (d, J =8.2 Hz, 1H), 7.22 (s, 1H), 7.04 (d, J = 8.2 Hz, 1H), 4.00 (s, 3H), 3.97 (s, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 158.24, 156.72, 151.94, 150.60, 149.92, 149.67, 148.86, 143.90, 135.84, 135.34, 129.51, 126.82, 125.05, 123.97, 120.15, 111.79, 110.39, 56.06 ppm.
- HRMS m/z [M+H]+ calcd for C19H15N3O2S 350.0958, found 350.0951.
- N-(6-Bromoisothiazolo[4,3-b]pyridin-3-yl)nicotinamide (19)
- To a mixture of 6-bromoisothiazolo[4,3-b]pyridin-3-amine (115 mg, 0.5 mmol, 1.0 eq.) and nicotinoyl chloride hydrochloride (115 mg, 0.6 mmol, 1.2 eq.) in dichloromethane (5 mL), trimethylamine (0.5 mL) was added. The mixture was stirred at room temperature for 2 h. After concentration under reduced pressure, the residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compounds 110 mg (0.33 mmol, 66%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3) δ: 9.18 (d, J = 1.8 Hz, 1H), 9.08 (d, J = 1.8 Hz, 1H), 8.88 (br., 1H), 8.83 (d, J = 4.8 Hz, 1H), 8.53 (d, J = 1.8 Hz, 1H), 8.24 (d, J = 8.0 Hz, 1H), 7.49 (dd, J = 8.0 & 4.8 Hz, 1H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 164.4, 163.7, 148.6, 147.9, 138.7, 135.3, 132.0, 128.7, 125.7, 123.9, 121.9, 114.6 ppm.
- N-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)nicotinamide (20)
- The title compound was synthesized from intemediate 19 (100 mg, 0.3 mmol, 1.0 eq.) and 3,4-dimethoxyphenylboronic acid (66 mg, 0.36 mmol, 1.2 eq.), Pd(PPh3)4 (4.2 mg, 0.005 mmol, 0.02 eq.) and K2CO3 (124 mg, 0.9 mmol, 3 eq.) using general Suzuki coupling procedure (80 °C, 4 h). The crude residue was purified by silica gel column (acetone in dichloromethane from 0 to 20% in 15 min) to yield the title compounds 82 mg (0.21 mmol, 71%) as yellowish solid.
- 1H NMR (300 MHz, CDCl3+CD3OD) δ: 9.21 (d, J = 2.0 Hz, 1H), 8.79 (s, 2H), 8.43 (m, 2H), 7.61 (dd, J = 8.1 Hz, 5.0 Hz, 1H), 7.31 (dd, J = 8.3 Hz, 1.8 Hz, 1H), 7.23 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 3.98 (s, 3H), 3.96 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3+CD3OD) δ: 165.38, 152.34, 150.55, 149.70, 148.53, 145.77, 140.57, 138.00, 136.38, 130.79, 129.65, 127.94, 125.72, 123.98, 120.69, 115.36, 111.92, 110.33, 55.81, 55.77 ppm. HRMS m/z [M+H]+ calcd for C20H16N4O3S 393.1016, found 393.1019.
- 6-(3,4-Dimethoxyphenyl)-3-(2-(pyridin-3-yl)ethyl)isothiazolo[4,3-b]pyridine (22)
- To a solution of compound 21 (200 mg, 0.54 mmol, 1.0 eq.) in a mixture of EtOH/THF (10 mL, in a ratio of 9:1), palladium hydroxide on carbon 20 wt. % was (100 mg) was added. The air from the flask was evacuated and filled with hydrogen using a balloon. This procedure was repeated 3 times and the reaction mixture was stirred under hydrogen at 60 °C for 3 days. After disappearance of the starting material as monitored by TLC, the mixture was cooled down to room temperature and the volatiles were evaporated in vacuo. The crude residue was purified by silica gel flash chromatography using a mixture of dichloromethane and acetone (in a ratio of 9:1) as mobile phase and a second time using hexane and acetone (7:3), affording the title compound as a beige solid in 20% yield (40.4 mg, 0.11 mmol).
- 1H NMR (500 MHz, CDCl3) δ: 3.27 (t, J = 7.6 Hz, 2H, CH2), 3.79 (t, J = 7.7 Hz, 2H, CH2), 3.96 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 7.03 (d, J = 8.3 Hz, 1H, arom H), 7.19 (d, J = 2.1 Hz, 1H, arom H), 7.23 (dd, J = 7.7, 4.8 Hz, 1H, arom H), 7.28 (dd, J = 8.3, 2.3 Hz, 1H, arom H), 7.52–7.57 (m, 1H, arom H), 8.13 (d, J = 2.0 Hz, 1H, arom H), 8.54–8.46 (m, 2H, arom H), 9.02 (d, J = 2.0 Hz, 1H, arom H) ppm. 13C NMR (126 MHz, CDCl3) δ: 27.54 (CH2), 33.68 (CH2), 56.05 (OCH3), 110.50 (CH), 111.78 (CH), 120.13 (CH), 123.44 (CH), 125.08 (CH), 129.92 (C), 135.46 (C), 135.83 (C), 135.99 (CH), 145.14 (C), 148.07 (CH), 149.64 (C), 149.79 (C), 149.97 (CH), 150.53 (CH), 155.74 (C), 163.66 (C) ppm. HRMS m/z [M+H]+ calcd for C21H19N3O2S 378.1273, found 378.1271.
- (Z)-6-(3,4-Dimethoxyphenyl)-3-(2-methoxy-2-(pyridin-3-yl)vinyl)isothiazolo[4,3-b]pyridine (23a)
- To the solution of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine 21 (187 mg, 0.5 mmol, 1.0 eq.) in methanol (5 mL) at room temperature, potassium tert-butoxide (112 mg, 1.0 mmol, 2.0 eq.) was added. The resulting mixture was stirred under reflux for 2 h. After cooling down to room temperature, the mixture was concentrated under reduced pressure. The residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (150 mg, 0.37 mmol, 74%).
- 1H NMR (300 MHz,) δ: 8.99 (s, 1H), 8.95 (s, 1H), 8.69 (d, J = 4.5 Hz, 1H), 8.16 (s, 1H), 7.96 (d, J = 7.8 Hz, 1H), 7.42 (dd, J = 7.8 & 4.5 Hz, 1H), 7.36 (s, 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.21 (s, 1H), 7.02 (d, J = 8.3 Hz, 1H), 3.99 (s, 3H), 3.96 (s, 3H), 3.90 (s, 3H) ppm.
