Discovery and Evaluation of Novel Sulfonamide Derivatives Targeting Aromatase in ER+ Breast Cancer
Abstract
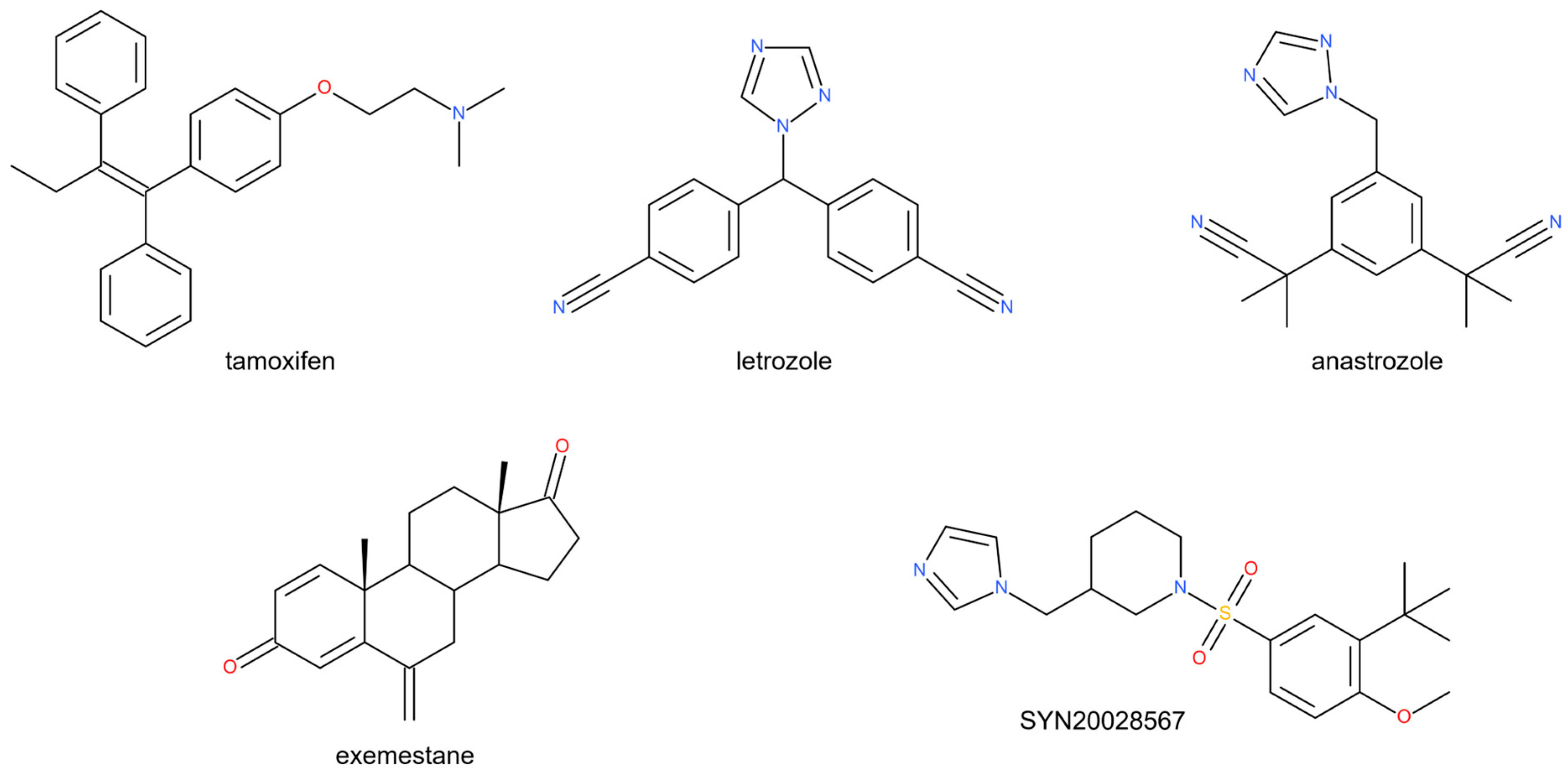
1. Introduction
2. Results and Discussion
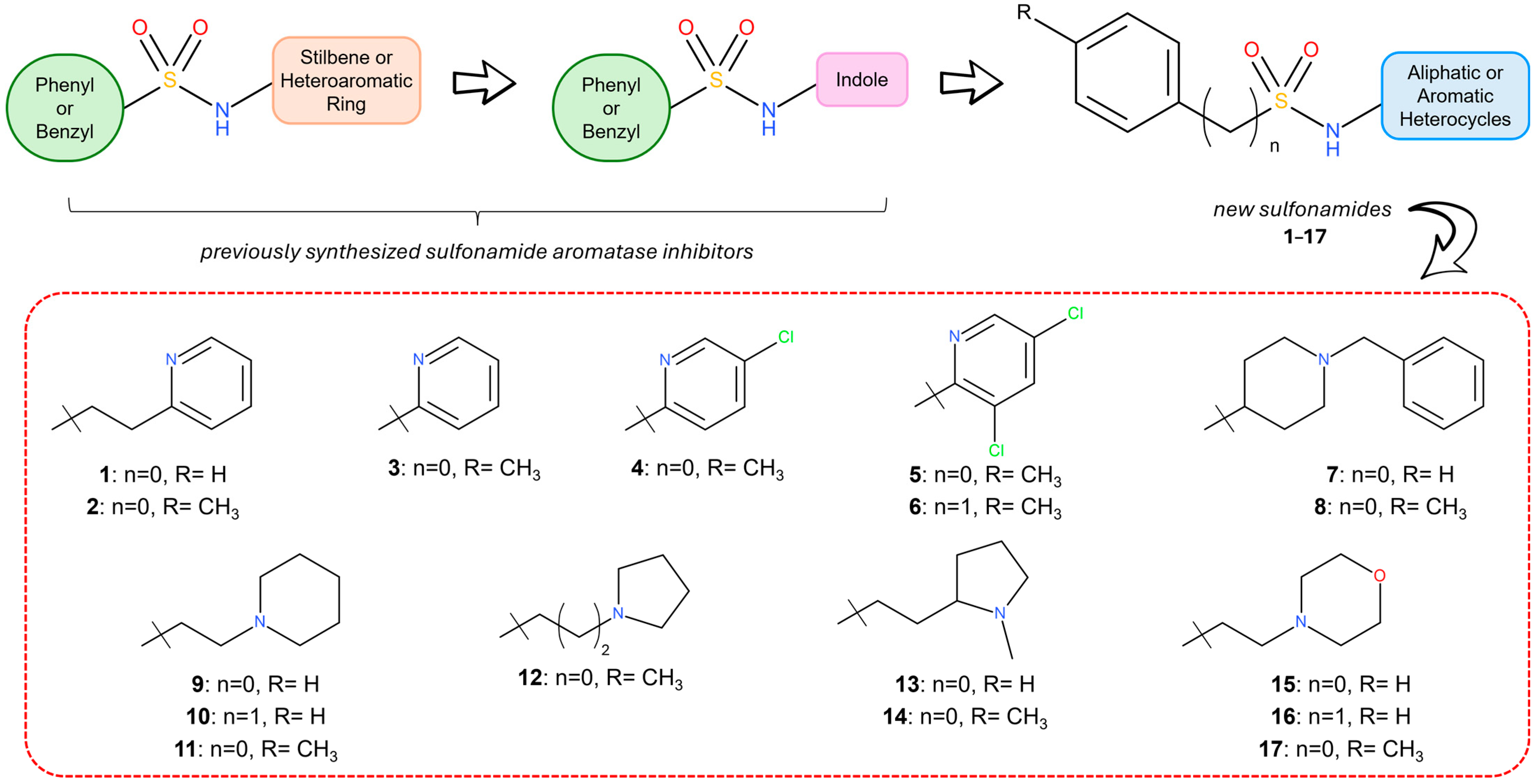
2.1. Structural Design
2.2. Chemistry
2.3. Biological Evaluation
2.3.1. In Vitro Aromatase Inhibition
2.3.2. Cell Viability and Cellular IC50
2.4. The Computational Study
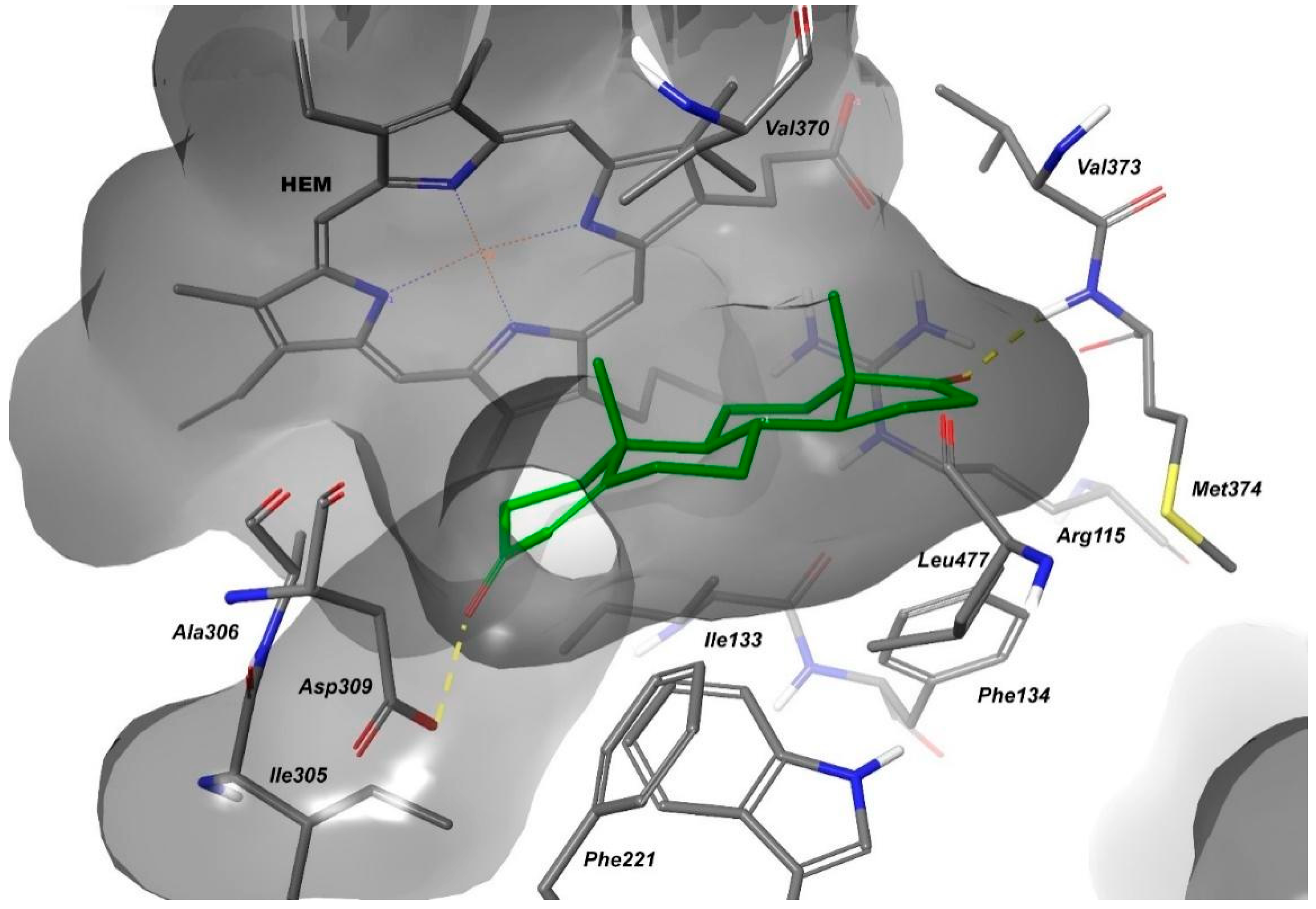
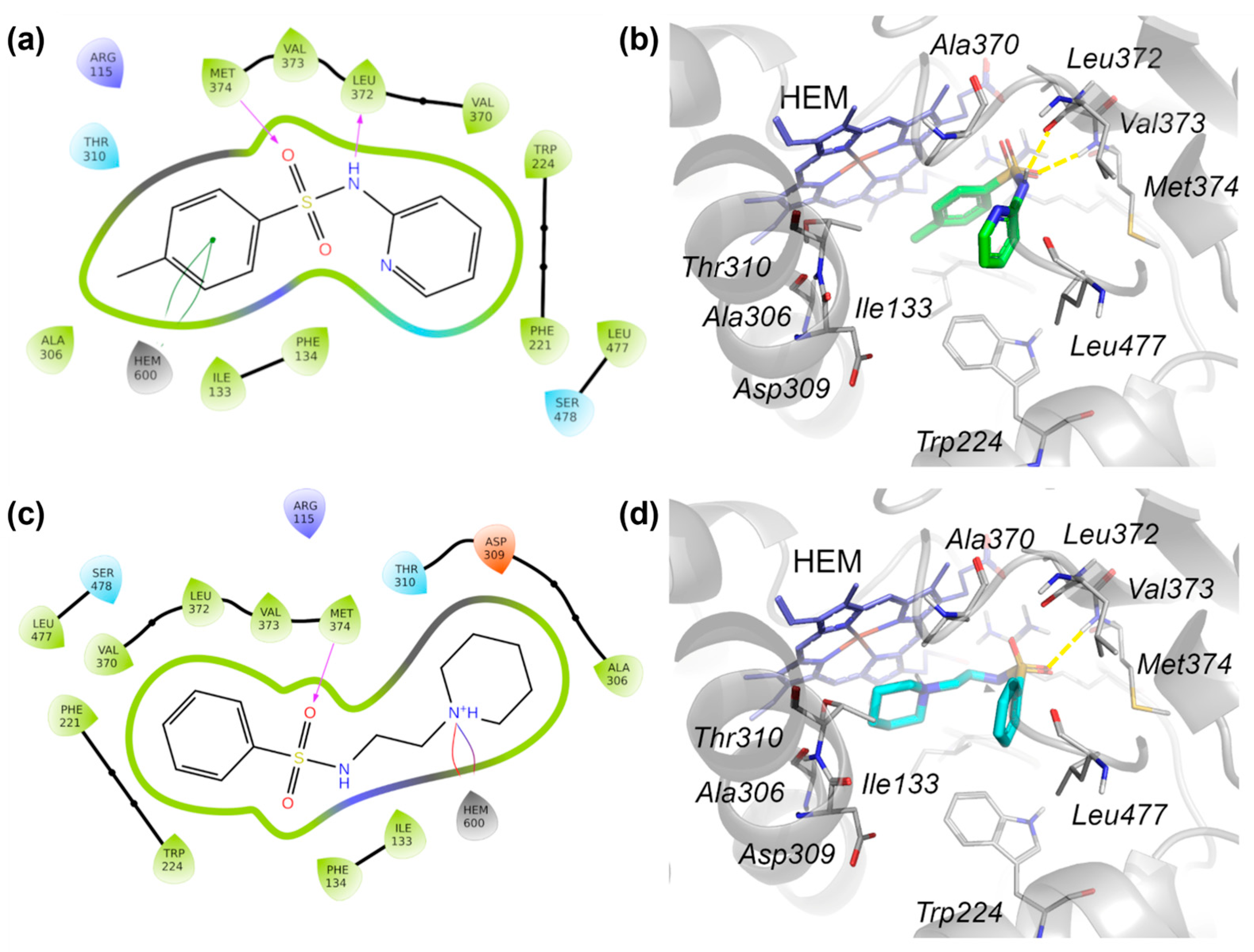
