Fluoroquinolone-Associated Disability: A Rodent Model Reveals Transient Neuropsychiatric and Persistent Gastrointestinal Effects of Low-Dose Ciprofloxacin
Abstract
1. Introduction
2. Results
2.1. Ciprofloxacin Only Causes a Transient Anxiety-like Phenotype
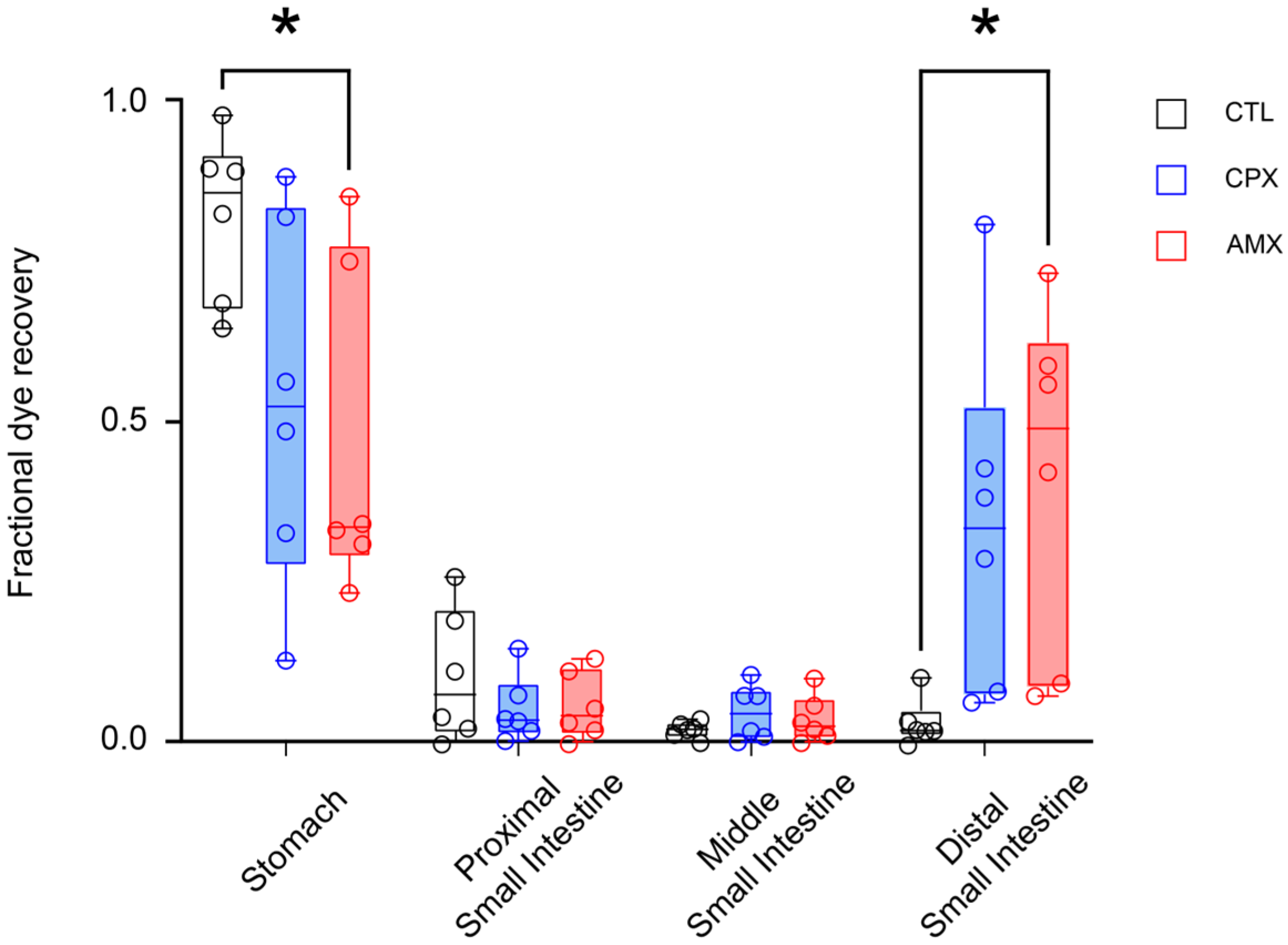
2.2. Ciprofloxacin Accelerates Transit Similarly to Amoxicillin
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Treatment
4.3. Behavior
4.3.1. The Elevated Plus Maze Test
4.3.2. The Marble Burying Test
4.3.3. The Open Field Test
4.4. Gastrointestinal Transit
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FQs | Fluoroquinolones |
| CDC | Centers for Disease Control and Prevention |
| FDA | Food and Drug Administration |
| FQAD | Fluoroquinolone Associated Disability |
| GI | Gastrointestinal |
| CNS | Central Nervous System |
| GABA | γ-aminobutyric acid |
| CPX | Ciprofloxacin |
| NMDA | (N-methyl-D-aspartate) |
| ACh | Acetylcholine |
| DMV | Dorsal Motor Nucleus of the vagus |
| LC | Locus Coeruleus |
| VNS | Vagus Nerve Stimulation |
| CTL | Control |
| AMX | Amoxicillin |
| PSI | Proximal Small Intestine |
| MSI | Middle Small Intestine |
| DSI | Distal Small Intestine |
| LMM | Linear Mixed effects Model |
| EMMs | Estimated Marginal Means |
References
- Fluoroquinolones|A.R. & Patient Safety Portal. Available online: https://arpsp.cdc.gov/profile/antibiotic-use/fluoroquinolones (accessed on 25 August 2021).
