Borylated Five-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis, and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
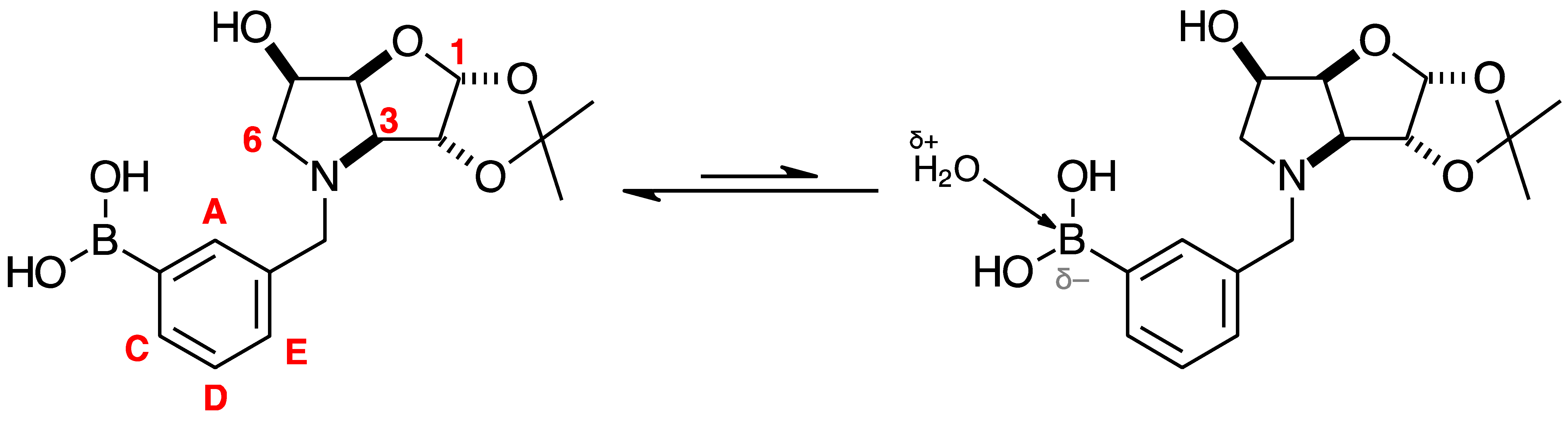
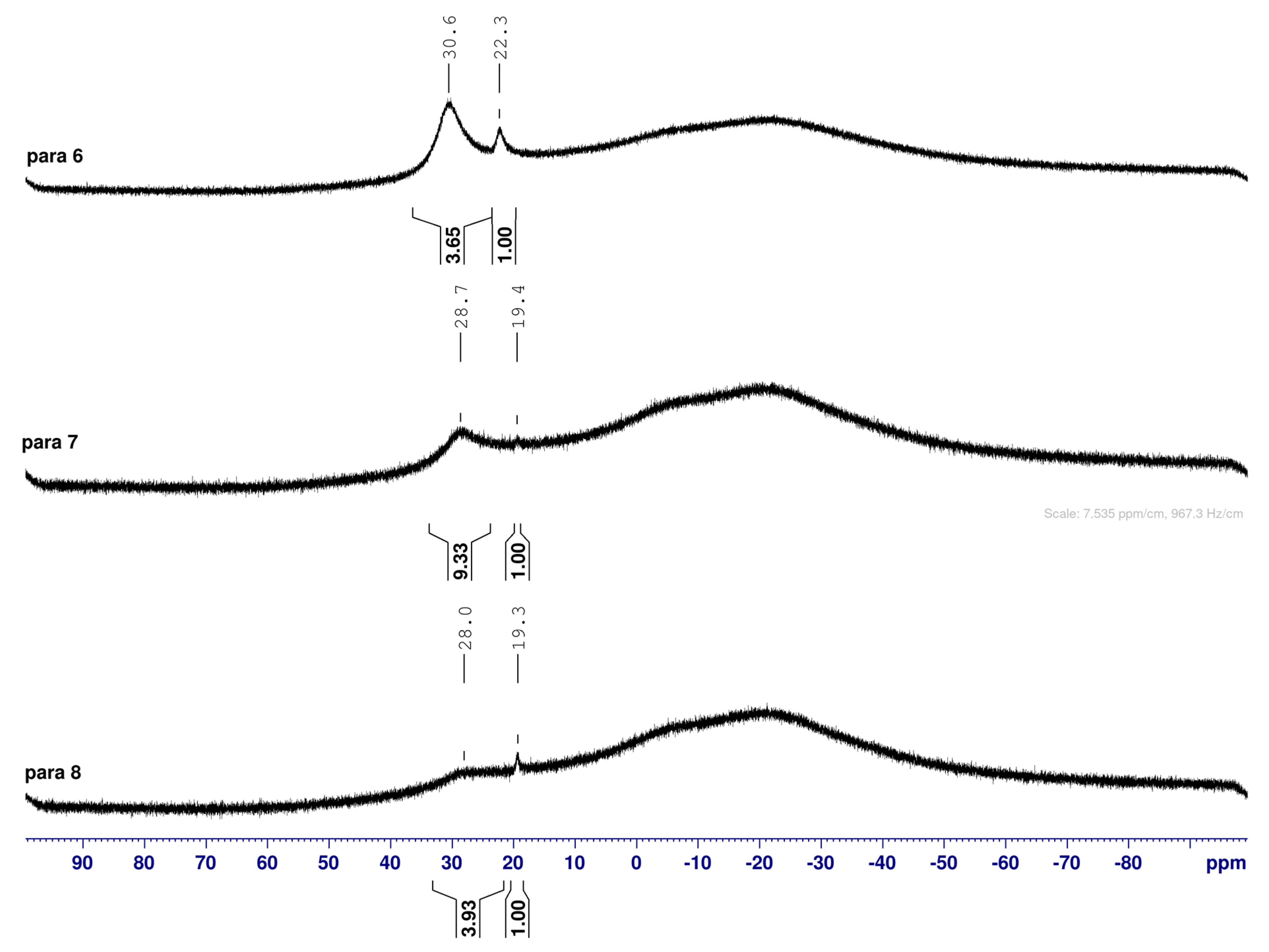
2.2. 11B-NMR Data Analysis
| Compound (Deuterated Solvent) | Chemical Shifts | Compound (Deuterated Solvent) | Chemical Shifts | Compound (Deuterated Solvent) | Chemical Shifts | ||||
|---|---|---|---|---|---|---|---|---|---|
| Signal Integration Ratio | Signal Integration Ratio | Signal Integration Ratio | |||||||
| Signal Shape | Signal Shape | Signal Shape | |||||||
| Geometry | Geometry | Geometry | |||||||
 para 6 (CDCl3) | 30.6 | 22.3 | 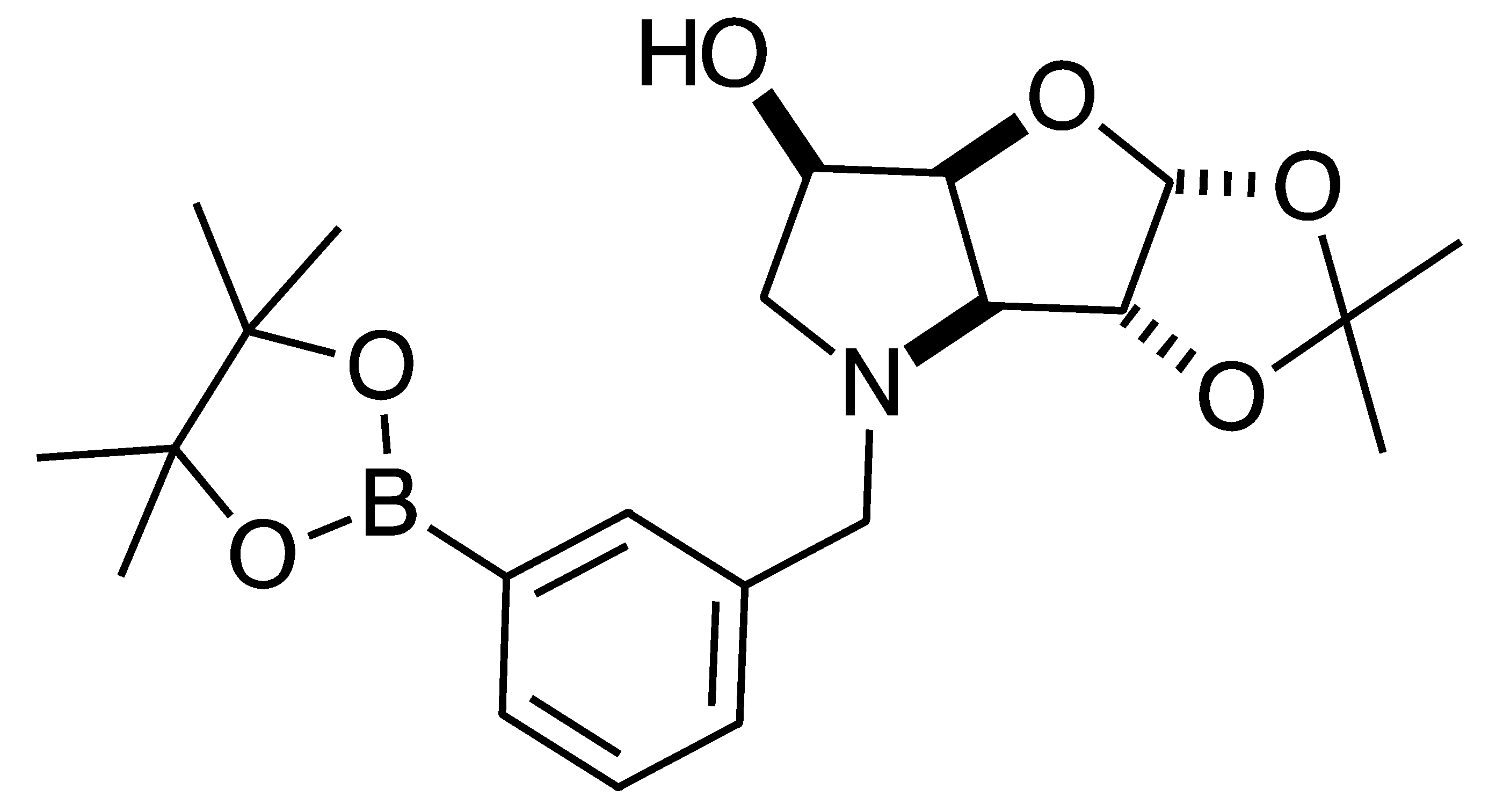 meta 2 (CDCl3) | 30.8 |  ortho 2 (CDCl3) | 31.0 | 22.3 | ||
| 3.7 | 1.0 | NA | 6.4 | 1.0 | |||||
| broad | sharp | Broad | sharp | sharp | |||||
| Boronate ester (trig) | Boronate ester (partially tet) | Boronate ester (trig) | Boronate ester (trig) | Boronate ammonium (partially tet) | |||||
 para 7 (D2O) α-fur/β-fur, 1.0:0.4 | 28.7 | 19.4 | 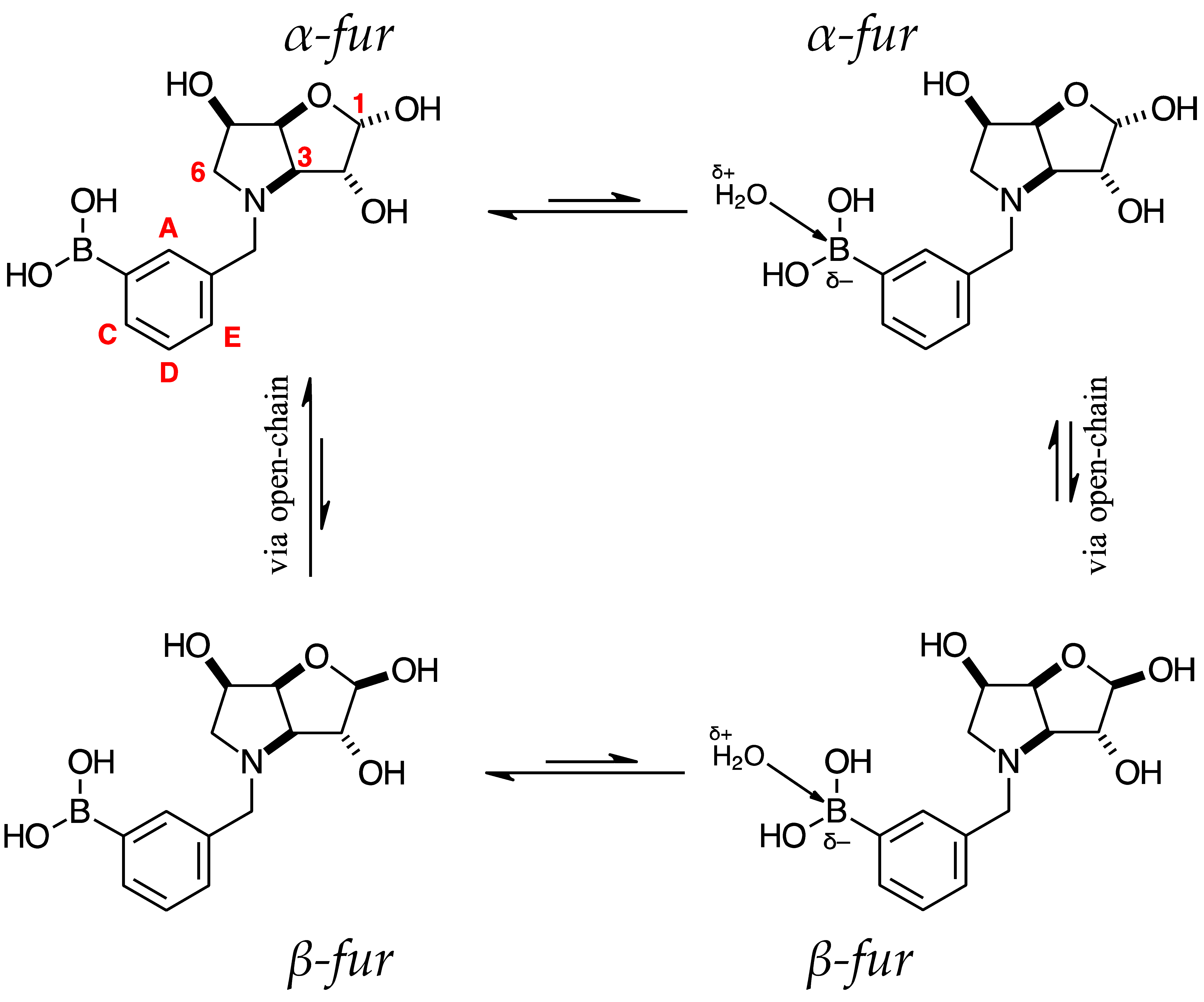 meta 3 (D2O) α-fur/β-fur/open-chain, 1.0:0.5:0.002 | 28.6 | 19.2 | 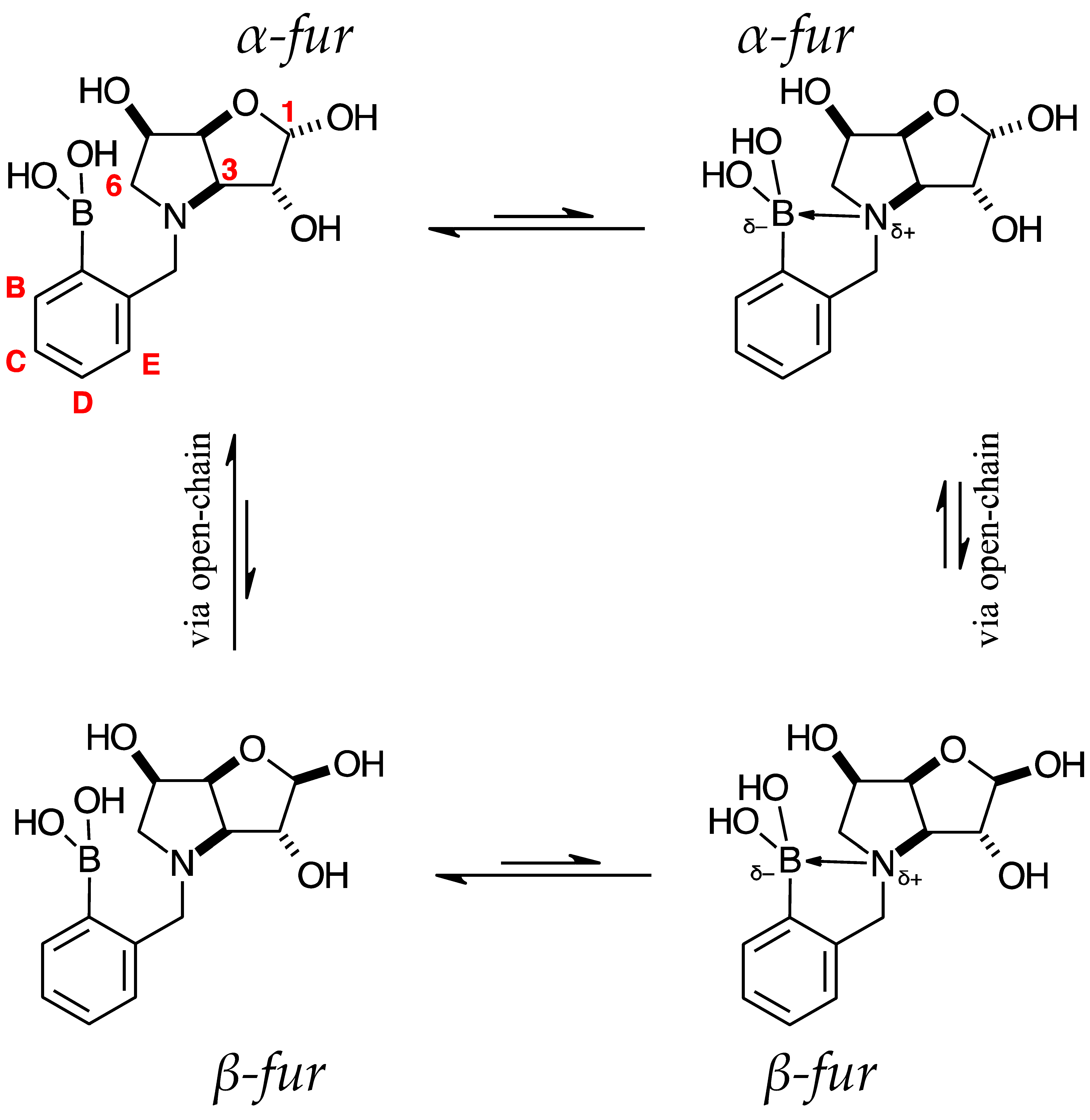 ortho 3 (D2O) α-fur:β-fur, 1.0:0.7 | 28.0 | 19.3 | |
