Development of Orally Disintegrating Tablets of Standardized Rhodiola rosea Extract
Abstract
1. Introduction
1.1. Stress and Adaptogens
1.2. Rhodiola rosea as Plant Raw Material
- Phenylpropanoids—rosavin;
- Phenylethanoid derivatives—salidroside and tyrosol;
- Flavonoids—tricine and rodionine;
- Monoterpene derivatives—rosidrol;
- Triterpenes—daucosterol;
1.3. Orally Disintegrating Tablets (ODTs)
1.4. Commercial Rhodiola rosea Products
2. Results
2.1. Herbal Drug Dry Extract
2.2. Development of ODT Formulation and Evaluation of Taste-Masking Capacity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Standardized RR Dry Extract Used as Drug (Active Ingredient) in ODTs
3.3. Powder Mixture Intended for Compression
3.4. Tableting and Testing of ODTs with Content of Standardized RR Dry Extract
3.5. Evaluation of Taste Acceptability (Palatability Test)
3.6. HPLC-UV Analytical Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ODTs | Orally disintegrating tablets |
| RR | Rhodiola rosea root and rhizome |
| HPLC | High-performance liquid chromatography |
| LOQ | Limit of quantification |
References
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions—Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef]
- Wal, A.; Wal, P.; Rai, A.K.; Tiwari, R.; Prajapati, S.K. Chapter 34—Adaptogens With a Special Emphasis on Withania somnifera and Rhodiola rosea. In Nutrition and Enhanced Sports Performance, 2nd ed.; Bagchi, D., Nair, S., Sen, C.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 407–418. [Google Scholar]
- Panossian, A.; Efferth, T. Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals 2022, 15, 1051. [Google Scholar] [CrossRef]
- EMEA. Reflection Paper on the Adaptogenic Concept; EMA: London, UK, 2008. [Google Scholar]