- 13C NMR (150 MHz, CDCl3 + CD3OD) δ: 157.26, 156.32, 154.34, 150.53, 149.63, 149.63, 148.42, 144.29, 135.96, 134.48, 130.12, 129.42, 124.94, 123.59, 120.11, 111.77, 110.52, 104.07, 58.09, 56.07 ppm. HRMS m/z [M+H]+ calcd for C22H19N3O3S 406.1220, found 406.1208.
- ((Z)-2-((2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)oxy)-N,N-dimethylethan-1-amine (23b)
- To a solution of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (187 mg, 0.5 mmol, 1.0 eq.) in 2-(dimethylamino)ethan-1-ol (4 mL) at room temperature, potassium tert-butoxide (112 g, 1.0 mmol, 2.0 eq.) was added. The resulting mixture was stirred at 80 °C for 2 h. The mixture was cooled down to room temperature and concentrated under reduced pressure. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (167 mg, 0.36 mmol, 72%).
- 1H NMR (300 MHz, CDCl3) δ: 8.99 (s, 1H), 8.97 (d, J = 1.8 Hz, 1H), 8.68 (d, J = 4.0 Hz, 1H), 8.15 (d, J = 1.8 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.42 (dd, J = 7.8 & 4.8 Hz, 1H), 7.31 (m, 2H), 7.20 (d, J = 1.8 Hz, 1H), 7.02 (d, J = 8.3 Hz, 1H), 4.10 (t, J = 6.0 Hz, 2H), 3.99 (s, 3H), 3.96 (s, 3H), 2.89 (t, J = 6.0 Hz, 2H), 2.32 (s, 6H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 156.47, 156.33, 154.25, 150.45, 149.71, 149.6, 148.48, 144.40, 135.94, 134.58, 130.13, 129.92, 124.91, 123.52, 120.10, 111.76, 110.51, 104.11, 68.99, 59.14, 56.06, 45.94 ppm.
- HRMS m/z [M+H]+ calcd for C25H26N4O3S 463.1798, found 463.1795.
- (Z)-4-(2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)morpholine (23c)
- A suspension of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine (187 mg, 0.5 mmol, 1.0 eq.) in morpholine (5 mL) was heated at 80 °C for 3h. The mixture was cooled down to room temperature and concentrated under reduced pressure to dryness. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (140 mg, 0.30 mmol, 60%).
- 1H NMR (300 MHz, CDCl3) δ: 8.86 (m, 2H), 8.66 (s, 1H), 7.95 (s, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.53 (dd, J = 7.7 & 5.0 Hz, 1H), 7.23 (d, J = 8.3 Hz, 1H), 7.16 (s, 1H), 7.00 (d, J = 8.3 Hz, 1H), 6.79 (s, 1H), 3.96 (s, 3H), 3.94 (s, 3H), 3.79 (m, 4H), 3.23 (m, 4H) ppm. 13C NMR (75 MHz, CDCl3) δ: 162.4, 154.36, 151.80, 151.42, 150.35, 149.60, 148.00, 144.05, 137.50, 135.94, 131.71, 130.27, 125.10, 124.71, 119.94, 111.71, 110.42, 94.86, 66.62, 66.45, 56.04, 56.02, 48.38 ppm. HRMS m/z [M+H]+ calcd for C25H24N4O3S 461.1642, found 461.1637.
- (Z)-2-((2-(6-(3,4-Dimethoxyphenyl)isothiazolo[4,3-b]pyridin-3-yl)-1-(pyridin-3-yl)vinyl)thio)ethan-1-ol (23d)
- The mixture of 6-(3,4-dimethoxyphenyl)-3-(pyridin-3-ylethynyl)isothiazolo[4,3-b]pyridine 187 mg, 0.5 mmol, 1.0 eq.), 2-mercaptoethan-1-ol (156 mg, 2.0 mmol, 4.0 eq.) and K2CO3 (280 mg, 2.0 mmol, 4.0 eq.) in THF (5 mL) was heated under reflux stirred for 2 h. The mixture was cooled down to room temperature and concentrated under reduced pressure to dryness. The resulted residue was purified by silica gel flash chromatography (from 0 to 20% acetone in dichloromethane over 15 min) to yield the title compound as a yellowish solid (167 mg, 0.37 mmol, 74%).
- 1H NMR (300 MHz, CDCl3) δ: 8.93 (s, 1H), 8.80 (d, J = 4.6 Hz, 1H), 8.66 (s, 1H), 7.99 (s, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.64 (s, 1H), 7.49 (dd, J = 7.7 & 5.0 Hz, 1H), 7.20 (d, J = 8.3 Hz, 1H), 7.13 (s, 1H), 6.98 (d, J = 8.3 Hz, 1H), 3.96 (s, 3H), 3.95 (m, 2H), 3.94 (s, 3H), 3.16 (m, 3H) ppm.
- 13C NMR (75 MHz, CDCl3) δ: 157.65, 154.50, 151.05, 150.04, 149.83, 149.62, 149.54, 143.95, 141.95, 136.94, 136.21, 133.84, 129.62, 124.86, 120.05, 114.41, 111.73, 110.35, 60.51, 56.05, 35.41 ppm.
- HRMS m/z [M+H]+ calcd for C23H21N3O3S2 452.1097, found 452.1092.