- Serwacki, P.; Gajda, M.; Świątek-Kwapniewska, W.; Wałaszek, M.; Nowak, K.; Wójkowska-Mach, J. Re-Evaluating the Suitability of Using Fluoroquinolones in the Treatment of Infections in the Context of FQ Consumption and Correlating Changes to Microorganism Resistance Levels in EU/EEA Countries between 2016 and 2021. Naunyn. Schmiedebergs Arch. Pharmacol. 2024, 397, 795–805. [Google Scholar] [CrossRef]
- Gootz, T.D.; Barrett, J.F.; Sutcliffe, J.A. Inhibitory Effects of Quinolone Antibacterial Agents on Eucaryotic Topoisomerases and Related Test Systems. Antimicrob. Agents Chemother. 1990, 34, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hangas, A.; Aasumets, K.; Kekäläinen, N.J.; Paloheinä, M.; Pohjoismäki, J.L.; Gerhold, J.M.; Goffart, S. Ciprofloxacin Impairs Mitochondrial DNA Replication Initiation through Inhibition of Topoisomerase 2. Nucleic Acids Res. 2018, 46, 9625–9636. [Google Scholar] [CrossRef]
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxid. Med. Cell. Longev. 2017, 2017, 8023935. [Google Scholar] [CrossRef]
- Keeney, K.M.; Yurist-Doutsch, S.; Arrieta, M.-C.; Finlay, B.B. Effects of Antibiotics on Human Microbiota and Subsequent Disease. Annu. Rev. Microbiol. 2014, 68, 217–235. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Baker, C.A. Fluoroquinolone Toxicity Profiles: A Review Focusing on Newer Agents. Clin. Infect. Dis. 1999, 28, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K. Mechanisms of Quinolone Phototoxicity. Toxicol. Lett. 1998, 102–103, 369–373. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, P.D.; Sturkenboom, M.C.J.M.; Herings, R.M.C.; Leufkens, H.M.G.; Rowlands, S.; Stricker, B.H.C. Increased Risk of Achilles Tendon Rupture with Quinolone Antibacterial Use, Especially in Elderly Patients Taking Oral Corticosteroids. Arch. Intern. Med. 2003, 163, 1801–1807. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Yang, Y.-M. Fluoroquinolone-Associated Tendinopathy. Chang Gung Med. J. 2011, 34, 461–467. [Google Scholar]
- Wise, B.; Peloquin, C.; Choi, H.; Lane, N.; Zhang, Y. Impact of Age, Sex, Obesity, and Steroid Use on Quinolone-Associated Tendon Disorders. Am. J. Med. 2012, 125, 1228.e23–1228.e28. [Google Scholar] [CrossRef]
- Arabyat, R.M.; Raisch, D.W.; McKoy, J.M.; Bennett, C.L. Fluoroquinolone-Associated Tendon-Rupture: A Summary of Reports in the Food and Drug Administration’s Adverse Event Reporting System. Expert Opin. Drug Saf. 2015, 14, 1653–1660. [Google Scholar] [CrossRef]
- Guzzardi, D.G.; Teng, G.; Kang, S.; Geeraert, P.J.; Pattar, S.S.; Svystonyuk, D.A.; Belke, D.D.; Fedak, P.W.M. Induction of Human Aortic Myofibroblast-Mediated Extracellular Matrix Dysregulation: A Potential Mechanism of Fluoroquinolone-Associated Aortopathy. J. Thorac. Cardiovasc. Surg. 2019, 157, 109–119.e2. [Google Scholar] [CrossRef]