2.4.1. The Molecular Docking Analysis of the Compounds
2.4.2. The Pharmacokinetic Profile
2.5. Searching for a Second Target Related to Breast Cancer
2.5.1. Database Construction
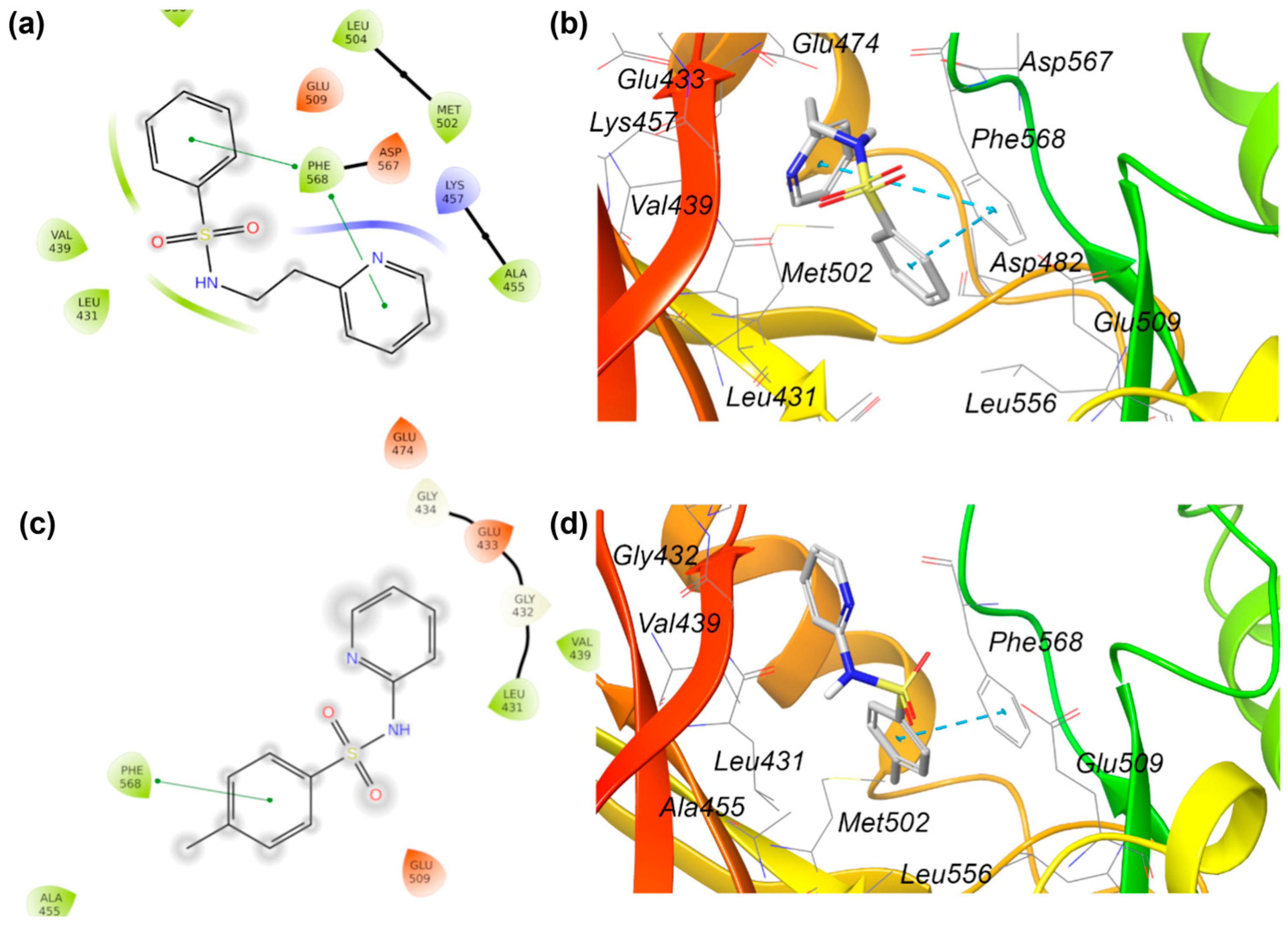
2.5.2. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. The General Procedure for the Synthesis of Compounds 1–2 and 7–17 (Method A)
3.1.2. The General Procedure for the Synthesis of Compounds 3–6 (Method B)
3.2. The Biological Assay
3.2.1. The Cell Culture
3.2.2. The Aromatase Inhibition Assay
