Isoliquiritigenin as a Neuronal Radiation Mitigant: Mitigating Radiation-Induced Anhedonia Tendency Targeting Grik3/Grm8/Grin3a via Integrated Proteomics and AI-Driven Discovery
Abstract
1. Introduction
2. Results
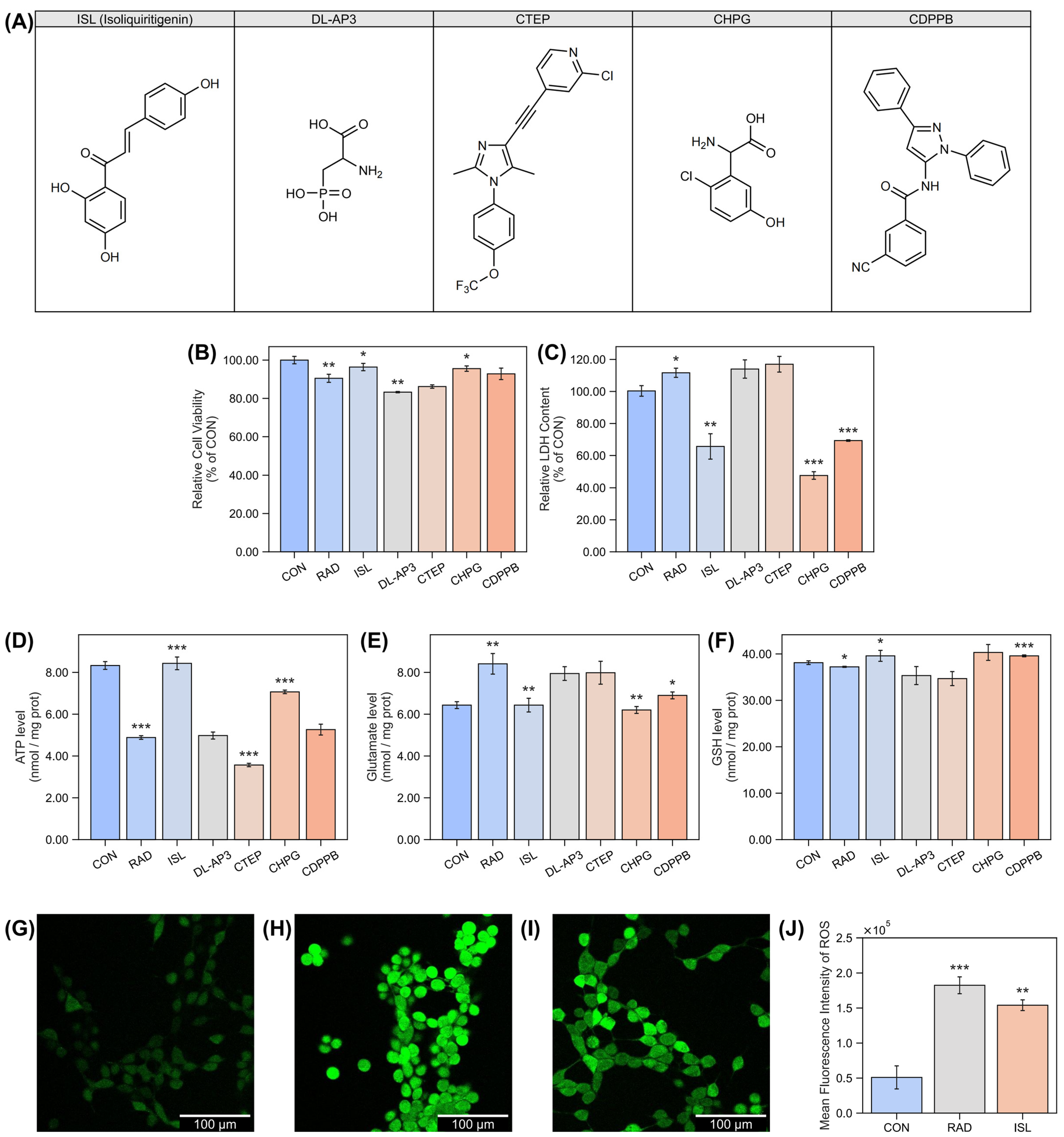
2.1. Isoliquiritigenin’s Protection Effect Against Radiation-Induced Damage at the Cellular Level
2.2. Isoliquiritigenin’s Radiation Mitigation Effect Against Radiation Damage at the Animal Level
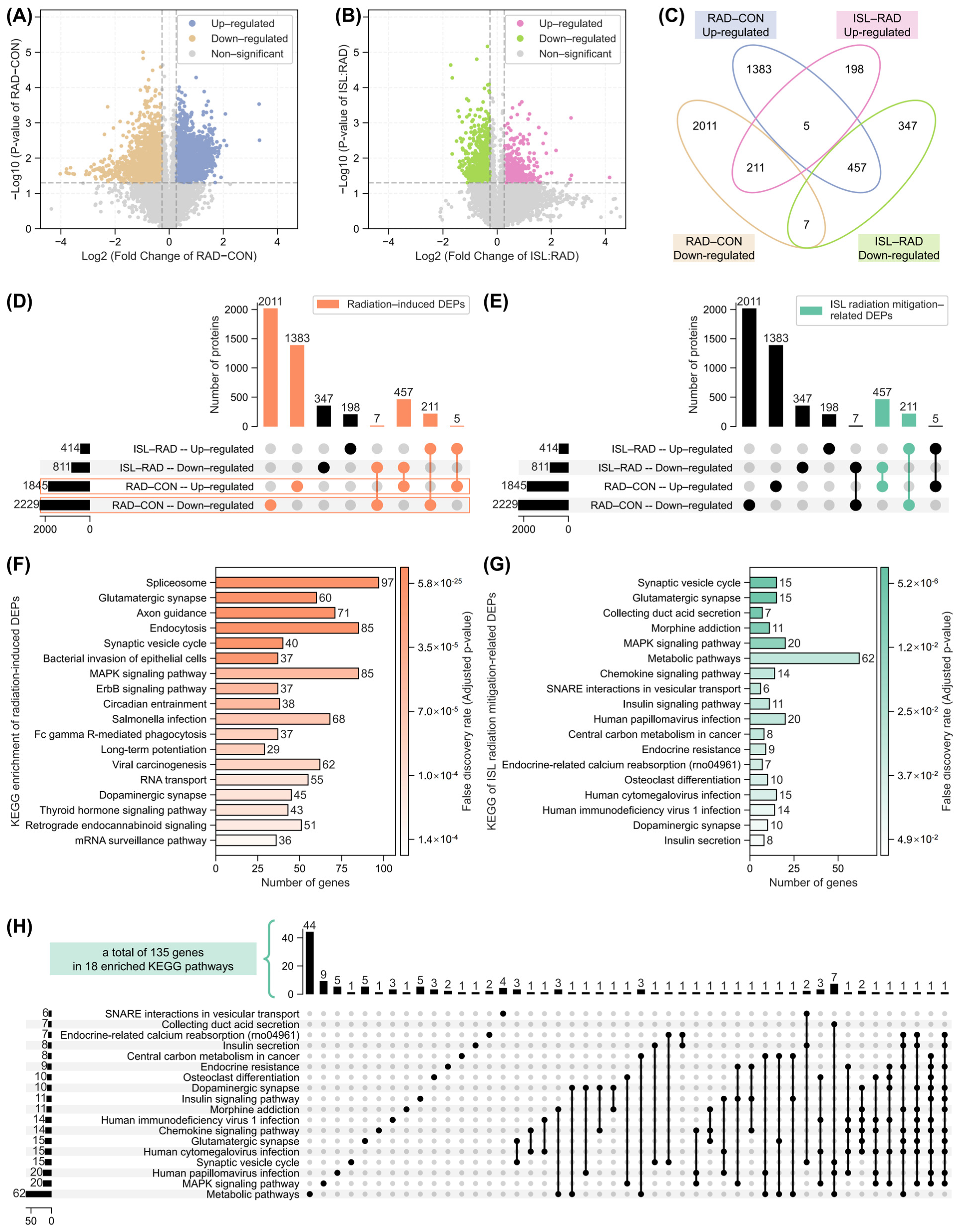
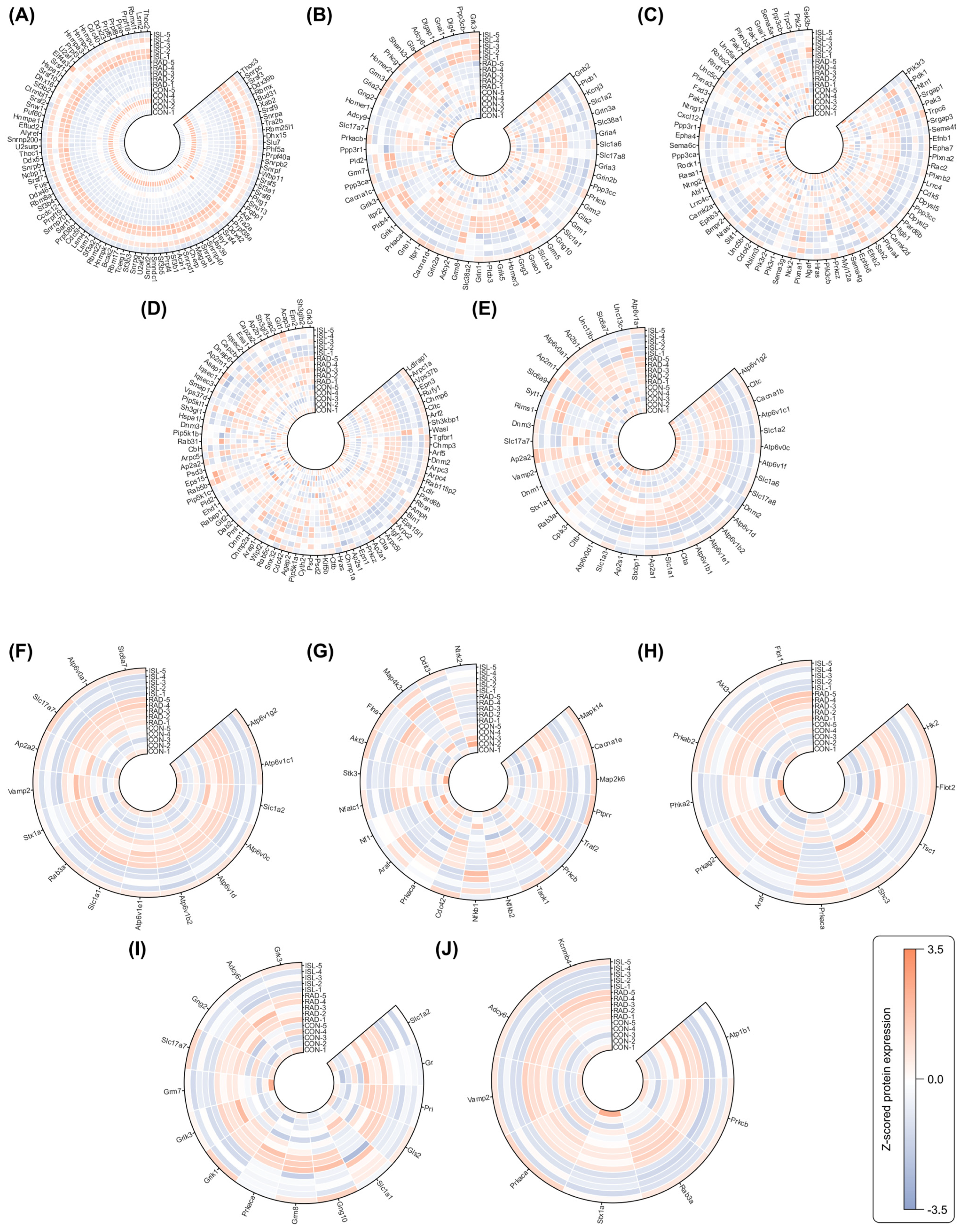
2.3. Analysis of Isoliquiritigenin-Related Radiation Mitigation Mechanism Based on Proteomics
2.4. WGCNA of Co-Expression Proteins Related to Isoliquiritigenin’s Radiation Mitigation
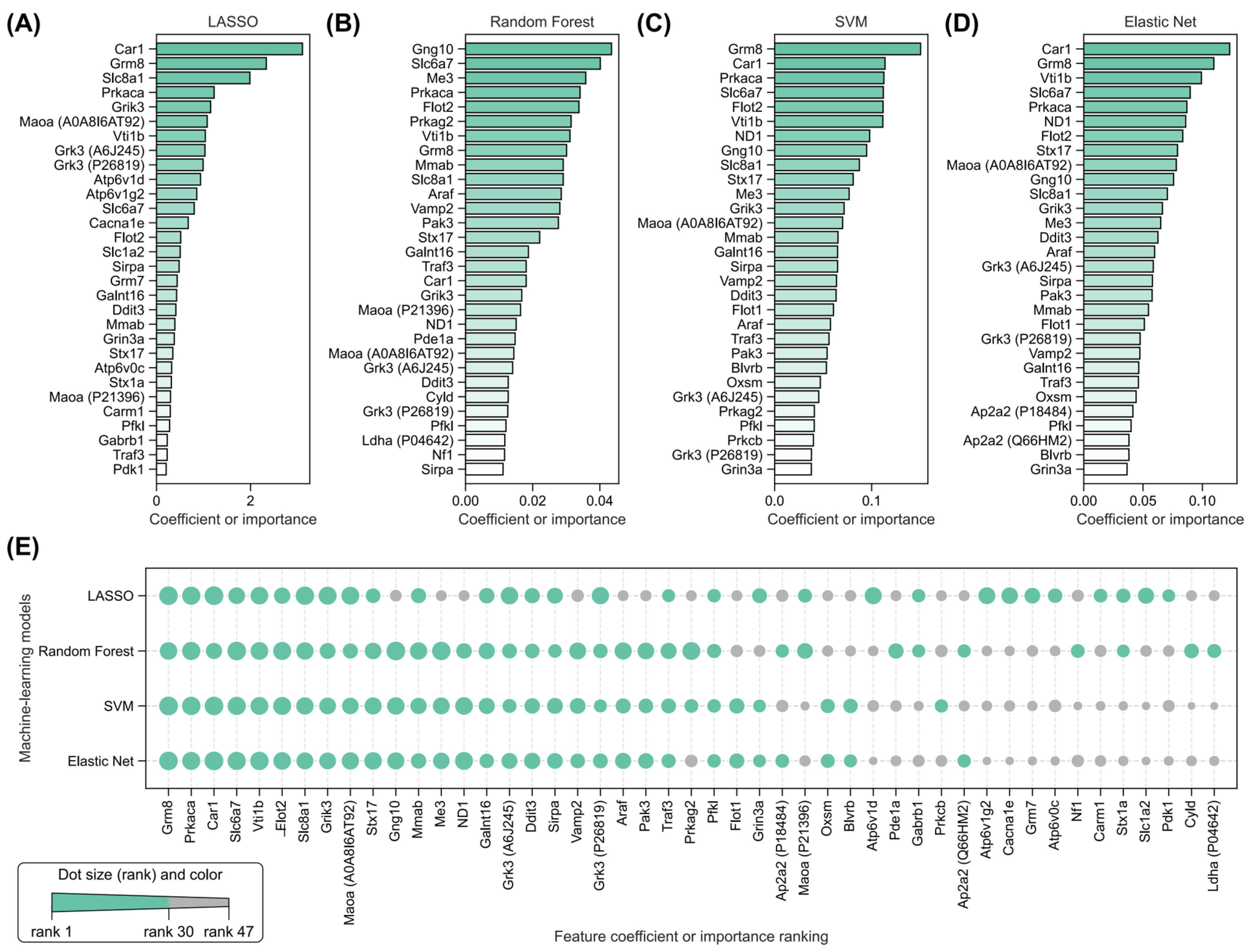
2.5. Identification of Key Radiation-Induced and Isoliquiritigenin Radiation Mitigation-Related Proteins Using Four Machine Learning Models
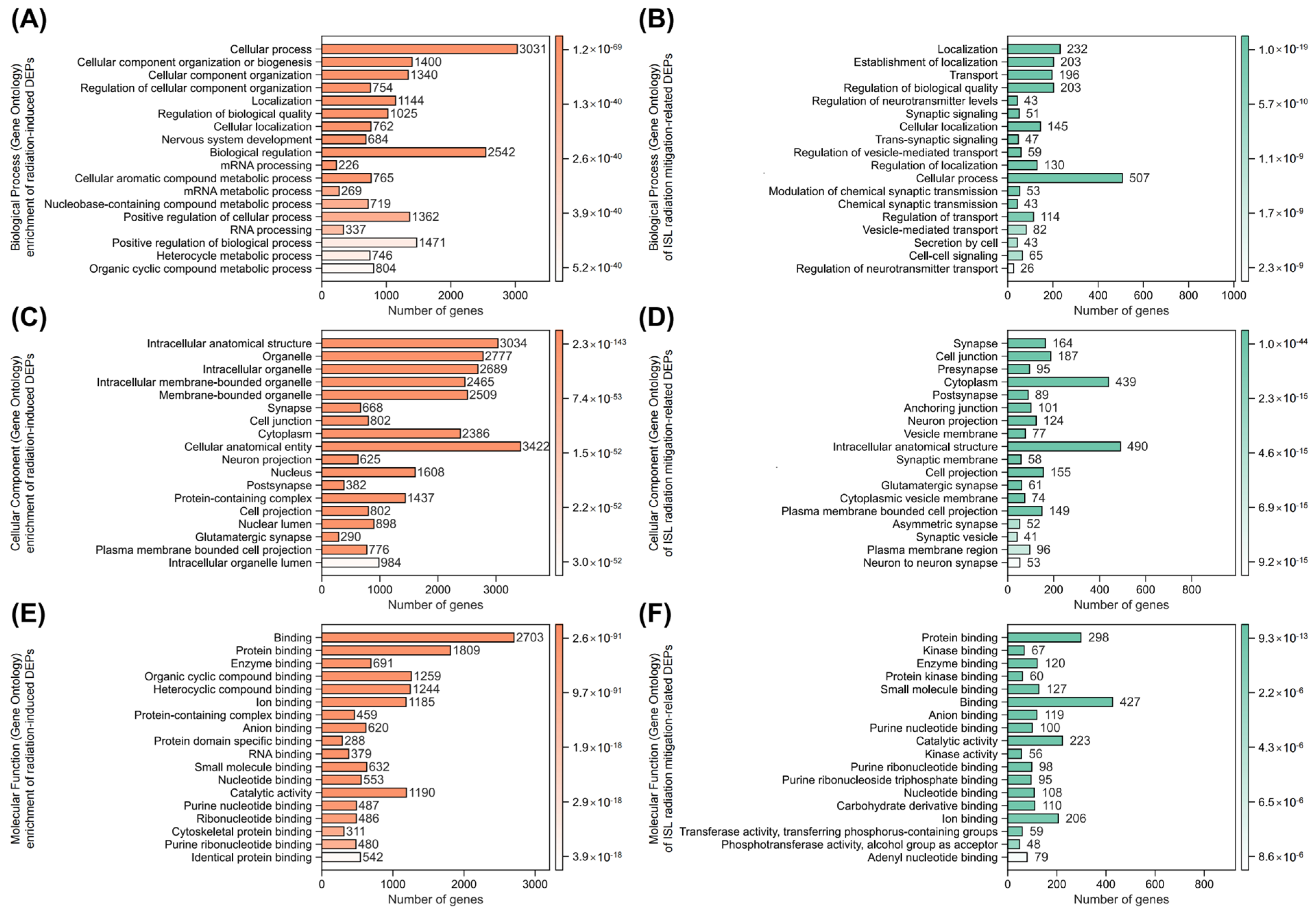
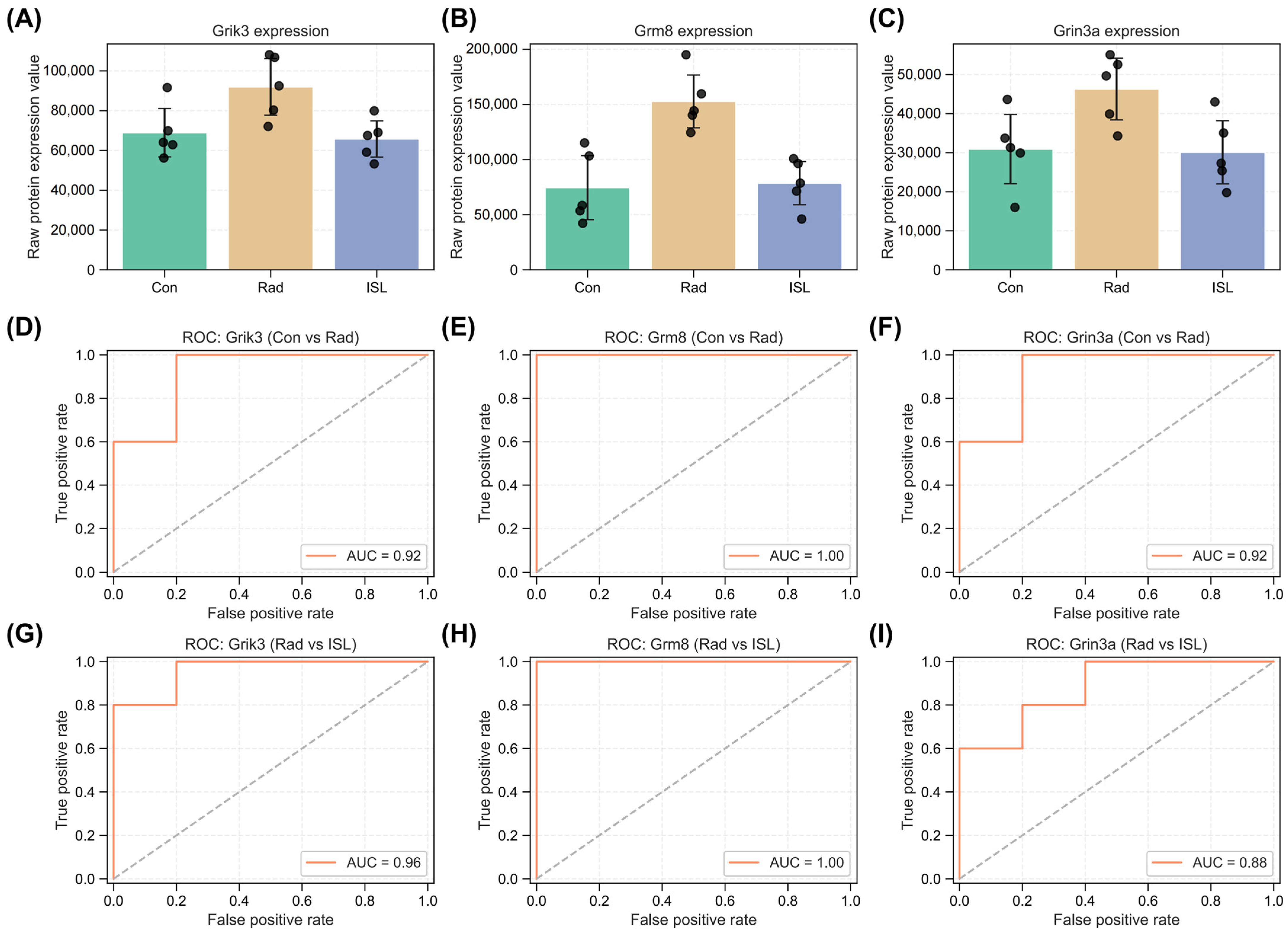
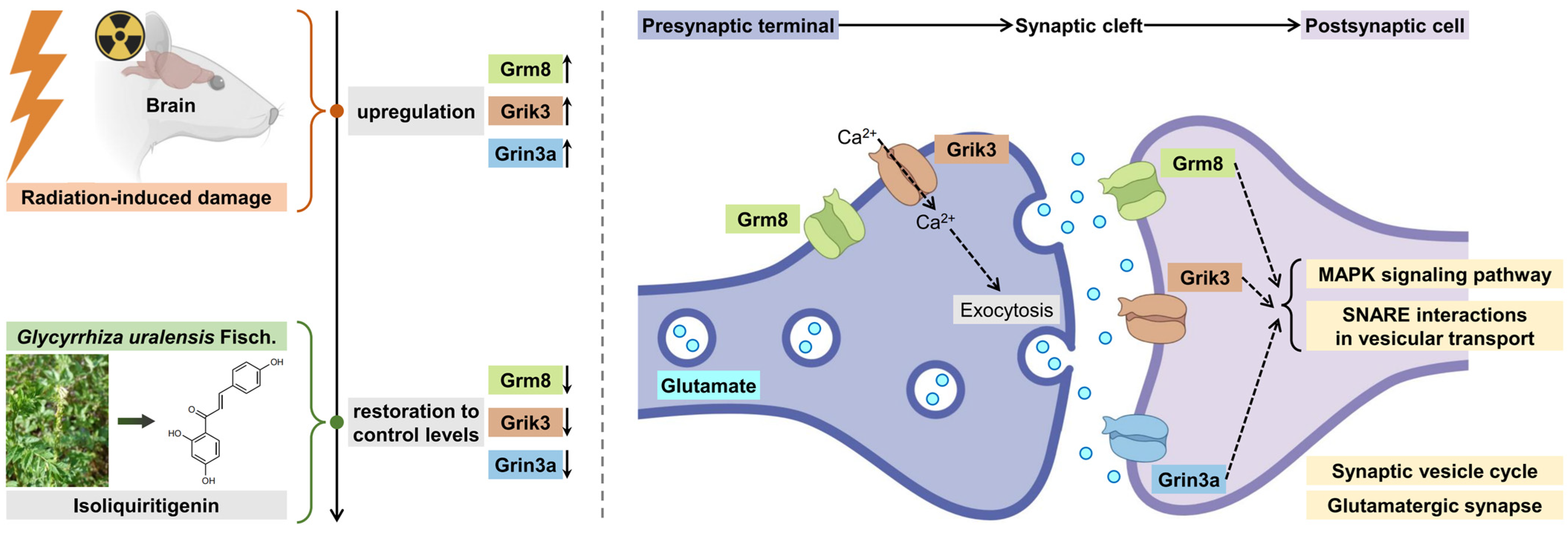
2.6. Identification of Key Isoliquiritigenin Radiation Mitigation-Related Targets Based on Protein–Protein Interaction Networks, KEGG/GO Enrichment and Functional Annotation
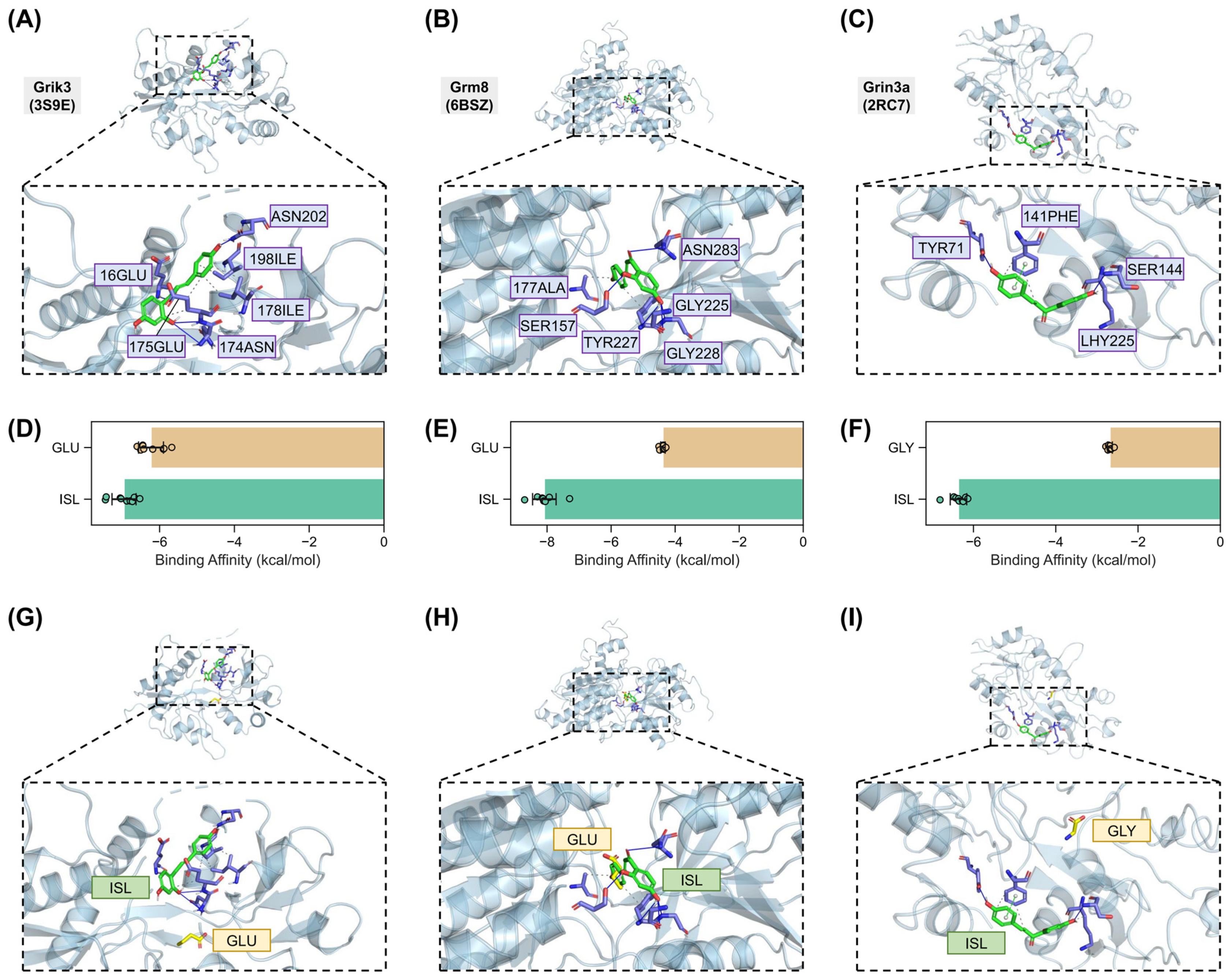
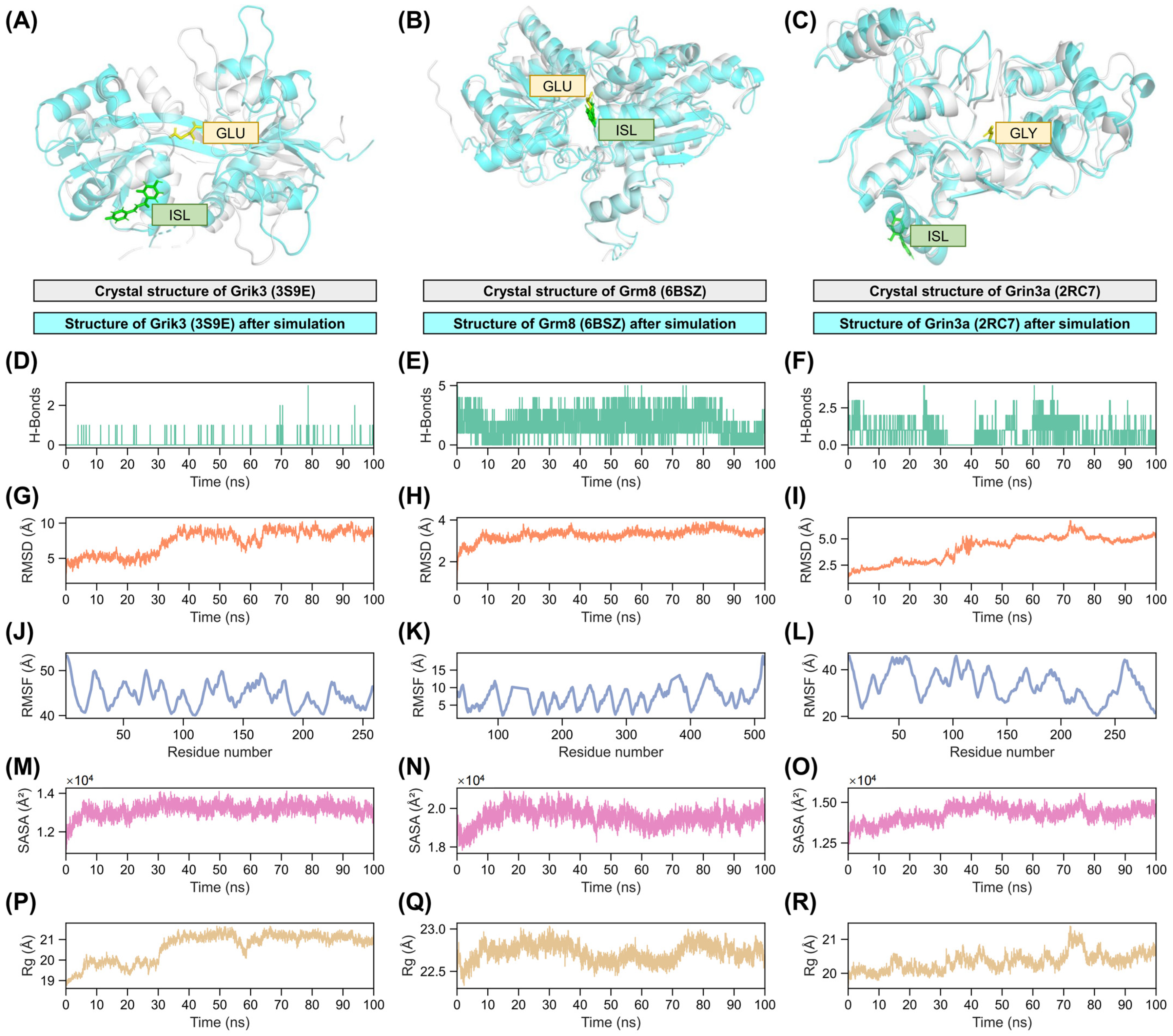
2.7. Validation of Interactions Between Isoliquiritigenin and Grik3, Grm8, Grin3a
3. Discussion
4. Materials and Methods
4.1. Cellular Studies
4.1.1. Cellular Culture and Treatment
4.1.2. Cell Viability Assay (CCK-8)
4.1.3. Lactate Dehydrogenase (LDH) Measurement
4.1.4. ATP Quantification
4.1.5. Measurement of Glutamate
4.1.6. Reduced Glutathione (GSH) Quantification
4.1.7. Measurement of Reactive Oxygen Species (ROS)
4.2. Animal Studies
4.2.1. Animal Model of Radiation
4.2.2. Sucrose Preference Test
4.2.3. Histopathological Analysis
4.2.4. Measurement of Inflammatory Factors IL-1β in the Brain Tissue
4.2.5. Golgi-Cox Staining Analysis
4.2.6. RT-qPCR Analysis
4.2.7. 4D-DIA Proteomic Analysis
- 4D-DIA proteomics data acquisition
- Quality control
- Differential expression analysis for proteomic
- Pathway enrichment analysis
4.2.8. Weighted Gene Correlation Network Analysis (WGCNA)
4.2.9. Four Machine Learning Models for Identifying Key Proteins
4.2.10. Protein–Protein Interaction Network Analysis
4.3. Molecular Docking
4.4. Molecular Dynamic Analysis
4.5. Statistic Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaffray, D.A.; Knaul, F.; Baumann, M.; Gospodarowicz, M. Harnessing Progress in Radiotherapy for Global Cancer Control. Nat. Cancer 2023, 4, 1228–1238. [Google Scholar] [CrossRef]