- Kinase assays
- Antiviral screening
- Cell lines for antiviral and viability assays
- Virus constructs
- Virus production
- Measuring antiviral activity
- Cell viability assays
- Antitumoral assays
- Cell culture
- Cell proliferation assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zolov, S.N.; Bridges, D.; Zhang, Y.; Lee, W.-W.; Riehle, E.; Verma, R.; Lenk, G.M.; Converso-Baran, K.; Weide, T.; Albin, R.L.; et al. In Vivo, Pikfyve Generates PI(3,5)P2, Which Serves as Both a Signaling Lipid and the Major Precursor for PI5P. Proc. Natl. Acad. Sci. USA 2012, 109, 17472–17477. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Lang, M.J.; Weisman, L.S. Phosphatidylinositol 3,5-bisphosphate: Regulation of cellular events in space and time. Biochem. Soc. Trans. 2016, 44, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Ríos, P.; Weisman, L.S. Roles of PIKfyve in multiple cellular pathways. Curr. Opin. Cell Biol. 2022, 76, 102086. [Google Scholar] [CrossRef] [PubMed]
- Rameh, L.E.; Blind, R.D. 25 Years of PI5P. Front. Cell. Dev. Biol. 2023, 11, 1272911. [Google Scholar] [CrossRef]
- Jefferies, H.B.; Cooke, F.T.; Jat, P.; Boucheron, C.; Koizumi, T.; Hayakawa, M.; Kaizawa, H.; Ohishi, T.; Workman, P.; Waterfield, M.D.; et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008, 9, 164–170. [Google Scholar] [CrossRef]
- Hayakawa, N.; Noguchi, M.; Takeshita, S.; Eviryanti, A.; Seki, Y.; Nishio, H.; Yokoyama, R.; Noguchi, M.; Shuto, M.; Shima, Y.; et al. Structure-activity relationship study, target identification, and pharmacological characterization of a small molecular IL-12/23 inhibitor, APY0201. Bioorg. Med. Chem. 2014, 22, 3021–3029. [Google Scholar] [CrossRef]
- Cai, X.; Xu, Y.; Cheung, A.K.; Tomlinson, R.C.; Alcázar-Román, A.; Murphy, L.; Billich, A.; Zhang, B.; Feng, Y.; Klumpp, M.; et al. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 2013, 20, 912–921. [Google Scholar] [CrossRef]
- Sano, O.; Kazetani, K.; Funata, M.; Fukuda, Y.; Matsui, J.; Iwata, H. Vacuolin-1 inhibits autophagy by impairing lysosomal maturation via PIKfyve inhibition. FEBS Lett. 2016, 590, 1576–1585. [Google Scholar] [CrossRef]
- Drewry, D.H.; Potjewyd, F.M.; Bayati, A.; Smith, J.L.; Dickmander, R.J.; Howell, S.; Taft-Benz, S.; Min, S.M.; Hossain, M.A.; Heise, M.; et al. Identification and Utilization of a Chemical Probe to Interrogate the Roles of PIKfyve in the Lifecycle of β-Coronaviruses. J. Med. Chem. 2022, 65, 12860–12882. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Wei, Y.; Wei, H.; Yuan, X.; Xiong, B.; Tang, M.; Yang, T.; Yang, Z.; Ye, H.; et al. Discovery of Potent and Selective Phosphatidylinositol 3-Phosphate 5-Kinase (PIKfyve) Inhibitors as Methuosis Inducers. J. Med. Chem. 2024, 67, 165–179. [Google Scholar] [CrossRef]
