Characterizing Population Pharmacokinetics of Vatiquinone in Healthy Volunteers and Patients with Friedreich’s Ataxia
Abstract
1. Introduction
2. Results
2.1. Pharmacokinetic (PK) Sampling and Demographics of Analysis Population
2.2. Exploratory Data Analysis of PK Concentrations
2.3. PopPK Modeling Analysis
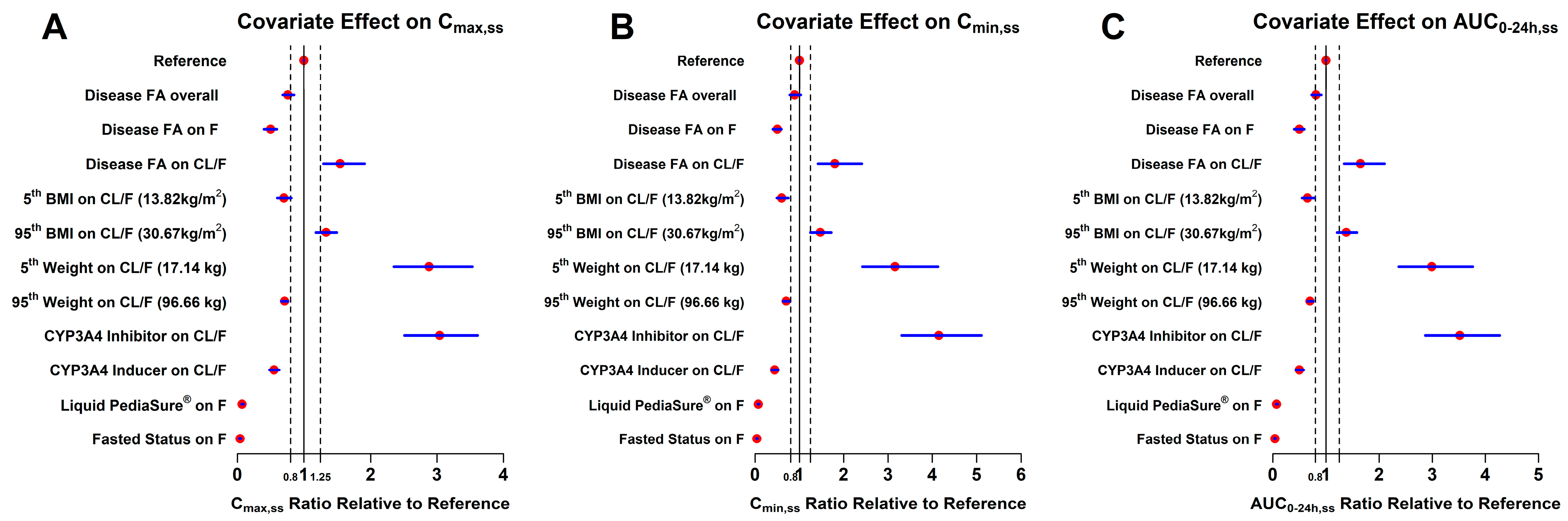
2.4. Visual Predictive Check (VPC) and Forest Plots
2.5. Internal Validation
3. Discussion
4. Materials and Methods
4.1. Software
4.2. Ethics
4.3. Clinical Study Population and Design
4.4. PK Data Handling and Imputation
4.5. Exploratory Data Analysis
4.6. PopPK Model Structure
4.7. Covariate Assessment
4.8. Final Model Evaluation, Illustration of Covariate Effects, and Internal Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NORD. Friedreich’s Ataxia. 2025. Available online: https://rarediseases.org/rare-diseases/friedreichs-ataxia/ (accessed on 4 July 2025).
