Network Pharmacology and Experiment Verification-Based Strategy for Exploring the Mechanisms of Shuqing Granule in the Treatment of COVID-19
Abstract
1. Introduction
2. Results
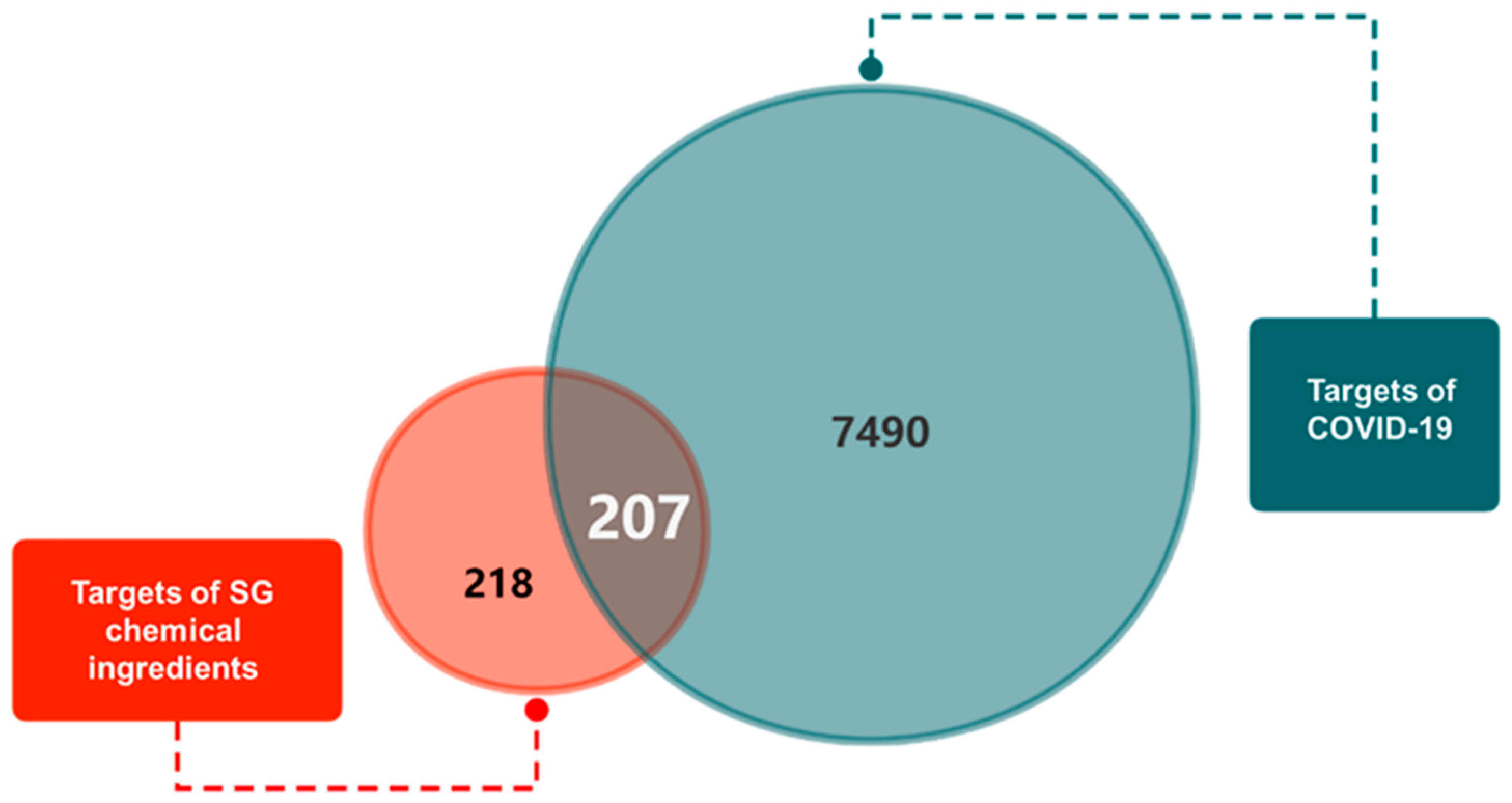
2.1. Obtaining the Chemical Ingredients and Corresponding Targets of SG and COVID-19-Related Genes
2.2. Construction of “SG Chemical Ingredient-Overlapping Target-COVID-19” Interaction Network and Identification of Key Ingredients
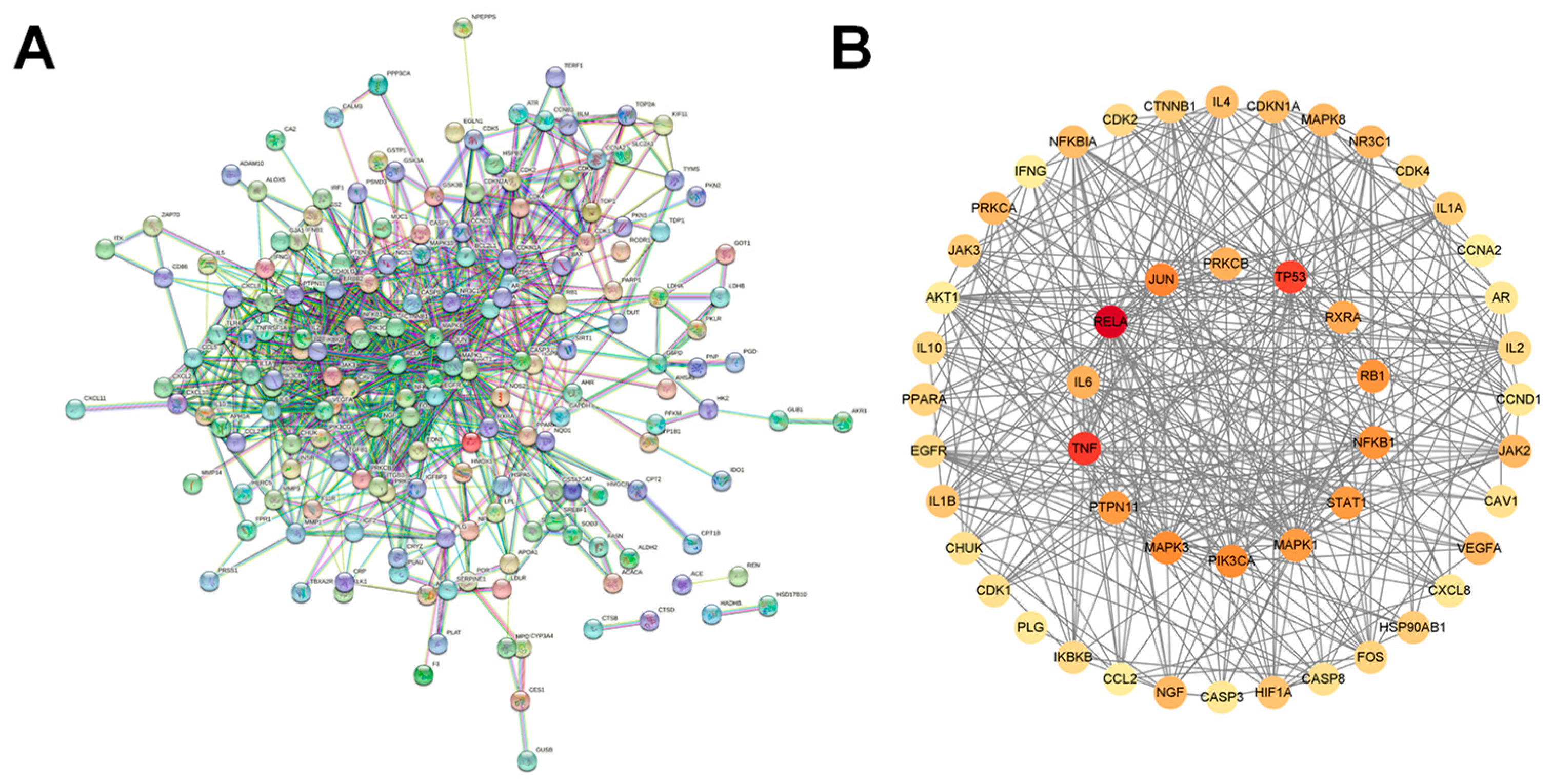
2.3. Protein–Protein Interaction Network (PPI) Construction and Identification of Core Targets
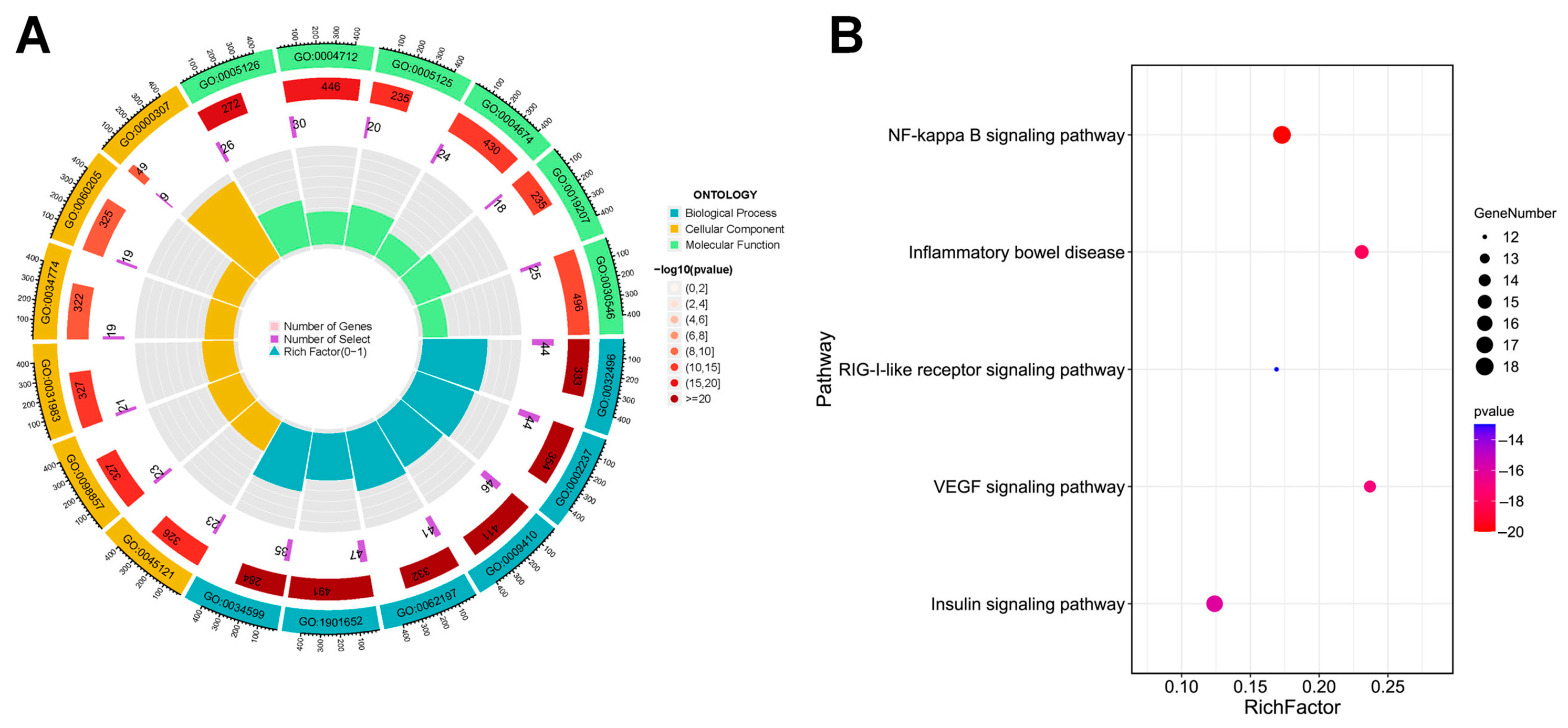
2.4. GO and KEGG Pathway Enrichment Analysis
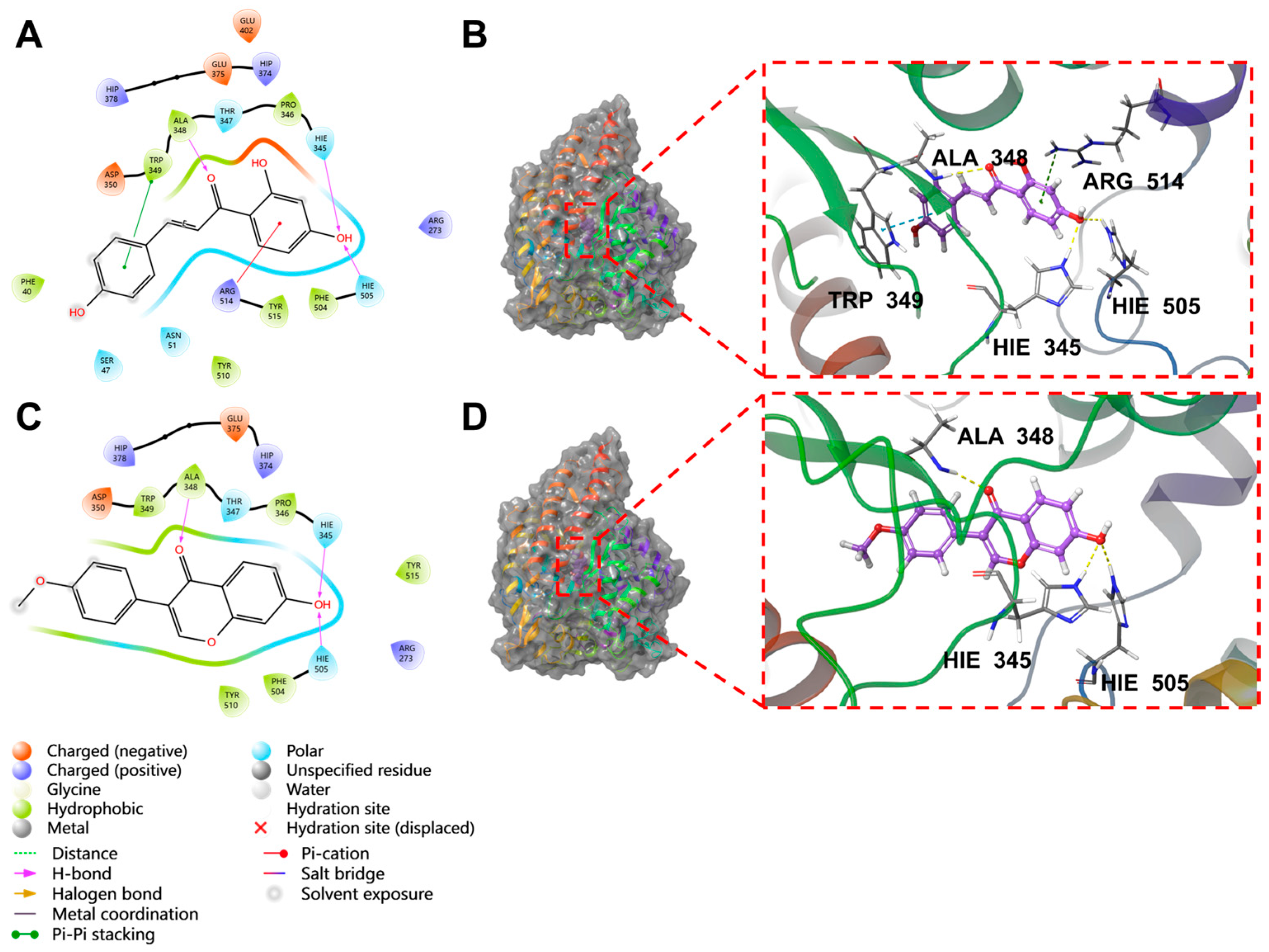
2.5. Molecular Docking Results
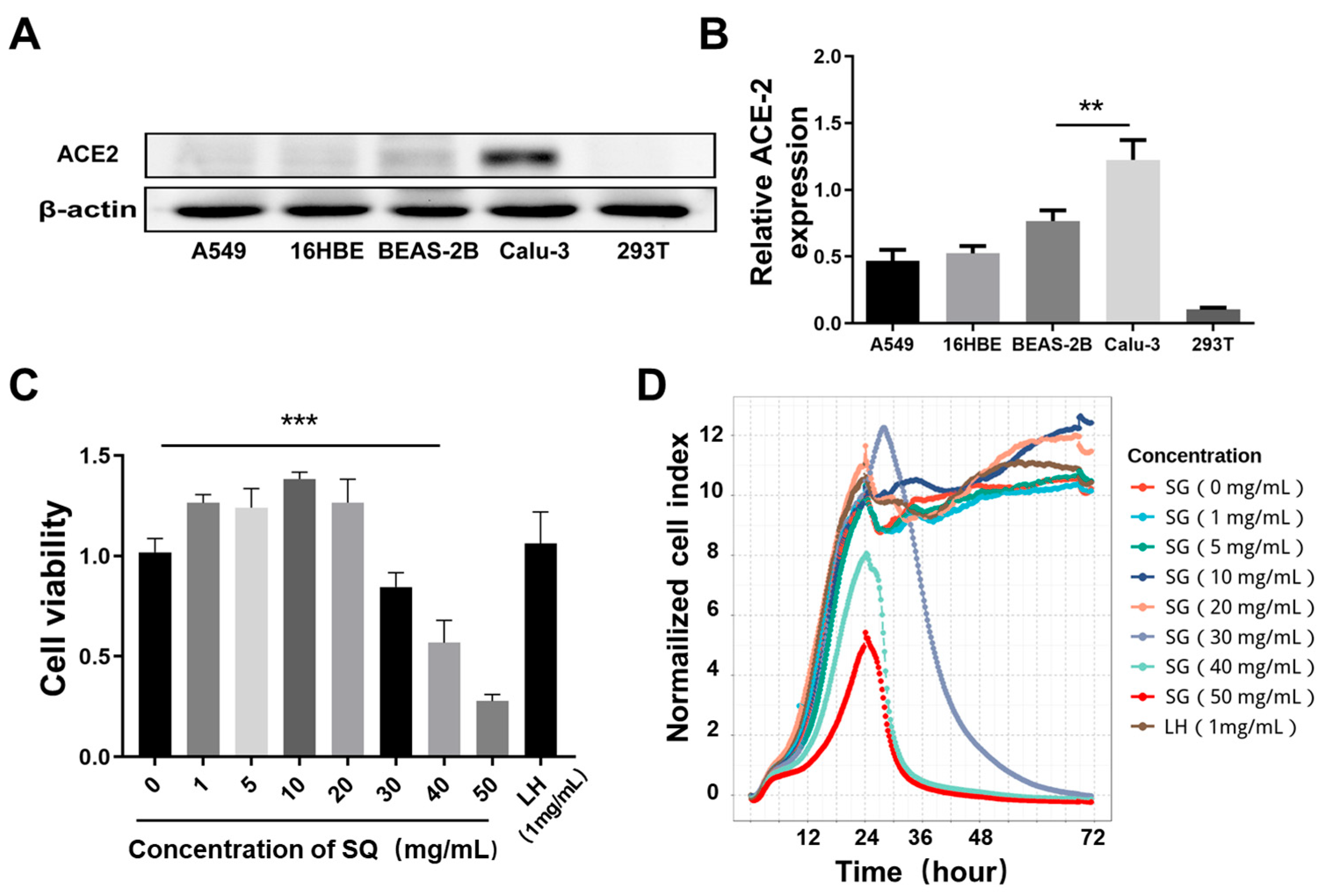
2.6. Screening of Cells with High Expression of ACE2
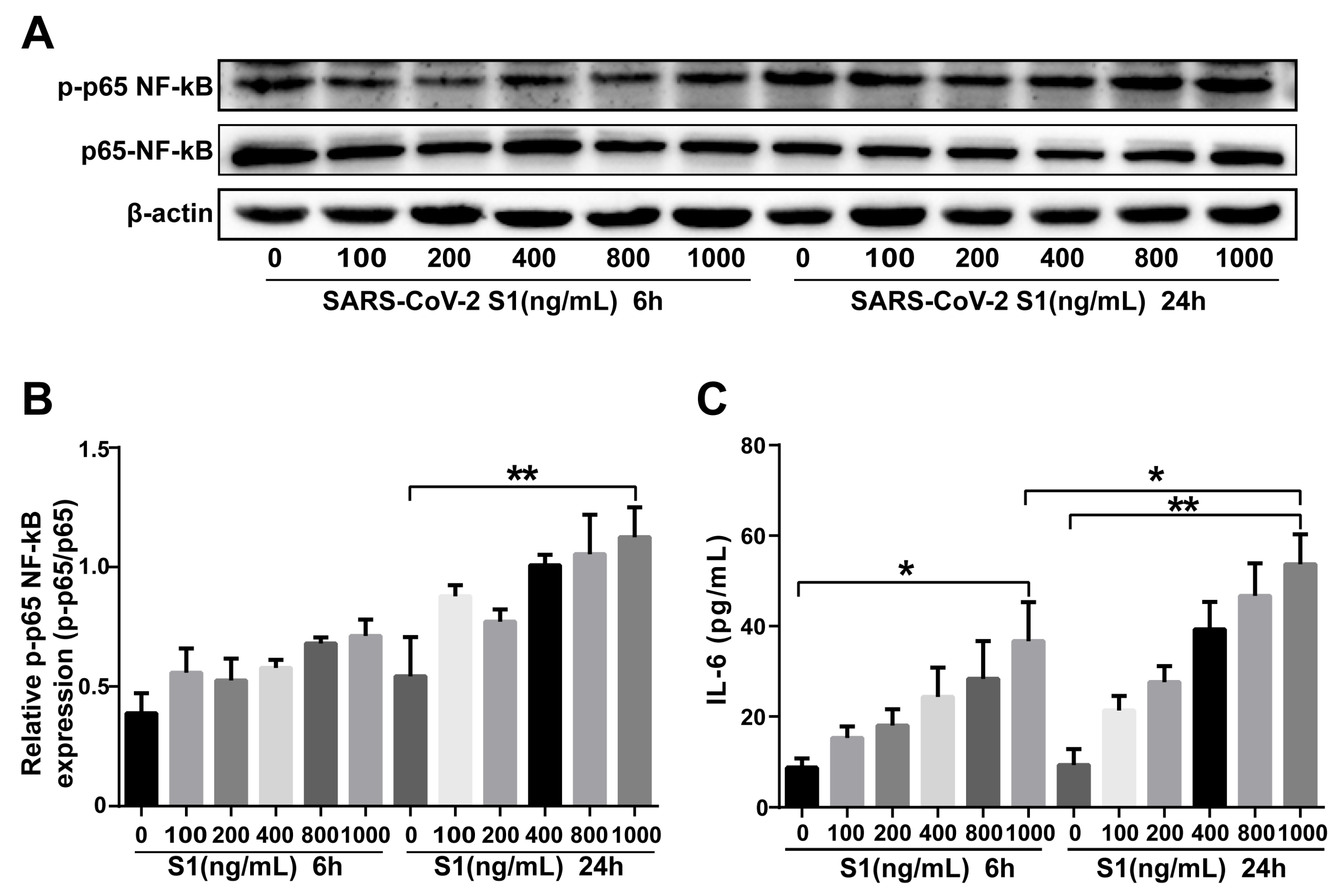
2.7. SARS-CoV-2 S1 Protein Stimulation of Calu-3 Cells Induces Inflammation
2.8. SG Inhibited Expression of ACE2 in Calu-3 Cells
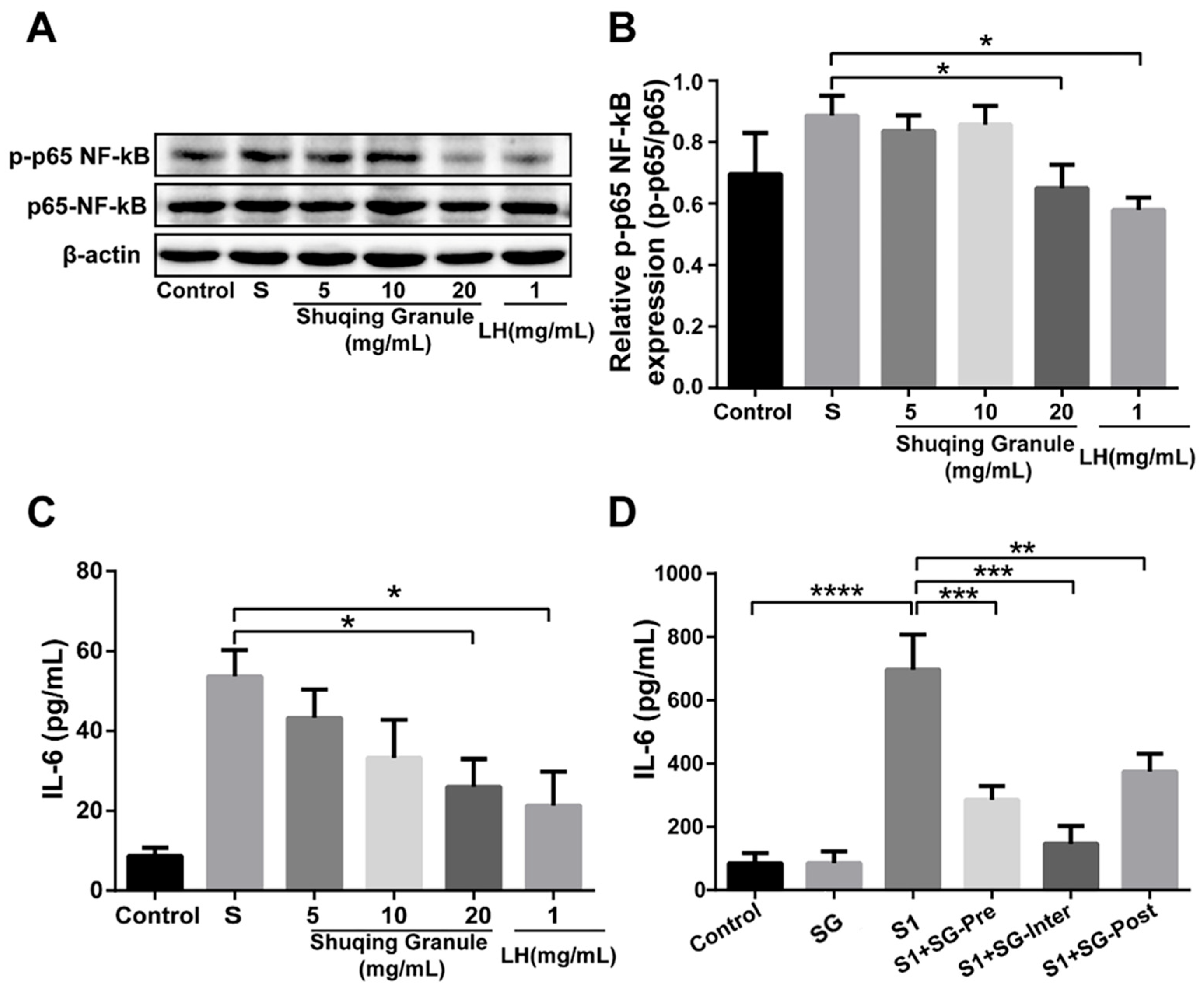
2.9. SG Inhibited the Phosphorylation Level of NF-κB p65 and the Secretion of IL-6
3. Discussion
4. Materials and Methods
4.1. Obtaining the Chemical Ingredients and Corresponding Targets of SG and COVID-19-Related Genes
4.2. Construction of “Chemical Ingredient-Overlapping Target-COVID-19” Interaction Network and Identification of Key Ingredients
4.3. Construction of Protein–Protein Interaction Network (PPI) and Identification of Core Targets
4.4. Enrichment Analysis
4.5. Molecular Docking
4.6. Drugs, Cells, and Animals
4.7. Cytotoxicity Assay
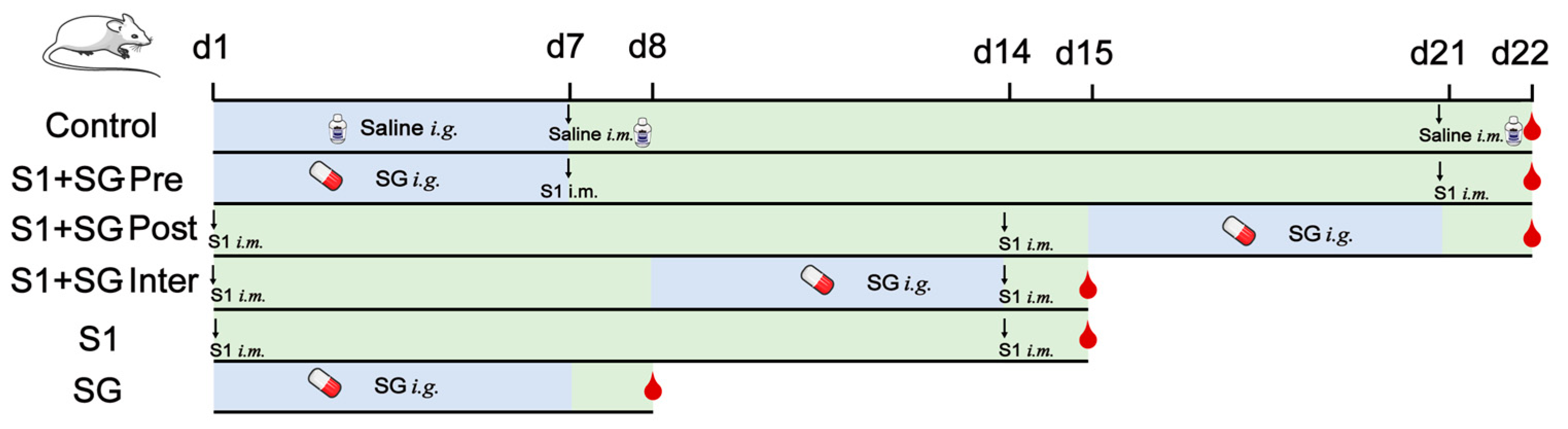
4.8. Murine Model Establishment
4.9. Enzyme-Linked Immunosorbent Assays (ELISAs)
4.10. RT-PCR Analysis
4.11. Western Blot
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 Immunity from Other Coronavirus Infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 2023, 257, 115503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection. Nutrients 2023, 15, 3443. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Ma, Y.; Luo, C.; Huang, J.; Zhang, T.; Yin, F. Summary of the COVID-19 epidemic and estimating the effects of emergency responses in China. Sci. Rep. 2021, 11, 717. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Z.; Wu, H.; Wang, S.; Wang, Y.; Wang, Y.; Xu, J.; Wang, L.; Qi, F.; Fang, M.; et al. Evaluation of the anti-neuraminidase activity of the traditional Chinese medicines and determination of the anti-influenza A virus effects of the neuraminidase inhibitory TCMs in vitro and in vivo. J. Ethnopharmacol. 2011, 137, 534–542. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, D.; Song, S.; Yu, Q.; Shi, C.; Xing, X.; Luo, J.B. Comparative Study on the Antivirus Activity of Shuang-Huang-Lian Injectable Powder and Its Bioactive Compound Mixture against Human Adenovirus III In Vitro. Viruses 2017, 9, 79. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shi, S.; Zhang, J.; Tian, J. Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): A systematic review and meta-analysis. Pharmacol. Res. 2020, 158, 104896. [Google Scholar] [CrossRef]
- Xia, Q.D.; Xun, Y.; Lu, J.L.; Lu, Y.C.; Yang, Y.Y.; Zhou, P.; Hu, J.; Li, C.; Wang, S.G. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Prolif. 2020, 53, e12949. [Google Scholar] [CrossRef]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; van de Merwe, R.C.; Louie, R.; Bracken, O.V.; Devine, O.P.; Goldstein, D.R.; Uddin, M.; Akbar, A.N.; Gilroy, D.W. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 2020, 21, 615–625. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Zhang, Y.; Hu, J.; Shi, Y.; Wang, F. The antiviral activity of Shu Qing granules against enterovirus 71 in vitro. J. Hum. Univ. CM 2013, 33, 3+7. (In Chinese) [Google Scholar]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.W.; Cheng, Y.; Zhang, J.; Jiang, X.M.; Wang, L.; Deng, J.; Wang, P.H. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J. Med. Virol. 2020, 92, 2693–2701. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P.; et al. Respiratory Impairment Predicts Response to IL-1 and IL-6 Blockade in COVID-19 Patients with Severe Pneumonia and Hyper-Inflammation. Front. Immunol. 2021, 12, 675678. [Google Scholar] [CrossRef] [PubMed]