- Wujanto, C.; Vellayappan, B.; Chang, E.L.; Chao, S.T.; Sahgal, A.; Lo, S.S. Radiotherapy to the Brain: What Are the Consequences of This Age-Old Treatment? Ann. Palliat. Med. 2021, 10, 93652–93952. [Google Scholar] [CrossRef]
- Harary, P.M.; Rajaram, S.; Chen, M.S.; Hori, Y.S.; Park, D.J.; Chang, S.D. Genomic Predictors of Radiation Response: Recent Progress towards Personalized Radiotherapy for Brain Metastases. Cell Death Discov. 2024, 10, 501. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Eisbruch, A. Organ-Sparing Radiation Therapy for Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2011, 8, 639–648. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-Particle Therapy in Cancer: Clinical Uses and Future Perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.X.; Tang, F.R. Radiation-Induced Neuropathological Changes in the Oligodendrocyte Lineage with Relevant Clinical Manifestations and Therapeutic Strategies. Int. J. Radiat. Biol. 2022, 98, 1519–1531. [Google Scholar] [CrossRef]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of Radiotherapy-Associated Cognitive Disability in Patients with Brain Tumours. Nat. Rev. Neurol. 2017, 13, 52–64. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, C.; Wang, T.; Shuai, P.; Wu, W.; Huang, S.; Li, Y.; Zhao, P.; Zeng, C.; Yi, L. Drug Protection against Radiation-Induced Neurological Injury: Mechanisms and Developments. Arch. Toxicol. 2025, 99, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Gurung, P. A Comprehensive Review of Sensors of Radiation-Induced Damage, Radiation-Induced Proximal Events, and Cell Death. Immunol. Rev. 2025, 329, e13409. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Zhang, M.; Yang, J.; Lian, P.; Cheng, X.; Qin, J. Radiation Exposure Induced Blood–Brain Barrier Injury via Mitochondria-Mediated Sterile Inflammation. Adv. Sci. 2025, 12, e02356. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Yang, T.-T.; Liu, L.; Peng, X.-C.; Qian, F.; Tang, F.-R. Ependyma in Neurodegenerative Diseases, Radiation-Induced Brain Injury and as a Therapeutic Target for Neurotrophic Factors. Biomolecules 2023, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, B.; Zheng, H.; Xing, Y.; Chen, G.; Zhou, P.; Qian, L.; Min, Y. Advances of Nanomedicine in Radiotherapy. Pharmaceutics 2021, 13, 1757. [Google Scholar] [CrossRef]
