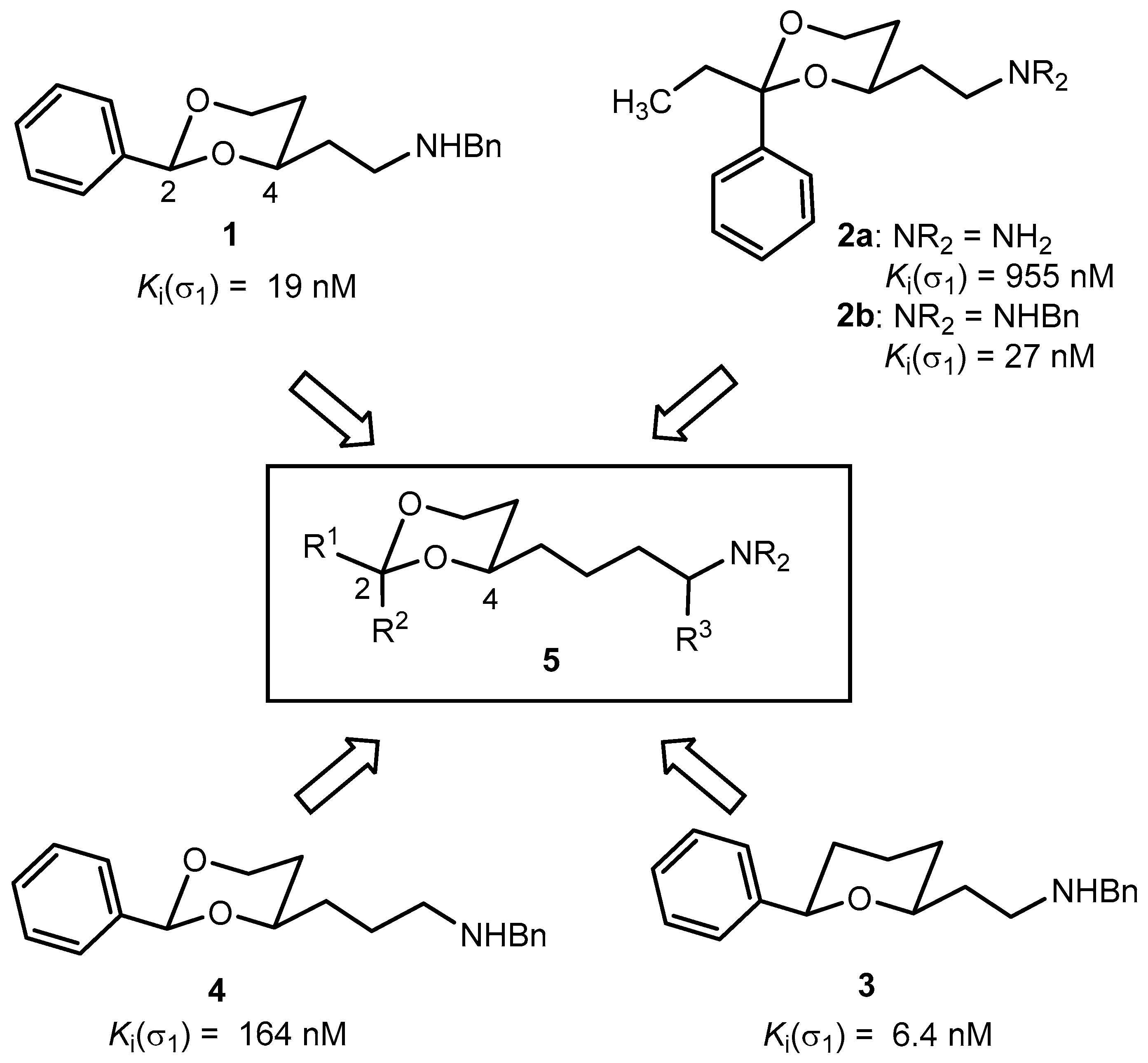
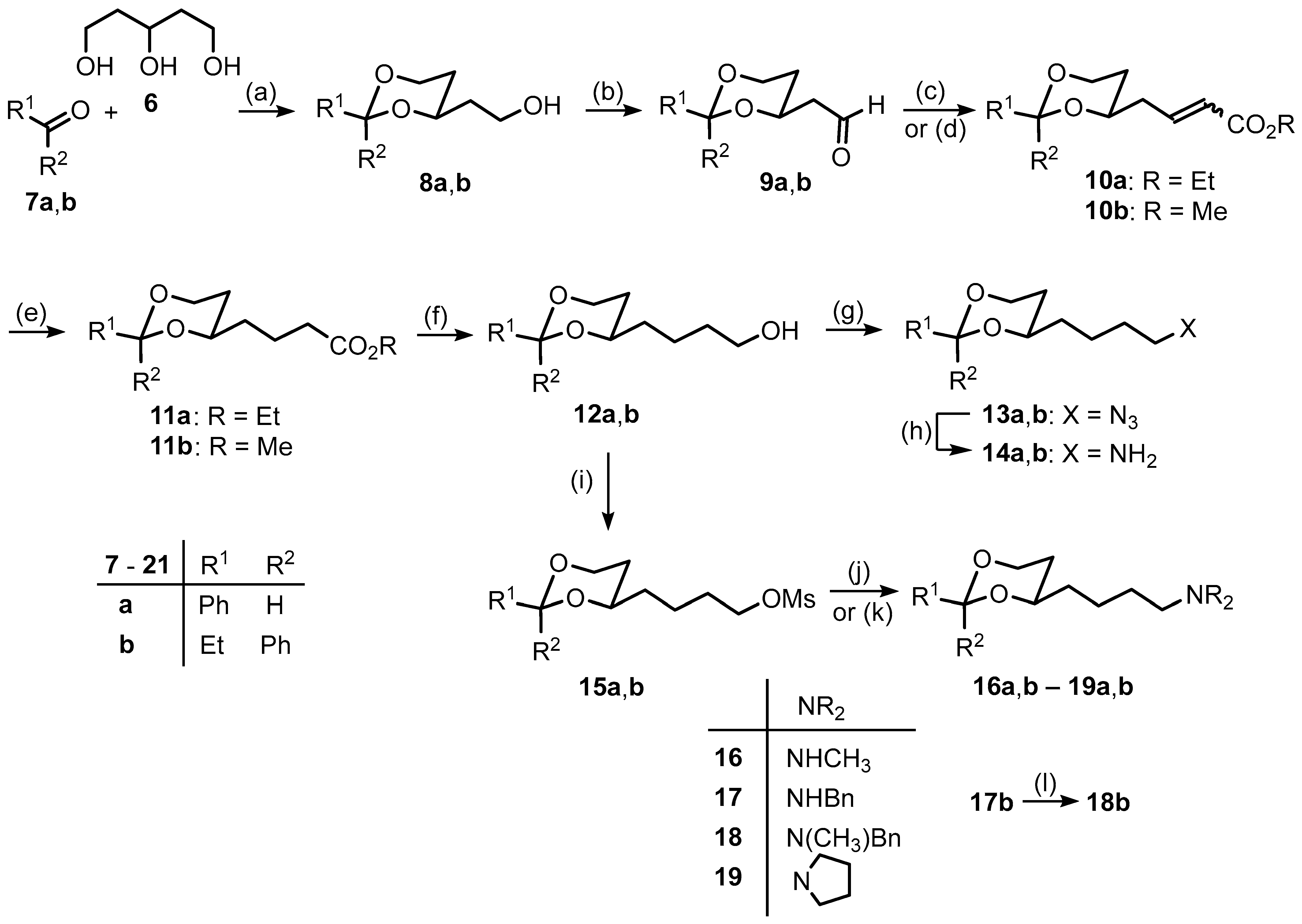
3.1. Synthetic Procedures
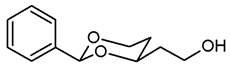
![Pharmaceuticals 18 01300 i007 Pharmaceuticals 18 01300 i007]()
Pentane-1,3,5-triol (6, 1.69 g, 14 mmol), benzaldehyde (7a, 3.5 mL, 35 mmol) and p-toluenesulfonic acid (75 mg) were dissolved in CH2Cl2 abs. (60 mL) and heated to reflux for 16 h, using an inverse Dean-Stark apparatus. For workup, the reaction mixture was washed with saturated aqueous solution of NaHCO3 (3 × 20 mL), dried (K2CO3) and the solvent was removed in vacuo. The residue was purified by flash column chromatography (Ø 6 cm, cyclohexane:thyl acetate = 1:1, length 20 cm, fraction 65 mL, Rf = 0.28). Colorless oil, yield 2.27 g (78%). C12H16O3, Mr = 208.3. MS (EI): m/z [%] = 208 (M, 33), 207 (M-H, 100), 177 (M-CH2OH, 13), 105 (PhCO, 47), 77 (Ph, 42). IR (neat): ͠ν [cm−1] = 3420 (O-H), 3066, 3034 (Ar-H), 2947, 2857 (C-H), 1098 (C-O-C), 749, 697 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.54 (dtd, J = 13.1/2.5/1.4 Hz, 1H, 5-Heq), 1.79–1.99 (m, 3H, CH2-CH2-OH and 5-Hax), 2.11 (dd, J = 6.1/4.7 Hz, 1H, CH2-OH), 3.79–3.90 (m, 2H, CH2-OH), 3.99 (td, J = 11.7/2.6 Hz, 1H, 6-Hax), 4.09–4.16 (m, 1H, 4-Hax), 4.28 (ddd, J = 11.4/5.0/1.1 Hz, 1H, 6-Heq), 5.54 (s, 1H, 2-Hax), 7.30–7.40 (m, 3H, Ar-H), 7.46–7.48 (m, 2H, Ar-H).HPLC (method MeOH): purity 96.8%, tR = 12.27 min.
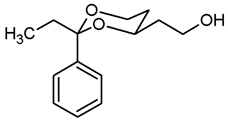
![Pharmaceuticals 18 01300 i008 Pharmaceuticals 18 01300 i008]()
Pentane-1,3,5-triol (6, 2.01 g, 16.6 mmol), propiophenone (7b 4.4 mL, 33.2 mmol) and p-toluenesulfonic acid (75 mg) were dissolved in toluene (90 mL) and heated to reflux for 1.5 h, using a Dean-Stark apparatus filled with molecular sieves 4 Å. For workup, the reaction mixture was washed with saturated aqueous solution of NaHCO3 (3 × 30 mL) and with brine (1 × 30 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 8 cm, cyclohexane:ethyl acetate = 7:3, length 17 cm, fraction 65 mL, Rf = 0.24). Colorless oil, yield 3.51 g (90%). C14H20O3, Mr = 236.3. MS (EI): m/z [%] = 237 (M + H, 24), 207 (M-CH3-CH2, 100), 105 (Ph-CO, 67). IR (neat): ͠ν [cm−1] = 3420 (O-H), 2926, 2875 (C-H), 756, 703 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.25–1.30 (m, 1 H, 5-Heq), 1.69–1.89 (m, 3 H, 5-Hax, CH2-CH2-OH), 1.75 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 2.58 (s, 1 H, O-H), 3.81 (td, J = 12.0/2.5 Hz, 1 H, 6-Hax), 3.87–3.91 (m, 3 H, 6-Heq, CH2-OH), 3.93–4.00 (m, 1 H, 4-Hax), 7.29–7.42 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.9%, tR = 17.54 min.