| 9.3 | 1.0 | 6.5 | 1.0 | 4.0 | 1.0 | ||||
| broad | sharp | broad | sharp | broad | sharp | ||||
| Boronic acid (trig) | Boronate (partially tet) | Boronic acid (trig) | Boronate (partially tet) | Boronic acid (trig) | Boronate (partially tet) | ||||
 para 8 (D2O) | 28.0 | 19.3 |  meta 4 (D2O) | 27.8 | 19.3 | 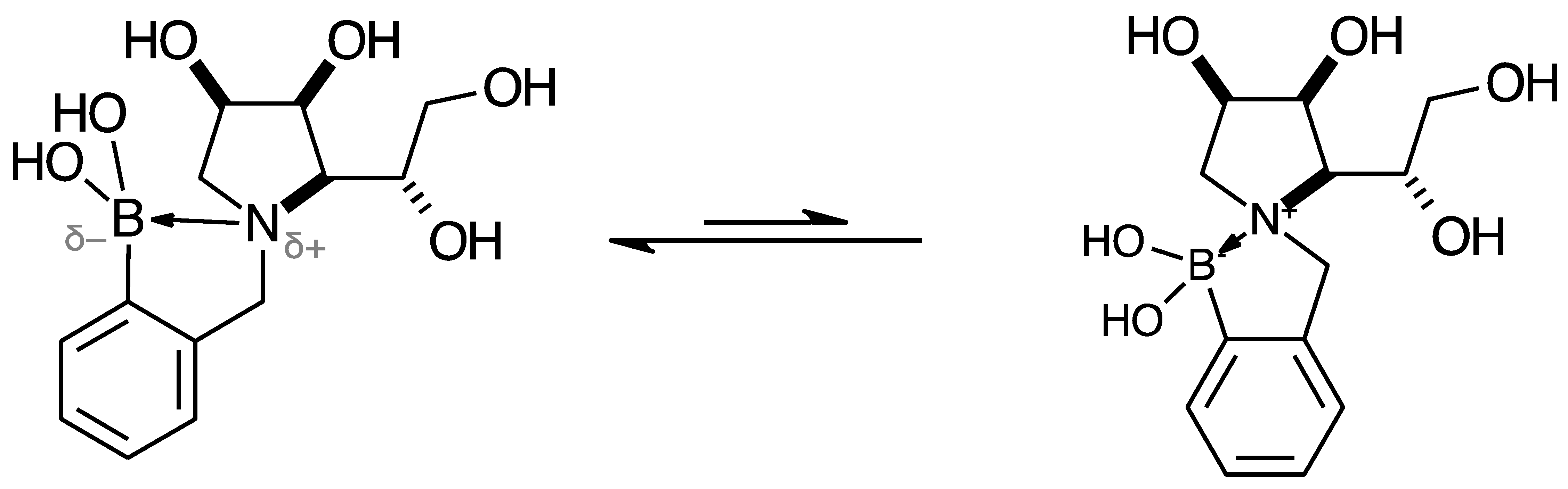 ortho 4 (D2O) | 28.3 | 19.4 | 12.3, 11.0 |
| 3.9 | 1.0 | 1.7 | 1.0 | 1.1 | 6.1 | 1.2, 1.0 | |||
| broad | sharp | broad | sharp | broad | sharp | Sharp, merging | |||
| Boronic acid (trig) | Boronate (partially tet) | Boronic acid (trig) | Boronate (partially tet) | Boronic acid (trig) | Boronate ammonium | ||||
| (partially tet) | (tet) | ||||||||
 meta 5 (MeOD) | 28.6 | 18.6 |  ortho 5 (D2O) | 29.5 | 19.3 | ||||
| 8.6 | 1.0 | 4.5 | 1.0 | ||||||
| sharp | sharp | broad | sharp | ||||||
| Boronic acid (trig) | Boronate (partially tet) | Boronic acid (trig) | Boronate (partially tet) | ||||||
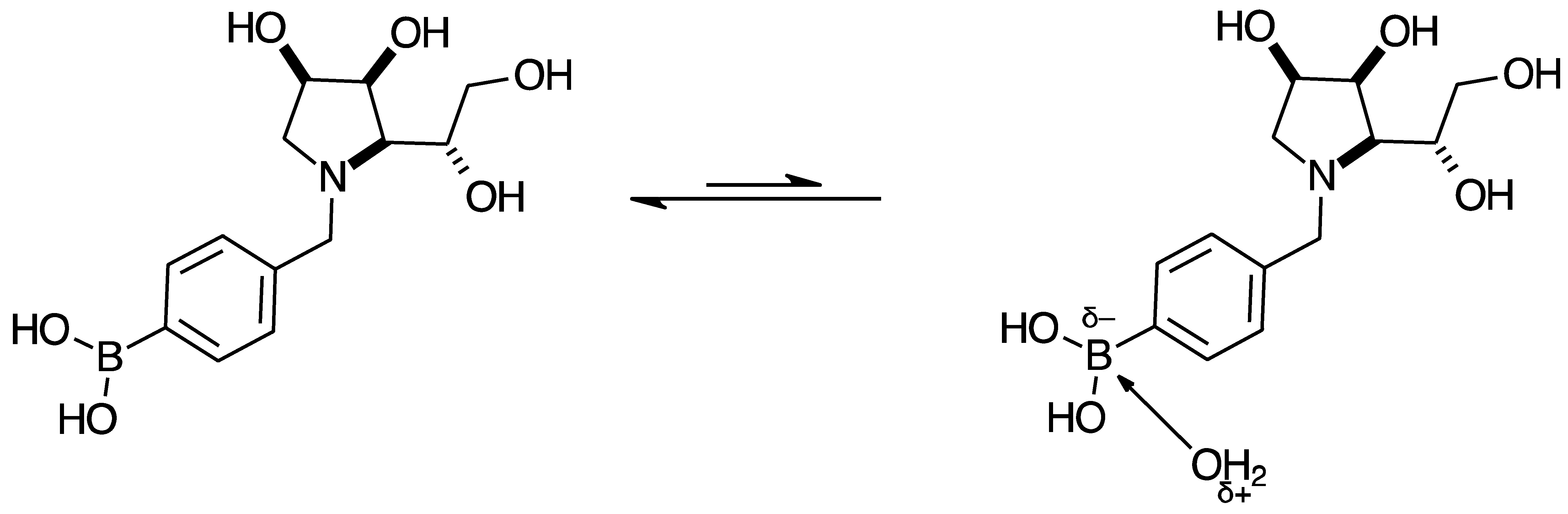
2.3. Observations on NMR Features for Borylated and Non-Borylated Compounds
2.3.1. Signal Broadening in the 13C-NMR Spectra
- The ArCquat-B signals in para 6, para 7, and para 8 were not discernible. These signals are broadened due to the presence of the quadrupolar B nuclei one bond away.