- Panossian, A.; Hamm, R.; Wikman, G.; Efferth, T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine 2014, 21, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Bawa, A.S.; Khanum, F. Anti-inflammatory activity of Rhodiola rosea—“a second-generation adaptogen”. Phytother. Res. 2009, 23, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Juanson Arabit, J.G.; Courville, R.; Kiani, D.; Chaston, J.M.; Nguyen, C.D.; Jena, N.; Liu, Z.Y.; Tata, P.; Van Etten, R.A. The impact of Rhodiola rosea on biomarkers of diabetes, inflammation, and microbiota in a leptin receptor-knockout mouse model. Sci. Rep. 2022, 12, 10581. [Google Scholar] [CrossRef]
- Liang, K.; Ma, S.; Luo, K.; Wang, R.; Xiao, C.; Zhang, X.; Gao, Y.; Li, M. Salidroside: An Overview of Its Promising Potential and Diverse Applications. Pharmaceuticals 2024, 17, 1703. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- EMEA. Rhodiolae Roseae Rhizoma Et Radix-Herbal Medicinal Product; EMA: London, UK, 2014. [Google Scholar]
- Parisi, A.; Tranchita, E.; Duranti, G.; Ciminelli, E.; Quaranta, F.; Ceci, R.; Cerulli, C.; Borrione, P.; Sabatini, S. Effects of chronic Rhodiola rosea supplementation on sport performance and antioxidant capacity in trained male: Preliminary results. J. Sports Med. Phys. Fit. 2010, 50, 57–63. [Google Scholar]
- Polumackanycz, M.; Konieczynski, P.; Orhan, I.E.; Abaci, N.; Viapiana, A. Chemical Composition, Antioxidant and Anti-Enzymatic Activity of Golden Root (Rhodiola rosea L.) Commercial Samples. Antioxidants 2022, 11, 919. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola rosea Extract by Supercritical CO(2)-Extraction and HPLC-ESI-MS/MS Spectrometry. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pióro-Jabrucka, E.; Czupa, W.; Synowiec, A.; Gniewosz, M.; Costa, R.; Mondello, L.; Węglarz, Z. Antioxidant and Antibacterial Activity of Roseroot (Rhodiola rosea L.) Dry Extracts. Molecules 2018, 23, 1767. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejiofor, P.; Dey, E.S. Extraction of rosavin from Rhodiola rosea root using supercritical carbon dioxide with water. J. Supercrit. Fluids 2009, 50, 29–32. [Google Scholar] [CrossRef]
- Langeder, J.; Grienke, U. A supercritical fluid workflow for the quality assessment of herbal drugs and commercial preparations from Rhodiola rosea. Phytochem. Anal. 2021, 32, 982–991. [Google Scholar] [CrossRef]