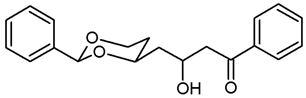
![Pharmaceuticals 18 01300 i009 Pharmaceuticals 18 01300 i009]()
Under N2 atmosphere, oxalyl chloride (0.51 mL, 6 mmol) was dissolved in CH2Cl2 abs. (25 mL) and cooled down to −78 °C. At this temperature, a solution of DMSO (0.85 mL, 12 mmol) in CH2Cl2 abs. (7.5 mL) was added very slowly (using a syringe pump, 7 mL/h) and the mixture was stirred for 15 min at −78 °C. Then, a solution of the alcohol 8a (1.043 g, 5 mmol) in CH2Cl2 abs. (7.5 mL) was added (using a syringe pump, 12 mL/h). The mixture was stirred for additional 45 min at −78 °C before NEt3 (3.5 mL, 25 mmol) was added. After warming to rt, n-hexane (40 mL) was added. The precipitate was filtered off using a glass suction filter and washed several times with Et2O. The filtrate was concentrated (600 mbar, 40 °C) and the filtration procedure was repeated once. The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 5.5 cm, cyclohexane:ethyl acetate = 8:2, length 20 cm, fraction 65 mL, Rf = 0.16). Colorless oil with sweet smell, yield 0.994 g (91%). C12H14O3, Mr = 206.3. MS (EI): m/z = 207 (M + H, 53), 205 (M-H, 76), 177 (M-CHO, 6), 105 (PhCO, 100), 77 (Ph, 81). IR (neat): ͠ν [cm−1] = 2961, 2921, 2856 (C-H), 2736 (C-Haldehyde), 1722 (C=O), 1099 (C-O), 752, 698 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.64 (dtd, J = 13.2/2.5/1.4 Hz, 1H, 5-Heq), 1.90 (dddd, J = 13.1/12.3/11.4/5.0 Hz, 1H, 5-Hax), 2.63 (ddd, J = 17.0/5.2/1.6 Hz, 1H, CH2-CHO), 2.83 (ddd, J = 17.0/7.3/2.1 Hz, 1H, CH2-CHO), 4.02 (td, J = 12.2/2.6 Hz, 1H, 6-Hax), 4.29 (ddd, J = 11.5/5.0/1.4 Hz, 1H, 6-Heq), 4.41–4.47 (m, 1 H, 4-Hax), 5.57 (s, 1H, 2-Hax), 7.33–7.38 (m, 3H, Ar-H), 7.45–7.48 (m, 2 H, Ar-H), 9.85 (t, J = 1.8 Hz, 1H, CHO). HPLC (method ACN): purity 90.4%, tR = 14.05 min. The substance was rather unstable and always used within three days after purification.
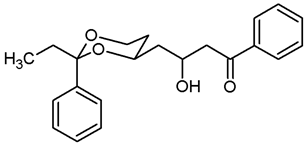
![Pharmaceuticals 18 01300 i010 Pharmaceuticals 18 01300 i010]()
Under N2 atmosphere, oxalyl chloride (0.87 mL, 10.15 mmol) was dissolved in CH2Cl2 abs. (26 mL) and cooled down to −78 °C. At this temperature, a solution of DMSO (1.44 mL, 20.30 mmol) in CH2Cl2 abs. (12 mL) was added very slowly (using a syringe pump, 9 mL/h) and the mixture was stirred for 15 min at −78 °C. Then a solution of the alcohol 8b (2.00 g, 8.46 mmol) in CH2Cl2 abs. (12 mL) was added (using a syringe pump, 18 mL/h). The mixture was stirred for additional 45 min at −78 °C before NEt3 (6.6 mL, 42.30 mmol) was added. After warming to rt, n-hexane (40 mL) was added. The precipitate was filtered off using a glass suction filter and washed several times with Et2O. The filtrate was concentrated (600 mbar, 40 °C) and the filtration procedure was repeated once. The solvent was removed in vacuo, and the residue was purified by flash column chromatography (6 cm, cyclohexane:ethyl acetate = 9.25:0.75, length 18 cm, fraction 65 mL, Rf = 0.09). Colorless solid, mp 55.8 °C, yield 1.76 g (89%). C14H18O3, Mr = 234.3. MS (EI): m/z [%] = 235 (M + H, 41), 205 (M-CH3-CH2, 100), 105 (Ph-CO, 52). IR (neat): ͠ν [cm−1] = 2972, 2930, 2877 (C-H), 2730 (C-Haldehyde), 1725 (C=O), 758, 704 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.34–1.39 (m, 1 H, 5-Heq), 1.73 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 1.79 (qd, J = 12.3/5.4 Hz, 1 H, 5-Hax), 2.50 (ddd, J = 16.5/4.4/1.6 Hz, 1 H, CH2-CHO), 2.73 (ddd, J = 16.5/8.0/2.5 Hz, 1 H, CH2-CHO), 3.83 (td, J = 11.9/2.5 Hz, 1 H, 6-Hax), 3.90 (ddd, J = 11.5/5.4/1.5 Hz, 1 H, 6-Heq), 4.22–4.29 (m, 1 H, 4-Hax), 7.29–7.33 (m, 1 H, Ar-H), 7.36–7.43 (m, 4 H, Ar-H), 9.91 (dd, J = 2.3/1.7 Hz, 1 H, CHO). HPLC (method ACN): purity 98.7%, tR = 18.15 min.
Under N2 atmosphere the commercially available P-ylide Ph3P=CH-CO2Et (1.23 g, 4.5 mmol) was dissolved in THF abs. (20 mL). A solution of the aldehyde 9a (619 mg, 3 mmol) in THF abs. (8 mL) was added, and the solution was diluted with THF to a total volume of 40 mL. The mixture was stirred for 24 h at rt and then heated to reflux for 2 h. Water (30 mL) and brine (15 mL) were added, and the mixture was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (1 × 40 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was adsorbed on silica gel and loaded on the column (Ø 5.5 cm, cyclohexane:ethyl acetate = 9:1, length 18 cm, fraction 65 mL).
(Z)-10a (Rf = 0.15): Colorless oil, yield 76 mg (9.1%). C16H20O4, Mr = 276.3. IR (neat): ͠ν [cm−1] = 2977, 2922, 2851 (C-H), 1714 (CO2R), 1646 (C=C), 1098 (C-O), 750, 697 (arom. monosubst). 1H NMR (CDCl3): δ [ppm] = 1.29 (t, J = 7.1 Hz, 3 H, CO2CH2CH3), 1.56 (dtd, J = 13.3/3.8/1.4 Hz, 1 H, 5-Heq), 1.89 (dddd, J = 13.1/12.3/11.4/5.1 Hz, 1 H, 5-Hax), 2.87–2.95 (m, 1 H, diox-CH2-CH=CH), 3.10 (dddd, J = 15.7/7.6/4.5/1.7 Hz, 1 H, diox-CH2-CH=CH), 3.97 (ddd, J = 12.3/11.6/2.6 Hz, 1 H, 6-Hax), 3.96–4.02 (m, 1 H, 4-Hax), 4.18 (q, J = 7.1 Hz, 2 H, CO2CH2CH3), 4.27 (ddd, J = 11.3/4.9/1.1 Hz, 1 H, 6-Heq), 5.52 (s, 1 H, 2-Hax), 5.88 (dt, J = 11.6/1.8 Hz, 1 H, CH=CH-CO2R), 6.45 (ddd, J = 11.6/7.7/6.9 Hz, 1 H, CH=CH-CO2R), 7.31–7.39 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 93.0%, tR = 20.90 min. Contamination with 2.9% of (E)-10a (tR = 20.35 min) was observed.
(E)-10a (Rf = 0.12: Colorless oil, yield 712 mg (86%). C16H20O4, Mr = 276.3. MS (ESI): m/z [%] = 277 (M + H, 10), 294 (M + NH4, 22), 299 (M + Na, 26), 575 (2 × M + Na, 20), 577 (M + Ph3P = O + Na, 100). IR (neat): ͠ν [cm−1] = 2979, 2853 (C-H), 1715 (CO2R), 1656 (C=C), 1099 (C-O), 751, 697 (arom. monosubst). 1H NMR (CDCl3): δ [ppm] = 1.29 (t, J = 7.1 Hz, 3 H, CO2CH2-CH3), 1.53–1.58 (m, 1 H, 5-Heq), 1.85 (dddd, J = 13.1/12.3/11.4/5.0 Hz, 1 H, 5-Hax), 2.46 (dddd, J = 14.7/7.4/5.7/1.4 Hz, 1 H, diox-CH2-CH=CH), 1.60 (dtd, J = 14.8/6.9/1.5 Hz, 1 H, diox-CH2-CH=CH), 3.96 (td, J = 12.0/2.6 Hz, 1 H, 6-Hax), 3.95–4.02 (m, 1 H, 4-Hax), 4.19 (q, J = 7.1 Hz, 2 H, CO2CH2-CH3), 4.28 (ddd, J = 11.4/5.0/1.2 Hz, 1 H, 6-Heq), 5.52 (s, 1 H, 2-Hax), 5.93 (dt, J = 15.7/1.4 Hz, 1 H, CH=CH-CO2R), 7.01 (dt, J = 15.6/7.3 Hz, 1 H, CH=CH-CO2R), 7.33–7.39 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 93.4%, tR = 20.34 min. Contamination with 1.7% of (Z)-10a (tR = 20.35 min) was observed.
Under N2 atmosphere the commercially available P-ylide Ph3P=CH-CO2CH3 (2.10 g, 6.27 mmol) was dissolved in THF abs. (20 mL). A solution of the aldehyde 9b (983 mg, 4.18 mmol) in THF abs. (10 mL) was added, and the solution was diluted with THF to a total volume of 65 mL. The mixture was stirred for 21 h at rt. Additional P-ylide (699 mg, 2.09 mmol) and THF abs. (10 mL) were added, and the mixture was stirred for another 2 h. Then, water (30 mL) and brine (15 mL) were added, and the mixture was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was adsorbed on silica gel and given on the column (Ø 5.5 cm, cyclohexane:ethyl acetate = 9.5:0.5, length 17 cm, fraction 65 mL).
(Z)-10b (Rf = 0.22): Colorless oil, yield 69 mg (5.7%). C17H22O4, Mr = 290.4. MS (ESI): m/z [%] = 313 (M + Na, 100). IR (neat): ͠ν [cm−1] = 2946, 2873 (C-H), 1721 (CO2R), 1647 (C=C), 758, 705 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.81 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.29–1.33 (m, 1 H, 5-Heq), 1.74 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 1.71–1.82 (m, 1 H, 5-Hax), 2.84 (dtd, J = 15.4/7.3/1.8 Hz, 1 H, diox-CH2-CH=CH), 2.96 (dddd, J = 15.6/7.4/4.5/1.8 Hz, 1 H, diox-CH2-CH=CH), 3.71 (s, 3 H, CO2CH3), 3.74–3.81 (m, 1 H, 6-Hax), 3.75–3.84 (m, 1 H, 4-Hax), 3.88 (ddd, J = 11.4/5.1/1.3 Hz, 1 H, 6-Heq), 5.93 (dt, J = 11.6/1.7 Hz, 1 H, CH=CH-CO2CH3), 6.51 (dt, J = 11.6/7.3 Hz, 1 H, CH=CH-CO2CH3), 7.26–7.40 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.0%, tR = 22.07 min.
(E)-10b (Rf = 0.15): Colorless solid, mp 66.2 °C, yield 1.09 g (90%). C17H22O4, Mr = 290.4. MS (ESI): m/z [%] = 291 (M + H, 25), 313 (M + Na, 100). IR (neat): ͠ν [cm−1] = 2980, 2943 (C-H), 1719 (CO2R), 1662 (C=C), 759, 702 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.81 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.28–1.32 (m, 1 H, 5-Heq), 1.74 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 1.67–1.77 (m, 1 H, 5-Hax), 2.33–2.40 (m, 1 H, CH2-CH=CH), 2.49 (dtd, J = 14.3/7.0/1.2 Hz, 1 H, CH2-CH=CH), 3.75 (s, 3 H, CO2CH3), 3.74–3.83 (m, 2 H, 6-Hax + 4-Hax), 3.88 (ddd, J = 11.4/5.0/1.2 Hz, 1 H, 6-Heq), 5.92 (d broad, J = 15.7 Hz, 1 H, CH=CH-CO2CH3), 7.03 (dt, J = 15.7/7.4 Hz, 1 H, CH=CH-CO2CH3), 7.25–7.40 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.0%, tR = 21.68 min.