- Investigators, R.-C.; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Liu, W.; Yang, Y.; Zhang, M.; Ye, Y.; Zhu, W.; Zhou, C.; Zhai, H.; Xu, Z.; et al. The S1′-S3′ Pocket of the SARS-CoV-2 Main Protease Is Critical for Substrate Selectivity and Can Be Targeted with Covalent Inhibitors. Angew. Chem. Int. Ed. Engl. 2023, 62, e202309657. [Google Scholar] [CrossRef]
- Syed, U.; Subramanian, A.; Wraith, D.C.; Lord, J.M.; McGee, K.; Ghokale, K.; Nirantharakumar, K.; Haroon, S. Incidence of immune-mediated inflammatory diseases following COVID-19: A matched cohort study in UK primary care. BMC Med. 2023, 21, 363. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Li, J.; Zheng, H.; Li, H.; Lai, Q.; Chen, Y.; Qin, L.; Zuo, Y.; Guo, L.; et al. Engagement of the G3BP2-TRIM25 Interaction by Nucleocapsid Protein Suppresses the Type I Interferon Response in SARS-CoV-2-Infected Cells. Vaccines 2022, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Gunther, S.; Rancic, J.; Cavagna, P.; Renaud, B.; Gendron, N.; Mousseaux, E.; Hua-Huy, T.; Reverdito, G.; Planquette, B.; et al. VEGF-A plasma levels are associated with impaired DLCO and radiological sequelae in long COVID patients. Angiogenesis 2023, 27, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Mantzoros, C.S. Cross-talk between SARS-CoV-2 infection and the insulin/IGF signaling pathway: Implications for metabolic diseases in COVID-19 and for post-acute sequelae of SARS-CoV-2 infection. Metabolism 2022, 134, 155267. [Google Scholar] [CrossRef] [PubMed]
- Breznik, J.A.; Rahim, A.; Zhang, A.; Ang, J.; Stacey, H.D.; Bhakta, H.; Clare, R.; Liu, L.M.; Kennedy, A.; Hagerman, M.; et al. Early Omicron infection is associated with increased reinfection risk in older adults in long-term care and retirement facilities. EClinicalMedicine 2023, 63, 102148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Song, X.Q. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wan, S.Y.; Gong, S.S.; Liu, J.C.; Li, F.; Kou, J.P. Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin. J. Nat. Med. 2020, 18, 881–889. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin. Med. J. 2014, 127, 1344–1350. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, M.; Yan, Y.; Wang, S.; Peng, T.; Li, Y.; Chai, Z. Molecular Mechanism of Huoxiang(Pogostemonis Herba) in Treatment of Corona Virus Disease 2019(COVID-19) Based on Network Pharmacology and Molecular Docking. J. Pract. Tradit. Chin. Intern. Med. 2020, 34, 120–122. [Google Scholar]
- Xu, L.; Huang, X.; Li, Y.; Song, Y.; Zeng, Z.; Liu, Z.; Zhou, X.; Lin, F. Study on the Potential Mechanism of Huopuxialing Decoction and its “Peitushengjin” Theory in the Treatment of COVID-19 Based on Network Pharmacology. Pharmacol. Clin. Chin. Mater. Med. 2020, 36, 85–90. [Google Scholar]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther. 2020, 214, 107618. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Yao, W.; Ju, W. The intervention effect investigation of Chinese medicine monomer on cytokine storm induced by COVID-19 based on interleukin-6 receptor. Chin. J. Hosp. Pharm. 2020, 40, 1182–1188. [Google Scholar]
- Ren, J.L.; Zhang, A.H.; Wang, X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef]
- Yang, J.; Tian, S.; Zhao, J.; Zhang, W. Exploring the mechanism of TCM formulae in the treatment of different types of coronary heart disease by network pharmacology and machining learning. Pharmacol. Res. 2020, 159, 105034. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Huang, J.; Huang, S.; He, D.; Peng, W.; Zhang, S. A Network Pharmacology Analysis of the Active Components of the Traditional Chinese Medicine Zuojinwan in Patients with Gastric Cancer. Med. Sci. Monit. 2020, 26, e923327. [Google Scholar] [CrossRef]
- Li, J.; Ye, M.; Gao, J.; Zhang, Y.; Zhu, Q.; Liang, W. Systematic Understanding of Mechanism of Yi-Qi-Huo-Xue Decoction Against Intracerebral Hemorrhagic Stroke Using a Network Pharmacology Approach. Med. Sci. Monit. 2020, 26, e921849. [Google Scholar] [CrossRef]
