Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo[3,4-d]Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents
Abstract
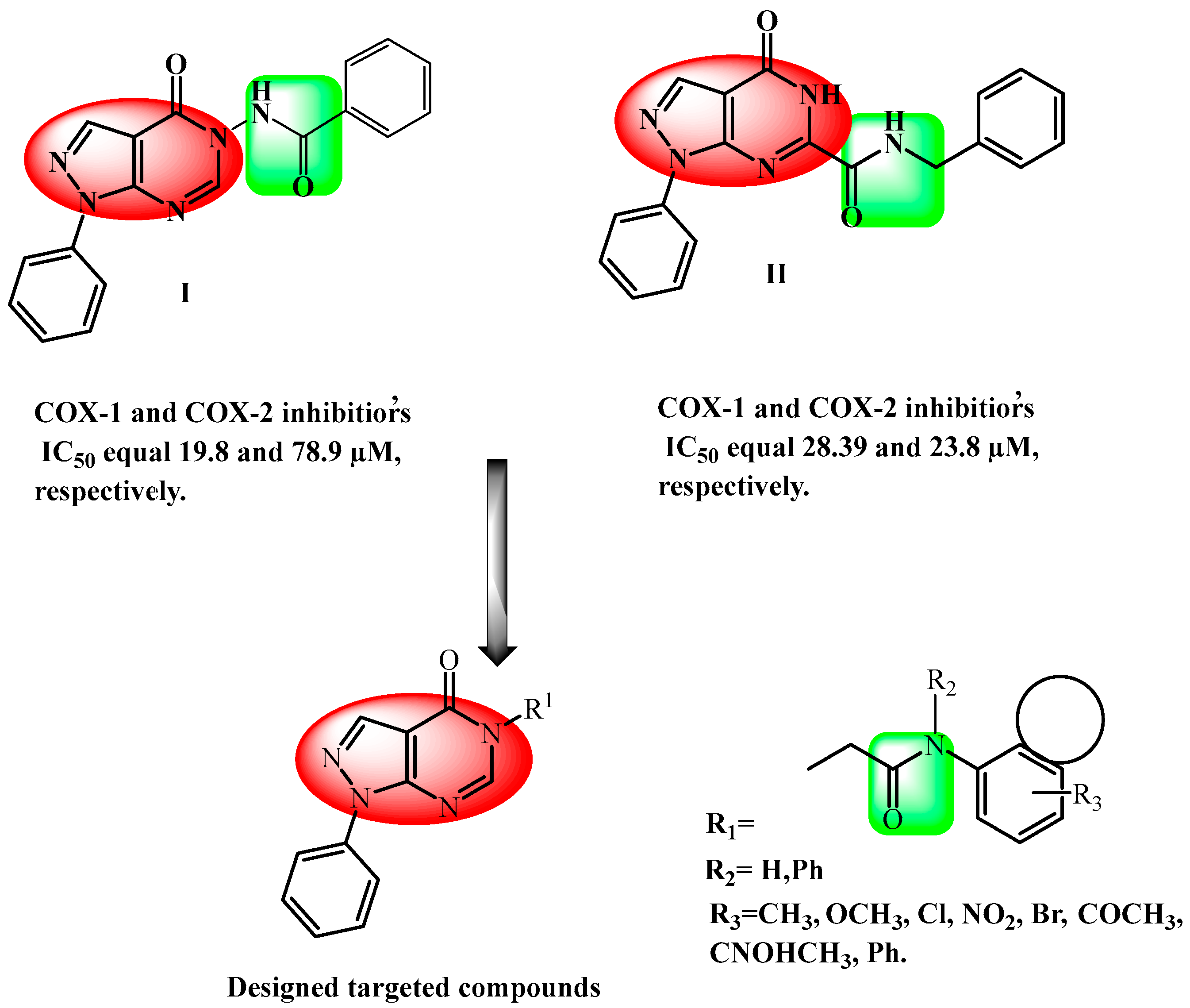
1. Introduction
2. Results and Discussion
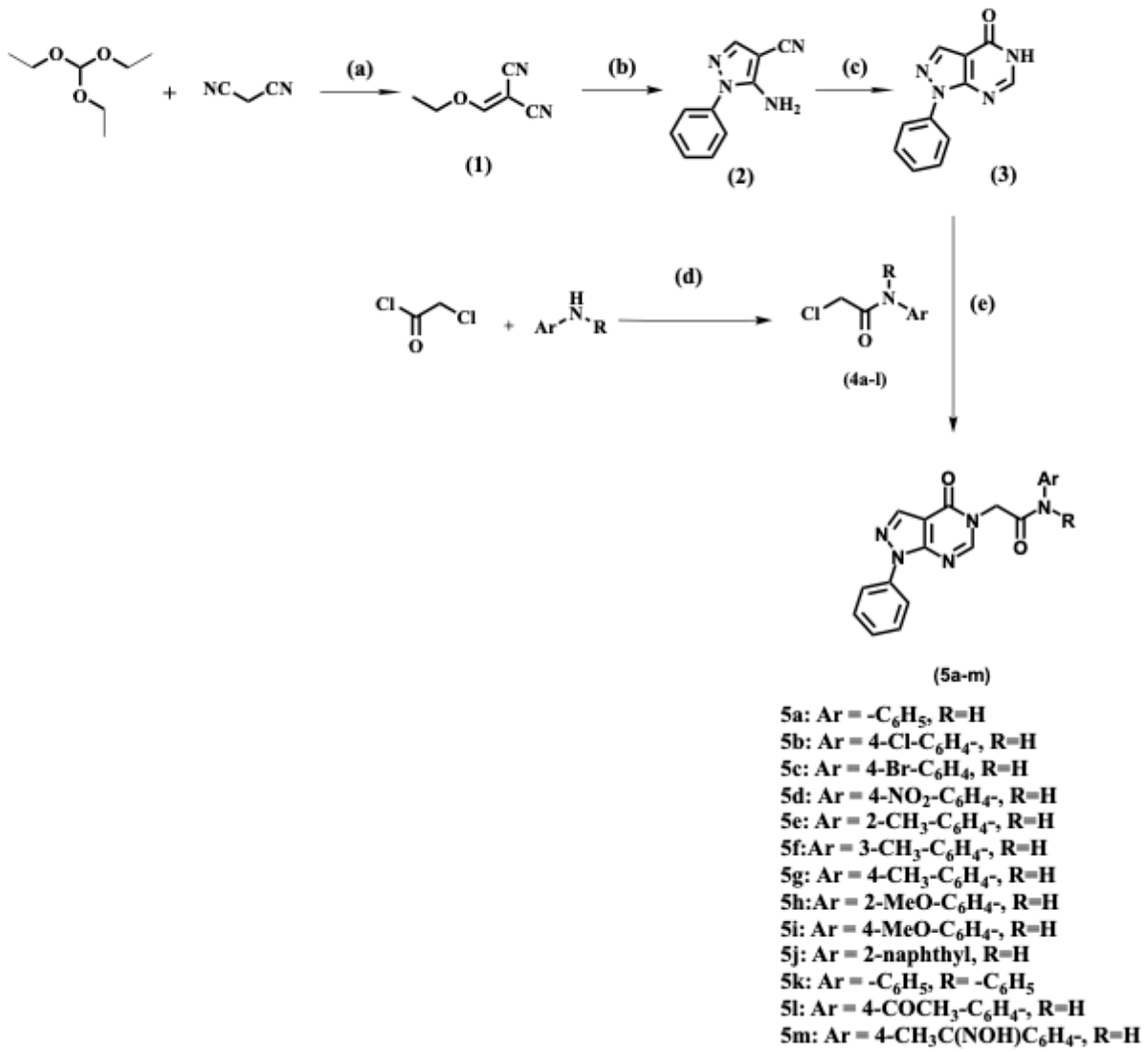
2.1. Chemistry
2.2. Evaluation of Biological Activities
2.2.1. In Vitro Cyclooxygenase Inhibitory Assay
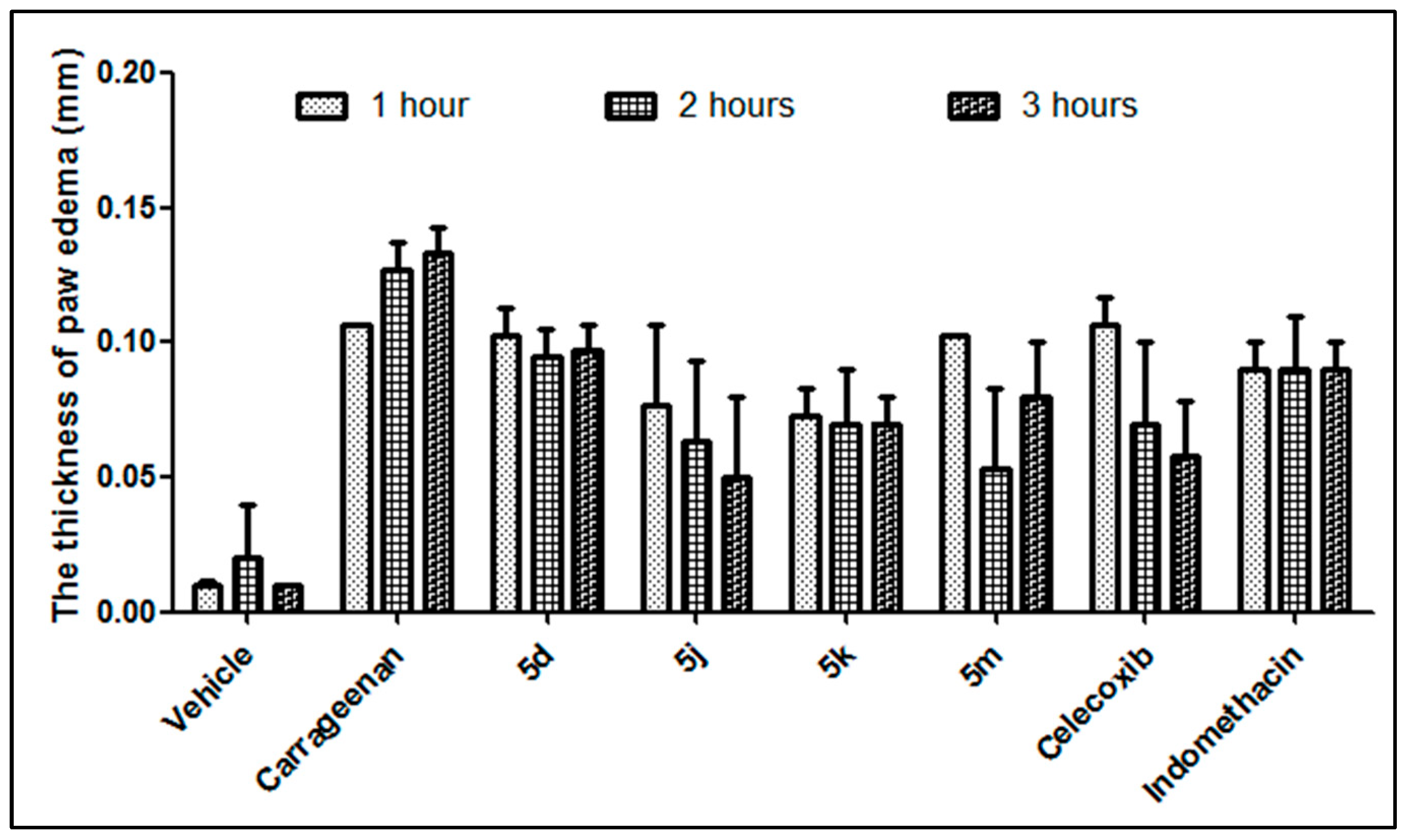
2.2.2. In Vivo Carrageenan-Induced Paw Edema
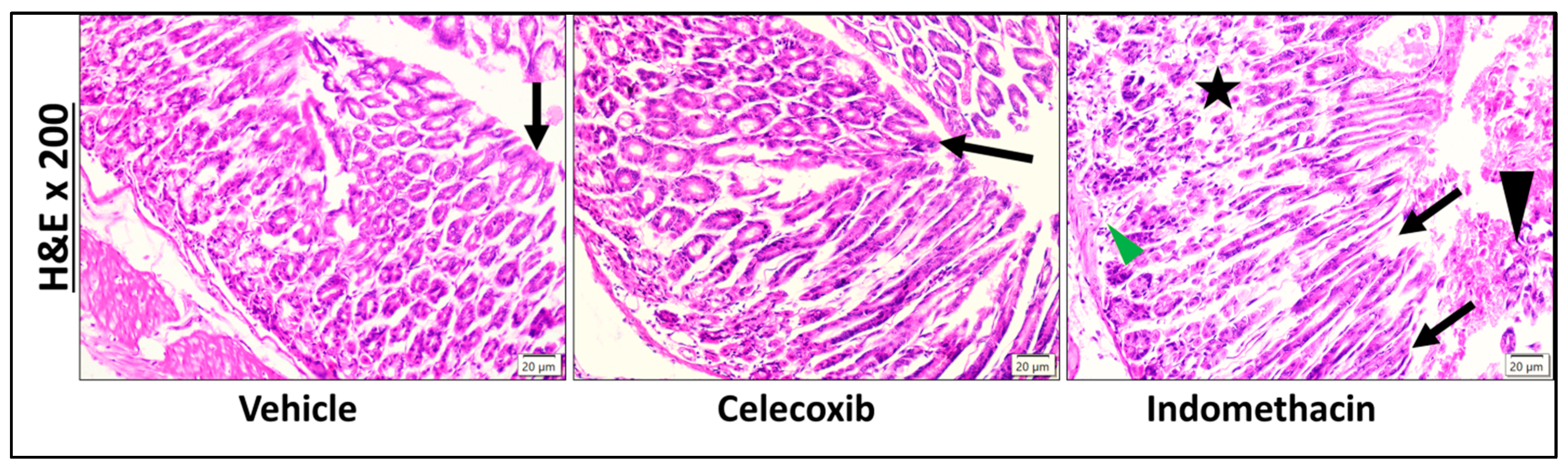
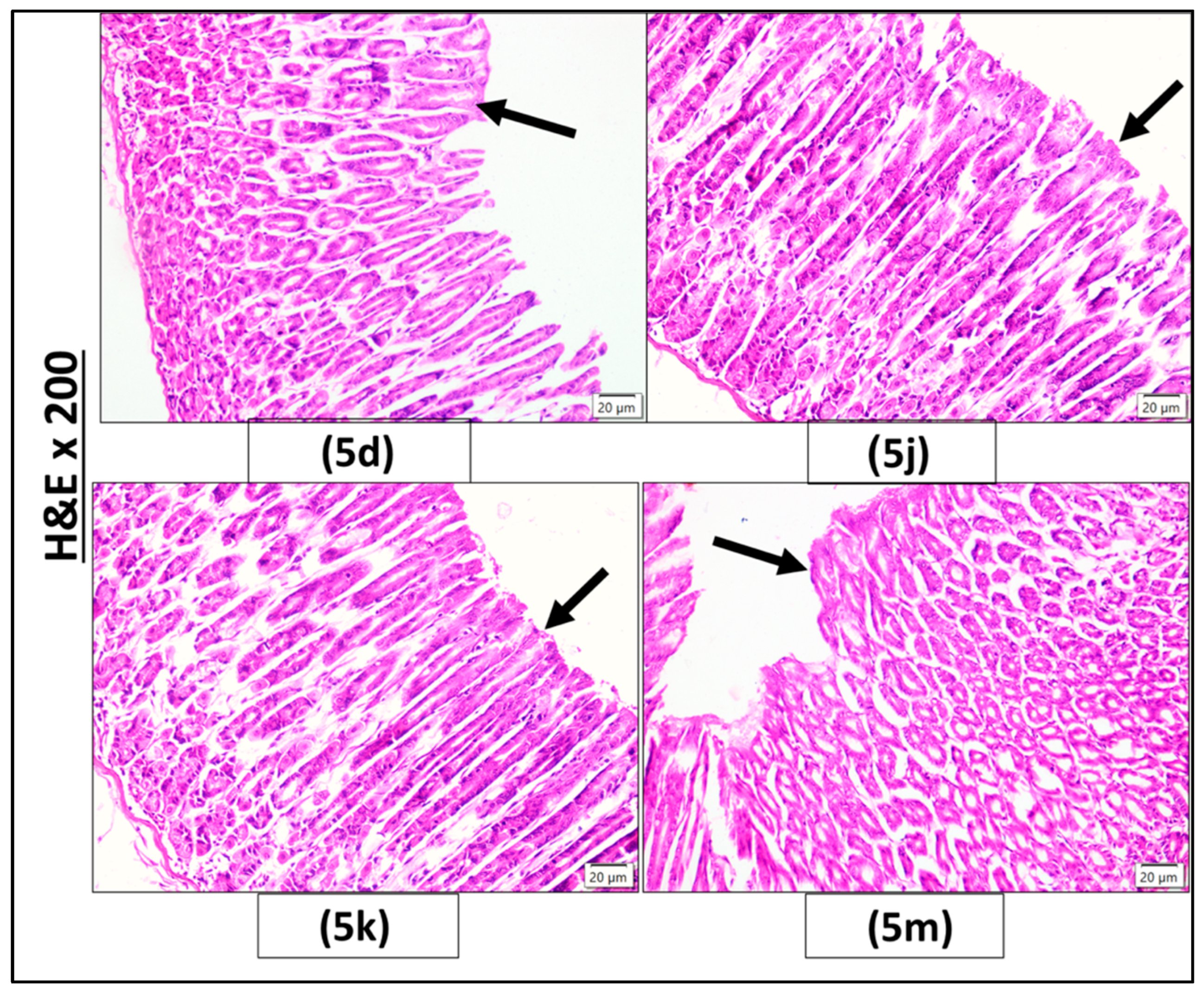
2.2.3. Histopathological Investigation
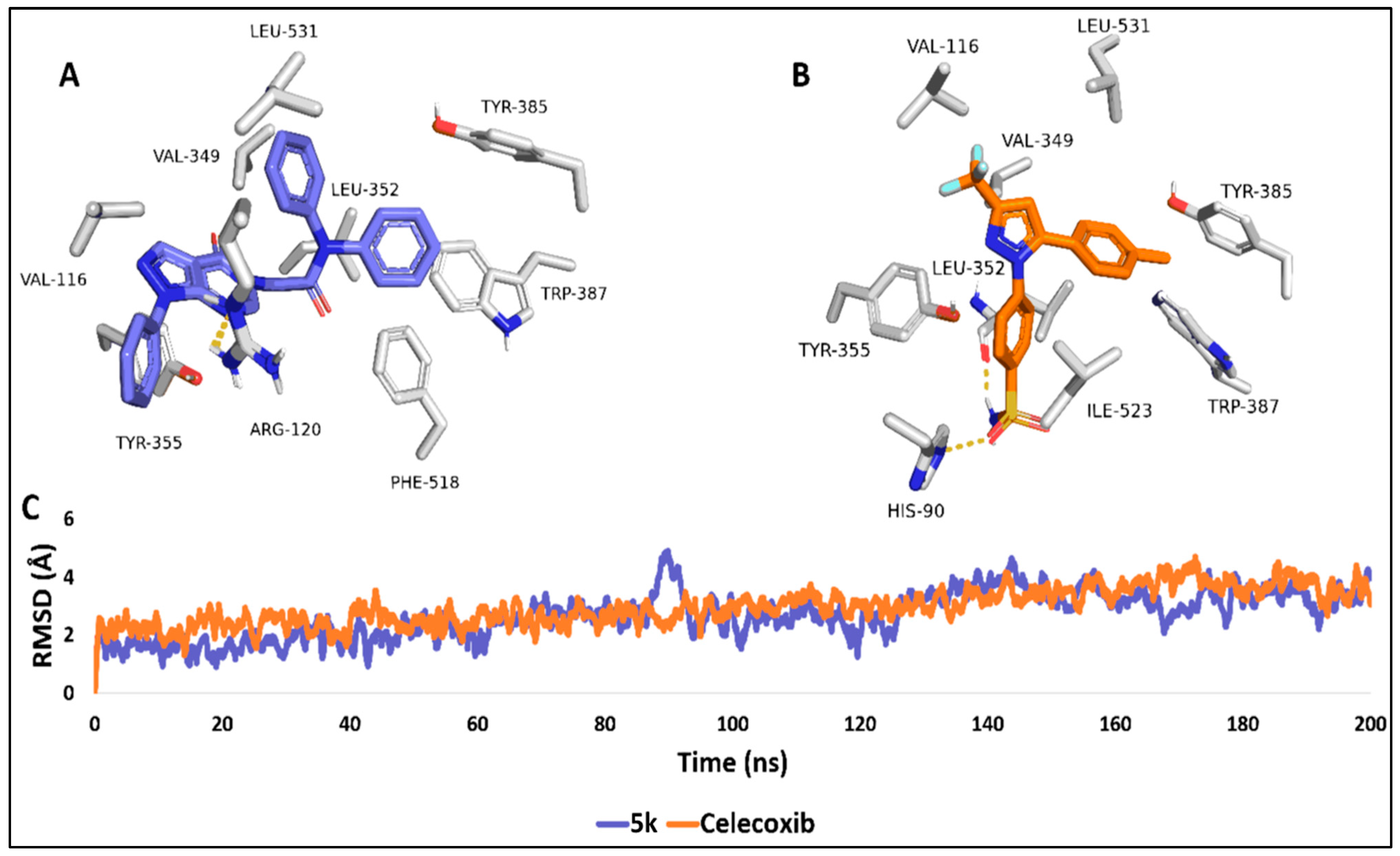
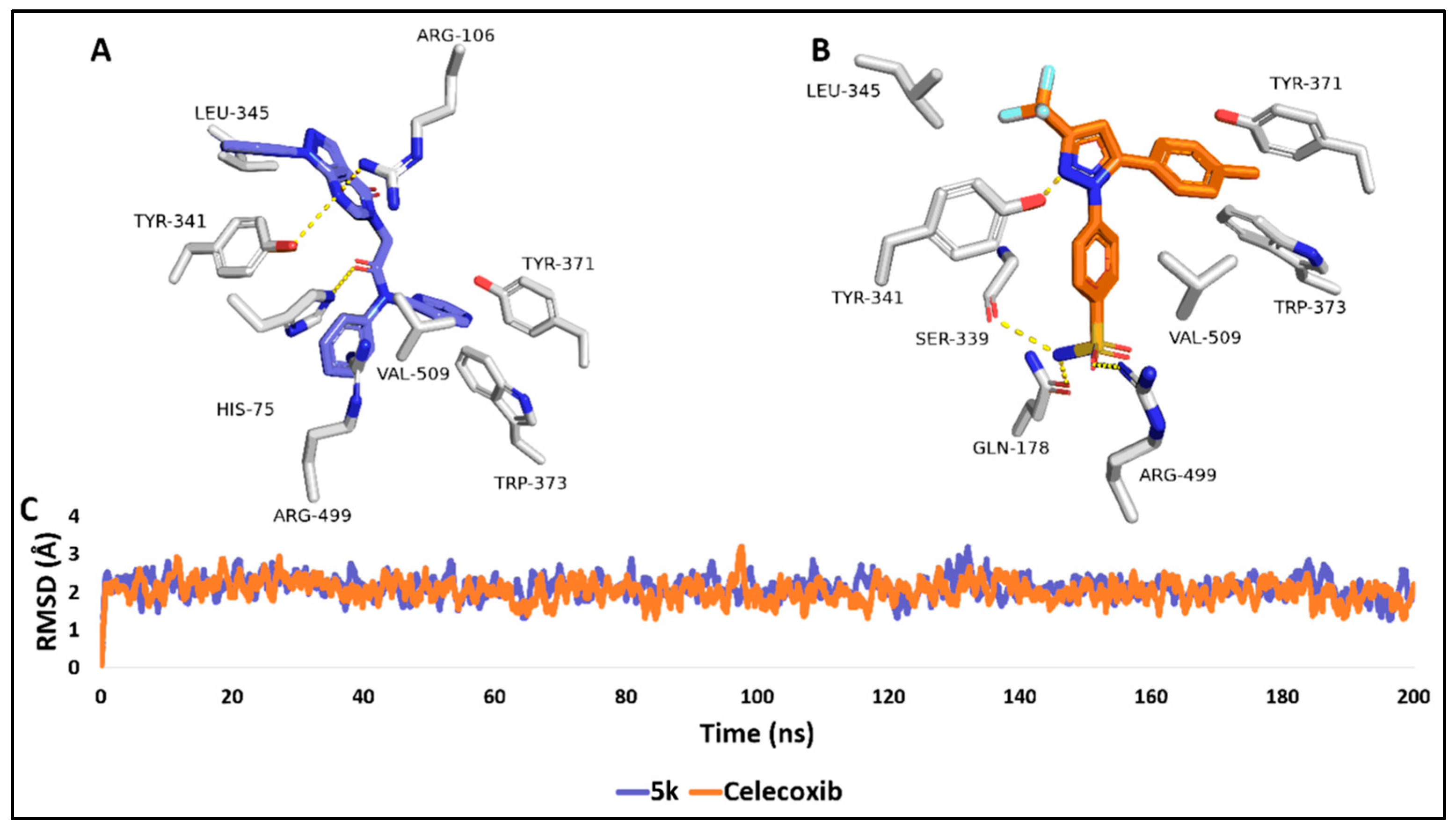
2.3. Molecular Modelling Study
Target Identification
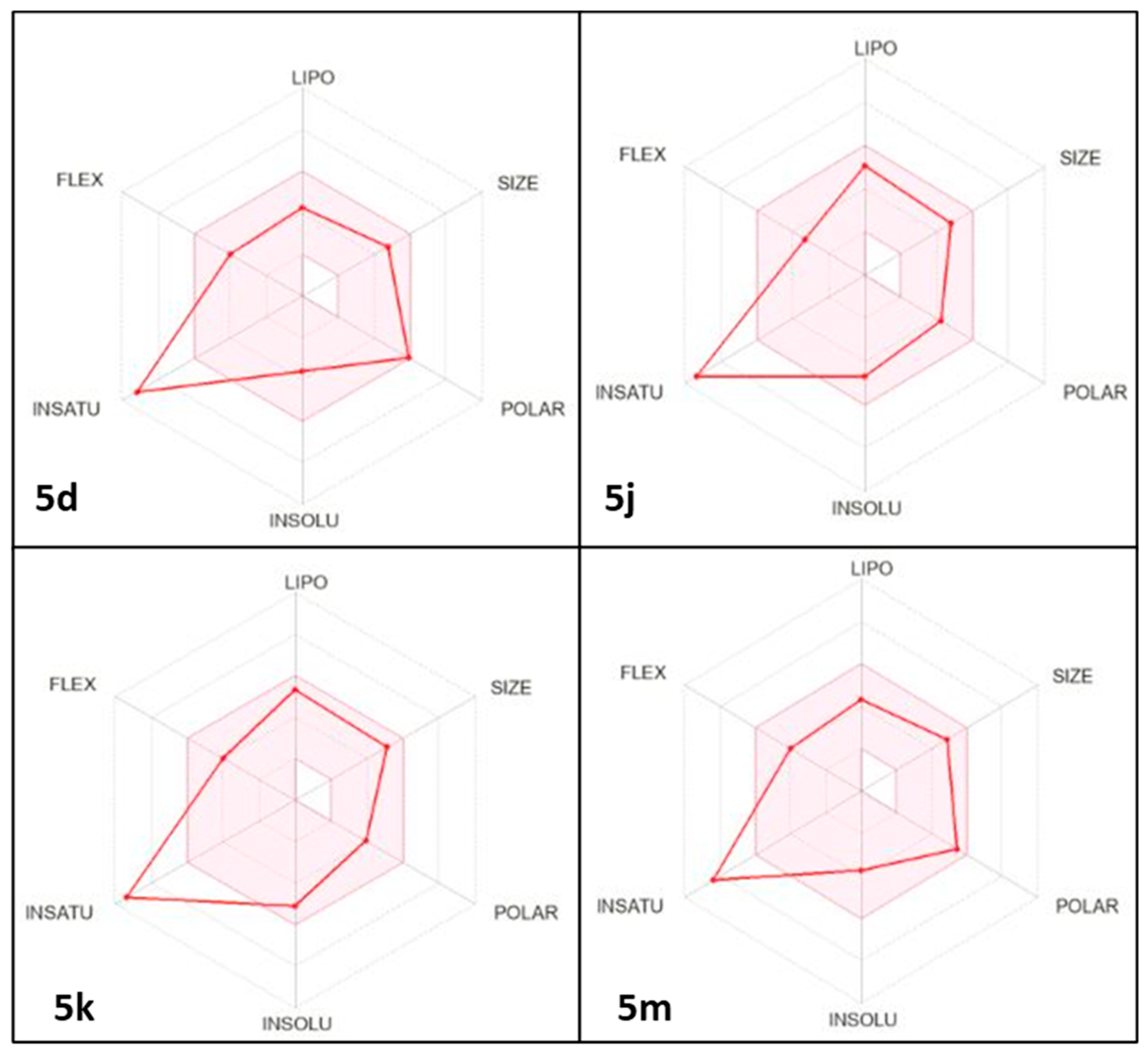
2.4. Predicted Physicochemical Properties and Toxicity Parameters
3. Experimental Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of 2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N-Phenylacetamide Derivatives (5a-m)
2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N-Phenyl Acetamide. (5a)
N-(4-Chlorophenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5b)
N-(4-Bromophenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5c)
N-(4-Nitrophenyl)-2-(4-oxo-1-phenyl-1,4-dihydro-5H-pyrazolo[3,4-d]pyrimidin-5-yl) Acetamide. (5d)
2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N-(o-Tolyl) Acetamide. (5e)
2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N-(m-Tolyl) Acetamide. (5f)
2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N-(p-Tolyl) Acetamide. (5g)
N-(2-Methoxyphenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5h)
N-(4-Methoxyphenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5i)
N-(Naphthalen-2-yl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5j)
2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)-N, N-Diphenyl Acetamide. (5k)
N-(4-Acetylphenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5l) [62]
N-(4-(1-(Hydroxyimino)Ethyl)Phenyl)-2-(4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetamide. (5m) [62]
3.2. Biology
3.2.1. In Vitro Cyclooxygenase (COX) Inhibition Assay
3.2.2. In Vivo Carrageenan-Induced Paw Edema
3.2.3. ELISA Assay for Tumor Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6)
3.2.4. Histopathological Examination
3.2.5. Animals
3.2.6. Blood Collection
3.2.7. Ethical Approval
3.2.8. Statistical Analysis
3.3. Molecular Docking
3.3.1. Molecular Dynamics Simulation
3.3.2. Binding Free Energy Calculations
3.4. Physicochemical and Pharmacokinetic Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serdar, O. Inflammation: Complexity and significance of cellular and molecular responses. J. Acute Disease 2024, 13, 3–7. [Google Scholar]