![Pharmaceuticals 18 01300 i013 Pharmaceuticals 18 01300 i013]()
The α,β-unsaturated ester (E/Z)-10a (900 mg, 3.25 mmol) was dissolved in methanol abs. (75 mL). Pd/C (10% Pd, 90 mg, 10% m/m) and ammonium formate (1.01 g, 16.25 mmol) were added. After purging with N2, the mixture was heated to reflux for 2 h, then cooled down to rt and filtered through Celite®. The solvent was removed in vacuo. In order to remove excess ammonium formate, the residue was dissolved in water (80 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine and dried (K2CO3). The solvent was removed in vacuo, and the remaining oil was purified by flash column chromatography (Ø 4 cm, n-hexane:ethyl acetate = 9:1, length 18 cm, fraction 30 mL, Rf = 0.12). Colorless oil, yield 833 mg (92%). C16H22O4, Mr = 278.3. MS (EI): m/z [%] = 278 (M, 19), 155 (M-PhCHO-H2O + H, 100), 91 (Ph-CH2, 70). IR (neat): ͠ν [cm−1] = 2952, 2849 (C-H), 1732 (C=O), 750, 698 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.25 (t, J = 7.1 Hz, 3 H, CO2CH2CH3), 1.51–1.91 (m, 6 H, 5-Hax+eq, diox-(CH2)2), 2.35 (t, J = 7.4 Hz, 2 H, CH2CO2Et), 3.82–3.88 (m, 1 H, 4-Hax), 3.96 (td, J = 11.9/2.5 Hz, 1 H, 6-Hax) 4.13 (q, J = 7.1 Hz, 2 H, CO2CH2CH3), 4.27 (dd broad, J = 11.4/4.9 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.30–7.38 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 97.1%, tR = 20.34 min.
![Pharmaceuticals 18 01300 i014 Pharmaceuticals 18 01300 i014]()
The α,β-unsaturated ester (E/Z)-11b (600 mg, 2.17 mmol) was dissolved in methanol abs. (50 mL). Pd/C (10% Pd, 60 mg, 10% m/m) and ammonium formate (808 mg, 13.02 mmol) were added. After purging with N2, the mixture was heated to reflux for 3 h (until the IR-band at 1648 cm−1 was gone), then cooled down to rt and filtered through Celite®. The solvent was removed in vacuo. In order to remove excess ammonium formate, the residue was dissolved in water (60 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (1 × 30 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 4 cm, cyclohexane:ethyl acetate = 9:1, length 17 cm, fraction 30 mL, Rf = 0.20). Colorless oil, yield 580 mg (92%). C17H24O4, Mr = 292.4. MS (EI): m/z [%] = 292 (M, 18), 263 (M-CH2CH3, 18), 81 (100). IR (neat): ͠ν [cm−1] = 2945, 2871 (C-H), 1738 (C=O), 758, 705 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.24–1.29 (m, 1 H, 5-Heq), 1.41–1.50 (m, 1 H, diox-(CH2)2), 1.56–1.78 (m, 3 H, 5-Hax, diox-(CH2)2), 1.73 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 1.85–1.96 (m, 1 H, diox-(CH2)2), 2.31–2.43 (m, 2 H, CH2CO2CH3), 3.61–3.70 (m, 1 H, 4-Hax), 3.68 (s, 3 H, CO2CH3), 3.77 (td, J = 11.6/2.6 Hz, 1 H, 6-Hax), 3.87 (ddd, J = 11.4/5.0/1.3 Hz, 1 H, 6-Heq), 7.27–7.40 (m, 5 H, Ar-H). HPLC (method ACN): purity > 99.9%, tR = 21.67 min.
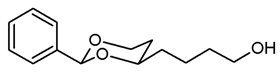
![Pharmaceuticals 18 01300 i015 Pharmaceuticals 18 01300 i015]()
Under N2 atmosphere, a solution of the saturated ester 11a (650 mg, 2.33 mmol) in THF abs. (40 mL) was cooled down to 0 °C. LiBH4 (4 M solution in THF, 2.3 mL, 9.2 mmol) was added slowly. The mixture was stirred at rt for 17 h and then heated to reflux for 2 h. For workup, the mixture was cooled down to rt and 0.1 M HCl (60 mL) was added dropwise. When H2 formation was finished, the mixture was saturated with NaCl and extracted with ethyl acetate (2 × 30 mL). The combined organic layers were washed with saturated aqueous solution of NaHCO3 (30 mL) and with brine (30 mL). After drying (K2CO3), the solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 4 cm, cyclohexane:ethyl acetate = 7:3, length 17 cm, fraction 30 mL, Rf = 0.12). Colorless oil, yield 532 mg (97%). C14H20O3, Mr = 236.3. MS (EI): m/z [%] = 235 (M-H, 17), 219 (M-H2O + H, 27), 105 (PhCO, 73), 91 (PhCH2, 57). IR (neat): ͠ν [cm−1] = 3410 (O-H), 2938, 2860 (C-H), 1103 (C-O), 750, 697 (arom. monosubst.) 1H NMR (CDCl3): δ [ppm] = 1.42–1.74 (m, 7 H, 5-Heq, diox-(CH2)3), 1.82 (tdd, J = 12.5/11.3/5.0 Hz, 1 H, 5-Hax), 3.66 (t, J = 6.3 Hz, 2 H, CH2-OH), 3.81–3.87 (m, 1 H, 4-Hax), 3.96 (td, J = 11.9/2.6 Hz, 1 H, 6-Hax), 4.27 (ddd, J = 11.4/5.0/1.2 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.30–7.38 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). A signal for the OH proton is not seen in the spectrum. HPLC (method ACN): purity 95.2%, tR = 16.90 min.
![Pharmaceuticals 18 01300 i016 Pharmaceuticals 18 01300 i016]()
Under N2 atmosphere, a solution of the saturated ester 11b (500 mg, 1.71 mmol) in THF abs. (25 mL) was cooled down to 0 °C. LiBH4 (4 M solution in THF, 6.84 mL, 6.84 mmol) was added slowly. The mixture was stirred at rt for 17 h and then heated to reflux for 3 h. For workup, the mixture was cooled down to rt and 0.1 M HCl (60 mL) was added dropwise. When H2 formation was finished, the mixture was extracted with ethyl acetate (2 × 30 mL). The combined organic layers were washed with saturated aqueous solution of NaHCO3 (30 mL) and with brine (30 mL). After drying (K2CO3), the solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 4 cm, cyclohexane:ethyl acetate = 7:3, length 17 cm, fraction 30 mL, Rf = 0.19). Colorless solid, mp 49.3 °C, yield 434 mg (96%). C16H24O3, Mr = 264.4. MS (EI): m/z [%] = 265 (M + H, 4), 247 (M + H-H2O, 10), 235 (M-CH2CH3, 18), 113 (C7H13O, 100), 105 (Ph-CO, 45). IR (neat): ͠ν [cm−1] = 3368 (O-H), 2927, 2866 (C-H), 767, 704 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.25–1.30 (m, 1 H, 5-Heq), 1.38–1.51 (m, 2 H, diox-(CH2)3), 1.58–1.70 (m, 5 H, 5-Hax, diox-(CH2)3), 1.74 (q, J = 7.4 Hz, 2 H, diox-CH2-CH3), 3.62–3.67 (m, 1 H, 4-Hax), 3.69 (t, J = 6.2 Hz, 2 H, CH2-OH), 3.77 (td, J = 12.0/2.5 Hz, 1 H, 6-Hax), 3.87 (ddd, J = 11.4/5.1/1.3 Hz, 1 H, 6-Heq), 7.27–7.40 (m, 5 H, Ar-H). A signal for the OH proton is not seen in the spectrum. HPLC (method ACN): purity 99.9%, tR = 19.60 min.
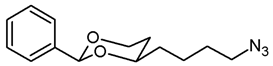
![Pharmaceuticals 18 01300 i017 Pharmaceuticals 18 01300 i017]()
The alcohol 12a (100 mg, 0.42 mmol) was dissolved in dry toluene (molecular sieves 4 Å, 4 mL). Zn(N3)2 · 2 pyridine 80 (97 mg, 0.315 mmol) and PPh3 (220 mg, 0.84 mmol) were added followed by a small amount of toluene (2 mL). Under N2 atmosphere, diisopropyl azodicarboxylate (0.16 mL, 0.84 mmol) was added dropwise and the mixture was stirred at rt for 5 h. The mixture was filtered through Celite®, the solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 3 cm, cyclohexane:ethyl acetate = 19:1, length 18 cm, fraction 10 mL, Rf = 0.18). Colorless oil, yield 74 mg (67%). C14H19N3O2, Mr = 261.3. MS (ESI): m/z [%] = 284 (M + Na, 82), 545 (2 × M + Na, 32). IR (neat): ͠ν [cm−1] = 2944, 2860 (C-H), 2091 (N3), 1105 (C-O), 751, 698 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.42–1.73 (m, 7 H, 5-Heq, diox-(CH2)3), 1.82 (tdd, J = 12.9/11.3/5.0 Hz, 1 H, 5-Hax), 3.29 (t, J = 6.6 Hz, 2 H, CH2-N3), 3.80–3.87 (m, 1 H, 4-Hax), 3.96 (td, J = 12.0/2.7 Hz, 1 H, 6-Hax), 4.27 (ddd, J = 11.4/5.0/1.1 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.30–7.38 (m, 3 H, Ar-H), 7.47–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 96.5%, tR = 21.75 min.
![Pharmaceuticals 18 01300 i018 Pharmaceuticals 18 01300 i018]()
The alcohol 12b (120 mg, 0.45 mmol) was dissolved in dry toluene (molecular sieves 4 Å, 7 mL). Zn(N3)2·2 pyridine 80 (97 mg, 0.315 mmol) and PPh3 (220 mg, 0.84 mmol) were added followed by a small amount of toluene (2 mL). Under N2 atmosphere, diisopropyl azodicarboxylate (0.18 mL, 0.91 mmol) was added dropwise and the mixture was stirred at rt for 5 h. The mixture was filtered through Celite®, the solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 3.5 cm, cyclohexane:ethyl acetate = 19:1, length 20 cm, fraction 20 mL, Rf = 0.19). Colorless oil, yield 76 mg (58%). C16H23N3O2, Mr = 289.4. MS (EM, APCI): m/z = calculated for C16H24N3O2 290.1863, found 290.1933. IR (neat): ͠ν [cm−1] = 2940, 2867 (C-H), 2091 (N3), 58, 705 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.24–1.29 (m, 1 H, 5-Heq), 1.40–1.70 (m, 7 H, 5-Hax, diox-(CH2)3), 1.74 (q, J = 7.4 Hz, 2 H, diox-CH2-CH3), 3.31 (t, J = 6.6 Hz, 2 H, CH2-N3), 3.61–3.68 (m, 1 H, 4-Hax), 3.77 (td, J = 11.5/2.4 Hz, 1 H, 6-Hax), 3.88 (ddd, J = 11.4/5.1/1.4 Hz, 1 H, 6-Heq), 7.26–7.41 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.7%, tR = 23.72 min.
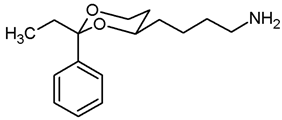
![Pharmaceuticals 18 01300 i019 Pharmaceuticals 18 01300 i019]()
In a 25 mL Schlenk flask, the azide 13a (64 mg, 0.24 mmol) was dissolved in dry ethyl acetate (molecular sieves 4 Å, 10 mL). Pd/C (10% Pd, 7 mg, 11% m/m) was added. The mixture was reacted with H2 at atmospheric pressure (using a rubber balloon). After 2 h, the H2 balloon was totally deflated and refilled with H2. The reaction was continued for additional 3 h. Afterwards, the mixture was filtered through Celite®. To eluate the basic amine from Celite®, it was necessary to wash with a basic eluent (see fc). The filtrate was concentrated in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, NH3 (16 mL) + MeOH (100 mL) + CH2Cl2 (ad 2000 mL), length 18 cm, fraction 10 mL, Rf = 0.06). Colorless oil, yield 47 mg (84%). C14H21NO2, Mr = 235.3. MS (EM, ESI): m/z = calculated for C14H22NO2 236.1645, found 236.1645. IR (neat): ͠ν [cm−1] = 3303, 3034, 2931 (N-H, C-H), 1104 (C-O), 749, 697 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.40–1.73 (m, 9 H, 5-Heq, diox-(CH2)3, NH2), 1.81 (tdd, J = 12.5/11.4/5.0 Hz, 1 H, 5-Hax), 2.71 (t, J = 6.6 Hz, 2 H, CH2-NH2), 3.80–3.86 (m, 1 H, 4-Hax), 3.95 (td, J = 12.0/2.5 Hz, 1 H, 6-Hax), 4.27 (ddd, J = 11.3/4.9/1.0 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.29–7.38 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 99.6%, tR = 12.77 min.