- Du, J.; Zhou, Y.; Li, Y.; Xia, J.; Chen, Y.; Chen, S.; Wang, X.; Sun, W.; Wang, T.; Ren, X.; et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020, 32, 101483. [Google Scholar] [CrossRef]
- Cotticelli, M.G.; Xia, S.; Lin, D.; Lee, T.; Terrab, L.; Wipf, P.; Huryn, D.M.; Wilson, R.B. Ferroptosis as a Novel Therapeutic Target for Friedreich’s Ataxia. J. Pharmacol. Exp. Ther. 2019, 369, 47–54. [Google Scholar] [CrossRef]
- La Rosa, P.; Petrillo, S.; Fiorenza, M.T.; Bertini, E.S.; Piemonte, F. Ferroptosis in Friedreich’s Ataxia: A Metal-Induced Neurodegenerative Disease. Biomolecules 2020, 10, 1551. [Google Scholar] [CrossRef]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and Efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann. Neurol. 2021, 89, 212–225. [Google Scholar] [CrossRef]
- Turchi, R.; Faraonio, R.; Lettieri-Barbato, D.; Aquilano, K. An Overview of the Ferroptosis Hallmarks in Friedreich’s Ataxia. Biomolecules 2020, 10, 1489. [Google Scholar] [CrossRef]
- Kahn-Kirby, A.H.; Amagata, A.; Maeder, C.I.; Mei, J.J.; Sideris, S.; Kosaka, Y.; Hinman, A.; Malone, S.A.; Bruegger, J.J.; Wang, L.; et al. Targeting ferroptosis: A novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy. PLoS ONE 2019, 14, e0214250. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Taylor-Fishwick, D.A.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 2011, 50, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharm. 1981, 9, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.S.; Fiedler-Kelly, J. Introduction to Population Pharmacokinetic/Pharmacodynamic Analysis with Nonlinear Mixed Effects Models; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Brocks, D.R.; Davies, N.M. Lymphatic Drug Absorption via the Enterocytes: Pharmacokinetic Simulation, Modeling, and Considerations for Optimal Drug Development. J. Pharm. Pharm. Sci. 2018, 21, 254s–270s. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.; Palin, K.J.; Davis, S.S. Lipid vehicles for intestinal lymphatic drug absorption. J. Pharm. Pharmacol. 1987, 39, 55–56. [Google Scholar] [CrossRef]
- Holm, R.; Porter, C.J.; Edwards, G.A.; Mullertz, A.; Kristensen, H.G.; Charman, W.N. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur. J. Pharm. Sci. 2003, 20, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rysanek, P.; Grus, T.; Sima, M.; Slanar, O. Lymphatic Transport of Drugs after Intestinal Absorption: Impact of Drug Formulation and Physicochemical Properties. Pharm. Res. 2020, 37, 166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yan, B.; Gao, L.; Tang, H.; Fan, Y.; Anderson, S.N.; Affleck, R.; Burns, D.J. Compound transfer efficiency from polystyrene surfaces: Application to microarrayed compound screening. J. Biomol. Screen. 2005, 10, 293–303. [Google Scholar] [CrossRef][Green Version]
- Hummel, J.; McKendrick, S.; Brindley, C.; French, R. Exploratory assessment of dose proportionality: Review of current approaches and proposal for a practical criterion. Pharm. Stat. 2009, 8, 38–49. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentre, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Mentre, F. Evaluation of different tests based on observations for external model evaluation of population analyses. J. Pharmacokinet. Pharmacodyn. 2010, 37, 49–65. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, M.; Marsot, A. External Evaluation of Population Pharmacokinetic Models for Precision Dosing: Current State and Knowledge Gaps. Clin. Pharmacokinet. 2023, 62, 533–540. [Google Scholar] [CrossRef]
- Moons, K.G.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef]
- Taylor, Z.L.; Poweleit, E.A.; Paice, K.; Somers, K.M.; Pavia, K.; Vinks, A.A.; Punt, N.; Mizuno, T.; Girdwood, S.T. Tutorial on model selection and validation of model input into precision dosing software for model-informed precision dosing. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1827–1845. [Google Scholar] [CrossRef]
- Lee, L.; Flach, S.; Xue, H.; Arivelu, L.; Golden, L.; Kong, R.; Darpo, B. Lack of Concentration-QTc Relationship and Cardiac Risk With Vatiquinone Therapeutic and Supratherapeutic Doses. Clin. Pharmacol. Drug Dev. 2024, 13, 1227–1238. [Google Scholar] [CrossRef]
- Ahn, J.E.; Karlsson, M.O.; Dunne, A.; Ludden, T.M. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J. Pharmacokinet. Pharmacodyn. 2008, 35, 401–421. [Google Scholar] [CrossRef]
- FDA. Population Pharmacokinetics Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics (accessed on 25 February 2022).
