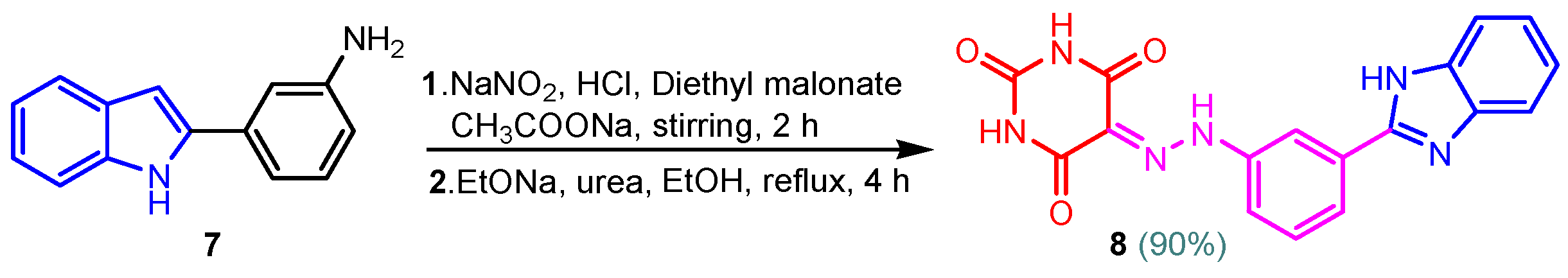Abstract
Background: Heterocyclic compounds represent a key class of compounds in medicinal chemistry. Both benzimidazoles and pyrimidines are essential heterocycles in medicinal chemistry, with various therapeutic properties. Recent literature presents a series of hybrid heterocyclic compounds, as their medicinal properties are generally improved compared to those of single heterocyclic rings. Methods: A literature search was conducted across relevant scientific literature from peer-reviewed sources, using keywords, including “benzimidazole”, “pyrimidine”, “Biginelli”, “benzimidazole-pyrimidine hybrids”, “anticancer”, “antiviral”, “antimicrobial”, and “anti-inflammatory”. Results: In this review, benzimidazole–pyrimidine hybrids are reported as anticancer, antimicrobial, antiviral, anti-inflammatory, analgesic, antiulcer, antidepressant, anti-Alzheimer’s, or antioxidant agents, with activities even better than those of existing drugs. The IC50 values for these anticancer hybrids are in the nanomolar range, which signifies potent anticancer agents. It can be mentioned here that the anticancer hybrid Abemaciclib, as a CDK4/6 inhibitor for the treatment of certain types of breast cancer, was approved in 2017. The antimicrobial activity of these hybrids proved especially potent against a broad variety of infections, with MIC values in the range of µM or even nM. Moreover, these hybrids exhibited good antiviral properties against SARS-CoV-2, HIV-1, and the hepatitis C virus. The hybrids also functioned as JAK3 inhibitors, COX-1 inhibitors, and MAO-A inhibitors. Conclusions: This review presents synthesis methods of benzimidazole–pyrimidine hybrids, their medicinal properties, and SAR studies reported in the last 20 years. For almost every therapeutic activity, SAR studies have revealed the essential presence of a substituent on the aromatic rings or between the two benzimidazole and pyrimidine nuclei.
1. Introduction
Since the medicinal properties of hybrid compounds are typically better than those of simple heterocyclic compounds, which only contain one type of heterocycle in the molecule, organic compounds with two or more distinct heterocyclic rings are being reported more frequently in current medicinal chemistry as possible therapeutic compounds [1,2,3,4,5]. Thus, hybrids created by combining two well-known pharmacophores have novel, peculiar characteristics, with fantastic properties that are determined by the unique structure of the newly created molecule [6]. This new design is highlighted in the efficacy of the new drug defined by the physicochemical parameters, namely absorption, distribution, the mechanism of interaction between the hybrid and its cellular target, metabolism, excretion, and toxicity [7,8,9]. Recent articles and reviews report a series of hybrid compounds with diverse therapeutic properties, such as coumarin–triazole [10], metronidazole–berberine [11], quinoline–triazole [12], benzimidazole–triazoles [13], benzimidazole–pyrazole [14,15,16], benzimidazole–morpholine [17], and of course many others.
Benzimidazole, or 1H-benzimidazole, a bicyclic heterocyclic aromatic compound in which a benzene ring is fused to the “4” and “5” positions of an imidazole ring, is a crucial part of vitamin B12, and only the imidazole ring scaffold is present in several natural compounds, such as histidine and purines. Due to its presence in many drugs, benzimidazole is also a key component in medicinal chemistry [17]. Recent articles indicate various routes for the synthesis of benzimidazoles, such as the coupling of 1,2-diaminobenzenes with carboxylic acids (Phillips–Ladenburg reaction), the coupling of 1,2-diaminobenzenes with aldehydes and ketones (Weidenhagen reaction), or the rearrangement of quinoxalinones [14,16].
The pyrimidine ring is present in vitamin B1 (thiamine) and several natural compounds, such as the pyrimidine bases uracil, thymine, and cytosine, which are fundamental building blocks for DNA and RNA synthesis and are essential for cellular functions [18]. Various methods for the synthesis of pyrimidines are studied in the literature, such as two-component cycloadditions, like the [5 + 1] annulation of enamidines, [4 + 2] cycloadditions, [3 + 3] cycloadditions, three-component cycloadditions, or the Biginelli reaction [18,19].
In this review, starting from the fact that both the pyrimidine [18,19,20] and the benzimidazole [21,22,23] nuclei are found as key nuclei in drugs with various therapeutic applications, such as anticancer [24,25], antibacterial [26,27], antifungal [28,29], antiviral [30,31], antidiabetic [32,33,34], antiulcer [35,36], antioxidant [37,38], anti-Alzheimer’s [39,40], antidepressant [41,42], anti-Parkinson’s [43,44], anticonvulsant [45,46], and anti-inflammatory [47,48], we aimed to review the synthetic methods of pyrimidine–benzimidazole hybrids, the medicinal properties of aforementioned hybrids, as well as the reported structure–property relationships (SAR). When required, a number of examples from the literature were provided for compounds with superior biological activity, along with a description of the several studies that were conducted on them (in vitro, in vivo, in silico).
To the best of our knowledge, this is the first review to examine the synthesis and therapeutic properties of pyrimidine–benzimidazole hybrids.
This review’s database search strategy involved using keywords that appear in the title, such as “pyrimidine”, “benzimidazole”, “benzimidazole-pyrimidine hybrids”, “Biginelli reaction”, “anticancer”, “anti-inflammatory”, “antimicrobial”, “antiviral”, and so forth, or therapeutic properties, across a variety of websites, including ACS Publications, PubMed, MDPI, Science Direct, Springer, The Royal Society Chemistry, and Taylor & Francis. Articles from the past ten years have often been chosen.
2. Anticancer Benzimidazole–Pyrimidine Hybrids
Cancer remains one of the most relentless contemporary diseases, increasingly common in people of all ages, social statuses, and lifestyles [49]. Finding new anticancer compounds remains a target of medicinal chemistry, for which nitrogen heterocyclic compounds constitute an advantageous choice, proven by recent studies [50].
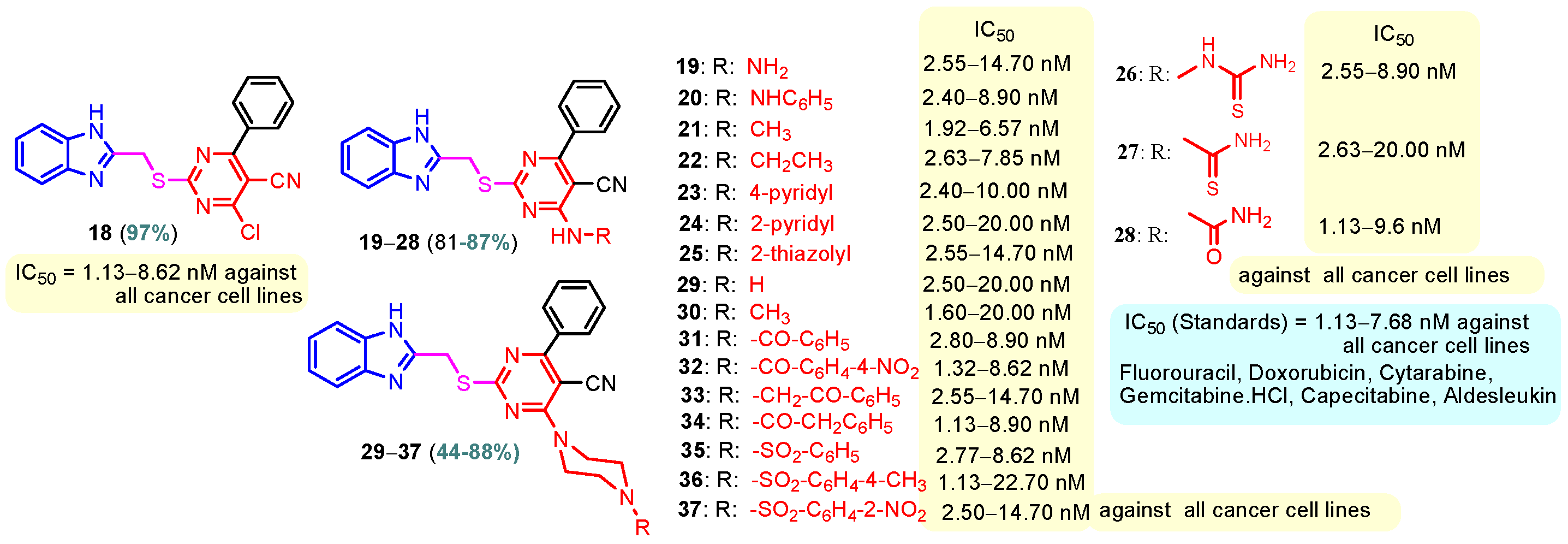
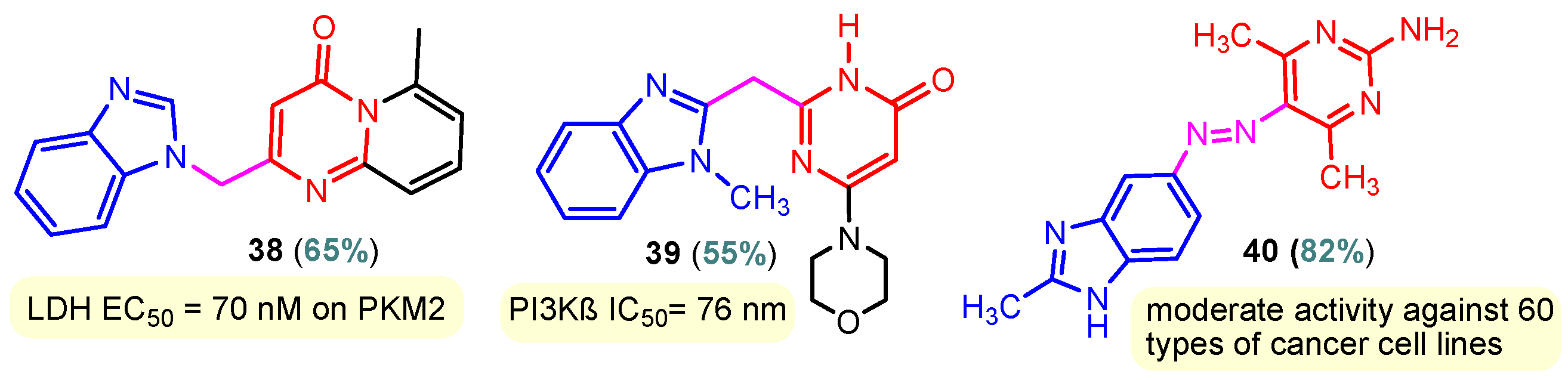
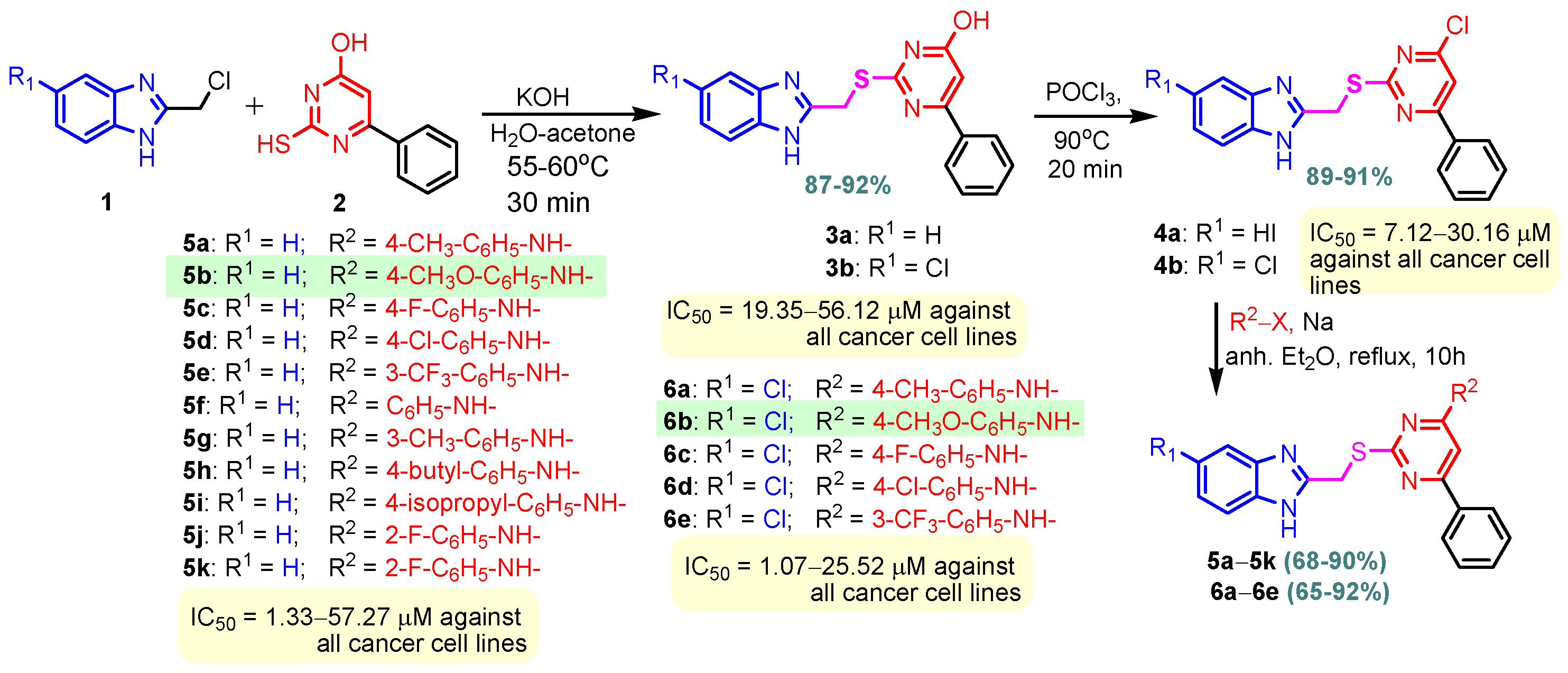
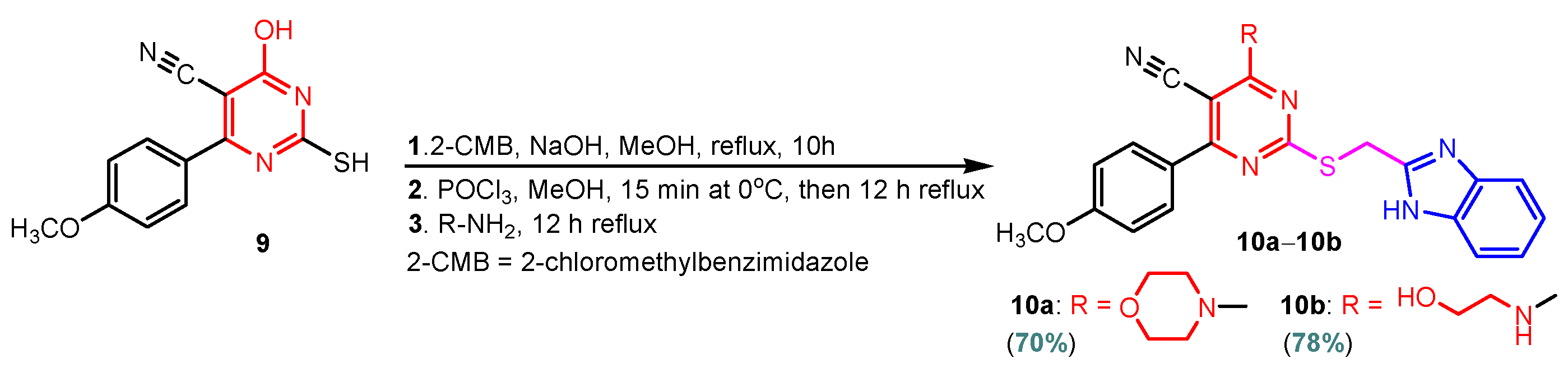
Benzimidazole–pyrimidine compounds represent a good choice in the treatment of cancer, as proven by recent studies conducted on the hybrids discussed in this article. Abemaciclib (Figure 1), the newest benzimidazole–pyrimidine hybrid CDK4/6 inhibitor for the treatment of certain types of breast cancer, approved in 2017, is the drug that demonstrates the efficacy of the presence of the two heterocyclic rings in its structure [51,52,53,54]. Goetz et al. (2024) demonstrated in analyses from the monarchE study that protocol-mandated dose reductions from 150 mg to 100 mg or 50 mg for patients with node-positive, hormone receptor-positive, human epidermal growth factor 2-negative, high-risk early breast cancer did not impair the effectiveness of adjuvant abemaciclib [55]. In another study, monarchE has demonstrated that abemaciclib added to standard adjuvant ET aromatase inhibitors [AIs] and/or antiestrogens with or without ovarian suppression (ET), significantly improves Invasive Disease-Free Survival (IDFS) in women and men with hormone receptor-positive HR+, hormone receptor-negative HER2, and node-positive early breast cancer (EBC), at high risk of early recurrence [56,57,58,59]. Kalinsky et al. (2024) found Abemaciclib plus Fulvestrant significantly improved PFS (Progression-Free Survival) after disease progression on previous CDK4/6i (cyclin-dependent kinase 4/6 inhibitors) + ET in patients with HR+, HER2– ABC, providing these patients with an extra alternative for targeted treatment [60]. Shao et al. (2014) reported the synthesis of anticancer hybrids 3–6, from 2-(chloromethyl)-1H-benzo[d]imidazole 1 and 2-mercapto-6- phenylpyrimidin-4-ol 2 in three steps (Scheme 1) [61]. Higher yields are observed for compounds 6a–6e compared to compounds 5a–5k due to the presence of the chlorine atom in the “5” position of the benzimidazole nucleus. All compounds had good anticancer activity when tested in vitro on four human cancer cell lines, including MCF-7 (human breast cancer cell line), MGC-803 (human gastric cancer cell line), EC-9706 (human esophageal cancer cell line), and SMMC-7721 (human liver cancer cell line) using the MTT assay method, as can be seen from the IC50 (concentration required to achieve 50% inhibition of the tumor growth) values in Scheme 1 for the hybrids and the standard 5-fluorouracil (5-FU). The most active compounds were 5a–5b and 6a–6b, with IC50 values of 1.33–20.50 µM and 1.07–19.28 µM, respectively. This suggests that the anticancer activities were aided by the addition of small electron-donating groups, such as CH3 or OCH3, at the para-position of the phenyl ring [61]. Abdelgawad et al. (2019) reported the synthesis of 2,4,6-trione 8 in two steps from 3-(1H-indol-2-yl)benzenamine 7 (Scheme 2) [62]. Compound 8 revealed moderate activity against breast carcinoma (MCF-7), non-small cell lung cancer (A549), human prostate cancer (PC-3), human pancreatic cancer (PaCa-2), and colorectal adenocarcinoma (HT-29) cell lines with IC50 values of 4.3–8.8 μM. This result marks hybrid 8 as a target for further development in the field of anticancer agents [62]. Compounds 10a and 10b were synthesized from 4-hydroxy-2-mercapto-6- (4-methoxyphenyl)pyrimidine-5-carbonitriles 9 in three steps: reaction with 2-chloromethyl benzimidazole at reflux for 10 h in methanol with the formation of the first benzimidazole–pyrimidine hybrids, synthesis of the chlorinated pyrimidine derivative by reaction with phosphoryl chloride, followed by reaction with amines, morpholine or 2-aminoethanol (Scheme 3). As can be seen, the presence of the 2-aminoethanol group on the pyrimidine nucleus in compound 10a favored a better reaction yield (78%) compared to the presence of the N-morpholino group in compound 10b, resulting in a yield of 70%. The most active compound 10a exhibited broad-spectrum cytotoxic activity against 25 cancer cell lines, with growth inhibitory activity of 88.84%, 79.89%, and 84.19% against HOP-92 (non-small cell lung cancer), A498 (renal cancer), and T-47D (breast cancer cell line), respectively. Compound 10b exhibited 61.80% growth inhibitory activity against the MOLT-4 (leukemia) cell line [63]. Reaction of enone 11 with an appropriately substituted N-arylguanidinium nitrate 12 and sodium hydroxide, at reflux in propan-2-ol, generated benzimidazole–pyrimidine hybrids 13a–13j with yields of 24–56% (Scheme 4). The presence of the morpholino group in compound 13d considerably improved the reaction yield, to 53%, as did the methoxy group in 13f (51.5%). The hydroxy group in compound 13g improved the yield the most, at 56%. The presence of chlorine led to a dramatic decrease in yield to 24% for compound 13i, while the simultaneous presence of hydroxy and methoxy groups in compound 13j led to a decrease in yield to 34% compared to 13g (56%). Hybrids 13a–13j inhibited at least four cancer-related protein kinases, namely Aurora B, PLK1, FAK, and VEGFR2. It should be noted that the most potent protein kinase inhibitor in the series, 13j, inhibited several cancer cell lines of the NCI panel in submicromolar concentrations, as can be seen in Table 1. The data in Table 1 show that the presence of a small oxygen-containing substituent at position “3” or “4” of the phenyl ring (derivatives 13f–13j) is important for kinase inhibition. Only low action is displayed by compounds with a bigger substituent (13d, 13e) or those without such a substituent (13a–13c). The decreased inhibitory activity of the derivatives 13d and 13e can be explained by the observation that substituents greater than methoxy may interfere with the pillar-like structure generated by Leu59 and Arg136 at the pocket entrance due to the alignment of this pose [64]. Zheng et al. (2013) synthesized a series of thirteen benzimidazole–pyrimidine compounds, having the imidazole ring as the connecting bridge between the two target rings (Scheme 5) [65]. All compounds were tested against the cancer cell lines, human acute monocytic leukemia cell line U937, human chronic myeloid leukemia cell line K562, human non-small cell lung cancer A549, and human colon cancer LoVo and HT29, and analyzed for Aurora A/B kinase inhibitory activity in vitro. Compounds 17a–17d showed similar potency to AT-9283 (IC50 ranged from 0.40 to 0.6 µM), with IC50 values ranging from 0.30 to 0.80 µM, as shown in Scheme 5. The good antitumor activity of compounds 17a–17e was correlated with the small volume of the substituent at the “2” position, such as methyl, methylthio, and methyl sulfonyl, of the pyrimidine nucleus [65]. Following a synthesis procedure similar to Shao and Haoran, Abdel-Mohsen and co-workers (2010) reported benzimidazole–pyrimidine carbonitriles 18–37 (Figure 2) of potent antitumor activity against 12 cell lines namely, Cervical carcinoma (KB), Ovarian carcinoma (SK OV-3), CNS cancer (SF-268), Non-small lung cancer (NCI H460), Colon adenocarcinoma (RKOP27), Leukemia (HL60, U937, K562), Melanoma (G361, SK-MEL-28), and Neuroblastoma (GOTO, NB-1). The MTT test was employed in accordance with the Mosmann method to determine the anticancer activity. Figure 2 reports the concentration values required to achieve 50% inhibition of the tumor growth of the tested compounds and of the standards (IC50 (nM)) [66]. Guo et al. (2013) synthesized benzimidazole–pyrimidine hybrid 38 by the reaction between benzimidazole and 2-(chloromethyl)-6-methyl-4H-pyrido [1,2-a]pyrimidin-4-one in DMF and K2CO3 as a catalyst [67]. Compound 38 activated Pyruvate Kinase M2 (PKM) in Huh7 cells (human liver cancer cell line), with an EC50 value of 70 nM, with a novel binding mode to the PKM2 protein (Figure 3) [67]. Certal et al. (2012) reported a new series of benzimidazole–pyrimidone hybrids as potent and selective PI3Kβ inhibitors [68]. Compound 39, resulting from the reaction between sodium [4-(morpholin-4-yl)-6-oxo-1,6-dihydropyrimidin-2-yl] acetate and N-methyl-1,2-phenylenediamine in pyridine with a yield of 55%, showed significant activity and selectivity for PI3Kβ, with an IC50 value of 76 nM. The antitumor activity of compound 39 in the human PTEN-deficient PC3 prostate carcinoma tumor model was investigated, xenografted subcutaneously in SCID mice. Hybrid 39 decreased tumor growth by 45% on day 32 at the end of therapy after being given orally to PC3 tumor-bearing mice at a dose of 300 mg/kg twice a day for nine days [68].
Figure 1.
Structural formula of the anticancer drug Abemaciclib.
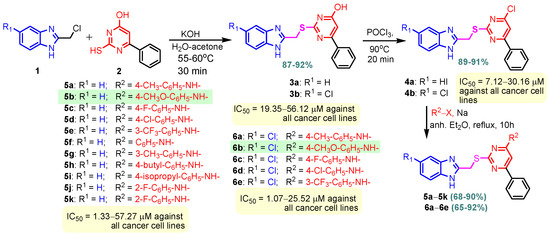
Scheme 1.
Synthesis of benzimidazole–pyrimidine hybrids 3–6.
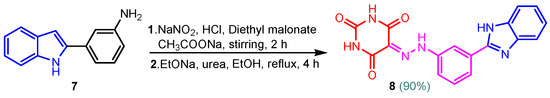
Scheme 2.
Synthesis of benzimidazole–pyrimidine hybrid 8.

Scheme 3.
Synthesis of benzimidazole–pyrimidine hybrids 10a–10b.
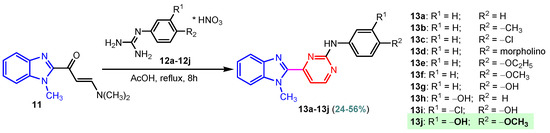
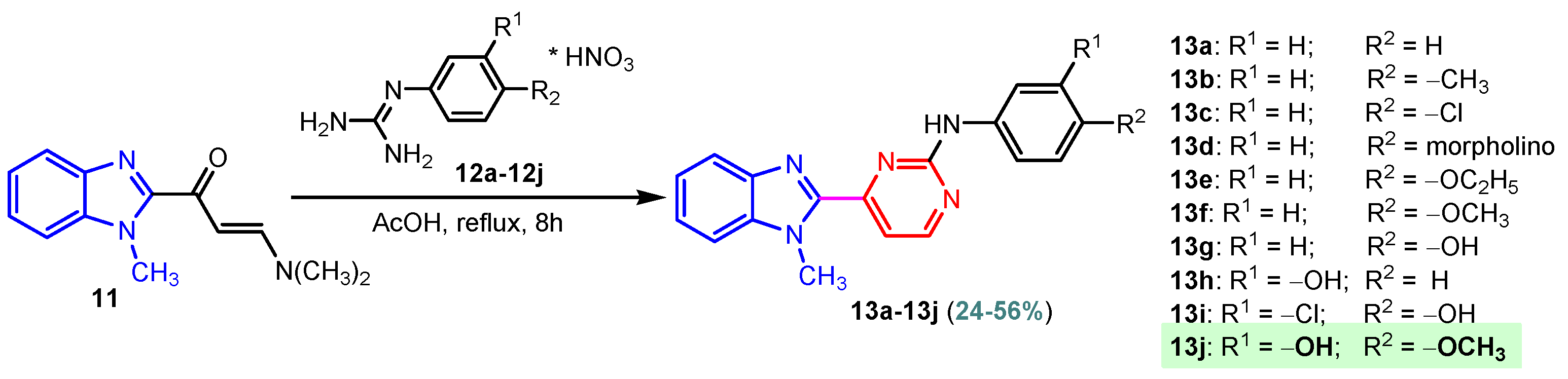
Scheme 4.
Synthesis of benzimidazole–pyrimidine hybrids 13a–13j.

Table 1.
Protein kinase inhibition by 13a–13j compared to standard agents sorafenib and sunitinib.