- Edwards, D.; Heufelder, A.; Zimmermann, A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms–results of an open-label study. Phytother. Res. 2012, 26, 1220–1225. [Google Scholar] [CrossRef]
- Suksawat, T.; Brniak, W.; Łyszczarz, E.; Wesoły, M.; Ciosek-Skibińska, P.; Mendyk, A. Orodispersible Dosage Forms with Rhinacanthin-Rich Extract as a Convenient Formulation Dedicated to Pediatric Patients. Pharmaceuticals 2024, 17, 994. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Orally Disintegrating Tablets; Food and Drug Administration Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2008. [Google Scholar]
- EDQM. Tablets. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- Chinwala, M. Recent Formulation Advances and Therapeutic Usefulness of Orally Disintegrating Tablets (ODTs). Pharmacy 2020, 8, 186. [Google Scholar] [CrossRef]
- Ghourichay, M.P.; Kiaie, S.H.; Nokhodchi, A.; Javadzadeh, Y. Formulation and Quality Control of Orally Disintegrating Tablets (ODTs): Recent Advances and Perspectives. BioMed Res. Int. 2021, 2021, 6618934. [Google Scholar] [CrossRef]
- Ivanovska, V.; Rademaker, C.M.A.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric Drug Formulations: A Review of Challenges and Progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef]
- Kelly, J.; D’Cruz, G.; Wright, D. Patients with dysphagia: Experiences of taking medication. J. Adv. Nurs. 2010, 66, 82–91. [Google Scholar] [CrossRef]
- Ozon, E.A.; Novac, M.; Gheorghe, D.; Musuc, A.M.; Mitu, M.A.; Sarbu, I.; Anuta, V.; Rusu, A.; Petrescu, S.; Atkinson, I.; et al. Formation and Physico-Chemical Evaluation of Nifedipine-hydroxypropyl-β-cyclodextrin and Nifedipine-methyl-β-cyclodextrin: The Development of Orodispersible Tablets. Pharmaceuticals 2022, 15, 993. [Google Scholar] [CrossRef]
- Sanjay, L.R.; Ashokbhai, M.K.; Ghatole, S.; Roy, S.; Kashinath, K.P.; Kaity, S. Strategies for beating the bitter taste of pharmaceutical formulations towards better therapeutic outcomes. RSC Pharm. 2024, 2, 59–81. [Google Scholar] [CrossRef]