- Khateeb, S.; Hassan, A.I. Insights into Nanostructured Lipid Carriers of Etoricoxib for Mitigating Radiation-Induced Lung Inflammation and Exploring Anti-Inflammatory Mechanisms in Rats. Fundam. Clin. Pharmacol. 2025, 39, e70014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, W.; Yang, X.; Lu, T.; Liu, Y. Isoliquiritigenin, a Potential Therapeutic Agent for Treatment of Inflammation-Associated Diseases. J. Ethnopharmacol. 2024, 318, 117059. [Google Scholar] [CrossRef]
- Mamedov, N.A.; Egamberdieva, D. Phytochemical Constituents and Pharmacological Effects of Licorice: A Review. In Plant and Human Health, Volume 3: Pharmacology and Therapeutic Uses; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–21. ISBN 978-3-030-04408-4. [Google Scholar]
- Simmler, C.; Hajirahimkhan, A.; Lankin, D.C.; Bolton, J.L.; Jones, T.; Soejarto, D.D.; Chen, S.-N.; Pauli, G.F. Dynamic Residual Complexity of the Isoliquiritigenin–Liquiritigenin Interconversion During Bioassay. J. Agric. Food Chem. 2013, 61, 2146–2157. [Google Scholar] [CrossRef]
- Mustafa, A.M.; El-Shiekh, R.A.; Esmail, M.M.; Hassan, E.; Senna, M.M.; Ebid, N.; Elgindy, A.M. Surveying the Therapeutic Potentials of Isoliquiritigenin (ISL): A Comprehensive Review. Chem. Biodivers. 2025, e202500456. [Google Scholar] [CrossRef]
- Gao, Y.; Lv, X.; Yang, H.; Peng, L.; Ci, X. Isoliquiritigenin Exerts Antioxidative and Anti-Inflammatory Effects via Activating the KEAP-1/Nrf2 Pathway and Inhibiting the NF-κB and NLRP3 Pathways in Carrageenan-Induced Pleurisy. Food Funct. 2020, 11, 2522–2534. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of Isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yung, K.K.-L.; Ko, J.K.-S. Therapeutic Intervention in Cancer by Isoliquiritigenin from Licorice: A Natural Antioxidant and Redox Regulator. Antioxidants 2022, 11, 1349. [Google Scholar] [CrossRef]
- Gao, M.; Cai, Q.; Si, H.; Shi, S.; Wei, H.; Lv, M.; Wang, X.; Dong, T. Isoliquiritigenin Attenuates Pathological Cardiac Hypertrophy via Regulating AMPKα in Vivo and in Vitro. J. Mol. Histol. 2022, 53, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hba, S.; Ghaddar, S.; Wahnou, H.; Pinon, A.; El Kebbaj, R.; Pouget, C.; Sol, V.; Liagre, B.; Oudghiri, M.; Limami, Y. Natural Chalcones and Derivatives in Colon Cancer: Pre-Clinical Challenges and the Promise of Chalcone-Based Nanoparticles. Pharmaceutics 2023, 15, 2718. [Google Scholar] [CrossRef]