- Wang, M.; Chen, S.; He, X.; Yuan, Y.; Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 2024, 17, 13. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.J.O. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Ayman, R.; Abusaif, M.S.; Radwan, A.M.; Elmetwally, A.M.; Ragab, A. Development of novel pyrazole, imidazo [1, 2-b] pyrazole, and pyrazolo [1, 5-a] pyrimidine derivatives as a new class of COX-2 inhibitors with immunomodulatory potential. Eur. J. Med. Chem. 2023, 249, 115138. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, A.A.; Bao, K.; Lupardus, P.; Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug. Discov. 2021, 20, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Kalinkovich, A. Resolution of chronic inflammation, restoration of epigenetic disturbances and correction of dysbiosis as an adjunctive approach to the treatment of atopic dermatitis. Cells 2024, 13, 1899. [Google Scholar] [CrossRef]
- Soares, C.L.; Wilairatana, P.; Silva, L.R.; Moreira, P.S.; Barbosa, N.M.; da Silva, P.R.; Coutinho, H.D.; de Menezes, I.R.; Felipe, C.F. Biochemical aspects of the inflammatory process: A narrative review. Biomed. Pharmacother. 2023, 168, 115764. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The role of pro-inflammatory cytokines in the pathogenesis of cardiovascular disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Chen, Z.; Balachandran, Y.L.; Chong, W.P.; Chan, K.W. Roles of cytokines in Alzheimer’s disease. Int. J. Mol. Sci. 2024, 25, 5803. [Google Scholar] [CrossRef]
- Song, K. Current development status of cytokines for cancer immunotherapy. Biomol. Ther. 2024, 32, 13. [Google Scholar] [CrossRef]
- Sheppe, A.E.; Edelmann, M.J. Roles of eicosanoids in regulating inflammation and neutrophil migration as an innate host response to bacterial infections. Infect. Immun. 2021, 89, e0009521. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Farooq, M.; Isham, I.M.; Ali, A.; Hassan, M.S.; Herath-Mudiyanselage, H.; Ranaweera, H.A.; Najimudeen, S.M.; Abdul-Careem, M.F. Cyclooxygenase-2/prostaglandin E2 pathway regulates infectious bronchitis virus replication in avian macrophages. J. Gen. Virol. 2024, 105, 001949. [Google Scholar] [CrossRef] [PubMed]
- Kobesy, M.R.; Mohamed, M.F.; Mohassab, A.M.; Beshr, E.A. Pyrazolo [3, 4-d] pyrimidine Scaffold: Synthetic Strategies and Biological Activities. Sphinx J. Pharm. Med. Sci. 2025, 9, 21–43. [Google Scholar] [CrossRef]
- Qandeel, N.A.; El-Damasy, A.K.; Sharawy, M.H.; Bayomi, S.M.; El-Gohary, N.S. Synthesis, in vivo anti-inflammatory, COX-1/COX-2 and 5-LOX inhibitory activities of new 2, 3, 4-trisubstituted thiophene derivatives. Bioorg. Chem. 2020, 102, 103890. [Google Scholar] [CrossRef]