- Saikia, S.; Bordoloi, M. Molecular Docking: Challenges, Advances and its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Luo, G.; Ye, D.; She, M.; Sun, N.; Lu, Y.J.; Zheng, J. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of Toujie Quwen Granules against coronavirus disease 2019 pneumonia. Phytomedicine 2021, 85, 153401. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, R.; Zhou, Z.; Tang, X.; Lin, J.; Wang, L.; Zhou, X.; Shen, T. A network pharmacology based approach for predicting active ingredients and potential mechanism of Lianhuaqingwen capsule in treating COVID-19. Int. J. Med. Sci. 2021, 18, 1866–1876. [Google Scholar] [CrossRef]
- Rutwick Surya, U.; Praveen, N. A molecular docking study of SARS-CoV-2 main protease against phytochemicals of Boerhavia diffusa Linn. for novel COVID-19 drug discovery. Virusdisease 2021, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef]
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.; Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020, 16, e1009128. [Google Scholar] [CrossRef] [PubMed]
- Corpetti, C.; Del Re, A.; Seguella, L.; Palenca, I.; Rurgo, S.; De Conno, B.; Pesce, M.; Sarnelli, G.; Esposito, G. Cannabidiol inhibits SARS-Cov-2 spike (S) protein-induced cytotoxicity and inflammation through a PPARgamma-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother. Res. 2021, 35, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Pere, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Stukas, S.; Cooper, J.; Thiara, S.; Chen, L.Y.C.; Biggs, C.M.; Hay, K.; Lee, A.Y.Y.; Shojania, K.; Abdulla, A.; et al. Amelioration of COVID-19-related cytokine storm syndrome: Parallels to chimeric antigen receptor-T cell cytokine release syndrome. Br. J. Haematol. 2020, 190, e150–e154. [Google Scholar] [CrossRef]
- England, J.T.; Abdulla, A.; Biggs, C.M.; Lee, A.Y.Y.; Hay, K.A.; Hoiland, R.L.; Wellington, C.L.; Sekhon, M.; Jamal, S.; Shojania, K.; et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2021, 45, 100707. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.S.