- ring-opening/-closing tautomerism
- Hydrogen bonding. Intramolecular or intermolecular hydrogen bonding in lactols can also influence relaxation times and contribute to line broadening.
- Conformational exchanges. The cyclic structure of lactols can adopt multiple conformations, causing additional dynamic averaging of carbon signals. These processes cause the carbon nuclei to experience different chemical environments on the NMR timescale (102–104 s−1), leading to broadened or averaged signals.
 | ||||||||||
| Compound: 1,4-Dideoxy-1,4-Imino- | 1H-NMR Chemical Shifts (δ, ppm), Multiplicity, and Coupling Constants (J, Hz) for Nucleus: | |||||||||
| H-1 | H-1′ | H-2 | H-3 | H-4 | H-5 | H-6 | H-6′ | ArCH2 | ArCHs | |
| N-benzyl-1,4-dideoxy-1,4-imino-d-allitol # [64] | 3.51, dd J 12.8 and 4.3 | δ 3.28, m | 4.42, app-s | δ 4.24, m | δ 3.58, dd J 6.3 and 2.5 | δ 4.24, m | δ 3.40, m | δ 3.28, m | δ 3.40, m NA | δ 7.37, m (5Hs) |
| N-benzyl-1,4-dideoxy-1,4-imino-d-galactitol (CD3OD) [65] | δ 2.86, m | δ 2.71, dd JH-1′,H-1 10.7 JH-1′,H-2 4.4 | δ 3.95–3.84, m | δ 4.09, m | δ 2.91, dd JH-4,H-5 4.6 JH-4,H-3 2.7 | δ 3.95–3.84, m | δ 3.72, dd JH-6,H-6′ 11.1 JH-6,H-5 5.6 | δ 3.68, dd JH-6′,H-6 11.1 JH-6′,H-5 6.2 | δ 4.20, d J 13.6 δ 3.52, d J 13.6 | δ 7.39–7.20, m |
| N-benzyl-1,4-dideoxy-1,4-imino-d-glucitol.HCl [64] | δ 3.27, d JH-1,H-1′ 13.2 | δ 3.71, dd JH-1′,H-1 13.2 JH-1′,H-2 4.2 | δ 4.31, m | δ 4.16, d | δ 3.60, br s | δ 3.79, m | δ 3.60, br s | δ 4.46, d J 13.0 δ 4.31, m | δ 7.37, m | |
| N-benzyl-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose [CDCl3] 3 | δ 5.99, d JH-1,H-2 3.5 | NA | δ 4.51, d JH-2,H-1 3.5 | δ 3.26, d JH-3,H-4 5.6 | δ 4.83, t JH-4,H-3/H-5 5.8 | δ 4.15, app-dddd JH-5,H-6/H-4/OH 5.8 JH-5,H-6′ 2.0 | δ 2.93, dd JH-6,H-6′ 10.8 JH-6,H-5 2.1 | δ 2.43, dd JH-6′,H-6 10.8 JH-6′,H-5 5.5 | δ 3.95, d JHa,Hb 13.4 δ 3.49, d JHb,Ha 13.4 | δ 7.36–7.24, m |
| N-benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 α-fur | δ 5.51, d JH-1,H-2 4.4 | NA | δ 4.48–4.43, m, obscured | δ 4.31, dd JH-3,H-4 8.2 JH-3,H-2 6.1 | δ 4.98, d J 8.3 | δ 4.48–4.43, m obscured | δ 3.66–3.60, m | δ 3.57, dd JH-6′,H-6 12.4 JH-6′,H-5 3.6 | δ 4.57, d JHa,Hb 13.0 δ 4.49, d JHb,Ha 13.0 | δ 7.60–7.47, m |
| N-benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 β-fur | δ 5.36, d J H-1,H-2 2.3 | NA | δ 4.57–4.53, m obscured | δ 4.23, dd JH-3,H-4 7.0 JH-3,H-2 2.6 | δ 5.00, app-d JH-4,H-3 8.2 | δ 4.57–4.53, m obscured | δ 3.81, dd JH-6,H-6′ 12.2 JH-6,H-5 4.9 | δ 3.62–3.55, m obscured | δ 4.63, d JHa,Hb 12.9 δ 4.40, d JHb,Ha 12.9 | δ 7.60–7.47, m |
| N-benzyl-1,4-dideoxy-1,4-imino-l-gulitol 5 | δ 3.59, dd JH-1,H-1′ 12.0 JH-1,H-2 7.0 | δ 3.28, dd JH-1′,H-1 12.1 JH-1′,H-2 9.1 | δ 4.55, dddd JH-2,H-1′ 9.3 JH-2,H-1/H-3 6.9 J 3.6 | δ 4.42, ddd JH-3,H-2 7.1 JH-3,H-4 4.2 J 3.0 | δ 3.84, dd JH-4,H-5 9.3 JH-4,H-3 4.0 | δ 4.38, ddd JH-5,H-4 8.5 JH-5,H-6′ 4.7 JH-5,H-6 3.2 | δ 3.85, dd JH-6,H-6′ 12.6 JH-6,H-5 3.2 | δ 3.70, dd JH-6′,H-6 12.8 JH-6′,H-5 4.8 | δ 4.79, obscured. δ 4.29, d JHb,Ha 12.8 Hz | δ 7.61–7.52, m |
| para 6 [CDCl3] boronic acid | δ 5.99, d JH-1,H-2 3.5 | NA | δ 4.52, d JH-2,H-1 3.5 | δ 3.25, d JH-3,H-4 5.5 | δ 4.80, t JH-4,H-3/H-5 5.8 | δ 4.13, app-dddd JH-5,H-6/H-4/OH 6.0 JH-5,H-6′ 2.3 | δ 2.90, dd JH-6′,H-6 10.8 JH-6′,H-5 2.3 | δ 2.42, dd JH-6,H-6′ 10.8 JH-6,H-5 5.6 | δ 3.95, d JHa,Hb 13.6 δ 3.51, d JHb,Ha 13.6 | δ 7.77, d J 7.9 δ 7.28, d J 7.9 |
| para 7 α-fur boronic acid | δ 5.51, d JH-1,H-2 4.3 | NA | δ 4.49–4.44, m | δ 4.32, dd JH-3,H-4 8.1 JH-3,H-2 6.3 | δ 4.99, app-d partially obscured JH-4,H-3 8.2 | δ 4.49–4.44, m | δ 3.65–3.60, m | δ 3.57, dd JH-6′,H-6 12.2 JH-6′,H-5 3.4 | δ 4.59, d JHa,Hb 13.0 δ 4.51, d JHb,Ha 13.0 | δ 7.87, d J 7.9 δ 7.59, d J 7.9 |
| para 7 β-fur boronic acid | δ 5.36, d JH-1,H-2 2.2 | NA | δ 4.36–4.29, obscured | δ 4.22, dd J 6.9 J 2.4 | δ 5.00, app-d partially obscured J 8.5 | δ 4.56–4.53 obscured | δ 3.82, dd JH-6,H-6′ 12.6 JH-6,H-5 5.0 | δ 3.62–3.50 partially obscured JH-6′,H-5 5.2 | δ 4.65, d JHa,Hb 13.0 δ 4.42, d JHb,Ha 13.0 | δ 7.88, d J 7.9 δ 7.60, d J 7.9 |
| para 8 boronic acid | δ 3.55, dd JH-1,H-1′ 12.0 JH-1,H-2 6.9 | δ 3.25, dd JH-1′,H-1 12.2 JH-1′,H-2 9.2 | δ 4.54–4.50, m | δ 3.79, app-d J 3.2 | δ 4.40–4.34, m | δ 3.82, app-dd JH-5,H-6 8.9 JH-5,H-6′ 5.1 | δ 4.40–4.34, m | δ 3.66, dd JH-6′,H-6 12.0 JH-6′,H-5 5.2 | δ 4.83, obscured. δ 4.27, d JHb,Ha 13.0 | δ 7.85, dd 3JArH,ArH 8.0 5JArH,ArH 1.6 δ 7.57, dd 3JArH,ArH 8.0 5JArH,ArH 1.7 |
| N-benzyl-1,4-dideoxy-1,4-imino-d-mannitol.HCl # [66] | δ 3.64, dd J 12.0, 7.2 | δ 3.38, dd J 12.0, 7.2 | δ 4.54–4.47, m | δ 3.89–3.78, m | δ 4.54–4.47, m | δ 3.96, q J 5.0 | δ 3.89–3.78, m, 2H | δ 4.61, d JHa,Hb 13.0 δ 4.36, d JHb,Ha 13.0 | δ 7.58–7.51, m | |
| N-benzyl-1,4-dideoxy-1,4-imino-l-mannitol [67] | δ 2.83, dd JH-1,H-1′ 11.4 JH-1,H-2 6.6 | δ 2.76, dd JH-1′,H-1 11.4 JH-1′’,H-2 6.6 | δ 4.13, dt JH-2,H-1/H-1′ 6.6 JH-2,H-3 4.6 | δ 4.34–4.29, m | δ 3.01–2.97, m | δ 3.93, dt JH-5,H-6 6.3 JH-5,H-6′ 3.7 | δ 3.79, dd JH-6,H-6′ 11.8 JH-6,H-5 3.7 | δ 3.72, dd JH-6′,H-6 11.8 JH-6′,H-5 6.3 | δ 3.89, d JHa,Hb 13.3 δ 3.56, d JHb,Ha 13.3 | δ 7.43–7.34, m |
| N-benzyl-1,4-dideoxy-1,4-imino-d-talitol.HCl [68] | δ 3.28, dd JH-1,H-1′ 12.9 JH-1,H-2 3.9 | δ 3.20, dd JH-1′,H-1 12.9 JH-1′,H-2 4.2 | δ 4.21, q | δ 4.11, dd JH-3,H-4 6.3 JH-3,H-2 4.2 | δ 3.53, m | δ 3.80, m | δ 3.53, m | δ 3.44, dd JH-6,H-6′ 12.3 JH-6,H-5 4.9 | δ 4.26, d J 13.3 Other signal obscured by HOD signal | δ 7.30, m |
