Histology of Pompia Peel and Bioactivity of Its Essential Oil: A New Citrus-Based Approach to Skin Regeneration
Abstract
1. Introduction
2. Results
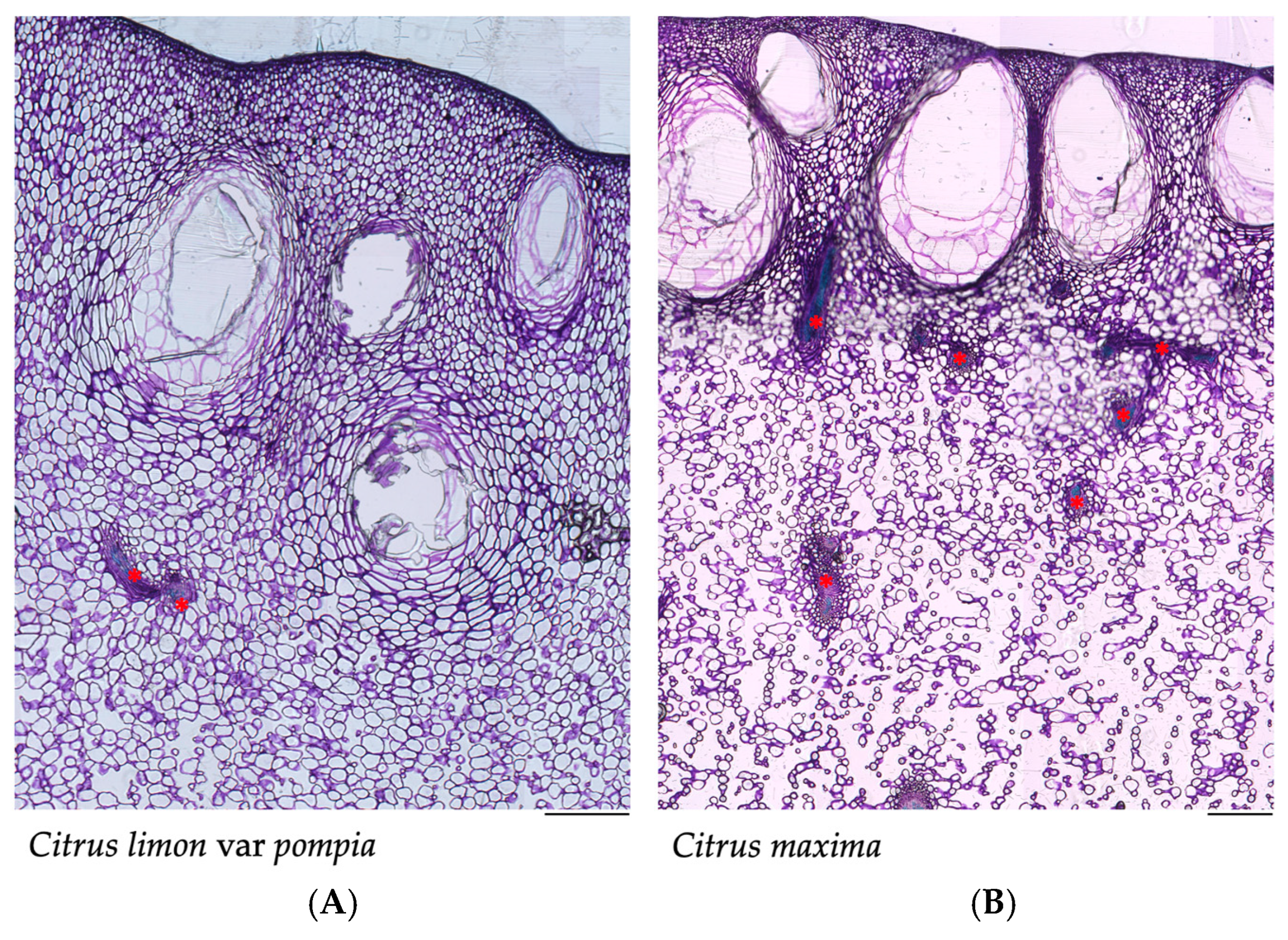
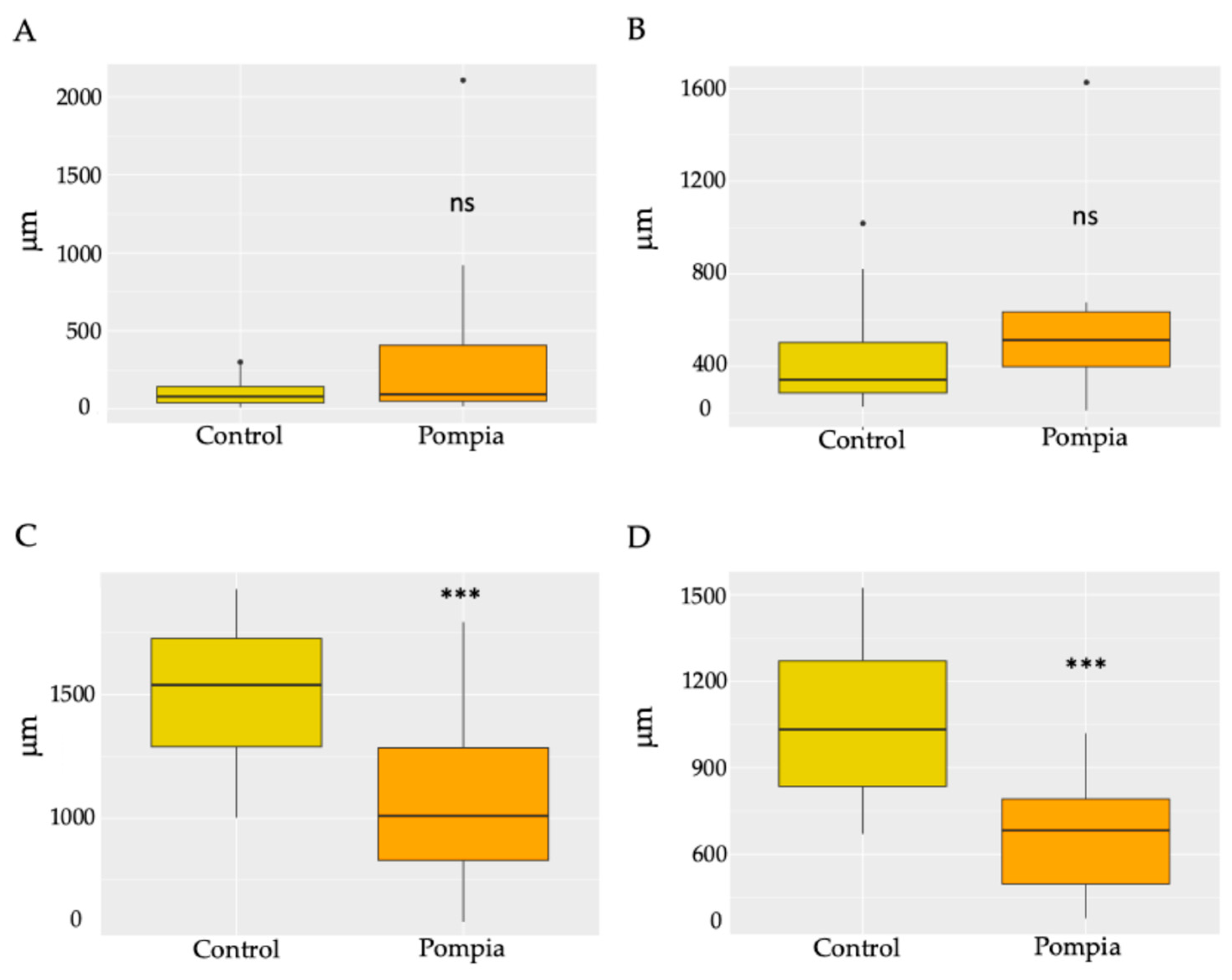
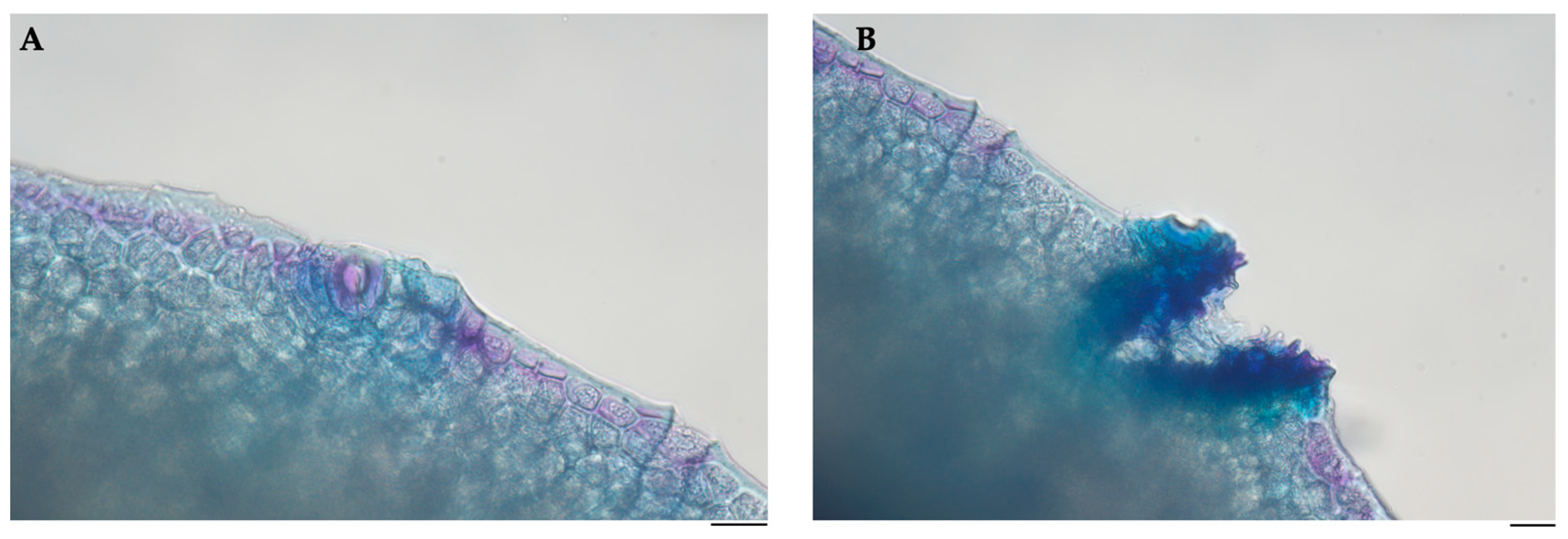
2.1. Morphological and Histological Profile of Pompia Fruit
2.2. Essential Oil Analysis
2.3. Biological Activities
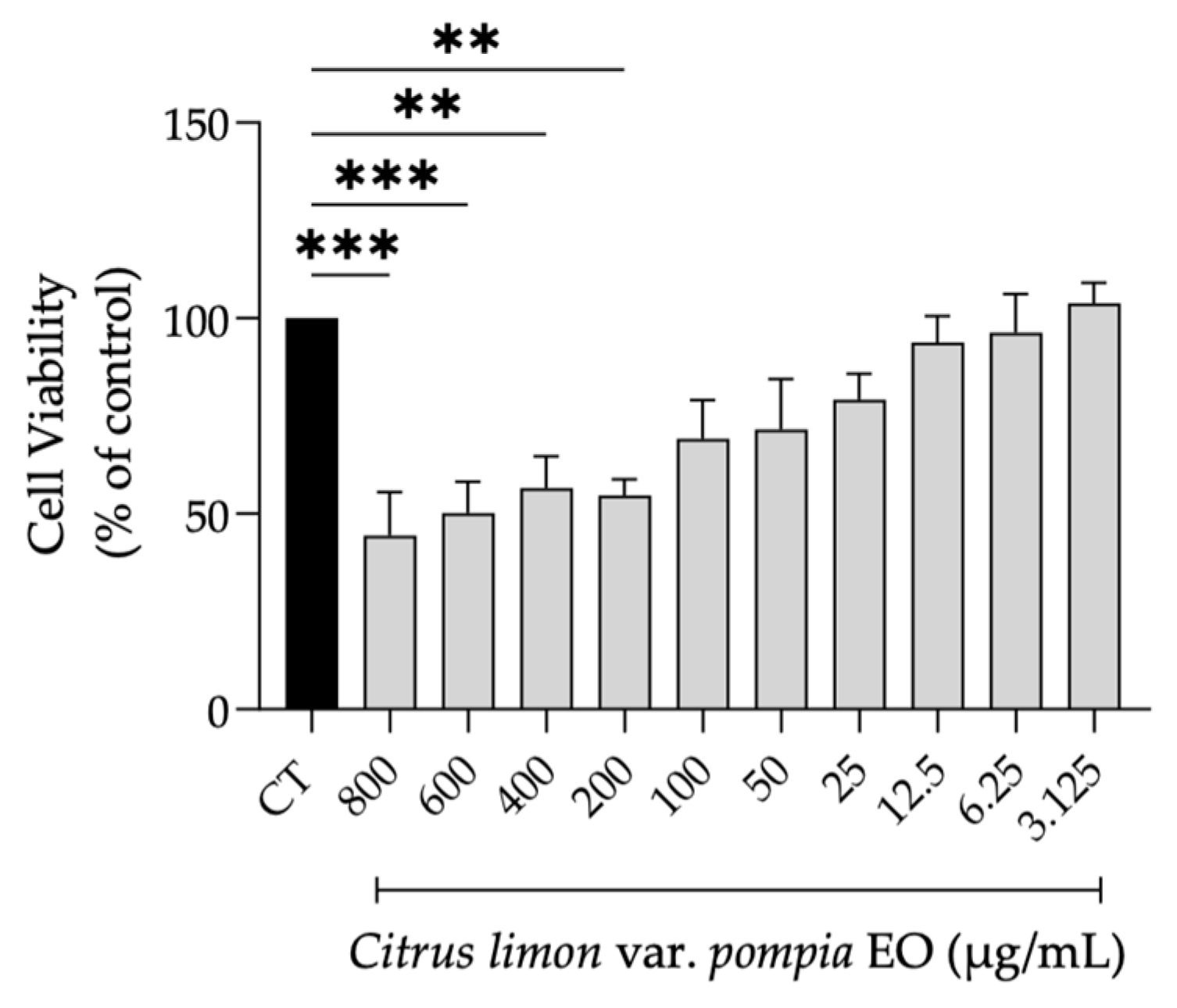
2.3.1. Safety Profile of Pompia Essential Oil
2.3.2. Effect of Pompia Essential Oil on Cell Migration