3.2.3. The Cytotoxicity Assay
3.3. The Computational Study
3.3.1. Molecular Docking
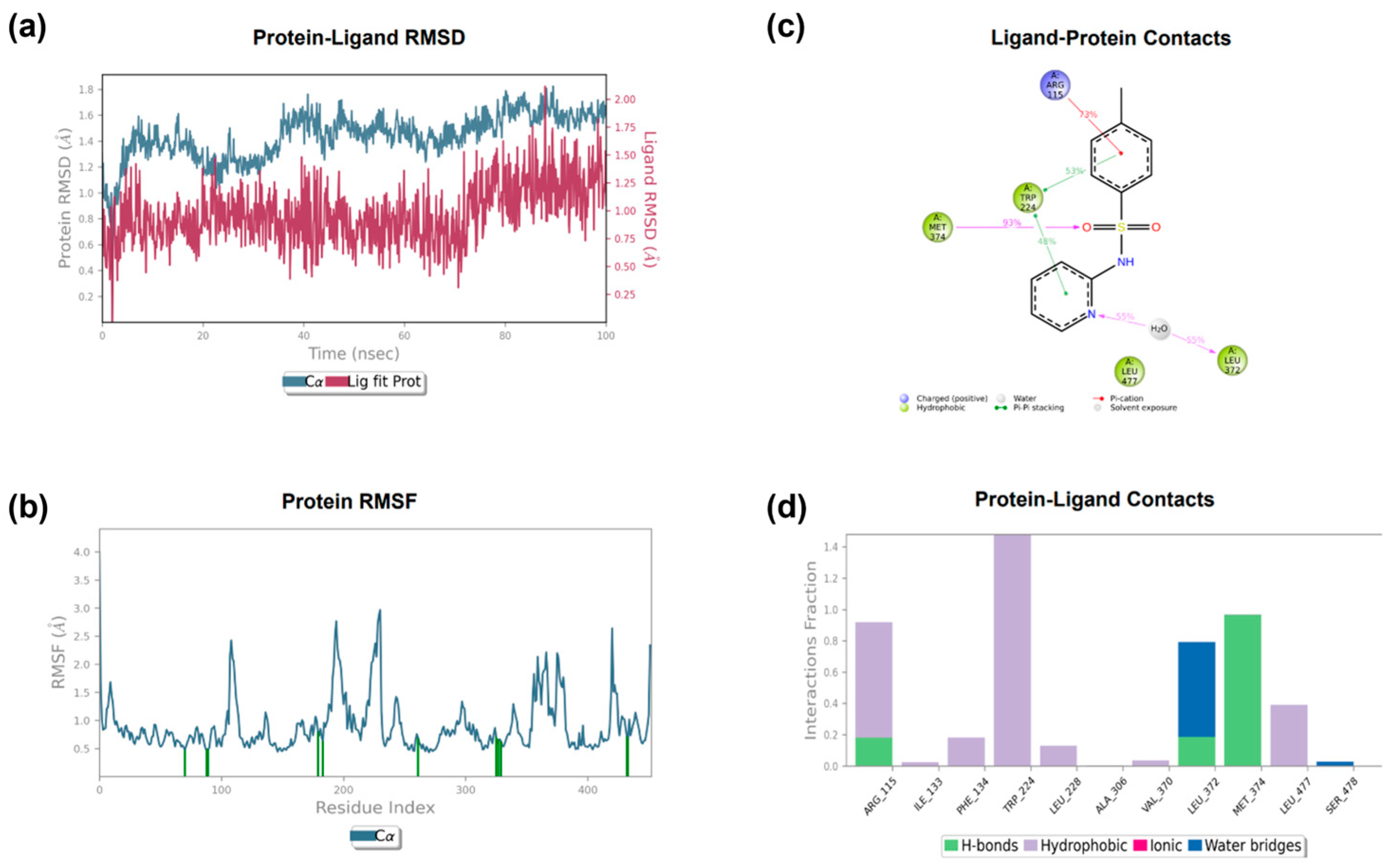
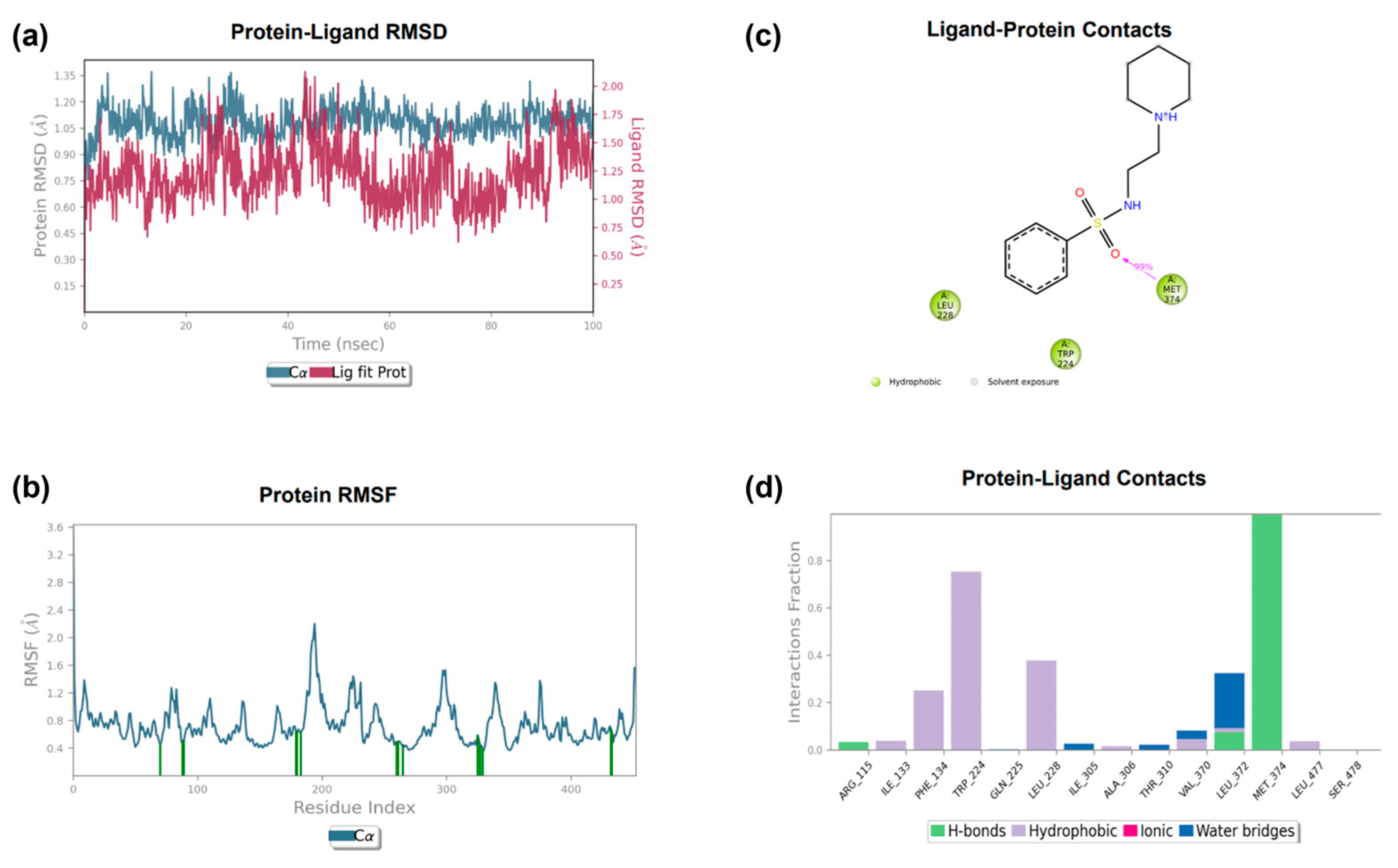
3.3.2. Molecular Dynamics
3.3.3. ADME Parameters and Drug-like Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Aromatase Inhibitor |
| EGFR | Epidermal Growth Factor Receptor |
| ER | Estrogen Receptor |
| FSH | Follicle-Stimulating Hormone |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| LTR | Letrozole |
| PDB | Protein Data Bank |
| PR | Progesterone Receptor |
| PTK2B | Protein Tyrosine Kinase 2 Beta |
References
- Zhang, Y.; Ji, Y.; Liu, S.; Li, J.; Wu, J.; Jin, Q.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; et al. Global burden of female breast cancer: New estimates in 2022, temporal trend and future projections up to 2050 based on the latest release from GLOBOCAN. J. Natl. Cancer Cent. 2025, 5, 287–296. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 15 May 2025).