- Arshad, U.; Chasseloup, E.; Nordgren, R.; Karlsson, M.O. Development of visual predictive checks accounting for multimodal parameter distributions in mixture models. J. Pharmacokinet. Pharmacodyn. 2019, 46, 241–250. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, K.M.; Patel, K.; Nieforth, K.; Kirkpatrick, C.M.J. A Regression Approach to Visual Predictive Checks for Population Pharmacometric Models. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 678–686. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Mouksassi, M.S.; Holford, N.; Al-Huniti, N.; Freedman, I.; Hooker, A.C.; John, J.; Karlsson, M.O.; Mould, D.R.; Perez Ruixo, J.J.; et al. Model Evaluation of Continuous Data Pharmacometric Models: Metrics and Graphics. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Menon-Andersen, D.; Yu, B.; Madabushi, R.; Bhattaram, V.; Hao, W.; Uppoor, R.S.; Mehta, M.; Lesko, L.; Temple, R.; Stockbridge, N.; et al. Essential pharmacokinetic information for drug dosage decisions: A concise visual presentation in the drug label. Clin. Pharmacol. Ther. 2011, 90, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Beal, S.L.; Sheiner, L.B. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodyn. 2001, 28, 171–192. [Google Scholar] [CrossRef]
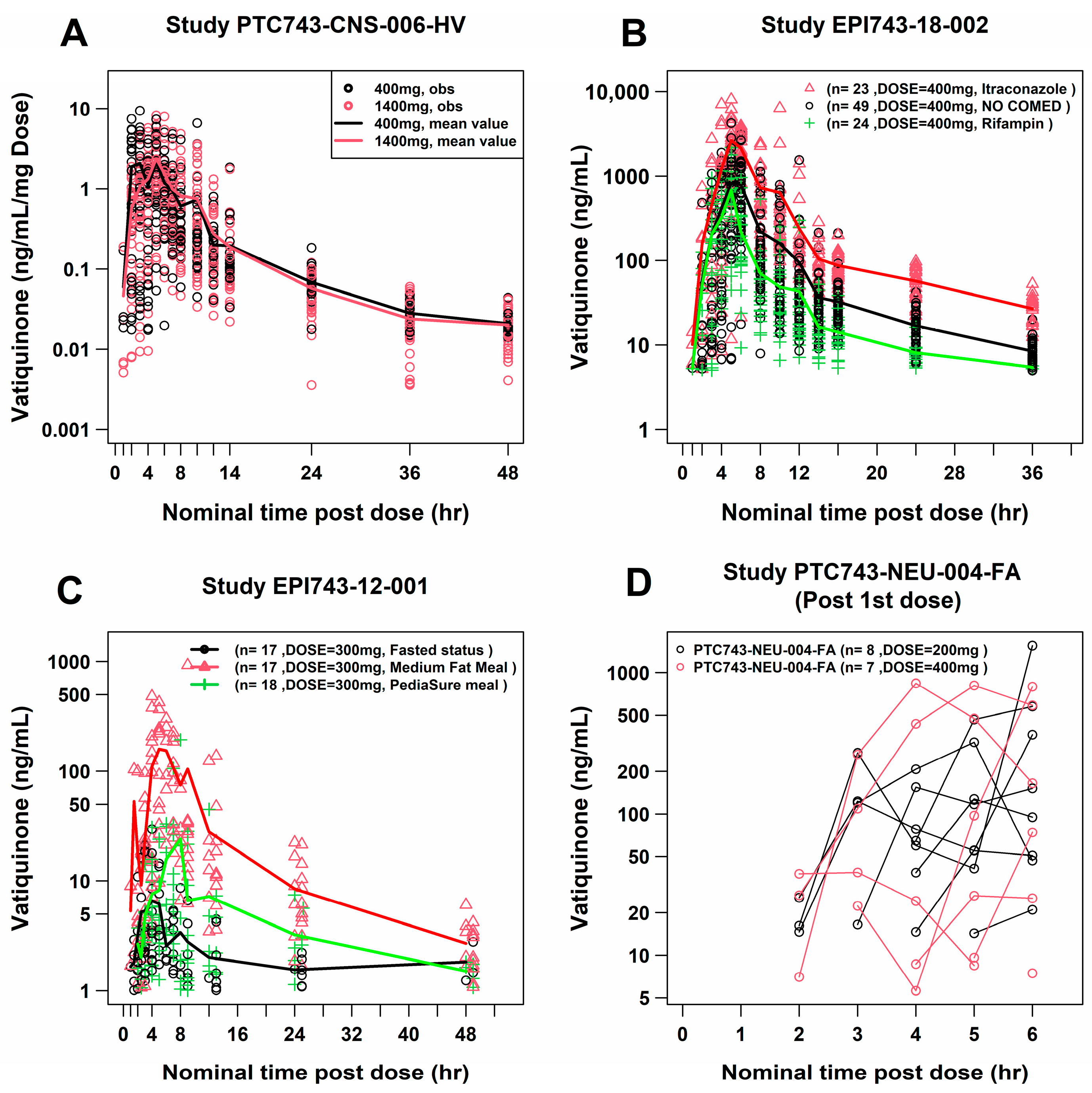
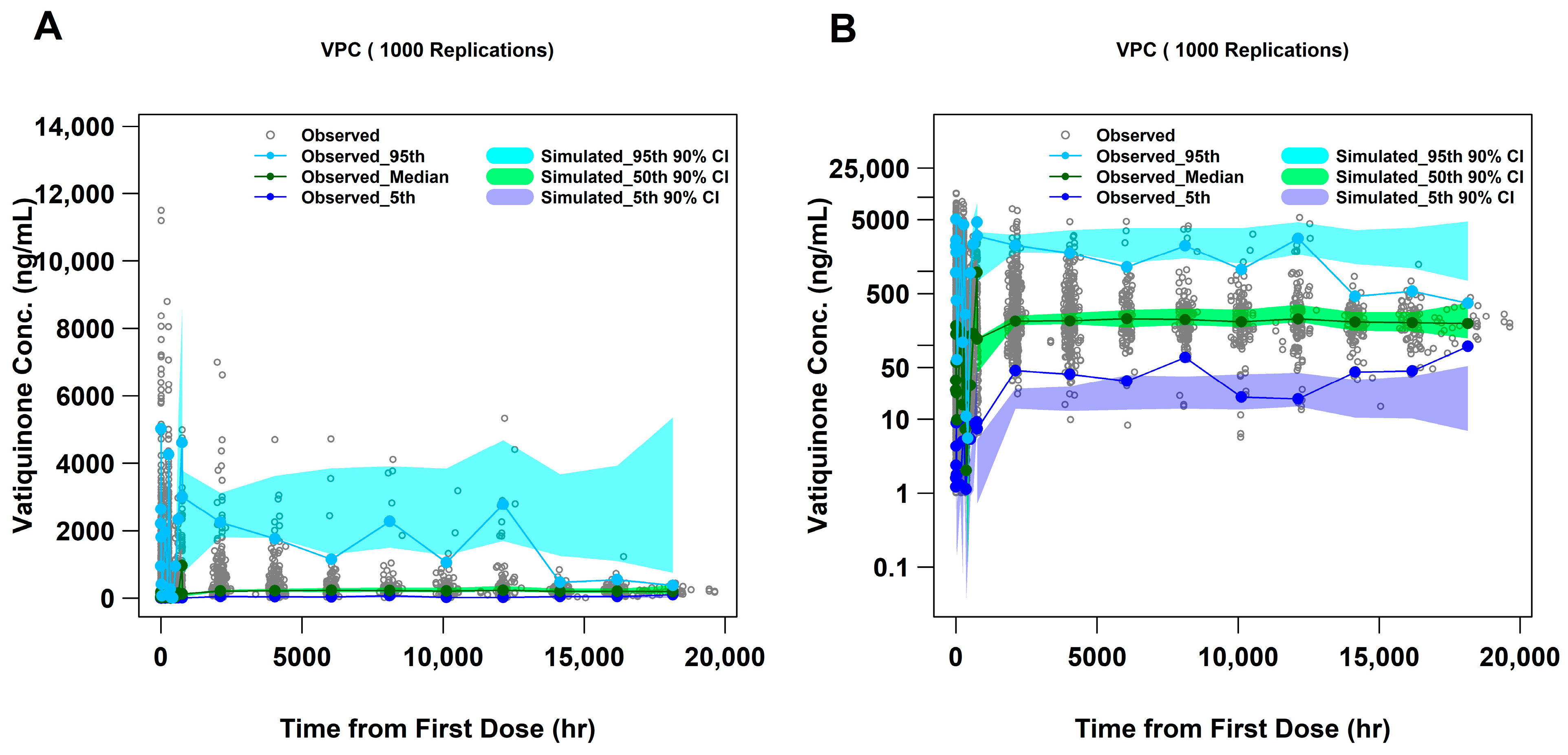


| Study | Population | Participants, n a | PK Samples, n b |
|---|---|---|---|
| EPI743-12-001 | Adult/HV | 18 | 333 |
| EPI743-18-002 | Adult/HV | 49 | 997 |
| PTC743-NEU-004-FA | Adult/HV | 16 | 466 |
| PTC743-CNS-006-HV | Adult/HV | 33 | 795 |
| EPI-2010-006 | Adult/FA | 42 | 700 |
| PTC743-NEU-003-FA | Adult/pediatric/FA | 126 | 972 |
| PTC743-NEU-005-FA | Pediatric/FA | 5 | 34 |
| PTC743-MIT-001-EP | Adult/pediatric/MD | 54 | 311 |