| Compound: 1,4-Dideoxy-1,4-Imino- | 13C-NMR Chemical Shifts (δ, ppm) (in D2O Unless Stated Otherwise) for Nucleus: | Melting Points (°C) | Optical Rotation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | ArCH2 | ArCquat1 | ArCquat-B) | ArCHs | Temp (°C) | [α]D (°) | ||
| N-benzyl-1,4-dideoxy-1,4-imino-d-allitol.HCl # [64] | δ 58.4, t | δ 70.7, d | δ 71.4, d | δ 70.3, d | δ 69.1, d | δ 63.0, t | δ 62.4, t | δ 130.3 | NA | δ 130.6 δ 131.5 δ 131.9 | NA | 20 | +23.1 (c 0.72, H2O) |
| N-benzyl-1,4-dideoxy-1,4-imino-l-allitol [69] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 110–111 | 20 | −25.5 (c 1.07, H2O) |
| N-benzyl-1,4-dideoxy-1,4-imino-d-galactitol (CD3OD) [65] | δ 58.9 | 75.7 | 79.2 | 73.3 | 71.2 | δ 63.7 | δ 60.7 | δ 138.6 | NA | δ 128.4 δ 127.9 δ 126.7 | 133–135 | NA | −25.5 (c 1.0, CHCl3) |
| N-benzyl-1,4-dideoxy-1,4-imino-d-glucitol.HCl # [64] | δ 59.9, t | 74.8, d | 76.9, d | 70.0, d | 68.8, d | 63.6, t | 61.4, t | Not reported | NA | δ 130.5 δ 131.4 δ 131.9 | NA | 20 | −31.9 (c 0.68, H2O) |
| N-benzyl-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose [CDCl3] 3 | δ 107.6 | 84.1 | 72.6 | 83.1 | 69.6 | 61.3 | 58.1 | 137.7 | NA | δ 128.9 δ 128.5 δ 127.4 | NA | 22 | –14.0 (c 1.0 in CHCl3) |
| N-benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 α-fur | δ 99.0 (31 Hz) | 72.6 | 72.2 | 78.8 (32 Hz) | 67.4 | 60.5–58.8 (171 Hz) | 60.0–58.2 (177 Hz) | 142.4 | NA | δ 133.1 δ 131.1 δ 129.3 | NA | 25 | 0.06 (c 0.005, MeOH) |
| N-benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 β-fur | 103.2 | 68.6 (21 Hz) | 72.8 | 80.7 | 67.5 | 57.5 | 59.8–58.8 (102 Hz) | 139.4 | NA | 130.8 130.4 129.5 | |||
| N-benzyl-1,4-dideoxy-1,4-imino-l-gulitol 5 | 53.1 | 68.9 | 70.2 | 70.1 | 68.6 | 63.1 | 61.6 | Not discernible | NA | 131.0 130.2 129.3 | NA | 25 | −0.04 (c 0.08, MeOH) [herein] |
| para 6 [CDCl3] boronic acid | 107.6 | 84.0 | 72.5 | 83.1 | 69.6 | 61.2 | 58.1 | 140.8 | not discernible | 135.0 128.2 | NA | 25 | 0.13 (c 0.16 in MeOH) |
| para 7 α-fur boronic acid | 99.0 (46 Hz) | 72.6 (38 Hz) | 72.3 (18 Hz) | 78.8 (56 Hz) | 67.5 | 60.5–58.8 (173 Hz) | 58.8–58.1 (76 Hz). | 143.0 | not discernible | 134.3 130.5 | NA | 19 | +0.15 (c 0.047, MeOH) |
| para 7 β-fur boronic acid | 103.2 (28 Hz) | 72.3 (18 Hz) | 72.9 (19 Hz) | 80.7 | 67.4 | 60.5–58.8 (173 Hz) | Not discernible | not discernible | 134.5 130.2 | ||||
| para 8 boronic acid | 53.2 | 68.9 | 70.3 | 70.3 | 63.1 | 61.5 | 134.5 | not discernible | 134.3 130.4 | NA | 19 | −0.03 (c 0.04, H2O) | |
| N-benzyl-1,4-dideoxy-1,4-imino-d-mannitol.HCl [66] | 55.2 | 68.4 | 70.9 | 67.7 | 68.5 | 62.6 | 58.3 | 131.0 | NA | 130.4 129.4 129.0 | NA | 26 | −25.2 (c 0.27, CH3OH) |
| N-benzyl-1,4-dideoxy-1,4-imino-l-mannitol [67] | 55.8 | 70.0 | 72.8 | 66.3 | 71.2 | 63.7 | 59.7 | 137.1 | NA | 130.3 129.0 128.3 | 108–109 | 21 | +37.7 (c 1.20, H2O) |
| N-benzyl-1,4-dideoxy-1,4-imino-d-talitol.HCl # [68] | 56.0, t | 70.7 | 73.9 | 70.6 | 73.4 | 64.5, t | 63.6, t | 130.9 | NA | 130.4 131.3 132.1 | NA | 20 | −10.1 (c 0.94, H2O) |
2.3.2. Electronic Effects of B on the Aromatic Ring
Comparison of Compounds 3 and para 6
Comparison of Compounds 4 and para 7
Comparison of Compounds 5 and para 8
2.4. 1H-, 13C- and 2D NMR Data Analysis
2.4.1. NMR Analysis of N-Benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4 (Figures S2–S5)
2.4.2. NMR Analysis of N-Benzyl-1,4-dideoxy-1,4-imino-l-gulitol 5 (Figures S6 and S7)
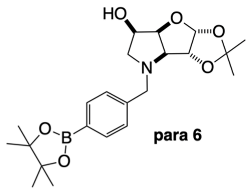
2.4.3. NMR Analysis of N-(4-Methylphenylboronic acid pinacol ester)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose para 6 (Figure S8, Scheme 2, Table 4)
- The presence of two signals points to an equilibrium between the trigonal planar B atom and several slightly quaternised B species visible on the NMR timescale. The integration between these two signals is ~3.7:1.0. This is reflected in the 1H-NMR, where the sum of integrations of the minor species mirrors this ratio. The hypothesis is that the pinacol B atom may be forming a partial dative bond with the Cl atom of the NMR solvent in such a way as to flick back and forth between the trigonal planar para 6 and a series of slightly quaternised species paratet 6 (Scheme 2). This phenomenon was also observed in another project in our research laboratory, where a slight pink tinge appeared upon dissolving a structurally related molecule in CDCl3.
- The signal at 22 ppm could arise from an interaction between the aromatic π-system of para 6 and the antibonding C-D σ-bond of a solvent molecule (Scheme 2). To our knowledge, this phenomenon has not been reported in the literature for borylated systems, and any resultant effects on the 11B-NMR are also yet to be reported. What has been reported in the literature is that aromatic rings can act as donors to result in electron density transfer to small molecules such as chloroform [81,82]. This interaction would shift the location of the boron signal in the 11B-NMR. However, it would be expected that this interaction would have a deshielding effect on the B and to find another signal downfield from 30.2 ppm. There is potentially another phenomenon coupled to this that shifts electron density back onto the B atom. The proximity of one of the Cl atoms to the empty p-orbital of boron may be in the correct orientation and distance for a lone pair to complex into that empty p-orbital to make the B atom more d- and the Cl atom involved more δ+.