- Wang, K.; Zhai, Y.; Yu, Z.; Zhang, X.; Zhou, S.; Fan, J. Inhibitory Mechanisms of Baicalein, Genistein, and Isoliquiritigenin on α-Amylase: Multispectral Characterization and Molecular Simulations Reveal Binding Interactions and Conformational Changes. Int. J. Biol. Macromol. 2025, 319, 145468. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, T.; Han, C.; Liu, Y.; Zhong, X.; Cao, Y.; Deng, Y. Potential of Dragon’s Blood as a Space Radiation Protectant Especially on Brain-Liver Bystander Effect. Space Sci. Technol. 2022, 2022. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s Blood: Botany, Chemistry and Therapeutic Uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Liang, J.; Mei, S.; Qiao, X.; Pan, W.; Zhao, Y.; Shi, S.; Zhai, Y.; Wen, H.; Wu, G.; Jiang, C. A Botanical Medicine Dragon’s Blood Exhibited Clinical Antithrombosis Efficacy Similar to Low Molecular Weight Heparin. Sci. China Life Sci. 2021, 64, 1691–1701. [Google Scholar] [CrossRef]
- Mistry, R.; Prabhu, G.; Godwin, M.; Challiss, R.A.J. Stimulatory Effects of the Putative Metabotropic Glutamate Receptor Antagonist L-AP3 on Phosphoinositide Turnover in Neonatal Rat Cerebral Cortex. Br. J. Pharmacol. 1996, 117, 1309–1317. [Google Scholar] [CrossRef]
- Milanese, M.; Bonifacino, T.; Torazza, C.; Provenzano, F.; Kumar, M.; Ravera, S.; Zerbo, A.R.; Frumento, G.; Balbi, M.; Nguyen, T.P.N.; et al. Blocking Glutamate mGlu5 Receptors with the Negative Allosteric Modulator CTEP Improves Disease Course in SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Br. J. Pharmacol. 2021, 178, 3747–3764. [Google Scholar] [CrossRef]
- Ugolini, A.; Corsi, M.; Bordi, F. Potentiation of NMDA and AMPA Responses by the Specific mGluR5 Agonist CHPG in Spinal Cord Motoneurons. Neuropharmacology 1999, 38, 1569–1576. [Google Scholar] [CrossRef]
- Horio, M.; Fujita, Y.; Hashimoto, K. Therapeutic Effects of Metabotropic Glutamate Receptor 5 Positive Allosteric Modulator CDPPB on Phencyclidine-Induced Cognitive Deficits in Mice. Fundam. Clin. Pharmacol. 2013, 27, 483–488. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, Z.; Yang, S.; Chen, Y.; Bao, S. Changes in Myelin Basic Protein and Demyelination in the Rat Brain within 3 Months of Single 2-, 10-, or 30-Gy Whole-Brain Radiation Treatments. J. Neurosurg. 2008, 109, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.A.; Ma, H.S.W.; Hansen, K.S.; Perks, J.; Kent, M.S.; Fragoso, R.C.; Marcu, L. The Effect of Radiation Dose on the Onset and Progression of Radiation-Induced Brain Necrosis in the Rat Model. Int. J. Radiat. Biol. 2017, 93, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, S.; Liu, J.; Zhou, H.; Liu, Z.; Xin, Y.; Suo, W.Z. An Experimental Study of Acute Radiation-Induced Cognitive Dysfunction in a Young Rat Model. Am. J. Neuroradiol. 2010, 31, 383–387. [Google Scholar] [CrossRef]