- Halliwell, R.F.; Davey, P.G.; Lambert, J.J. Antagonism of GABAA Receptors by 4-Quinolones. J. Antimicrob. Chemother. 1993, 31, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Charter, M.; Kelly, S.; Varma, T.R.; Jacobson, I.; Freeman, A.; Precious, E.; Lambert, J. Ciprofloxacin and Sparfloxacin Penetration into Human Brain Tissue and Their Activity as Antagonists of GABAA Receptor of Rat Vagus Nerve. Antimicrob. Agents Chemother. 1994, 38, 1356–1362. [Google Scholar] [CrossRef]
- Green, M.A.; Halliwell, R.F. Selective Antagonism of the GABA(A) Receptor by Ciprofloxacin and Biphenylacetic Acid. Br. J. Pharmacol. 1997, 122, 584–590. [Google Scholar] [CrossRef] [PubMed]
- De Sarro, G.; Nava, F.; Calapai, G.; De Sarro, A. Effects of Some Excitatory Amino Acid Antagonists and Drugs Enhancing Gamma-Aminobutyric Acid Neurotransmission on Pefloxacin-Induced Seizures in DBA/2 Mice. Antimicrob. Agents Chemother. 1997, 41, 427–434. [Google Scholar] [CrossRef]
- Schmuck, G.; Schürmann, A.; Schlüter, G. Determination of the Excitatory Potencies of Fluoroquinolones in the Central Nervous System by an In Vitro Model. Antimicrob. Agents Chemother. 1998, 42, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Moghimi, A.; Mollazadeh, S.; Rassouli, F.B.; Shiee, R.; Khalilzade, M.A. The Effect of Ciprofloxacin Injection on Genetically Absence Prone (Wag/Rij) Rat’s Electroencephalogram Characteristics. Basic Clin. Neurosci. 2013, 4, 31–35. [Google Scholar]
- Ilgin, S.; Can, O.D.; Atli, O.; Ucel, U.I.; Sener, E.; Guven, I. Ciprofloxacin-Induced Neurotoxicity: Evaluation of Possible Underlying Mechanisms. Toxicol. Mech. Methods 2015, 25, 374–381. [Google Scholar] [CrossRef]
- Kaur, K.; Fayad, R.; Saxena, A.; Frizzell, N.; Chanda, A.; Das, S.; Chatterjee, S.; Hegde, S.; Baliga, M.S.; Ponemone, V.; et al. Fluoroquinolone-Related Neuropsychiatric and Mitochondrial Toxicity: A Collaborative Investigation by Scientists and Members of a Social Network. J. Community Support. Oncol. 2016, 14, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.S. Peripheral Neuropathy Associated with Fluoroquinolones. Ann. Pharmacother. 2001, 35, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.K. Peripheral Neuropathy and Guillain-Barré Syndrome Risks Associated with Exposure to Systemic Fluoroquinolones: A Pharmacovigilance Analysis. Ann. Epidemiol. 2014, 24, 279–285. [Google Scholar] [CrossRef]
- Golomb, B.A.; Koslik, H.J.; Redd, A.J. Fluoroquinolone-Induced Serious, Persistent, Multisymptom Adverse Effects. BMJ Case Rep. 2015, 2015, bcr2015209821. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, M.-T.G.; Chen, Y.-S.; Lee, S.-H.; Chen, Y.-S.; Chen, S.-C.; Chang, S.-C. Risk of Aortic Dissection and Aortic Aneurysm in Patients Taking Oral Fluoroquinolone. JAMA Intern. Med. 2015, 175, 1839–1847. [Google Scholar] [CrossRef]
- Singh, S.; Nautiyal, A. Aortic Dissection and Aortic Aneurysms Associated with Fluoroquinolones: A Systematic Review and Meta-Analysis. Am. J. Med. 2017, 130, 1449–1457.e9. [Google Scholar] [CrossRef]