![Pharmaceuticals 18 01300 i020 Pharmaceuticals 18 01300 i020]()
In a 25 mL Schlenk flask the azide 13b (65 mg, 0.22 mmol) was dissolved in dry ethyl acetate (molecular sieves 4 Å, 10 mL). Pd/C (10% Pd, 6.5 mg, 10% m/m) was added. The mixture was reacted with H2 at atmospheric pressure (using a rubber balloon). After 2 h and after 3 h, the H2 balloon was totally deflated and refilled with H2. The reaction was stopped after 4.5 h by filtration through Celite®. To eluate the basic amine from Celite®, it was necessary to wash with a basic eluent (see fc). The filtrate was concentrated in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, NH3 (16 mL) + MeOH (100 mL) + CH2Cl2 (ad 2000 mL), length 18 cm, fraction 10 mL, Rf = 0.11). Colorless solid, mp 83.0 °C, yield 37 mg (63%). C16H25NO2, Mr = 263.4. MS (EM, ESI): m/z = calculated for C16H24NO2 264.1958, found 264.1963. IR (neat): ͠ν [cm−1] = 2972, 2935, 2864 (C-H), 760, 706 (arom. monosubst.) 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.24–1.30 (m, 1 H, 5-Heq), 1.35–1.73 (m, 9 H, 5-Hax, diox-(CH2)3, NH2), 1.74 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 2.73 (t, J = 6.7 Hz, 2 H, CH2-NH2), 3.59–3.68 (m, 1 H, 4-Hax), 3.76 (td, J = 11.5/2.5 Hz, 1 H, 6-Hax), 3.87 (ddd, J = 11.4/5.2/1.4 Hz, 1 H, 6-Hax), 7.27–7.41 (m, 5 H, Ar-H). HPLC (method ACN): purity 98.3%, tR = 15.40 min.
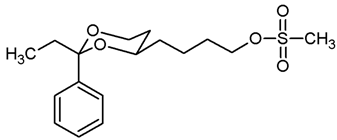
![Pharmaceuticals 18 01300 i021 Pharmaceuticals 18 01300 i021]()
Under N2 atmosphere, the alcohol 12a (451 mg, 1.91 mmol) was dissolved in CH2Cl2 abs. (30 mL) and treated with NEt3 (0.79 mL, 5.73 mmol). The mixture was cooled down to 0 °C. At this temperature, methanesulfonyl chloride (0.22 mL, 2.87 mmol) was added dropwise and the mixture was stirred at rt for 4 h. Then, CH2Cl2 (30 mL) was added, and the mixture was washed with 0.5 M NaOH (2 × 25 mL) and with saturated aqueous solution of NH4Cl (1 × 25 mL). The organic layer was dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 4 cm, cyclohexane:ethyl acetate = 7:3, length 18 cm, fraction 30 mL, Rf = 0.21). Colorless oil, yield 578 mg (96%). C15H22O5S, Mr = 314.4. MS (EI): m/z [%] = 314 (M, 41), 105 (PhCO, 37), 91 (PhCH2, 35). IR (neat): ͠ν [cm−1] = 2924, 2856 (C-H), 1350, 1170 (RO-SO2R), 1104 (C-O), 752, 699 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.50–1.87 (m, 8 H, 5-Hax+eq, diox-(CH2)3), 2.98 (s, 3 H, SO2CH3), 3.81–3.87 (m, 1 H, 4-Hax), 3.96 (td, J = 12.0/2.6 Hz, 1 H, 6-Hax), 4.24 (t, J = 6.5 Hz, 2 H, CH2-OSO2CH3), 4.27 (ddd, J = 11.4/5.0/1.2 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.30–7.38 (m, 3 H, Ar-H), 7.47–7.49 (m, 2 H, Ar-H). HPLC (method ACN): purity 97.4%, tR = 19.72 min.
![Pharmaceuticals 18 01300 i022 Pharmaceuticals 18 01300 i022]()
Under N2 atmosphere, the alcohol 12b (250 mg, 0.95 mmol) was dissolved in CH2Cl2 abs. (30 mL) and treated with NEt3 (0.40 mL, 2.85 mmol). The mixture was cooled down to 0 °C. At this temperature, methanesulfonyl chloride (0.11 mL, 1.43 mmol) was added dropwise and the mixture was stirred at rt for 4 h. Then, CH2Cl2 (30 mL) was added, and the mixture was washed with 0.5 M NaOH (2 × 25 mL) and with saturated aqueous solution of NH4Cl (1 × 25 mL). The organic layer was dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 3 cm, cyclohexane:ethyl acetate = 8:2, length 17 cm, fraction 20 mL, Rf = 0.11). Colorless oil, yield 314 mg (97%). C17H26O5S, Mr = 342.5. MS (EI): m/z [%] = 343 (M + H, 1), 313 (M-CH2CH3, 17), 105 (PhCO, 28), 95 (100). IR (neat): ͠ν [cm−1] = 2941, 2871 (C-H), 1352, 1171 (RO-SO2R), 759, 705 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.24–1.29 (m, 1 H, 5-Heq), 1.42–1.84 (m, 7 H, 5-Hax, diox-(CH2)3), 1.73 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 3.02 (s, 3 H, SO2CH3), 3.62–3.68 (m, 1 H, 4-Hax), 3.77 (td, J = 11.5/2.5 Hz, 1 H, 6-Hax), 3.88 (ddd, J = 11.4/5.1/1.4 Hz, 1 H, 6-Heq), 4.27 (t, J = 6.5 Hz, 2 H, CH2-O-SO2CH3), 7.27–7.41 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.9%, tR = 21.43 min.
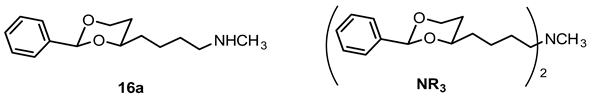
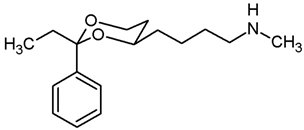
N-Methyl-4-(cis-2-phenyl-1,3-dioxan-4-yl)butan-1-amine (16a) and N-methyl-N,N-bis[4-(cis-2-phenyl-1,3-dioxan-4-yl)butyl]amine (NR3).
Mesylate 15a (86 mg, 0.27 mmol) was dissolved in a solution of methylamine in ethanol (33% m/m, 8 mL). The mixture was heated to reflux for 3 h, using a reflux apparatus equipped with a rubber balloon to avoid loss of gaseous amine. Ethanol was removed under reduced pressure. The residue was dissolved in CH2Cl2 (20 mL) and washed with 1 M NaOH (2 × 10 mL). The organic layer was dried (K2CO3) and the solvent was removed in vacuo, giving a pale yellow oil which was purified by flash column chromatography (Ø 2 cm, gradient cyclohexane:ethyl acetate + 1% N,N-dimethylethanamine → ethyl acetate:MeOH 5:1 + 2.5% N,N-dimethylethanamine, length 17 cm, fraction 10 mL). The first eluent gave the dialkylated tertiary amine NR3 (Rf = 0.26), the second eluent gave the monoalkylated secondary amine 16a (Rf = 0.11).
Secondary amine 16a: Colorless oil, yield 48 mg (70%). C15H23NO2, Mr = 249.4. MS (EM, APCI): m/z = calculated for C15H24NO2 250.1802, found 250.1790. IR (neat): ͠ν [cm−1] = 2929, 2854, 2790 (C-H), 1103 (C-O), 750, 697 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.37–1.75 (m, 7 H, 5-Heq, diox-(CH2)3)), 1.75–1.85 (m, 1 H, 5-Hax), 2.01 (s broad, 1 H, NH), 2.44 (s, 3 H, N-CH3), 2.63 (t, J = 6.9 Hz, 2 H, CH2-N-CH3), 3.80–3.86 (m, 1 H, 4-Hax), 3.95 (td, J = 11.9/2.5 Hz, 1 H, 6-Hax), 4.26 (dd broad, J = 11.3/4.3 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.29–7.38 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 97.5%, tR = 12.54 min. The substance was sensitive to heat and air.
Tertiary amine NR3: Colorless oil, yield 15 mg (24%). C29H41NO4, Mr = 467.6. MS (EM, ESI): m/z = calculated for C29H42NO4 468.3108, found 468.3110. IR (neat): ͠ν [cm−1] = 2941, 2854, 2786 (C-H), 1103 (C-O), 749, 696 (arom. monosubst.) 1H NMR (CDCl3): δ [ppm] = 1.36–1.74 (m, 14 H, 2 × 5-Heq, 2 × diox-(CH2)3), 1.80 (tdd, J = 12.7/11.3/5.0 Hz, 2 H, 2 × 5-Hax), 2.21 (s, 3 H, N-CH3), 2.33 (t, J = 7.0 Hz, 4 H, CH2-N-CH2), 3.79–3.84 (m, 2 H, 2 × 4-Hax), 3.95 (td, J = 12.0/2.6 Hz, 2 H, 2 × 6-Hax), 4.26 (dd broad, J = 11.3/4.9 Hz, 2 H, 2 × 6-Heq), 5.49 (s, 2 H, 2 × 2-Hax), 7.29–7.38 (m, 6 H, Ar-H), 7.48–7.50 (m, 4 H, Ar-H). HPLC (method ACN): purity 98.4%, tR = 20.15 min.
![Pharmaceuticals 18 01300 i024 Pharmaceuticals 18 01300 i024]()
Mesylate 15b (58 mg, 0.17 mmol) was dissolved in a solution of methylamine in ethanol (33% m/m, 5 mL). The mixture was heated to reflux for 5 h, using a reflux apparatus equipped with a rubber balloon to avoid loss of gaseous amine. Ethanol was removed under reduced pressure. The residue was dissolved in CH2Cl2 (10 mL) and washed with 1 M NaOH (2 × 10 mL). The organic layer was dried (K2CO3) and the solvent was removed in vacuo, giving a pale yellow oil which was purified by flash column chromatography (Ø 2 cm, NH3 (16 mL) + MeOH (100 mL) + CH2Cl2 (ad 2000 mL), length 17 cm, fraction 10 mL, Rf = 0.11). Colorless oil, yield 35 mg (74%). C17H27NO2, Mr = 277.4. MS (EM, ESI): m/z = calculated for C17H28NO2 278.2115, found 278.2112. IR (neat): ͠ν [cm−1] = 2928, 2863, 2788 (C-H), 757, 704 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.25–1.29 (m, 1 H, 5-Heq), 1.36–1.73 (m, 8 H, 5-Hax, diox-(CH2)3, NH), 1.74 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 2.46 (s, 3 H, N-CH3), 2.62 (t, J = 7.0 Hz, 2 H, CH2-N-CH3), 3.61–3.67 (m, 1 H, 4-Hax), 3.73–3.80 (m, 1 H, 6-Hax), 3.87 (ddd, J = 11.4/5.0/1.4 Hz, 1 H, 6-Heq), 7.26–7.40 (m, 5 H, Ar-H). HPLC (method ACN): purity 98.9%, tR = 16.33 min.