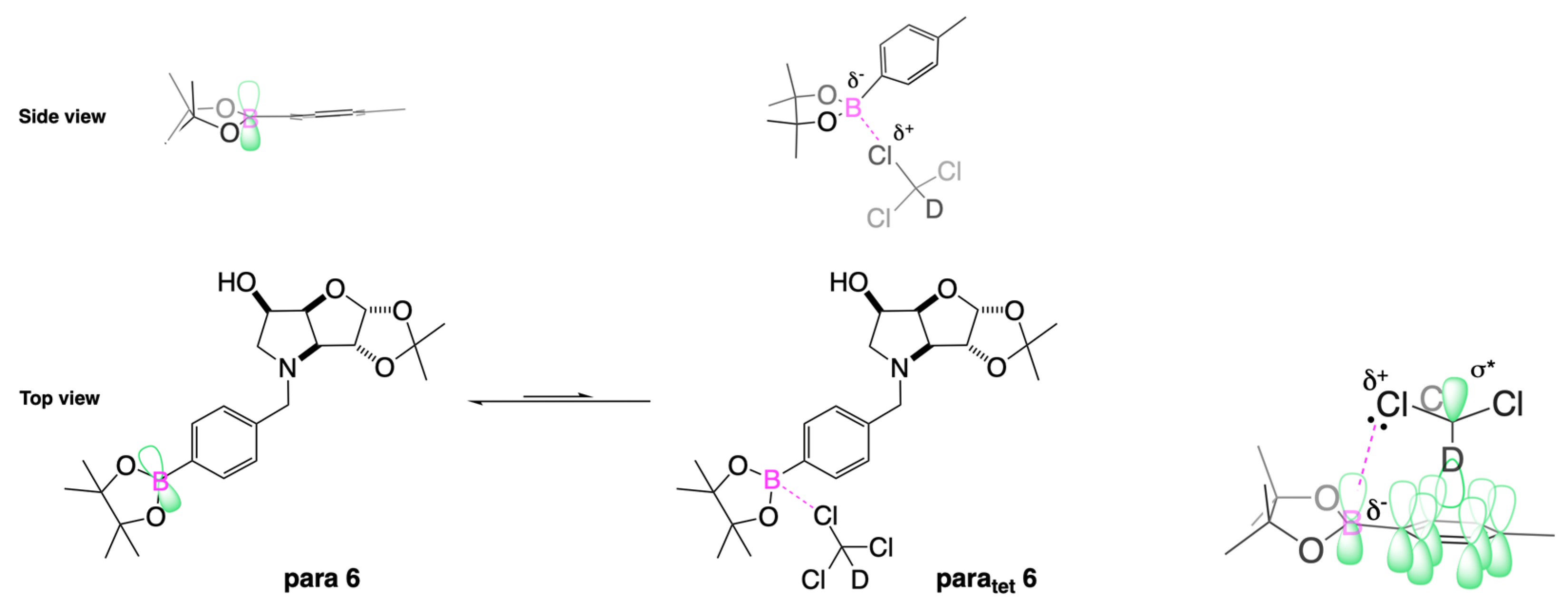
| Solvent | Chemical Shift, ppm | Proposed Assignment |
|---|---|---|
| CDCl3 | 30 (1.00) | Boronate ester (trigonal planar) |
| 22 (0.35) | Solvent CDCl3 → aromatic π-system or CDCl3 → B p-orbital (partially tetrahedral) | |
| MeOD | 32 (1.00) | Boronate ester (trigonal planar) |
| 18 (0.06) | Solvent MeOD → B p-orbital (partially tetrahedral) | |
| MeOD acidified with HCl to pH 1 | 29.8 (1.00) | Boronate ester (trigonal planar) |
| 19.3 (0.12) | Solvent MeOD → B p-orbital (partially tetrahedral) |
2.4.4. NMR Analysis of N-(4-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-d-gulofuranose para 7 (Figures S9–S12)
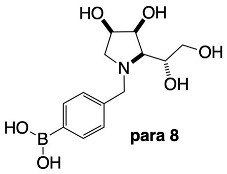
2.4.5. NMR Analysis of N-(4-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-l-gulitol para 8 (Figures S13–S16)
3. Materials and Methods
3.1. Biological Assays
3.1.1. Glycosidase Inhibition
- N-Benzyl-1,4-dideoxy-1,4-imino-d-allitol [69]. The parent iminosugar 1,4-dideoxy-1,4-imino-l-allitol has a relatively broad selectivity in glycosidase inhibition. However, a narrower selectivity is observed upon N-benzylation from predominantly an inhibitor of α-d-mannosidase to one that selectively inhibits α-l-fucosidase (76%), with weak/no inhibitions for α-d-mannosidase (Golgi II, 11% and lysosomal acidic, 7%), β-d-mannosidase (0%) and β-d-N-acetylhexosaminidase (35%).
- N-Benzyl-1,4-dideoxy-1,4-imino-d-galactitol [65]. Aldose reductase is recognised as an important checkpoint of the main biochemical abnormalities affecting diabetic tissues. When screened against aldose reductase and α-d-glucosidase, this iminosugar displays an inhibition of 32.6% and 93.2% respectively. A marked increase in efficacy was detected through the addition of the benzyl group compared to the parent iminosugar. The IC50 was found to be 40.6 μM towards α-d-glucosidase.
- N-Benzyl-1,4-dideoxy-1,4-imino-d-mannitol.HCl. This is by far the most studied in its inhibition against glycosidases [66,83,84]. N-Aralkylation with short alkyl chains delivers mostly inactivity [83]. However, inhibition towards β-d-glucosidase, β-d-galactosidase, and β-d-glucuronidase increases as the N-alkyl chain is lengthened [66]. In particular, for N-Benzyl-1,4-dideoxy-1,4-imino-d-mannitol.HCl, the following inhibitions are observed: α-d-glucosidase (rice) (34.2%), α-d-glucosidase (rat intestinal maltase) (16.6%), α-d-glucosidase (yeast) (6.5%), β-d-glucosidase (almond) (6.1%), β-d-glucosidase (bovine liver) (9.5%), β-d-glucosidase (human lysosome) (4.7%), α-d-galactosidase (coffee bean) (8.8%), β-d-galactosidase (bovine liver) (7.9%), α-d-mannosidase (Jack bean) (27%), β-d-mannosidase (snail) (4.4%), α-l-fucosidase (bovine kidney) (8.4%), trehalase (porcine kidney) (0%), β-d-glucuronidase (E. coli) (9.8%), α-l-rhamnosidase (P. decumbens) (11.5%), amyloglucosidase (A. niger) (11.4%) [66]. Screening against α-d-mannosidases shows significant inhibition towards lysosomal acidic (34%), neutral (44%), and Golgi II (72%) [83]. Screening against α-d-mannosidases: fruit fly lysosomal acidic shows an IC50 = 1.5 × 10−3 M and fruit fly Golgi II an IC50 = 6.9 × 10−4 M [84].
- N-Benzyl-1,4-dideoxy-1,4-imino-d-talitol.HCl [85]. This iminosugar was screened against β-d-galactosidase (E. coli), α-d-galactosidase (coffee bean), and α-d-mannosidase (Jack bean), displaying little inhibition. N-Arylation with a borylated benzyl resulted in a significant increase in inhibition of β-d-galactosidase (E. coli) (44–55%), and less pronounced increases in inhibition of α-d-galactosidase (coffee bean) (<5%), α-d-mannosidase (Jack bean) (10%) [85].
Glycosidase Inhibitions (Table 6)
Glycosidase Inhibitions (Table 7)
3.1.2. Cancer Screening (Table 8)
| Compound | α-d-Glucosidase | β-d- Glucosidase | α-d- Galactosidase | β-d- Galactosidase | α-d- Mannosidase | β-d- Mannosidase | α-l- Rhamnosidase | α-l- Fucosidase | β-d- Glucuronidase | α,α- Trehalase | Amyloglucosidase | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | Yeast | Rat Intestinal Maltase | Almond | Bovine Liver | Coffee Beans | Bovine Liver | Jack Bean | Snail | P. decumbens | Bovine Kidney | E. coli | Bovine Liver | Porcine Kidney | A. niger | |
| BSH | a NI b (0%) | a NI b (6.9%) | a NI b (0%) | a NI b (0%) | a NI b (15%) | a NI b (12.3%) | a NI b (0%) | a NI b (7.9%) | a NI b (19.2%) | a NI b (0.2%) | a NI b (0%) | a NI b (6%) | a NI b (19.6%) | a NI b (4.2%) | a NI b (0%) |
| 10B-BSH | a NI b (0%) | a NI b (5.6%) | a NI b (0%) | a NI b (0%) | a NI b (11.9%) | a NI b (3.9%) | a NI b (22.3%) | a NI b (7.7%) | a NI b (15%) | a NI b (0%) | a NI b (6.5%) | a NI b (3.2%) | a NI b (12.5%) | a NI b (2.3%) | a NI b (0%) |
| BPA | c NI d (0%) | c NI d (0%) | c NI d (0%) | c NI d (0%) | c NI d (0%) | c NI d (4.3%) | c NI d (10.9%) | c NI d (1.1%) | c NI d (2.1%) | c NI d (0%) | c NI d (0%) | c NI d (0.5%) | c NI d (7.4%) | c NI d (0%) | c NI d (0%) |
| 10B-BPA | c NI d (0%) | c NI d (0%) | c NI d (0%) | c NI d (0%) | c NI d (14.2%) | c NI d (1.2%) | c NI d (0%) | c NI d (0%) | c NI d (0.3%) | c NI d (0%) | c NI d (0%) | c NI d (2.4%) | c NI d (0%) | c NI d (0%) | c NI d (0%) |
| 3 | c NI d (0%) | c NI d (4.7%) | c NI d (0%) | c NI d (0%) | c NI d (31.9%) | c NI d (9.5%) | c NI d (26.1%) | c NI d (0.9%) | c NI d (2.7%) | c NI d (0.5%) | c NI d (0%) | c NI d (2.4%) | c NI d (0%) | c NI d (0%) | c NI d (0%) |
| 4 | a NI b (1.3%) | a NI b (0%) | a NI b (22.4%) | a NI b (27.4%) | a NI b (16.4%) | a NI b (1.8%) | 133 | a NI b (7.5%) | a NI b (1.0%) | a NI b (0%) | a NI b (0.4%) | a NI b (7.0%) | a NI b (7.0%) | a NI b (0%) | a NI b (0.2%) |
| 5 | c NI d (0%) | c NI d (2.9%) | c NI d (12.9%) | c NI d (0%) | c NI d (3.3%) | c NI d (1.5%) | c NI d (0%) | c NI d (4.4%) | c NI d (6.9%) | c NI d (1.5%) | NA | c NI d (0%) | c NI d (3.7%) | c NI d (0%) | c NI d (2.1%) |
| para 6 | c NI d (0%) | c NI d (3.4%) | c NI d (0%) | c NI d (0%) | c NI d (13%) | c NI d (1.6%) | c NI d (22.3%) | c NI d (0%) | c NI d (0%) | c NI d (1.4%) | c NI d (0%) | c NI d (3.2%) | c NI d (0%) | c NI d (0%) | c NI d (0%) |
| para 7 | a NI b (0%) | a NI b (0%) | a NI b (0%) | a NI b (43.6%) | a NI b (38.8%) | a NI b (0%) | 218 | a NI b (0%) | a NI b (4.1%) | a NI b (15.8%) | a NI b (0.8%) | a NI b (17.8%) | a NI b (3.5%) | a NI b (0%) | a NI b (2.7%) |
| para 8 | a NI b (8.2%) | a NI b (5.6%) | a NI b (10.5%) | a NI b (28%) | a NI b (27.1%) | a NI b (1.4%) | 501 | a NI b (0%) | a NI b (6.9%) | a NI b (26.3%) | a NI b (1.7%) | a NI b (22.9%) | a NI b (6.0%) | a NI b (0%) | a NI b (3.6%) |
| Compound | Appearance | α-d-Glucosidase | β-d-Glucosidase | α-d-Mannosidase | N-Acetyl-β-d- glucosaminidase | N-Acetyl-β-d- hexosaminidase | β-d-Glucuronidase | ||
|---|---|---|---|---|---|---|---|---|---|
| Yeast | Bacillus | Rat Intestine | Almond | Jack Bean | Bovine Kidney | Rat Intestine | Bovine Liver | ||
| BSH | In solution | 59 | 48.1 | NA | 47.3 | −18.7 | 37.1 | NA | 31.6 |
| 10B-BSH | In solution | 65.9 | 53 | NA | 49.9 | −16.8 | 40.9 | NA | 44.1 |
| BPA | Some in solution with undissolved sediment | 2.9 | 19.9 | NA | 3.9 | 0.6 | 6.7 | NA | −0.7 |
| 10B-BPA | Some in solution with undissolved sediment | 3.4 | 19.5 | NA | 3 | −0.7 | 6.3 | NA | −1 |
| 3 | In solution | −89 | −43.2 | NA | −8.3 | −30.6 | −0.1 | NA | −0.2 |
| 4 | In solution | −79 | −37.9 | NA | 58.1 | −12.1 | −9.1 | NA | −4.3 |
| 5 | In solution | 5.5 | NA | −1.3 | 17.1 | NA | NA | −1.5 | −2.2 |
| para 6 | Completely insoluble | NA | NA | NA | NA | NA | NA | NA | NA |
| para 7 | In solution | 4.2 | NA | −5.4 | 68.1 | NA | NA | 4.4 | 1.1 |
| para 8 | In solution | 9.7 | NA | ND | 23.9 | NA | NA | 2.6 | 2.4 |
| Compound | HT29 | U87 | MCF-7 | A2780 | H460 | A431 | Du145 | BE2-C | SJ-G2 | MIA-Pa-Ca2 | MCF10A |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colon Carcinoma | Glioblastoma | Breast Carcinoma | Ovarian Carcinoma | Lung Carcinoma | Skin Carcinoma | Prostate Carcinoma | Neuroblastoma | Glioblastoma | Pancreatic Carcinoma | Breast (Normal) | |
| BSH | * 3 ± 2 | * <0 | * 15 ± 3 | * 2 ± 5 | * 8 ± 2 | * <0 | * 0 ± 8 | * 10 ± 6 | * 3 ± 8 | * 2 ± 6 | * 8 ± 3 |
| 10B-BSH | * 5 ± 1 | * 0 ± 2 | * 5 ± 3 | * 5 ± 4 | * 4 ± 2 | * <0 | *7 ± 7 | * 8 ± 7 | * 1 ± 9 | * 2 ± 4 | * 13 ± 4 |
| BPA | * 14 ± 0 | * <0 | * <0 | * 4 ± 1 | * 7 ± 8 | * 4 ± 6 | * 19 ± 10 | * 13 ± 10 | * 5 ± 8 | * 3 ± 3 | * 4 ± 1 |
| 10B-BPA | * 15 ± 4 | * <0 | * 1 ± 3 | * 8 ± 4 | * 8 ± 5 | * 4 ± 4 | * 15 ± 9 | * 10 ± 6 | * 5 ± 10 | * 11 ± 3 | * <0 |
| 3 | * 10 ± 0 | * 5 ± 4 | * 7 ± 2 | * 11 ± 3 | * 9 ± 3 | * 7 ± 4 | * <0 | * 10 ± 6 | * 10 ± 10 | * 7 ± 5 | * 9 ± 4 |
| 4 | * 11 ± 3 | * 8 ± 4 | * 9 ± 5 | * 15 ± 4 | * 8 ± 5 | * 11 ± 5 | * 0 ± 9 | * 12 ± 6 | * 12 ± 10 | * 8 ± 6 | * 14 ± 4 |
| 5 | 2 ± 3 | NA | NA | 13 ± 6 | * 0 ± 1 | * 2 ± 4 | NA | NA | NA | 6 ± 1 | * 3 ± 2 |
| 5 | >50 | NA | NA | >50 | >50 | >50 | NA | NA | NA | >50 | >50 |
| para 6 | * 8 ± 5 | * 7 ± 4 | * 3 ± 4 | * 14 ± 4 | * 10 ± 4 | * 6 ± 7 | * <0 | * 5 ± 4 | * 9 ± 7 | * 9 ± 5 | * 14 ± 4 |
| para 7 | 15 ± 3 | NA | NA | 27 ± 2 | 9 ± 6 | 9 ± 5 | NA | NA | NA | 14 ± 0 | 4 ± 2 |
| para 7 | >50 | NA | NA | >50 | >50 | >50 | NA | NA | NA | >50 | >50 |