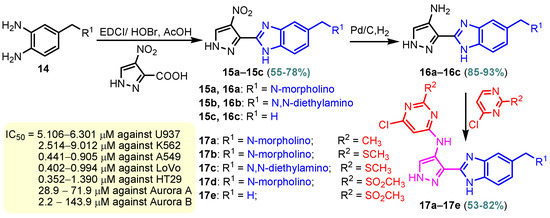
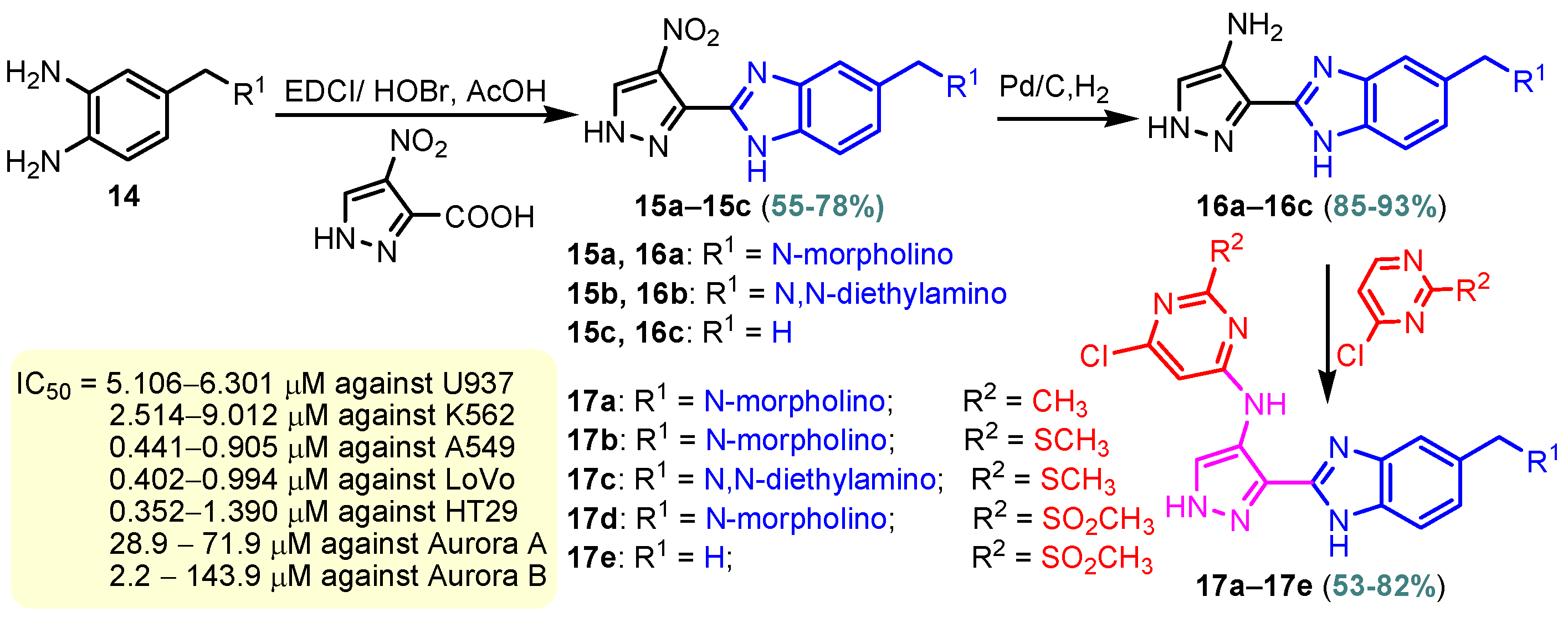
Scheme 5.
Synthesis of benzimidazole–pyrimidine hybrids 17a–17j.
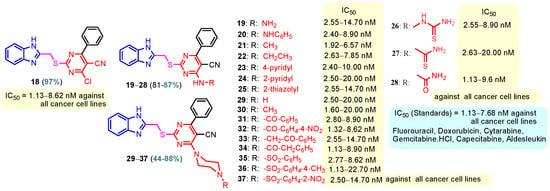
Figure 2.
Anticancer benzimidazole–pyrimidine hybrids 18–37.
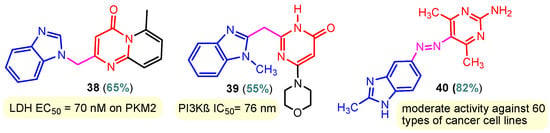
Figure 3.
Anticancer benzimidazole–pyrimidine hybrids 38–40.
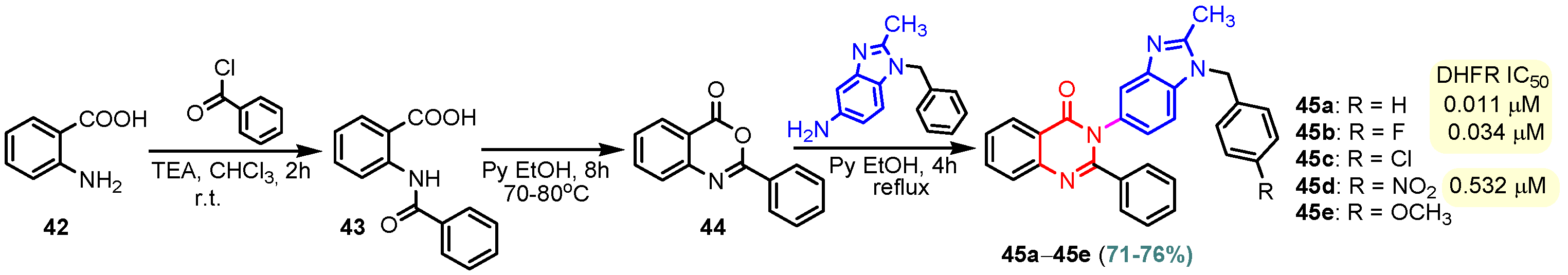
Compound 40 was synthesized by the reaction of (E)-4-hydroxy-3-((E)-(2-methyl- 1H-benzo[d] imidazol-5-yl)diazenyl)pent-3-en-2-one and guanidine at reflux for 10 h in acetic acid. A moderate cytotoxic activity was determined for hybrid 40 against 60 types of human cancer cell lines, including leukemia, non-small cell lung cancer, melanoma, colon cancer, CNS cancer, ovarian cancer, renal cancer, prostate cancer, and breast cancer [69]. Chen et al. (2013) reported effects 2-(2-(4-(pyrimidin-2-yl)piperazin-1-yl) ethyl)-1H-anthra [1,2-d]imidazole-6,11-dione 41 on cytotoxicity by MTT assay and repressing hTERT (human telomerase reverse transcriptase) expression activity by SEAP (Secreted Alkaline Phosphatase) assay (Table 2) [70]. Compound 41 affected SEAP expression without significantly affecting the proliferation of treated H1299 cells; therefore, it could selectively repress hTERT expression. The cytotoxic effect of this compound on normal human diploid fibroblasts IMR90 was also determined. Compound 41 showed an IC50 value at 100 µM against IMR90, suggesting that it did not affect the overall growth of normal cells [70]. Compounds 45a–45e were achieved in three steps from anthranilic acid 42 with yields of 76–80% (Scheme 6). The best result was observed for compound 45a (IC50 = 0.011 μM), which showed a dihydrofolate reductase inhibitory activity (DHFR) comparable or even superior to methotrexate (IC50 = 0.02 μM). The presence of electron-withdrawing functional groups in position “4” of the benzene nucleus led to the loss of DHFR activity for molecule 45c or the decrease of DHFR activity for 45d (IC50 = 0.532 μM). Also, the presence of an electron-donating group -OCH3 in compound 45e led to the loss of DHFR activity. It is noted that compounds 45c, 45d, and 45e, which had chloro, nitro, or methoxy groups at position “4” in the phenyl ring, showed less activity towards DHFR than compound 45a, which had a simple phenyl ring, and compound 45b (IC50 = 0.034µM), which was fluoro substituted. Using UV–visible and fluorescence spectroscopy, the initial interaction studies of compound 45a with calf thymus DNA showed that 45a successfully intercalated with ct-DNA to generate 45a. DNA complex that is further corroborated by research on ethidium bromide displacement. The binding interactions of compound 45a with bovine serum albumin (BSA) demonstrated that hydrogen bonds and van der Waals forces played important roles in the strong association of compound 14.BSA [71,72].

Table 2.
Cytotoxicity by MTT assay and repression of human reverse telomerase expression activity by SEAP assay for hybrid 41.
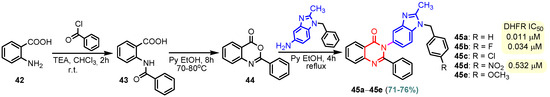
Scheme 6.
Synthesis of benzimidazole–pyrimidine hybrids 45a–45e.
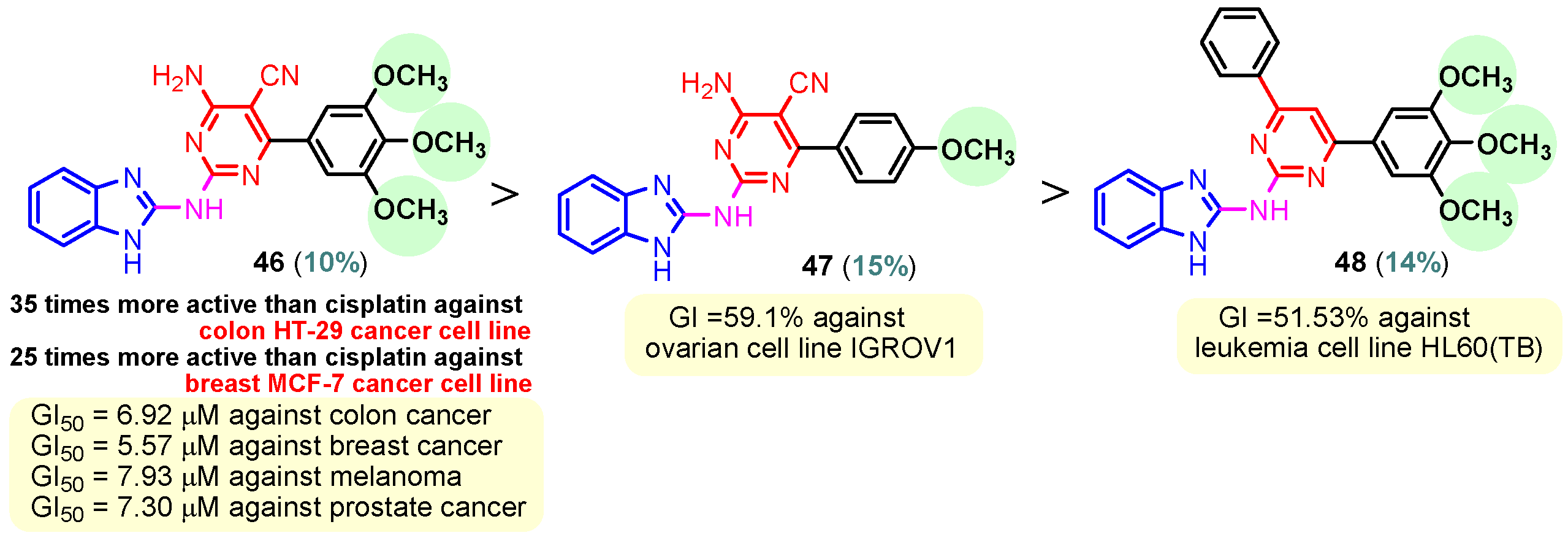
To synthesize hybrids 46–48, Ismail et al. (2020) followed a similar process to Zheng [65]. Compound 46 with three methoxy groups (Figure 4) on the phenyl ring proved the strongest antitumor activity, displaying 35 times the activity of cisplatin against colon HT-29 and 25 times its activity against breast MCF-7 cancer cell lines. With GI50 values of 6.92 µM against colon cancer, 7.93 µM against melanoma, 7.30 µM against prostate cancer, and 5.57 µM against breast cancer, hybrid 46 also demonstrated broad-spectrum anticancer activity against seven cancer panels, particularly colon, melanoma, prostate, and breast cancers. With only one methoxyl group, compound 47 demonstrated anticancer activity against the ovarian cell line IGROV1, with a growth inhibition (GI) of 59.1%. With a GI of 51.53%, compound 48, which likewise has three methoxy groups on the phenyl ring connected to pyrimidine, exhibits anticancer action against the leukemia cell line HL60(TB) [73]. Sana et al. (2021) reported the synthesis of benzimidazole–pyrimidine hybrid 52 in two steps, starting from pyrimidin-2-amine 49 and 2-(trifluoromethyl)- benzimidazol-5-amine 50, through the intermediate hybrid 51, as seen in Scheme 7 [74]. Compound 52 demonstrated the best antitumor activity among a series of synthesized benzimidazole–pyrimidine hybrids. Thus, at concentrations between 2.21 and 7.29 μM, hybrid 52 had the highest cytotoxic activity against the human lung cancer cell line A549. Additionally, 52 demonstrated the most promising anticancer action against the A549 cell line (IC50 = 2.21 ± 0.12 μM) by superior microtubule disruption, reduced mitochondrial membrane potential that caused DNA damage, colony-forming ability, and cellular migratory impairment. These characteristics cause tubulin polymerization to be inhibited (IC50 = 5.72 ± 0.51 μM) in the G2/M phase, which stops cell proliferation. Structure–activity relationship (SAR) investigations revealed that the hybrids with amine linkage exhibited higher anticancer efficiency against all tested cell types. A sterically hindered trifluoromethyl substituent at the C2-position of the benzimidazole ring, as in compound 52, enhanced in vitro cytotoxicity against the A549 cell line [74,75].
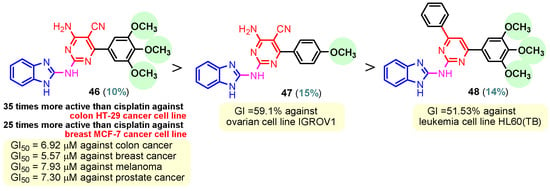
Figure 4.
Anticancer benzimidazole–pyrimidine hybrids 46–48.
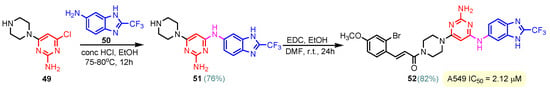
Scheme 7.
Synthesis of benzimidazole–pyrimidine hybrid 52.
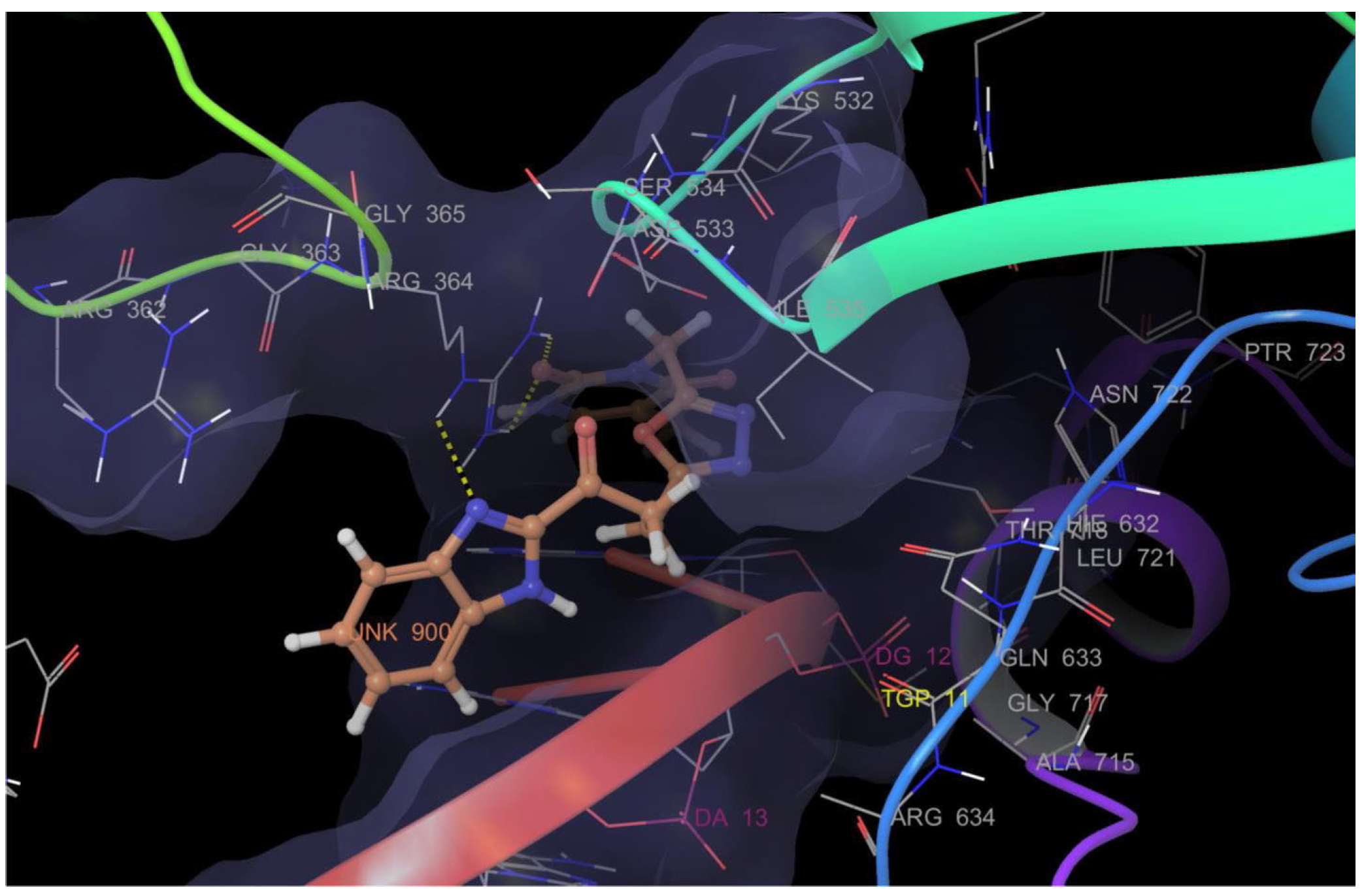
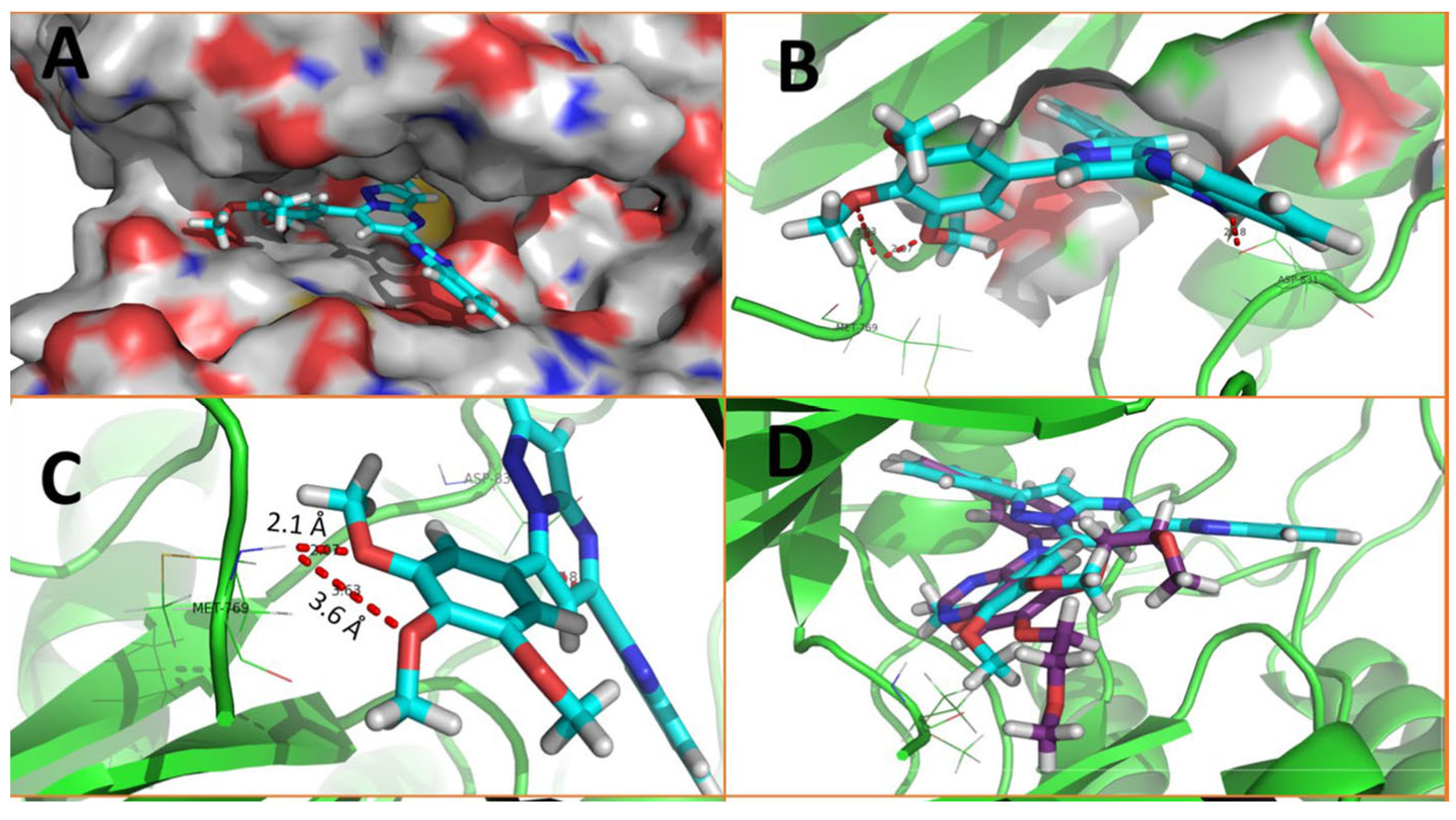
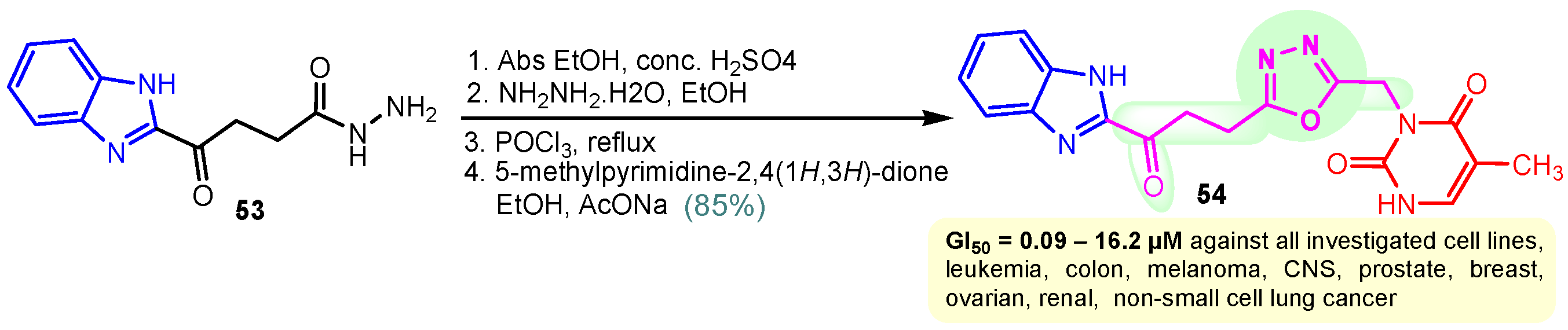
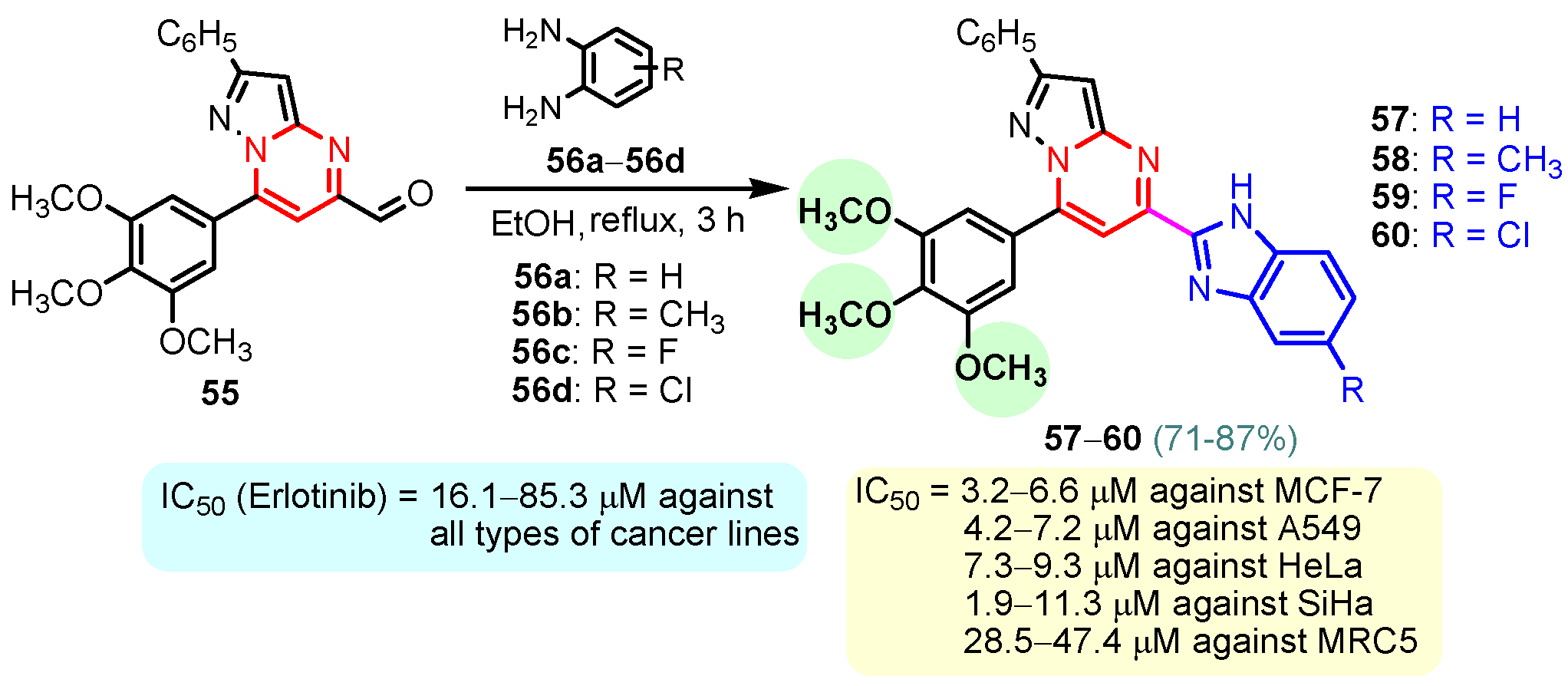
Rashid et al. (2019) synthesized dione 54 in four steps, starting from 4-oxobutanehydrazide 53 (Scheme 8) [76]. Compound 54 displayed remarkable cytotoxic potential against all investigated cell lines, leukemia, colon, melanoma, CNS, prostate, breast, ovarian, renal, non-small cell lung cancer, with GI50 values obtained between 0.09 and 16.2 µM falling within the sensitive range. The antiproliferative effects of oxadiazole conjugate 54 were found to be more potent than those of thiadiazole, triazolo-thiadiazines, and triazolo-thiadiazoles, according to the scientists [76]. Docking of hybrid 54 into the enzyme active site yielded a number of molecular interactions showing hydrogen bond, π interactions, and hydrophobic interactions between the drug and enzyme, which are considered to be accountable for the affinity of compound 54. In the hydrogen bond interaction between the carboxyl group (C=O) of the side chain residue of Arg 364 (1.73 Å) and the nitrogen (–N–) of the imidazole ring of compound 54, the latter functions as a hydrogen bond acceptor and the former as a donor. Additionally, the carbonyl group (C=O) of hybrid 54 acts as the hydrogen bond acceptor in the second hydrogen bond interaction, and an amino group (N–H) of the side chain residue of Arg 364 (2.39 Å) is a hydrogen bond donor (Figure 5) [77]. Bagul et al. (2023) synthesized a series of benzimidazole-bridged pyrazolo[1,5-a]pyrimidine 57–60 by reaction between pyrazolo[1,5-a]pyrimidine-5-carboxylate 55 and substituted benzene-1,2-diamines 56a–56d (Scheme 9) [78]. Antiproliferative activity, ranging from 3.2 to 47.4 μM, was observed against panel of cancer cell lines which included MCF-7 (breast cancer), A549 (lung cancer), HeLa (cervical cancer), SiHa (cervical cancer), and significant anticancer activity against cell lines MCF-7, A549, and HeLa (IC50 = 3.2–9.3 µM). Also, hybrids 57–60 were found to be less cytotoxic to normal lung fibroblast MRC5 cells. The binding pose for hybrid 57 (Figure 6A,B) shows that the molecule binds well in the ATP binding site. The C-2 phenyl ring was buried in the hydrophobic specificity pocket enclosed by Ala719 (1.87 Å), Ile720 (2.99 Å), Lys721 (2.72 Å), Glu738 (4.23 Å), Leu764 (2.25 Å), Ile765 (2.93 Å), and Thr766 (2.16 Å) amino acids. These interactions in the hydrophobic specificity pocket are important for attaining the specificity among the other kinases. The superimposed pose of hybrid 57 with cocrystal ligand erlotinib (Figure 6D) showed that the C-2 phenyl ring of 57 overlapped with the phenylacetylene group of Erlotinib. The C–7 phenyl ring was found oriented towards the hinge region, where 3-methoxy (2.07 Å) and 4-methoxy (3.63 Å) formed hydrogen bonds with the backbone NH of Met769 [78].