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Yu, H.; Wu, C.; Yan, Q.; Wang, Y.; Yang, M.; Xie, H.; Wu, T.; Yang, H.; et al. Nimbolide targeting SIRT1 mitigates intervertebral disc degeneration by reprogramming cholesterol metabolism and inhibiting inflammatory signaling. Acta Pharm. Sin. B 2023, 13, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, K.; Wang, G.; Guan, X.; Pang, Z.; Guo, Y.; Yuan, Y.; Ran, N.; Liu, Y.; Wang, F. Protective effect of amygdalin on epithelial-mesenchymal transformation in experimental chronic obstructive pulmonary disease mice. Phytother. Res. 2019, 33, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Zhang, C.; Liu, D.; Zhang, T. Analysis on chemical constituents from Glycyrrhizae Radix et Rhizoma by HPLC-Q-TOF-MS. Chin. Tradit. Herb. Drugs. 2016, 12, 2061–2068. (In Chinese) [Google Scholar]
- He, Y.; Tu, Z.; Zou, A.; Liu, H.; Gao, L.; Li, Y.; Li, C. Identification of chemical constituents in Gancao Xiexin Decoction by HPLC-QTOF/MS. Drugs & Clinic. 2021, 11, 2246–2254. (In Chinese) [Google Scholar]
- Jiang, C.; Xiu, J.; Cui, S.; Xiu, H.; Xia, Z.; Yang, H. Mechanism of Yishen Huashi Granules in treatment of IgA nephropathy based on HPLC-Q-TOF-MS/MS and network pharmacology. Chin. Tradit. Herb. Drugs 2021, 21, 6576–6585. (In Chinese) [Google Scholar]
- Li, N.; Ren, Y.; Zhang, C.; Zhong, G.; Xiu, L.; Liu, H.; Chen, S.; Chen, F.; Li, M.; Liao, W. Research progress on chemical constituents and pharmacological effects of different varieties of Glycyrrhizae Radix et Rhizoma and predictive analysis of quality markers. Chin. Tradit. Herb. Drugs. 2021, 24, 7680–7692. (In Chinese) [Google Scholar]
- Zhao, P.; Jiao, L.; Zhang, Z.; Yang, X.; Xu, J.; Lian, Y.; Eerdun, B. Study of the differences of flavonoids compounds in Glycyrrhiza residues from different countries by UPLC-QTOF-MS. China Food Addit. 2017, 3, 111–116. (In Chinese) [Google Scholar]
- Zhou, Y.; Wang, M.; Liao, X.; Zhu, X.; Peng, S.; Ding, L. Rapid Identification of Compounds in Glycyrrhiza Uralensis by Liquid Chromatography/Tandem Mass Spectrometry. Chin. J. Anal. Chem. 2004, 2, 174–178. (In Chinese) [Google Scholar]