![Pharmaceuticals 18 01300 i025 Pharmaceuticals 18 01300 i025]()
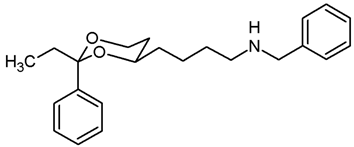
Mesylate 15a (107 mg, 0.34 mmol) was dissolved in dry acetonitrile (molecular sieves 3 Å, 10 mL). K2CO3 (140 mg, 1.02 mmol) and freshly distilled benzylamine (48 μL, 0.44 mmol) were added, and the mixture was heated to reflux for 16 h. To complete the reaction, additional benzylamine (24 μL, 0.22 mmol) was added, and the mixture was heated to reflux for another 2 h. For workup, the solvent was removed in vacuo, and the residue was transferred into a separatory funnel with 0.1 M HCl and a small amount of ethyl acetate. The pH value was adjusted to 3–4 by adding a few drops of 0.5 M HCl. The mixture was quickly extracted with ethyl acetate. The organic layer contained small amounts of educt 15a but no product 17a (TLC-control) and was discarded. The aqueous layer was alkalized with 2 M NaOH and again extracted with ethyl acetate (3 × 10 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 7:3 + 1% N,N-dimethylethanamine, length 8 cm, fraction 8 mL, Rf = 0.09). Colorless oil, yield 36 mg (32%). C21H27NO2, Mr = 325.4. MS (EI): m/z [%] = 326 (M + H, 2), 248 (M-Ph, 4), 106 (PhCHO, 64), 91 (PHCH2, 100). IR (neat): ͠ν [cm−1] = 3030, 2928, 2853 (C-H), 1105 (C-O), 746, 696 (arom. monosubst.) 1H NMR (CDCl3): δ [ppm] = 1.38–1.71 (m, 7 H, 5-Heq, diox-(CH2)3), 1.80 (tdd, J = 12.5/11.3/5.0 Hz, 1 H, 5-Hax), 2.65 (t, J = 6.9 Hz, 2 H, CH2-NHBz), 3.79 (s, 2 H, Ph-CH2-N), 3.80–3.85 (m, 1 H, 4-Hax), 3.95 (td, J = 12.9/2.6 Hz, 1 H, 6-Hax), 4.26 (ddd, J = 11.3/4.9/1.0 Hz, 1 H, 6-Heq), 5.49 (s, 1 H, 2-Hax), 7.22–7.38 (m, 8 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). A signal for the NH proton is not seen in the spectrum. HPLC (method ACN): purity 98.2%, tR = 17.69 min.
![Pharmaceuticals 18 01300 i026 Pharmaceuticals 18 01300 i026]()
Mesylate 15b (100 mg, 0.29 mmol) was dissolved in dry acetonitrile (molecular sieves 3 Å, 10 mL). K2CO3 (120 mg, 0.87 mmol) and freshly distilled benzylamine (48 μL, 0.44 mmol) were added, and the mixture was heated to reflux for 8 h. Additional benzylamine (48 μL, 0.44 mmol) was added, and the mixture was heated to reflux for another 17 h overnight. For workup, the solvent was removed in vacuo, and the residue was transferred into a separatory funnel with water and a small amount of ethyl acetate. The pH value was adjusted to 9–10 with 1 M NaOH and the mixture was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2.5 cm, cyclohexane:ethyl acetate = 8:2 + 0.5% N,N-dimethylethanamine, length 18 cm, fraction 10 mL, Rf = 0.15). Colorless oil, yield 84 mg (82%). C23H31NO2, Mr = 353.5. MS (EM, ESI): m/z = calculated for C23H32NO2 354.2428, found 354.2424. IR (neat): ͠ν [cm−1] = 3026, 2928, 2862 (C-H), 757, 699 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.23–1.28 (m, 1 H, 5-Heq), 1.37–1.71 (m, 8 H, 5-Hax, diox-(CH2)3, NH), 1.73 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 2.66 (t, J = 7.0 Hz, 2 H, CH2-NHBz), 3.59–3.66 (m, 1 H, 4-Hax), 3.73–3.79 (m, 1 H, 6-Hax), 3.81 (s, 2 H, N-CH2-Ph), 3.87 (ddd, J = 11.4/5.1/1.4 Hz, 1 H, 6-Heq), 7.23–7.39 (m, 10 H, Ar-H). HPLC (method ACN): purity 99.2%, tR = 19.16 min.
![Pharmaceuticals 18 01300 i027 Pharmaceuticals 18 01300 i027]()
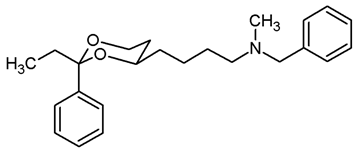
Mesylate 15a (80 mg, 0.25 mmol) was dissolved in dry acetonitrile (molecular sieves 3 Å, 10 mL). K2CO3 (104 mg, 0.75 mmol) and N-methylbenzylamine (48 μL, 0.44 mmol) were added, and the mixture was heated to reflux for 7 h. To complete the transformation, additional N-methylbenzylamine (48 μL, 0.44 mmol) was added, and the mixture was heated to reflux for another 16 h. For workup, the solvent was removed in vacuo, and the residue was transferred into a separatory funnel with water and ethyl acetate. The pH value was adjusted to 9–10 with 2 M NaOH. The mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were dried (K2CO3), the solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, gradient CH2Cl2:MeOH = 97.5:2.5 → CH2Cl2:MeOH = 95:5, length 19 cm, fraction 10 mL, Rf = 0.30 (CH2Cl2:MeOH = 95:5)). Colorless oil, yield 68 mg (80%). C22H29NO2, Mr = 339.5. MS (EM, APCI): m/z = calculated for C22H30NO2 340.2271, found 340.2313. IR (neat): ͠ν [cm−1] = 2941, 2842, 2785 (C-H), 740, 696 (arom. monosubst). 1H NMR (CDCl3): δ [ppm] = 1.36–1.72 (m, 7 H, 5-Heq, diox-(CH2)3), 1.80 (tdd, J = 12.7/11.3/5.0 Hz, 1 H, 5-Hax), 2.20 (s, 3 H, N-CH3), 2.39 (t, J = 7.0 Hz, 2 H, diox-(CH2)3-CH2-N), 3.49 (s, 2 H, N-CH2-Ph), 3.79–3.85 (m, 1 H, 4-Hax), 3.95 (td, J = 12.0/2.6 Hz, 1 H, 6-Hax), 4.27 (ddd, J = 11.3/4.9/0.9 Hz, 1 H, 6-Heq), 5.50 (s, 1 H, 2-Hax), 7.22–7.38 (m, 8 H, Ar-H), 7.49–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 98.3%, tR = 18.33 min.
![Pharmaceuticals 18 01300 i028 Pharmaceuticals 18 01300 i028]()
Benzylamine 17b (50 mg, 0.14 mmol) was dissolved in CH2Cl2 abs. (7 mL). A solution of formalin (37%, stab. with 10–15% MeOH, 210 μL, 2.8 mmol) and NaBH(OAc)3 (95%, 62.5 mg, 0.28 mmol) were added, and the mixture was stirred at rt overnight. CH2Cl2 (10 mL) and water (15 mL) were added, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine (1 × 15 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, CH2Cl2:MeOH = 96:4, length 20 cm, fraction 10 mL, Rf = 0.14). Colorless resin, yield 47 mg (91%). C24H33NO2, Mr = 367.5. MS (EM, APCI): m/z = calculated for C24H34NO2 368.2584, found 368.2625. IR (neat): ͠ν [cm−1] = 2940, 2865, 2786 (C-H), 757, 699 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.26 (d broad, J = 12.8 Hz, 1 H, 5-Heq), 1.50–1.69 (m, 7 H, 5-Hax, diox-(CH2)3), 1.74 (q, J = 7.4 Hz, 2 H, diox-CH2-CH3), 2.23 (s, 3 H, N-CH3), 2.40–2.46 (m, 2 H, diox-(CH2)3-CH2-N), 3.53 (s broad, 2 H, N-CH2-Ph), 3.59–3.66 (m, 1 H, 4-Hax), 3.76 (td, J = 12.0/2.5 Hz, 1 H, 6-Hax), 3.87 (dd broad, J = 11.3/4.9 Hz, 1 H, 6-Heq), 7.23–7.40 (m, 10 H, Ar-H). HPLC (method ACN): purity 99.5%, tR = 20.01 min.
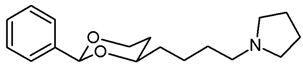
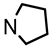
![Pharmaceuticals 18 01300 i029 Pharmaceuticals 18 01300 i029]()
Mesylate 15a (80 mg, 0.25 mmol) was dissolved in dry acetonitrile (molecular sieves 3 Å, 10 mL). K2CO3 (104 mg, 0.75 mL) and freshly distilled pyrrolidine (31 μL, 0.375 mmol) were added, and the mixture was heated to reflux for 14.5 h. For workup, the solvent was removed in vacuo, and the residue was transferred into a separatory funnel with water and a small amount of ethyl acetate. The pH value was adjusted to 9-10 with 2 M NaOH. The mixture was extracted with ethyl acetate (3 × 20 mL) and with CH2Cl2 (3 × 20 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, NH3 (16 mL) + MeOH (100 mL) + CH2Cl2 (ad 2000 mL), length 18 cm, fraction 8 mL, Rf = 0.12). The eluent was prepared as follows: In a 2 L volumetric flask, MeOH (100 mL) and NH3 (16 mL) were mixed, CH2Cl2 (1.5 L) was added, and the mixture was gently swirled. The mixture cooled down by itself. It was warmed up to rt and then diluted with CH2Cl2 to a total volume of 2 L. Colorless solid, mp 50–51 °C, yield 53 mg (81%). C18H27NO2, Mr = 289.4. MS (EI): m/z [%] = 288 (M-1, 10), 84 (CH2=N(CH2-CH2)2, 100).
IR (neat): ͠ν [cm−1] = 2925, 2859, 2786 (C-H), 1105 (C-O), 762, 701 (arom. monosubst.).
1H NMR (CDCl3): δ [ppm] = 1.38–1.85 (m, 12 H, 5-Hax+eq, diox-(CH2)3, N(CH2-CH2)2), 2.47 (t, J = 7.5 Hz, 2 H, CH2-Npyrrolidine), 2.50–2.55 (m, 4 H, N-(CH2-CH2)2), 3.80–3.86 (m, 1 H, 4-Hax), 3.95 (td, J = 12.2/2.6 Hz, 1 H, 6-Hax), 4.24 (ddd, J = 11.3/4.9/1.0 Hz, 1 H, 6-Heq), 5.49 (s, 1 H, 2-Hax), 7.29–7.38 (m, 3 H, Ar-H), 7.48–7.50 (m, 2 H, Ar-H). HPLC (method ACN): purity 98.0%, tR = 15.07 min.
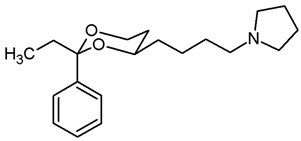
![Pharmaceuticals 18 01300 i030 Pharmaceuticals 18 01300 i030]()
Mesylate 15b (100 mg, 0.29 mmol) was dissolved in dry acetonitrile (molecular sieves 3 Å, 10 mL). K2CO3 (120 mg, 0.87 mL) and freshly distilled pyrrolidine (46 μL, 0.435 mmol) were added, and the mixture was heated to reflux for 17 h. For workup, the solvent was removed in vacuo, and the residue was transferred into a separatory funnel with water and a small amount of ethyl acetate. The pH value was adjusted to 9–10 with 2 M NaOH. The mixture was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine (1 × 20 mL) and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 8:2 + 0.5% N,N-dimethylethanamine, length 18 cm, fraction 10 mL, Rf = 0.11 (cyclohexane:ethyl acetate = 7:3 + 0.5% N,N-dimethylethanamine)). Colorless oil, yield 86 mg (94%). C20H31NO2, Mr = 317.5. MS (EI): m/z [%] = 318 (M + H, 34), 84 (CH2=N(CH2-CH2)2, 100). IR (neat): ͠ν [cm−1] = 2930, 2866, 2784 (C-H), 757, 704 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.25–1.29 (m, 1H, 5-Heq), 1.33–1.83 (m, 11 H, 5-Hax, diox-(CH2)3, N(CH2-CH2)2), 1.73 (q, J = 7.5 Hz, 2 H, diox-CH2-CH3), 2.45–2.49 (m, 2 H, CH2-Npyrrolidine), 2.50–2.55 (m, 4 H, N(CH2-CH2)2), 3.60–3.67 (m, 1 H, 4-Hax), 3.73–3.79 (m, 1 H, 6-Hax), 3.87 (ddd, J = 11.3/5.0/1.3 Hz, 1 H, 6-Heq), 7.26–7.40 (m, 5 H, Ar-H). HPLC (method ACN): purity 99.7%, tR = 17.47 min.