- Brinckmann, J.A.; Cunningham, A.B.; Harter, D.E.V. Running out of time to smell the roseroots: Reviewing threats and trade in wild Rhodiola rosea L. J. Ethnopharmacol. 2021, 269, 113710. [Google Scholar] [CrossRef]
- Kołtun-Jasion, M.; Czerwiec, K.; Parzonko, A.; Bakiera, A.; Ożarowski, M.; Kiss, A.K. Comprehensive profiling of Rhodiola rosea roots and corresponding products: Phytochemical insights and modulation of neuroinflammation in BV2 microglial cell model. Front. Pharmacol. 2025, 16, 1608767. [Google Scholar] [CrossRef]
- EDQM. Rhodiola root and rhizome. In European Pharmacopoeia, 12th ed.; European Directorate for the Quality of Medicines & HealthCare, Ed.; Council of Europe: Strasbourg, France, 2025. [Google Scholar]
- Tsvetov, N.; Paukshta, O.; Fokina, N.; Volodina, N.; Samarov, A. Application of Natural Deep Eutectic Solvents for Extraction of Bioactive Components from Rhodiola rosea (L.). Molecules 2023, 28, 912. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Mamat, H.; Jusoh, W.M.S.W.; Idham, Z.; Yunus, M.A.C.; Irianto, I. Influence of particle size in supercritical carbon dioxide extraction of roselle (Hibiscus sabdariffa) on bioactive compound recovery, extraction rate, diffusivity, and solubility. Sci. Rep. 2023, 13, 10871. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi; Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Zheng, L.; He, P.; Tan, J.; Cai, J.; Wu, M.; Ye, X. Rosavin: Research Advances in Extraction and Synthesis, Pharmacological Activities and Therapeutic Effects on Diseases of the Characteristic Active Ingredients of Rhodiola rosea L. Molecules 2023, 28, 7412. [Google Scholar] [CrossRef]
- Ajdert, P.; Jan, L.; Burman, R. Liquid chromatographic method for the quantification of salidroside and cinnamyl alcohol glycosides for quality control of golden root (Rhodiola rosea L.). J. Appl. Res. Med. Aromat. Plants 2022, 26, 100364. [Google Scholar] [CrossRef]