- Pasternak, B.; Inghammar, M.; Svanström, H. Fluoroquinolone Use and Risk of Aortic Aneurysm and Dissection: Nationwide Cohort Study. BMJ 2018, 360, k678. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Zhang, L.; Luo, W.; Ren, P.; Azares, A.R.; Wang, Y.; Zhang, C.; Coselli, J.S.; Shen, Y.H. Effect of Ciprofloxacin on Susceptibility to Aortic Dissection and Rupture in Mice. JAMA Surg. 2018, 153, e181804. [Google Scholar] [CrossRef]
- Sommet, A.; Bénévent, J.; Rousseau, V.; Chebane, L.; Douros, A.; Montastruc, J.-L.; Montastruc, F. What Fluoroquinolones Have the Highest Risk of Aortic Aneurysm? A Case/Non-Case Study in VigiBase®. J. Gen. Intern. Med. 2019, 34, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Sodhi, M.; Ganjizadeh-Zavareh, S.; Carleton, B.; Kezouh, A.; Brophy, J.M. Oral Fluoroquinolones and Risk of Mitral and Aortic Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 1444–1450. [Google Scholar] [CrossRef]
- Marchant, J. When Antibiotics Turn Toxic. Nature 2018, 555, 431–433. [Google Scholar] [CrossRef]
- Freeman, M.Z.; Cannizzaro, D.N.; Naughton, L.F.; Bove, C. Fluoroquinolones-Associated Disability: It Is Not All in Your Head. NeuroSci 2021, 2, 235–253. [Google Scholar] [CrossRef]
- Yarrington, M.E.; Anderson, D.J.; Dodds Ashley, E.; Jones, T.; Davis, A.; Johnson, M.; Lokhnygina, Y.; Sexton, D.J.; Moehring, R.W. Impact of FDA Black Box Warning on Fluoroquinolone and Alternative Antibiotic Use in Southeastern US Hospitals. Infect. Control Hosp. Epidemiol. 2019, 40, 1297–1300. [Google Scholar] [CrossRef]
- Bennett, A.C.; Bennett, C.L.; Witherspoon, B.J.; Knopf, K.B. An Evaluation of Reports of Ciprofloxacin, Levofloxacin, and Moxifloxacin-Association Neuropsychiatric Toxicities, Long-Term Disability, and Aortic Aneurysms/Dissections Disseminated by the Food and Drug Administration and the European Medicines Agency. Expert Opin. Drug Saf. 2019, 18, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, S.; Hersh, A.L.; Shapiro, D.J.; Fleming-Dutra, K.E.; Pavia, A.T.; Hicks, L.A. Opportunities to Improve Fluoroquinolone Prescribing in the United States for Adult Ambulatory Care Visits. Clin. Infect. Dis. 2018, 67, 134–136. [Google Scholar] [CrossRef]
- Cannizzaro, D.; Naughton, L.; Freeman, M.; Bennett, C.L.; Martin, L.; Bove, C. A New Criterion for Fluoroquinolone-Associated Disability Diagnosis: Functional Gastrointestinal Disorders. Medicina 2021, 57, 1371. [Google Scholar] [CrossRef]
- Reviglio, V.E.; Hakim, M.A.; Song, J.K.; O’Brien, T.P. Effect of Topical Fluoroquinolones on the Expression of Matrix Metalloproteinases in the Cornea. BMC Ophthalmol. 2003, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Her, Y.F.; Maher, L.J. Nonantibiotic Effects of Fluoroquinolones in Mammalian Cells. J. Biol. Chem. 2015, 290, 22287–22297. [Google Scholar] [CrossRef]
- Jun, C.; Fang, B. Current Progress of Fluoroquinolones-Increased Risk of Aortic Aneurysm and Dissection. BMC Cardiovasc. Disord. 2021, 21, 470. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Hsu, C.-C.; Chen, H.-C.; Hsu, Y.-H.; Lin, M.-S.; Wu, C.-W.; Pang, J.-H.S. Ciprofloxacin-Mediated Inhibition of Tenocyte Migration and down-Regulation of Focal Adhesion Kinase Phosphorylation. Eur. J. Pharmacol. 2009, 607, 23–26. [Google Scholar] [CrossRef]