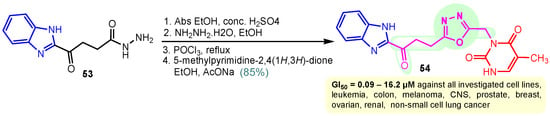
Scheme 8.
Synthesis of benzimidazole–pyrimidine hybrid 54.
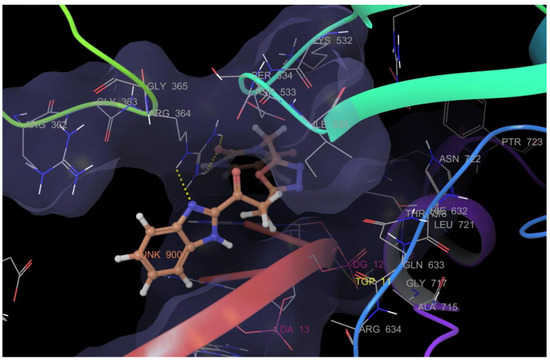
Figure 5.
Binding mode of hybrid 54 into the binding sites of topoisomerase enzyme (PDB code: 1SC7) showing hydrogen bond (yellow dotted lines) with Arg 364 and π interaction with Arg 364.
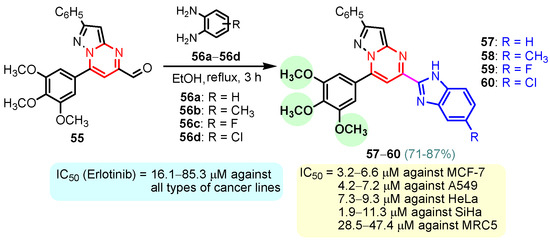
Scheme 9.
Synthesis of benzimidazole–pyrimidine hybrids 57–60.
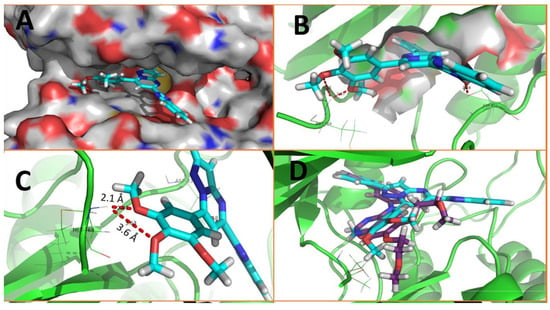
Figure 6.
Docking poses on EGFR (A) Binding pose of 57 with 3D structure; (B) Binding pose of 57 with EGFR specificity region; (C) Binding pose of 57 with surface structure; (D) Binding pose of 57 with hydrogen bonds.
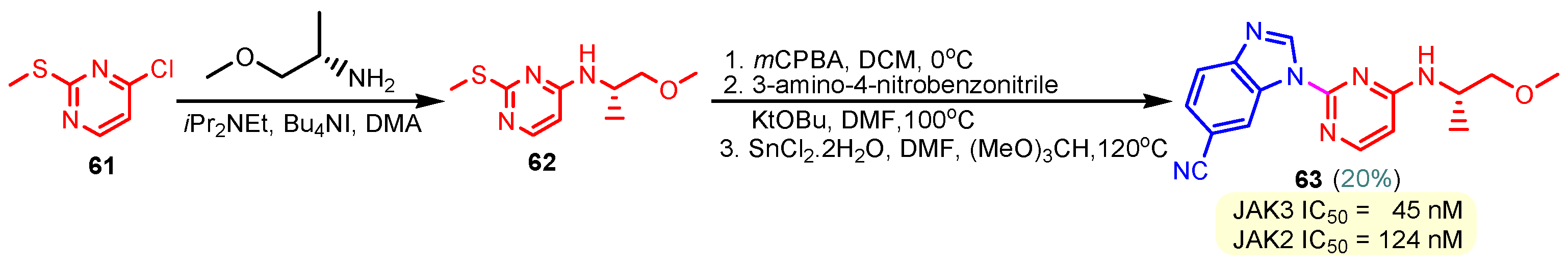
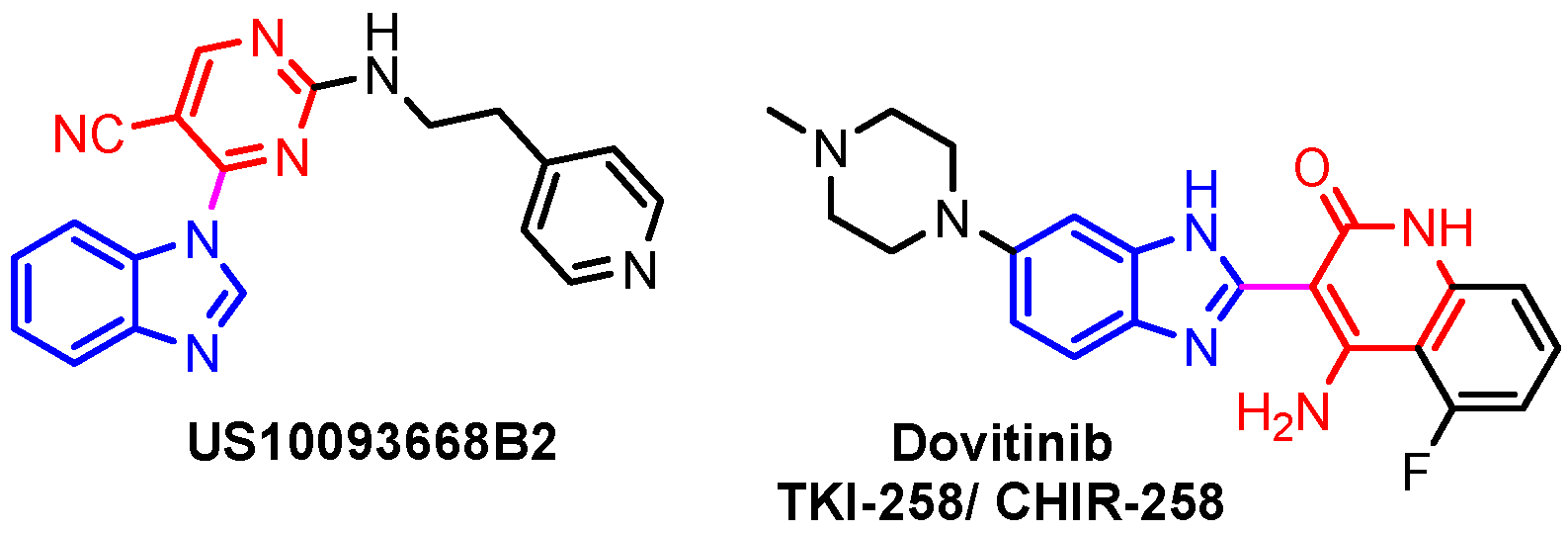
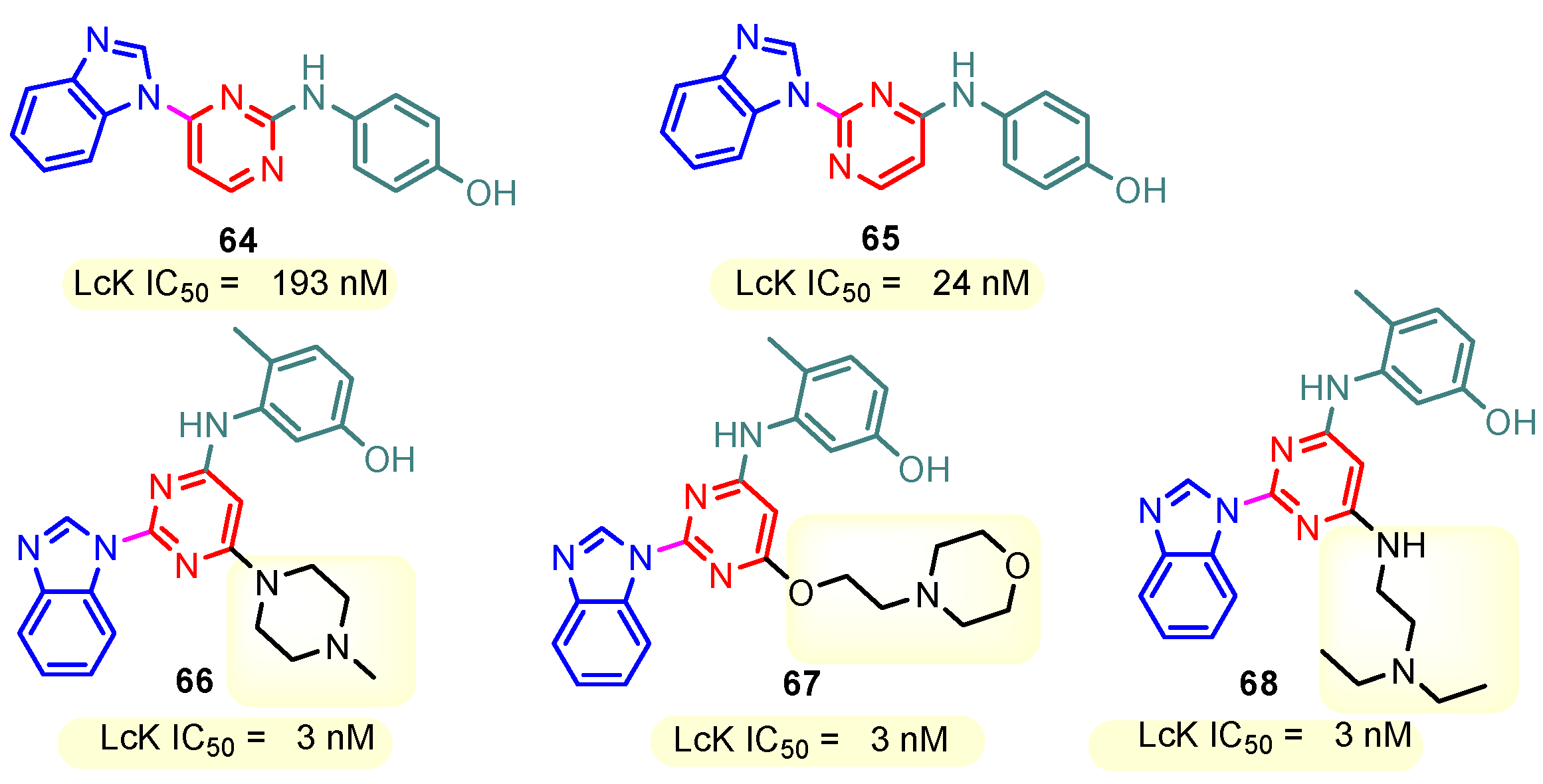
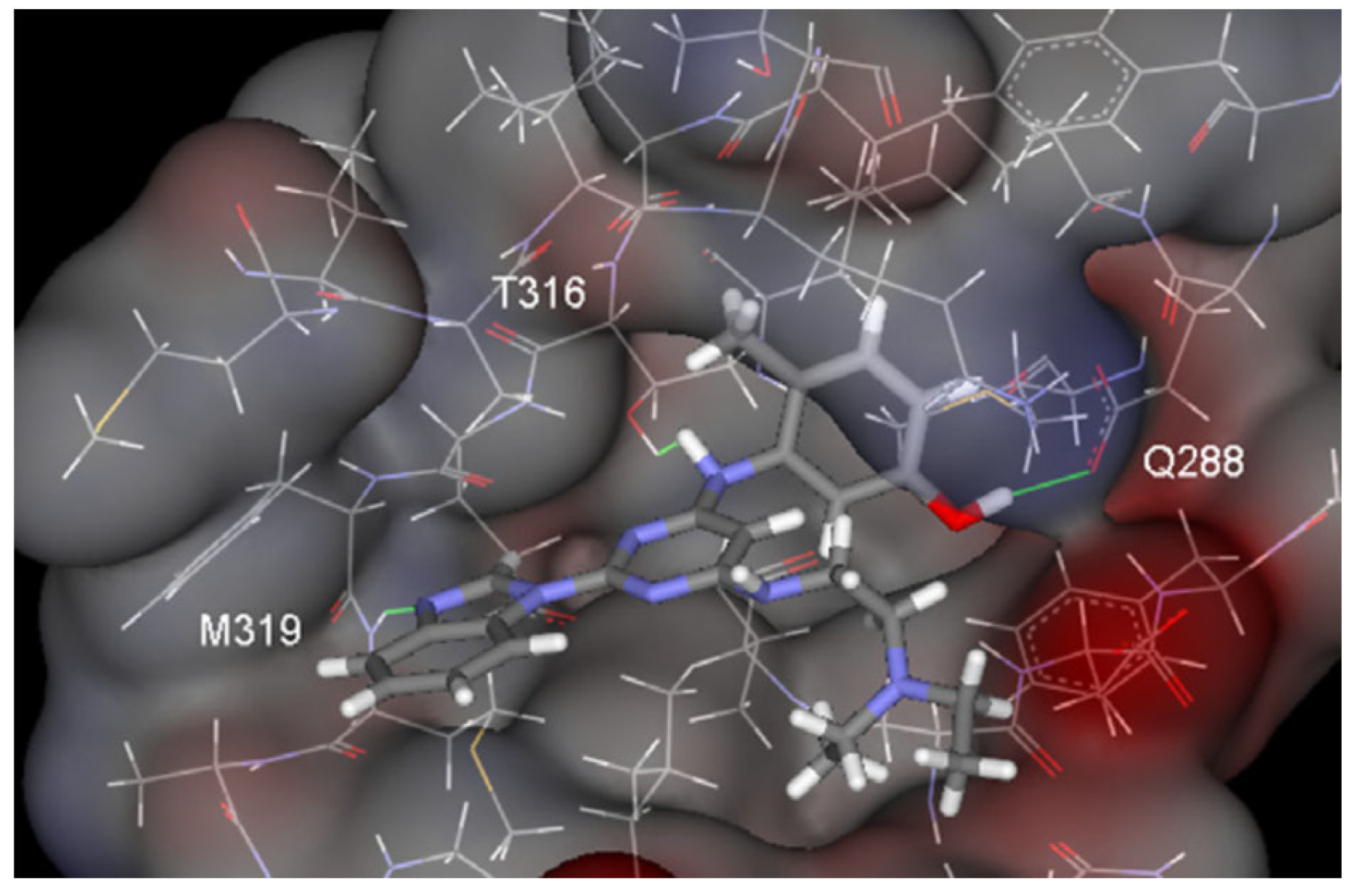
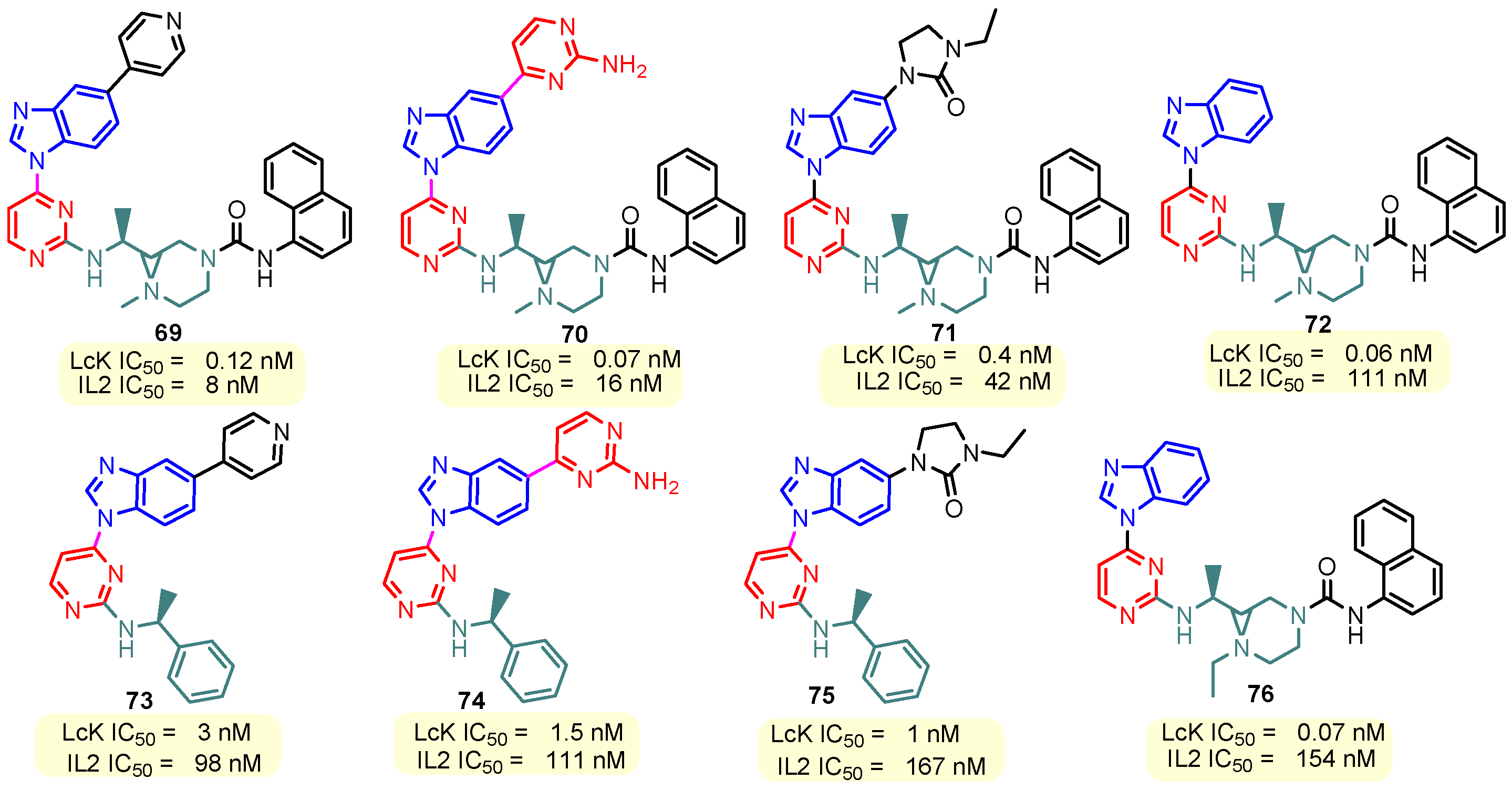
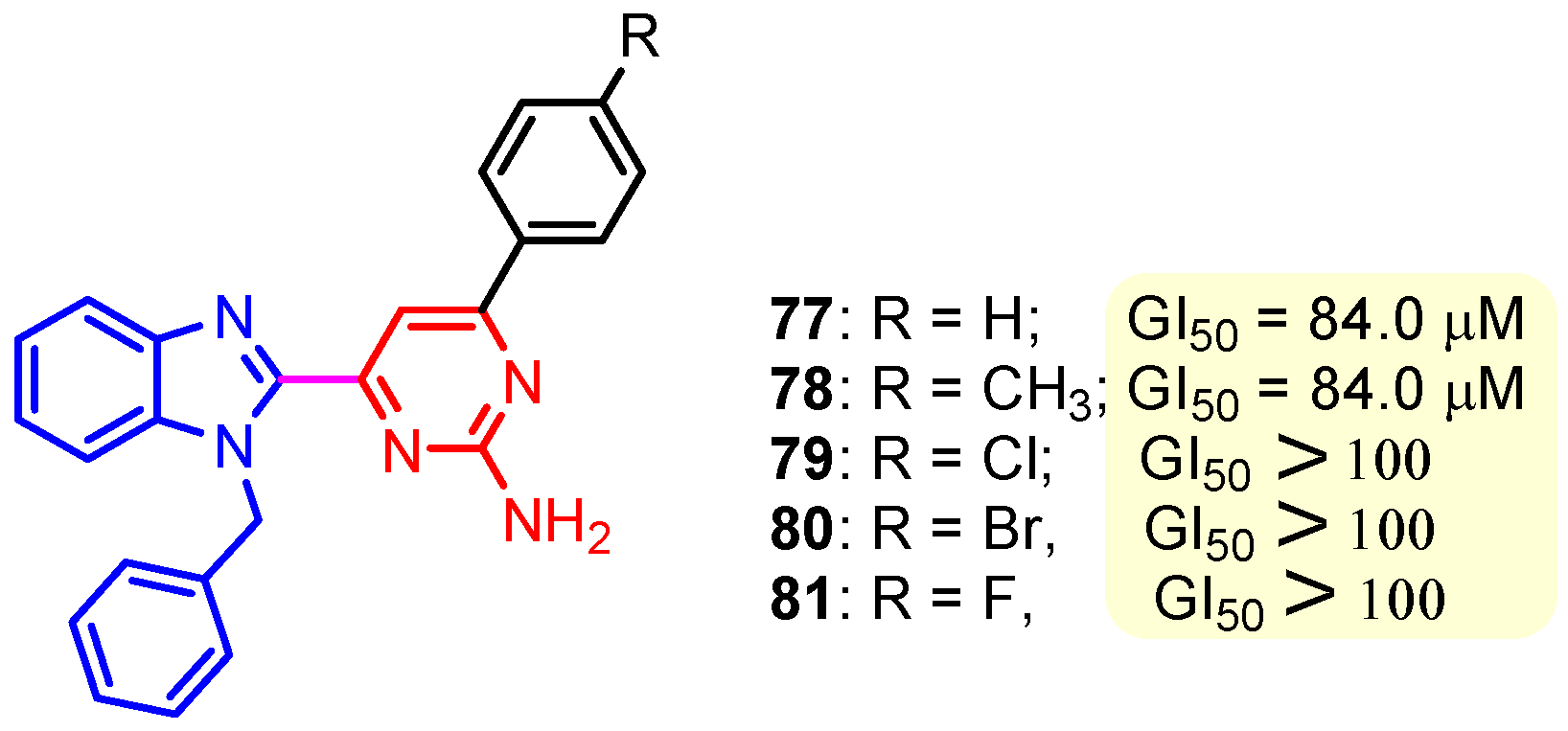
This hydrogen bond is vital for EGFR inhibitors and is present in all the drug molecules and ATP as well [79,80,81]. Venugopal et al. reported compound US10093668B2 that inhibited Mitogen-Activated Protein Kinase (MAPK) and was effective in acute myeloid leukemia (Figure 7) [82]. MAPK-interacting kinases (MNKs) are involved in the phosphorylation of initiation factor 4E (eIF4E), where their protein pathway MNK1/2 eIF4E is overexpressed in cancer cells [83,84]. Dovitinib (TKI-258/CHIR-258), a pan receptor tyrosine kinase (RTK) inhibitor, primarily targets fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), fms-like tyrosine kinase 3 (FLT3), and Proto-Oncogene Receptor Tyrosine Kinase (c-KIT) [85]. Dovitinib has demonstrated antitumor activity in pre-clinical models of several cancers and is currently in clinical trials for renal, prostate, adenoid cystic, gastrointestinal, urothelial, thyroid, pancreatic, breast, glioblastoma, and non-small lung cancers [86]. Yadav et al. recently demonstrated the efficacy of Dovitinib in the systemic treatment of mice harboring prostate cancer xenograft tumors [87]. The formation and function of immune cells depend on the protein tyrosine kinase Janus kinase 3 (JAK3), which is involved in cytokine receptor signaling. Immune cell cancers and immunodeficiency can result from aberrant JAK3 activity, especially brought on by mutations. Because cytokine signaling pathways are frequently dysregulated in cancer cells, JAK3 plays a part in cancer [88]. Chen et al. (2006) synthesized hybrid 63 in four steps starting from 4-chloro-2-(methylthio)pyrimidine 61 through the intermediate 2-(methylthio)pyrimidin-4-amine 62 (Scheme 10) [89]. Compound 63 showed low nanomolar IC50 activity, of 45 nM against JAK3 and of 124 nM against JAK2. The inhibitory activity of hybrid 63 against JAK3 and JAK2 was assessed by the kinase-Glo luminescent assay with Tofacitinib as a reference [89]. Sabat et al. (2006) reported compounds 64 and 65 as inhibitors of lymphocyte-specific kinase (Lck) with IC50 values of 193 and 24 nM, respectively [90]. In the same study, the series of compounds 66, 67, and 68 showed an excellent potency, with LcK IC50 of 3 nM (the key phenolic residue is colored in green, and the 6-pyrimidine substituent essential for anticancer activity is highlighted in yellow in Figure 8) [90]. Mostly present in cells of the myeloid and B-lymphocyte lineages, Hck (hematopoietic cell kinase) is a protein tyrosine kinase that is a member of the Src family. It is essential to the growth and spread of several cancers, as well as signaling pathways that are important in immune cell function, such as cytokine and Fc receptor signaling. Additionally, Hck may be a therapeutic target for Bcr/Abl-chronic myeloid leukemia and HIV infections. To establish the binding mode of these molecules, an X-ray crystallographic structure of 68 with Hck was obtained (Figure 9). Inhibitor 68 orients itself in the Hck enzyme such that the benzimidazole N–H bonds are associated through hydrogen bonds with the amide N–H of Met319, and the phenolic OH hydrogen bonds with the carboxyl of Glu 288. An additional interaction may occur between the O–H of the Thr316 and the aniline N–H on the phenol substituent. The 4-methyl group on the phenol substituent appears to sit in a small hydrophobic groove formed in part by the CH3 of Thr316. Thus, the phenolic OH optimally interacts with Glu288 [90]. Hunt et al. (2009) developed a family of benzimidazole–pyrimidine hybrids 69–76, as subnanomolar inhibitors of Lck and also low-nanomolar inhibitors of cellular IL2 release (Figure 10) [91]. As can be seen, compound 69 is a potent inhibitor of both Lck activity and cellular IL2 release. The strongest inhibitor of Lck activity in this series, hybrid 72 (IC50 = 0.06 nM), only inhibits cellular IL2 production at a concentration of 111 nM, which represents a nearly 2000-fold shift in potency from the enzyme to the cell. Compounds 69, 70, and 71, with IL2 values of 8, 16, and 42 nM, respectively, showed much less dramatic shifts in potency from the enzyme to the cell, as compared to the parent piperazine 72, with IL of 111 nM. Hybrid 76, N-ethyl substituted, has a similar behavior to 72, while hybrids 73, 74, and 75 have excellent LcK IC50 values of 3, 1.5, and 1 nM, respectively [91]. Padhy et al. (2019) reported synthesis of hybrids 77–81 (Figure 11) from 2-acetylbenzimidazole in three steps: (1) Claisen–Schmidt condensation of 2-acetylbenzimidazole with substituted aromatic aldehydes in presence of NaOH; (2) nucleophilic substitution of chalcones previously synthesized with benzyl chloride to obtain N-benzyl substituted benzimidazole chalcones; and (3) condensation of the previous chalcones with guanidine hydrochloride [92]. The in vitro anticancer activities (cell viability assay) of all compounds were evaluated by SRB assay against the human breast cancer cell line MDA-MB-231 [92]. Compounds 77 (GI50 = 84.0 μM) and 78 (GI50 = 39.6 μM) exhibited weak activity compared to the standard drug adriamycin (GI50 = 0.04 μM). Unexpectedly, the halogeno-substituted compounds 79, 80, and 81 showed IC50 > 100 µM [92].
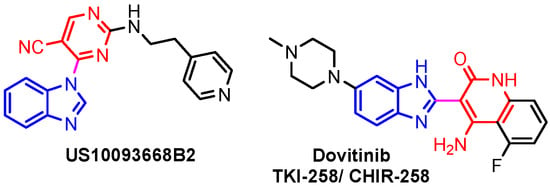
Figure 7.
Structure of benzimidazole–pyrimidine anticancer hybrids.
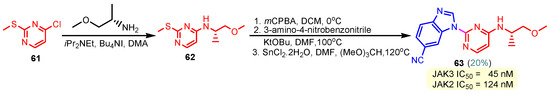
Scheme 10.
Synthesis of hybrid 63 as an inhibitor of JAK3 and JAK2.
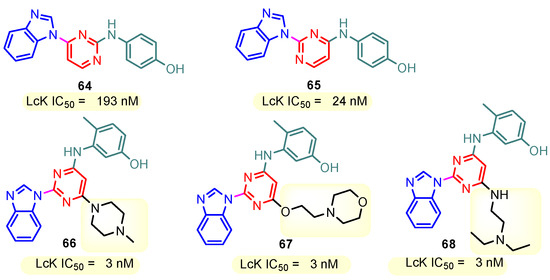
Figure 8.
Structure of benzimidazole–pyrimidine LCK inhibitors 64–68.
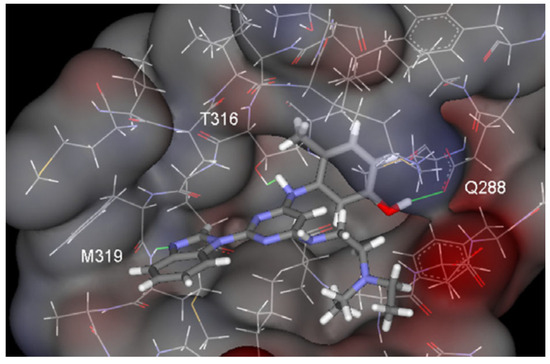
Figure 9.