- Huang, P.; Einav, S.; Asquith, C.R.M. PIKfyve: A lipid kinase target for COVID-19, cancer and neurodegenerative disorders. Nat. Rev. Drug Discov. 2021, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Gayle, S.; Landrette, S.; Beeharry, N.; Conrad, C.; Hernandez, M.; Beckett, P.; Ferguson, S.M.; Mandelkern, T.; Zheng, M.; Xu, T.; et al. Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 2017, 129, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.L.; Chou, Y.Y.; Rothlauf, P.W.; Liu, Z.; Soh, T.K.; Cureton, D.; Case, J.B.; Chen, R.E.; Diamond, M.S.; Whelan, S.P.J.; et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 20803–20813. [Google Scholar] [CrossRef]
- Nelson, E.A.; Dyall, J.; Hoenen, T.; Barnes, A.B.; Zhou, H.; Liang, J.Y.; Michelotti, J.; Dewey, W.H.; DeWald, L.E.; Bennett, R.S.; et al. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Negl. Trop. Dis. 2017, 11, e0005540. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Ferreira, A.; Mariën, J.; Delay, C.; Lee, E.; Trojanowski, J.Q.; Moechars, D.; Annaert, W.; De Muynck, L. PIKfyve activity is required for lysosomal trafficking of tau aggregates and tau seeding. J. Biol. Chem. 2021, 296, 100636. [Google Scholar] [CrossRef]
- Hung, S.T.; Linares, G.R.; Chang, W.H.; Eoh, Y.; Krishnan, G.; Mendonca, S.; Hong, S.; Shi, Y.; Santana, M.; Kueth, C.; et al. PIKFYVE Inhibition Mitigates Disease in Models of Diverse Forms of ALS. Cell 2023, 186, 786–802.e28. [Google Scholar] [CrossRef]
- Rameh, L.E.; Tolias, K.F.; Duckworth, B.C.; Cantley, L.C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 1997, 390, 192–196. [Google Scholar] [CrossRef]
- Bulley, S.J.; Clarke, J.H.; Droubi, A.; Giudici, M.L.; Irvine, R.F. Exploring phosphatidylinositol 5-phosphate 4-kinase function. Adv. Biol. Regul. 2015, 57, 193–202. [Google Scholar] [CrossRef]
- Arora, G.K.; Palamiuc, L.; Emerling, B.M. Expanding role of PI5P4Ks in cancer: A promising druggable target. FEBS Lett. 2022, 596, 3–16. [Google Scholar] [CrossRef]
- Clarke, J.H.; Giudici, M.L.; Burke, J.E.; Williams, R.L.; Maloney, D.J.; Marugan, J.; Irvine, R.F. The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P-binding site. Biochem. J. 2015, 466, 359–367. [Google Scholar] [CrossRef]
- Boffey, H.K.; Rooney, T.P.C.; Willems, H.M.G.; Edwards, S.; Green, C.; Howard, T.; Ogg, D.; Romero, T.; Scott, D.E.; Winpenny, D.; et al. Development of selective phosphatidylinositol 5-phosphate 4-kinase γ inhibitors with a non-ATP-competitive, allosteric binding mode. J. Med. Chem. 2022, 65, 3359–3370. [Google Scholar] [CrossRef] [PubMed]