- EDQM. Chromatographic Separation Techniques. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- Desai, P.M.; Er, P.X.; Liew, C.V.; Heng, P.W. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech 2014, 15, 1093–1104. [Google Scholar] [CrossRef]
- Ali, A.T.; Nasir, F.; Hidayatullah, T.; Pervez, S.; Rabqa Zainab, S.; Gohar, S.; Ur Rahman, A.; Khattak, M.A.; Alasmari, F.; Neau, S.H.; et al. Quality by Design Formulation Approach for the Development of Orodispersible Tablets of Dexlansoprazole. Drug Des. Dev. Ther. 2025, 19, 4163–4181. [Google Scholar] [CrossRef] [PubMed]
- Sutthapitaksakul, L.; Thanawuth, K.; Dass, C.R.; Sriamornsak, P. Optimized Taste-Masked Microparticles for Orally Disintegrating Tablets as a Promising Dosage Form for Alzheimer’s Disease Patients. Pharmaceutics 2021, 13, 1046. [Google Scholar] [CrossRef]
- Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. [Google Scholar] [CrossRef] [PubMed]
- Yoo, O.; von Ungern-Sternberg, B.S.; Lim, L.Y. Paediatric Medicinal Formulation Development: Utilising Human Taste Panels and Incorporating Their Data into Machine Learning Training. Pharmaceutics 2023, 15, 2112. [Google Scholar] [CrossRef]
- Maheshwari, R.; Todke, P.; Kuche, K.; Raval, N.; Tekade, R.K. Chapter 17-Micromeritics in Pharmaceutical Product Development. In Dosage Form Design Considerations; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 599–635. [Google Scholar]
- EDQM. Herbal Drug Extracts. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Loss on Drying. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EMEA. Guideline on Declaration of Herbal Substances and Herbal Preparations in Herbal Medicinal Products/Traditional Herbal Medicinal Products; EMEA: London, UK, 2010. [Google Scholar]
- Fu, Y.; Yang, S.; Jeong, S.H.; Kimura, S.; Park, K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 433–476. [Google Scholar] [CrossRef] [PubMed]