Key hydrogen bonds between 68 and Hck.
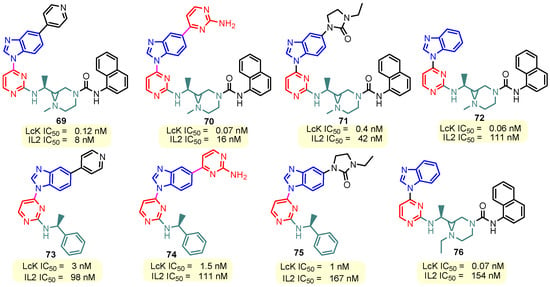
Figure 10.
Structure of benzimidazole–pyrimidine LcK and IL2 inhibitors.
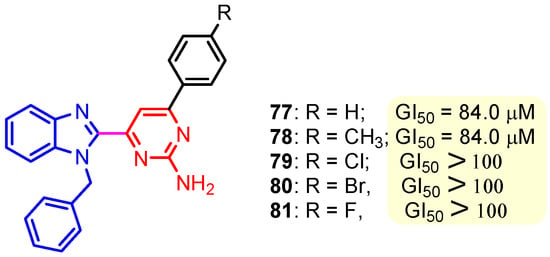
Figure 11.
Structure of benzimidazole–pyrimidine anticancer hybrids 77–81.
3. Antimicrobial Benzimidazole–Pyrimidine Hybrids
Events in recent years have demonstrated the need to find new antimicrobial compounds that prevent the unwanted effects that occur with the frequent use of classic antibiotics, as well as antibiotic resistance, and, also, the urgency of more in-depth studies regarding the structure–property relationship in order to generate more effective compounds against a much wider range of pathogens [93]. Research in recent years has shown greater efficacy in antimicrobial compounds with multiple heterocyclic rings, as well as various SAR studies on these hybrids [94]. In the following, we will present studies on benzimidazole–pyrimidine hybrids with antimicrobial properties.
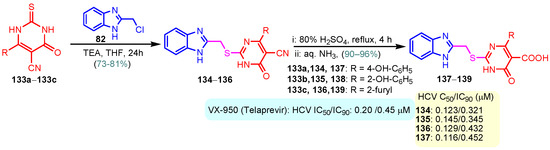
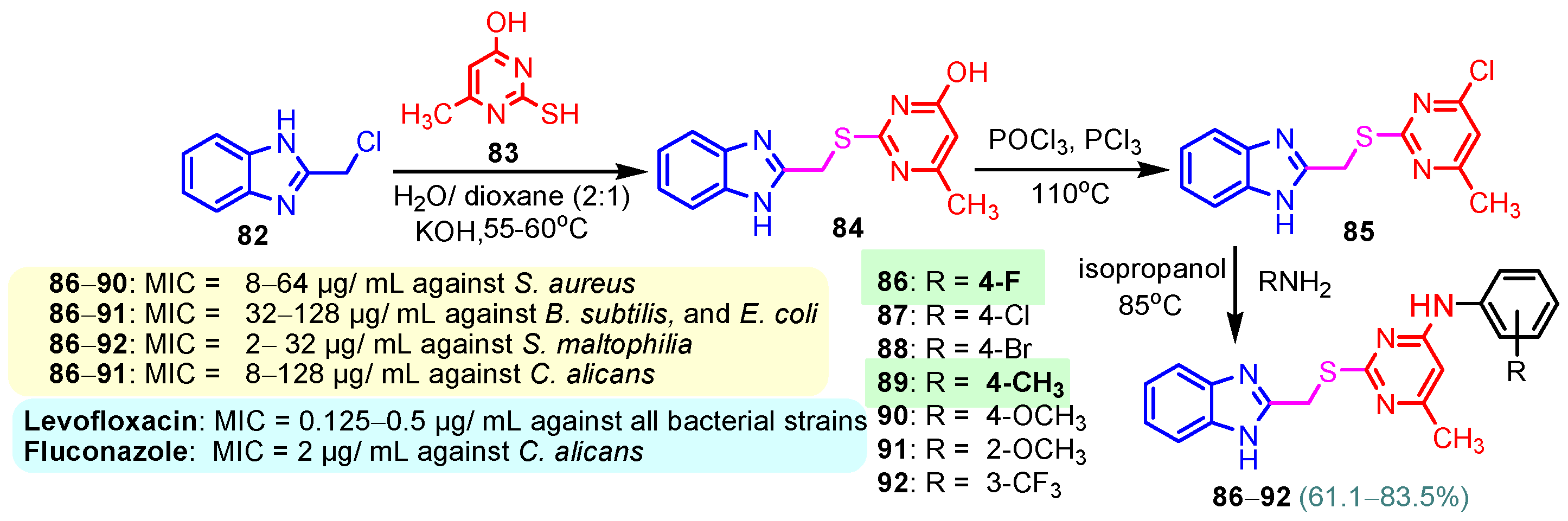
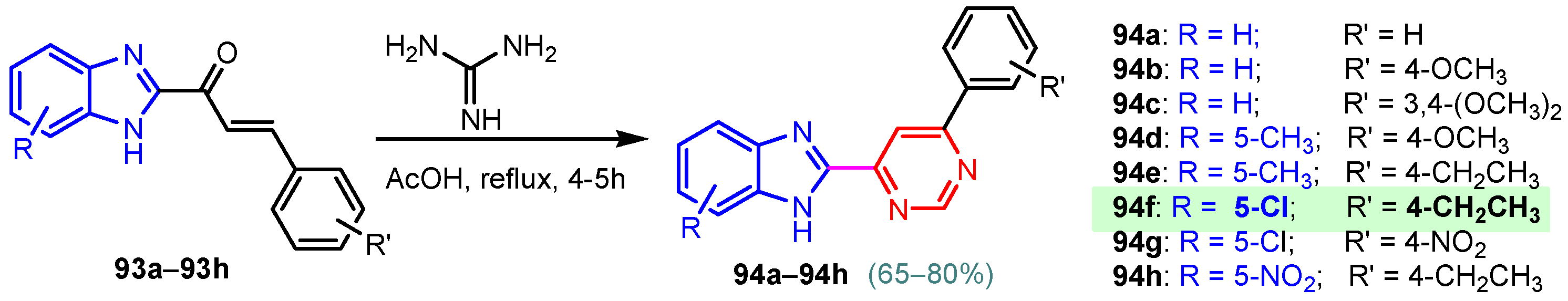
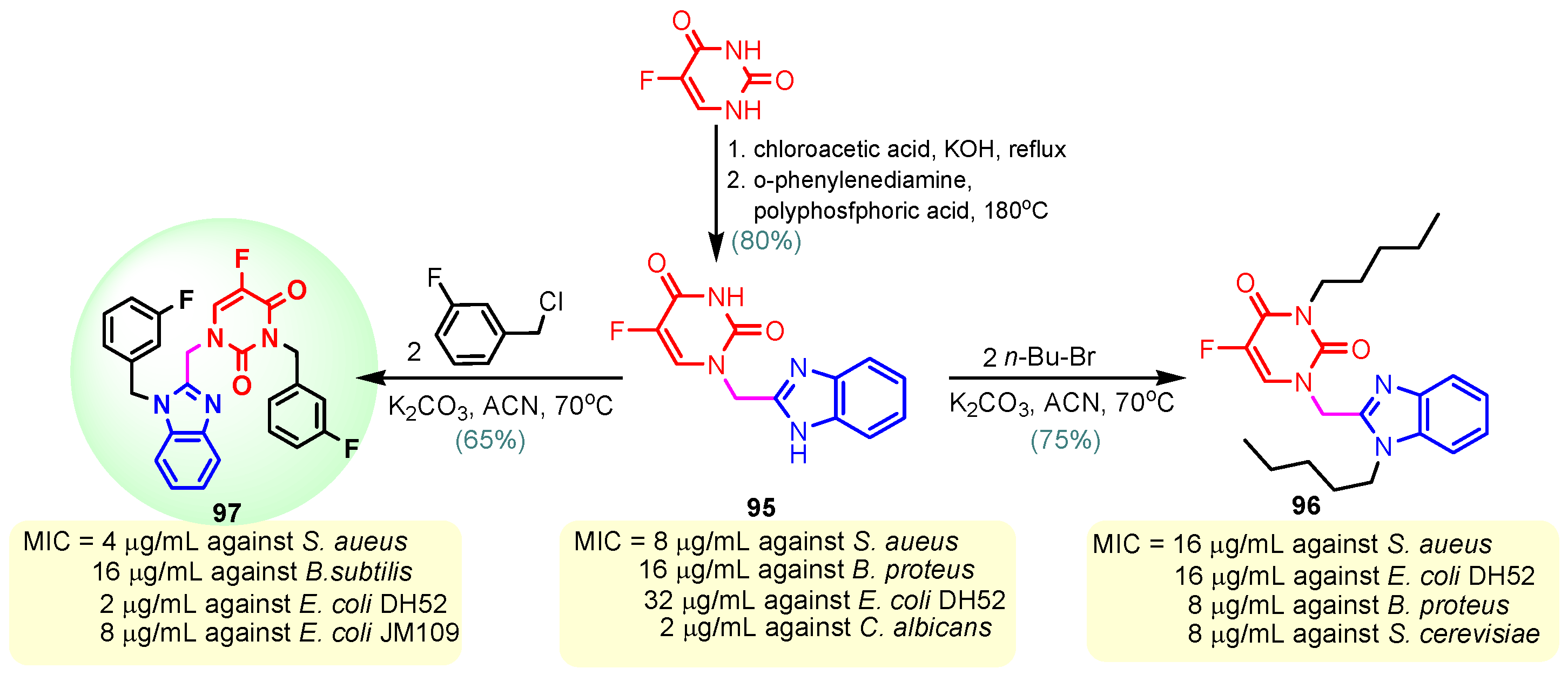
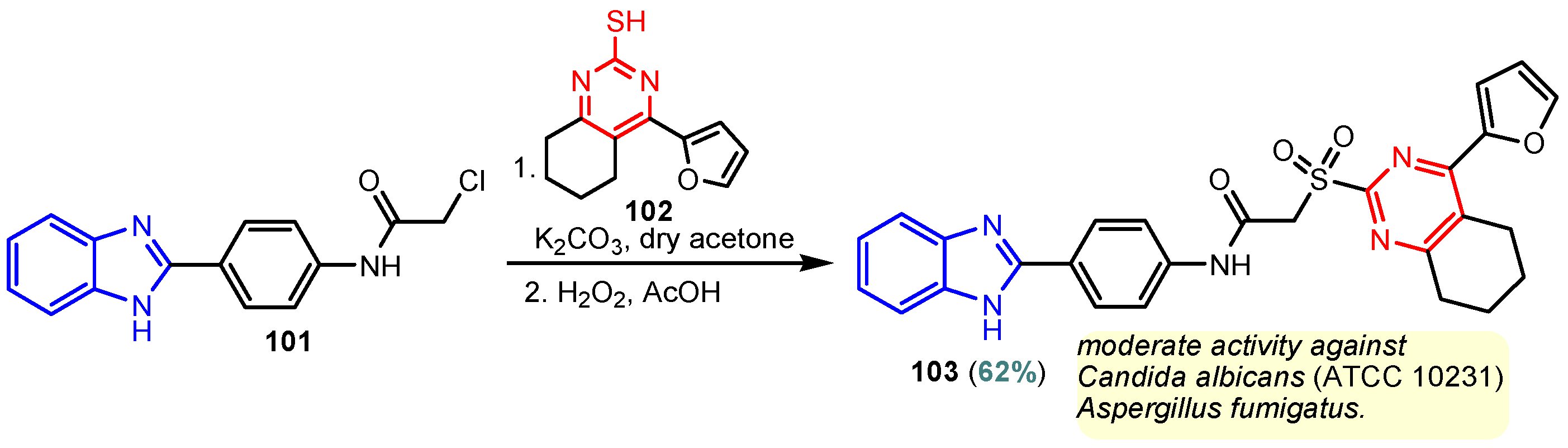
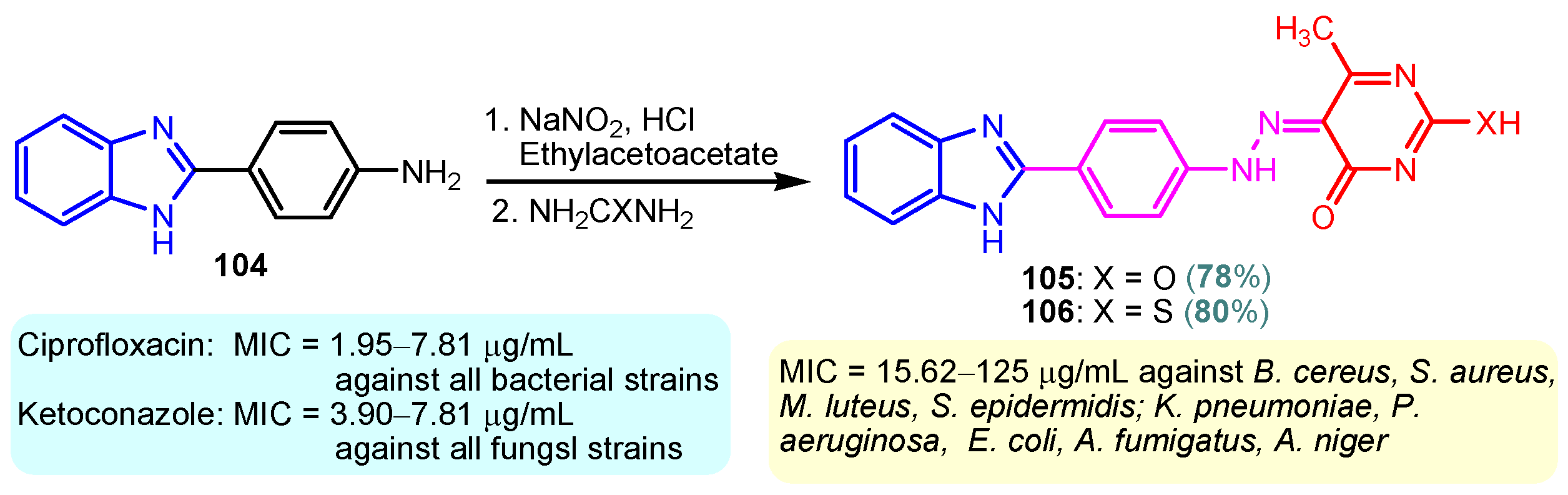
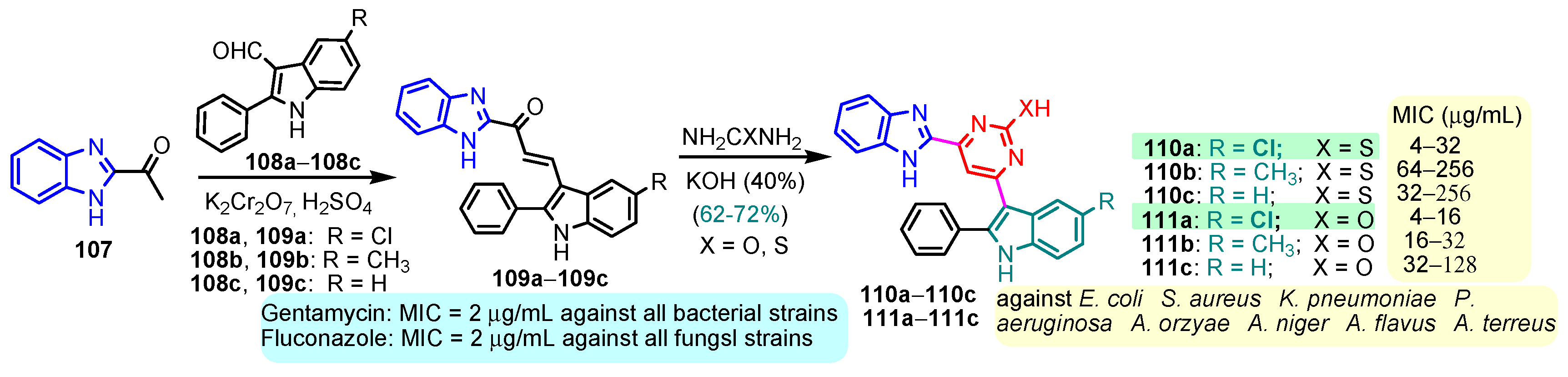
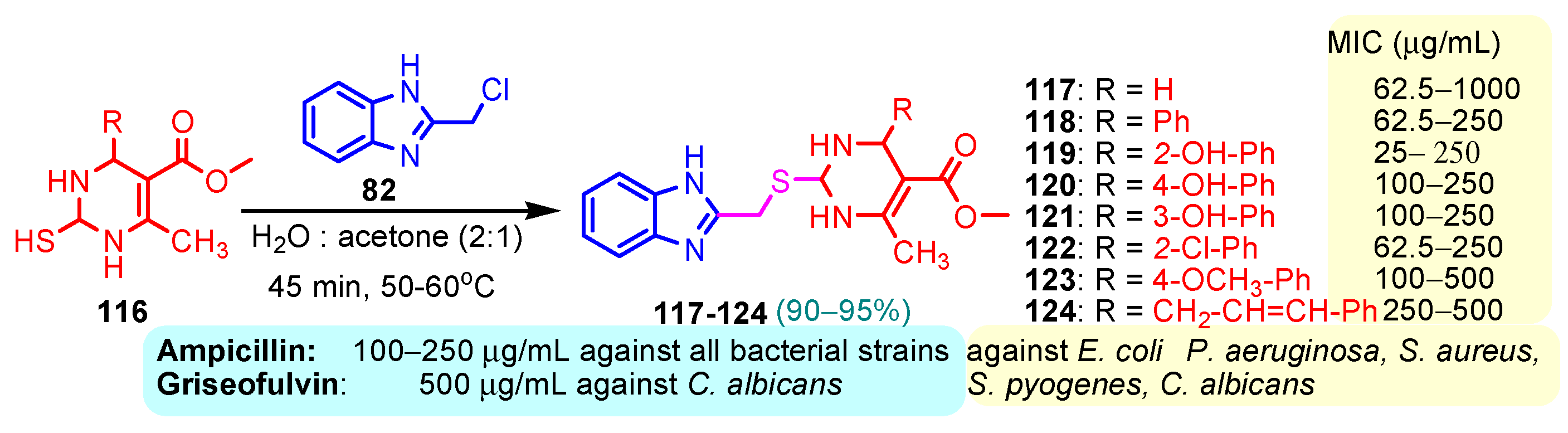
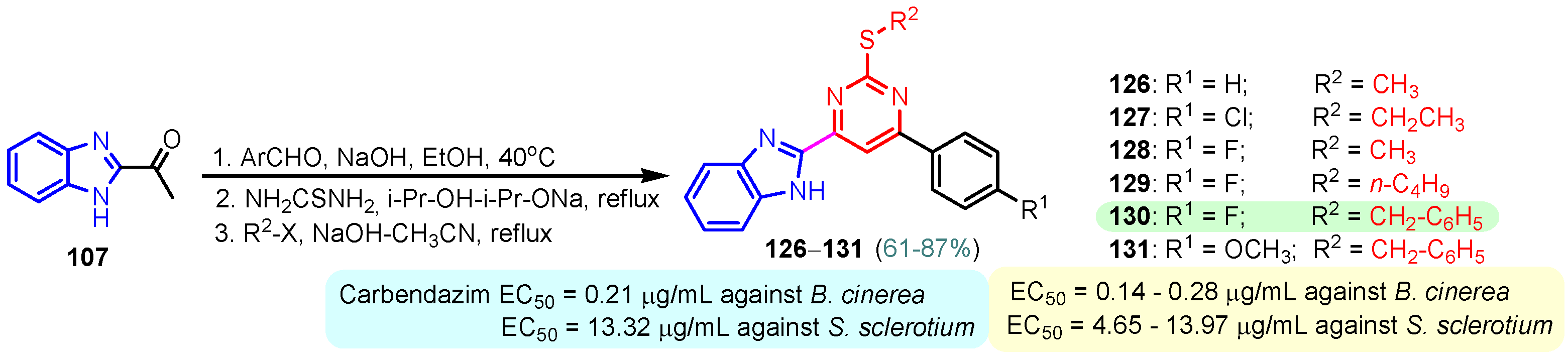
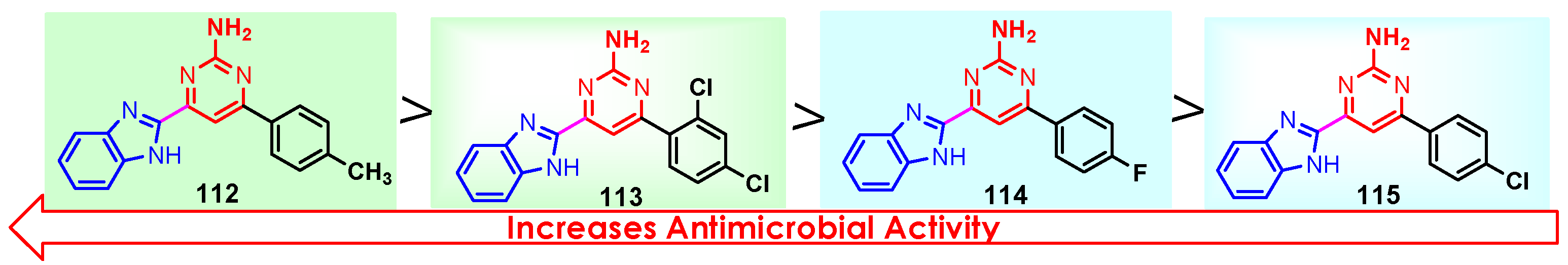
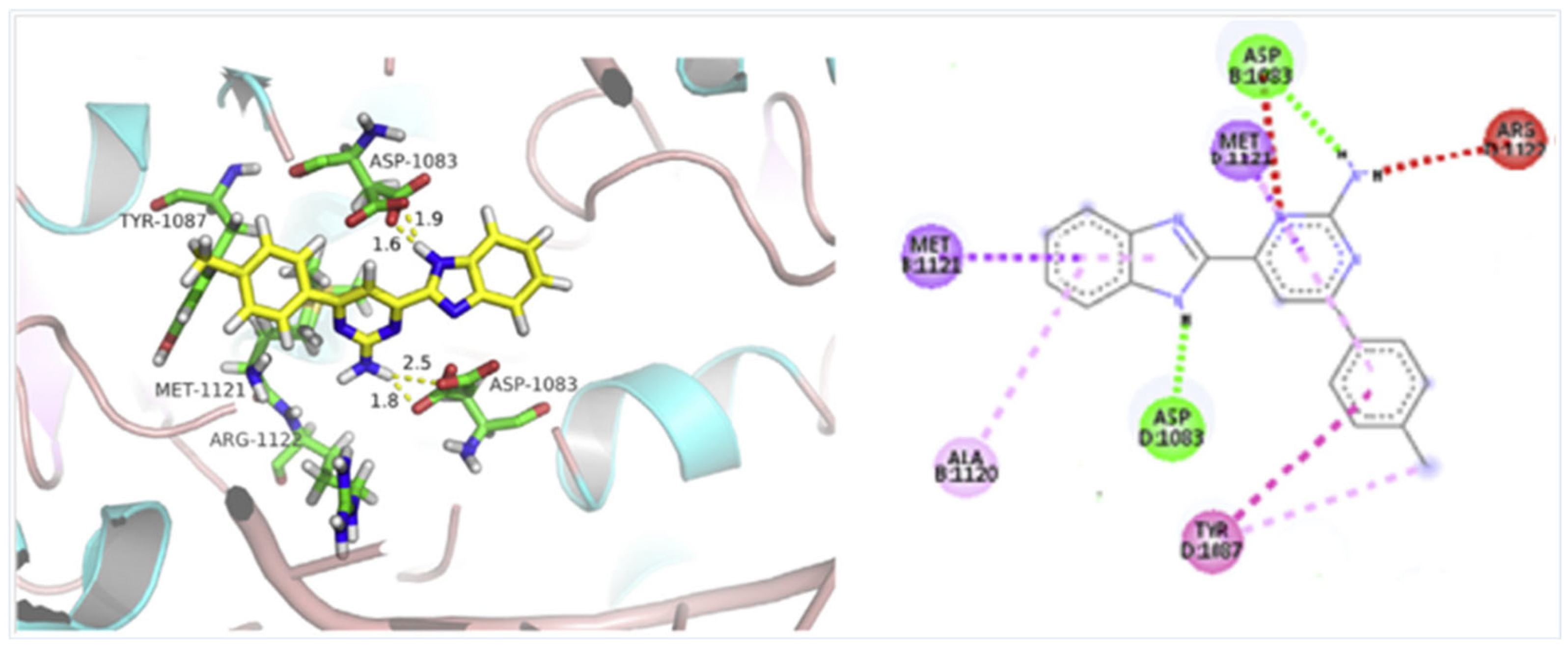
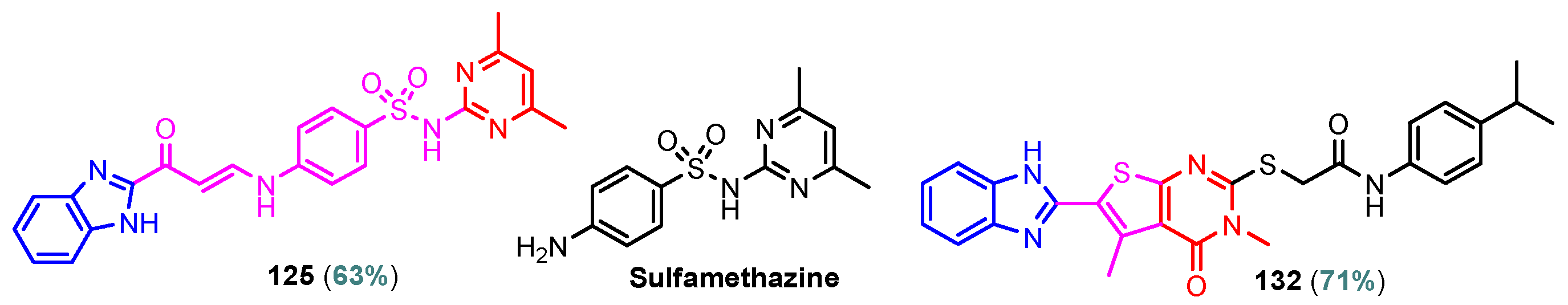
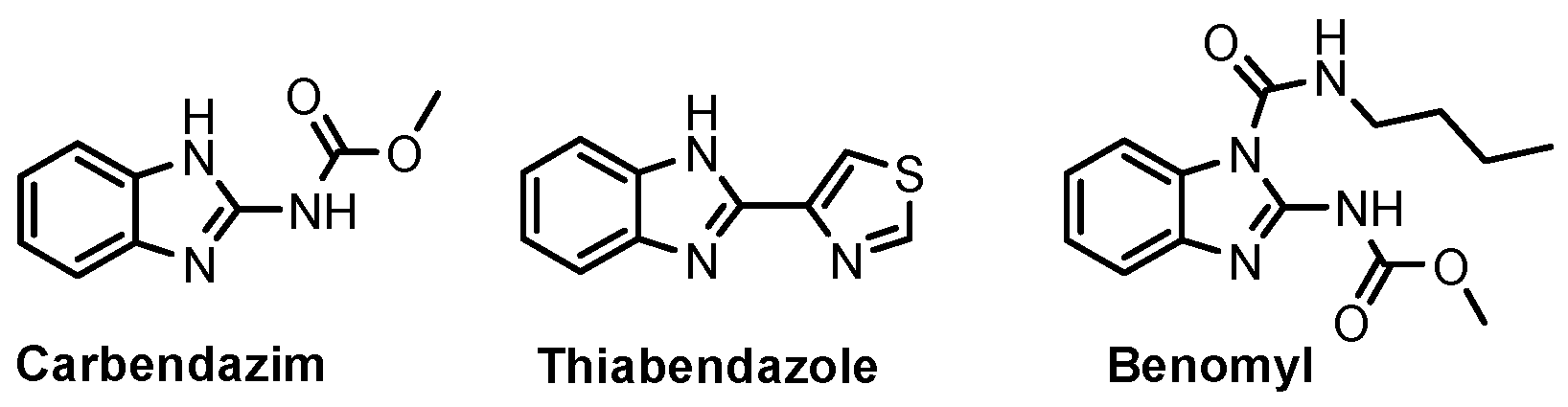
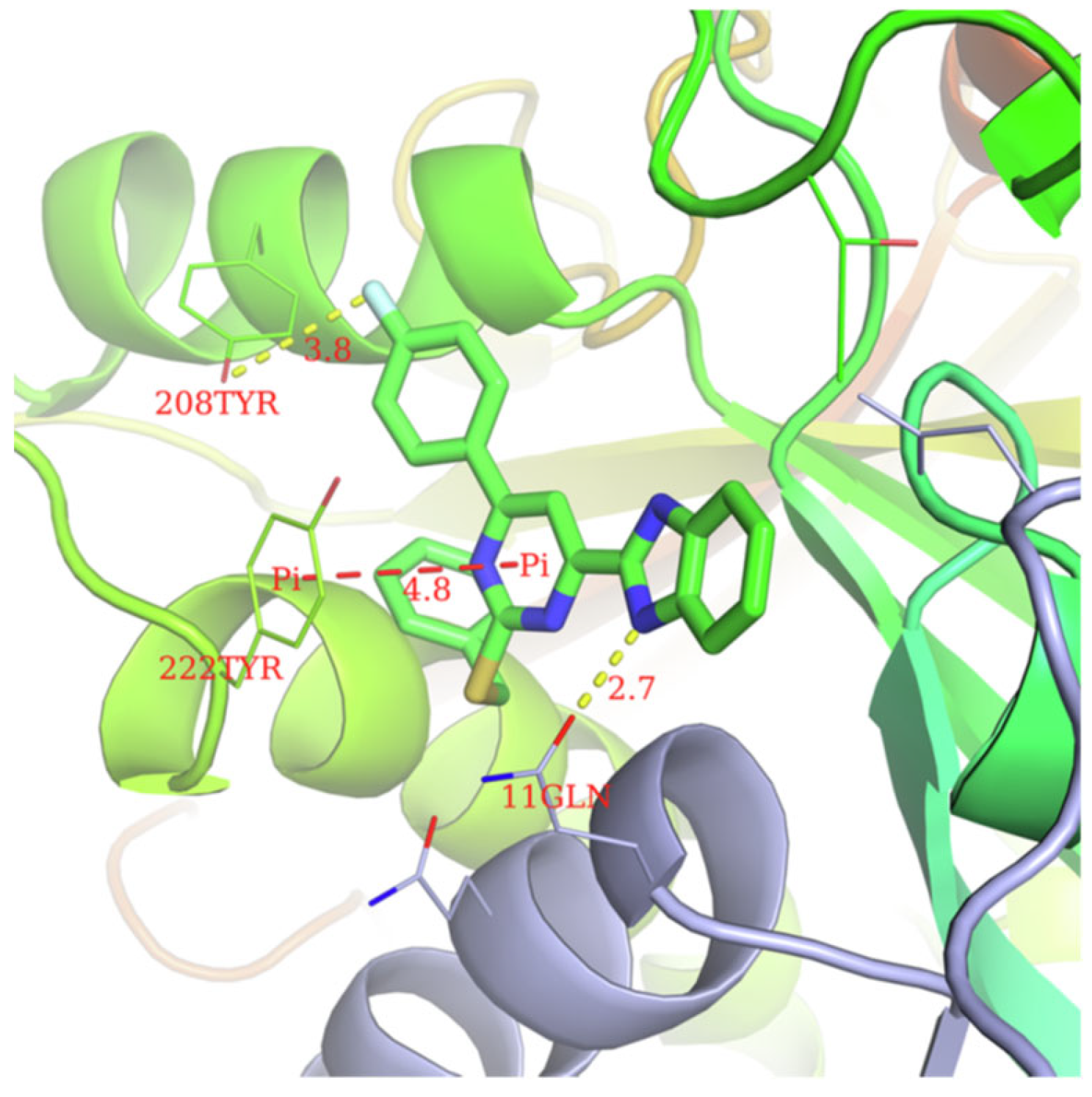
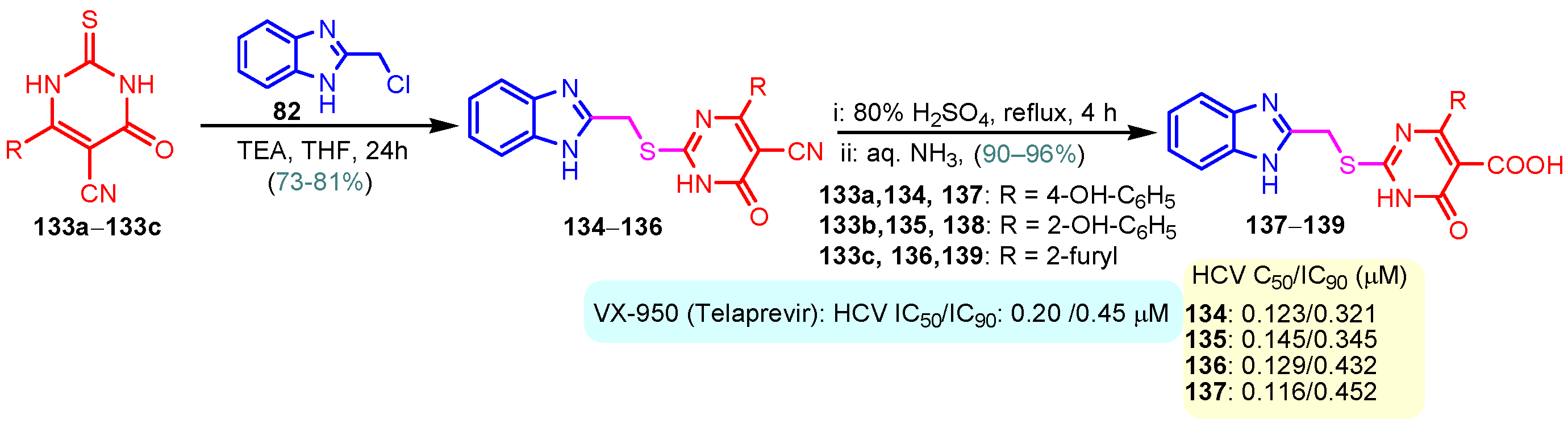
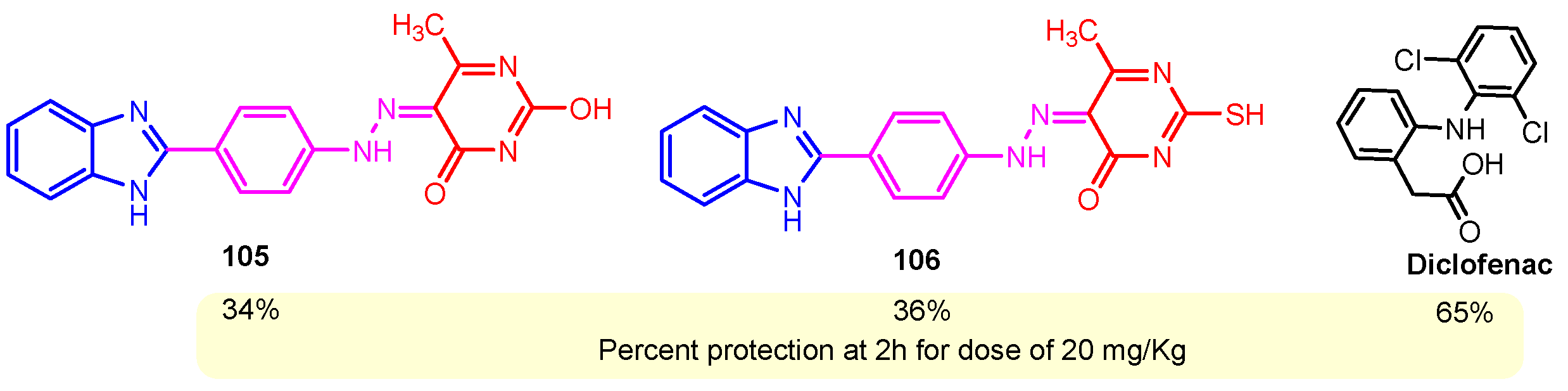
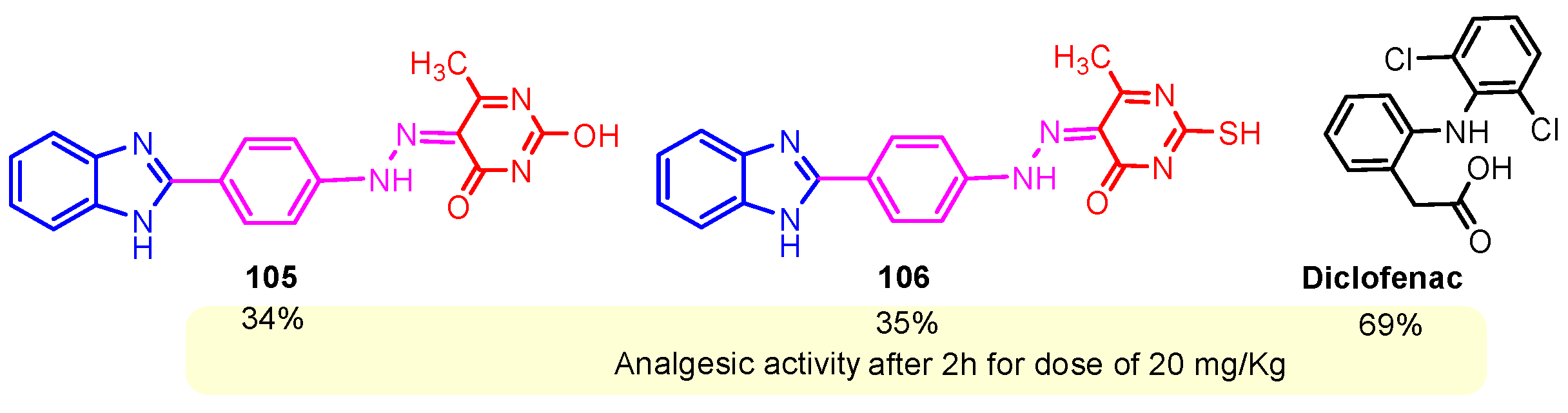
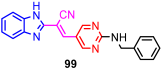
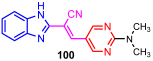
According to Chen et al. (2014), hybrids 86–92 were synthesized in three steps from 2-(chloromethyl)-benzimidazole 82 and 2-mercapto-6-methylpyrimidin-4-ol 83 [95]. The intermediate 84 was halogenated with POCl3 and PCl3 at 110 °C, yielding hybrid 85 (Scheme 11) [95]. The reaction of compound 85 with various aromatic amines led to hybrids 86–92, which were tested against four bacterial strains (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Stenotrophomonas maltophilia) and one fungal strain (Candida albicans) in vitro, with levofloxacin as a positive control drug for bacterial strains and fluconazole as a positive control drug for fungi. All compounds exhibited inhibitory activity against S. maltophilia with MICs ranging from 2 to 32 µg/mL, as shown in Scheme 11, and hybrids 86 to 90 exhibited antimicrobial properties against all species of Gram-positive, Gram-negative bacteria, and fungi used in the study. For compounds 86 and 89, we observe the best antimicrobial activities, which means that the presence of fluorine and methyl substituents in position “4” of the phenyl nucleus was the most beneficial for their bioactivity. The cytotoxic activity of the same compounds showed that only compounds 86 and 89 exhibited enhanced activities against MGC-803 (human gastric cancer cell line), with IC50 values of 5.77 µM and 7.39 µM, respectively, compared with 5-Fu (IC50 = 8.13 µM) in vitro [95]. Kunduru et al. (2014) synthesized benzimidazole-pyrimidine hybrids 94a–94h by the reaction of substituted 3-phenylpropenones 93a–93h with guanidine, at reflux in acetic acid for 4–5 h (Scheme 12) [96]. A maximum reaction yield of 80% is observed for compound 94d with the methyl group in the “5” position of the benzene ring and the methoxy group in the “4” position of the benzene nucleus. A slightly lower yield (73%) is given by compound 94c with two methoxy groups grafted onto the benzene nucleus. Antibacterial activity of the synthesized compounds was screened against B. subtilus, S. aureus, E. coli, and K. antifungal activity against F. oxysporum and A. niger (Scheme 12). It was noted that compound 94f has excellent activity against all bacterial strains compared to Steptomicyn and Fluconazole as standards. Hybrid 94b displayed high activity against B. subtilus and good activity against E. coli and K. pneumoniae, and 94c shows good activity against all organisms except E. coli [96]. Starting from 5-fluorouracil, Fang et al. synthesized a number of benzimidazole–pyrimidine hybrids as a new class of potential antibacterial agents (Scheme 12). Compound 97 with 3-fluorobenzyl groups exhibited superior antibacterial activity to chloromycin against S. aureus, B. subtilis, E. coli DH52, and E. coli JM109. Additionally, its anti-MRSA activity (MIC = 2 µg/mL) was eight-fold higher than that of chloromycin (MIC = 16 µg/mL) and four-fold higher than that of norfloxacin (MIC = 8 µg/mL). Of all the newly synthesized benzimidazole compounds, compound 97 was the most effective against the Gram-negative bacteria Bacillus typhi (MIC = 8 µg/mL). It was found that intermediate 95 displayed good antimicrobial activities against most of the tested bacterial and fungal strains, as seen in Scheme 13. With MIC values of 2 µg/mL against C. albicans and 8 µg/mL against S. aureus, intermediate 95 was found to have strong antibacterial activity against the majority of the tested bacterial and fungal species. Additionally, the di-n-pentyl-substituted hybrid 96 has good antibacterial activity against S. cerevisiae (MIC = 8 µg/mL) and B. proteus (MIC = 8 µg/mL) [97]. AlNeyadi et al. (2017) reported the synthesis of benzimidazole–pyrimidine acrylonitrile hybrids 98–100 by reaction between 2-(1H-benzo[d]imidazol-2-yl)acetonitrile and 2-substituted pyrimidine-5-carbaldehyde in piperidine at 25 °C in 81–89% yields [98]. Hybrid 98 exhibited good antibacterial activity against both Gram-positive and Gram-negative bacteria with a MIC ranging from 9 to 13 µg/mL. Compounds 99 and 100 exhibited moderate antibacterial activity against the tested organisms with MICs of 11.1 to 25 µg/mL (Table 3) [98]. Hessein et. al. (2016) reported the synthesis of hybrid 103 in two steps, from chloroacetamide 101 and quinazoline-2-thiol 102 (Scheme 14). Compound 103 had moderate activity against two fungal strains, Candida albicans (ATCC 10231) and Aspergillus fumigatus [99]. Chikkula and Sundararajan (2017) reported the synthesis of two benzimidazole–pyrimidine hybrids, 105 and 106, in two steps, starting from benzenamine 104 (Scheme 15) [100]. The antibacterial activity of the compounds was evaluated against four Gram-positive bacteria, Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 9144, Micrococcus luteus ATCC 4698, and Staphylococcus epidermidis ATCC 155, and three Gram-negative bacteria, Klebsiella pneumoniae ATCC 11298, Pseudomonas aeruginosa ATCC 2853, and Escherichia coli ATCC 25922. The antifungal activities of the synthesized compounds were evaluated against two fungi, Aspergillus fumigatus ATCC 46645 and Aspergillus niger ATCC 9029. The antimicrobial activity of compound 105 was, in most cases, twice as good as that of compound 106, but four to eight times weaker than that of the standard compounds considered, Ciprofloxacin and Ketoconazole, as can be seen in Scheme 15 [100]. Saundane and Mathada (2016) reported the synthesis of indole grafted benzimidazole–pyrimidine hybrids 110 and 111 from ethanone 107 and substituted 2-phenyl-1H-indole-3-carbaldehydes 108a–108c, via intermediate chalcones 109a–109c (Scheme 16) [101]. All compounds had good antimicrobial activity on bacterial strains, Escherichia coli MTCC 723, Staphylococcus aureus ATCC 29513, Klebsiella pneumonia NCTC 13368, and Pseudomonas aeruginosa MTCC 1688, using Gentamycin as a reference, and on fungal strains, Aspergillus oryzae MTCC 3567T, Aspergillus niger MTCC 281, Aspergillus flavus MTCC 1973, and Aspergillus terreus MTCC 1782, using Fluconazole as a reference, by the serial dilution method, with a MIC ranging from 4 to 16 µg/mL for the best compounds, 110a and 111a (Scheme 16). It was found that compound 111a, which had –OH group position “2” of the pyrimidine nucleus and was substituted with chlorine on the indole nucleus, exhibited the highest biological activity [101]. Liu et al. (2018) obtained hybrids 112–115 in 58–72% yields, also using an intermediate benzimidazole chalcone (Figure 12) [102]. Compounds 112–115 were evaluated for their antimicrobial activities in vitro against four Gram-positive bacteria, Staphylococcus aureus ATCC25923, Methicillin-Resistant Staphylococcus aureus N315, Bacillus subtilis, and Micrococcus luteus ATCC4698, six Gram-negative bacteria, Bacillus proteus ATCC13315, Escherichia coli DH52, Pseudomonas aeruginosa, Bacillus typhi, Escherichia coli JM109, and Shigella dysenteriae, and five fungi, Candida albicans ATCC76615, Candida mycoderma, Candida utilis, Saccharomyces cerevisiae, and Aspergillus flavus, using the standard two-fold serial dilution method in 96-well micro-test plates (Table 4). Based on their antimicrobial activity, 4-methyl-substituted hybrid 112 had the strongest antibacterial activity, followed by 2,4-dichloro-substituted hybrid 113, 4-fluoro-substituted compound 114, and 4-chloro-substituted compound 115, which had the lowest activity. Analyzing hybrid 112’s antibacterial activity required a flexible ligand receptor docking study. The interaction of compound 112 with the gyrase–DNA receptor is shown in Figure 13. The gyrase residue ASP1083 was in close proximity to the NH group of the benzimidazole ring via hydrogen bonds, with distances of 1.6 Å and 1.9 Å, respectively. The ability of compound 112 to form hydrogen bonds with the residue ASP1083 through the NH2 group’s hydrogen atom demonstrated the significance of NH and NH2 groups in biological activity. Additionally, the aromatic fragment of compound 112 had electrostatic interactions with the gyrase residues ARG1122, MET1121, ALA1120, TYR1087, and ASP1083. Compound 112 may have strong inhibitory activity against the tested strains due to cooperative binding that may be advantageous for stabilizing the compound–enzyme–DNA complex [102]. Khan et al. (2021) reported the synthesis of hybrids 117–124 by the reaction between 2-chloromethylbenzimidazole 82 and 6-substituted tetrahydropyrimidines 116 (Scheme 17) [103]. All compounds had extremely strong antimicrobial activity against Gram-negative strains E. coli, P. aeruginosa, and Gram-positive strains S. aureus, S. pyogenes, compared to Ampicillin as a standard, and a fungus, C. albicans, with Griseofulvin as a reference, as seen in Scheme 17. The MIC of compound 117 against E. coli was 62.5 µg/mL, which was much more potent than Ampicillin, while hybrids 119, 121, and 122 were equipotent with the standard, at 100 µg/mL. P. aeruginosa was sensitive to all derivatives at 62.5, 100, and 250 µg/mL, but not to Ampicillin. Staphylococcus aureus was sensitive to compounds 117, 118, 120, 121, and 123, at 200, 100, 100, 100, and 200 µg/mL, respectively; therefore, they are more potent than ampicillin, with MIC = 250 µg/mL. Compounds 118–122 exhibited two-fold greater antifungal activity against C. albicans compared to Griseofulvin, with MIC values of 250 µg/mL [103]. Zaghary et al. (2021) reported the synthesis of hybrid 125 by refluxing a mixture of sulfamethazine and (E)-1-(1H-benzo[d]imidazol-2-yl)-3- (dimethylamino)prop-2-en-1-one in acetic acid for two hours (Figure 14) [104]. Compound 125 had antimicrobial activity comparable to standard Ciprofloxacin against the microbial strains Staphylococcus aureus ATCC 29213, B. subtilis ATCC6633, B. Cerrus MTCC 1305, E. coli ATCC 2592, and Pseudomonas aeruginosa ATCC 27953, considering the average diameter of inhibition zones [104]. Sun et al. (2021) reported the synthesis of 2-(2-alkylthio-6-phenylpyrimidin-4-yl)-1H- benzimidazoles 126–131 in three steps, from ethanone 107, namely (1) aldol condensation with different aldehydes in NaOH solution to yield α,β-unsaturated ketones; (2) cyclization of previous ketones with thiourea in sodium isopropyl-isopropanol at reflux to afford substituted pyrimidine-2-thiols; and (3) previous key intermediates reacted with various alkyl halides or benzyl halides under the catalysis of NaOH in acetonitrile, with the formation of hybrids 126–131 in yields of 61–87% (Scheme 18) [105]. With EC50 values ranging from 0.14 to 0.28 μg/mL, compounds 126–131 demonstrated exceptional fungicidal activity against B. cinerea, proving that their activities were on par with or higher than carbendazim (EC50 = 0.21 μg/mL). Compounds 127, 129, 130, and 131 displayed notable fungicidal activities against S. sclerotiorum, with EC50 values of 4.65–13.97 μg/mL, which illustrated that their activities were also comparable or higher than that of carbendazim (EC50 = 13.32 μg/mL, Scheme 18). Structure–activity relationship (SAR) studies revealed that for S. sclerotiorum, the antifungal activity towards the R2 decreases in the order C6H4CH2 > n-C4H9 > CH3 when R1 is H, F, or OCH3 [105]. The authors chose the β-tubulin protein from B. cinerea as the biological target for docking, because benzimidazole derivatives used as agricultural fungicides (e.g., carbendazim, thiabendazole, benomyl—Figure 15) are inhibitors of β-tubulin [106]. The amino acid sequence of the B. cinerea protein (VERSION: AXO78835.1) was obtained from the NCBI protein database [107], and then the homology modeling was carried out using the Caenorhabditis elegans microtubule (PDB: 6e88) as a template [108], where their sequence alignment showed a 78.75% identity. The constructed 3D model of β-tubulin protein was used as the receptor, and 130 was used as the ligand to perform the docking study [109], with the result shown in Figure 16. As illustrated in Figure 16, the fluorine atom of the benzene ring may form a hydrogen bond with the OH of Tyr-208 residue at a distance of 3.8 Å, while the NH moiety of the benzimidazole ring may form a hydrogen bond with the carbonyl oxygen atom of the Gln-11 residue at a distance of 2.7 Å. Furthermore, the pyrimidine ring of 130 and the benzene ring of Tyr-222 developed a weak π-π interaction. The β-tubulin protein and 130 had a strong affinity because of the two hydrogen bonds and one π-π interaction. This could explain the superior fungicidal activity of compound 130. Vlasov et al. (2021) reported the synthesis of benzimidazole–pyrimidine acetamide 132 by reaction between 6-(1H-benzo[d]imidazol-2-yl)-3,5-dimethyl-2-thioxo-2,3-dihydro thieno[2,3-d]pyrimidin-4(1H)-one and 2-chloro-N-(4-isopropylphenyl)acetamide in dimethyl formamide for 3.5 h [110]. Hybrid 132 was more active than the Streptomycin standard against Gram-positive bacteria, S. aureus ATCC 25923, B. subtilis ATCC 6633, and demonstrated similar activity to the reference against Gram-negative bacteria, E. coli ATCC 25922, P. vulgaris ATCC 4636, P. aeruginosa ATCC 27853, and also inhibited the growth of the C. albicans strain, as evidenced by the average diameter (mm) of the growth inhibition zone [110].
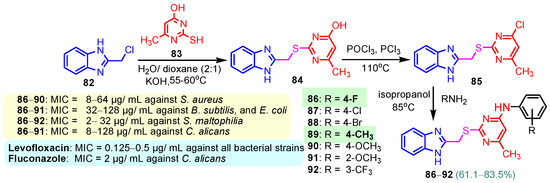
Scheme 11.
Synthesis of antimicrobial hybrids 86–92.
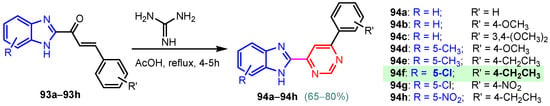
Scheme 12.
Synthesis of antimicrobial hybrids 94a–94h.
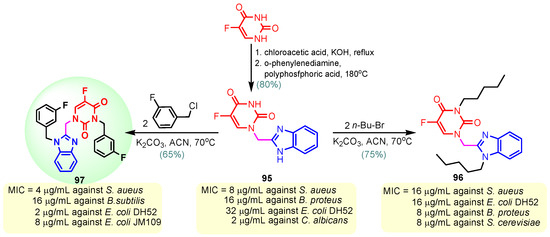
Scheme 13.
Synthesis of antimicrobial hybrids 95–97.

Table 3.
Minimum inhibitory concentration (MIC) of hybrids 98–100.
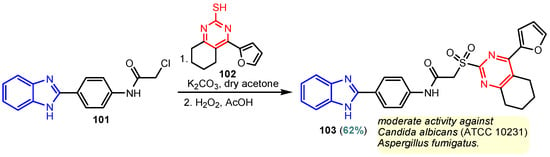
Scheme 14.
Synthesis of antimicrobial hybrid 103.
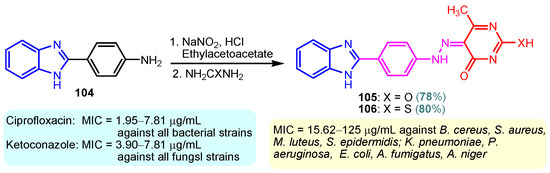
Scheme 15.
Synthesis of antimicrobial hybrids 105–106.
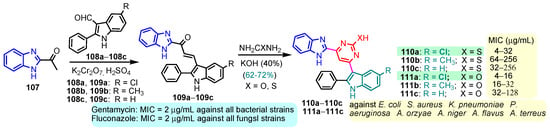
Scheme 16.
Synthesis of antimicrobial hybrids 110a–110c and 111a–111c.
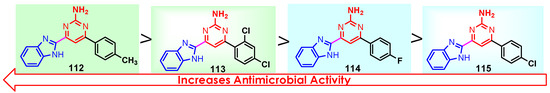
Figure 12.
Structures of antimicrobial hybrids 112–115.

Table 4.
MIC (Minimum inhibitory concentration) in μg/mL of compounds 112–115 and standard drugs.
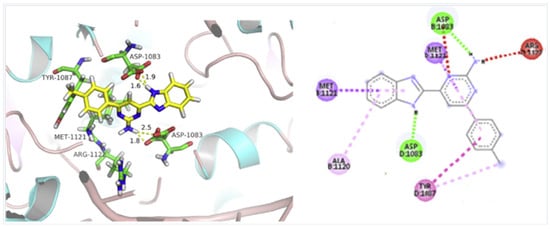
Figure 13.
Three-dimensional conformation of compound 112 docked in bacterial gyrase–DNA complex.
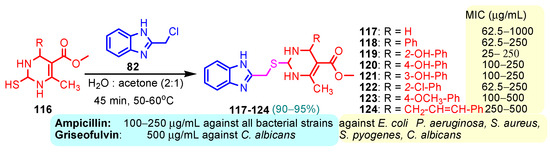
Scheme 17.
Synthesis of antimicrobial hybrids 117–124.
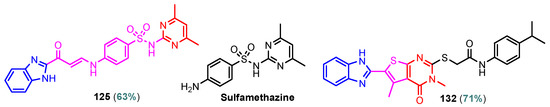
Figure 14.
Structures of antimicrobial compounds 104, 111, and sulfamethazine.
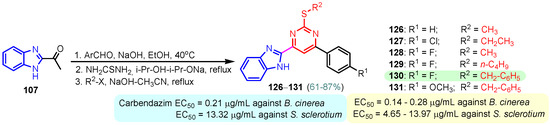
Scheme 18.
Synthesis of antimicrobial hybrids 126–131.
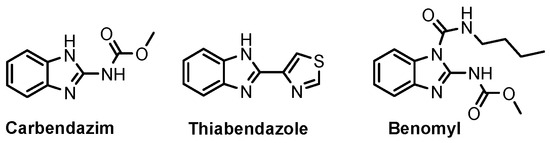
Figure 15.
Benzimidazole fungicides.
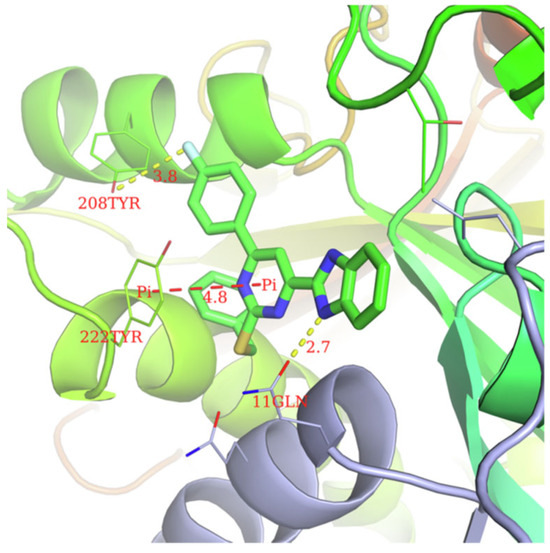
Figure 16.
Docking mode of compound 130 and the target protein.