- Lawrence, J.W.; Claire, D.C.; Weissig, V.; Rowe, T.C. Delayed Cytotoxicity and Cleavage of Mitochondrial DNA in Ciprofloxacin-Treated Mammalian Cells. Mol. Pharmacol. 1996, 50, 1178–1188. [Google Scholar] [CrossRef]
- Nadanaciva, S.; Dillman, K.; Gebhard, D.F.; Shrikhande, A.; Will, Y. High-Content Screening for Compounds That Affect mtDNA-Encoded Protein Levels in Eukaryotic Cells. J. Biomol. Screen. 2010, 15, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci. Transl. Med. 2013, 5, 192ra85. [Google Scholar] [CrossRef] [PubMed]
- Stakenborg, N.; Giovangiulio, M.D.; Boeckxstaens, G.E.; Matteoli, G. The Versatile Role of The Vagus Nerve in the Gastrointestinal Tract. EMJ Gastroenterol. 2013, 1, 106–114. [Google Scholar] [CrossRef]
- Baker, E.; Lui, F. Neuroanatomy, Vagal Nerve Nuclei. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Altschuler, S.M.; Escardo, J.; Lynn, R.B.; Miselis, R.R. The Central Organization of the Vagus Nerve Innervating the Colon of the Rat. Gastroenterology 1993, 104, 502–509. [Google Scholar] [CrossRef]
- Gillis, R.A.; Dezfuli, G.; Bellusci, L.; Vicini, S.; Sahibzada, N. Brainstem Neuronal Circuitries Controlling Gastric Tonic and Phasic Contractions: A Review. Cell. Mol. Neurobiol. 2021, 42, 333–360. [Google Scholar] [CrossRef]
- Travagli, R.A.; Anselmi, L. Vagal Neurocircuitry and Its Influence on Gastric Motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef]
- Fornai, M.; Antonioli, L.; Colucci, R.; Tuccori, M.; Blandizzi, C. Pathophysiology of Gastric Ulcer Development and Healing: Molecular Mechanisms and Novel Therapeutic Options. Peptic Ulcer Dis. 2011, 7, 113–142. [Google Scholar]
- Travagli, R.A.; Gillis, R.A.; Rossiter, C.D.; Vicini, S. Glutamate and GABA-Mediated Synaptic Currents in Neurons of the Rat Dorsal Motor Nucleus of the Vagus. Am. J. Physiol. 1991, 260, G531–G536. [Google Scholar] [CrossRef]
- Sivarao; Krowicki; Hornby. Role of GABAA Receptors in Rat Hindbrain Nuclei Controlling Gastric Motor Function. Neurogastroenterol. Motil. 1998, 10, 305–313. [Google Scholar] [CrossRef]
- Travagli, R.A.; Gillis, R.A. Hyperpolarization-Activated Currents, IH and IKIR, in Rat Dorsal Motor Nucleus of the Vagus Neurons in Vitro. J. Neurophysiol. 1994, 71, 1308–1317. [Google Scholar] [CrossRef]
- Eglen, R.M. Muscarinic Receptors and Gastrointestinal Tract Smooth Muscle Function. Life Sci. 2001, 68, 2573–2578. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M. Nitric Oxide and Its Role as a Non-adrenergic, Non-cholinergic Inhibitory Neurotransmitter in the Gastrointestinal Tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef]
- Rogers, R.C.; Hermann, G.E.; Travagli, R.A. Brainstem Pathways Responsible for Oesophageal Control of Gastric Motility and Tone in the Rat. J. Physiol. 1999, 514 Pt 2, 369–383. [Google Scholar] [CrossRef]