| Overall | 343 | 4608 |
| Theta/Parameter (Units) | Estimate | ASE | %RSE | 90% CI | ||
|---|---|---|---|---|---|---|
| 1 FK0 | 0.744 | 0.021 | 2.823 | 0.710; 0.779 | ||
| 2 Ka (1/h) | 0.200 | 0.016 | 8.000 | 0.173; 0.228 | ||
| 3 TK0 (hour) | 6.034 | 0.102 | 1.690 | 5.866; 6.203 | ||
| 4 TLAG1 (hour) | 2.787 | 0.044 | 1.579 | 2.715; 2.859 | ||
| 5 V/F (L) | 180.748 | 21.38 | 11.829 | 145.471; 216.025 | ||
| 6 CL/F (L/h) | 162.721 | 10.14 | 6.232 | 145.990; 179.452 | ||
| 7 V2/F (L) | 4852.69 | 773.404 | 15.938 | 3576.573; 6128.807 | ||
| 8 Q/F (L/h) | 67.896 | 6.136 | 9.037 | 57.772; 78.019 | ||
| 10 Itraconazole on CL/F | −1.446 | 0.175 | −12.102 | −1.735; −1.158 | ||
| 11 Rifampin on CL/F | 0.704 | 0.099 | 14.063 | 0.541; 0.867 | ||
| 12 Liquid PediaSure® on FK0 | −2.671 | 0.130 | −4.867 | −2.886; −2.457 | ||
| 13 Fasted statuses on FK0 | −3.324 | 0.167 | −5.024 | −3.599; −3.050 | ||
| 14 BWT on CL/F | 0.915 | 0.123 | 13.443 | 0.712; 1.118 | ||
| 15 Disease FA on CL/F | −0.406 | 0.082 | −20.197 | −0.541; −0.272 | ||
| 16 Disease FA on FK0 | −0.501 | 0.057 | −11.377 | −0.595; −0.408 | ||
| 17 BMI on CL/F | −0.975 | 0.249 | −25.538 | −1.386; −0.564 | ||
| Residual Variability | Estimate | ASE | %RSE | 90% CI | ||
| 9 Additive residual | 1.062 | 0.011 | 1.036 | 1.043; 1.081 | ||
| IIV | Estimate | ASE | (%CV) | (Shrinkage) | ||
| IIV–CL/F | 0.191 | 0.025 | 45.880 | 24.370 | ||
| OFV | 5488.13 | Condition number | 57.856 | |||
| Covariate | Reference Population | Ratio Relative to Reference Mean Value (90% CI) | Clinical Relevance d | |||
|---|---|---|---|---|---|---|
| Cmax,ss | Cmin,ss | AUC0–24h,ss | ||||
| Reference | HV | 1 (1; 1) | 1 (1; 1) | 1 (1; 1) | -- | |
| Disease FA on exposure a | HV | 0.76 (0.68; 0.85) | 0.89 (0.78; 1.03) | 0.81 (0.73; 0.91) | Most likely | |