5. Anti-Inflammatory Benzimidazole–Pyrimidine Hybrids
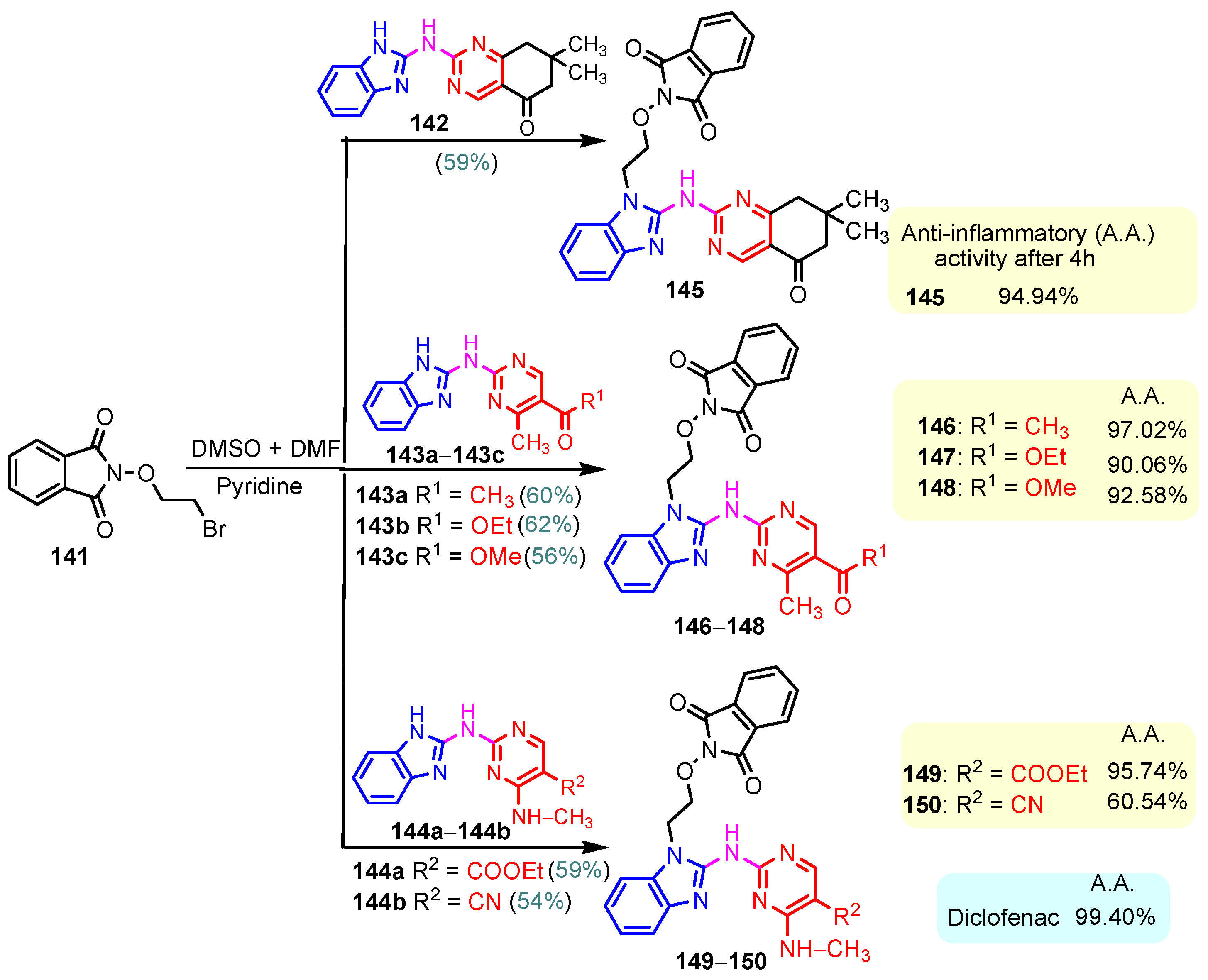
One of the most often prescribed medications in the world is non-steroidal anti-inflammatory drugs, or NSAIDs. The majority of anti-inflammatory medications currently used in clinical settings are becoming outdated because of their possible negative effects. They are turning out to be very dangerous to utilize for extended periods of time. As a result, new anti-inflammatory drugs have been created recently, and many of them are currently in advanced clinical testing. Benzimidazole–pyrimidine compounds with anti-inflammatory activities reported in the literature are presented below [114]. Chikkula and Sundararajan (2017) studied the anti-inflammatory activity of compounds 105 and 106 (Scheme 15) [100]. The carrageenan-induced paw edema test was performed using Wistar rats, and the obtained results are shown in Figure 18. The anti-inflammatory activity of thio-compound 106 was slightly better than that of oxo-compound 105, and both were half as effective as the standard Diclofenac [100]. Prajapat and Telasara (2016) reported the synthesis of a series of benzimidazole–pyrimidine 142–150 compounds containing alkoxy phthalimide [115]. Thus, by the reaction of isoindoline-1,3-dione 141 with benzimidazole–pyrimidine hybrids 142, 143a–143c, and 144a–144b, the target hybrids 145–150 resulted (Scheme 20) [115]. The best yield of 62% correlates with the presence of the carboethoxy group in the “5” position of the pyrimidine ring in compound 143b, while for compound 143c with the carbomethoxy group in the same position, the yield decreased to 56%. The presence of the acetyl group in the same position of the pyrimidine ring in 143a led to a yield of 60%. The hybrids 145–150 showed significant anti-inflammatory activity against carrageenan-induced rat paw edema. On screening, compounds 145, 146, and 149 exhibited good anti-inflammatory activity, whereas compounds 147, 148, and 150 exhibited moderate anti-inflammatory activity when compared to the reference diclofenac (Scheme 20) [115]. By blocking the activity of lymphocyte-specific kinase (Lck), hybrid 68 demonstrated a strong anti-inflammatory effect, according to Sabat et al. (Figure 8) [90,116]. Compound 68 exhibited strong anti-inflammatory properties and was effective at 0.054 mM for IL-2 cytokine inhibition and 3 nM for Lck kinase inhibition [90].
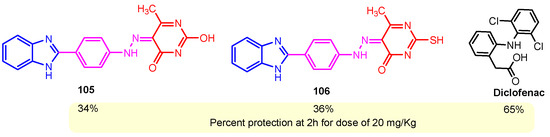
Figure 18.
Percent anti-inflammatory activity (Carrageenan-induced paw edema test in rats) of the compounds 105, 106, and standard Diclofenac.
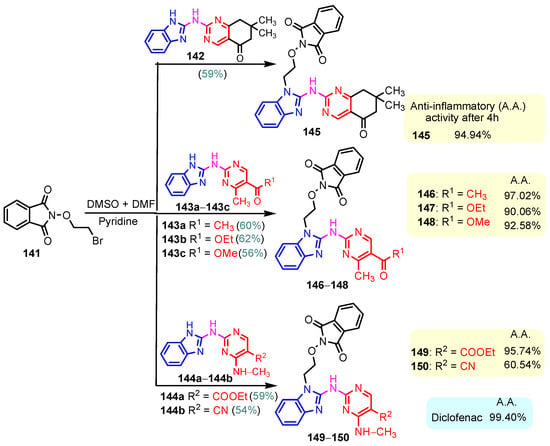
Scheme 20.
Synthesis of anti-inflammatory hybrids 145–150.
Chen et al. (2006) found hybrid 63 (Scheme 10) as a potent inhibitor of Janus kinase 3 (JAK3) with an IC50 activity of 45 nM [89]. SAR studies revealed that the nitrile group at the benzimidazole’s “6” position had a role in the high level of JAK3 inhibition [89]. Hybrids 69–76 were identified by Hunt et al. (2009) as inhibitors of Lck and inhibitors of cellular IL2 release (Figure 10) [91]. Compound 69 exhibits strong inhibition of cellular Lck activity and IL2 release, with IC50 values of 0.12 and 8 nM, respectively. Hybrid 72, the most potent Lck activity inhibitor in this series (IC50 = 0.06 nM), barely stops cells from producing IL2 at 111 nM. Also, hybrids 73, 74, and 75 have strong LcK IC50 values of 3, 1.5, and 1 nM, respectively [91]. Abdelgawad et al. (2019) reported the anti-inflammatory activity of compound 8 by determining the inhibitory activity of cyclooxygenase (COX-1, COX-2) and phospholipase A2-V (sPLA2-V) [62]. The results showed that hybrid 8 has a strong inhibitory activity against COX-1 (IC50 = 2.76 μM), a moderate activity against COX-2 (IC50 = 7.47 μM), and a moderate inhibitory activity against secretory phospholipase A2-V (sPLA2-V), with an IC50 of 7.51 μM [62].
6. Analgesic Benzimidazole–Pyrimidine Hybrids
The International Association for the Study of Pain (IASP) defines pain as an unpleasant emotional and sensory experience connected to actual or potential tissue damage. It is unethical and morally wrong to ignore pain [117,118]. Chikkula and Sundararajan (2017) reported analgesic activity of compounds 105 and 106 [100]. Compounds generally exhibited moderate analgesic activity at 30 min of reaction time; activity rose in the first hour, peaked in the second, and then declined in the third (Figure 19). These compounds exhibited a mild analgesic response compared to the standard drug Diclofenac [100].
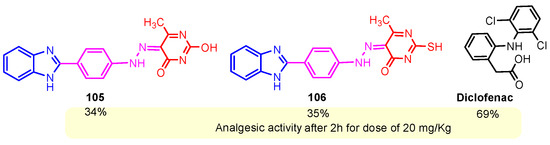
Figure 19.
Analgesic activity of hybrids 105, 106, and the standard diclofenac.
7. Antiulcer Benzimidazole–Pyrimidine Hybrids
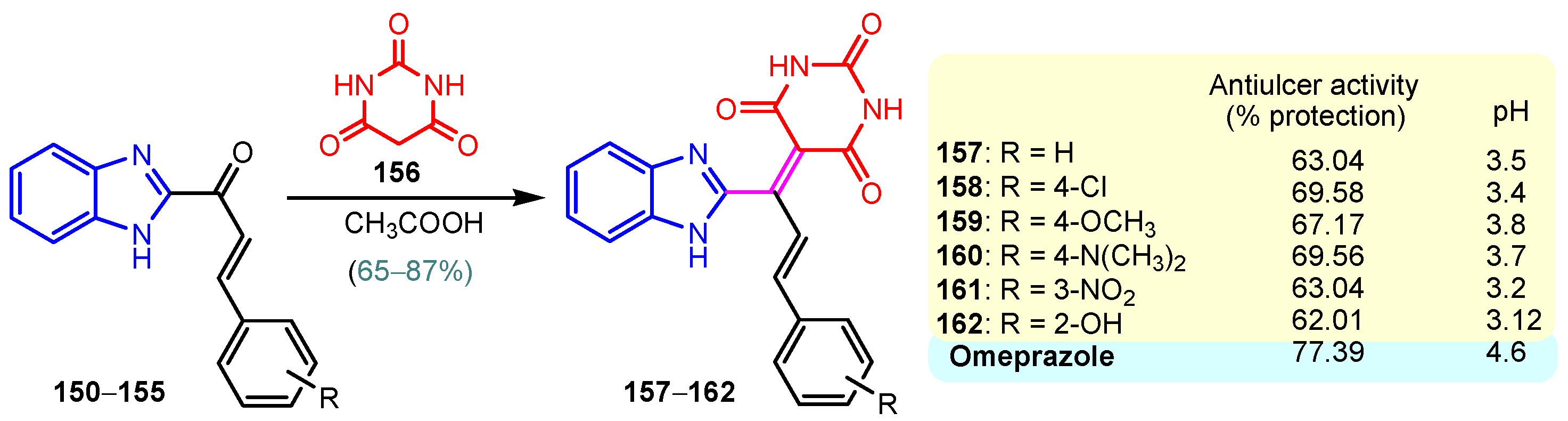
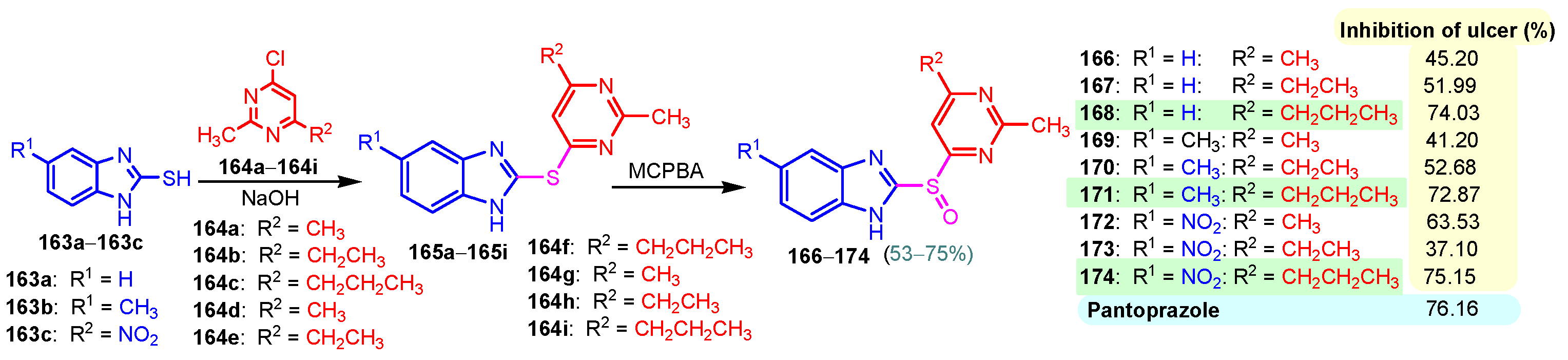
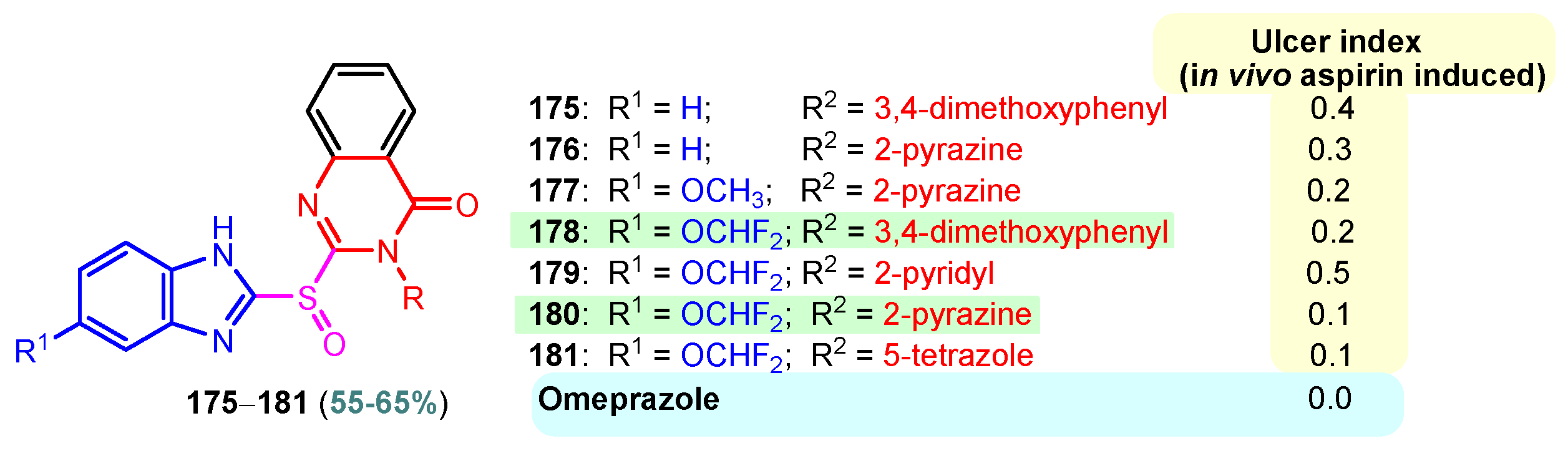
A chronic condition that affects up to 10% of people worldwide is peptic ulcer disease. Peptic ulcers are caused by a decline in mucosal defenses and the presence of gastric juice pH. The two main causes of the disruption of mucosal resistance to injury are Helicobacter pylori (H. pylori) infection and non-steroidal anti-inflammatory drugs (NSAIDs). Both the benzimidazole nucleus and the pyrimidine nucleus are known from the literature to have antiulcer properties [14,119,120]. The synthesis and antiulcer properties of the benzimidazole–pyrimidine hybrids will be discussed below. Mathew et al. (2013) reported the synthesis of 2-substituted benzimidazole-pyrimidine-2,4,6-triones 157–162 by refluxing a mixture of enone 150–155 and pyrimidine-trione 156 in acetic acid (Scheme 21) [121]. Compounds 158, 160, and 159 showed the best percentage protection of 69.58, 69.56, and 67.17%, respectively, at a dose of 50 mg/kg b.w. when compared to the standard omeprazole (77.37%, 2 mg/kg body weight). The total acidity and pH values from Scheme 21 revealed that hybrids 157–162 had a good protective effect on the gastric mucosa in ulcers. The mice treated with each of the hybrids 157–162 showed no damage to the stomach mucosa, according to electron microscope scanning of stomach specimens. Hence, each derivative demonstrated good mucomembranous protection [121]. Farhan and Farooqui (2021) reported synthesis of benzimidazole–sulfinyl–pyrimidines 166–174 by the reaction of 5-substituted benzimidazole-2-thiols 163a–163c with 6-substituted 4-chloro-2-methyl pyrimidines 164a–164i in basic medium of sodium hydroxide, and oxidation of intermediates 166a–166i with meta-chloroperoxybenzoic acid (MCPBA) (Scheme 22) [122]. All the compounds showed 30 to 70% inhibition of ulcers. It was found that the compounds 166 (45.20%), 169 (41.20%), and 173 (37.10%) exhibited lowest antiulcer activity, while the compounds 167 (51.99%) and 170 (52.68%) showed slightly significant antiulcer activity compared to the standard Pantoprazole (76.16}, and compounds 168 (74.03%), 171 (72.87%), and 174 (75.15%) showed highly significant antiulcer activity compared to the standard drug. The ulcerogenic activity increases in the following order: 173 < 169 < 166 < 167 < 170 < 172 < 171 < 168 < 174 < Pantoprazole. This means that the presence of the n-propyl group at the “5” position of the benzimidazole ring is essential for the high ulcerogenic activity, as in the compounds with the best activities, 174, 168, and 171 [122]. Patil et al. (2010) used a similar synthetic route to obtain compounds 175–181 (Figure 20) [123]. Compounds 178 and 180 showed the most potent activity as compared to Omeprazole at the dose level of 10 and 20 mg/kg, while compounds 175, 176, 177, 179, and 181 showed moderate activity at the same doses [123].
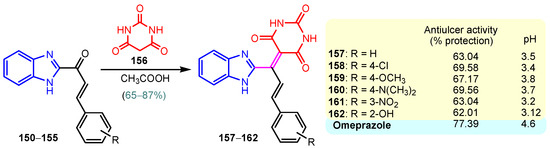
Scheme 21.
Synthesis of antiulcer hybrids 157–162.
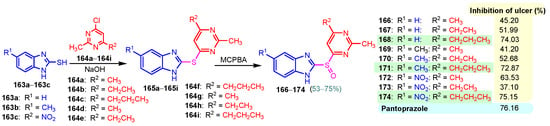
Scheme 22.
Synthesis of antiulcer hybrids 166–174.
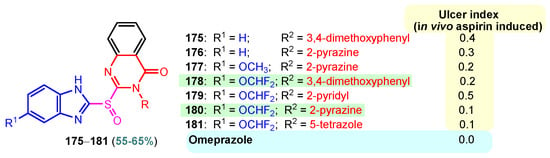
Figure 20.
Antiulcer benzimidazole–pyrimidines 176–181.
8. Antidepressant Benzimidazole–Pyrimidine Hybrids
According to the World Health Organization, depression is the primary cause of disability globally, affecting an estimated 300 million people. Furthermore, the presence of depression raises the risk of various illnesses like epilepsy, Alzheimer’s disease, stroke, cardiovascular disease, etc., considerably [124]. Finding new antidepressant compounds that better satisfy the increasingly varied needs that emerge globally is therefore crucial. A complex enzyme system in the central nervous system, monoamine oxidase (MAO) specifically catalyzes the deamination or inactivation of biogenic amines. MAO inhibitors increase the intracellular concentration of endogenous amines by inhibiting their deamination, which seems to be the cause of their antidepressant action. Mathew et al. (2016) reported the antidepressant activity of compounds 157–162 (Scheme 21) as MAO inhibitors [125]. All compounds showed good antidepressant activity when compared to the standard Clomipiramine at a dose level of 20 mg/kg (Table 6). The compound 160 had the best MAO-A inhibitory activity, significantly reducing the duration of immobility times at a 50 mg/kg dose level when compared to the standard Clomipiramine. The compounds 160, 159, and 162 significantly reduced the duration of immobility times to 62.58%, 57.23%, and 55.35% at a 50 mg/kg dose level when compared to the standard drug. The results revealed that the electron-donating groups, such as dimethyl amino, methoxy, and hydroxyl groups in the phenyl nucleus of the compounds, significantly enhanced the antidepressant activity when compared to (1H-benzimidazol-2-yl)-3-phenylprop-2-]pyrimidine-2,4,6 (1H,3H,5H)-triones having no substituents or electron-withdrawing groups on the phenyl ring system. According to molecular docking studies, the phenyl ring’s high lipophilic group may have an additional hydrophobic binding area that significantly increases the pharmacological action of the CNS antidepressant [125].

Table 6.
Antidepressant activity and neurotoxicity screening of compounds 157–162.
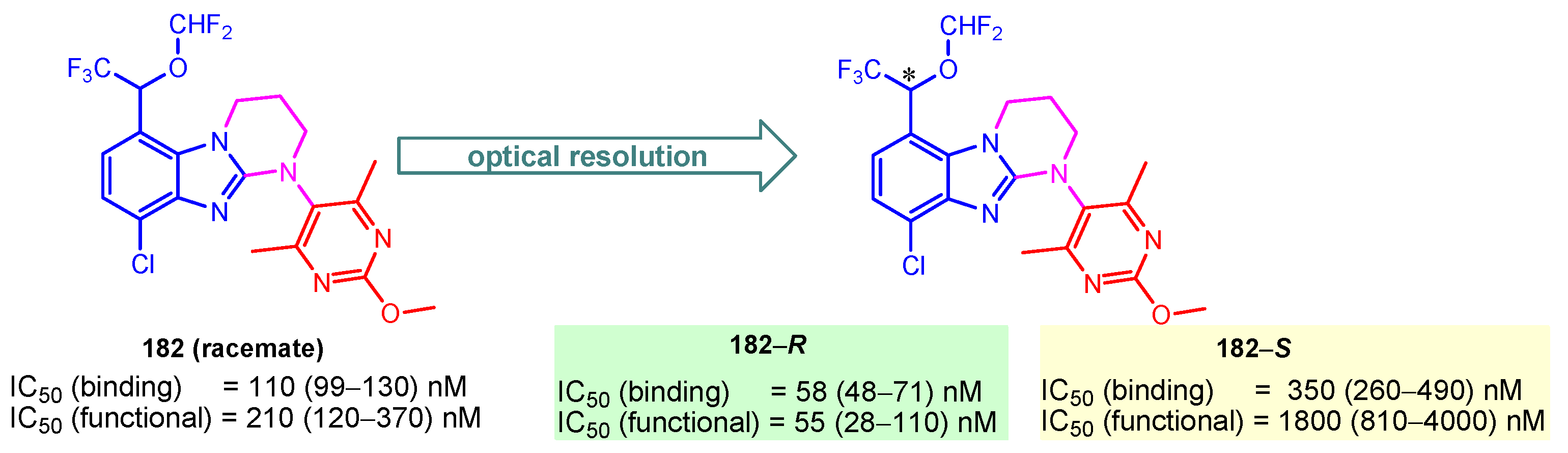
Corticotropin-releasing factor 1 (CRF1) receptor antagonists are promising targets for stress-related disorders. Kojima et al. (2018) reported compound 182 as a CRF1 receptor antagonist with IC50(binding) of 110 nM and IC50(functional) of 210 nM for the racemate [126]. Also, they determined that enantiomer 182-R successfully binds CRF1 receptors in the brain and exhibits the potential to be further examined for clinical studies (Figure 21) [126].
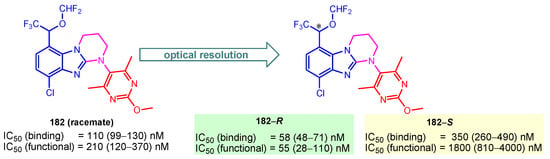
Figure 21.
IC50 values and 95% confidence intervals of chiral 182–R and 182–S as CRF1 receptor antagonists. * denotes asymmetric carbon atom.
9. Anti-Alzheimer’s Benzimidazole–Pyrimidine Hybrids
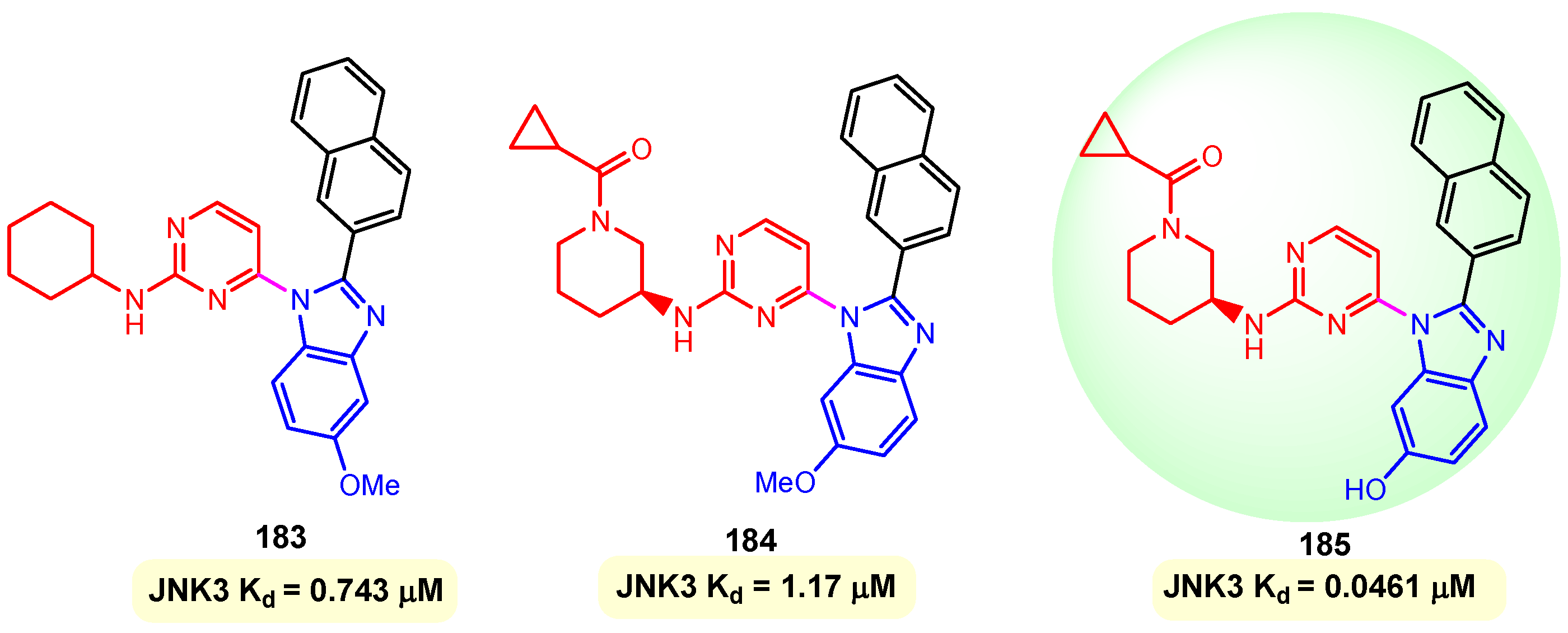
JNKs, or c-Jun N-terminal kinases, were first discovered to be kinases that phosphorylate and bind to c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. The three genes JNK1 (four isoforms), JNK2 (four isoforms), and JNK3 (two isoforms) produce the ten isoforms of the c-Jun N-terminal kinases. The JNK3s are regarded as degenerative signal transducers in pathological conditions. During the last two decades, a number of reports have supported JNK as a good therapeutic target for neurodegenerative diseases such as Alzheimer’s and Parkinsonian diseases, in addition to ischemic injury. The direct toxicity of β-amyloid contributes to the neuronal dysfunction and loss observed in Alzheimer’s disease. β-Amyloid-induced cell death is attenuated in cortical neurons from JNJ3-null mice, and JNK3 mediates this cell death through the activation of c-Jun and the enhanced expression of Fas ligand, a protein that plays a role in programmed cell death (apoptosis) and has been implicated in the pathogenesis of Alzheimer’s disease (AD). Kim et al. (2013) reported benzimidazole–pyrimidine hybrids 183–185 as inhibitors of c-Jun N-terminal kinases, JNK3 [127]. The synthesis of hybrids 183–185 has as an essential step, the reaction between 5-substituted 2-(naphthalen-2-yl)-1H-benzo [d]imidazoles and 4-chloro-2-(methylthio)pyrimidines on a palladium catalyst, in toluene with the formation of benzimidazole–pyrimidine hybrids [127]. Their activities were evaluated through measurement of Kd using SPR (Surface Plasmon Resonance), JNK3 kinase assay, and cell viability of human neuroblastoma cells. Compounds 183, 184, and 185 showed strong affinities to JNK3 of 0.743, 1.17, and 0.0461 µM, respectively (Figure 22). The best compound, 185, was confirmed as a potent and selective JNK3 inhibitor in cells, dramatically reducing phosphorylation of c-Jun. Thus, a dose-dependent decrease in tumor necrosis factor TNFα-mRNA levels by hybrid 185 with an IC50 of 1.09 µM was observed in the study [127].