- Elhawary, N.A.; Ekram, S.N.; Sembawa, H.A.; Tashkandi, E.; Bannani, S.; Azher, Z.A.; Almuqati, R.M.; Attieh, R.; Sindi, I.A.; Almutrafi, M.; et al. Descriptive epidemiology of female breast cancer around the world: Incidence, mortality, and sociodemographic risks and disparities. Int. J. Environ. Health Res. 2025, 16, 1–15. [Google Scholar] [CrossRef]
- Igissinov, N.; Toguzbayeva, A.; Khamidullina, Z.; Telmanova, Z.; Bilyalova, Z.; Kudaibergenova, I.; Muratbekova, S.; Igissinova, G.; Rustemova, K.; Kulmirzayeva, D.; et al. Epidemiology of Breast Cancer Mortality in Kazakhstan, trends and Geographic Distribution. Asian Pac. J. Cancer Prev. 2023, 24, 3361. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e102737. [Google Scholar] [CrossRef]
- American Cancer Society, W.C.O. Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html (accessed on 15 May 2025).
- Li, Y.; Zhang, H.; Jiang, T.; Li, P. Role of Estrogen Receptor-Positive/Negative Ratios in Regulating Breast Cancer. Evid. Based Complement. Alternat. Med. 2022, 2022, 7833389. [Google Scholar] [CrossRef]
- Xia, S.; Lin, Q. Estrogen Receptor Bio-Activities Determine Clinical Endocrine Treatment Options in Estrogen Receptor-Positive Breast Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221090351. [Google Scholar] [CrossRef]
- Hyder, T.; Marino, C.C.; Ahmad, S.; Nasrazadani, A.; Brufsky, A.M. Aromatase Inhibitor-Associated Musculoskeletal Syndrome: Understanding Mechanisms and Management. Front. Endocrinol. 2021, 12, 713700. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23, 382–392. [Google Scholar] [CrossRef]
- Gonnelli, S.; Petrioli, R. Aromatase inhibitors, efficacy and metabolic risk in the treatment of postmenopausal women with early breast cancer. Clin. Interv. Aging 2008, 3, 647. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Holota, S.; Lesyk, R.; Wujec, M. Aromatase Inhibitors as a Promising Direction for the Search for New Anticancer Drugs. Molecules 2024, 29, 346. [Google Scholar] [CrossRef] [PubMed]
- Słopień, R.; Męczekalski, B. Aromatase inhibitors in the treatment of endometriosis. Prz. Menopauzalny. 2016, 15, 43. [Google Scholar] [CrossRef]
- Peitsidis, P.; Tsikouras, P.; Laganà, A.S.; Laios, A.; Gkegkes, I.D.; Iavazzo, C. A Systematic Review of Systematic Reviews on the Use of Aromatase Inhibitors for the Treatment of Endometriosis: The Evidence to Date. Drug Des. Devel. Ther. 2023, 17, 1329. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.; Aguiar, I.J.M.; Matheus, G.; Dups Talah, B.A.; Morales Meirelles, L.; Moretti, N.R.; Ayoub Silva, L.; De Souza Wagner, P.H.; Alves Kelly, F.; Aquino de Moraes, F.C. Cardiovascular risks associated with aromatase inhibitors versus tamoxifen in breast cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2025, 43, 12018. [Google Scholar] [CrossRef]
- Bell, S.G.; Dalton, L.; McNeish, B.L.; Fang, F.; Henry, N.L.; Kidwel, K.M.; McLean, K. Aromatase inhibitor use, side effects and discontinuation rates in gynecologic oncology patients. Gynecol. Oncol. 2020, 159, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Liu, Y.; Zhang, J.; Zheng, L.; Zheng, M. Adverse Event Profiles of the Third-Generation Aromatase Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS. Biomedicines 2024, 12, 1708. [Google Scholar] [CrossRef]
- Ghosh, D.; Griswold, J.; Erman, M.; Pangborn, W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 2009, 457, 219–223. [Google Scholar] [CrossRef]
- Azevedo-Barbosa, H.; Dias, D.F.; Franco, L.L.; Hawke, J.A.; Carvalho, D.T. From Antibacterial to Antitumour Agents: A Brief Review on The Chemical and Medicinal Aspects of Sulfonamides. Mini. Rev. Med. Chem. 2020, 20, 2052–2066. [Google Scholar] [CrossRef]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.Y.; Qin, H.L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef]
- Elsayad, K.A.; Elmasry, G.F.; Mahmoud, S.T.; Awadallah, F.M. Sulfonamides as anticancer agents: A brief review on sulfonamide derivatives as inhibitors of various proteins overexpressed in cancer. Bioorg. Chem. 2024, 147, 107409. [Google Scholar] [CrossRef]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042. [Google Scholar] [CrossRef] [PubMed]