- Rooney, T.P.C.; Aldred, G.G.; Boffey, H.K.; Willems, H.M.G.; Edwards, S.; Chawner, S.J.; Scott, D.E.; Green, C.; Winpenny, D.; Skidmore, J.; et al. The Identification of Potent, Selective, and Brain Penetrant PI5P4Kγ Inhibitors as In Vivo-Ready Tool Molecules. J. Med. Chem. 2023, 66, 804–821. [Google Scholar] [CrossRef]
- Sivakumaren, S.C.; Shim, H.; Zhang, T.; Ferguson, F.M.; Lundquist, M.R.; Browne, C.M.; Seo, H.S.; Paddock, M.N.; Manz, T.D.; Jiang, B.; et al. Targeting the PI5P4K Lipid Kinase Family in Cancer Using Covalent Inhibitors. Cell. Chem. Biol. 2020, 27, 525–537. [Google Scholar] [CrossRef]
- Karim, M.; Mishra, M.; Lo, C.W.; Saul, S.; Cagirici, H.; Gourdelier, M.; Ghita, L.; Ojha, A.; Tran, D.H.N.; Agrawal, A.; et al. PIP4K2C inhibition reverses autophagic flux impairment induced by SARS-CoV-2. Nat. Commun. 2025, 16, 6397. [Google Scholar] [CrossRef]
- Kovackova, S.; Chang, L.; Bekerman, E.; Neveu, G.; Barouch-Bentov, R.; Chaikuad, A.; Heroven, C.; Šála, M.; De Jonghe, S.; Knapp, S.; et al. Selective Inhibitors of Cyclin G Associated Kinase (GAK) as Anti-Hepatitis C Agents. J. Med. Chem. 2015, 58, 3393–3410. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, G.; Liu, Q. A mild copper-mediated Glaser-type coupling reaction under the novel CuI/NBS/DIPEA promoting system. Tetrahedron Lett. 2009, 50, 4033–4036. [Google Scholar] [CrossRef]
- Han, G.; Tamaki, M.; Hruby, V.J. Fast, efficient and selective deprotection of the tert-butoxycarbonyl (Boc) group using HCl/dioxane (4 m). J. Pept. Res. 2001, 58, 338–341. [Google Scholar] [CrossRef]
- Wouters, R.; Pu, S.Y.; Froeyen, M.; Lescrinier, E.; Einav, S.; Herdewijn, P.; De Jonghe, S. Cyclin G-associated kinase (GAK) affinity and antiviral activity studies of a series of 3-C-substituted isothiazolo[4,3-b]pyridines. Eur. J. Med. Chem. 2019, 163, 256–265. [Google Scholar] [CrossRef]
- Clarke, J.H.; Irvine, R.F. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem. J. 2013, 454, 49–57. [Google Scholar] [CrossRef]
- Sharma, G.; Guardia, C.M.; Roy, A.; Vassilev, A.; Saric, A.; Griner, L.N.; Marugan, J.; Ferrer, M.; Bonifacino, J.S.; DePamphilis, M.L. A family of PIKFYVE inhibitors with therapeutic potential against autophagy-dependent cancer cells disrupt multiple events in lysosome homeostasis. Autophagy 2019, 15, 1694–1718. [Google Scholar] [CrossRef]