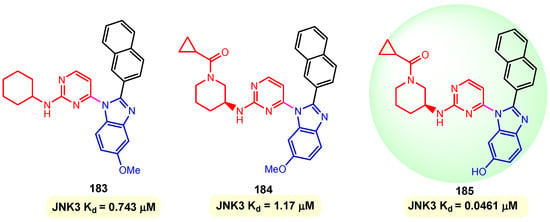
Figure 22.
Structures of hybrids 183–185 and their affinities (Kd) against JNK3.
10. Antioxidant Benzimidazole–Pyrimidine Hybrids
Antioxidants are substances that can prevent or reduce harm to cells. These molecules are produced by the body in response to environmental stressors and other factors. These compounds, also known as “free-radical scavengers,” are necessary for cells to survive in their internal processes. Antioxidant supplements may help reduce age-related glaucoma-related vision loss in older adults [128].
Abdelgawad et al. (2019) evaluated the free radical scavenging effect of hybrid 8 using a colorimetric test, the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging test with trolox as a standard [62]. The results of inhibitory effects at different concentrations of 10, 50, and 100 µM, respectively, showed that compound 8 exhibited a good scavenger effect against the DPPH radical [62].
11. Solubility of Benzimidazole–Pyrimidine Hybrids
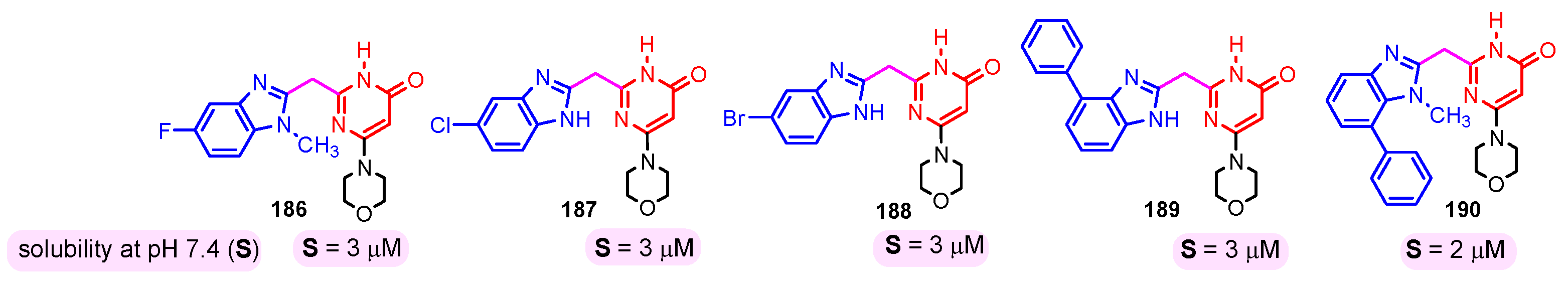
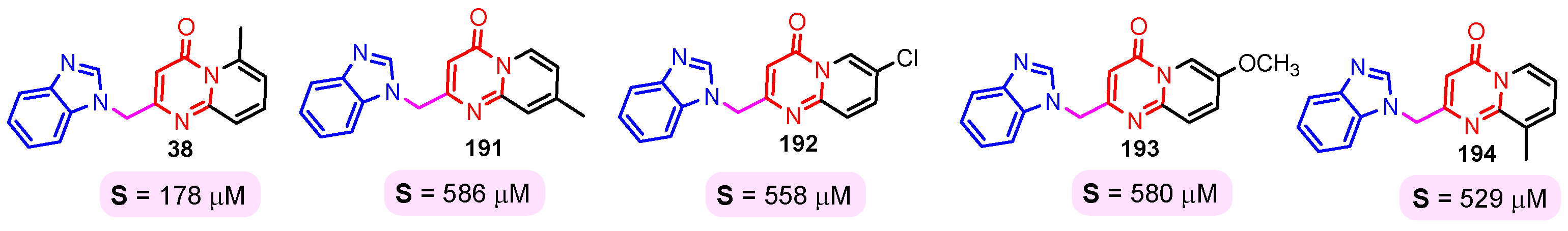
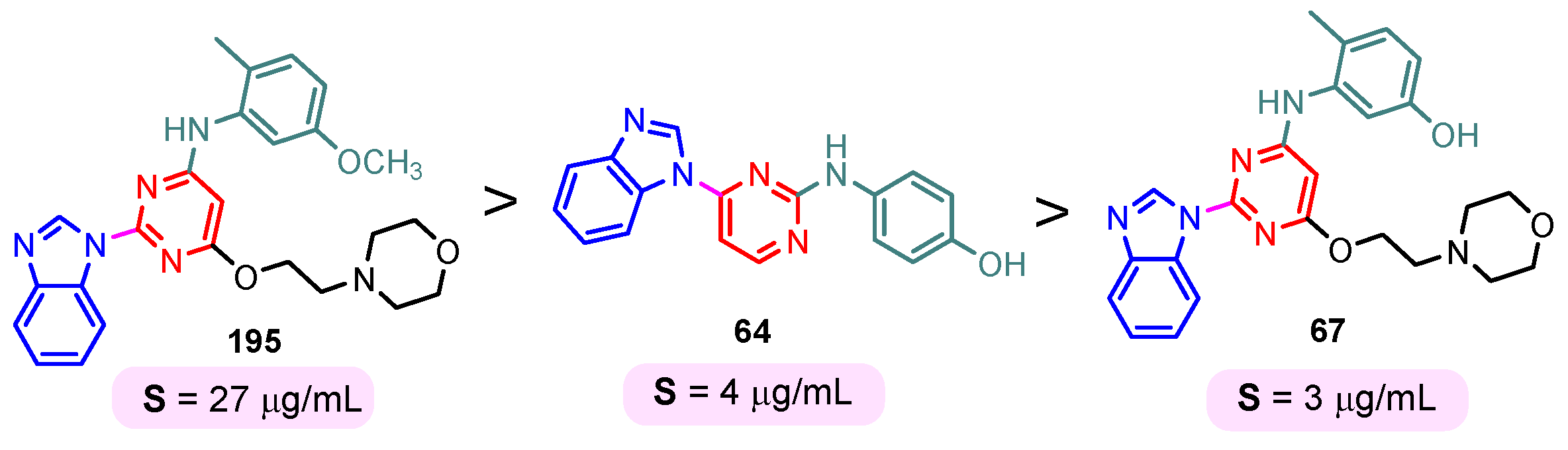
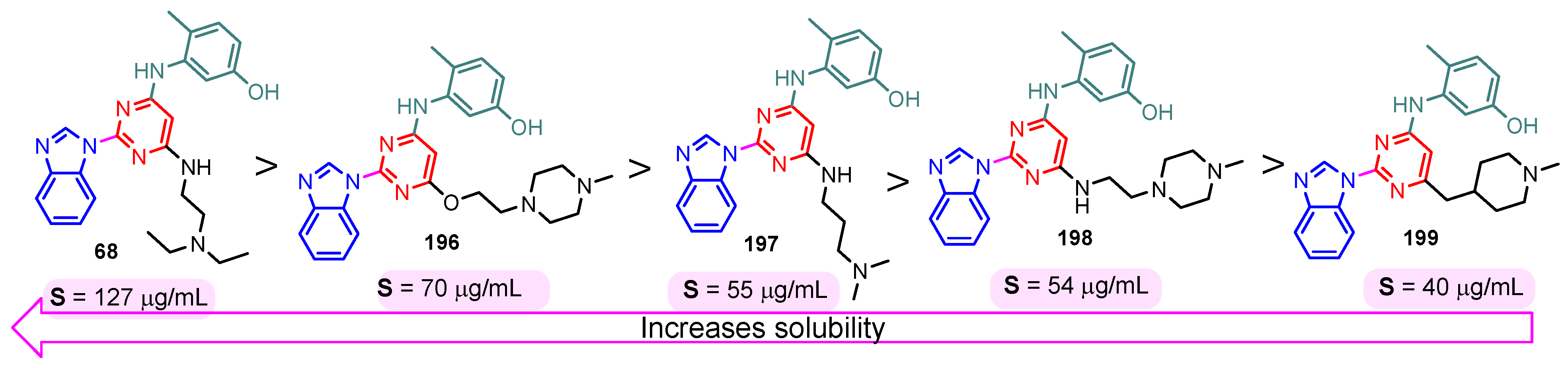
The solubility of the compounds is essential for further pharmacological studies, pharmacokinetic properties, and bioavailability of the compounds. In general, the aqueous solubility of these hybrids is particularly poor. Certal (2012) studied benzimidazole– pyrimidine hybrids 186–190 with surprisingly low aqueous solubility, less than 50 µM, at pH 7.4 (Figure 23) [68]. This solubility was correlated with strong crystal packing with intermolecular hydrogen bonds and hydrophobic interactions [68]. Guo et al. (2013) report anticancer hybrids 38, 191–194 with good aqueous solubility of 178–586 µM at pH 7.4 (Figure 24) [67]. In general, small substituents such as methyl, chloro, or methoxy groups improved solubility and also their potency as PKM2 activators [67]. A more detailed study on the solubility of benzimidazole–pyrimidine hybrids was carried out by Sabat et al. [90]. The researchers focused on improving the poor aqueous solubility of compound 64 (solubility of 4 µg/mL) by functionalizing it. The synthesized hybrid 67 had an even poorer solubility of 3 µg/mL (Figure 25). Methylation of its phenolic group improved the solubility nine-fold in hybrid 195 (27 µg/mL). The best solubility in this series was found for compound 68 (127 µg/mL) substituted in the 6-position with (N,N-diethylamino) ethylamino, followed by compound 196, (4-methylpiperazinyl)ethoxy substituted with a solubility of 70 µg/mL. The presence of the secondary amino group in position “6” of the pyrimidine ring and a hydrocarbon chain attached to it was found to be essential for the improved solubility of the compounds 68, 196–199 (Figure 26). In conclusion, from this series of compounds, hybrids 68, 196–199, had good solubilities of 40–127 µg/mL, and could be further studied for intrinsic clearance and bioavailability [90]. In most cases, the increase in the solubility of the hybrids is accompanied by a decrease in therapeutic activity.
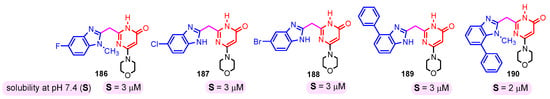
Figure 23.
Benzimidazole–pyrimidine hybrids with low aqueous solubility.
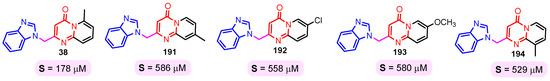
Figure 24.
Benzimidazole–pyrimidine hybrids 38, and 191–194 with good aqueous solubility.
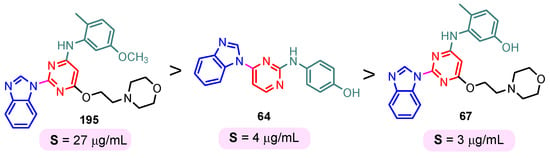
Figure 25.
Benzimidazole–pyrimidine hybrids with poor aqueous solubility.
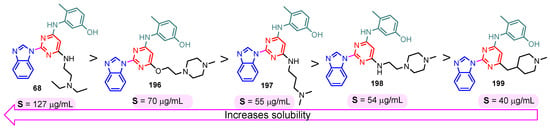
Figure 26.
Benzimidazole–pyrimidine hybrids 68, and 196–199 with good aqueous solubility.
12. Current Challenges and Future Prospects
Current challenges in the synthesis of benzimidazole–pyrimidine hybrids include the following:
1. Optimization of reaction conditions for high yields and selectivities. From this review, many syntheses with modest yields of 10–20% or even lower are observed. Therefore, finding new strategies to generate these hybrids is of utmost importance. Studies report various reaction conditions for optimizing yields, such as temperature, solvent, catalyst, and synthesis strategies. A very important role in a synthesis is played by the substituents grafted onto molecules, in the benzimidazole, pyrimidine, or other nuclei present, both through the electronic effects they generate and through the variation in solubility depending on the presence of one or more substituents.
From the presentation of the syntheses of benzimidazole–pyrimidine hybrids, it is observed that the presence of certain substituents, such as hydroxy, thiol, amino, methyl, methoxy, ethoxy, fluorine, trifluoromethyl, led to higher yields compared to molecules that did not contain these substituents. The yield of the synthesis reactions of benzimidazole hybrids is significantly improved by the presence of fluorine, chlorine, amino, methyl, and trifluoromethyl substituents on the benzene ring, while the presence of the same halogen on a phenyl ring linked to one of the rings leads to a substantial decrease in the yield. Methyl, amino, aminophenyl, thiomethyl, and sulfinylmethyl groups directly linked to the pyrimidine ring lead to an increase in the reaction yield. The presence of a cyano group on the benzimidazole ring leads to a strong decrease in the yield. The simultaneous presence of cyano and amino groups on the pyrimidine ring leads to a dramatic decrease in the yield. Hydroxy or morpholino groups, grafted on a phenyl ring, improve the reaction yield, while the simultaneous presence of hydroxy and methoxy groups leads to a dramatic decrease in the yield.
2. Addressing solubility issues for most hybrids, since, as shown above, in general, the problem of aqueous solubility of hybrids is essential, especially in therapeutic formulations. To obtain more water-soluble hybrids, it is necessary to functionalize the rings present in the molecule, both benzimidazole and pyrimidine, or other rings present, and among the substituents that help in this regard, those with amino, hydroxy, ether, or alkylyl, methyl, ethyl, or propyl groups stand out.
3. Understanding structure–activity relationships (SARs) to increase therapeutic potential. Many of the studies mentioned try to achieve a correlation between the architecture of a molecule, the presence of certain substituents, and therapeutic activity. The conclusions are not always uniform. For example, if initially an improved biological activity is noted by the presence of a halogen, fluorine, chlorine, bromine, or iodine, in many of the studies, there is no improvement in the therapeutic activity of benzimidazole–pyrimidine hybrids by their presence. Most likely, in this case, the presence of a halogen leads to a decrease in its solubility, not to a therapeutic improvement. Methylene, sulfidomethylene, amino, diazo, or carbonyl bridges between the two benzimidazole and pyrimidine rings typically increase the biological activity of anticancer drugs, according to SAR studies. It has been demonstrated that the presence of saturated heterocyclic substituents, such as morpholine or piperazine, as well as methoxy and methyl groups on a heterocycle or phenyl ring, is very advantageous for a variety of anticancer compounds. The S=O group between the two cores of pyrimidine and benzimidazole, the grafted alkyl groups—particularly the n-propyl to the pyrimidinone nitrogen—and the substituents -OCH3 and -OCF3 from the “5” position of the benzimidazolic ring are noted as being crucial for enhanced therapeutic activity. Similar to anticancer compounds, the antibacterial action of compounds is enhanced when a linker, such as methylene, thio, amino, or another heterocycle, is present between the two benzimidazole and pyrimidine rings. SAR studies showed that the high degree of JAK3 inhibition was caused by the presence of the nitrile group at the “6” position of the benzimidazole.
4. Computational methods such as DFT are also used to predict and optimize molecular properties and biological activity. The design of hybrid molecules with therapeutic properties through density functional theory is a widely used method, with good results. The receptor–ligand interaction establishes the active centers of the target molecule to be synthesized, and thus, the functional groups and their positions. Of particular importance in this regard are the hydrogen bonds that are established between the hydroxy or amino groups grafted on the hybrids and the amino acid residues in the receptor protein. More hydrogen bonds mean a stronger interaction and a superior therapeutic activity.
5. The role of chalcogens both within and outside the ring could be beneficial in improving the therapeutic activity through the generated chalcogen–receptor interaction. Chalcogens can be easily bound or assimilated by certain receptor structures due to their similarity to important biochemical structures, such as enzymes.
6. Future prospects focus on improving the efficacy, bioavailability, and safety of the compounds for the development of new pharmaceutical products with superior qualities to those currently known. In this sense, all the aspects discussed above are included here, especially the finding of hybrids with better aqueous solubilities and pharmaceutical formulations that would enhance their therapeutic action. The aim is to design, project, synthesize, and test the compounds and improve their characteristics by structural modification, respectively, grafting of substituents marked to be beneficial for a potential therapeutic activity.
13. Conclusions
In this review, the importance of benzimidazole–pyrimidine hybrids was highlighted by discussing various schemes for obtaining them, the tested medicinal properties, as well as the SAR studies performed. It is observed that most of the presented hybrids are potential anticancer agents, with remarkable properties, constituting future drugs to treat different types of cancer. SAR studies for anticancer compounds show that the presence of methylene, sulfidomethylene, amino, diazo, or carbonyl bridges between the two benzimidazole and pyrimidine rings generally improves their biological activity. Also, from the docking studies, it was seen that the presence of an additional heterocycle, such as 1,3,4-oxadiazole, considerably improved the anticancer activity of the hybrids. The presence of methoxy and methyl groups on one heterocycle or phenyl ring on various anticancer molecules has proven particularly beneficial, as has the presence of saturated heterocyclic substituents, such as morpholine or piperazine. The IC50 values for many of the reported anticancer hybrids are in the nanomolar range, which signifies particularly potent anticancer agents. The antimicrobial activity of the presented hybrids was particularly effective against a very wide range of pathogens, Gram-positive, Gram-negative bacteria, and fungi, with very good values of the minimum inhibitory concentrations, in the order of µM or µg/mL. SAR and docking studies have shown for many cases a more efficient interaction of antimicrobial molecules when they have electron-withdrawing substituents, such as F, Cl, Br, or OH, grafted onto the aromatic nuclei. These results are consistent with SAR studies reported in the literature for other heterocyclic compounds [129,130,131]. As in the case of anticancer molecules, the presence of a linker, methylene, thio, amino, or another heterocycle between the two benzimidazole and pyrimidine rings improves the antimicrobial activity of the compounds. The hybrids also had a nice range of antiviral activities against SARS-CoV-2, hepatitis C virus (HCV), and HIV-1. The benzimidazole–pyrimidine hybrids had very good anti-inflammatory activities, with IC50 values in the nanomolar and subnanomolar range. A series of compounds were JAK3 inhibitors and inhibitors of cellular IL2 release. SAR studies revealed that the nitrile group at the benzimidazole’s “6” position had a role in the high level of JAK3 inhibition. Compounds with strong inhibitory activity against COX-1 were also reported. Antiulcer activity of the hybrids discussed was close to the benzimidazole-containing standards, omeprazole and pantoprazole. It is highlighted the importance of the S=O group between the two cores benzimidazole and pyrimidine, as well as of the grafted alkyl groups, especially of the n-propyl to the pyrimidinone nitrogen, and of the substituents -OCH3 and -OCF2 from the “5” position of the benzimidazolic ring. The antidepressant activity of these compounds is remarkable. A number of compounds were MAO-A inhibitors and CRF1 receptor antagonists. According to molecular docking studies, the phenyl ring’s high lipophilic group may have an additional hydrophobic binding area that significantly increases the pharmacological action of the CNS antidepressant. The benzimidazole–pyrimidine hybrids have been shown to be selective JNK3 inhibitors in cells, and thus are potential anti-Alzheimer’s agents. The analgesic and antioxidant properties of these hybrids are also reported. These encouraging results motivate more research in this area. Many of the findings are only the start of future research directions that could transform medicinal chemistry. For upcoming generations of researchers, chemists, pharmacists, and biochemists, we hope this review will be a helpful resource.
Author Contributions
Conceptualization, M.M.; methodology, M.M. and C.Z.; validation, M.M. and C.Z.; resources, M.M.; data curation, M.M. and C.Z.; writing—original draft preparation, M.M.; writing—review and editing, M.M.; visualization, C.Z.; supervision, M.M. and C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in support of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murtazaeva, Z.; Nasrullaev, A.; Buronov, A.; Gaybullaev, S.; Nie, L.; Numonov, S.; Khushnazarov, Z.; Turgunov, D.; Kuryazov, R.; Zhao, J.; et al. Imidazole Hybrids: A Privileged Class of Heterocycles in Medicinal Chemistry with New Insights into Anticancer Activity. Molecules 2025, 30, 2245. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Roszczenko, P.; Holota, S.; Szewczyk, O.K.; Dudchak, R.; Bielawski, K.; Bielawska, A.; Lesyk, R. 4-Thiazolidinone-Bearing Hybrid Molecules in Anticancer Drug Design. Int. J. Mol. Sci. 2022, 23, 13135. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Marinescu, M.; Constantinescu, C.; Mitu, B.; Ion, V.; Ionita, I.; Dinescu, M.; Emandi, A. Nonlinear optical studies on 4-(ferrocenylmethylimino)-2-hydroxybenzoic acid thin films deposited by matrix-assisted pulsed laser evaporation (MAPLE). Appl. Surf. Sci. 2016, 374, 206–212. [Google Scholar] [CrossRef]
- Soliman, M.M.; Elwahy, A.H.M.; Sayed, A.M.; Ibrahim, M.; Dawoud, M.A.; Mohamed Ali, S.H.; Nady, M.S.; Hassan, N.A.; Saad, W.; Abdelhamid, I.A. Synthesis and antimicrobial evaluation of a new hybrid bis-cyanoacrylamide -based-piperazine containing sulphamethoxazole moiety against rheumatoid arthritis-associated pathogens. Naunyn Schmiedeberg’s Arch. Pharmacol. 2025, 398, 8587–8617. [Google Scholar] [CrossRef]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef]
- Mok, Z.H. The effect of particle size on drug bioavailability in various parts of the body. Pharm. Sci. Adv. 2024, 2, 100031. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Chandrakanth, M.; Thomas, N.M.; Arya, C.G.; Fabitha, K.; Banothu, J. Coumarin–1,2,4-triazole hybrids: Recent advances in synthesis and medicinal applications. J. Mol. Struct. 2024, 1299, 137197. [Google Scholar] [CrossRef]
- Ansari, M.F.; Tan, Y.M.; Sun, H.; Li, S.; Zhou, C.-H. Unique iminotetrahydroberberine-corbelled metronidazoles as potential membrane active broad-spectrum antibacterial agents. Bioorg. Med. Chem. Let. 2022, 76, 129012. [Google Scholar] [CrossRef]
- Yadav, J.; Kaushik, C.P. Quinoline-1,2,3-triazole hybrids: Design, synthesis, antimalarial and antimicrobial evaluation. J. Mol. Struct. 2024, 1316, 138882. [Google Scholar] [CrossRef]
- Marinescu, M. Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review. Antibiotics 2023, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Synthesis of Antimicrobial Benzimidazole–Pyrazole Compounds and Their Biological Activities. Antibiotics 2021, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Tarcomnicu, I.; Marinescu, M.; Nitulescu, G.M.; Tatia, R.; Moldovan, L.; Popa, M.; Chifiriuc, M.C. New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1094. [Google Scholar] [CrossRef]
- Adardour, M.; Ait Lahcen, M.; Oubahmane, M.; Ettahiri, W.; Hdoufane, I.; Bouamama, H.; Alanazi, M.M.; Cherqaoui, D.; Taleb, M.; Garcia, E.Z.; et al. Design, Synthesis, Molecular Modeling and Biological Evaluation of Novel Pyrazole Benzimidazolone Derivatives as Potent Antioxidants. Pharmaceuticals 2023, 16, 1648. [Google Scholar] [CrossRef]
- Marinescu, M.; Cinteza, L.O.; Marton, G.I.; Chifiriuc, M.C.; Popa, M.; Stanculescu, I.; Zalaru, C.M.; Stavarache, C.E. Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases. BMC Chem. 2020, 14, 45. [Google Scholar] [CrossRef]
- Díaz-Fernández, M.; Calvo-Losada, S.; Quirante, J.-J.; Sarabia, F.; Algarra, M.; Pino-González, M.-S. Catalyzed Methods to Synthesize Pyrimidine and Related Heterocyclic Compounds. Catalysts 2023, 13, 180. [Google Scholar] [CrossRef]
- Patil, S.B. Recent medicinal approaches of novel pyrimidine analogs: A review. Heliyon 2023, 9, e16773. [Google Scholar] [CrossRef]
- Nammalwar, B.; Bunce, R.A. Recent Advances in Pyrimidine-Based Drugs. Pharmaceuticals 2024, 17, 104. [Google Scholar] [CrossRef]
- Mahurkar, N.D.; Gawhale, N.D.; Lokhande, M.N.; Uke, S.J.; Kodape, M.M. Benzimidazole: A versatile scaffold for drug discovery and beyond—A comprehensive review of synthetic approaches and recent advancements in medicinal chemistry. Res. Chem. 2023, 6, 101139. [Google Scholar] [CrossRef]
- Aroua, L.M.; Alminderej, F.M.; Almuhaylana, H.R.; Alosaimia, A.H.; Medini, F.; Mohammed, H.A.; Almahmoud, S.A.; Khan, R.A.; Mekni, N.H. Benzimidazole(s): Synthons, bioactive lead structures, total synthesis, and the profiling of major bioactive categories. RSC Adv. 2025, 15, 7571–7608. [Google Scholar] [CrossRef] [PubMed]
- Ahmadfilab, P.; Pordel, M.; Kheirkhahnia, S.; Ziaei, S. Advances in benzimidazole coordination chemistry: From synthetic innovations to emerging therapeutic potentials. J. Mol. Struct. 2025, 1342, 142646. [Google Scholar] [CrossRef]
- Youssif, B.G.M.; Morcoss, M.M.; Bräse, S.; Abdel-Aziz, M.; Abdel-Rahman, H.M.; Abou El-Ella, D.A.; Abdelhafez, E.S.M.N. Benzimidazole-Based Derivatives as Apoptotic Antiproliferative Agents: Design, Synthesis, Docking, and Mechanistic Studies. Molecules 2024, 29, 446. [Google Scholar] [CrossRef]
- Reymova, F.; Sever, B.; Topalan, E.; Sevimli-Gur, C.; Can, M.; Tuyun, A.F.; Başoğlu, F.; Ece, A.; Otsuka, M.; Fujita, M.; et al. Design, Synthesis, and Mechanistic Anticancer Evaluation of New Pyrimidine-Tethered Compounds. Pharmaceuticals 2025, 18, 270. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm Activities of Halogenated Pyrimidines Against Enterohemorrhagic Escherichia coli O157:H7. Int. J. Mol. Sci. 2025, 26, 1386. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.V.; Tănase, M.A.; Soare, A.C.; Tablet, C.; Bala, D.; Cinteza, L.O.; Diţu, L.M.; Gifu, I.C.; Petcu, C. Synthesis, Characterization, DFT Study and Antifungal Activities of Some Novel 2-(Phenyldiazenyl)phenol Based Azo Dyes. Materials 2022, 15, 8162. [Google Scholar] [CrossRef]
- Balaes, T.; Mangalagiu, V.; Antoci, V.; Amariucai-Mantu, D.; Diaconu, D.; Mangalagiu, I.I. Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods. Pharmaceuticals 2025, 18, 495. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Kamat, V. Pyrimidines: A New Versatile Molecule in the Drug Development Field, Scope, and Future Aspects. Pharmaceuticals 2024, 17, 1258. [Google Scholar] [CrossRef]
- Georgiou, E.A.; Paraskevas, K.; Koutra, C.; Persoons, L.; Schols, D.; De Jonghe, S.; Kostakis, I.K. Exploring 4,7-Disubstituted Pyrimido [4,5-d]pyrimidines as Antiviral and Anticancer Agents. Molecules 2024, 29, 5549. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Wang, B.; Zhang, D.; Zhao, L.; Bei, Z.; Song, Y. Design, Synthesis, and Biological Evaluation of Benzimidazole Derivatives as Potential Lassa Virus Inhibitors. Molecules 2023, 28, 1579. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Iqbal, S.; Shah, M.; Rehman, W.; Khan, S.; Rasheed, L.; Rahim, F.; Dera, A.A.; Kehili, S.; Elkaeed, E.B.; et al. Synthesis of Novel Benzimidazole-Based Thiazole Derivatives as Multipotent Inhibitors of α-Amylase and α-Glucosidase: In Vitro Evaluation along with Molecular Docking Study. Molecules 2022, 27, 6457. [Google Scholar] [CrossRef] [PubMed]
- Rafique, I.; Maqbool, T.; Rutjes, F.P.J.T.; Irfan, A.; Jardan, Y.A.B. Anti-Diabetic Activities and Molecular Docking Studies of Aryl-Substituted Pyrazolo [3,4-b]pyridine Derivatives Synthesized via Suzuki Cross-Coupling Reaction. Pharmaceuticals 2024, 17, 1326. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.A. Pyrimidine derivatives as multifaceted antidiabetic agents: A comprehensive review of structure-activity relationships, mechanisms, and clinical potential. Eur. J. Med. Chem. 2025, 296, 117859. [Google Scholar] [CrossRef]
- Rostami, H.; Haddadi, M.H. Benzimidazole derivatives: A versatile scaffold for drug development against Helicobacter pylori-related diseases. Fundam. Clin. Pharmacol. 2022, 36, 930–943. [Google Scholar] [CrossRef]
- Vyas, A.; Sahu, B.; Pathania, S.; Nandi, N.K.; Chauhan, G.; Asati, V.; Kumar, B. An insight on medicinal attributes of pyrimidine scaffold: An updated review. J. Heterocycl. Chem. 2023, 60, 1081–1121. [Google Scholar] [CrossRef]
- Keshk, R.M.; Salama, Z.A.; Elsaedany, S.K.; ElRehim, E.M.A.; Beltagy, D.M. Synthesis, antimicrobial, anti-inflammatory, antioxidant and cytotoxicity of new pyrimidine and pyrimidopyrimidine derivatives. Sci. Rep. 2025, 15, 9328. [Google Scholar] [CrossRef]
- Rudrapal, M.; Mojammil, M.; Farooque, A.; Ansari, M.; de Oliveira, A.M.; Khan, J. Synthesis, toxicity and antioxidant activity of phenolic benzimidazole derivatives: In vitro and in silico studies. Chem. Phys. Imp. 2025, 10, 100875. [Google Scholar] [CrossRef]
- Othman, M.; Hayat, S.; Rahim, F.; Taha, M.; Sajid, M.; Khan, S.; Iqbal, W.; Shah, S.A.A.; Fareid, M.A.; Aboelnaga, S.M.; et al. New benzimidazole based Schiff bases as potent anti-alzheimer agents: Synthesis, bio-evaluation and molecular docking study. J. Mol. Struct. 2024, 1309, 138058. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, Q.; LI, B.-Q.; Zhang, D.; Chui, R.H.; Zhang, L.L.; Zhang, Q.; Ma, L.Y. Progress in the study of anti-Alzheimer’s disease activity of pyrimidine-containing bioactive molecules. Eur. J. Med. Chem. 2025, 285, 117199. [Google Scholar] [CrossRef]