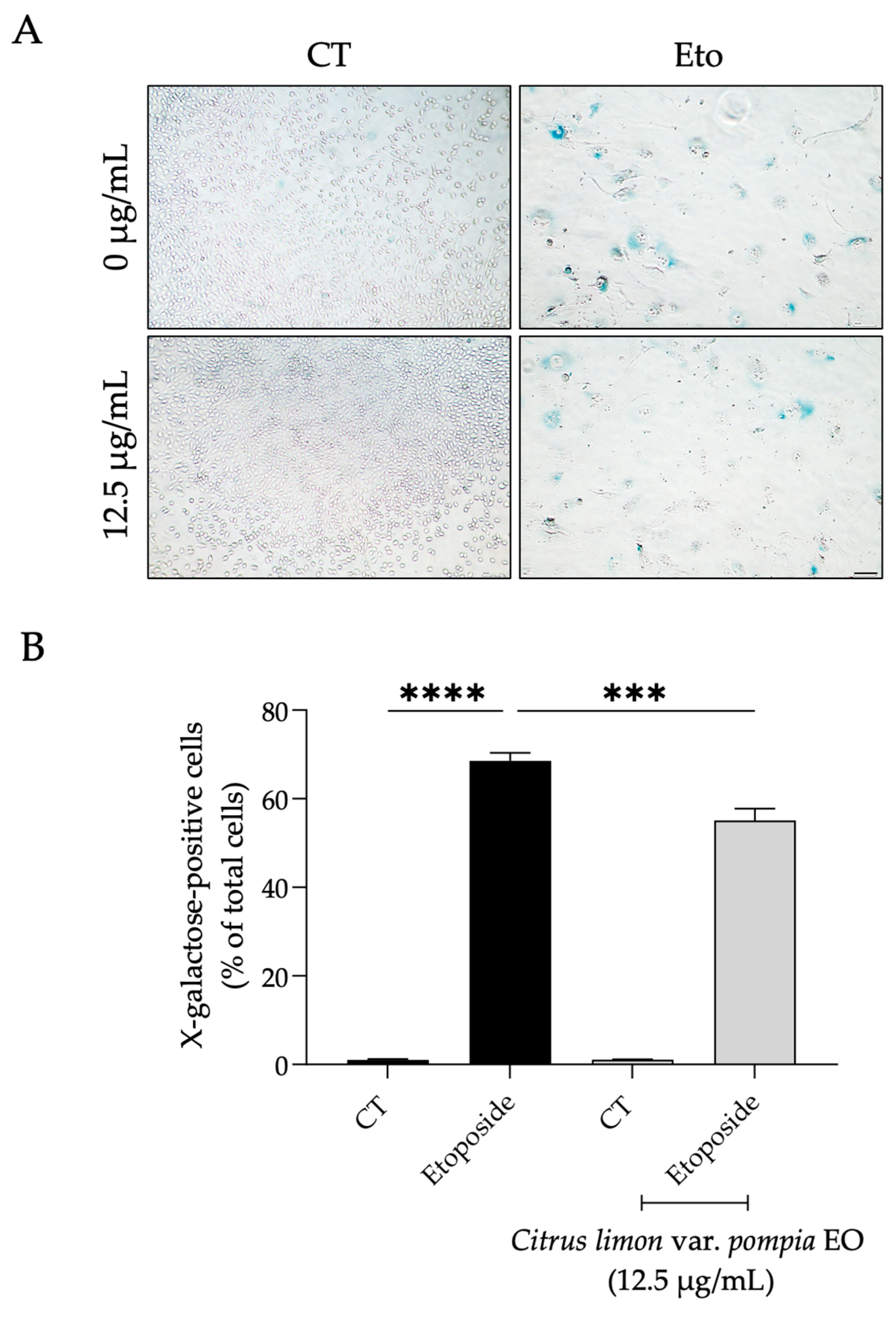
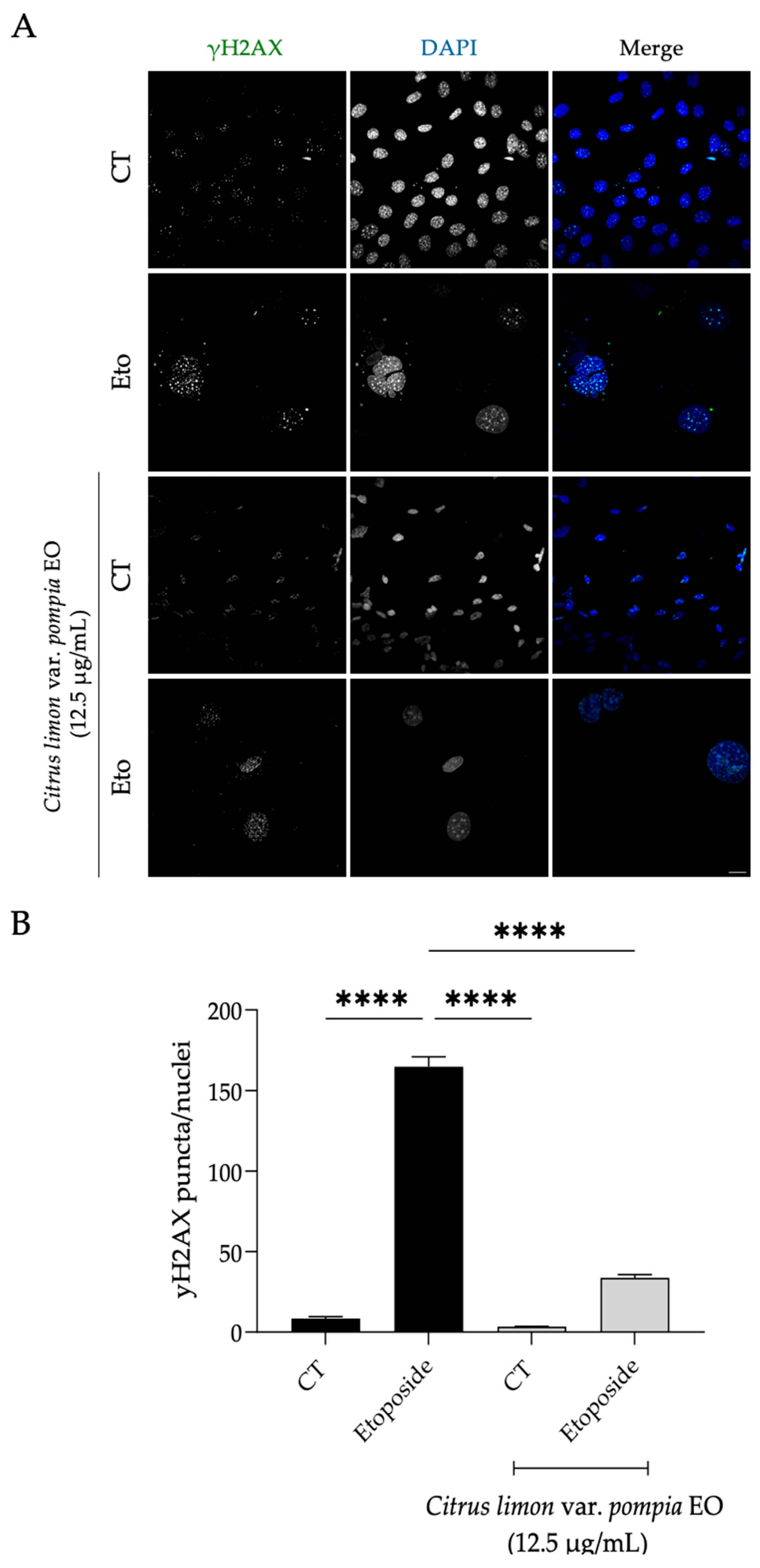
2.3.3. Effect of Pompia Essential Oil on Cellular Senescence
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation
4.2. Histological Analysis
4.3. GC-MS Analysis
4.4. Cell Culture
4.5. Cell Viability
4.6. Scratch Wound Assay
4.7. Cellular Senescence
4.8. Senescence-Associated β-Galactosidase Activity
4.9. γ.H2AX Immunofluorescence Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dell’Arca, A.M. Agricoltura di Sardegna; Vincenzo Orsino: Napoli, Italy, 1780; p. 326. [Google Scholar]