| Disease FA on FK0 | HV | 0.50 (0.4; 0.59) | 0.50 (0.40; 0.59) | 0.50 (0.4; 0.59) | Yes | |
| Disease FA on CL/F | HV | 1.54 (1.29; 1.91) | 1.80 (1.42; 2.41) | 1.65 (1.34; 2.1) | Yes | |
| BMI b | (5th, 13.82 kg/m2) | 21.6 kg/m2 | 0.70 (0.60; 0.81) | 0.60 (0.49; 0.75) | 0.65 (0.55; 0.78) | Yes |
| (95th, 30.67 kg/m2) | 21.6 kg/m2 | 1.33 (1.18; 1.49) | 1.47 (1.25; 1.72) | 1.38 (1.21; 1.58) | Most likely | |
| BWT b | (5th, 17.14 kg) | 65 kg | 2.88 (2.35; 3.53) | 3.16 (2.42; 4.13) | 2.99 (2.37; 3.76) | Yes |
| (95th, 96.66 kg) | 65 kg | 0.71 (0.66; 0.76) | 0.70 (0.63; 0.77) | 0.70 (0.65; 0.76) | Yes | |
| Itraconazole (CYP3A4 inhibitor) | Vatiquinone monotherapy | 3.04 (2.51; 3.61) | 4.15 (3.31; 5.11) | 3.52 (2.87; 4.27) | Yes | |
| Rifampin (CYP3A4 inducer) | Vatiquinone monotherapy | 0.55 (0.48; 0.63) | 0.44 (0.37; 0.52) | 0.50 (0.43; 0.58) | Yes | |
| Liquid PediaSure® meal | Medium-fat meal c | 0.07 (0.06; 0.09) | 0.07 (0.06; 0.09) | 0.07 (0.06; 0.09) | Yes | |
| Fasted status | Medium-fat meal c | 0.04 (0.03; 0.05) | 0.04 (0.03; 0.05) | 0.04 (0.03; 0.05) | Yes | |
| Parameter | Typical Prediction (PRED), Mean (90% CI) | Individual Prediction (IPRED), Mean (90% CI) | Prespecified Criteria a |
|---|---|---|---|
| PE (%) | 4.55 (−18.8, 38.0) | 4.48 (−22.8, 39.1) | <±10% |
| APE (%) | 11.90 (0.616, 38.4) | 14.90 (0.901, 40.5) | <20% |
| RMSE (%) | 0.84 (0.627, 1.06) | 0.90 (0.711, 1.13) | <10% |
| MPE (%) | 4.55 (1.59, 7.78) | 4.48 (1.12, 8.00) | <±10% |
| MAPE (%) | 11.90 (9.98, 14.2) | 14.90 (12.7, 17.4) | <20% |
| Study | Population b | Description | Dose Regimen | Plasma Sampling |
|---|---|---|---|---|