- EDQM. Powder Flow. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Bulk Density of Powders. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Uniformity of Mass of Single-Dose Preparations. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Resistance to Crushing of Tablets. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Disintegration of Tablets and Capsules. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EDQM. Friability of Uncoated Tablets. In European Pharmacopoeia, 12th ed.; European Pharmacopoeia (EDQM, Council of Europe): Strasbourg, France, 2025. [Google Scholar]
- EMEA. Guideline on Validation of Analytical Procedures; EMA: Amsterdam, The Netherlands, 2023; p. 33. [Google Scholar]
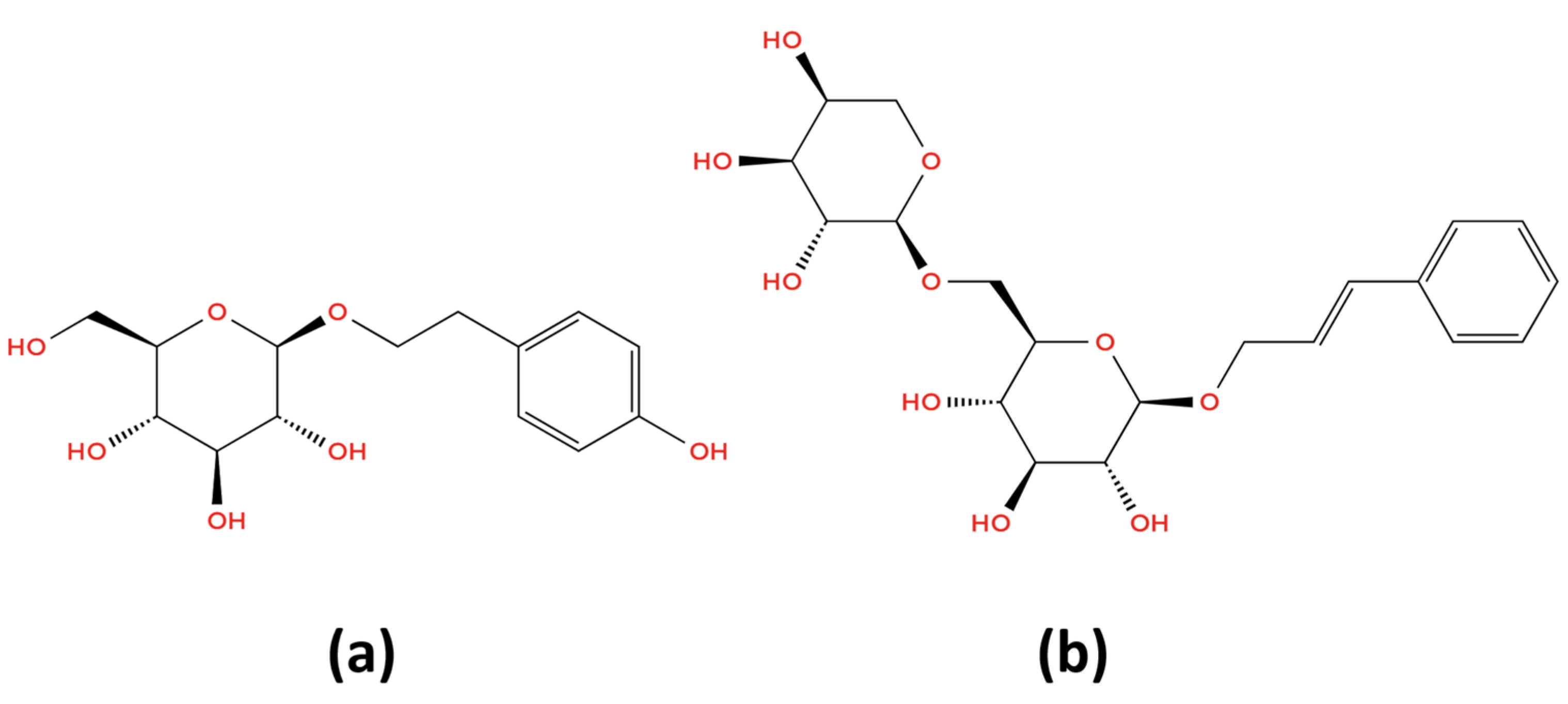
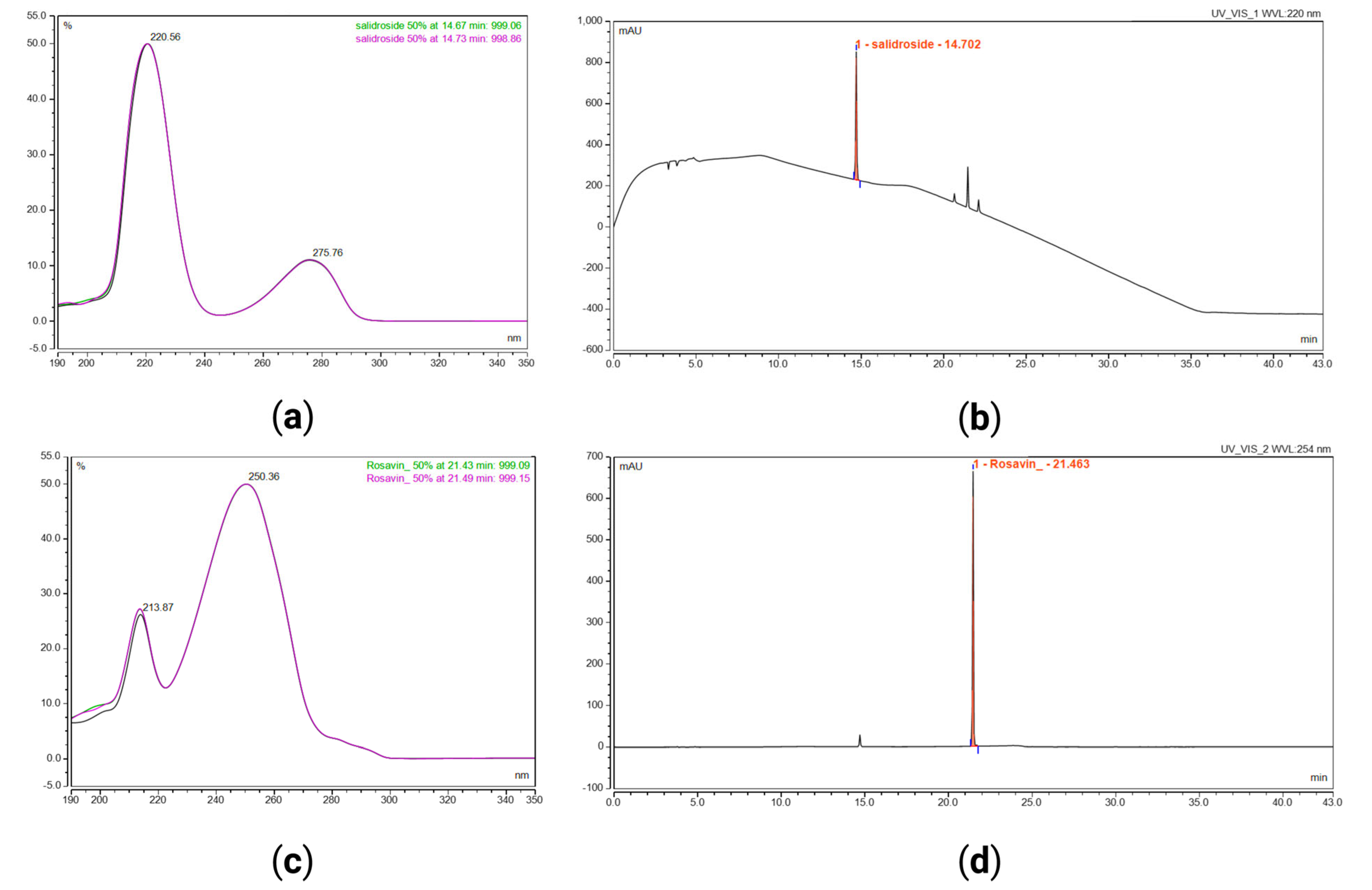
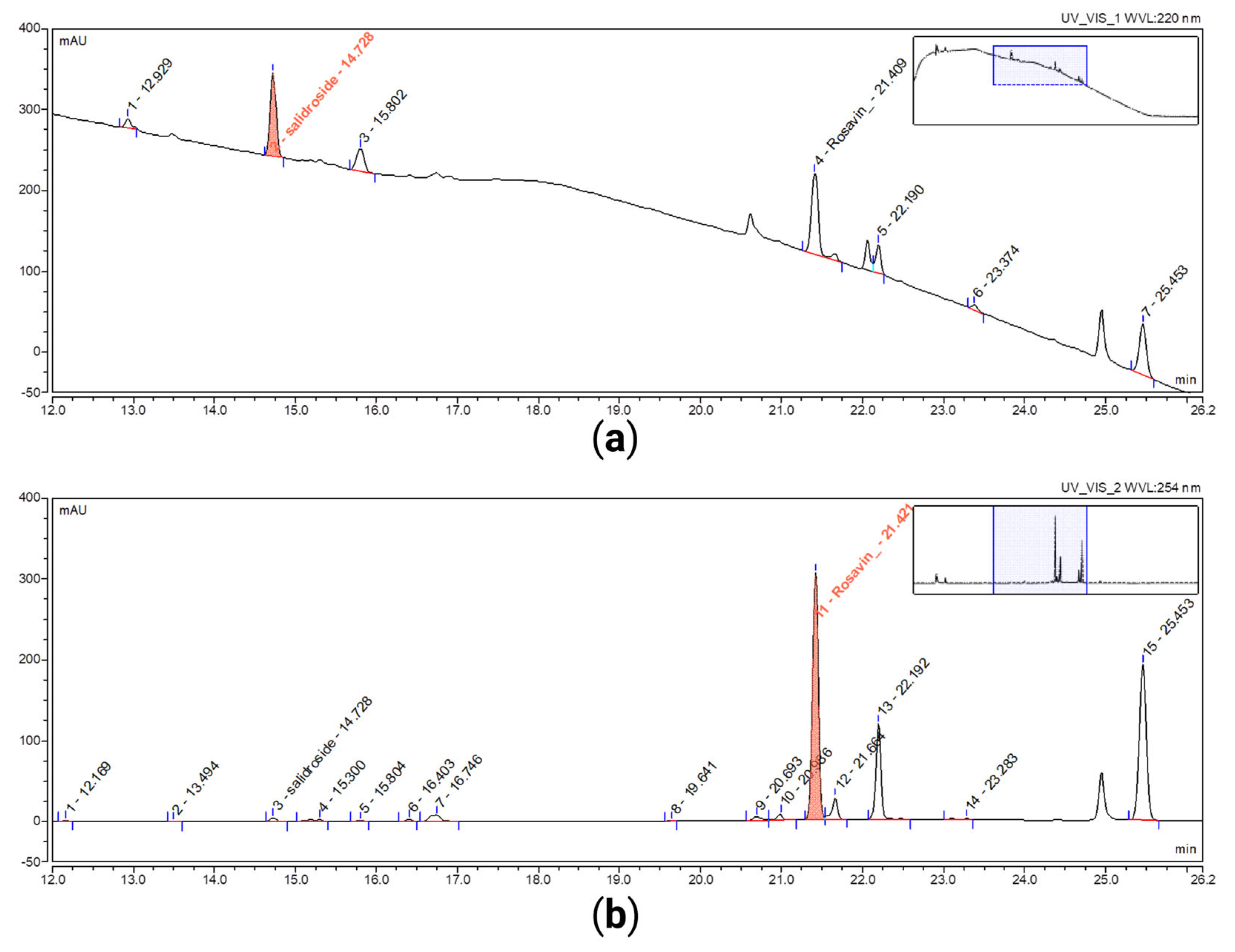
| Objective | Parameter | Acceptance Criteria | Result, Rosavin | Result, Salidroside | Observations |
|---|---|---|---|---|---|
| Specificity | Resolution | Rs ≥ 1.5 | 2.0 | 5.8 | n = 16 |
| Peak purity match factor | PPMF ≥ 950 | 984 | 985 | n = 16 | |
| Selectivity | Symmetry factor | 0.8 ≤ As ≤ 1.8 | 0.9 | 0.9 | n = 74 |
| Capacity factor | 2 ≤ k’ ≤ 10 | 6 | 3 | n = 74 | |
| Linearity | Correlation coefficient | R ≥ 0.997 | 0.99998 | 0.99998 | n = 20 |
| Standard Error | - | 0.11 | 0.13 | n = 20 | |
| Intercept | - | 0.0577 | 0.0941 | n = 20 | |
| Slope | - | 168.49 | 88.92 | n = 20 | |
| Residual plot | No trend | No trend | No trend | n = 20 | |
| Quantification limit | Limit of quantification | LOQ ≤ 0.01 mg/mL | 0.00011 mg/mL | 0.0014 mg/mL | n = 3 |
| Signal-to-noise ratio | S/N ≥ 10 | 24 | 19 | n = 3 | |
| Repeatability | Area | RSD ≤ 1% | 0.1 | 0.1 | n = 6 |
| Retention time | RSD ≤ 1% | 0.02 | 0.04 | n = 12 | |
| Content | RSD ≤ 5% | 0.6 | 1.7 | n = 6 | |
| Intermediate precision | Retention time | RSD ≤ 1% | 0.1 | 0.2 | n = 24 |
| Content | RSD ≤ 5% | 3.0 | 4.3 | n = 8 | |
| Accuracy | Recovery (80% level) | 95–105% | 96 | 98 | n = 3 |
| Recovery (100% level) | 95–105% | 97 | 98 | n = 3 |
| ODT | Formulation Variables (Ingredient %) | Taste Evaluation (Score 1–12) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Herbal Drug Extract | Sweetener, Flavor Enhancer | Disintegrant | Glidant, Lubricant | ||||||