- Cherchi Paba, F. Evoluzione Storica Dell’attività Agricola, Caccia e Pesca in Sardegna; Regione Autonoma della Sardegna, Assessorato Industriale e Commercio, Ed. Fossataro: Cagliari, Italy, 1974. [Google Scholar]
- Moris, G.G. Flora Sardoa: Seu Historia Plantarum in Sardinia et Adjacentibus Insulis vel Sponte Nascentium vel ad Utilitatem Latius Excultarum ex Regio Typographeo; Ex Regio Typographeo: Parma, Italy, 1837–1859; Volumes 1–3. [Google Scholar]
- Camarda, I.; Mazzola, P.; Brundu, A.; Fenu, G.; Lombardo, G.; Palla, F. Un agrume nella storia della Sardegna: Citrus limon var. pompia Camarda var. nova. Quad. Bot. Amb. Appl. 2013, 24, 109–118. [Google Scholar]
- Luro, F.; Viglietti, G.; Marchi, E.; Costantino, G.; Scarpa, G.M.; Tomi, F.; Paoli, M.; Curk, F.; Ollitrault, P. Genetic, morphological and chemical investigations reveal the genetic origin of Pompia (C. medica tuberosa Risso & Poiteau)—An old endemic Sardinian citrus fruit. Phytochemistry 2019, 168, 112083. [Google Scholar]
- Istat. Coltivazioni: Superfici e Produzioni-Dati Complessivi-Prov. 2022. Available online: http://www.istat.it (accessed on 21 March 2025).
- Posadino, A.M.; Maccioccu, P.; Eid, A.H.; Giordo, R.; Pintus, G.; Fenu, G. Citrus limon var. pompia Camarda var. nova: A Comprehensive Review of Its Botanical Characteristics, Traditional Uses, Phytochemical Profile, and Potential Health Benefits. Nutrients 2024, 16, 2619. [Google Scholar] [CrossRef]
- Slow Food Foundation. Available online: https://www.fondazioneslowfood.com/en/slow-food-presidia/pompia/ (accessed on 21 March 2025).