- Xia, F.; Fascianelli, V.; Vishwakarma, N.; Ghinger, F.G.; Kwon, A.; Gergues, M.M.; Lalani, L.K.; Fusi, S.; Kheirbek, M.A. Understanding the Neural Code of Stress to Control Anhedonia. Nature 2025, 637, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R. A Review of Proton Therapy—Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Bellozi, P.M.Q.; Gomes, G.F.; da Silva, M.C.M.; de Lima, I.V.A.; Batista, C.R.Á.; de Carneiro Junior, W.O.; Dória, J.G.; Vieira, É.L.M.; Vieira, R.P.; de Freitas, R.P.; et al. A Positive Allosteric Modulator of mGluR5 Promotes Neuroprotective Effects in Mouse Models of Alzheimer’s Disease. Neuropharmacology 2019, 160, 107785. [Google Scholar] [CrossRef]
- Stoppel, D.C.; McCamphill, P.K.; Senter, R.K.; Heynen, A.J.; Bear, M.F. mGluR5 Negative Modulators for Fragile X: Treatment Resistance and Persistence. Front. Psychiatry 2021, 12, 718953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, Glutamate Receptors, and Downstream Signaling Pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Pan, M.; Li, Q.; Song, J.; Zhang, R. The Role of Extracellular Glutamate Homeostasis Dysregulated by Astrocyte in Epileptic Discharges: A Model Evidence. Cogn. Neurodynamics 2024, 18, 485–502. [Google Scholar] [CrossRef]
- Magdaleno Roman, J.Y.; González, C.C. Glutamate and Excitotoxicity in Central Nervous System Disorders: Ionotropic Glutamate Receptors as a Target for Neuroprotection. Neuroprotection 2024, 2, 137–150. [Google Scholar] [CrossRef]
- Kalivas, P.W. The Glutamate Homeostasis Hypothesis of Addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, T.; Zhang, M.; Gao, J.; Huang, R.; Huang, X.; Chen, J.; Cheng, L.; Zhang, L.; Huang, Z.; et al. Daphnetin Ameliorates Neuropathic Pain via Regulation of Microglial Responses and Glycerophospholipid Metabolism in the Spinal Cord. Pharmaceuticals 2024, 17, 789. [Google Scholar] [CrossRef]
- Guan, R.; Xue, Z.; Huang, K.; Zhao, Y.; He, G.; Dai, Y.; Liang, M.; Wen, Y.; Ye, X.; Liu, P.; et al. α-Ketoglutarate Attenuates Oxidative Stress-Induced Neuronal Aging via Modulation of the mTOR Pathway. Pharmaceuticals 2025, 18, 1080. [Google Scholar] [CrossRef]
- Ma, J.Z.; Payne, T.J.; Li, M.D. Significant Association of Glutamate Receptor, Ionotropic N-Methyl-d-Aspartate 3A (GRIN3A), with Nicotine Dependence in European- and African-American Smokers. Hum. Genet. 2010, 127, 503–512. [Google Scholar] [CrossRef]