| Dextrose | Mannitol | Aspartame | Citric Acid | Croscarmellose Sodium | Talc | Magnesium Stearate | |||
| R-1 | 20.0 | - | 77.5 | - | - | - | 2.0 | 0.5 | 3 |
| R-2 | 20.0 | 77.5 | - | - | - | - | 2.0 | 0.5 | 3 |
| R-3 | 20.0 | 38.8 | 38.7 | - | - | - | 2.0 | 0.5 | 3 |
| R-4 | 20.0 | - | 74.5 | - | - | 3.0 | 2.0 | 0.5 | 3 |
| R-5 | 20.0 | 74.5 | - | - | - | 3.0 | 2.0 | 0.5 | 4 |
| R-6 | 20.0 | 37.3 | 37.2 | - | - | 3.0 | 2.0 | 0.5 | 5 |
| R-7 | 30.0 | 64.5 | - | - | - | 3.0 | 2.0 | 0.5 | 1 |
| R-8 | 20.0 | 73.5 | - | - | 1.0 | 3.0 | 2.0 | 0.5 | 6 |
| R-9 | 20.0 | 36.8 | 36.7 | - | 1.0 | 3.0 | 2.0 | 0.5 | 5 |
| R-10 | 30.0 | 63.5 | - | - | 1.0 | 3.0 | 2.0 | 0.5 | 2 |
| R-11 | 30.0 | 31.8 | 31.7 | - | 1.0 | 3.0 | 2.0 | 0.5 | 2 |
| R-12 | 20.0 | 72.5 | - | - | 2.0 | 3.0 | 2.0 | 0.5 | 7 |
| R-13 | 20.0 | 75.5 | - | - | 1.0 | 1.0 | 2.0 | 0.5 | 6 |
| R-14 | 20.0 | 74.5 | - | - | 2.0 | 1.0 | 2.0 | 0.5 | 8 |
| R-15 | 20.0 | 71.5 | - | - | 1.0 | 5.0 | 2.0 | 0.5 | 4 |
| R-16 | 20.0 | 70.5 | - | - | 2.0 | 5.0 | 2.0 | 0.5 | 4 |
| R-17 | 20.0 | 66.2 | - | 7.3 | 2.0 | 2.0 | 2.0 | 0.5 | 9 |
| R-18 | 20.0 | 65.3 | - | 7.2 | 2.0 | 3.0 | 2.0 | 0.5 | 10 |
| R-19 | 20.0 | 68.0 | - | 7.5 | 2.0 | 2.0 | - | 0.5 | 9 |
| R-20 | 20.0 | 67.1 | - | 7.4 | 2.0 | 3.0 | - | 0.5 | 10 |
| Parameter (mean ± SD, n = 3) | R-12 | R-13 | R-14 | R-15 | R-16 | R-17 | R-18 | R-19 | R-20 |
|---|---|---|---|---|---|---|---|---|---|
| Disintegration time (s) | 17 ± 13.2 | 36 ± 6.1 | 44 ± 12.5 | 15 ± 6.8 | 32 ± 11.5 | 24 ± 9.0 | 43 ± 28.7 | 20 ± 8.7 | 110 ± 62.1 |
| Uniformity of mass (mg) | 202.2 ± 7.7 | 201.3 ± 3.8 | 200.3 ± 4.3 | 202.7± 5.9 | 201.8 ± 3.9 | 201.9 ± 0.7 | 198.5 ± 8.2 | 199.4± 5.8 | 202.4 ± 4.8 |
| Hardness (N) | 41.5 ± 10.6 | 45.5 ± 0.7 | 43.3 ± 6.8 | 32.3 ± 0.1 | 35.3 ± 4.7 | 39.3 ± 6.7 | 21.0 ± 10.1 | 27.0 ± 2.8 | 36.0 ± 9.8 |
| Parameter (Mean ± SD) | Powder Blend (20% Standardized RR Dry Extract) | ODTs (100 mg Standardized RR Dry Extract) (n = 10) |
|---|---|---|
| Angle of repose (°) (n = 3) | 37.0 ± 16.3 | - |
| Hausner ratio (HR) | 1.21 | - |