| para 8 | 5 ± 2 | NA | NA | 14 ± 7 | 1 ± 2 | 0 ± 5 | NA | NA | NA | 11 ± 1 | 1 ± 0 |
| para 8 | >50 | NA | NA | >50 | >50 | >50 | NA | NA | NA | >50 | >50 |
3.2. Inhibition Experimental
3.2.1. Glycosidase Inhibition Experimental (Table 6)
3.2.2. Glycosidase Inhibition Experimental (Table 7)
3.2.3. Cancer Screening Experimental (Table 8)
3.2.4. Numbering System
3.2.5. General Chemical Characterisation Experimental
3.2.6. Reagents and Solvents
3.3. Chemistry Experimental
3.3.1. N-Benzyl-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose 3
3.3.2. N-Benzyl-3,6-dideoxy-3,6-imino-d-gulofuranose 4
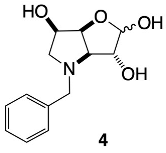
3.3.3. N-Benzyl-1,4-dideoxy-1,4-imino-l-gulitol 5

3.3.4. N-(4-Methylphenyl boronic acid pinacol ester)-3,6-dideoxy-3,6-imino-1,2-O-isopropylidene-α-d-gulofuranose para 6
3.3.5. N-(4-Methylphenyl boronic acid)-3,6-dideoxy-3,6-imino-d-gulofuranose para 7

3.3.6. N-(4-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-l-gulitol para 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Petri, G.L.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Topics Curr. Chem. 2021, 379, 34–80. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. (Eds.) Iminosugars: From Synthesis to Therapeutic Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Binaeizadeh, M.R.; Iraji, A.; Larijani, B.; Saeedi, M.; Mahdavi, M. A review on α-glucosidase inhibitory activity of first row transition metal complexes: A futuristic strategy for treatment of type 2 diabetes. RSC Adv. 2022, 12, 12011–12052. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Wadhwa, R. Alpha Glucosidase Inhibitors. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557848/ (accessed on 9 May 2025).
- Liu, S.K.; Hao, H.; Bian, Y.; Ge, Y.X.; Lu, S.; Xie, H.X.; Wang, K.M.; Tao, H.; Yuan, C.; Zhang, J.; et al. Discovery of New α-Glucosidase Inhibitors: Structure-Based Virtual Screening and Biological Evaluation. Front. Chem. 2021, 9, 639279. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Wei, J.C.C.; Lin, M.C.; Hsu, C.C.; Hwu, C.M. Long-term outcomes of adding alpha-glucosidase inhibitors in insulin-treated patients with type 2 diabetes. BMC Endocr. Dis. 2021, 21, 25. [Google Scholar] [CrossRef]
- Tsunoda, T.; Samadi, A.; Burade, S.; Mahmud, T. Complete biosynthetic pathway to the antidiabetic drug acarbose. Nat. Commun. 2022, 13, 3455. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 2018, 4, 27. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Kuo, C.L.; Lelieveld, L.T.; Boer, D.E.C.; Lienden, M.J.C.V.D.; Overkleeft, H.S.; Artola, M. Glycosphingolipids and lysosomal storage disorders as illustrated by gaucher disease. Curr. Opin. Chem. Biol. 2019, 53, 204–215. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Fernández, J.M.G.; Mellet, C.O. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: Lessons from Gaucher, GM1-gangliosidosis and Fabry diseases. Chem. Commun. 2016, 52, 5497–5515. [Google Scholar] [CrossRef]
- Parenti, G. Treating lysosomal storage diseases with pharmacological chaperones: From concept to clinics. EMBO Mol. Med. 2009, 1, 268–279. [Google Scholar] [CrossRef]
- Pastores, G.M.; Hughes, D.A. Lysosomal Storage Disorders and Malignancy. Diseases 2017, 5, 8. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T.; Ohira, M. Biomarkers for Lysosomal Storage Disorders with an Emphasis on Mass Spectrometry. Int. J. Mol. Sci. 2020, 21, 2704. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; D’Alonzo, D.; Fenza, M.D.; Gregorio, E.D.; Tamanini, A.; Lippi, G.; Dechecchi, M.C.; Guaragna, A. Synthesis and Therapeutic Applications of Iminosugars in Cystic Fibrosis. Int. J. Mol. Sci. 2020, 21, 3353. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Jenkinson, S.F.; Saville, A.W.; Alonzi, D.S.; Wormald, M.R.; Butters, T.D.; Norez, C.; Becq, F.; Blériot, Y.; Adachi, I.; et al. Cystic fibrosis and diabetes: isoLAB and isoDAB, enantiomeric carbon-branched pyrrolidine iminosugars. Tetrahedron Lett. 2010, 51, 4170–4174. [Google Scholar] [CrossRef]
- Pucci, M.; Duca, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models. Cancers 2022, 14, 2128. [Google Scholar] [CrossRef]
- Marimuthu, S.; Batra, S.K.; Ponnusamy, M.P. Pan-cancer analysis of altered glycosyltransferases confers poor clinical outcomes. Clin. Transl. Disc. 2022, 2, e100–e105. [Google Scholar] [CrossRef]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef]
- Du, Y.; Ye, H.; Gill, T.; Wang, L.; Guo, F.; Cuconati, A.; Guo, J.T.; Block, T.M.; Chang, J.; Xu, X. N-Alkyldeoxynojirimycin derivatives with novel terminal tertiary amide substitution for treatment of bovine viral diarrhea virus (BVDV), Dengue, and Tacaribe virus infections. Bioorg. Med. Chem. Lett. 2013, 23, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Soengas, R.G.; Jenkinson, S.F.; Evinson, E.L.; Nash, R.J.; Fleet, G.W.J. Synthesis of three branched iminosugars [(3R,4R,5S)-3-(hydroxymethyl)piperidine-3,4,5-triol, (3R,4R,5R)-3-(hydroxymethyl)piperidine-3,4,5-triol and (3S,4R,5R)-3-(hydroxymethyl)piperidine-3,4,5-triol] and a branched trihydroxynipecotic acid [(3R,4R,5R)-3,4,5-trihydroxypiperidine-3-carboxylic acid] from sugar lactones with a carbon substituent at C-2. Tetrahedron Asymm. 2012, 23, 401–408. [Google Scholar] [CrossRef]
- Liang, P.H.; Cheng, W.C.; Lee, Y.L.; Yu, H.P.; Wu, Y.T.; Lin, Y.L.; Wong, C.H. Novel Five-Membered Iminocyclitol Derivatives as Selective and Potent Glycosidase Inhibitors: New Structures for Antivirals and Osteoarthritis. ChemBioChem 2006, 7, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.; Soloveva, V.; Warren, T.; Guo, F.; Wu, S.; Lu, H.; Guo, J.; Su, Q.; Shen, H.; et al. Enhancing the antiviral potency of ER α-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo. Antivir. Res. 2018, 150, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gill, T.; Wang, L.; Du, Y.; Ye, H.; Qu, X.; Guo, J.T.; Cuconati, A.; Zhao, K.; Block, T.M.; et al. Design, synthesis, and biological evaluation of N-alkylated deoxynojirimycin (DNJ) derivatives for the treatment of dengue virus infection. J. Med. Chem. 2012, 55, 6061–6075. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.B.; Collin, M.; Karlsson, G.B.; James, W.; Butters, T.D.; Davis, S.J.; Gordon, S.; Dwek, R.A.; Platt, F.M. The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J. Virol. 1995, 69, 5791–5797. [Google Scholar] [CrossRef]
- Terrault, N.A. Benefits and risks of combination therapy for hepatitis B. Hepatology 2009, 49, S122–S128. [Google Scholar] [CrossRef]
- Miller, J.L.; Lachica, R.; Sayce, A.C.; Williams, J.P.; Bapat, M.; Dwek, R.; Beatty, P.R.; Harris, E.; Zitzmann, N. Liposome-mediated delivery of iminosugars enhances efficacy against dengue virus in vivo. Antimicrob. Agents Chemother. 2012, 56, 6379–6386. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Ye, Q.; Chi, H.; Guo, Z.; Chen, J.; Wu, M.; Fan, B.; Li, B.; Qin, C.F.; et al. Rational Development of Hypervalent Glycan Shield-Binding Nanoparticles with Broad-Spectrum Inhibition against Fatal Viruses Including SARS-CoV-2 Variants. Adv. Sci. 2022, 10, 2202689. [Google Scholar] [CrossRef]
- Dwek, R.A.; Butters, T.D.; Platt, F.M.; Zitzmann, N. Targeting Glycosylation as a Therapeutic Approach. Nat. Rev. Drug Disc. 2002, 1, 65–75. [Google Scholar] [CrossRef]
- Chang, J.; Block, T.M.; Guo, J.T. Antiviral therapies targeting host ER α-glucosidases: Current status and future directions. Antivir. Res. 2013, 99, 251–260. [Google Scholar] [CrossRef]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antivir. Res. 2011, 89, 26–34. [Google Scholar] [CrossRef]
- Prichard, K.; Campkin, D.; O’Brien, N.; Kato, A.; Fleet, G.W.J.; Simone, M. Biological Activities of 3,4,5-Trihydroxypiperidines and their O- and N-Alkylated Derivatives. Chem. Biol. Drug Des. 2018, 92, 1171–1197. [Google Scholar] [CrossRef]
- Clemente, F.; Matassini, C.; Cardona, F. Reductive Amination Routes in the Synthesis of Piperidine Imino Sugars. Eur. J. Org. Chem. 2020, 2020, 4447–4462. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Catarzi, S.; Matassini, C.; D’Adamio, G.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Human Acid β-Glucosidase Inhibition by Carbohydrate Derived Iminosugars: Towards New Pharmacological Chaperones for Gaucher Disease. ChemBioChem 2015, 16, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Meng, A.; Liu, C. Multivalent Pyrrolidine Iminosugars: Synthesis and Biological Relevance. Molecules 2022, 27, 5420. [Google Scholar] [CrossRef]
- Denavit, V.; St-Gelais, J.; Tremblay, T.; Giguère, D. Exploring the Chemistry of Non-sticky Sugars: Synthesis of Polyfluorinated Carbohydrate Analogues of D-Allopyranose. Chem. Eur. J. 2019, 25, 9272–9279. [Google Scholar] [CrossRef]
- Conforti, I.; Marra, A. Iminosugars as glycosyltransferase inhibitors. Org. Biomol. Chem. 2021, 19, 5439–5475. [Google Scholar] [CrossRef]
- Horne, G. Iminosugars: Therapeutic Applications and Synthetic Considerations. In Topics in Medicinal Chemistry 12; Seeberger, P., Rademacher, C., Eds.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2014; pp. 23–51. [Google Scholar]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W.J. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef]