- Mohanraj, N.; Joshi, N.S.; Poulose, R.; Patil, R.R.; Santhoshkumar, R.; Kumar, A.; Waghmare, G.P.; Saha, A.K.; Haider, S.Z.; Markandeya, Y.S.; et al. A Proteomic Study to Unveil Lead Toxicity-Induced Memory Impairments Invoked by Synaptic Dysregulation. Toxicol. Rep. 2022, 9, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.R.; Lahiri, S.; Rajamma, U. Glutamate Mediated Signaling in the Pathophysiology of Autism Spectrum Disorders. Pharmacol. Biochem. Behav. 2012, 100, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Sprengel, R.; Eltokhi, A. Ionotropic Glutamate Receptors (and Their Role in Health and Disease). In Neuroscience in the 21st Century; Springer: Cham, Switzerland, 2022; pp. 57–86. ISBN 978-3-030-88832-9. [Google Scholar]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A Monoamine Oxidase and Serotonin Are Coordinately Involved in Depressive Disorders: From Neurotransmitter Imbalance to Impaired Neurogenesis. J. Neural Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Devor, D.C. (Eds.) Studies of Epithelial Transporters and Ion Channels: Ion Channels and Transporters of Epithelia in Health and Disease—Vol. 3; Physiology in Health and Disease; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-55453-8. [Google Scholar]
- Turnham, R.E.; Scott, J.D. Protein Kinase A Catalytic Subunit Isoform PRKACA.; History, Function and Physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef]
- Leung, C.C.Y.; Wong, Y.H. Role of G Protein-Coupled Receptors in the Regulation of Structural Plasticity and Cognitive Function. Molecules 2017, 22, 1239. [Google Scholar] [CrossRef]
- Rojas, A.; Dingledine, R. Ionotropic Glutamate Receptors: Regulation by G-Protein-Coupled Receptors. Mol. Pharmacol. 2013, 83, 746–752. [Google Scholar] [CrossRef]
- Mulligan, T.; Blaser, H.; Raz, E.; Farber, S.A. Prenylation-Deficient G Protein Gamma Subunits Disrupt GPCR Signaling in the Zebrafish. Cell. Signal. 2010, 22, 221–233. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, S.; Zhao, J.; Zheng, G.; Han, Y.; Zhang, Y.; Qin, Y.; Yang, C.; Xiong, Q.; Chu, X.; et al. Mechanism Exploration of Dietary Supplement Astaxanthin on Improving Atherosclerosis through an Integrated Strategy Encompassing Artificial Intelligence Virtual Screening and Experimental Validation. J. Agric. Food Chem. 2025, 73, 11265–11287. [Google Scholar] [CrossRef] [PubMed]