- Hou, J.; Xi, Z.; Niu, J.; Li, W.; Wang, X.; Liang, C.; Sun, H.; Fang, D.; Xie, S. Inhibition of PIKfyve using YM201636 suppresses the growth of liver cancer via the induction of autophagy. Oncol. Rep. 2019, 41, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gardner, C.L.; Watson, A.M.; Ryman, K.D.; Klimstra, W.B. Stable, high-level expression of reporter proteins from improved alphavirus expression vectors to track replication and dissemination during encephalitic and arthritogenic disease. J. Virol. 2014, 88, 2035. [Google Scholar] [CrossRef]
- Chiem, K.; Vasquez, D.M.; Park, J.; Platt, R.N.; Anderson, T.; Walter, M.R.; Kobie, J.J.; Ye, C.; Martinez-Sobrido, L. Generation and Characterization of recombinant SARS-CoV-2 expressing reporter genes. J. Virol. 2021, 95, e02209-20. [Google Scholar] [CrossRef]
- Saul, S.; Karim, M.; Ghita, L.; Huang, P.; Chiu, W.; Durán, V.; Lo, C.; Kumar, S.; Bhalla, N.; Leyssen, P.; et al. Anticancer pan-ErbB inhibitors reduce inflammation and tissue injury and exert broad-spectrum antiviral effects. J. Clin. Investig. 2023, 133, e169510. [Google Scholar] [CrossRef]
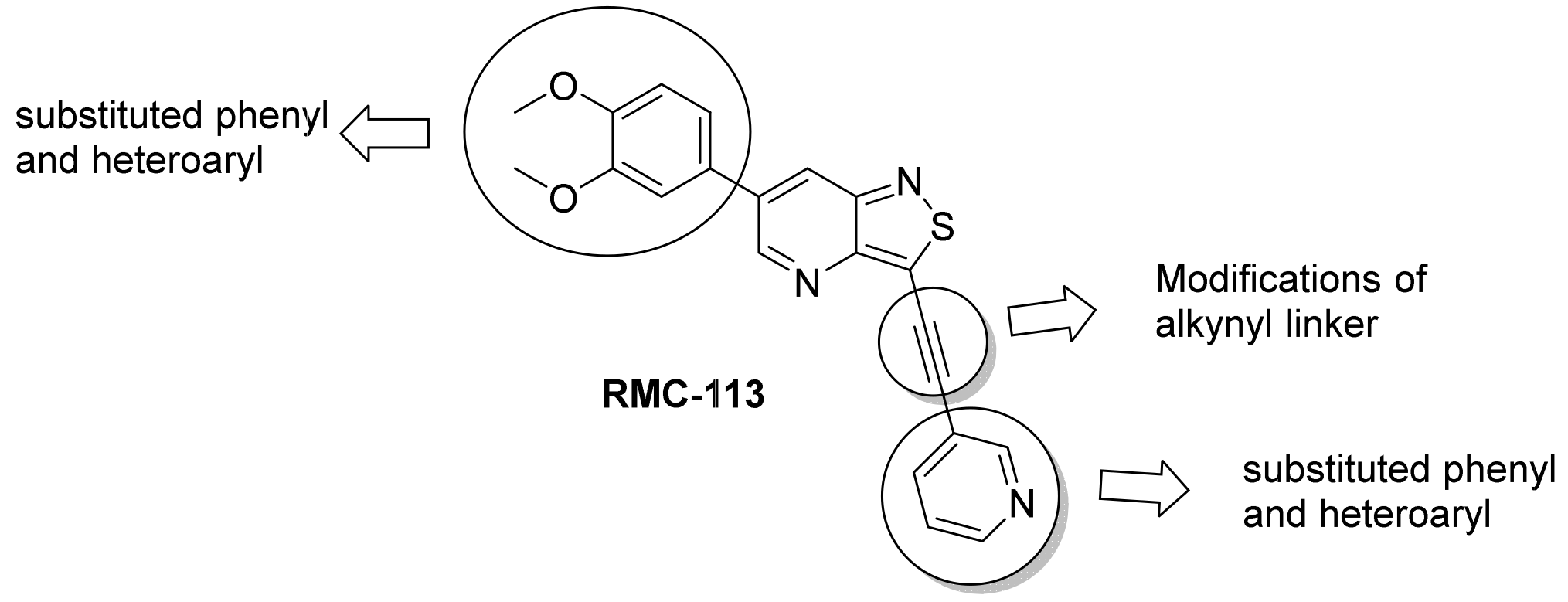
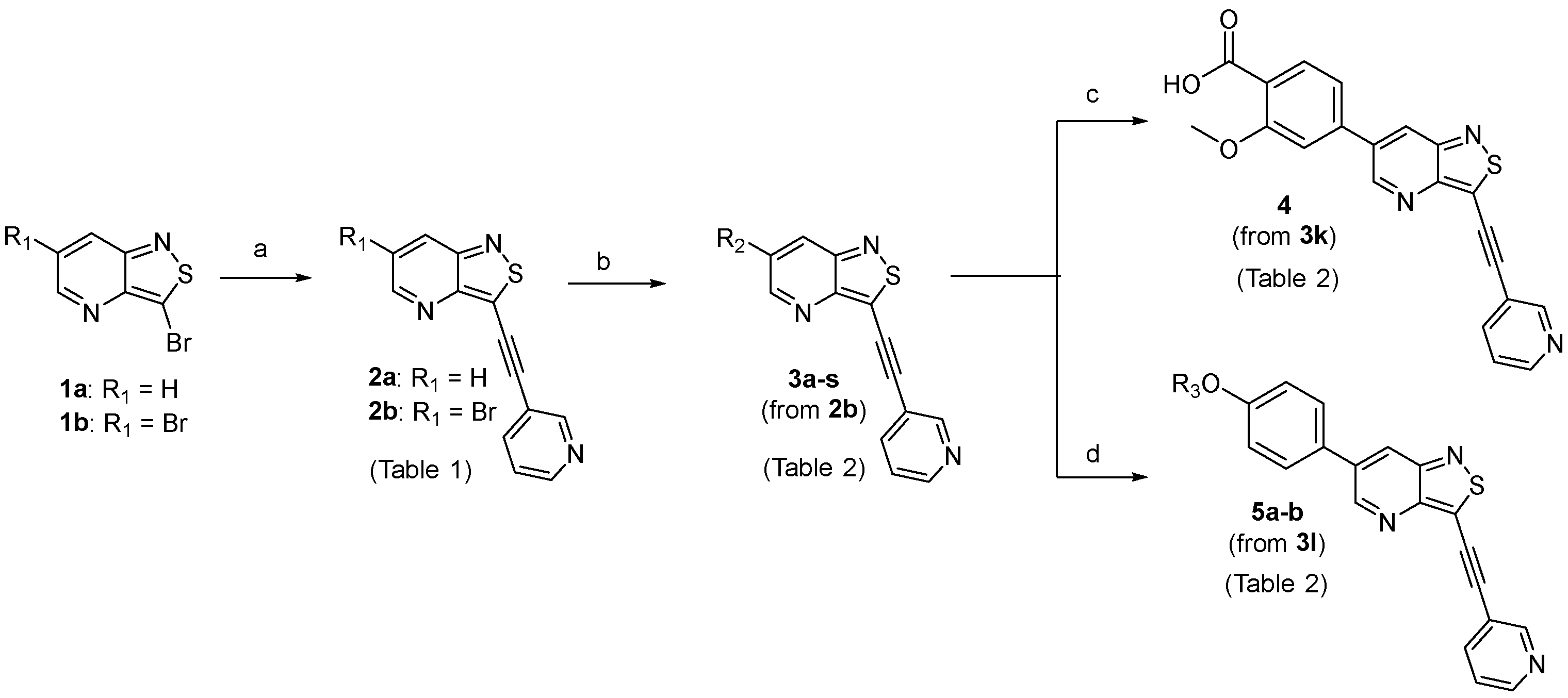
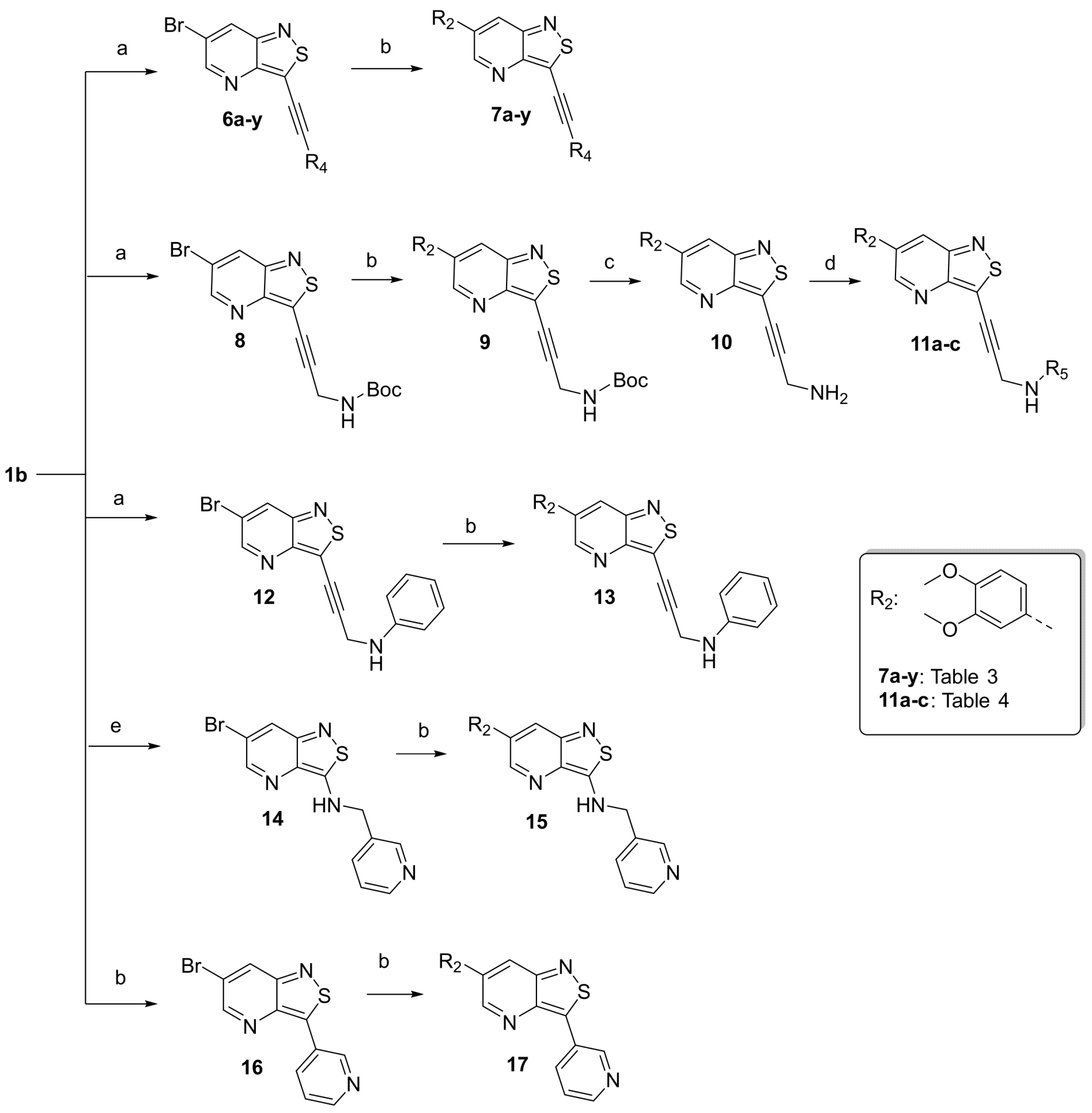
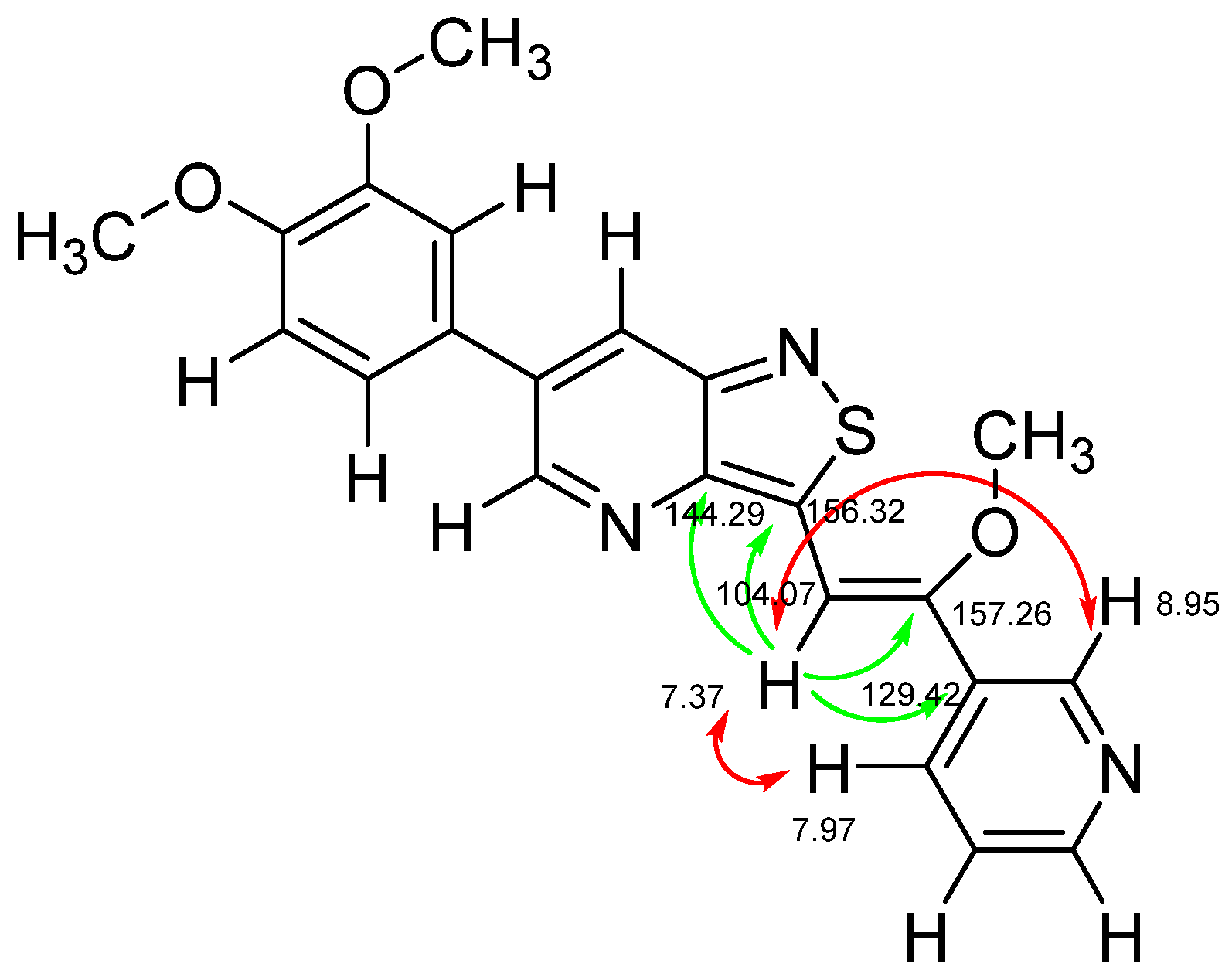
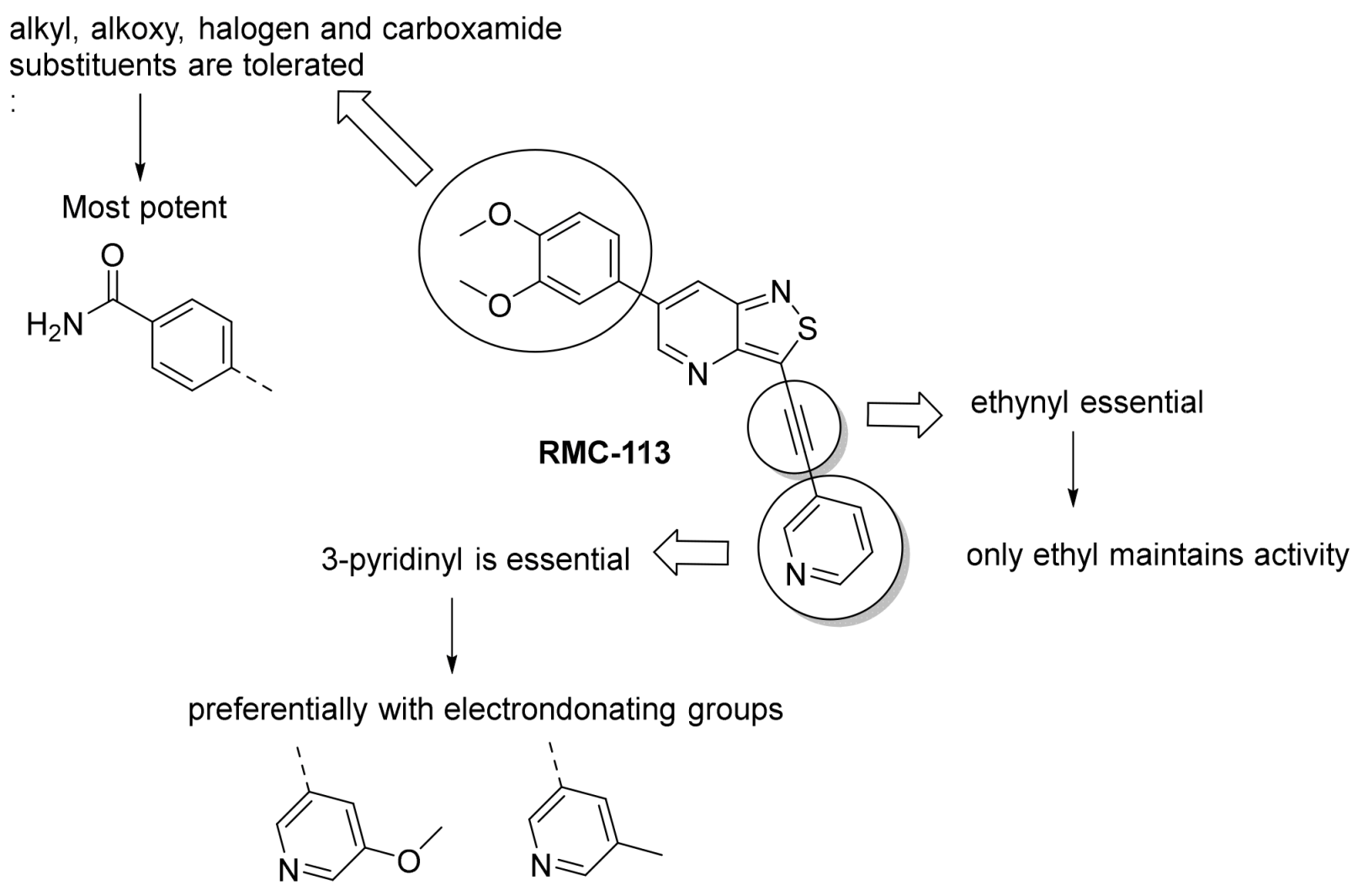
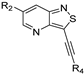 | |||
| Cmpd# | R2 | R4 | IC50 (µM) |
| 21 (RMC-113) | 3,4-dimethoxyphenyl | 3-pyridyl | 0.0080 |
| 2a | H | 3-pyridyl | 1.15 |
| 7a | 3,4-dimethoxyphenyl | n-propyl | 0.85 |
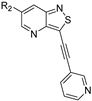 | ||
| Cmpd# | R2 | IC50 (µM) |
| 21 (RMC-113) |  | 0.0080 |
| 3a | 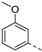 | 0.0068 |
| 3b | 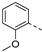 | 0.0068 |
| 3c | 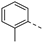 | 0.0082 |
| 3d | 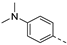 | 0.0022 |
| 3e | 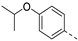 | 0.020 |
| 3f |  | 0.0028 |
| 3g |  | 0.010 |
| 3h |  | 0.0088 |
| 3i | 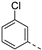 | 0.021 |
| 3j | 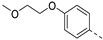 | 0.0021 |
| 4 |  | 0.049 |
| 5a |  | 0.0071 |
| 5b |  | 0.0055 |
| 3m |  | 0.0016 |
| 3n |  | 0.0035 |
| 3o |  | 0.025 |
| 3p |  | 0.020 |
| 3q |  | 0.018 |
| 3r |  | 0.0061 |
| 3s |  | 0.0019 |
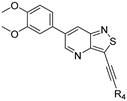 | ||
| Cmpd# | R4 | IC50 (µM) |
| 7b | Ph | 0.059 |
| 7c | 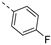 | 0.25 |
| 7d | 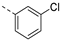 | 0.039 |
| 7e | 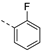 | 0.062 |
| 7f | 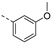 | 0.021 |
| 7g | 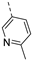 | 0.36 |
| 7h | 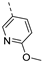 | 0.59 |
| 7i | 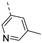 | 0.002 |
| 7j | 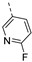 | 0.12 |
| 7k | 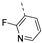 | 0.029 |