- Trostchansky, A.; Wood, I.; Rubbo, H. Regulation of arachidonic acid oxidation and metabolism by lipid electrophiles. Prostaglandins Other Lipid Mediat. 2021, 152, 106482. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.A.; Ahmed, E.M.; Tharwat, T.; Mahmoud, Z. NSAIDs between past and present; a long journey towards an ideal COX-2 inhibitor lead. RSC Adv. 2024, 14, 30647–30661. [Google Scholar] [CrossRef]
- Mohsin, N.U.A.; Aslam, S.; Ahmad, M.; Irfan, M.; Al-Hussain, S.A.; Zaki, M.E. Cyclooxygenase-2 (COX-2) as a target of anticancer agents: A review of novel synthesized scaffolds having anticancer and COX-2 inhibitory potentialities. Pharmaceuticals 2022, 15, 1471. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi-Rabbani, M.; Abdolghaffari, A.H.; Ghesmati, M.; Amini, A.; Zarghi, A. Selective COX-2 inhibitors as anticancer agents: A patent review (2018–2023). Expert Opin. Ther. Pat. 2024, 34, 733–757. [Google Scholar]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Abdel-Aziz, M.; Dalby, K.N.; Kaoud, T.S. Novel quinoline incorporating 1, 2, 4-triazole/oxime hybrids: Synthesis, molecular docking, anti-inflammatory, COX inhibition, ulceroginicity and histopathological investigations. Bioorganic Chem. 2017, 75, 242–259. [Google Scholar]
- Arfeen, M.; Srivastava, A.; Srivastava, N.; Khan, R.A.; Almahmoud, S.A.; Mohammed, H.A. Design, classification, and adverse effects of NSAIDs: A review on recent advancements. Bioorganic Med. Chem. 2024, 112, 117899. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Rodrigues, I.; Napoleão, L.; Lira, L.; Marques, D.; Veríssimo, M.; Andrade, J.P.; Dourado, M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biomed. Pharmacother. 2022, 150, 112958. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Muscara, M.J.D.; Disease, L. Selective cyclo-oxygenase-2 inhibitors: Cardiovascular and gastrointestinal toxicity. Dig. Liver Dis. 2000, 33, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; McLachlan, A.J.; Day, R.O.; Williams, K.M. Clinical pharmacokinetics and pharmacodynamics of celecoxib: A selective cyclo-oxygenase-2 inhibitor. Clin. Pharmacokinet. 2000, 38, 225–242. [Google Scholar] [CrossRef]
- Gong, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenetics Genom. 2012, 22, 310–318. [Google Scholar] [CrossRef]
- Lavie, C.J.; Howden, C.W.; Scheiman, J.; Tursi, J. Upper gastrointestinal toxicity associated with long-term aspirin therapy: Consequences and prevention. Curr. Probl. Cardiol. 2017, 42, 146–164. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Mohamed, M.F.; Allam, R.M.; Nafady, A.; Mohamed, S.K.; Gouda, A.E.; Beshr, E.A. Design, synthesis, and molecular docking of novel pyrazole-chalcone analogs of lonazolac as 5-LOX, iNOS, and tubulin polymerization inhibitors with potential anticancer and anti-inflammatory activities. Bioorganic Chem. 2022, 129, 106171. [Google Scholar] [CrossRef]