- Posadino, A.M.; Maccioccu, P.; Giordo, R.; Spissu, Y.; Eid, A.H.; Barberis, A.; Pintus, G.; Fenu, G. Exploring the Medicinal Potential of Citrus limon var. pompia Camarda var. nova: From Phytochemicals to Therapeutic Applications. Food Biosci. 2025, 66, 106255. [Google Scholar] [CrossRef]
- Fenu, G.; Carai, A.; Foddai, M.; Azara, E.; Careddu, S.; Usai, M. Composition and seasonal variation of Citrus monstruosa essential oil from Sardinia. Int. J. Essent. Oil Ther. 2010, 4, 23–25. [Google Scholar]
- Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential Oil Composition and Biological Activity of “Pompia”, a Sardinian Citrus Ecotype. Molecules 2019, 24, 908. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’Hallewin, G.; Zaru, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Pinna, R.; Filigheddu, E.; Juliano, C.; Palmieri, A.; Manconi, M.; D’hallewin, G.; Petretto, G.; Maioli, M.; Caddeo, C.; Manca, M.L.; et al. Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics 2019, 11, 234. [Google Scholar] [CrossRef]
- Rosa, A.; Nieddu, M.; Petretto, G.L.; Sarais, G. Chemical composition and in vitro bioactivity of essential oil obtained from the flavedo of ‘Pompia’, an ancient Sardinian fruit. J. Essent. Oil Res. 2019, 31, 390–399. [Google Scholar] [CrossRef]
- Rosa, A.; Petretto, G.L.; Maldini, M.; Tirillini, B.; Chessa, M.; Pintore, G.; Sarais, G. Chemical characterization, antioxidant and cytotoxic activity of hydroalcoholic extract from the albedo and flavedo of Citrus limon var. pompia Camarda. J. Food Meas. Charact. 2023, 17, 627–635. [Google Scholar] [CrossRef]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.-E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Montoro, P.; Jerković, I.; Atzeri, A.; Marijanović, Z.; Serreli, G.; Piacente, S.; Tuberoso, C.I.G. First characterization of Pompia intrea candied fruit: The headspace chemical profile, polar extract composition and its biological activities. Food Res. Int. 2019, 120, 620–630. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef]
- Danzi, D.; Ladu, G.; Veltkamp Prieto, C.; Garitas Bullon, A.; Petretto, G.L.; Fancello, F.; Venditti, T. Effectiveness of essential oil extracted from pompia leaves against Penicillium digitatum. J. Sci. Food Agric. 2020, 100, 3639–3647. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Aleksic, I.; Barac, A.; Arsic-Arsenijevic, V.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathog. Dis. 2021, 74, 102. [Google Scholar] [CrossRef]
- Gao, S.; Kang, H.; An, X.; Cheng, Y.; Chen, H.; Chen, Y.; Li, S. Non-destructive Storage Time Prediction of Newhall Navel Oranges Based on the Characteristics of Rind Oil Glands. Front. Plant Sci. 2022, 13, 811630. [Google Scholar] [CrossRef]
- Pal, D.; Malik, S.K.; Kumar, S.; Choudhary, R.; Sharma, K.C.; Chaudhury, R. Genetic Variability and Relationship Studies of Mandarin (Citrus reticulata Blanco) Using Morphological and Molecular Markers. Agric. Res. 2013, 2, 236–245. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Voo, S.S.; Grimes, H.D.; Lange, B.M. Assessing the Biosynthetic Capabilities of Secretory Glands in Citrus Peel. Plant Physiol. 2012, 159, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.G.; Klieber, A.; Sedgley, M. The Relationship between Oil Gland and Fruit Development in Washington Navel Orange (Citrus sinensis L. Osbeck). Ann. Bot. 2001, 88, 1039–1047. [Google Scholar] [CrossRef]
- Wang, H.; Ren, J.; Zhou, S.; Duan, Y.; Zhu, C.; Chen, C.; Liu, Z.; Zheng, Q.; Xiang, S.; Xie, Z.; et al. Molecular regulation of oil gland development and biosynthesis of essential oils in Citrus spp. Science 2024, 383, 659–666. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon, L. Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 2019, 10, 1167. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, M.; Rattanpal, H.S.; Arora, A.; Sharma, V.; Singh, S. Physiological and biochemical characterisation of split and healthy Daisy mandarin (Citrus reticulata Burm.) fruits. N. Z. J. Crop Hortic. Sci. 2024, 53, 1061–1078. [Google Scholar] [CrossRef]
- La Spada, P.; Dominguez, E.; Continella, A.; Heredia, A.; Gentile, A. Factors influencing fruit cracking: An environmental and agronomic perspective. Front. Plant Sci. 2024, 15, 1343452. [Google Scholar] [CrossRef] [PubMed]
- Linjing, Z.; Mingzhu, G. Cracking mechanism of Prunus salicina and related preventions. Acta Hortic. Sin. 2006, 33, 699. [Google Scholar]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef]
- Palmas, L.; Aroffu, M.; Petretto, G.L.; Escribano-Ferrer, E.; Díez-Sales, O.; Usach, I.; Peris, J.-E.; Marongiu, F.; Ghavam, M.; Fais, S.; et al. Entrapment of Citrus limon var. pompia Essential Oil or Pure Citral in Liposomes Tailored as Mouthwash for the Treatment of Oral Cavity Diseases. Pharmaceuticals 2020, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, X.; Liu, C.; Liu, L. Exploring the Potential of Lemon Peel Extracts in Cosmetics: Chemical Composition and Bioactive Properties. J. Microbiol. Biotechnol. 2025, 35, e2412042. [Google Scholar] [CrossRef]
- Ishfaq, M.; Akhtar, B.; Muhammad, F.; Sharif, A.; Akhtar, M.F.; Hamid, I.; Sohail, K.; Muhammad, H. Antioxidant and Wound Healing Potential of Essential Oil from Citrus reticulata Peel and Its Chemical Characterization. Curr. Pharm. Biotechnol. 2021, 22, 1114–1121. [Google Scholar] [CrossRef]