- Baraa, G.A.; Khulood, S.; Alaa, S.; Ahmed, A.-T. Sulfonamide derivatives: Synthesis and applications. Int. J. Front. Chem. Pharm. Res. 2024, 4, 1. [Google Scholar] [CrossRef]
- Kharb, R.; Haider, K.; Neha, K.; Yar, M.S. Aromatase inhibitors: Role in postmenopausal breastcancer. Arch Pharm. 2020, 353, e2000081. [Google Scholar] [CrossRef] [PubMed]
- Caporuscio, F.; Rastelli, G.; Imbriano, C.; Del Rio, A. Structure-Based Design of Potent Aromatase Inhibitors by High-Throughput Docking. J. Med. Chem. 2011, 52, 12. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; De Filippis, B.; Gallorini, M.; Ammazzalorso, A.; Giampietro, L.; Maccallini, C.; Aturki, Z.; Donati, E.; Ibrahim, R.S.; Shawky, E.; et al. Synthesis, biological evaluation, and docking study of indole aryl sulfonamides as aromatase inhibitors. Eur. J. Med. Chem. 2020, 185, 111815. [Google Scholar] [CrossRef] [PubMed]
- Fantacuzzi, M.; Gallorini, M.; Gambacorta, N.; Ammazzalorso, A.; Aturki, Z.; Balaha, M.; Carradori, S.; Giampietro, L.; Maccallini, C.; Cataldi, A.; et al. Design, Synthesis and Biological Evaluation of Aromatase Inhibitors Based on Sulfonates and Sulfonamides of Resveratrol. Pharmaceuticals 2021, 14, 984. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, L.; Gallorini, M.; Gambacorta, N.; Ammazzalorso, A.; De Filippis, B.; Della Valle, A.; Fantacuzzi, M.; Maccallini, C.; Mollica, A.; Cataldi, A.; et al. Synthesis, structure-activity relationships and molecular docking studies of phenyldiazenyl sulfonamides as aromatase inhibitors. Eur. J. Med. Chem. 2021, 224, 13737. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Gallorini, M.; Fantacuzzi, M.; Gambacorta, N.; De Filippis, B.; Giampietro, L.; Maccallini, C.; Nicolotti, O.; Cataldi, A.; Amoroso, R. Design, synthesis and biological evaluation of imidazole and triazole-based carbamates as novel aromatase inhibitors. Eur. J. Med. Chem. 2021, 211, 113115. [Google Scholar] [CrossRef]
- Di Matteo, M.; Ammazzalorso, A.; Andreoli, F.; Caffa, I.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Nencioni, A.; Parenti, M.D.; et al. Synthesis and biological characterization of 3-(imidazol-1-ylmethyl) piperidine sulfonamides as aromatase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3192–3194. [Google Scholar] [CrossRef]
- Babalola, B.A.; Malik, M.; Olowokere, O.; Adebesin, A.; Sharma, L. Indoles in drug design and medicinal chemistry. Eur. J. Med. Chem. Rep. 2025, 13, 100252. [Google Scholar] [CrossRef]
- Vinod, A.; Chandra Mouli, H.M.; Jana, A.; Peraman, R. Unlocking therapeutic potential: Exploring indole scaffolds and their structural insights as pharmacophores in designing anti-breast cancer agents. Med. Chem. Res. 2024, 33, 1100–1132. [Google Scholar] [CrossRef]
- Osmaniye, D.; Levent, S.; Sağlık, B.N.; Karaduman, A.B.; Özkay, Y.; Kaplancıklı, Z.A. Novel imidazole derivatives as potential aromatase and monoamine oxidase-B inhibitors against breast cancer. New J. Chem. 2022, 46, 7442. [Google Scholar] [CrossRef]
- Evren, A.E.; Nuha, D.; Dawbaa, S.; Sağlık, B.N.; Yurttaş, L. Synthesis of novel thiazolyl hydrazone derivatives as potent dual monoamine oxidase-aromatase inhibitors. Eur. J. Med. Chem. 2022, 229, 114097. [Google Scholar] [CrossRef]
- Rani, S.; Raheja, K.; Luxami, V.; Paul, K. A review on diverse heterocyclic compounds as the privileged scaffolds in non-steroidal aromatase inhibitors. Bioorg. Chem. 2021, 113, 105017. [Google Scholar] [CrossRef]
- Bhatia, N.; Thareja, S. Aromatase inhibitors for the treatment of breast cancer: An overview (2019–2023). Bioorg. Chem. 2024, 151, 107607. [Google Scholar] [CrossRef] [PubMed]
- Sağlık, B.N.; Ilgın, S.; Özkay, Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur. J. Med. Chem. 2016, 124, 1026. [Google Scholar] [CrossRef]
- Osmaniye, D.; Görgülü, Ş.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Design, synthesis, in vitro and in silico studies of some novel thiazole-dihydrofuran derivatives as aromatase inhibitors. Bioorg. Chem. 2021, 114, 105123. [Google Scholar] [CrossRef]
- Schrödinger Release 2023-1; Maestro, Glide, Protein Preparation Wizard, Epik, QikProp, MacroModel, Desmond. Schrödinger, Inc.: New York, NY, USA, 2023.