![Pharmaceuticals 18 01300 i031 Pharmaceuticals 18 01300 i031]()
Under N2 atmosphere, iPr2NH (1.12 mL, 8 mmol) was dissolved in THF abs. (12 mL) and cooled to −78 °C. A solution of n-butyllithium (1.6 M in n-hexane, 5 mL, 8 mmol) was added dropwise and the mixture was stirred for 15 min at −78 °C. Acetophenone (1.21 mL, 10.4 mmol), dissolved in THF abs. (6 mL), was added dropwise. 5 min later, a solution of the aldehyde 9a (827 mg, 4 mmol) in THF abs. (6 mL) was added, and the reaction mixture was stirred for 45 min at −78 °C. Saturated aqueous solution of NH4Cl (25 mL) was added, and the cooling bath was removed, allowing the mixture to warm up. At rt, the reaction mixture was diluted with water (30 mL) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (1 × 30 mL), dried (K2CO3) and the solvent was removed in vacuo. The residue was purified by flash column chromatography (Ø 7 cm, cyclohexane:ethyl acetate = 7:3, length 20 cm, fraction 65 mL, Rf = 0.15) to give a solid, which was recrystallized from cyclohexane with a few drops of ethyl acetate. Colorless crystals, mp 82–83 °C, yield 905 mg (69%). C20H22O4 (Mr = 326.4). MS (ESI): m/z [%] = 675 (2 × M + Na, 100). IR (neat): ͠ν [cm−1] = 3492 (O-H) 2923, 2858 (C-H), 1676 (C=O), 1099 (C-O), 749, 689 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.56 (dtd, J = 13.3/2.4/1.3 Hz, 0.6H, 5-Heq), 1.62 (dtd, J = 13.3/2.3/1.4 Hz, 0.4H, 5-Heq), 1.81–2.01 (m, 3H, 5-Hax and diox-CH2-CH-OH), 3.09 (dd, J = 17.7/8.8 Hz, 0.6H, CH2-C=O), 3.15 (dd, J = 17.3/4.5 Hz, 0.4H, CH2-C=O), 3.25 (dd, J = 17.7/2.9 Hz, 0.6H, CH2-C=O), 3.26 (dd, J = 17.3/7.7 Hz, 0.4H, CH2-C=O), 3.56–3.57 (m, 1H, CHOH), 4.01 (td, J = 11.8/2.5 Hz, 1H, 6-Hax), 4.20–4.20 (m, 1H, CHOH), 4.29 (ddd, J = 11.4/5.0/1.1 Hz, 1H, 6-Heq), 4.49–4.60 (m, 1 H, 4-Hax), 5.57 (s, 1 H, 2-Hax), 7.32–7.60 (m, 8 H, Ar-H), 7.92–7.96 (m, 2 H, Ar-H). The ratio of diastereomer is 60: 40. HPLC (method ACN): purity 99.6%, tR = 19.87 min.
![Pharmaceuticals 18 01300 i032 Pharmaceuticals 18 01300 i032]()
Under N2 atmosphere, iPr2NH (2.05 mL, 14.52 mmol) was dissolved in THF abs. (22 mL) and cooled to −78 °C. A solution of n-butyllithium (1.6 M in n-hexane, 9.10 mL, 14.52 mmol) was added dropwise and the mixture was stirred for 15 min at −78 °C. Acetophenone (2.20 mL, 18.88 mmol), dissolved in THF abs. (11 mL), was added dropwise. 5 min later, a solution of the aldehyde 9b (1.70 g, 7.26 mmol) in THF abs. (11 mL) was added, and the reaction mixture was stirred for 80 min at −78 °C. Saturated aqueous solution of NH4Cl (50 mL) was added, and the cooling bath was removed, allowing the mixture to warm up. At rt, the reaction mixture was diluted with water (enough to dissolve all solids) and extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (1 × 30 mL), dried (K2CO3) and the solvent was removed in vacuo. Purification by flash column chromatography (Ø 6 cm, cyclohexane:ethyl acetate = 9:1, length 17 cm, fraction 65 mL, Rf = 0.09) gave a mixture of two diastereomers. Colorless solid, mp 122–129 °C, yield 2.16 g (84%). One of the diastereomers was isolated by recrystallisation from cyclohexane:ethyl acetate 95:5. Colorless crystals, mp 132 °C, yield 61% related to the fc isolated product. C22H26O4, Mr = 354.4. MS (EI): m/z [%] = 377 (M + Na, 100). IR (neat): ͠ν [cm−1] = 3530 (O-H), 3060, 2956, 2882 (C-H), 1669 (C=O), 752, 688 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 × 0.7 H, diox-CH2-CH3), 0.80 (t, J = 7.5 Hz, 3 × 0.7 H, diox-CH2-CH3), 1.26–1.39 (m, 1 H, 5-Heq), 1.71–1.89 (m, 5 H, diox-CH2-CH3, 5-Hax, diox-CH2-COH), 3.12 (dd, J = 17.0/5.3 Hz, 0.3 H, CH2-CO), 3.15 (dd, J = 17.7/8.3 Hz, 0.7 H, CH2-CO), 3.26 (dd, J = 17.6/3.5 Hz, 0.7 H, CH2-CO), 3.29 (dd, J = 17.0/7.0 Hz, 0.3 Hz, CH2-CO), 3.58–3.59 (m, 1 H, O-H), 3.77–3.92 (m, 2 H, 6-Hax+eq), 4.03–4.13 (m, 1 H, 4-Hax), 4.53–4.62 (m, 0.3 H, CH-OH), 4.64–4.73 (m, 0.7 H, CH-OH), 7.28–7.63 (m, 8 H, Ar-H), 7.97–8.00 (m, 2 H, Ar-H). The ratio of diastereomers is 70:30. HPLC (method ACN): purity 95.7%, tR = 21.00 min.
(2Z)-1-Phenyl-4-(cis-2-phenyl-1,3-dioxan-4-yl)but-2-en-1-one ((Z)-21a) and(2E)-1-Phenyl-4-(cis-2-phenyl-1,3-dioxan-4-yl)but-2-en-1-one ((E)-21a) and (3E)-1-Phenyl-4-(cis-2-phenyl-1,3-dioxan-4-yl)but-3-en-1-one (A).
Under N2 atmosphere, the β-hydroxyketone 20a (600 mg, 1.84 mmol) was dissolved in CH2Cl2 abs. (9 mL) and treated with NEt3 (1.54 mL, 11.04 mmol). The mixture was cooled to 0 °C. A solution of methanesulfonyl chloride (280 μL, 3.68 mmol) in CH2Cl2 abs. (1 mL) was added dropwise and the reaction mixture was stirred for 2 h at 0 °C. Water (10 mL) was added, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with 1 M HCl (2 × 20 mL) and with saturated aqueous solution of NaHCO3 (2 × 20 mL) and were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 4 cm, cyclohexane:ethyl acetate = 9:1, length 18 cm, fraction 30 mL).
(Z)-21a (Rf = 0.15): Colorless oil, yield 27 mg (2.3%). C20H20O3, Mr = 308.4. 1H NMR (CDCl3): δ [ppm] = 1.56–1.62 (m, 1 H, 5-Heq), 1.92 (qd, J = 12.5/5.0 Hz, m, 1 H, 5-Hax), 2.87–2.95 (m, 1 H, CH2-CH=CH), 3.06 (dddd, J = 15.8/7.4/4.3/1.7 Hz, 1 H, CH2-CH=CH), 3.98 (td, J = 12.0/2.7 Hz, 1 H, 6-Hax), 4.01–4.08 (m, 1 H, 4-Hax), 4.28 (ddd, J = 11.5/4.1/1.0 Hz, 1 H, 6-Heq), 5.54 (s, 1 H, 2-Hax), 6.57 (dt, J = 11.7/7.2 Hz, 1 H, CH=CH-CO), 6.95 (dt, 11.7/1.6 Hz, 1 H, CH=CH-C=O), 7.33–7.58 (m, 8 H, Ar-H), 7.94–7.96 (m, 2 H, Ar-H).
(E)-21a (Rf = 0.08): Colorless oil, yield 474 mg (84%). C20H20O3, Mr = 308.4. MS (ESI): m/z [%] = 331 (M + Na, 27), 639 (2 × M + Na, 100), 655 (2 × M + K, 76). IR (neat): ν [cm−1] = 3062, 2921, 2853 (C-H), 1669 (C=O), 1620 (C=C), 1100 (C-O), 749, 594 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.61 (dtd, J = 13.3/2.5/1.4 Hz, 1 H, 5-Heq), 1.90 (dddd, J = 13.2/12.4/11.3/5.1 Hz, 1 H, 5-Hax), 2.59 (dddd, J = 14.9/6.8/5.6/0.9 Hz, 1 H, CH2-CH=CH), 2.72 (dtd, J = 15.0/6.9/1.2 Hz, 1 H, CH2-CH=CH), 3.97 (td, J = 12.0/2.6 Hz, 1 H, 6-Hax), 4.03–4.09 (m, 1 H, 4-Hax), 4.30 (ddd, J = 11.5/5.0/1.3 Hz, 1 H, 6-Heq), 5.52 (s, 1 H, 2-Hax), 6.91 (dt, J = 15.5/1.0, 1 H, CH=CH-CO), 7.10 (dt, J = 15.4/7.1 Hz, 1 H, CH=CH-CO), 7.31–7.59 (m, 8 H, Ar-H), 7.92–7.94 (m, 2 H, Ar-H). HPLC (method ACN): purity 96.4%, tR = 21.63 min.
A (Rf = 0.12): Colorless oil, yield 29 mg (2.6%). C20H20O3, Mr = 308.4. 1H NMR (CDCl3): δ [ppm] = 1.63 (dtd, J = 13.3/2.5/1.5 Hz, 1 H, 5-Heq), 1.97 (dddd, J = 13.1/12.4/11.4/5.0 Hz, 1 H, 5-Hax), 3.76–3.79 (m, 2 H, CH2-CO), 4.00 (td, J = 12.0/2.5 Hz, 1 H, 6-Hax), 4.29 (ddd, J = 11.3/4.9/1.1 Hz, 1 H, 6-Heq), 4.40–4.45 (m, 1 H, 4-Hax), 5.57 (s, 1 H, 2-Hax), 5.76 (ddt, J = 15.7/6.1/3.1 Hz, 1 H, CH=CH-CH2-CO), 6.08 (dtd, J = 15.5/6.9/1.1 Hz, 1 H, CH=CH-CH2-CO), 7.30–7.60 (m, 8 H, Ar-H), 7.95–7.97 (m, 2 H, Ar-H).
![Pharmaceuticals 18 01300 i034 Pharmaceuticals 18 01300 i034]()
Under N2 atmosphere, the β-hydroxyketone 20b (1.776 g, 5.0 mmol) was dissolved in CH2Cl2 abs. (20 mL) and treated with NEt3 (4.17 mL, 30.1 mmol). The mixture was cooled to 0 °C. A solution of methanesulfonyl chloride (0.78 mL, 10.0 mmol) in CH2Cl2 abs. (2 mL) was added dropwise and the reaction mixture was stirred for 2.5 h at 0 °C. Water (30 mL) was added, and the organic layer was separated. The aqueous layer was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with 1 M HCl (2 × 30 mL) and with saturated aqueous solution of NaHCO3 (2 × 30 mL) and were dried (K2CO3). The solvent was removed in vacuo. Purification by flash column chromatography (Ø 5.5 cm, cyclohexane:ethyl acetate = 9.5:0.5, length 17 cm, fraction 65 mL) gave a mixture of (Z)-21b (Rf = 0.22) and (E)-21b (Rf = 0.15). Colorless oil, yield 1.567 g (93%). C22H24O3, Mr = 336.4. MS (ESI): m/z [%] = 337 (M + H, 45), 359 (M + Na, 100). IR (neat): ͠ν [cm−1] = 3059, 2928, 2875 (C-H), 1671 (C=O), 1622 (C=C), 758, 695 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.81 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.33–1.37 (m, 1 H, 5-Heq), 1.75 (q, J = 7.4 Hz, 2 H, diox-CH2-CH3), 1.72–1.83 (m, 1 H, 5-Hax), 3.45–2.52 (m, 0.87 H, CH2-CH=CH), 2.61 (dtd, J = 14.3/7.2/1.1 Hz, 0.87 H, CH2-CH=CH), 2.97–2.87 (m, 0.13 H, CH2-CH=CH), 2.90–2.97 (m, 0.13 H, CH2-CH=CH), 3.80 (td, J = 11.6/2.5 Hz, 1 H, 6-Hax), 3.84–3.90 (m, 1 H, 4-Hax), 3.91 (ddd, J = 11.5/5.0/1.4 Hz, 1 H, 6-Heq), 6.61 (dt, 11.7/7.2 Hz, 0.13 H, CH=CH-CO), 6.96 (dt, J = 15.5/1.1 Hz, 0.87 H, CH=CH-CO) 6.94–7.00 (m, 0.13 H, CH=CH-CO), 7.10 (dt, J = 15.3/7.2 Hz, 0.87 H, CH=CH-CO), 7.27–7.60 (m, 8 H, Ar-H), 7.94–7.97 (m, 2 H, Ar-H). The 1H NMR spectrum shows diastereomers (E)-21b:(Z)-21b in the ratio 87:13. HPLC (method ACN): 86.5%, tR = 23.3 min ((E)-21b), 11.5%, tR = 23.6 min ((Z)-21b).