- Kassab, A.E. Pyrazolo [3, 4-d] pyrimidine scaffold: A review on synthetic approaches and EGFR and VEGFR inhibitory activities. Arch. Der Pharm. 2023, 356, 2200424. [Google Scholar] [CrossRef]
- Baillache, D.J.; Unciti-Broceta, A. Recent developments in anticancer kinase inhibitors based on the pyrazolo [3, 4-d] pyrimidine scaffold. RSC Med. Chem. 2020, 11, 1112–1135. [Google Scholar] [CrossRef]
- Alharthy, R.D. Design and synthesis of novel pyrazolo [3, 4-d] pyrimidines: In vitro cytotoxic evaluation and free radical scavenging activity studies. Pharm. Chem. J. 2020, 54, 273–278. [Google Scholar] [CrossRef]
- Lee, J.A.; Kwon, Y.W.; Kim, H.R.; Shin, N.; Son, H.J.; Cheong, C.S.; Kim, D.J.; Hwang, O. A novel pyrazolo [3, 4-d] pyrimidine induces heme oxygenase-1 and exerts anti-inflammatory and neuroprotective effects. Mol. Cells 2022, 45, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Salem, I.M.; Al-Awadh, M.A.; Alharbi, A.S.; Abouzed, D.E.E.; Allam, R.M.; Ahmed, O.A.; Ibrahim, T.S.; Abuo-Rahma, G.E.D.A.; Mohamed, M.F. Exploration of anti-inflammatory activity of pyrazolo [3, 4-d] pyrimidine/1, 2, 4-oxadiazole hybrids as COX-2, 5-LOX and NO release inhibitors: Design, synthesis, in silico and in vivo studies. Bioorganic Chem. 2025, 156, 108181. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S. (Ed.) Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Quintela, J.M.; Peinador, C.; González, L.; Devesa, I.; Ferrándiz, M.L.; Alcaraz, M.J.; Riguera, R. 6-Dimethylamino 1H-pyrazolo [3, 4-d] pyrimidine derivatives as new inhibitors of inflammatory mediators in intact cells. Bioorganic Med. Chem. 2003, 11, 863–868. [Google Scholar] [CrossRef]
- Tageldin, G.N.; Fahmy, S.M.; Ashour, H.M.; Khalil, M.A.; Nassra, R.A.; Labouta, I.M. Design, synthesis and evaluation of some pyrazolo [3, 4-d] pyrimidines as anti-inflammatory agents. Bioorganic Chem. 2018, 78, 358–371. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Sayed, H.A.; Sharaky, M.; Diab, H.M.; Elwahy, A.H.M.; Abdelhamid, I.A. Synthesis, cytotoxicity, anti-inflammatory, anti-metastatic and anti-oxidant activities of novel chalcones incorporating 2-phenoxy-N-arylacetamide and thiophene moieties: Induction of apoptosis in MCF7 and HEP2 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 10091–10107. [Google Scholar] [CrossRef]
- Yusov, A.S.; Chashchina, S.V.; Mikhailovskii, A.G.; Rudakova, I.P. Synthesis and analgesic and anti-inflammatory activities of (3,3-dipropyl-6,7-dimethoxy-3,4-dihydroisoquinolin-1(2H)-ylidene)-acetamide hydrochlorides. Pharm. Chem. J. 2019, 53, 35–39. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Plescia, F.; Cascioferro, S.; Raimondi, M.V.; Plescia, S.; Cusimano, M.G. Pyrazolo [3, 4-d] pyrimidine derivatives as COX-2 selective inhibitors: Synthesis and molecular modelling studies. Int. J. Pharm. Med. Chem. 2009, 342, 321–326. [Google Scholar]