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750. [Google Scholar] [CrossRef]
- Murphy, R.B.; Philipp, D.M.; Friesner, R.A. A mixed quantum mechanics/molecular mechanics (QM/MM) method for large-scale modeling of chemistry in protein environments. J. Comp. Chem. 2000, 21, 1442. [Google Scholar] [CrossRef]
- Philipp, D.M.; Friesner, R.A. Mixed ab initio QM/MM modeling using frozen orbitals and tests with alanine dipeptide and tetrapeptide. J. Comp. Chem. 1999, 20, 1468. [Google Scholar] [CrossRef]
- Pillai, O.; Dhanikula, A.B.; Panchagnul, R. Drug delivery: An odyssey of 100 years. Curr. Opin. Chem. Biol. 2001, 5, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Kihlberg, J. Drug discovery beyond the rule of 5—Opportunities and challenges. Expert Opin. Drug Discov. 2017, 12, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Selitrennik, M.; Lev, S. PYK2 integrates growth factor and cytokine receptors signaling and potentiates breast cancer invasion via a positive feedback loop. Oncotarget 2015, 6, 22214–22226. [Google Scholar] [CrossRef]
- Gil-Henn, H.; Girault, J.A.; Lev, S. PYK2, a hub of signaling networks in breast cancer progression. Trends Cell Biol. 2024, 34, 312–326. [Google Scholar] [CrossRef]
- Shen, T.; Guo, Q. EGFR signaling pathway occupies an important position in cancer-related downstream signaling pathways of Pyk2. Cell Biol. Int. 2020, 44, 2–13. [Google Scholar] [CrossRef]
- Addisu, S.; Bekele, A.; Seifu, D.; Assefa, M.; Gemechu, T.; Hoenerhoff, M.J.; Merajver, S.D. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor A (VEGF-A) expressions in Ethiopian female breast cancer and their association with histopathologic features. PLoS ONE 2024, 19, e0308411. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, L.; Wang, N.; Xiong, Y.; Li, Y.; Gu, Y. Relationship of Epidermal Growth Factor Receptor Expression with Clinical Symptoms and Metastasis of Invasive Breast Cancer. J. Interferon Cytokine Res. 2018, 38, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Zhang, C.; Xue, L.; Yang, L. Expression and clinical significance of MAPK and EGFR in triple-negative breast cancer. Oncol. Lett. 2020, 19, 1842–1848. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, J.; Fan, Y.; Wang, Z.; Tian, R.; Ji, W.; Zhang, F.; Niu, R. TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol. Oncol. 2018, 12, 305–321. [Google Scholar] [CrossRef]
- Jeong, Y.; Bae, S.Y.; You, D.; Jung, S.P.; Choi, H.J.; Kim, I.; Lee, S.K.; Yu, J.; Kim, S.W.; Lee, J.E.; et al. EGFR is a Therapeutic Target in Hormone Receptor-Positive Breast Cancer. Cell Physiol. Biochem. 2019, 53, 805–819. [Google Scholar] [CrossRef]
- Silva Rocha, F.; da Silva Maués, J.H.; Brito Lins Pereira, C.M.; Moreira-Nunes, C.A.; Rodriguez Burbano, R.M. Analysis of Increased EGFR and IGF-1R Signaling and Its Correlation with Socio-Epidemiological Features and Biological Profile in Breast Cancer Patients: A Study in Northern Brazil. Breast Cancer 2021, 13, 325–339. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Gawade, R.L.; Kumar, D.; Kotmale, A.; Gonnade, R.G.; Stürzer, T. Crystal Engineering for Intramolecular π–π Stacking: Effect of Sequential Substitution of F on Molecular Geometry in Conformationally Flexible Sulfonamides. Cryst. Growth Des. 2019, 19, 5665–5678. [Google Scholar] [CrossRef]
- Aromatase (CYP19A) Inhibitor Screening Kit (Fluorometric) (Catalog No: K984-100) Manual; BioVision: Milpitas, CA, USA, 2023. Available online: https://www.biovision.com/documentation/datasheets/K984.pdf (accessed on 1 December 2024).
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu Rev. 2005, 11, 127. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 23, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]

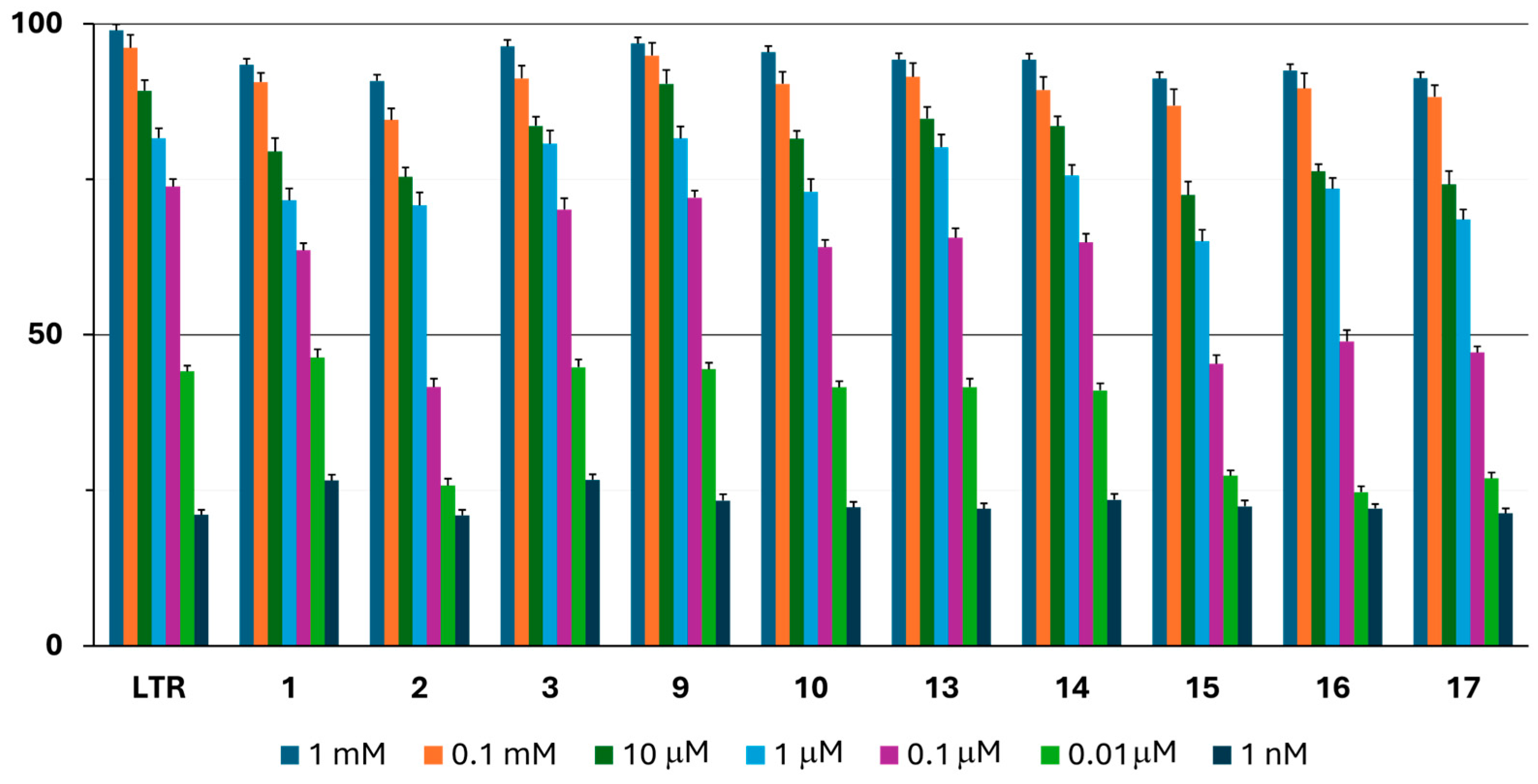


| Aromatase IC50 * (µM) | MCF7 IC50 * (µM) | NIH3T3 IC50 * (µM) | |
|---|---|---|---|
| 1 | 0.060 ± 0.002 | 7.511 ± 0.296 | 46.910 ± 1.994 |
| 2 | 0.248 ± 0.010 | 15.410 ± 0.356 | 51.368 ± 1.743 |
| 3 | 0.035 ± 0.001 | 2.679 ± 0.104 | 64.306 ± 1.832 |
| 4 | >100 | >100 | >100 |
| 5 | >100 | >100 | 76.582 ± 1.126 |
| 6 | >100 | 25.311 ± 0.945 | >100 |
| 7 | >1000 | 28.469 ± 0.514 | 44.275 ± 1.055 |
| 8 | >1000 | >100 | >100 |