| Carr’s index (CI) (%) | 17.5 | - |
| Bulk density (g/mL) | 0.60 | - |
| Tapped density (g/mL) | 0.73 | - |
| Taste score (1–12 scale) | 8 | |
| Mass uniformity (mg) | - | 499.2 ± 4.69 |
| Tablet thickness (mm) (n = 10) | - | 5.13 ± 0.018 |
| Tablet hardness (N) (n = 10) | - | 73 ± 4.8 |
| Friability (%) (n = 10) | - | 0.169 |
| Disintegration time (s) | - | 119.6 ± 19.5 |
| Acceptance value (AV) based on the mass variation | - | 11.25 |
| Pharmaceutical Ingredient | Declared mg/tab | Determined RR Active Marker Content mg/tab (Mean ± SD, n = 4) | ||
|---|---|---|---|---|
| Sample | Rosavin | Salidroside | ||
| RR dry extract (3% rosavin) | 100 | Preparation | 3.03 ± 0.02 | 1.57 ± 0.02 |
| Dextrose (sweetener) | 372.5 | |||
| Croscarmellose sodium (disintegrant) | 5 | |||
| Citric acid (flavor enhancer) | 10 | 6-month storage | 2.95 ± 0.13 | 1.65 ± 0.08 |
| Talc (glidant, lubricant) | 10 | |||
| Magnesium stearate (lubricant) | 2.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brante, O.; Bagons, R.T.; Niedra, S.; Mazurs, A.; Mauriņa, B.; Bernatoniene, J.; Logviss, K. Development of Orally Disintegrating Tablets of Standardized Rhodiola rosea Extract. Pharmaceuticals 2025, 18, 1328. https://doi.org/10.3390/ph18091328
Brante O, Bagons RT, Niedra S, Mazurs A, Mauriņa B, Bernatoniene J, Logviss K. Development of Orally Disintegrating Tablets of Standardized Rhodiola rosea Extract. Pharmaceuticals. 2025; 18(9):1328. https://doi.org/10.3390/ph18091328
Chicago/Turabian StyleBrante, Oxana, Rihards Talivaldis Bagons, Santa Niedra, Austris Mazurs, Baiba Mauriņa, Jurga Bernatoniene, and Konstantins Logviss. 2025. "Development of Orally Disintegrating Tablets of Standardized Rhodiola rosea Extract" Pharmaceuticals 18, no. 9: 1328. https://doi.org/10.3390/ph18091328
APA StyleBrante, O., Bagons, R. T., Niedra, S., Mazurs, A., Mauriņa, B., Bernatoniene, J., & Logviss, K. (2025). Development of Orally Disintegrating Tablets of Standardized Rhodiola rosea Extract. Pharmaceuticals, 18(9), 1328. https://doi.org/10.3390/ph18091328