- Abdelazeem, A.H.; Abdelatef, S.A.; El-Saadi, M.T.; Omar, H.A.; Khan, S.I.; McCurdy, C.R.; El-Moghazy, S.M. Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. Eur. J. Pharm. Sci. 2014, 62, 197–211. [Google Scholar] [CrossRef]
- Somakala, K.; Tariq, S.; Amir, M. Synthesis, evaluation and docking of novel pyrazolo pyrimidines as potent p38α MAP kinase inhibitors with improved anti-inflammatory, ulcerogenic and TNF-α inhibitory properties. Bioorganic Chem. 2019, 87, 550–559. [Google Scholar] [CrossRef]
- Souza, H.D.; Sousa, R.P.D.; Lira, B.F.; Vilela, R.F.; Borges, N.H.; Siqueira-Junior, J.P.D.; Lima, E.O.; Jardim, J.U.; Silva, G.A.D.; Barbosa-Filho, J.M.; et al. Synthesis, in silico study and antimicrobial evaluation of new selenoglycolicamides. J. Mol. Stru. 2019, 30, 188–197. [Google Scholar] [CrossRef]
- Salem, M.G.; Aziz, Y.M.A.; Elewa, M.; Nafie, M.S.; Elshihawy, H.A.; Said, M.M. Synthesis, molecular modeling, selective aldose reductase inhibition and hypoglycemic activity of novel meglitinides. Bioorganic Chem. 2021, 111, 104909. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, M.; Bahia, M.S.; Silakari, O. Synthesis, cytotoxic study and docking-based multidrug resistance modulator potential analysis of 2-(9-oxoacridin-10 (9H)-yl)-N-phenyl acetamides. Eur. J. Med. Chem. 2014, 80, 83–91. [Google Scholar] [CrossRef]
- Joshi, D.; Parikh, K. Synthesis and evaluation of novel benzimidazole derivatives as antimicrobial agents. Med. Chem. Res. 2014, 23, 1290–1299. [Google Scholar] [CrossRef]
- Abbas, R.F.; Allawi, A.G.; Abdulhassan, N.M.; Mahmoud, N.H. Spectrophotometric determination of paracetamol using a newly synthesized chromogenic reagent 4-[(2-amino-1, 3-thiazol-4-yl) amino] nitrobenzene. Egypt. J. Chem. 2020, 63, 4681–4693. [Google Scholar]
- Modh, R.P.; Kumar, S.P.; Jasrai, Y.T.; Chikhalia, K.H. Design, synthesis, biological evaluation, and molecular modeling of coumarin–piperazine derivatives as acetylcholinesterase inhibitors. Arch. Der Pharm. 2013, 346, 793–804. [Google Scholar] [CrossRef]
- Haggag, H.S.; Aboukhatwa, S.M.; Nafie, M.S.; Paul, A.; Sharafeldin, N.; Oliver, A.W.; El-Hamamsy, M.H. Design and synthesis of quinazolin-4-one derivatives as potential anticancer agents and investigation of their interaction with RecQ helicases. Bioorganic Chem. 2024, 144, 107086. [Google Scholar] [CrossRef]
- Chekryshkina, L.; Demin, A.; Tumashov, A.; Babikova, E.; Slepova, N.J. Synthesis and quantification of 2-(diethylamino)-N-(2-methylphenyl) acetamide nitrate. Russ. J. Org. Chem. 2019, 55, 1532–1538. [Google Scholar] [CrossRef]