- Thotala, D.; Karvas, R.M.; Engelbach, J.A.; Garbow, J.R.; Hallahan, A.N.; DeWees, T.A.; Laszlo, A.; Hallahan, D.E. Valproic Acid Enhances the Efficacy of Radiation Therapy by Protecting Normal Hippocampal Neurons and Sensitizing Malignant Glioblastoma Cells. Oncotarget 2015, 6, 35004–35022. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, P.; Sun, F.; Li, B.; Chen, Y.; Pei, S.; Zhang, Z.; Manzoor, R.; Deng, Y.; Sun, C.; et al. The Mechanism of Bystander Effect Induced by Different Irradiation in Human Neuroblastoma Cells. Acta Astronaut. 2020, 166, 599–606. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Wang, T.; Liang, L.; Chang, W.; Xia, H. Pharmacokinetics, Biodistribution and Bioavailability of Isoliquiritigenin after Intravenous and Oral Administration. Pharm. Biol. 2014, 52, 228–236. [Google Scholar] [CrossRef]
- Zeng, Z.; Ma, Y.; Hu, L.; Tan, B.; Liu, P.; Wang, Y.; Xing, C.; Xiong, Y.; Du, H. OmicVerse: A Framework for Bridging and Deepening Insights across Bulk and Single-Cell Sequencing. Nat. Commun. 2024, 15, 5983. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Lee, J.; Hitzenberger, M.; Rieger, M.; Kern, N.R.; Zacharias, M.; Im, W. CHARMM-GUI Supports the Amber Force Fields. J. Chem. Phys. 2020, 153, 035103. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E.I. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Prokai, L.; Rahlouni, F.; Zaman, K.; Nguyen, V.; Prokai-Tatrai, K. Proteomics Complementation of the Rat Uterotrophic Assay for Estrogenic Endocrine Disruptors: A Roadmap of Advancing High Resolution Mass Spectrometry-Based Shotgun Survey to Targeted Biomarker Quantifications. Int. J. Mol. Sci. 2021, 22, 1686. [Google Scholar] [CrossRef] [PubMed]
- Roffia, V.; De Palma, A.; Lonati, C.; Di Silvestre, D.; Rossi, R.; Mantero, M.; Gatti, S.; Dondossola, D.; Valenza, F.; Mauri, P.; et al. Proteome Investigation of Rat Lungs Subjected to Ex Vivo Perfusion (EVLP). Molecules 2018, 23, 3061. [Google Scholar] [CrossRef] [PubMed]



| UniprotID | Protein | FC (RAD–CON) | p-Value (RAD–CON) | FC (ISL–RAD) | p-Value (ISL–RAD) |
|---|---|---|---|---|---|
| A6JZR8 | Araf | 1.21679 | 0.02708 | 0.63141 | 0.00354 |
| Q3KRE3 | Gng10 | 1.99501 | 0.00005 | 0.55094 | 0.00182 |
| P42264 | Grik3 | 1.33416 | 0.03918 | 0.71525 | 0.01468 |
| Q9R1M7 | Grin3a | 1.49793 | 0.03213 | 0.65006 | 0.02106 |
| P70579 | Grm8 | 2.04935 | 0.00319 | 0.51491 | 0.00137 |
| A0A8I6AT92 | Maoa | 1.31883 | 0.02602 | 0.70331 | 0.00989 |
| P21396 | Maoa | 1.32761 | 0.01922 | 0.73558 | 0.01890 |
| P03889 | Mt-nd1 | 1.23889 | 0.01227 | 0.82758 | 0.01090 |
| A0A8I5YBQ9 | Prkaca | 1.49942 | 0.01078 | 0.69403 | 0.00127 |
| A6H9P0 | Slc8a1 | 1.53984 | 0.01354 | 0.58458 | 0.00404 |