![Pharmaceuticals 18 01300 i035 Pharmaceuticals 18 01300 i035]()
21a (250 mg, 0.81 mmol) was dissolved in methanol abs. (25 mL). Pd/C (10% Pd, 25 mg, 10% m/m) and ammonium formate (101 mg, 1.62 mmol) were added. After purging with N2, the mixture was heated to reflux for 2 h, then cooled down to rt and filtered through Celite®. The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 3 cm, cyclohexane:ethyl acetate = 9:1, length 18 cm, fraction 20 mL, Rf = 0.33 (cyclohexane:ethyl acetate = 8:2)). Colorless solid, mp 68.3 °C, yield 206 mg (82%). C20H22O3, Mr = 310.4. MS (ESI): m/z [%] = 643 (2 × M + Na, 100). IR (neat): ͠ν [cm−1] = 2916, 2855 (C-H), 1672 (C=O), 1093 (C-O), 756, 691 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.53–1.58 (m, 1 H, 5-Heq), 1.62–2.02 (m, 5 H, diox-CH2-CH2, 5-Hax), 3.04 (t, J = 7.2 Hz, 2 H, CH2-CO-Ph), 3.87–3.93 (m, 1 H, 4-Hax), 3.97 (td, J = 11.9/2.6 Hz, 1 H, 6-Hax), 4.27 (ddd, J = 11.4/4.9/1.1 Hz, 1 H, 6-Heq), 5.52 (s, 1 H, 2-Hax), 7.29–7.57 (m, 8H, Ar-H), 7.94–7.96 (m, 2 H, Ar-H). HPLC (method MeOH): purity 98.8%, tR = 18.61 min.
![Pharmaceuticals 18 01300 i036 Pharmaceuticals 18 01300 i036]()
The α,β-unsaturated ketone 21b (mixture of (E)-21b and (Z)-21b, 200 mg, 0.59 mmol) was dissolved in methanol abs. (10 mL). Pd/C (10% Pd, 20 mg, 10% m/m) and ammonium formate (73 mg, 1.18 mmol) were added. After purging with N2, the mixture was heated to reflux for 2 h, then cooled down to rt and filtered through Celite®. The solvent was removed in vacuo, and the residue was adsorbed on silica gel and given on the column (Ø 2 cm, cyclohexane:ethyl acetate = 9.5:0.5, length 18 cm, fraction 10 mL, Rf = 0.21). Colorless solid, mp 78–79 °C, yield 149 mg (75%). C22H26O3, Mr = 338.4. MS (ESI): m/z [%] = 361 (M + Na, 100). IR (neat): ͠ν [cm−1] = 3060, 2940, 2871 (C-H), 1682 C=O), 756, 705 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.79 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.27–1.32 (m, 1 H, 5-Heq), 1.38–1.61 (m, 3 H, 5-Hax, diox-CH2-CH2-CH2), 1.63 (q, J = 7.3 Hz, 2 H, diox-CH2-CH3), 1.70–1.96 (m, 2 H, diox-CH2-CH2-CH2), 2.93 (t, J = 7.3 Hz, 2 H, CH2-CO), 3.57–3.63 (m, 1 H, 4-Hax), 3.68 (td, J = 12.0/2.7 Hz, 1 H, 6-Hax), 3.77 (ddd, J = 11.3/5.1/1.3 Hz, 1 H, 6-Heq), 7.15–7.48 (m, 8 H, Ar-H), 7.86–7.88 (m, 2 H, Ar-H). HPLC (method ACN): purity 99.4%, tR = 23.12 min.
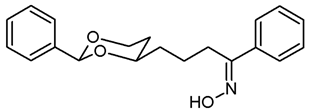
![Pharmaceuticals 18 01300 i037 Pharmaceuticals 18 01300 i037]()
The saturated ketone 22a (180 mg, 0.58 mmol) and hydroxylamine-HCl (180 mg, 2.6 mmol) were suspended in dry ethanol (molecular sieves 3 Å, 20 mL). NaOAc (95 mg, 1.16 mmol) was added, and the mixture was heated to reflux for 7 h. Ethanol was removed under reduced pressure, and the residue was transferred into a separatory funnel with CH2Cl2 (20 mL) and water (20 mL). The layers were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo giving the crude product, which was purified by flash column chromatography (Ø 3 cm, cyclohexane:ethyl acetate = 9:1, length 19 cm, fraction 20 mL, Rf = 0.05). Colorless solid, mp 86 °C, yield 147 mg (78%). C20H23NO3, Mr = 325.4. MS (ESI): m/z [%] = 325 (M, 100), 348 (M + Na, 75), 364 (M + K, 30). IR (neat): ͠ν [cm−1] = 3235 (O-H), 3065, 2925, 2850 (C-H), 1104 (C-O), 748, 693 (arom. monosubst). 1H NMR (CDCl3): δ [ppm] = 1.48–1.52 (m, 1 H, 5-Heq), 1.59–1.85 (m, 5 H, 5-Hax, diox-CH2-CH2), 2.81–2.92 (m, 2 H, CH2-C=N), 3.83–3.88 (m, 1 H, 4-Hax), 3.94 (td, J = 12.1/2.5 Hz, 1 H, 6-Hax), 4.25 (dd broad, J = 11.3/4.8 Hz, 1 H, 6-Heq), 5.49 (s, 1 H, 2-Hax), 7.30–7.37 (m, 6 H, Ar-H), 7.46 (dd, J = 7.7/1.7 Hz, 2 H, N=C-Ar-Hortho), 7.60–7.62 (m, 2 H, diox-Ar-Hortho), 8.45 (s, 1 H, C=N-O-H). HPLC (method MeOH): purity 98.5%, tR = 17.83 min.
(a) Reduction in oxime 23a
The oxime 23a (110 mg, 0.34 mmol) was dissolved in THF abs. (10 mL) and LiAlH4 (64.5 mg, 1.7 mmol) was added. The mixture was stirred for 19 h at rt and then heated to reflux for 4 h. After cooling to rt, ice-cooled water (10 mL) was added dropwise. When H2 formation was finished, the mixture was transferred into a separatory funnel, water (20 mL) and brine (10 mL) were added, and the mixture was extracted with CH2Cl2 (3 × 20 mL). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 6:4 + 1.5% N,N-dimethylethanamine, length 17 cm, fraction 10 mL, Rf = 0.11). Colorless oil, yield 56 mg (53%). HPLC (method ACN): purity 92%, tR = 16.76 min.
(b) Reductive amination of saturated ketone 22a
The saturated ketone 22a (60 mg, 0.19 mmol) and NH4OAc (223 mg, 2.90 mmol) were dissolved in methanol abs. (12 mmol) using ultrasound. NaBH3CN (95%, 64 mg, 0.97 mmol) was added, and the mixture was heated to reflux for 22 h. Water (30 mL) was added, and the pH value was adjusted to 10–11 with 5 M NaOH. The aqueous layer was extracted with CH2Cl2 (3 × 25 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 7:3 + 1% N,N-dimethylethanamine, length 18 cm, fraction 10 mL, Rf = 0.04). Colorless oil, yield 33.5 mg (56%). HPLC (method ACN): purity 92%, tR = 16.76 min. C20H25NO2, Mr = 311.4. MS (ESI): m/z [%] = 312 (M + H, 100). IR (neat): ͠ν [cm−1] = 3032, 2926, 2851 (C-H), 1103 (C-O), 750, 697 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.25–1.84 (m, 8 H, diox-(CH2)3, 5-Hax+eq), 3.74–3.84 (m, 1 H, 4-Hax), 3.87–3.97 (m, 2 H, 6-Hax, Ph-CH-N), 4.25 (dd broad, J = 11.3/5.0 Hz, 1 H, 6-Heq), 5.47 (s, 0.5 H, 2-Hax), 5.48 (s, 0.5 H, 2-Hax), 7.21–7.39 (m, 8 H, Ar-H), 7.43–7.43 (m, 2 H, Ar-H). Signals for the NH2 protons are not seen in the spectrum. HPLC (method ACN): purity 97.2%, tR = 16.76 min.
![Pharmaceuticals 18 01300 i039 Pharmaceuticals 18 01300 i039]()
The saturated ketone 22b (100 mg, 0.30 mmol) and NH4OAc (347 mg, 4.5 mmol) were dissolved in methanol abs. (20 mL) using ultrasound. NaBH3CN (95%, 64 mg, 0.97 mmol) was added, and the mixture was heated to reflux for 24 h, using a reflux apparatus equipped with a rubber balloon. Water (30 mL) was added, and the pH value was adjusted to 10–11 with 5 M NaOH. The aqueous layer was diluted with water and extracted with CH2Cl2 (3 × 25 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, CH2Cl2:methanol = 9.5:0.5, length 18 cm, fraction 10 mL, Rf = 0.07). Colorless oil, yield 46 mg (45%). C22H29NO2, Mr = 339.5. MS (EM, APCI): m/z = calculated for C22H30NO2 340.2271, found 340.2302. IR (neat): ͠ν [cm−1] = 2930, 2865 (C-H), 757, 701 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.77 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.20–1.24 (m, 1 H, 5-Heq), 1.25–1.74 (m, 9 H, diox-CH2-CH3, diox-(CH2)3, 5-Hax), 1.95 (s broad, 2 H, NH2), 3.54–3.62 (m, 1 H, 4-Hax), 3.73 (t broad, J = 12.0 Hz, 1 H, 6-Hax), 3.84 (dd broad, J = 11.4/5.0 Hz, 1 H, 6-Heq), 3.93 (t broad, J = 6.9 Hz, 1 H, Ph-CH-N), 7.22–7.38 (m, 10 H, Ar-H). HPLC (method ACN): purity 97.3%, tR = 18.03 min.
![Pharmaceuticals 18 01300 i040 Pharmaceuticals 18 01300 i040]()
The saturated ketone 22a (100 mg, 0.32 mmol) was dissolved in CH2Cl2 abs. (7 mL). Methylamine (2 M solution in THF, 0.24 mL, 0.48 mmol) and NaBH(OAc)3 (95%, 214 mg, 0.96 mmol) were added. The mixture was heated to reflux, using a reflux apparatus equipped with a rubber balloon to avoid loss of gaseous amine. Additional methylamine (2 M solution in THF, each 0.24 mL, 0.48 mmol) was added after 2 h, 3.5 h, 20 h, 24 h. Additional NaBH(OAc)3 (71.4 mg, 0.32 mmol) was added after 20 h. After 26 h, 1 M NaOH (15 mL) was added, and the layers were separated. The aqueous layer was extracted with CH2Cl2 (3 × 10 mL) and ethyl acetate (3 × 10 mL). The CH2Cl2 fraction and the ethyl acetate fraction were washed with brine (1 × 20 mL) separately, then combined and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 7:3 + 3% N,N-dimethylethanamine, length 18.5 cm, fraction 10 mL, Rf = 0.1). Colorless oil, yield 34 mg (33%). C21H27NO2, Mr = 325.4. MS (EI): m/z [%] = 325 (M, 2), 120 (Ph-CH=NH-CH3, 100). IR (neat): ͠ν [cm−1] = 2932, 2848, 2788 (C-H), 1103 (C-O), 750, 697 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.20–1.82 (m, 9 H, diox-(CH2)3, NH, 5-Hax+eq), 2.26 (s, 3 H, N-CH3), 3.45 (ddd, J = 8.2/6.1/2.4 Hz, 1 H, Ph-CH-N), 3.72–3.80 (m, 1 H, 4-Hax), 3.88–3.95 (m, 1H, 6-Hax), 4.23 (dd broad, J = 11.7/4.6 Hz, 1 H, 6-Heq), 5.45 (s, 0.5 H, 2-Hax), 5.46 (s, 0.5 H, 2-Hax), 7.23–7.37 (m, 8 H, Ar-H), 7.43–7.47 (m, 2 H, Ar H). HPLC (method MeOH): purity 98.1%, tR = 15.73 min.