- Fioravanti, R.; Proia, E.; Tyurenkov, I.N.; Kurkin, D.V.; Bakulin, D.A.; Kovalev, N.S.; Sheikin, D.S.; Kirillov, I.A.; Nawrozkij, M.B.; Vernigora, A.A.; et al. Pyrimidine thioethers: A novel class of antidepressant agents, endowed with anxiolytic, performance enhancing and nootropic activity. Eur. J. Med. Chem. 2023, 245, 114902. [Google Scholar] [CrossRef]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of benzimidazole-linked-1,3,4-oxadiazole carboxamides as GSK-3β inhibitors with in vivo antidepressant activity. Bioorg. Chem. 2018, 77, 393–401. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, M.; He, J.; Song, X.; Guo, J.; Gao, B.; Zhang, J.; Zhang, C.; Xie, Y. Discovery of novel benzimidazole derivatives as selective and reversible monoamine oxidase B inhibitors for Parkinson’s disease treatment. Eur. J. Med. Chem. 2024, 274, 116566. [Google Scholar] [CrossRef]
- Pant, S.; Kapri, A.; Nain, S. Pyrimidine analogues for the management of neurodegenerative diseases. Eur. J. Med. Chem. Rep. 2022, 6, 100095. [Google Scholar] [CrossRef]
- Vasil’ev, P.M.; Kalitin, K.Y.; Spasov, A.A.; Grechko, O.Y.; Poroikov, V.V.; Filimonov, D.A.; Anisimova, V.A. Prediction and Study of Anticonvulsant Properties of Benzimidazole Derivatives. Pharm. Chem. J. 2017, 50, 775–780. [Google Scholar] [CrossRef]
- Song, M.; Zhao, W.; Zhu, Y.; Liu, W.; Deng, X.; Huang, Y. Design, Synthesis, and Evaluation of Anticonvulsant Activities of New Triazolopyrimidine Derivatives. Front. Chem. 2022, 10, 925281. [Google Scholar] [CrossRef]
- Bano, S.; Nadeem, H.; Zulfiqar, I.; Shahzadi, T.; Anwar, T.; Bukhari, A.; Masaud, S.M. Synthesis and anti-inflammatory activity of benzimidazole derivatives; an in vitro, in vivo and in silico approach. Heliyon 2024, 10, e30102. [Google Scholar] [CrossRef]
- Tylińska, B.; Janicka-Kłos, A.; Gębarowski, T.; Nowotarska, P.; Plińska, S.; Wiatrak, B. Pyrimidine Derivatives as Selective COX-2 Inhibitors with Anti-Inflammatory and Antioxidant Properties. Int. J. Mol. Sci. 2024, 25, 11011. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen Containing Heterocycles as Anticancer Agents: A Medicinal Chemistry Perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Abemaciclib: First Global Approval. Drugs 2017, 77, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Lau, G.; Saraceni, M. Abemaciclib: The Newest CDK4/6 Inhibitor for the Treatment of Breast Cancer. Ann. Pharmacother. 2019, 53, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Davie, A.; Traoré, S.; Badreldin, W.; Torstensson, A.; Cakar, E. A Cost-Effectiveness Analysis of Abemaciclib in Combination with Adjuvant Endocrine Therapy for HR+, HER2–, Node-Positive, High-Risk Early Breast Cancer. Adv. Ther. 2025, 42, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Huober, J.; Garcia-Saenz, J.; Masuda, N.; Sohn, J.H.; Andre, V.A.M.; Barriga, S.; Cox, J.; Goetz, M. Management of Abemaciclib-Associated Adverse Events in Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Safety Analysis of MONARCH 2 and MONARCH 3. Oncologist 2021, 26, e53–e65. [Google Scholar] [CrossRef]
- Goetz, M.P.; Cicin, I.; Testa, L.; Tolaney, S.M.; Huober, J.; Guarner, V.; Johnston, S.R.D.; Martin, M.; Rastogi, P.; Harbeck, N.; et al. Impact of dose reductions on adjuvant abemaciclib efficacy for patients with high-risk early breast cancer: Analyses from the monarchE study. npj Breast Cancer 2024, 10, 34. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR1, HER22, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 34. [Google Scholar] [CrossRef]
- O’Keefe, K.; Desai, N.V.; Tan, A.R. Practical Guidance on Abemaciclib in Combination with Adjuvant Endocrine Therapy for Treating Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative High-Risk Early Breast Cancer. BCT 2024, 16, 517–527. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Guarneri, V.; Seo, J.H.; Cruz, J.; Abreu, M.H.; Takahashi, M.; Barrios, C.; McIntyre, K.; Wei, R.; Munoz, M. Long-term patient-reported outcomes from monarchE: Abemaciclib plus endocrine therapy as adjuvant therapy for HR+, HER2-, node-positive, high-risk, early breast cancer. Eur. J. Cancer 2024, 199, 113555. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, K.; Song, C.-G.; Ouyang, Q.; Liu, Z.; Liu, Q.; Feng, J.; Chiu, J.W.Y.; Tang, J.; Jiang, Z.; et al. Abemaciclib combined with endocrine therapy as adjuvant treatment for hormone-receptor-positive, HER2−, high-risk early breast cancer: 5-year Chinese population analysis of the phase III randomized monarchE study. Ther. Adv. Med. Oncol. 2024, 16, 1–12. [Google Scholar] [CrossRef]
- Kalinsky, K.; Bianchini, G.; Hamilton, E.; Graff, S.L.; Park, K.H.; Jeselsohn, R.; Demirci, U.; Martin, M.; Layman, R.M.; Hurvitz, S.A.; et al. Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial. J. Clin. Oncol. 2024, 43, 1101–1112. [Google Scholar] [CrossRef]
- Shao, K.P.; Zhang, X.-Y.; Chen, P.-J.; Xue, D.-Q.; He, P.; Ma, L.-Y.; Zheng, J.-X.; Zhang, Q.R.; Liu, H.M. Synthesis and biological evaluation of novel pyrimidine–benzimidazol hybrids as potential anticancer agents. Bioorg. Med. Chem. Let. 2014, 24, 3877–3881. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; Ahmad, W.; Al-Sanea, M.M.; Elshemy, H.A.H. New pyrimidine-benzoxazole/benzimidazole hybrids: Synthesis, antioxidant, cytotoxic activity, in vitro cyclooxygenase and phospholipase A2-V inhibition. Bioorg. Chem. 2019, 92, 103218. [Google Scholar] [CrossRef]
- Haoran, W.; Akhtar, W.; Nainwal, L.M.; Kaushik, S.M.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Synthesis and biological evaluation of benzimidazole pendant cyanopyrimidine derivatives as anticancer agents. J. Heterocycl. Chem. 2020, 57, 3350–3360. [Google Scholar] [CrossRef]
- Determann, R.; Dreher, J.; Baumann, K.; Preu, L.; Jones, P.G.; Totzke, F.; Schächtele, C.; Michael, H.G.; Kubbutat, M.H.G.; Kunick, C. 2-Anilino-4-(benzimidazol-2-yl)pyrimidines e A multikinase inhibitor scaffold with antiproliferative activity toward cancer cell lines. Eur. J. Med. Chem. 2012, 53, 254–263. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, M.; Ling, X.; Liu, Y.; Xue, Y.; An, L.; Gu, N.; Jin, M. Design, synthesis, quantum chemical studies and biological activity evaluation of pyrazole–benzimidazole derivatives as potent Aurora A/B kinase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.T.; Ragab, F.A.F.; Ramla, M.M.; El Diwani, H.I. Novel benzimidazole–pyrimidine conjugates as potent antitumor agents. Eur. J. Med. Chem. 2010, 45, 2336–2344. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Linton, A.; Jalaie, M.; Kephart, S.; Ornelas, M.; Pairish, M.; Greasley, S.; Richardson, P.; Maegley, K.; Hickey, M.; et al. Discovery of 2-((1H-benzo[d]imidazol-1-yl)methyl)-4H-pyrido [1,2-a]pyrimidin-4-ones as novel PKM2 activators. Bioorg. Med. Chem. Lett. 2013, 23, 3358–3363. [Google Scholar] [CrossRef]
- Certal, V.; Halley, F.; Virone-Oddos, A.; Delorme, C.; Karlsson, A.; Rak, A.; Thompson, F.; Filoche-Rommé, F.; El-Ahmad, Y.; Carry, J.-C.; et al. Discovery and Optimization of New Benzimidazole- and Benzoxazole- Pyrimidone Selective PI3Kβ Inhibitors for the Treatment of Phosphatase and TENsin homologue (PTEN) Deficient Cancers. J. Med. Chem. 2012, 55, 4788–4805. [Google Scholar] [CrossRef]
- El-Naem, S.I.; El-Nzhawy, A.O.; El-Diwani, H.I.; Hamid, A.O.A. Synthesis of 5-Substituted 2-Methylbenzimidazoles with Anticancer Activity. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 7–17. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, D.-M.; Chen, T.-C.; Lee, C.-C.; Hsieh, H.-H.; Huang, F.-C.; Huang, K.F.; Jih-Hwa Guh, J.-H.; Lin, J.-J.; Huang, H.-S. Structure-based design, synthesis and evaluation of novel anthra [1,2-d]imidazole-6,11-dione derivatives as telomerase inhibitors and potential for cancer polypharmacology. Eur. J. Med. Chem. 2013, 60, 29–41. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Pal, K. Quinazolinone-benzimidazole conjugates: Synthesis, characterization, dihydrofolate reductase inhibition, DNA and protein binding properties. J. Photochem. Photobiol. B Biol. 2017, 168, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Refaat, H.M. Versatile mechanisms of 2-substituted benzimidazoles in targeted cancer therapy. Fut. J. Pharm. Sci. 2020, 6, 41. [Google Scholar] [CrossRef]
- Ismail, M.M.F.; El-Sehrawi, H.; Elzahabi, H.S.A.; Shawer, T.; Ammar, Y.A. Synthesis and Antitumor Activity of Novel Hybrids of Pyrimidine/Benzimidazole Scaffolds. PAC 2020, 42, 2363–2377. [Google Scholar] [CrossRef]
- Sana, S.; Reddy, V.G.; Reddy, T.S.; Tokala, R.; Kumar, R.; Bhargava, S.K.; Shankaraiah, N. Cinnamide derived pyrimidine-benzimidazole hybrids as tubulin inhibitors: Synthesis, in silico and cell growth inhibition studies. Bioorg. Chem. 2021, 110, 104765. [Google Scholar] [CrossRef]
- Satija, G.; Sharma, B.; Madan, A.; Iqubal, A.; Shaquiquzzaman, M.; Akhter, M.; Parvez, S.; Khan, M.A.; Alam, M.M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heterocycl. Chem. 2022, 59, 22–66. [Google Scholar] [CrossRef]
- Rashid, M.; Husain, A.; Mishra, R.; Karim, S.; Khan, S.; Ahmad, M.; Al-wabel, N.; Husain, A.; Ahmad, A.; Khan, S.A. Design and synthesis of benzimidazoles containing substituted oxadiazole, thiadiazole and triazolothiadiazines as a source of new anticancer agents. Arab. J. Chem. 2019, 12, 3202–3224. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2023, 13, 478–497. [Google Scholar] [CrossRef]
- Bagul, C.; Rao, G.K.; Veena, I.; Kulkarni, R.; Tamboli, J.R.; Akunuri, R.; Shaik, S.P.; Pal-Bhadra, M.; Kamal, A. Benzimidazole-linked pyrazolo [1,5-a]pyrimidine conjugates: Synthesis and detail evaluation as potential anticancer agents. Mol. Div. 2023, 27, 1185–1202. [Google Scholar] [CrossRef]
- Warnault, P.; Yasri, A.; Coisy-Quivy, M.; Cheve, G.; Bories, C.; Fauvel, B.; Benhida, R. Recent advances in drug design of epidermal growth factor receptor inhibitors. Curr. Med. Chem. 2013, 20, 2043–2067. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Ferbinteanu, M.; Tarcomnicu, I.; Marinescu, M.; Moldovan, Z.; Nitulescu, G.M.; Tatia, R.; Popa, M. Synthesis, Spectroscopic Characterization, Structural Analysis, and Evaluation of Anti-Tumor, Antimicrobial, and Antibiofilm Activities of Halogenoaminopyrazoles Derivatives. Antibiotics 2024, 13, 1119. [Google Scholar] [CrossRef]
- Farag, B.; Zaki, M.E.A.; Elsayed, D.A.; Gomha, S.M. Benzimidazole chemistry in oncology: Recent developments in synthesis, activity, and SAR analysis. RSC Adv. 2025, 15, 18593. [Google Scholar] [CrossRef]
- Venugopal, S.; Balwinder Kaur, B.; Verma, A.; Wadhwa, P.; Magan, M.; Hudda, S.; Kakoty, V. Recent advances of benzimidazole as anticancer agents. Chem. Biol. Drug. Des. 2023, 102, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Díez, C.; Ferreras-Martín, R.; Carrión-Marchante, R.; González, V.M.; Martín, M.E. Deeping in the Role of the MAP-Kinases Interacting Kinases (MNKs) in Cancer. Int. J. Mol. Sci. 2020, 21, 2967. [Google Scholar] [CrossRef] [PubMed]
- Hagar, F.F.; Abbas, S.H.; Atef, E.; Abdelhamid, D.; Abdel-Aziz, M. Benzimidazole scaffold as a potent anticancer agent with different mechanisms of action (2016–2023). Mol. Div. 2025, 29, 1821–1849. [Google Scholar] [CrossRef]
- Lee, Y.; Bae, K.J.; Chon, J.; Kim, S.H.; Kim, S.A.; Kim, J. A Receptor Tyrosine Kinase Inhibitor, Dovitinib (TKI-258), Enhances BMP-2-Induced Osteoblast Differentiation In Vitro. Mol. Cells 2016, 39, 389–394. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Román-Gil, M.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef]
- Yadav, S.S.; Li, J.; Jennifer, A.; Stockert, J.A.; Herzog, B.; James O’Connor, J.; Garzon-Manco, L.; Parsons, R.; Tewari, A.K.; Yadav, K.K. Induction of Neuroendocrine Differentiation in Prostate Cancer Cells by Dovitinib (TKI-258) and its Therapeutic Implications. Transl. Oncol. 2017, 10, 357–366. [Google Scholar] [CrossRef]
- Liongue, C.; Ratnayake, T.; Basheer, F.; Ward, A.C. Janus Kinase 3 (JAK3): A Critical Conserved Node in Immunity Disrupted in Immune Cell Cancer and Immunodeficiency. Int. J. Mol. Sci. 2024, 25, 2977. [Google Scholar] [CrossRef]
- Chen, J.J.; Thakur, K.D.; Clark, M.P.; Laughlin, S.K.; George, K.M.; Bookland, R.G.; Davis, J.R.; Cabrera, E.J.; Easwaran, V.; De, B.; et al. Development of pyrimidine-based inhibitors of Janus tyrosine kinase 3. Bioorg. Med. Chem. Lett. 2006, 16, 5633–5638. [Google Scholar] [CrossRef]
- Sabat, M.; VanRens, J.C.; Laufersweiler, M.J.; Brugel, T.A.; Maier, J.; Golebiowski, A.; De, B.; Easwaran, V.; Hsieh, L.C.; Walter, R.L.; et al. The development of 2-benzimidazole substituted pyrimidine based inhibitors of lymphocyte specific kinase (Lck). Biorg. Med. Chem. Lett. 2006, 16, 5973–5977. [Google Scholar] [CrossRef]
- Hunt, J.A.; Beresis, R.T.; Goulet, J.L.; Holmes, M.A.; Hong, X.J.; Kovacs, E.; Mills, S.G.; Ruzek, R.D.; Wong, F.; Hermes, J.D.; et al. Disubstituted pyrimidines as Lck inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5440–5443. [Google Scholar] [CrossRef] [PubMed]
- Padhy, G.K.; Panda, J.; Behera, A.K. Synthesis and Characterization of Novel N-Benzylbenzimidazole Linked Pyrimidine Derivatives as Anticancer Agents. Ind. J. Pharm. Ed. Res. 2019, 53, 129–134. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Rohani, M. Antibiotic resistance and the alternatives to conventional antibiotics: The role of probiotics and microbiota in combating antimicrobial resistance. Microbiol. Res. 2023, 267, 127275. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I. Recent Advances: Heterocycles in Drugs and Drug Discovery. Int. J. Mol. Sci. 2024, 25, 9503. [Google Scholar] [CrossRef]
- Chen, P.-J.; Yang, A.; Gu, Y.-F.; Zhang, X.-S.; Shao, K.-P.; Xue, D.-Q.; He, P.; Jiang, T.-F.; Zhang, Q.-R.; Liu, H.M. Synthesis, in vitro antimicrobial and cytotoxic activities of novel pyrimidine–benzimidazol combinations. Bioorg. Med. Chem. Lett. 2014, 24, 2741–2743. [Google Scholar] [CrossRef]
- Kunduru, R.R.; Vanga, M.R.; Boche, S. Synthesis and Characterization of Some Novel 2-[(5-aryl)-4, 5-dihydro-1H-pyrazole-3-yl]-1H-benzimidazoles with Antimicrobial Activity. Int. J. Pharm. Sci. Drug Res. 2014, 6, 278–282. [Google Scholar] [CrossRef]
- Fang, X.-J.; Jeyakkumar, P.; Rao Avula, S.R.; Zhou, Q.; Zhou, C.-H. Design, synthesis and biological evaluation of 5-fluorouracil-derived benzimidazoles as novel type of potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2016, 26, 2584–2588. [Google Scholar] [CrossRef]
- AlNeyadi, S.S.; Salem, A.A.; Ghattas, M.A.; Atatreh, N.; Abdou, I.M. Antibacterial activity and mechanism of action of the benzazole acrylonitrile-based compounds: In vitro, spectroscopic, and docking studies. Eur. J. Med. Chem. 2017, 136, 280–282. [Google Scholar] [CrossRef]
- Hessein, S.A.; Fouad, S.A.F.; Raslan, R.R.; A Shemiss, N.A. Synthesis of some novel pyrrolidine, thiomorpholine, pyrimidine and pyridine derivatives containing benzimidazole moiety of expected antiviral and antimicrobial activity. Der Pharm. Chem. 2016, 8, 170–181. [Google Scholar]
- Chikkula, K.V.; Sundararajan, R. Analgesic, anti-inflammatory, and antimicrobial activities of novel isoxazole/pyrimidine/pyrazole substituted benzimidazole analogs. Med. Chem. Res. 2017, 26, 3026–3037. [Google Scholar] [CrossRef]
- Saundane, A.R.; Mathada, K.N. Synthesis, characterization, and biological evaluation of some new chalcones containing indole moiety and their derivatives. Monatsh. Chem. 2016, 147, 1291–1301. [Google Scholar] [CrossRef]
- Liu, H.-B.; Gao, W.-W.; Tangadanchu, V.K.R.; Zhou, C.-H.; Geng, R.-X. Novel aminopyrimidinyl benzimidazoles as potentially antimicrobial agents: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2018, 143, 66–84. [Google Scholar] [CrossRef]
- Khan, S.; Kale, M.; Nema, N. Novel pyrimidine-benzimidazole hybrids with antibacterial and antifungal properties and potential inhibition of SARS-CoV-2 main protease and spike glycoprotein. Dig. Chin. Med. 2021, 4, 102–119. [Google Scholar] [CrossRef]
- Zaghary, W.A.; Anwar, M.M.; Abd El-Karim, S.; Ghada, E.A.; Awad, G.E.A.; Hussein, G.K.; Mahfouz, N.M. Design, Synthesis and Molecular Docking of New Benzimidazole Derivatives of Potential Antimicrobial Activity as DNA Gyrase and Topoisomerase IV Inhibitors. Egypt. J. Chem. 2021, 64, 3817–3839. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Qian, P.; Li, Y.; Deng, H.; Ren, W.; Jiang, L. Synthesis and fungicidal activity of novel 2-(2-alkylthio-6-phenylpyrimidin-4-yl)-1H-benzimidazoles. Bioorg. Med. Chem. Lett. 2021, 47, 128210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, T.; Liang, S.; Liu, K.; Zhou, M.; Chen, C. Molecular mechanism of resistance of Fusarium fujikuroi to benzimidazole fungicides. FEMS Microbiol. Lett. 2014, 357, 77–84. [Google Scholar] [CrossRef]
- Duan, Y.B.; Yang, Y.; Li, M.X.; Li, T.; Fraaije, B.A.; Zhou, M.G. Development and application of a simple, rapid and sensitive method for detecting moderately carbendazim-resistant isolates in Botrytis cinerea. Ann. Appl. Biol. 2018, 172, 355–365. [Google Scholar] [CrossRef]
- Chaaban, S.; Jariwala, S.; Hsu, C.T.; Redemann, S.; Kollman, J.M.; Muller-Reichert, T.; Sept, D.; Bui, K.H.; Brouhard, G.J. The structure and dynamics of C. elegans tubulin reveals the mechanistic basis of microtubule growth. Dev. Cell 2018, 47, 191–204. [Google Scholar] [CrossRef]
- Rathi, P.C.; Ludlow, R.F.; Verdonk, M.L. Practical high-quality electrostatic potential surfaces for drug discovery using a graph-convolutional deep neural network. J. Med. Chem. 2020, 63, 8778–8790. [Google Scholar] [CrossRef]
- Vlasov, S.V.; Vlasova, O.D.; Severina, H.I.; Krolenko, K.Y.; Borysov, O.V.; Abu Sharkh, A.I.M.; Vlasov, V.S.; Georgiyants, V.A. Design, Synthesis and In Vitro Antimicrobial Activity of 6-(1H-Benzimidazol-2-yl)-3,5-dimethyl-4-oxo-2-thio-3,4-dihydrothieno [2,3-d]pyrimidines. Sci. Pharm. 2021, 89, 49. [Google Scholar] [CrossRef]
- De, A.; Sarkar, S.; Majee, A. Recent advances on heterocyclic compounds with antiviral properties. Chem. Heterocycl. Compd. 2021, 57, 410–416. [Google Scholar] [CrossRef]
- El Diwani, H.I.; Abdel-Mohsen, H.T.; Salama, I.; Ragab, F.A.-F.; Ramla, M.M.; Galal, S.A.; Abdalla, M.M.; Abdel-Wahab, A.; El Demellawy, M.A. Synthesis, Molecular Modeling, and Biological Evaluation of Novel Benzimidazole Derivatives as Inhibitors of Hepatitis C Virus RNA Replication. Chem. Pharm. Bull. 2014, 62, 856–866. [Google Scholar] [CrossRef]
- Selvam, P.; Lakr, D.R.; Pannecouque, C. Synthesis, Anti-viral and cytotoxicity studies of some novel N-substituted benzimidzole derivatives. Int. J. Pharm. Sci. Res. 2010, 1, 105–109. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef]
- Prajapat, P.; Talesara, G.L. Synthesis and Anti-inflammatory Screening of Some Mono and Bis-Alkoxyphthalimide Linked Benzimidazole and their Quinazoline and Pyrimidine Derivatives. J. Heterocycl. Chem. 2016, 53, 1603–1610. [Google Scholar] [CrossRef]
- Veerasamy, R.; Roy, A.; Karunakaran, R.; Rajak, H. Structure–Activity Relationship Analysis of Benzimidazoles as Emerging Anti-Inflammatory Agents: An Overview. Pharmaceuticals 2021, 14, 663. [Google Scholar] [CrossRef]
- Bułdyś, K.; Górnicki, T.; Kałka, D.; Szuster, E.; Biernikiewicz, M.; Markuszewski, L.; Sobieszczańska, M. What Do We Know about Nociplastic Pain? Healthcare 2023, 11, 1794. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Iovu, M.; Marinescu, M.; Tarcomnicu, I.; Nitulescu, G.M. Synthesis and biological screening of some novel 2-(1H-pyrazol-1-yl)-acetamides as lidocaine analogue. Ind. J. Chem. B 2014, 53B, 733–739. [Google Scholar]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Galil, E.; Amr, A.E.-G.E.; Al-Omar, M.A.; Abdalla, M.M. Biological Evaluations of some Synthesized Pyrimidothieno [2,3-b] Pyrimidine Candidates as Antiulcer Agents. Int. J. Pharm. 2015, 11, 840–845. [Google Scholar]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Synthesis and PASS-assisted in silico approach of some novel 2-substituted benzimidazole bearing a pyrimidine-2, 4,6(trione) system as mucomembranous protector. J. Pharm. BioAllied Sci. 2013, 5, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Farhan, K.R.; Farooqui, M.S. Synthesis and Pharmacological Evaluation of Novel Benzimidazole Derivatives as Antiulcer and H+ K+ ATPase Inhibitor. J. Adv. Med. Pharm. Sci. 2021, 23, 65173. [Google Scholar]
- Patil, A.; Ganguly, S.; Surana, S. Synthesis and antiulcer activity of 2-[5-substituted-1-H-benzo(d)imidazol-2-yl sulfinyl]methyl-3-substituted quinazoline-4-(3H)ones. J. Chem. Sci. 2010, 122, 443–450. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Chung, S.K.; Xu, B. New insights into anti-depression effects of bioactive phytochemicals. Pharm. Res. 2025, 212, 107566. [Google Scholar] [CrossRef]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Development of novel (1-H) benzimidazole bearing pyrimidine-trione based MAO-A inhibitors: Synthesis, docking studies and antidepressant activity. J. Saudi Chem. Soc. 2016, 20, S132–S139. [Google Scholar] [CrossRef]
- Kojima, T.; Mochizuki, M.; Takai, T.; Hoashi, Y.; Morimoto, S.; Seto, M.; Nakamura, M.; Kobayashi, K.; Sako, Y.; Tanaka, M.; et al. Discovery of 1,2,3,4-tetrahydropyrimido [1,2-a]benzimidazoles as novel class of corticotropin releasing factor 1 receptor antagonists. Bioorg. Med. Chem. 2018, 26, 2229–2250. [Google Scholar] [CrossRef]
- Kim, M.-h.; Lee, J.; Jung, K.; Kim, M.; Park, Y.-J.; Ahn, H.; Kwon, Y.H.; Hah, J.-M. Syntheses and biological evaluation of 1-heteroaryl-2-aryl-1H-benzimidazole derivatives as c-Jun N- terminal kinase inhibitors with neuroprotective effects. Bioorg. Med. Chem. 2013, 21, 2271–2285. [Google Scholar] [CrossRef]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Res. Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Marinescu, M.; Cinteza, L.O.; Marton, G.; Măruţescu, L.; Chifiriuc, M.C.; Constantinescu, C. Density functional theory molecular modeling and antimicrobial behaviour of selected 1,2,3,4,5,6,7,8-octahydroacridine-(10)-oxides. J. Mol. Struct. 2017, 1144, 14–23. [Google Scholar] [CrossRef]
- Potmischil, F.; Marinescu, M.; Nicolescu, A.; Deleanu, C.; Hillebrand, M. Hydroacridines: Part 29.15N NMR chemical shifts of 9-substituted 1,2,3,4,5,6,7,8-octahydroacridines and their N-oxides. Magn. Res. Chem. 2008, 46, 1141–1147. [Google Scholar] [CrossRef]
- Ion, V.; Matei, A.; Constantinescu, C.; Mitu, B.; Ionita, I.; Marinescu, M.; Dinescu, M.; Emandi, A. Octahydroacridine thin films grown by matrix-assisted pulsed laser evaporation for non linear optical applications. Mat. Sci. Semicon. Proc. 2015, 36, 78–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).