| Q9Z158 | Stx17 | 1.26830 | 0.00966 | 0.75174 | 0.00198 |
| P63045 | Vamp2 | 1.57046 | 0.00297 | 0.76330 | 0.01232 |
| P58200 | Vti1b | 1.23527 | 0.01934 | 0.82430 | 0.00324 |
| Receptor | Interaction | Amino Acid | Distance (Å) | H-A Distance (Å) |
|---|---|---|---|---|
| Grik3 | Hydrophobic interactions | 175 GLU | 3.40 | |
| Hydrophobic interactions | 175 GLU | 3.87 | ||
| Hydrophobic interactions | 178 ILE | 3.63 | ||
| Hydrophobic interactions | 178 ILE | 3.65 | ||
| Hydrophobic interactions | 198 ILE | 3.56 | ||
| Hydrogen Bonds | 16 GLU | 1.85 | ||
| Hydrogen Bonds | 174 ASN | 3.47 | ||
| Hydrogen Bonds | 175 GLU | 2.34 | ||
| Hydrogen Bonds | 202 ASN | 2.57 | ||
| Grm8 | Hydrophobic interactions | 177 ALA | 3.81 | |
| Hydrophobic interactions | 227 TYR | 3.87 | ||
| Hydrophobic interactions | 227 TYR | 3.99 | ||
| Hydrogen Bonds | 157 SER | 2.09 | ||
| Hydrogen Bonds | 225 GLY | 2.55 | ||
| Hydrogen Bonds | 227 TYR | 3.24 | ||
| Hydrogen Bonds | 228 GLY | 1.93 | ||
| Hydrogen Bonds | 283 ASN | 3.16 | ||
| Grin3a | Hydrophobic interactions | 141 PHE | 3.67 | |
| Hydrophobic interactions | 141 PHE | 3.57 | ||
| Hydrophobic interactions | 225 LYS | 3.98 | ||
| Hydrogen Bonds | 71 TYR | 3.20 | ||
| Hydrogen Bonds | 71 TYR | 2.87 | ||
| Hydrogen Bonds | 144 SER | 1.94 | ||
| PI stacking | 141 PHE | 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Cheng, S.; Zhang, H.; Li, B. Isoliquiritigenin as a Neuronal Radiation Mitigant: Mitigating Radiation-Induced Anhedonia Tendency Targeting Grik3/Grm8/Grin3a via Integrated Proteomics and AI-Driven Discovery. Pharmaceuticals 2025, 18, 1307. https://doi.org/10.3390/ph18091307
Li B, Cheng S, Zhang H, Li B. Isoliquiritigenin as a Neuronal Radiation Mitigant: Mitigating Radiation-Induced Anhedonia Tendency Targeting Grik3/Grm8/Grin3a via Integrated Proteomics and AI-Driven Discovery. Pharmaceuticals. 2025; 18(9):1307. https://doi.org/10.3390/ph18091307
Chicago/Turabian StyleLi, Boyang, Suqian Cheng, Han Zhang, and Bo Li. 2025. "Isoliquiritigenin as a Neuronal Radiation Mitigant: Mitigating Radiation-Induced Anhedonia Tendency Targeting Grik3/Grm8/Grin3a via Integrated Proteomics and AI-Driven Discovery" Pharmaceuticals 18, no. 9: 1307. https://doi.org/10.3390/ph18091307
APA StyleLi, B., Cheng, S., Zhang, H., & Li, B. (2025). Isoliquiritigenin as a Neuronal Radiation Mitigant: Mitigating Radiation-Induced Anhedonia Tendency Targeting Grik3/Grm8/Grin3a via Integrated Proteomics and AI-Driven Discovery. Pharmaceuticals, 18(9), 1307. https://doi.org/10.3390/ph18091307