| 7l | 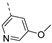 | 0.0033 |
| 7m | 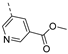 | 0.12 |
| 7n | 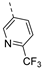 | 0.68 |
| 7o | 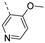 | 0.019 |
| 7p | 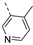 | 0.033 |
| 7q | 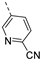 | >10 |
| 7r | 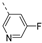 | 0.098 |
| 7s | 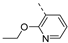 | 0.11 |
| 7t | 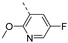 | >10 |
| 7u | 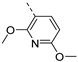 | 5.67 |
| 7v | 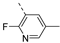 | 0.042 |
| 7w |  | 5.69 |
| 7x |  | 0.075 |
| 7y |  | 0.030 |
 | ||
| Cmpd# | R6 | IC50 (µM) |
| 17 |  | 0.602 |
| 15 |  | >10 |
| 20 |  | 4.29 |
| 22 |  | 0.033 |
| 11a | 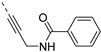 | 2.04 |
| 11b |  | 1.84 |
| 11c | 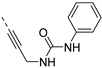 | >10 |
| 13 | 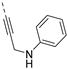 | 0.184 |
| 23a | 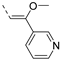 | 0.525 |
| 23b | 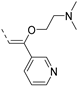 | 0.309 |
| 23c |  | 0.613 |
| 23d |  | 1.54 |
| Cmpd# | PIP4K2C KD (µM) | PIKfyve IC50 (µM) |
|---|---|---|
| 21 (RMC-113) | 0.046 | 0.008 |
| 3a | 0.190 | 0.0068 |
| 3i | 0.180 | 0.021 |
| 3f | 0.044 | 0.0028 |
| 3m | 0.041 | 0.0016 |
| 4 | 0.068 | 0.049 |
| 3s | 0.030 | 0.0019 |
| 7e | 0.280 | 0.062 |
| 7k | 0.066 | 0.029 |
| 7l | 0.039 | 0.0033 |
| 7q | >30 | >100 |
| 7w | 2 | 5.69 |
| 7o | 0.230 | 0.019 |
| 7r | 0.110 | 0.098 |
| VEEV (TC-83) | SARS-CoV2 | |||
|---|---|---|---|---|
| Cmpd# | EC50 (µM) a | CC50 (µM) b | EC50 (µM) a | CC50 (µM) b |
| 21 (RMC-113) | 0.75 ± 0.02 | >10 | 1.01 ± 0.04 | >20 |
| 3a | 6.88 ± 0.33 | >10 | <10 | <10 |
| 3h | 6.93 ± 0.13 | >20 | 1.34 ± 0.5 | >20 |
| 3f | 0.26 ± 0.08 | >20 | 2.74 ± 0.04 | >20 |
| 3m | 0.19 ± 0.001 | >20 | 1.82 ± 0.94 | >20 |
| 4 | 8.7 ± 1.7 | >10 | 0.36 ± 0.19 | >20 |
| 5a | 0.31 ± 0.01 | >10 | 5.63 ± 0.97 | >20 |
| 5b | 0.71 ± 0.09 | >10 | 1.42 ± 0.14 | >20 |
| 3s | 0.4 ± 0.08 | >10 | 5.65 ± 1.49 | >20 |
| 7e | 16.84 ± 1.25 | >20 | 0.55 ± 0.15 | 15.69 ± 1.34 |
| 7f | 4.79 ± 0.24 | >10 | <10 | <10 |
| 7k | 3.4 ± 0.08 | >10 | 1.83 ± 0.30 | >20 |
| 7l | 1.64 ± 0.05 | >10 | 4.92 ± 0.93 | >20 |
| 7m | 1.16 ± 0.001 | >10 | 2.56 ± 0.86 | >20 |
| 7w | 2.08 ± 0.1 | >10 | <10 | <10 |
| 7o | 0.92 ± 0.08 | 7.69 ± 0.001 | <10 | <10 |
| 7p | 4.01 ± 0.13 | >10 | 0.83 ± 0.06 | >20 |
| 7r | 1.85 ± 0.12 | >10 | 1.92 ± 0.52 | >20 |
| Apilimod | 1.35 ± 0.49 | >10 | 0.19 ± 0.00 | 18.35 ± 0.42 |
| CC50 (µM) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Capan-1 | HCT-116 | LN-229 | NCI-H460 | DND-41 | HL-60 | K-562 | Z-138 | |