- Bhushan, G.; Lim, L.; Bird, I.; Chothe, S.K.; Nissly, R.H.; Kuchipudi, S.V. Iminosugars with Endoplasmic Reticulum α-Glucosidase Inhibitor Activity Inhibit ZIKV Replication and Reverse Cytopathogenicity in vitro. Front. Microbiol. 2020, 11, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Zamoner, L.O.B.; Aragão-Leoneti, V.; Carvalho, I. Iminosugars: Effects of Stereochemistry, Ring Size, and N-Substituents on Glucosidase Activities. Pharmaceuticals 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Prichard, K.L.; Clarke, Z.; Houston, T.A.; Fleet, G.W.J.; Simone, M. Synthetic Pathways to 3,4,5-Trihydroxypiperidines from the Chiral Pool. Eur. J. Org. Chem. 2018, 2018, 6812–6829. [Google Scholar] [CrossRef]
- Winkler, D.A.; Holan, G. Design of Potential Anti-HIV Agents. 1. Mannosidase Inhibitors. J. Med. Chem. 1989, 32, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.W.J.; Karpas, A.; Dwek, R.A.; Fellows, L.E.; Tyms, A.S.; Petursson, S.; Namgoong, S.K.; Ramsden, N.G.; Smith, P.W.; Son, J.C.; et al. Inhibition of HIV replication by amino-sugar derivatives. FEBS Lett. 1988, 237, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Simone, M.; Edwards, A.A.; Tranter, G.E.; Fleet, G.W.J. C-3 Branched δ-3,5-cis- and trans-THF Sugar Amino Acids: Synthesis of the first generation of branched homooligomers. Amino Acids 2011, 41, 643–661. [Google Scholar] [CrossRef]
- Simone, M. Diastereoselective Synthesis of the Borylated D-Galactose Monosaccharide 3-Boronic-3-Deoxy-d-Galactose and Biological Evaluation in Glycosidase Inhibition and in Cancer for Boron Neutron Capture Therapy (BNCT). Molecules 2023, 28, 4321. [Google Scholar] [CrossRef] [PubMed]
- Campkin, D.M.; Shimadate, Y.; Bartholomew, B.; Bernhardt, P.V.; Nash, R.J.; Sakoff, J.A.; Kato, A.; Simone, M. Borylated 2,3,4,5-Tetrachlorophthalimide and Their 2,3,4,5-Tetrachlorobenzamide Analogues: Synthesis, Their Glycosidase Inhibition and Anticancer Properties in View to Boron Neutron Capture Therapy. Molecules 2022, 27, 3447. [Google Scholar] [CrossRef]
- Legge, W.J.; Shimadate, Y.; Sakoff, J.; Houston, T.A.; Kato, A.; Bernhardt, P.V.; Simone, M. Borylated methyl cinnamates: Green synthesis, characterization, crystallographic analysis and biological activities–in glycosidase inhibition and in cancer cells lines. Beilstein Arch. 2021, 20214. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Thompson, A.L.; Simone, M. Methyl 2-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)-4-nitrobenzoate. Acta Cryst. 2012, 68, o2429–o2430. [Google Scholar] [CrossRef]
- Simone, M. Borylated 5-Membered Ring Iminosugars: Detailed Nuclear Magnetic Resonance Spectroscopic Characterisation, and Method for Analysis of Anomeric and Boron Equilibria. Molecules 2025, 30, 1402. [Google Scholar] [CrossRef]
- Simone, M. Borylated Monosaccharide 3-Boronic-3-deoxy-d-galactose: Detailed NMR Spectroscopic Characterisation, and Method for Spectroscopic Analysis of Anomeric and Boron Equilibria. Int. J. Mol. Sci. 2024, 25, 12396. [Google Scholar] [CrossRef]
- Deng, J.P.; Yu, C.S. Recent Development of Radiofluorination of Boron Agents for Boron Neutron Capture Therapy of Tumor: Creation of 18F-Labeled C-F and B-F Linkages. Pharmaceuticals 2023, 16, 93. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Cheng, X.; Li, F.; Liang, L. Boron Neutron Capture Therapy: Clinical Application and Research Progress. Curr. Oncol. 2022, 29, 7868–7886. [Google Scholar] [CrossRef]
- Austin, G.N.; Baird, P.D.; Fleet, G.W.J.; Peach, J.M.; Smith, P.W.; Watkin, D.J. 3,6-Dideoxy-3,6-imino-1,2-O-isopropylidene-a-d-glucofuranose intermediate for the synthesis of hydroxylated pyrrolidines: Synthesis of 1,4-dideoxy-1,4-imino-l-gulitol, 1,4-dideoxy-1,4-imino-d-lyxitol, 2S,3S,4R-3,4-dihydroxyproline and (1S,2R,8S,RaR)-1,2,8-trihydroxyoctahydroindolizine [8-epi-swainsonine]. X-ray crystal structure of (1S,2R,8S,8aR)-1,2,8-trihydroxy-5-oxo-octahydroindolizine. Tetrahedron 1987, 43, 3095–3108. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. Chapter 1: Structure, properties, and preparation of boronic acid derivatives. In Overview of Their Reactions and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Buchner, G.S.; Murphy, R.D.; Buchete, N.V.; Kubelka, J. Dynamics of protein folding: Probing the kinetic network of folding–unfolding transitions with experiment and theory. Biochim. Biophys. Acta 2011, 1814, 1001–1020. [Google Scholar] [CrossRef]
- Alexandersson, E.; Nestor, G. Complete 1H and 13C NMR spectral assignment of D-glucofuranose. Carbohydr. Res. 2022, 511, 108477. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Lorenzo, F.D.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; Castro, C.D. Liquid-state NMR spectroscopy for complex carbohydrate structural analysis: A hitchhiker’s guide. Carbohydr. Polymers 2022, 277, 118885. [Google Scholar] [CrossRef]
- Anet, F.A.L.; Anet, R. Chapter 14: Conformational Processes in Rings. In Dynamic Nuclear Magnetic Resonance Spectroscopy; Jackman, L.M., Cotton, F.A., Eds.; Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Angyal, S.J.; Pickles, V.A. Equilibria between pyranoses and furanoses. Aust. J. Chem. 1972, 25, 1695–1710. [Google Scholar] [CrossRef]
- Synytsya, A.; Opíková, J.; Brus, J. 13C CP/MAS NMR Spectra of Pectins: A Peak-Fitting Analysis in the C-6 Region. Czech J. Food Sci. 2003, 21, 1–12. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Son, J.C. Polyhydroxylated pyrrolidines from sugar lactomes: Synthesis of 1,4-dideoxy-1,4-imino-d-glucitol from D-galactonolactone and syntheses of 1,4-dideoxy-1,4-imino-d-allitol, 1,4-dideoxy-1,4-imino-d-ribitol, and (2s,3r,4s)-3,4-dihydroxyproline from D-gulonolactone. Tetrahedron 1988, 44, 2637–2647. [Google Scholar] [CrossRef]
- Guazzelli, L.; D’Andrea, F.; Sartini, S.; Giorgelli, F.; Confini, G.; Quattrini, L.; Piano, I.; Gargini, C.; Motta, C.L. Synthesis and investigation of polyhydroxylated pyrrolidine derivatives as novel chemotypes showing dual activity as glucosidase and aldose reductase inhibitors. Bioorg. Chem. 2019, 92, 103298. [Google Scholar] [CrossRef]
- Yang, L.F.; Shimadate, Y.; Kato, A.; Li, Y.X.; Jia, Y.M.; Fleet, G.W.J.; Yu, C.Y. Synthesis and glycosidase inhibition of N-substituted derivatives of 1,4-dideoxy-1,4-imino-d-mannitol (DIM). Org. Biomol. Chem. 2020, 18, 999–1011. [Google Scholar] [CrossRef]
- Håkansson, A.E.; Ameijde, J.V.; Guglielmini, L.; Horne, G.; Nash, R.J.; Evinson, E.L.; Kato, A.; Fleet, G.W.J. Looking glass inhibitors: Synthesis of a potent naringinase inhibitor L-DIM [1,4-dideoxy-1,4-imino-l-mannitol], the enantiomer of DIM [1,4-dideoxy-1,4-imino-d-mannitol] a potent α-d-mannosidase inhibitor. Tetrahedron Asymm. 2007, 18, 282–289. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Son, J.C.; Green, D.S.C.; Bello, I.C.D.; Winchester, B. Synthesis from D-mannose of 1,4-dideoxy-1,4-imino-l-ribitol and of the α-mannosidase inhibitor 1,4-dideoxy-1,4-imino-d-talitol. Tetrahedron 1988, 44, 2649–2655. [Google Scholar] [CrossRef]
- Daher, S.A.; Fleet, G.; Namgoong, S.K.; Winchester, B. Change in specificity of glycosidase inhibition by N-alkylation of amino sugars. Biochem. J. 1989, 258, 613–615. [Google Scholar] [CrossRef]
- Snyder, H.R.; Konecky, M.S.; Lennarz, W.J. Aryl boronic acids. ii. aryl boronic anhydrides and their amine complexes. J. Am. Chem. Soc. 1958, 80, 3611–3615. [Google Scholar] [CrossRef]
- Chen, L.; Zou, X.; Zhao, H.; Xu, S. Copper-Catalyzed Asymmetric Protoboration of β-Amidoacrylonitriles and β-Amidoacrylate Esters: An Efficient Approach to Functionalized Chiral α-Amino Boronate Esters. Org. Lett. 2017, 19, 3676–3679. [Google Scholar] [CrossRef]
- Touchet, S.; Mace, A.; Roisnel, T.; Carreaux, F.; Bouillon, A.; Carboni, B. [3,3]-Sigmatropic Rearrangement of Boronated Allylcyanates: A New Route to α-Aminoboronate Derivatives and Trisubstituted Tetrahydrofurans. Org. Lett. 2013, 15, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Liu, Y.; Wu, W.; Zhou, Y.; Maw, H.H.; Bachovchin, W.W.; Bhat, K.L.; Bock, C.W. Synthesis and structural investigation of internally coordinated α-amidoboronic acids. J. Org. Chem. 2006, 71, 512–519. [Google Scholar] [CrossRef]
- Wrackmeyer, B. Nuclear magnetic resonance spectroscopy of boron compounds containing two-, three-and four-coordinate boron. Ann. Rep. NMR Spectr. 1988, 20, 61–203. [Google Scholar] [CrossRef]
- Weiss, J.W.; Bryce, D.L. A solid-state 11B NMR and computational study of boron electric field gradient and chemical shift tensors in boronic acids and boronic esters. J. Phys. Chem. A 2010, 114, 5119–5131. [Google Scholar] [CrossRef]
- Zhuo, J.C.; Soloway, A.H.; Beeson, J.C.; Ji, W.; Barnum, B.A.; Rong, F.G.; Tjarks, W.; Jordan, G.T.; Liu, J.; Shore, S.G. Boron-containing heterocycles: Syntheses, structures, and properties of benzoborauracils and a benzoborauracil nucleoside. J. Org. Chem. 1999, 64, 9566–9574. [Google Scholar] [CrossRef]
- 11B-NMR Chemical Shifts. Available online: https://www.chemistry.sdsu.edu/research/BNMR/ (accessed on 17 December 2023).