| EPI743-12-01 (NCT: NA) | HV, adults (n = 18) | Crossover, food effect study (fasted, liquid food PediaSure®, and medium-fat meals) | 300 mg, single dose, capsule | Intensive (pre-dose, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 48 h post dose) |
| EPI743-18-002 (NCT: NA) | HV, adults (n = 49) | Crossover, DDI study with itraconazole or rifampin, medium-fat meals | 400 mg, single dose, capsule | Intensive (Day 1 and 22: pre-dose, 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 24, and 36 h post dose) |
| PTC743-NEU-004-FA a (NCT: NA) | HV, adults (n = 16) | Part 1: 7 days TID dosing, medium-fat meals Part 2: 14C, single dose | 400 mg (n = 8), 200 mg (n = 8), multiple dose (TID), capsule | Intensive (Day 1 and 6: pre-dose, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 24 h post morning dose) |
| PTC743-CNS-006-HV (NCT: NA) | HV, adults (n = 33) | Run-in phase, 4 treatments crossover TQT study, medium-fat meals | 400 mg (n = 28), 1400 mg (n = 28), Placebo (n = 28), single dose, capsule | Intensive (Pre-dose, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 24, 36, and 48 h post dose) |
| EPI-2010-006 (NCT: 01728064) | FA patients, adults (n = 63) | Phase 2b 6-month safety/efficacy double-blind placebo controlled with 6-month extension, medium-fat meals | Placebo, 200 mg TID, 400 mg TID, multiple doses, capsule | Intensive (Day 1 and 3: pre-dose, 1, 2, 3, 4, 6, 8, 10, 11, 12 h post morning dose) |
| PTC743-NEU-003-FA (NCT: 04577352) | FA patients, pediatrics and adults, 8–67 years old (n = 146) | Randomized double-blind, placebo-controlled (72 weeks), medium-fat meals | 200 mg (TID) if ˂12 years old and body weight ˂ 25 kg or a dose of 400 mg (TID) if ≥12 years old and/or body weight ≥ 25 kg or placebo (TID), multiple doses, capsule | Sparse (pre-dose at each visit of Week 1, 12, 24, 36, 48, 60, 72, 84, and 96) |