- Cruz, M.T.; Murphy, E.C.; Sahibzada, N.; Verbalis, J.G.; Gillis, R.A. A Reevaluation of the Effects of Stimulation of the Dorsal Motor Nucleus of the Vagus on Gastric Motility in the Rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R291–R307. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, S.S. Fluoroquinolone-Induced Depression. Am. J. Psychiatry 1995, 152, 954–955. [Google Scholar] [CrossRef]
- Kandasamy, A.; Srinath, D. Levofloxacin-Induced Acute Anxiety and Insomnia. J. Neurosci. Rural Pract. 2012, 3, 212–214. [Google Scholar] [CrossRef]
- Haiping, L.; Ziqiang, J.; Qina, Z.; Yuhua, D. Adverse Reactions of Fluoroquinolones to Central Nervous System and Rational Drug Use in Nursing Care. Pak. J. Pharm. Sci. 2019, 32, 427–432. [Google Scholar] [PubMed]
- Morris, L.S.; McCall, J.G.; Charney, D.S.; Murrough, J.W. The Role of the Locus Coeruleus in the Generation of Pathological Anxiety. Brain Neurosci. Adv. 2020, 4, 2398212820930321. [Google Scholar] [CrossRef] [PubMed]
- Carreno, F.R.; Frazer, A. Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurother. J. Am. Soc. Exp. Neurother. 2017, 14, 716–727. [Google Scholar] [CrossRef]
- Magnon, V.; Dutheil, F.; Vallet, G.T. Benefits from One Session of Deep and Slow Breathing on Vagal Tone and Anxiety in Young and Older Adults. Sci. Rep. 2021, 11, 19267. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Décarie-Spain, L.; Hayes, A.M.R.; Lauer, L.T.; Kanoski, S.E. The Gut-Brain Axis and Cognitive Control: A Role for the Vagus Nerve. Semin. Cell Dev. Biol. 2024, 156, 201–209. [Google Scholar] [CrossRef]
- Chiusolo, F.; Capriati, T.; Erba, I.; Bianchi, R.; Ciofi Degli Atti, M.L.; Picardo, S.; Diamanti, A. Management of Enteral Nutrition in the Pediatric Intensive Care Unit: Prokinetic Effects of Amoxicillin/Clavulanate in Real Life Conditions. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 521–530. [Google Scholar] [CrossRef]
- Gomez, R.; Fernandez, S.; Aspirot, A.; Punati, J.; Skaggs, B.; Mousa, H.; Di Lorenzo, C. Effect of Amoxicillin/Clavulanate on Gastrointestinal Motility in Children. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Mohammed, A.E.; Eltom, K.H.; Albrahim, J.S.; Alburae, N.A. Evaluation of Antibiotic-Induced Behavioral Changes in Mice. Physiol. Behav. 2020, 223, 113015. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Emmerzaal, T.L.; Geenen, B.; Verweij, V.; van Bodegom, M.; Morava, E.; Kiliaan, A.J.; Kozicz, T. Early-Adolescent Antibiotic Exposure Results in Mitochondrial and Behavioral Deficits in Adult Male Mice. Sci. Rep. 2021, 11, 12875. [Google Scholar] [CrossRef]
- Lubasch, A.; Keller, I.; Borner, K.; Koeppe, P.; Lode, H. Comparative Pharmacokinetics of Ciprofloxacin, Gatifloxacin, Grepafloxacin, Levofloxacin, Trovafloxacin, and Moxifloxacin after Single Oral Administration in Healthy Volunteers. Antimicrob. Agents Chemother. 2000, 44, 2600–2603. [Google Scholar] [CrossRef]