| 9 | 0.032 ± 0.001 | 3.288 ± 0.119 | 58.241 ± 0.952 |
| 10 | 0.052 ± 0.002 | 6.201 ± 0.258 | 30.856 ± 1.028 |
| 11 | >100 | 43.617 ± 1.863 | 29.629 ± 0.812 |
| 12 | >1000 | 32.062 ± 1.166 | >100 |
| 13 | 0.046 ± 0.002 | 8.062 ± 0.319 | 49.630 ± 1.726 |
| 14 | 0.051 ± 0.001 | 10.505 ± 0.477 | 56.057 ± 1.930 |
| 15 | 0.337 ± 0.012 | 12.386 ± 0.417 | 39.276 ± 0.995 |
| 16 | 0.160 ± 0.006 | 19.579 ± 0.678 | 50.943 ± 1.510 |
| 17 | 0.206 ± 0.009 | 14.613 ± 0.522 | 43.057 ± 1.029 |
| LTR | 0.026 ± 0.001 | / | / |
| Doxorubicin | / | 1.940 ± 0.084 | >100 |
| Cmp | H-Bond | π-π Bond | Salt Bridge | Docking Score (kcal/mol) |
|---|---|---|---|---|
| 1 | SO-Met374 | Py-HEM | / | −7.423 |
| 3 | SO-Met374 NH-Leu372 | Ph-HEM | / | −7.280 |
| 9 | SO-Met374 | Ph-Trp224 | NH-HEM | −7.864 |
| 10 | SO-Met374 | Ph-Trp224 | NH-HEM | −8.147 |
| 13 | SO-Met374 | / | NH-HEM | −7.608 |
| 14 | SO-Met374 | / | NH-HEM | −7.570 |
| cmp | MW | donorHB | accptHB | QPlogPo/w | PSA | CNS | QPlogHERG | QPPCaco | QPlogBB | QPPMDCK | QPlogKhsa | RuleOfFive | hOralAbs | % hOralAbs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 262.33 | 1 | 5.5 | 1.56 | 61.48 | −2 | −4.51 | 1019.17 | −0.59 | 505.91 | −0.42 | 0.00 | 3.00 | 91.71 |
| 3 | 248.30 | 1 | 5.5 | 2.08 | 57.98 | 0 | −5.19 | 1284.06 | −0.45 | 661.61 | −0.22 | 0.00 | 3.00 | 92.91 |
| 9 | 268.37 | 1 | 6.5 | 2.97 | 53.65 | 1 | −4.22 | 362.05 | −0.03 | 182.52 | 0.17 | 0.00 | 3.00 | 78.71 |
| 10 | 282.40 | 1 | 6.5 | 2.83 | 52.98 | 0 | −4.18 | 500.69 | 0.03 | 259.12 | −0.15 | 0.00 | 3.00 | 83.43 |
| 13 | 268.37 | 1 | 6.5 | 0.22 | 56.13 | 1 | −4.46 | 311.97 | −0.10 | 157.74 | −0.79 | 0.00 | 3.00 | 77.74 |
| 14 | 282.40 | 1 | 6.5 | 1.39 | 56.14 | 1 | −4.35 | 312.40 | −0.12 | 158.05 | −0.38 | 0.00 | 3.00 | 79.28 |
| Gene Symbol | Description | Uniprot ID | |
|---|---|---|---|
| 1 | MEN1 | Menin 1 | O00255 |
| CDK2 | Cyclin-Dependent Kinase 2 | P24941 | |
| CYP17A1 | Cytochrome P450 Family 17 Subfamily A Member 1 | P05093 | |
| IRAK4 | Interleukin 1 Receptor-Associated Kinase 4 | Q9NWZ3 | |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 | P11511 | |
| PTK2B | Protein Tyrosine Kinase 2 Beta | Q14289 | |
| 3 | AKT1 | AKT Serine/Threonine Kinase 1 | P31749 |
| ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 | P04626 | |
| PTK2B | Protein Tyrosine Kinase 2 Beta | Q14289 | |
| 9 | EGFR | Epidermal Growth Factor Receptor | P00533 |
| 10 | TERT | Telomerase Reverse Transcriptase | O14746 |
| CASR | Calcium-Sensing Receptor | P41180 | |
| 13 | CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 | P11511 |
| 14 | ABCC1 | ATP-Binding Cassette Subfamily C Member 1 | P33527 |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 | P11511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Filippis, B.; Agamennone, M.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L.; Maccallini, C.; Sağlık, B.N.; De Simone, C.; Zuccarini, M.; Kaplancıklı, Z.A.; et al. Discovery and Evaluation of Novel Sulfonamide Derivatives Targeting Aromatase in ER+ Breast Cancer. Pharmaceuticals 2025, 18, 1206. https://doi.org/10.3390/ph18081206
De Filippis B, Agamennone M, Ammazzalorso A, Amoroso R, Giampietro L, Maccallini C, Sağlık BN, De Simone C, Zuccarini M, Kaplancıklı ZA, et al. Discovery and Evaluation of Novel Sulfonamide Derivatives Targeting Aromatase in ER+ Breast Cancer. Pharmaceuticals. 2025; 18(8):1206. https://doi.org/10.3390/ph18081206
Chicago/Turabian StyleDe Filippis, Barbara, Mariangela Agamennone, Alessandra Ammazzalorso, Rosa Amoroso, Letizia Giampietro, Cristina Maccallini, Begüm Nurpelin Sağlık, Chiara De Simone, Mariachiara Zuccarini, Zafer Asım Kaplancıklı, and et al. 2025. "Discovery and Evaluation of Novel Sulfonamide Derivatives Targeting Aromatase in ER+ Breast Cancer" Pharmaceuticals 18, no. 8: 1206. https://doi.org/10.3390/ph18081206
APA StyleDe Filippis, B., Agamennone, M., Ammazzalorso, A., Amoroso, R., Giampietro, L., Maccallini, C., Sağlık, B. N., De Simone, C., Zuccarini, M., Kaplancıklı, Z. A., & Fantacuzzi, M. (2025). Discovery and Evaluation of Novel Sulfonamide Derivatives Targeting Aromatase in ER+ Breast Cancer. Pharmaceuticals, 18(8), 1206. https://doi.org/10.3390/ph18081206