| PTC743-CNS-005-FA (NCT: 05485987) | Children with FA < 7 years old (n = 5) | An open-label, 72-week study to evaluate PK, safety, and efficacy of vatiquinone, medium-fat meals | 15 mg/kg if body weight < 13 kg and 200 mg if body weight ≥ 13 kg, TID, multiple doses, solution | Sparse (Week 4, 12, and 24: pre-dose, 1 to 3 h, and 3–6 h post morning dose) |
| PTC743-MIT-001-EP (NCT: 04378075) | Mitochondria disease, pediatric patients < 19 years old (n = 94) | Randomized double-blind, placebo-controlled (24 weeks) Open-label extension (48 weeks), PediaSure meals | 15 mg/kg if body weight < 13 kg, 200 mg if body weight ≥ 13 kg, multiple doses (TID), solution | Sparse (Day 1 and Week 24: pre-dose, 1, 3, 4, and 8 h post first dose; Week 48 and 72: 4 h post first dose) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Gao, L.; Lee, L.; Cherry, J.J.; Kong, R. Characterizing Population Pharmacokinetics of Vatiquinone in Healthy Volunteers and Patients with Friedreich’s Ataxia. Pharmaceuticals 2025, 18, 1339. https://doi.org/10.3390/ph18091339
Hu Y, Gao L, Lee L, Cherry JJ, Kong R. Characterizing Population Pharmacokinetics of Vatiquinone in Healthy Volunteers and Patients with Friedreich’s Ataxia. Pharmaceuticals. 2025; 18(9):1339. https://doi.org/10.3390/ph18091339
Chicago/Turabian StyleHu, Yongjun, Lan Gao, Lucy Lee, Jonathan J. Cherry, and Ronald Kong. 2025. "Characterizing Population Pharmacokinetics of Vatiquinone in Healthy Volunteers and Patients with Friedreich’s Ataxia" Pharmaceuticals 18, no. 9: 1339. https://doi.org/10.3390/ph18091339
APA StyleHu, Y., Gao, L., Lee, L., Cherry, J. J., & Kong, R. (2025). Characterizing Population Pharmacokinetics of Vatiquinone in Healthy Volunteers and Patients with Friedreich’s Ataxia. Pharmaceuticals, 18(9), 1339. https://doi.org/10.3390/ph18091339