![Pharmaceuticals 18 01300 i041 Pharmaceuticals 18 01300 i041]()
The saturated ketone 22b (80 mg, 0.24 mmol) was dissolved in CH2Cl2 abs. (7 mL). Methylamine (2 M solution in THF, 0.36 mL, 0.72 mmol) and NaBH(OAc)3 (95%, 161 mg, 0.72 mmol) were added. The mixture was heated to reflux, using a reflux apparatus equipped with a rubber balloon to avoid loss of gaseous amine. Additional NaBH(OAc)3 (53 mg, 0.24 mmol) was added after 9 h. Additional methylamine (2 M solution in THF, each 0.24 mL, 0.48 mmol) was added after 4.5 h, 9 h, 24 h. After 28 h, 1 M NaOH (15 mL) was added. The aqueous layer was diluted with water (ca. 10 mL) and then extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 9.5:0.5 + 2% N,N-dimethylethanamine, length 17.5 cm, fraction 10 mL, Rf = 0.21). Colorless oil, yield 46 mg (55%). C23H31NO2, Mr = 353.5. MS (EM, APCI): m/z = calculated for C23H32NO2 354.2428, found 354.2455. IR (neat): ͠ν [cm−1] = 2932, 2865, 2788 (C-H), 757, 701 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.76 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.18–1.78 (m, 11 H, diox-CH2-CH3, 5-Hax+eq, diox-(CH2)3, N-H), 2.28 (s, 0.5 × 3 H, N-CH3), 2.29 (0.5 × 3 H, N-CH3), 3.45–3.49 (m, 1 H, Ph-CH-N), 3.52–3.59 (m, 1 H, 4-Hax), 3.72 (t broad, J = 12.0 Hz, 1 H, 6-Hax), 3.83 (dd broad, J = 11.3/5.3 Hz, 1 H, 6-Heq), 7.22–7.37 (m, 10 H, Ar-H). HPLC (method ACN): purity 97.9%, tR = 18.36 min.
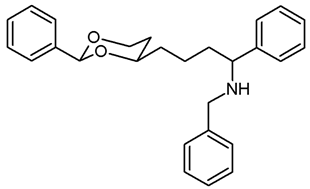
![Pharmaceuticals 18 01300 i042 Pharmaceuticals 18 01300 i042]()
The saturated ketone 22a (80 mg, 0.26 mmol) and freshly distilled benzylamine (42 μL, 0.39 mmol) were dissolved in CH2Cl2 abs. (5 mL). NaBH(OAc)3 (95%, 174 mg, 0.78 mmol) was added, and the mixture was heated to reflux. After 24 h, additional NaBH(OAc)3 (95%, 60 mg, 0.26 mmol) and benzylamine (42 μL, 0.39 mmol) were added, and the mixture was heated to reflux for another 8 h. Then, 1 M NaOH (15 mL) was added, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2.5 cm, cyclohexane:ethyl acetate = 9:1 + 0.5% N,N-dimethylethanamine, length 18 cm, fraction 10 mL, Rf = 0.06). Colorless oil, yield 49 mg (47%). C27H31NO2, Mr = 401.5. MS (ESI): m/z [%] = 402 (M + H, 100). IR (neat): ͠ν [cm−1] = 3029, 2930, 2848 (C-H), 1104 (C-O), 748, 696 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 1.16–1.79 (m, 9 H, 5-Hax+eq, diox-(CH2)3, N-H), 3.53 (d, J = 13.2 Hz, 1 H, N-CH2-Ph), 3.65 (d, J = 13.5 Hz, 1 H, N-CH2-Ph), 3.61–3.64 (m, 1 H, Ph-CH-N), 3.71–3.79 (m, 1 H, 4-Hax), 3.91 (td, J = 12.4/2.1 Hz, 1 H, 6-Hax), 4.23 (dd, J = 11.4/4.8 Hz, 1 H, 6-Heq), 5.45 (s, 0.5 H, 2-Hax), 5.46 (s, 0.5 H, 2-Hax), 7.23–7.37 (m, 13 H, Ar-H), 7.43–7.46 (m, 2 H, Ar-H). A signal for the NH proton is not seen in the spectrum. 13C NMR (CDCl3): δ [ppm] = 21.8 (0.5 C, diox-CH2-CH2), 21.9 (0.5 C, diox-CH2-CH2), 31.4 (0.5 C, C-5), 31.5 (0.5 C, C-5), 36.0 (0.5 C, diox-CH2), 36.1 (0.5 C, diox-CH2), 38.1 (1 C, diox-(CH2)2-CH2), 51.5 (1 C, Ph-CH2-N), 62.6 (1 C, Ph-CH-N-Bz), 67.2 (1 C, C-6), 77.1 (1 C, C-4), 101.2 (1 C, C-2), 126.3–128.8 (15 C, Ar), 139.0 (1 C, Arquart.), 140.3 (1 C, Arquart.), 143.8 (1 C, Arquart.). HPLC (method ACN): purity 97.6%, tR = 20.05 min.
The saturated ketone 22b (70 mg, 0.21 mmol) and freshly distilled benzylamine (69 μL, 0.63 mmol) were dissolved in CH2Cl2 abs. (5 mL). NaBH(OAc)3 (95%, 140 mg, 0.63 mmol) was added, and the mixture was heated to reflux for a total of 3 d. Additional NaBH(OAc)3 (95%, 47 mg, 0.21 mmol) was added after 24 h. Additional benzylamine (each 69 μL, 0.63 mmol) was added after 24 h and 2.5 d. After 3 d, 1 M NaOH (15 mL) was added, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 3 cm, cyclohexane:ethyl acetate = 9:1, length 16 cm, fraction 10 mL, Rf = 0.09).
Colorless oil, yield 75 mg (83%). C29H35NO2, Mr = 429.6. MS (ESI): m/z [%] = 430 (M + H, 100). IR (neat): ͠ν [cm−1] = 3026, 2928, 2865 (C-H), 757, 699 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.76 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 1.18 (d broad, J = 12.8 Hz, 1 H, 5-Heq), 1.20–1.81 (m, 7 H, diox-(CH2)3, 5-Hax), 1.70 (q, J = 7.5 Hz, 0.5 × 2 H, diox-CH2-CH3), 1.70 (q, J = 7.5 Hz, 0.5 × 2 H, diox-CH2-CH3), 3.49–3.57 (m, 1 H, 4-Hax), 3.54 (d, J = 13.2 Hz, 0.5 H, N-CH2-Ph), 3.54 (d, J = 13.2 Hz, 0.5 H, N-CH2-Ph), 3.64 (t, J = 7.0 Hz, 1 H, Ph-CH-N), 3.68 (d, J = 13.3 Hz, 1 H, N-CH2-Ph), 3.68–3.75 (m, 1 H, 6-Hax), 3.83 (dd broad, J = 11.3/5.0, 1 H, 6-Heq), 7.27–7.37 (m, 15 H, Ar-H). A signal for the NH proton is not seen in the spectrum. HPLC (method ACN): purity 97.4%, tR = 21.11 min.
![Pharmaceuticals 18 01300 i044 Pharmaceuticals 18 01300 i044]()
The saturated ketone 22a (70 mg, 0.23 mmol) and freshly distilled pyrrolidine (29 μL, 0.345 mmol) were dissolved in CH2Cl2 abs. (5 mL). NaBH(OAc)3 (95%, 154 mg, 0.69 mmol) was added, and the mixture was heated to reflux. After 4 h, additional NaBH(OAc)3 (95%, 51 mg, 0.23 mmol) and pyrrolidine (29 μL, 0.345 mmol) were added, and the mixture was heated to reflux for additional 20 h. Then, 1 M NaOH (15 mL) was added, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine and dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 9:1 + 0.5% N,N-dimethylethanamine, length 18 cm, fraction 10 mL, Rf = 0.06). Colorless oil, yield 30 mg (33%). C24H31NO2, Mr = 365.5. MS (EI): m/z [%] = 365 (M, 100), 160 (Ph-CH=N(CH2-CH2)4, 100). IR (neat): ͠ν [cm−1] = 2946, 2859, 2779 (C-H), 1103 (C-O), 749, 697 (arom. monosubst.) 1H NMR (CDCl3): δ [ppm] = 1.01–1.98 (m, 12 H, 5-Hax+eq, diox-(CH2)3, N(CH2CH2)2), 2.26–2.33 (m, 2 H, N(CH2CH2)2), 2.48–2.54 (m, 2 H, N(CH2CH2)2), 3.01–3.06 (m, 1 H, Ph-CH-N), 3.64–3.73 (m, 1 H, 4-Hax), 3.86 (t broad, J = 11.3 Hz, 1 H, 6-Hax), 5.19 (dd broad, J = 11.1/4.6 Hz, 1 H, 6-Heq), 5.40 (s, 0.5 H, 2-Hax), 5.41 (s, 0.5 H, 2-Hax), 7.19–7.34 (m, 8 H, Ar-H), 7.39–7.42 (m, 2 H, Ar-H). HPLC (method ACN): purity 99.6%, tR = 18.83 min.
![Pharmaceuticals 18 01300 i045 Pharmaceuticals 18 01300 i045]()
The saturated ketone 22b (70 mg, 0.21 mmol) and freshly distilled pyrrolidine (52 μL, 0.63 mmol) were dissolved in CH2Cl2 abs. (6 mL). NaBH(OAc)3 (95%, 141 mg, 0.63 mmol) was added, and the mixture was heated to reflux for a total of 4 d. Additional NaBH(OAc)3 (95%, 47 mg, 0.21 mmol) was added after 48 h. Additional pyrrolidine (each 52 μL, 0.63 mmol) was added after 24, 48 and 72 h. After 4 d, 1 M NaOH (15 mL) was added, the organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were dried (K2CO3). The solvent was removed in vacuo, and the residue was purified by flash column chromatography (Ø 2 cm, cyclohexane:ethyl acetate = 9:1 + 0.5% N,N-dimethylethanamine, length 19 cm, fraction 10 mL, Rf = 0.12). Colorless oil, yield 65 mg (83%). C26H35NO2, Mr = 393.5. MS (EM, ESI): m/z = calculated for C27H35NO2 394.2741, found 394.2740. IR (neat): ͠ν [cm−1] = 2943, 2867, 2780 (C-H), 757, 702 (arom. monosubst.). 1H NMR (CDCl3): δ [ppm] = 0.74 (t, J = 7.5 Hz, 3 H, diox-CH2-CH3), 0.99–2.05 (m, 14 H, 5-Hax+eq, diox-CH2-CH3, diox-(CH2)3, N(CH2-CH2)2), 2.37, 2.48, 2.56 (s broad each, 4 H, N(CH2-CH2)2, 3.04–3.08 (m, 1 H, Ph-CH-Npyrolidine), 3.44–3.62 (m, 1 H, 4-Hax), 3.69 (“tt”, J = 11.4/2.5 Hz, 1 H, 6-Hax), 3.79–3.85 (m, 1 H, 6-Heq), 7.20–7.39 (m, 10 H, Ar-H). HPLC (method ACN): purity 99.9%, tR = 19.98 min.