- Shirvani, P.; Fayyazi, N.; Van Belle, S.; Debyser, Z.; Christ, F.; Saghaie, L.; Fassihi, A. Design, synthesis, in silico studies, and antiproliferative evaluations of novel indolin-2-one derivatives containing 3-hydroxy-4-pyridinone fragment. Bioorganic Med. Chem. Lett. 2022, 70, 128784. [Google Scholar] [CrossRef]
- Forrest, J.; Liddell, D.A.; Tucker, S.H. The oxidation of diphenylamine and its N-derivatives. Part I. Oxidation of >N· CH2R to >N· CO·R. J. Chem. Soc. (Resumed) 1946, 454–456. [Google Scholar] [CrossRef]
- Patel, N.B.; Shaikh, A.R. Synthesis of new 1, 3-oxazolyl-7-chloroquinazolin-4 (3H) ones and evaluation of their antimicrobial activities. Acta Pol. Pharm. 2011, 68, 223–230. [Google Scholar] [PubMed]
- Luis, J.A.d.S.; Souza, H.D.d.S.; Lira, B.F.; Alves, F.d.S.; de Athayde-Filho, P.F.; Lima, T.K.d.S.; Rocha, J.C.; Junior, F.J.B.M.; Scotti, L.; Scotti, M.T. Combined structure-and ligand-based virtual screening aiding discovery of selenoglycolicamides as potential multitarget agents against Leishmania species. J. Mol. Struct. 2019, 1198, 126872. [Google Scholar] [CrossRef]
- Rafiq, M.; Nazir, Y.; Ashraf, Z.; Rafique, H.; Afzal, S.; Mumtaz, A.; Hassan, M.; Ali, A.; Afzal, K.; Yousuf, M.R. Synthesis, computational studies, tyrosinase inhibitory kinetics and antimelanogenic activity of hydroxy substituted 2-[(4-acetylphenyl) amino]-2-oxoethyl derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 1562–1572. [Google Scholar] [CrossRef]
- Consalvi, S. Synthesis and biological evaluation of 1, 5-diphenylpyrrole derivatives as COX-2 selective inhibitors and NO-releasing agents and development of a novel BRD9 chemical probe. Ph.D. Thesis, Sapienza Università Editrice, Rome, Italy, 2020. [Google Scholar]
- Özdemir, A.; Temel, H.E. COX inhibitory profiles of a series of thiadiazole-benzothiazole hybrids. Eur. J. Life Sci. 2024, 3, 9–15. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, K.; Peng, J.; Li, J.; Luo, L.; Liu, M.; Chen, Y.; Xiang, Z.; Xiong, P.; Liu, L.; et al. Chemical characterization of extracts of leaves of Kadsua coccinea (Lem.) AC Sm. by UHPLC-Q-exactive orbitrap mass spectrometry and assessment of their antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2022, 149, 112828. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D.J.P. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef]
- El-Saghier, A.M.; Abosella, L.; Hamed, A.M.; Elakesh, E.O.; Abuo-Rahma, G.E.; Abdellattif, M.H.; Aziz, H.A. New norfloxacin analogues based on N-4-piperazinyl-(3-arylidene/alkylidene acrylonitrile) moieties: Design, synthesis, antibacterial evaluation, and in silico studies. Monatshefte Chem.-Chem. Mon. 2025, 7, 1–8. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, X.; Yu, W.; Hu, X.; Zhang, Y.; Yang, K.; You, Z.; Liu, Z.; Qiao, X.; Song, Y. Pyrazolo[3,4-d]pyrimidine-based dual HDAC/Topo II inhibitors: Design, synthesis, and biological evaluation as potential antitumor agents. J. Mol. Struct. 2023, 1272, 134221. [Google Scholar] [CrossRef]