- D’Alessio, P.A.; Mirshahi, M.; Bisson, J.F.; Bene, M.C. Skin repair properties of d-Limonene and perillyl alcohol in murine models. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2014, 13, 29–35. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, T.H.; Ansari, M.N.; Ahmad, S.F. Enhanced Wound Healing by Topical Administration of D Limonene in Alloxan Induced Diabetic Mice through Reduction of pro-Inflammatory Markers and Chemokine Expression. BMC Genom. 2014, 15, P29. [Google Scholar] [CrossRef][Green Version]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-Coated Poly(lactic acid) Nanofibres Loaded with Essential Oils for Wound Healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Li, L.; Cai, Y.; Kang, X.; Li, P. Carboxymethyl chitosan-based electrospun nanofibers with high citral-loading for potential anti-infection wound dressings. Int. J. Biol. Macromol. 2022, 209, 344–355. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Cellular Senescence in Acute and Chronic Wound Repair. Cold Spring Harb. Perspect. Biol. 2022, 14, a041221. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Chang, H.-C.; Chao, Y.-Y.; Cheng, H.-L.; Lien, W.-C.; Wang, C.-Y. Etoposide Triggers Cellular Senescence by Inducing Multiple Centrosomes and Primary Cilia in Adrenocortical Tumor Cells. Cells 2021, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Karpinich, N.O.; Tafani, M.; Rothman, R.J.; Russo, M.A.; Farber, J.L. The Course of Etoposide-Induced Apoptosis from Damage to DNA and P53 Activation to Mitochondrial Release of Cytochromec. J. Biol. Chem. 2002, 277, 16547–16552. [Google Scholar] [CrossRef] [PubMed]
- El Kady, W.M.; Ayoub, I.M.; El Mehrate, A.K.; Emad, M.; Tarek, M.; El Gdeily, A.; Mohamed, E.R.; Medhat, R.; Mahmoud, O.; Gad, A.M.; et al. Valorization of Citrus peels: GC/MS-based metabolites profiling, multivariate analysis, and antiaging potential. Arch. Pharm. 2024, 357, e2300742. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Lall, N.; Anina Lambrechts, I.; Reid, A.-M.; Maphutha, J.; Nel, M.; Hassan, A.H.; Khalid, A.; Abdalla, A.N.; Van, B.L.; et al. In Vitro and In Silico Pharmacological and Cosmeceutical Potential of Ten Essential Oils from Aromatic Medicinal Plants from the Mascarene Islands. Molecules 2022, 27, 8705. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Elhady, S.S.; Bannan, D.F.; Malatani, R.T.; Gad, H.A. Citrus reticulata Leaves Essential Oil as an Antiaging Agent: A Comparative Study between Different Cultivars and Correlation with Their Chemical Compositions. Plants 2022, 11, 3335. [Google Scholar] [CrossRef]
- Li, G.; Xiang, S.; Pan, Y.; Long, X.; Cheng, Y.; Han, L.; Zhao, X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021, 8, 689094. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Sousa, C.; Alves-Silva, J.; Salgueiro, L. Plant Monoterpenes and Essential Oils as Potential Anti-Ageing Agents: Insights from Preclinical Data. Biomedicines 2024, 12, 365. [Google Scholar] [CrossRef]
- Secerli, J.; Erdem, O.; Bacanlı, M. Antiaging Effects of Limonene in Ageing-Induced HaCaT Cells. Genet. Appl. 2023, 7, 27–34. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.-P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Salsabila, D.; Wardani, R.; Hasanah, N.; Tafrihani, A.; Zulfin, U.; Ikawati, M.; Meiyanto, E. Cytoprotective properties of citronella oil (Cymbopogon nardus (L.) Rendl.) and lemongrass oil (Cymbopogon citratus (DC.) Stapf) through attenuation of senescent-induced chemotherapeutic agent doxorubicin on Vero and NIH-3T3 Cells. Asian Pac. J. Cancer Prev. 2023, 24, 1667–1675. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 7th ed.; Directorate for the Quality of Medicines & HealthCare of the Council of Europe: Strasbourg, France, 2010; ISBN 978-92-871-6700-2. [Google Scholar]
- Liang, S.J.; Wu, H.; Lun, X.; Lu, D.W. Secretory cavity development and its relationship with the accumulation of essential oil in fruits of Citrus medica L. var. sarcodactylis (Noot.) Swingle. J. Integr. Plant Biol. 2006, 48, 573–583. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Maccioni, D.; Cocco, E.; Gonçalves, M.J.; Porcedda, S.; Piras, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants 2023, 12, 1247. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Maccioni, A.; Falconieri, D.; Porcedda, S.; Gonçalves, M.J.; Alves-Silva, J.M.; Silva, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Chemical composition and biological activity of essential oil of Teucrium scordium L. subsp. scordioides (Schreb.) Arcang. (Lamiaceae) from Sardinia Island (Italy). Nat. Prod. Res. 2022, 36, 5828–5835. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. In Epidermal Cells: Methods in Molecular Biology; Turksen, K., Ed.; Humana: New York, NY, USA, 2019; Volume 2109, pp. 225–229. [Google Scholar]
- Alves-Silva, J.M.; Cocco, E.; Piras, A.; Gonçalves, M.J.; Silva, A.; Falconieri, D.; Porcedda, S.; Cruz, M.T.; Maxia, A.; Salgueiro, L. Unveiling the Chemical Composition and Biological Properties of Salvia cacaliifolia Benth. Essential Oil. Plants 2023, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 March 2024).