- Zhao, X.; Zhang, J.; Ma, X.; Wang, Z.; Liu, A. A review of the chemical composition of different medicinal parts of lotus. Chin. J. Inf. Tradit. Chin. Med. 2012, 1, 106–109. (In Chinese) [Google Scholar]
- Cai, S.; Zhao, H.; Jia, M.; Zhao, X.; Chi, Y.; Zhang, W.; Wang, H.; Di, L. Quality evaluation of fried Glycyrrhizae Radix et Rhizoma pieces by HPLC fingerprint and multicomponent quantitative analysis. Chin. J. Chin. Mater. Med. 2021, 1, 118–124. (In Chinese) [Google Scholar]
- Ji, X. Screening of COX-2 Inhibitory Components of RadixTrachelospermum Jasminoides and Its Effect on InflammatoryPain and Analgesia Study on the Therapeutic Effect of Epilepsy. Master’s Thesis, Shaanxi University of Chinese Medicine, Xianyang, China, 2021. (In Chinese). [Google Scholar]
- Fang, S. Bioactive Constituents of Flavonoids in the Herb Residues of Licorice. Master’s Thesis, Nanjing University of Chinese Medicine, Nanjing, China, 2016. (In Chinese). [Google Scholar]
- Hua, M.; Zhou, Q.; Jiang, H.; Dai, Y.; Shi, D.; Wang, P.; Zhang, L.; Zhou, J. Rapid Screening and Identification of Antioxidant Active Components in Glycyrrhiza uralensis Decoction Pieces. China Pharmacy. 2021, 2, 176–181. (In Chinese) [Google Scholar]
- Deng, Z.; Liu, J.; Xiong, S.; Han, L. Metabolite Identification of Morusin in Rats. Food and Drug. 2021, 4, 296–300. (In Chinese) [Google Scholar]

| No. | Chemical Ingredients | Degree | Source | Chemical Structure |
|---|---|---|---|---|
| 1 | Quercetin | 726 | Mulberry leaf, Lugen | 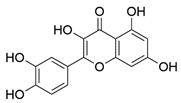 |
| 2 | Kaempferol | 231 | Mulberry leaf, Lugen | 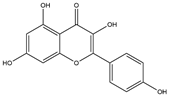 |
| 3 | Acetic Acid | 109 | Mulberry leaf | 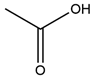 |
| 4 | Aceton | 61 | Mulberry leaf | 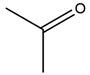 |
| 5 | Eugenol | 57 | Mulberry leaf |  |
| 6 | Naringenin | 55 | Licorice | 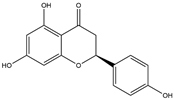 |
| 7 | Calcium sulfate | 52 | Shigao | 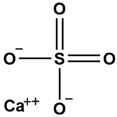 |
| 8 | Salicylic Acid | 47 | Daqingye | 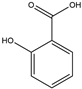 |
| 9 | Formononetin | 40 | Licorice |  |
| 10 | Indirubin | 35 | Daqingye |  |