- Ibrahim, T.S.; Moustafa, A.H.; Almalki, A.J.; Allam, R.M.; Althagafi, A.; Md, S.; Mohamed, M.F. Novel chalcone/aryl carboximidamide hybrids as potent anti-inflammatory via inhibition of prostaglandin E2 and inducible NO synthase activities: Design, synthesis, molecular docking studies and ADMET prediction. J. Enzym. Inhib. Med. Chem. 2021, 36, 1067–1078. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Oxford, UK, 2018. [Google Scholar]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J.; et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate, having a shorter and more favorable human half-life. Bioorganic Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, H.S.; Hu, Z. Using PyMOL as a platform for computational drug design. Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Saidi, A.E.; Bouzidi, N.; Ziane, M.; Gherib, M.; Rahila, C.; Mioc, M. In silico and in vitro studies: Investigating the chemical composition, DFT, molecular docking, and dynamic simulation of Satureja candidissima (Munby) Briq essential oil as a potential antibacterial agent. J. Biomol. Struct. Dyn. 2024, 43, 4043–4062. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Ahmed, A.K.M.; Beshr, E.A.M.; Salem, I.M.; Ahmed, O.A.A.; Ibrahim, T.S.; Elsayed Abouzed, D.E.; Mahmoud, A.M.; Mohamed, M.F.A. Novel chalcone candidates as potential in vitro and in vivo anti-inflammatory agents: Synthesis, in silico docking, multitarget bioevaluation and molecular dynamic simulation. Bioorganic Chem. 2025, 161, 108540. [Google Scholar] [CrossRef]


| Compound ID | IC50 μM a ± SEM | SI b | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| 5d | 14.08 ± 0.47 | 0.49 ± 0.017 | 28.73 |
| 5j | 6.403 ± 0.22 | 0.398 ± 0.014 | 16.09 |
| 5k | 25.47 ± 0.86 | 0.266 ± 0.009 | 95.75 |
| 5m | 57.31 ± 1.93 | 2.342 ± 0.08 | 24.47 |
| Celecoxib | 28.92 ± 0.93 | 0.293 ± 0.01 | 98.70 |
| Indomethacin | 0.0892 ± 0.003 | 0.202 ± 0.007 | 0.441 |
| Compound ID | 1 h | 2 h | 3 h |
|---|---|---|---|
| 5d | 3.43% | 21.26% | 27.32% |
| 5j | 28.35% | 50.13% | 62.41% |
| 5k | 31.46% | 44.88% | 54.89% |
| 5m | 3.43% | 58.01% | 39.85% |
| Celecoxib | 0.31% | 44.88% | 56.14% |
| Indomethacin | 15.89% | 29.13% | 32.33% |
| Compound ID | iLOGP | XLOGP3 | WLOGP | MLOGP | Silicos-IT LogP | Consensus Log P |
|---|---|---|---|---|---|---|
| 5d | 2.09 | 1.92 | 1.94 | 1.61 | −0.70 | 1.37 |
| 5j | 2.96 | 3.34 | 3.18 | 3.17 | 2.47 | 3.03 |
| 5k | 3.52 | 3.84 | 3.95 | 3.83 | 2.43 | 3.51 |