- Borghammer, P.; Van Den Berge, N. Brain-First versus Gut-First Parkinson’s Disease: A Hypothesis. J. Park. Dis. 2019, 9, S281–S295. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Chan, P.; Stoessl, A.J. The Underlying Mechanism of Prodromal PD: Insights from the Parasympathetic Nervous System and the Olfactory System. Transl. Neurodegener. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.J.; Civitelli, L.; Hu, M.T. The Gut-Brain Axis in Early Parkinson’s Disease: From Prodrome to Prevention. J. Neurol. 2025, 272, 413. [Google Scholar] [CrossRef]
- Forster, E.R.; Green, T.; Elliot, M.; Bremner, A.; Dockray, G.J. Gastric Emptying in Rats: Role of Afferent Neurons and Cholecystokinin. Am. J. Physiol. 1990, 258, G552–G556. [Google Scholar] [CrossRef]
- Brennan, I.M.; Little, T.J.; Feltrin, K.L.; Smout, A.J.P.M.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Dose-Dependent Effects of Cholecystokinin-8 on Antropyloroduodenal Motility, Gastrointestinal Hormones, Appetite, and Energy Intake in Healthy Men. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1487–E1494. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Levin, F.; Hellström, P.M.; Schmidt, P.T. Ghrelin Stimulates Motility in the Small Intestine of Rats through Intrinsic Cholinergic Neurons. Regul. Pept. 2004, 121, 25–30. [Google Scholar] [CrossRef]
- Fujino, K.; Inui, A.; Asakawa, A.; Kihara, N.; Fujimura, M.; Fujimiya, M. Ghrelin Induces Fasted Motor Activity of the Gastrointestinal Tract in Conscious Fed Rats. J. Physiol. 2003, 550, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Nduka, S.O.; Okonta, M.J.; Esimone, C.O. Effects of Zingiber Officinale on the Plasma Pharmacokinetics and Lung Penetrations of Ciprofloxacin and Isoniazid. Am. J. Ther. 2013, 20, 507–513. [Google Scholar] [CrossRef]
- Saraçoğlu, A.; Temel, H.E.; Ergun, B.; Colak, O. Oxidative Stress-Mediated Myocardiotoxicity of Ciprofloxacin and Ofloxacin in Juvenile Rats. Drug Chem. Toxicol. 2009, 32, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Quesenberry, K.; Carpenter, J. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Elsevier: St. Louis, MO, USA, 2014; Volume 55. [Google Scholar]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Trullas, R.; Skolnick, P. Differences in Fear Motivated Behaviors among Inbred Mouse Strains. Psychopharmacology 1993, 111, 323–331. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble Burying and Nestlet Shredding as Tests of Repetitive, Compulsive-like Behaviors in Mice. J. Vis. Exp. JoVE 2013, 82, 50978. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. JoVE 2015, 96, e52434. [Google Scholar] [CrossRef]
- Souza, M.a.N.; Souza, M.H.L.P.; Palheta, R.C.; Cruz, P.R.M.; Medeiros, B.A.; Rola, F.H.; Magalhães, P.J.C.; Troncon, L.E.A.; Santos, A.A. Evaluation of Gastrointestinal Motility in Awake Rats: A Learning Exercise for Undergraduate Biomedical Students. Adv. Physiol. Educ. 2009, 33, 343–348. [Google Scholar] [CrossRef]
- Jiang, Y.; Babic, T.; Travagli, R.A. Sex Differences in GABAergic Neurotransmission to Rat DMV Neurons. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G476–G483. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 20 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.5.1. 2022; Available online: https://www.R-project.org/ (accessed on 20 May 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