- Nanalysis. Boron NMR Spectroscopy. Available online: https://www.nanalysis.com/nmready-blog/2016/10/17/boron-nmr-spectroscopy (accessed on 17 December 2023).
- Wrackmeyer, B. Organoboranes and tetraorganoborates studied by 11B and 13C NMR spectroscopy and DFT calculations. Z. Naturforsch. 2015, 70, 421–424. [Google Scholar] [CrossRef]
- León-Negrete, A.; Villamil-Ramos, R.; Sánchez-Portillo, P.; González-Hernández, A.; Barba, V. Six-Membered Heterocyclic Boronate Esters. Synthesis and Structural Analysis. J. Mex. Chem. Soc. 2022, 66, 421–432. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. The interaction of benzene with chloro-and fluoromethanes: Effects of halogenation on CH/π interaction. J. Phys. Chem. A 2002, 106, 4423–4428. [Google Scholar] [CrossRef]
- Tamres, M. Aromatic Compounds as Donor Molecules in Hydrogen Bonding. J. Am. Chem. Soc. 1952, 74, 3375–3378. [Google Scholar] [CrossRef]
- Winchester, B.; Daher, S.A.; Carpenter, N.C.; Bello, I.C.D.; Choi, S.S.; Fairbanks, A.J.; Fleet, G.W.J. The structural basis of the inhibition of human α-mannosidases by azafuranose analogues of mannose. Biochem. J. 1993, 290, 743–749. [Google Scholar] [CrossRef]
- Šesták, S.; Bella, M.; Klunda, T.; Gurská, S.; Dzubáck, P.; Wöls, F.; Wilson, I.B.H.; Sladek, V.; Hajdúch, M.; Poláková, M.; et al. N-Benzyl Substitution of Polyhydroxypyrrolidines: The Way to Selective Inhibitors of Golgi a-Mannosidase II. ChemMedChem 2018, 13, 373–383. [Google Scholar] [CrossRef]
- Johnson, L.L.; Houston, T.A. A drug targeting motif for glycosidase inhibitors: An iminosugar–boronate shows unexpectedly selective β-galactosidase inhibition. Tetrahedron Lett. 2002, 43, 8905–8908. [Google Scholar] [CrossRef]
- Cox, T.M. Gaucher’s Disease; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Mena-Barragán, T.; García-Moreno, M.I.; Sevšek, A.; Okazaki, T.; Nanba, E.; Higaki, K.; Martin, N.I.; Pieters, R.J.; Fernández, J.M.G.; Mellet, C.O. Probing the Inhibitor versus Chaperone Properties of sp2-Iminosugars towards Human β-Glucocerebrosidase: A Picomolar Chaperone for Gaucher Disease. Molecules 2018, 23, 927. [Google Scholar] [CrossRef] [PubMed]
- Martín-Banderas, L.; Holgado, M.A.; Durán-Lobato, M.; Infante, J.J.; Álvarez-Fuentes, J.; Fernández-Arévalo, M. Role of Nanotechnology for Enzyme Replacement Therapy in Lysosomal Diseases. A Focus on Gaucher’s Disease. Curr. Med. Chem. 2016, 23, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Zhang, S.; Smith, A.C.; Callahan, J.W. Impaired Elastic-Fiber Assembly by Fibroblasts from Patients with Either Morquio B Disease or Infantile GM1-Gangliosidosis Is Linked to Deficiency in the 67-kD Spliced Variant of β-Galactosidase. Am. J. Hum. Genet. 2000, 67, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Glenister, A.; Chen, C.K.J.; Renfrew, A.K.; Simone, M.; Hambley, T.W. Warburg Effect Targeting Cobalt(III) Cytotoxin Chaperone Complexes. J. Med. Chem. 2021, 64, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Glenister, A.; Simone, M.; Hambley, T.W. A Warburg effect targeting vector designed to increase the uptake of compounds by cancer cells demonstrates glucose and hypoxia dependent uptake. PLoS ONE 2019, 14, e0217712. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Müller, M.; Semenza, G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim. Biophys. Acta 1978, 506, 136–154. [Google Scholar] [CrossRef]
- McNaught, A.D. IUPAC and IUBMB Joint Commission on Biochemical Nomenclature; Nomenclature of Carbohydrates. Pure Appl. Chem. 1996, 68, 1919–2008. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Bruker. NMR Software TopSpin. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html?utm_source=Advertising&utm_medium=GoogleAd&utm_campaign=BBIO-Software-Cross-All-TopSpin-H2-2025&gad_source=1&gad_campaignid=15149563659&gbraid=0AAAAADsq6H3hmr5YRBHOpUv-a-tmY2e0U&gclid=EAIaIQobChMIvr208NXjjgMVzy57Bx1-LwL3EAAYASAAEgKW-vD_BwE (accessed on 30 June 2025).
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/AU/en/product/aldrich/dwk231700006?srsltid=AfmBOooBqu8eAIi5jtc28sWdih3PbRDScnsOaxbYMVB8PVNLSPe6lWxm (accessed on 20 February 2025).
- Corning. Available online: https://ecatalog.corning.com/life-sciences/b2c/US/en/General-Labware/Tubes/Tubes-Storage/PYREX®-Tube%2C-NMR%2C-5-mm-Diameter/p/pyrexTubeNMR5mmDiameter (accessed on 20 February 2025).
- Bruker. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr/nmr-probes.html (accessed on 20 February 2025).
- Macho, J.M.; Blue, R.M.; Lee, H.W.; MacMillan, J.B. Boron NMR as a Method to Screen Natural Product Libraries for B-Containing Compounds. Org. Lett. 2022, 24, 3161–3166. [Google Scholar] [CrossRef]
- Boron Molecular. A Fine Chemicals Manufacturer. Available online: https://www.boronmolecular.com (accessed on 21 April 2025).
| Iminosugar | Glycosidase/s Most Inhibited |
|---|---|
| N-Benzyl-1,4-dideoxy-1,4-imino- | |
| d-allitol [69] | α-l-fucosidase (76%) |
| d-galactitol [65] | α-d-glucosidase (93.2%), IC50 = 40.6 μM |
| d-mannitol.HCl [66,83,84] | α-d-mannosidases (lysosomal acidic, 34%), (neutral, 44%), and (Golgi II, 72%) |
| d-talitol.HCl [85] | NI |
| N-(2-Methylphenyl boronic acid)-1,4-dideoxy-1,4-imino-d-talitol.HCl [85] | β-d-galactosidase (E. coli) (44–55%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prichard, K.; Yamamoto, S.; Shimadate, Y.; Yoshimura, K.; Bartholomew, B.; Gilbert, J.; Sakoff, J.; Nash, R.; Kato, A.; Simone, M. Borylated Five-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis, and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1. Pharmaceuticals 2025, 18, 1302. https://doi.org/10.3390/ph18091302
Prichard K, Yamamoto S, Shimadate Y, Yoshimura K, Bartholomew B, Gilbert J, Sakoff J, Nash R, Kato A, Simone M. Borylated Five-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis, and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1. Pharmaceuticals. 2025; 18(9):1302. https://doi.org/10.3390/ph18091302
Chicago/Turabian StylePrichard, Kate, Suzuka Yamamoto, Yuna Shimadate, Kosuke Yoshimura, Barbara Bartholomew, Jayne Gilbert, Jennette Sakoff, Robert Nash, Atsushi Kato, and Michela Simone. 2025. "Borylated Five-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis, and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1" Pharmaceuticals 18, no. 9: 1302. https://doi.org/10.3390/ph18091302
APA StylePrichard, K., Yamamoto, S., Shimadate, Y., Yoshimura, K., Bartholomew, B., Gilbert, J., Sakoff, J., Nash, R., Kato, A., & Simone, M. (2025). Borylated Five-Membered Ring Iminosugars: Synthesis, Spectroscopic Analysis, and Biological Evaluation for Glycosidase Inhibition and Anticancer Properties for Application in Boron Neutron Capture Therapy (BNCT)—Part 1. Pharmaceuticals, 18(9), 1302. https://doi.org/10.3390/ph18091302