| RI | RI (Litt) | Compound | % Area ± SD |
|---|---|---|---|
| 933 | 932 | α-Pinene | 0.45 ± 0.008 |
| 973 | 969 | Sabinene | 0.05 ± 0.002 |
| 977 | 974 | β-Pinene | 0.05 ± 0.001 |
| 986 | 986 | 6-Methyl-5-hepten-2-one | 0.04 ± 0.002 |
| 991 | 988 | Myrcene | 1.71 ± 0.047 |
| 1003 | 998 | n-Octanal | 0.03 ± 0.002 |
| 1006 | 1002 | α-Phellandrene | 0.08 ± 0.002 |
| 1026 | 1024 | Limonene | 89.17 ± 0.939 |
| 1036 | 1038 | cis-Ocimene | 0.17 ± 0.031 |
| 1047 | 1047 | trans-Ocimene | 0.76 ± 0.023 |
| 1058 | 1054 | γ-Terpinene | 0.04 ± 0.001 |
| 1069 | 1063 | n-Octanol | 0.04 ± 0.005 |
| 1089 | 1086 | Terpinolene | 0.05 ± 0.001 |
| 1100 | 1095 | Linalool | 0.47 ± 0.029 |
| 1104 | 1100 | n-Nonanal | 0.56 ± 0.033 |
| 1153 | 1148 | Citronellal | 0.22 ± 0.018 |
| 1183 | 1177 | (E)-Isocitral | 0.05 ± 0.004 |
| 1191 | 1186 | α-Terpineol | 0.16 ± 0.008 |
| 1228 | 1223 | Citronellol | 0.82 ± 0.109 |
| 1241 | 1235 | Neral | 1.24 ± 0.093 |
| 1254 | 1249 | Geraniol | 0.35 ± 0.057 |
| 1271 | 1264 | Geranial | 1.63 ± 0.170 |
| 1274 | 1269 | Perilla aldehyde | 0.06 ± 0.001 |
| 1365 | 1359 | Neryl acetate | 0.17 ± 0.023 |
| 1385 | 1379 | Geranyl acetate | 0.12 ± 0.020 |
| 1420 | 1420 | β-Caryophyllene | 0.10 ± 0.013 |
| 1436 | 1432 | α-trans-Bergamotene | 0.43 ± 0.046 |
| 1457 | 1454 | E-β-Farnesene | 0.03 ± 0.004 |
| 1493 | 1496 | Valencene | 0.27 ± 0.031 |
| 1497 | 1500 | Bicyclogermacrene | 0.08 ± 0.010 |
| 1509 | 1505 | β-Bisabolene | 0.59 ± 0.064 |
| Total identified | 99.97 | ||
| Total monoterpenes | 92.53 | ||
| Total sesquiterpenes | 1.50 | ||
| Total oxygenated | 5.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, E.; Giorgi, G.; Marsigliesi, V.; Mura, F.; Alves-Silva, J.M.; Zuzarte, M.; Salgueiro, L.; Ghiani, V.; Sanjust, E.; Falconieri, D.; et al. Histology of Pompia Peel and Bioactivity of Its Essential Oil: A New Citrus-Based Approach to Skin Regeneration. Pharmaceuticals 2025, 18, 1256. https://doi.org/10.3390/ph18091256
Cocco E, Giorgi G, Marsigliesi V, Mura F, Alves-Silva JM, Zuzarte M, Salgueiro L, Ghiani V, Sanjust E, Falconieri D, et al. Histology of Pompia Peel and Bioactivity of Its Essential Oil: A New Citrus-Based Approach to Skin Regeneration. Pharmaceuticals. 2025; 18(9):1256. https://doi.org/10.3390/ph18091256
Chicago/Turabian StyleCocco, Emma, Giulia Giorgi, Valeria Marsigliesi, Francesco Mura, Jorge M. Alves-Silva, Mónica Zuzarte, Lígia Salgueiro, Valentina Ghiani, Enrico Sanjust, Danilo Falconieri, and et al. 2025. "Histology of Pompia Peel and Bioactivity of Its Essential Oil: A New Citrus-Based Approach to Skin Regeneration" Pharmaceuticals 18, no. 9: 1256. https://doi.org/10.3390/ph18091256
APA StyleCocco, E., Giorgi, G., Marsigliesi, V., Mura, F., Alves-Silva, J. M., Zuzarte, M., Salgueiro, L., Ghiani, V., Sanjust, E., Falconieri, D., Maccioni, D., Valletta, A., Brasili, E., & Maxia, A. (2025). Histology of Pompia Peel and Bioactivity of Its Essential Oil: A New Citrus-Based Approach to Skin Regeneration. Pharmaceuticals, 18(9), 1256. https://doi.org/10.3390/ph18091256