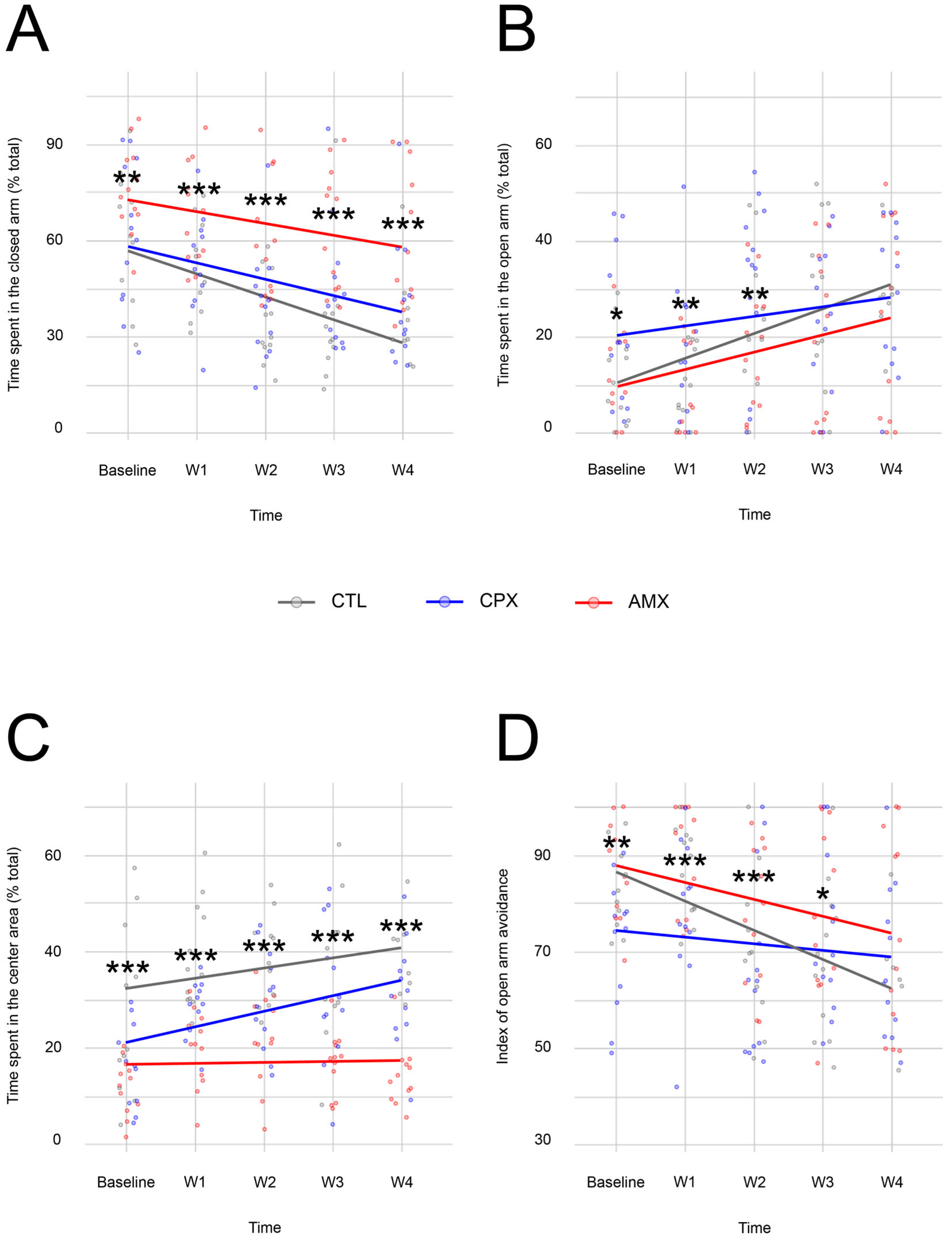
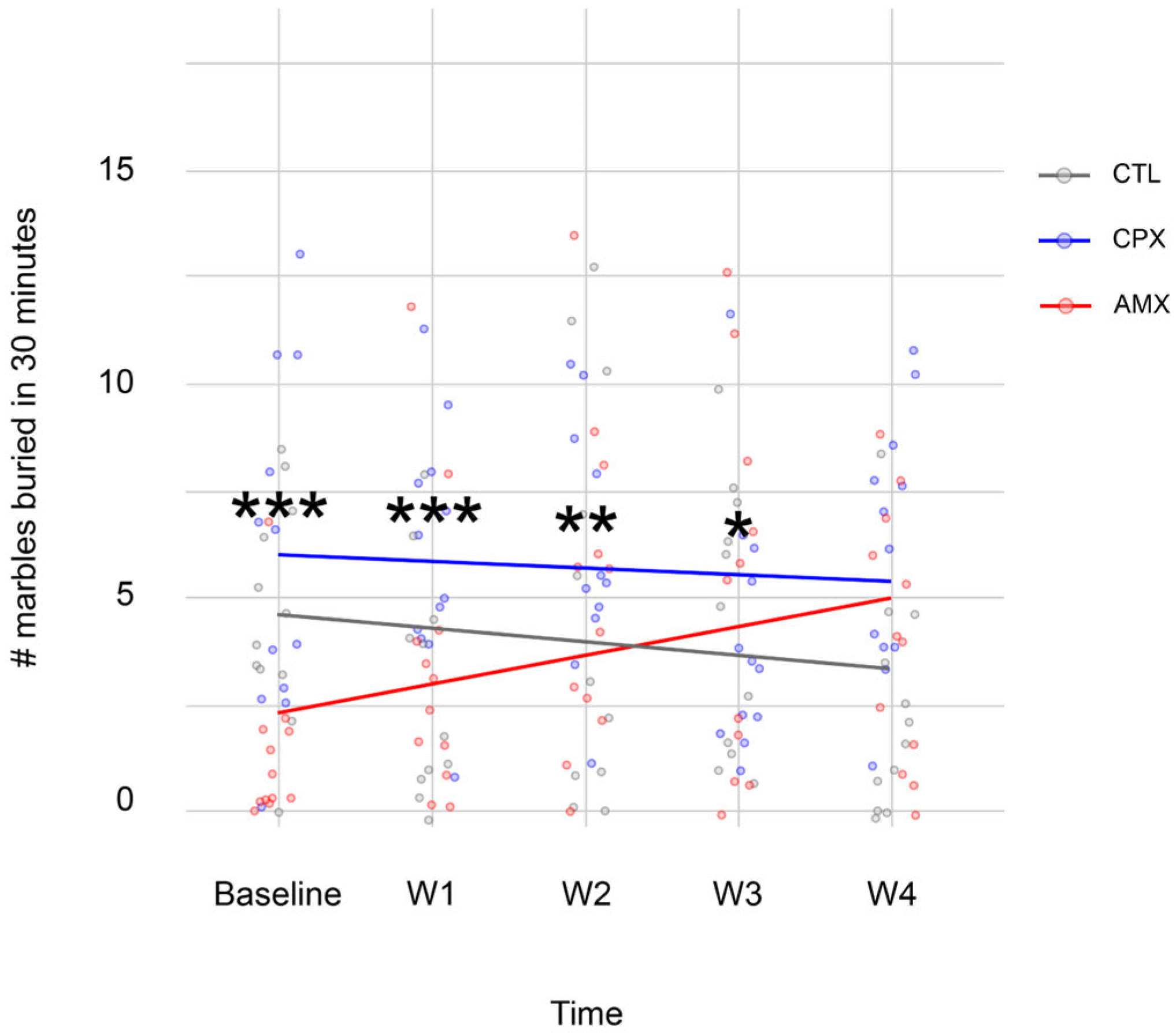
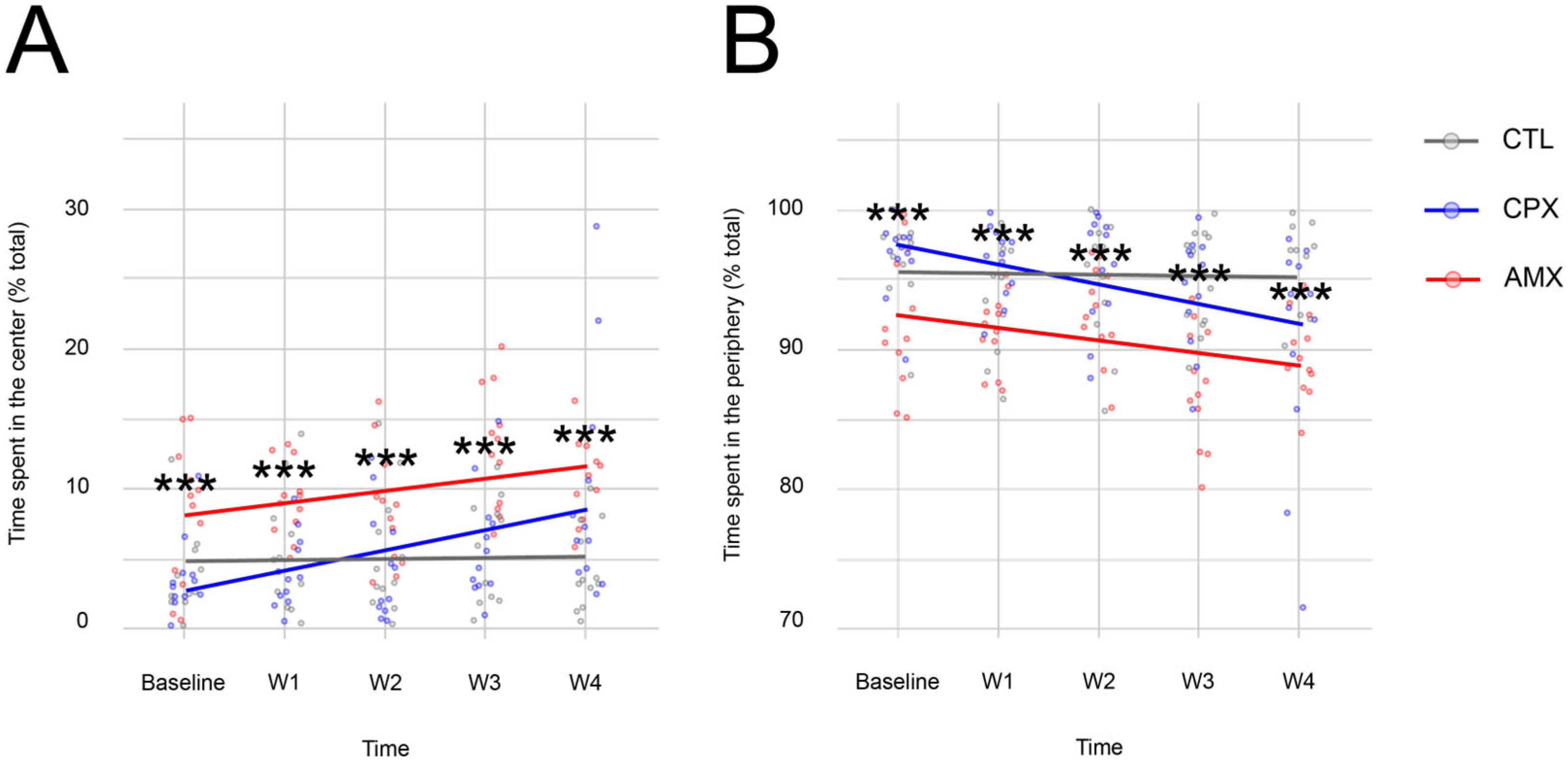
| Open Arm | ||
| t-value | p-value | |
| Intercept | 2.746 | 0.008 |
| CPX | 2.574 | 0.011 |
| CTL | 0.200 | 0.842 |
| Time | 2.971 | 0.003 |
| CPX and time | −0.973 | 0.332 |
| CTL and time | 0.822 | 0.412 |
| Closed arm | ||
| t-value | p-value | |
| Intercept | 16.457 | <2−16 |
| CPX | −2.817 | 0.005 |
| CTL | −2.984 | 0.003 |
| Time | −2.422 | 0.017 |
| CPX and time | −0.740 | 0.460 |
| CTL and time | −1.657 | 0.100 |
| Central Neutral Square | ||
| t-value | p-value | |
| Intercept | 7.044 | 2.37−10 |
| CPX | 1.415 | 0.159 |
| CTL | 4.806 | 3.55−6 |
| Time | 0.195 | 0.846 |
| CPX and time | 2.339 | 0.021 |
| CTL and time | 1.441 | 0.152 |
| Index of Open Arm Avoidance | ||
| t-value | p-value | |
| Intercept | 24.544 | <2−16 |
| CPX | −3.139 | 0.002 |
| CTL | −0.287 | 0.774 |
| Time | −2.913 | 0.004 |
| CPX and time | 1.245 | 0.215 |
| CTL and time | −1.326 | 0.187 |
| Center | ||
| t-value | p-value | |
| Intercept | 7.796 | 2.22−10 |
| CPX | −4.659 | 6.57−6 |
| CTL | −2.895 | 0.004 |
| Time | 2.679 | 0.008 |
| CPX and time | 1.138 | 0.257 |
| CTL and time | −1.748 | 0.082 |
| Periphery | ||
| t-value | p-value | |
| Intercept | 91.535 | <2−16 |
| CPX | 4.558 | 1.01−5 |
| CTL | 2.735 | 0.007 |
| Time | −2.801 | 0.006 |
| CPX and time | −1.142 | 0.255 |
| CTL and time | 1.847 | 0.067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getaneh, B.; Kerler, J.; Ganzorig, M.; Muhl, C.; Miller, A.; Efiom-Ekaha, I.; Belai, B.; Bove, C. Fluoroquinolone-Associated Disability: A Rodent Model Reveals Transient Neuropsychiatric and Persistent Gastrointestinal Effects of Low-Dose Ciprofloxacin. Pharmaceuticals 2025, 18, 1277. https://doi.org/10.3390/ph18091277
Getaneh B, Kerler J, Ganzorig M, Muhl C, Miller A, Efiom-Ekaha I, Belai B, Bove C. Fluoroquinolone-Associated Disability: A Rodent Model Reveals Transient Neuropsychiatric and Persistent Gastrointestinal Effects of Low-Dose Ciprofloxacin. Pharmaceuticals. 2025; 18(9):1277. https://doi.org/10.3390/ph18091277
Chicago/Turabian StyleGetaneh, Bitseat, Jacqueline Kerler, Maral Ganzorig, Courtney Muhl, Alden Miller, Ini Efiom-Ekaha, Bethy Belai, and Cecilia Bove. 2025. "Fluoroquinolone-Associated Disability: A Rodent Model Reveals Transient Neuropsychiatric and Persistent Gastrointestinal Effects of Low-Dose Ciprofloxacin" Pharmaceuticals 18, no. 9: 1277. https://doi.org/10.3390/ph18091277
APA StyleGetaneh, B., Kerler, J., Ganzorig, M., Muhl, C., Miller, A., Efiom-Ekaha, I., Belai, B., & Bove, C. (2025). Fluoroquinolone-Associated Disability: A Rodent Model Reveals Transient Neuropsychiatric and Persistent Gastrointestinal Effects of Low-Dose Ciprofloxacin. Pharmaceuticals, 18(9), 1277. https://doi.org/10.3390/ph18091277