| 21 (RMC-113) | 0.81 ± 0.11 | 0.82 ± 0.37 | 1.62 ± 0.50 | 2.52 ± 1.09 | 1.75 ± 1.02 | 1.47 ± 0.45 | 4.24 ± 0.11 | 0.77 ± 0.06 |
| 3g | 0.73 ± 0.23 | 0.81 ± 0.16 | 4.39 ± 0.94 | 1.46 ± 0.36 | 3.06 ± 1.71 | 1.51 ± 0.71 | 5.18 ± 0.48 | 1.03 ± 0.20 |
| 3b | 1.02 ± 0.09 | 1.02 ± 1.24 | 3.28 ± 1.24 | 2.97 ± 0.75 | 3.92 ± 0.72 | 1.48 ± 1.13 | 5.27 ± 0.59 | 1.81 ± 0.69 |
| 3m | 2.41 ± 0.94 | 0.34 ± 0.78 | 3.23 ± 0.78 | 2.9 ± 1.72 | 2.25 ± 0.19 | 1.0 ± 0.57 | 6.53 ± 0.46 | 0.96 ± 0.30 |
| 7i | 14.6 ± 2.97 | ≥74.0 | >100 | >100 | >100 | >100 | >100 | 70.2 ± 2.55 |
| 7l | 1.79 ± 1.20 | 2.45 ± 0.85 | 5.15 ± 2.05 | 5.38 ± 0.56 | 5.25 ± 0.64 | 1.80 ± 0.00 | 5.70 ± 1.27 | 1.85 ± 0.07 |
| 7p | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 12.3 ± 1.77 |
| Apilimod | <0.02 | <0.02 | 0.08 ± 0.09 | <0.02 | 0.08 ± 0.02 | 0.17 ± 0.02 | 0.33 ± 0.34 | 0.06 ± 0.00 |
| Vacuolin-1 | 0.90 ± 0.13 | 1.87 ± 0.75 | 6.35 ± 1.80 | 1.46 ± 0.16 | 24.13 ± 1.21 | 26.43 ± 1.48 | 17.21 ± 3.08 | 16.6 ± 1.31 |
| YM-201636 | 0.17 ± 0.05 | 1.08 ± 1.23 | 0.16 ± 0.03 | 0.81 ± 0.09 | 0.38 ± 0.09 | 0.31 ± 0.11 | 1.24 ± 0.28 | 0.17 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalebic, D.; Gao, L.-J.; Martinez-Gualda, B.; Karim, M.; Saul, S.; Tran, D.H.N.; Rozenski, J.; Persoons, L.; Schols, D.; Dehaen, W.; et al. Structure–Activity Relationship Study of 3-Alkynyl-6-aryl-isothiazolo[4,3-b]pyridines as Dual Inhibitors of the Lipid Kinases PIKfyve and PIP4K2C. Pharmaceuticals 2025, 18, 1341. https://doi.org/10.3390/ph18091341
Kalebic D, Gao L-J, Martinez-Gualda B, Karim M, Saul S, Tran DHN, Rozenski J, Persoons L, Schols D, Dehaen W, et al. Structure–Activity Relationship Study of 3-Alkynyl-6-aryl-isothiazolo[4,3-b]pyridines as Dual Inhibitors of the Lipid Kinases PIKfyve and PIP4K2C. Pharmaceuticals. 2025; 18(9):1341. https://doi.org/10.3390/ph18091341
Chicago/Turabian StyleKalebic, Demian, Ling-Jie Gao, Belén Martinez-Gualda, Marwah Karim, Sirle Saul, Do Hoang Nhu Tran, Jef Rozenski, Leentje Persoons, Dominique Schols, Wim Dehaen, and et al. 2025. "Structure–Activity Relationship Study of 3-Alkynyl-6-aryl-isothiazolo[4,3-b]pyridines as Dual Inhibitors of the Lipid Kinases PIKfyve and PIP4K2C" Pharmaceuticals 18, no. 9: 1341. https://doi.org/10.3390/ph18091341
APA StyleKalebic, D., Gao, L.-J., Martinez-Gualda, B., Karim, M., Saul, S., Tran, D. H. N., Rozenski, J., Persoons, L., Schols, D., Dehaen, W., Einav, S., & De Jonghe, S. (2025). Structure–Activity Relationship Study of 3-Alkynyl-6-aryl-isothiazolo[4,3-b]pyridines as Dual Inhibitors of the Lipid Kinases PIKfyve and PIP4K2C. Pharmaceuticals, 18(9), 1341. https://doi.org/10.3390/ph18091341