| 5m | 2.62 | 2.02 | 2.23 | 2.09 | 1.61 | 2.12 |
| Compound ID | ESOL Solubility (mg/mL) | ESOL Class | Ali Solubility (mg/mL) | Ali Class | Silicos-IT Solubility (mg/mL) | Silicos-IT Class |
|---|---|---|---|---|---|---|
| 5d | 9.59 × 10−2 | Soluble | −2.33 × 10−2 | Moderately soluble | 2.63 × 10−3 | Moderately Soluble |
| 5j | 8.22 × 10−3 | Moderately Soluble | 7.28 × 10−5 | Moderately soluble | 1.36 × 10−5 | Poorly soluble |
| 5k | 3.34 × 10−3 | Moderately Soluble | 3.59 × 10−3 | Moderately soluble | 1.14 × 10−5 | Poorly soluble |
| 5m | 7.29 × 10−2 | Soluble | 3.42 × 10−4 | Moderately soluble | 8.27 × 10−4 | Moderately Soluble |
| Compound ID | GI Absorption | BBB Permanent | Pgp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Log Kp (cm/s) |
|---|---|---|---|---|---|---|---|---|---|
| 5d | High | No | No | No | No | Yes | No | No | −7.32 |
| 5j | High | No | Yes | Yes | Yes | Yes | No | No | −6.34 |
| 5k | High | Yes | No | No | No | Yes | No | No | −6.14 |
| 5m | High | No | No | No | No | Yes | No | No | −7.31 |
| Compound ID | Lipinski Violations | Ghose Violations | Veber Violations | Egan Violation | Muegge Violations | Bioavailability Score |
|---|---|---|---|---|---|---|
| 5d | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 5j | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 5k | 0 | 0 | 0 | 0 | 0 | 0.55 |
| 5m | 0 | 0 | 0 | 0 | 0 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshdi, M.; Mohamed, M.F.A.; Beshr, E.A.M.; Aziz, H.A.; Gebril, S.M.; Bräse, S.; Mohassab, A.M. Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo[3,4-d]Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents. Pharmaceuticals 2025, 18, 1326. https://doi.org/10.3390/ph18091326
Roshdi M, Mohamed MFA, Beshr EAM, Aziz HA, Gebril SM, Bräse S, Mohassab AM. Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo[3,4-d]Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents. Pharmaceuticals. 2025; 18(9):1326. https://doi.org/10.3390/ph18091326
Chicago/Turabian StyleRoshdi, Mostafa, Mamdouh F. A. Mohamed, Eman A. M. Beshr, Hossameldin A. Aziz, Sahar M. Gebril, Stefan Bräse, and Aliaa M. Mohassab. 2025. "Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo[3,4-d]Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents" Pharmaceuticals 18, no. 9: 1326. https://doi.org/10.3390/ph18091326
APA StyleRoshdi, M., Mohamed, M. F. A., Beshr, E. A. M., Aziz, H. A., Gebril, S. M., Bräse, S., & Mohassab, A. M. (2025). Design, Synthesis, In Silico Docking, Multitarget Bioevaluation and Molecular Dynamic Simulation of Novel Pyrazolo[3,4-d]Pyrimidinone Derivatives as Potential In Vitro and In Vivo Anti-Inflammatory Agents. Pharmaceuticals, 18(9), 1326. https://doi.org/10.3390/ph18091326