| 11 | 7-Methoxy-2-methyl isoflavone | 34 | Licorice | 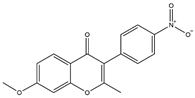 |
| 12 | Isoliquiritigenin | 34 | Licorice | 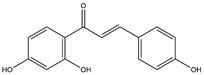 |
| 13 | Anethole | 33 | Mulberry leaf | 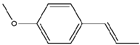 |
| 14 | Dbp | 32 | Mulberry leaf | 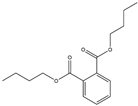 |
| 15 | Shinpterocarpin | 30 | Licorice | 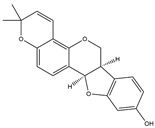 |
| No. | Ingredient | XP GScore | MMGBSA dG Bind |
|---|---|---|---|
| 1 | Isoliquiritigenin | −6.005 | −44.40 |
| 2 | Formononetin | −5.958 | −40.39 |
| 3 | Quercetin | −5.892 | −29.34 |
| 4 | Kaempferol | −5.161 | −30.97 |
| 5 | Indirubin | −4.900 | −32.40 |
| 6 | Naringenin | −4.471 | −31.22 |
| 7 | Shinpterocarpin | −4.337 | −35.88 |
| 8 | 7-Methoxy-2-methylisoflavone | −2.252 | −30.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zheng, H.; An, Y.; Song, Y.; Liu, T.; Zhou, Z.; Liu, C.; Wang, G.; Wang, F. Network Pharmacology and Experiment Verification-Based Strategy for Exploring the Mechanisms of Shuqing Granule in the Treatment of COVID-19. Pharmaceuticals 2025, 18, 1216. https://doi.org/10.3390/ph18081216
Guo X, Zheng H, An Y, Song Y, Liu T, Zhou Z, Liu C, Wang G, Wang F. Network Pharmacology and Experiment Verification-Based Strategy for Exploring the Mechanisms of Shuqing Granule in the Treatment of COVID-19. Pharmaceuticals. 2025; 18(8):1216. https://doi.org/10.3390/ph18081216
Chicago/Turabian StyleGuo, Xiaoping, Haoyu Zheng, Yiming An, Yuemeng Song, Tianqi Liu, Zhengjie Zhou, Chuangui Liu, Guoqiang Wang, and Fang Wang. 2025. "Network Pharmacology and Experiment Verification-Based Strategy for Exploring the Mechanisms of Shuqing Granule in the Treatment of COVID-19" Pharmaceuticals 18, no. 8: 1216. https://doi.org/10.3390/ph18081216
APA StyleGuo, X., Zheng, H., An, Y., Song, Y., Liu, T., Zhou, Z., Liu, C., Wang, G., & Wang, F. (2025). Network Pharmacology and Experiment Verification-Based Strategy for Exploring the Mechanisms of Shuqing Granule in the Treatment of COVID-19. Pharmaceuticals, 18(8), 1216. https://doi.org/10.3390/ph18081216







