1. Introduction
Bladder cancer ranks among the most prevalent malignancies of the urinary tract, with significant clinical and economic burdens globally [
1]. Preliminary studies indicate that non-muscle-invasive bladder cancer (NMIBC) accounts for 70–75% of cases, muscle-invasive bladder cancer (MIBC) comprises 20–25%, while metastatic disease represents approximately 5% [
2,
3,
4]. Despite the high treatment costs, clinical outcomes for bladder cancer remain suboptimal. Within 5 years, 31–78% of NMIBC patients experience recurrence, while 10–15% progress to MIBC, resulting in a 36–48% reduction in 5-year survival rates [
5]. Cisplatin (CDDP)-based chemotherapy remains the first-line treatment for muscle-invasive bladder cancer (MIBC) patients. However, due to the high tumor heterogeneity characteristic of bladder cancer, approximately 40% of patients fail to derive clinical benefit from conventional therapy and even experience persistent disease progression [
6]. Based on the side effects associated with chemotherapy (e.g., high nephrotoxicity), patients who tolerate this treatment regimen usually receive only short-term treatment. Therefore, the development of new platinum-based chemotherapeutic agents with fewer toxicities but better therapeutic efficacy deserves our more attention, and Pt(IV) prodrugs have come onto the stage. The Pt(IV) prodrug design paradigm promises to address this clinical challenge through rational axial ligand modification, enabling simultaneous delivery of multimodal payloads while preserving the DNA-damaging platinum core.
Cyclooxygenase-2 (COX-2) is an inducible enzyme correlated with inflammatory diseases and cancer [
7]. Normally, the expression of COX-2 in tissues is low, while in inflammation, tumor and other conditions, the expression of COX-2 is significantly elevated [
8]. Studies have shown that high COX-2 expression in bladder cancer correlates with higher stage grading of the tumor and worse prognosis of advanced bladder cancer [
9,
10,
11]. COX-2 also modulates arachidonic acid metabolites to help cancer cells resist apoptosis, cause inflammation, and grow new blood vessels [
12,
13]. Thus, COX inhibitors, as known as nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce inflammation by inhibiting COX-2 expression, thereby reducing tumor growth and lowering cancer risk and mortality [
14,
15,
16,
17]. The pervious study had shown that the Pt(IV) prodrug DNP, using the NSAID naproxen (NPX) as an axial ligand, has high cytotoxicity and anti-inflammatory properties in the treatment of breast cancer [
18]. Given the pronounced inflammatory infiltration characteristic of bladder cancer pathogenesis, we performed a preliminary investigation into the anti-tumor activity of the Pt(IV), DNP, marking the first application of this prodrug in bladder cancer models.
3. Discussion
Bladder cancer is a high-grade urological tumor of high malignancy with high mortality and recurrence rates [
24]. Over the past three decades, bladder cancer clinical management has evolved from radical cystectomy to platinum-based neoadjuvant chemotherapy combined with radiotherapy, representing a paradigm shift in treatment strategies. However, key prognostic indicators such as the five-year survival rate have not improved significantly [
25,
26]. Previous studies have reported that traditional divalent platinum-based chemotherapeutic agents (e.g., cisplatin, carboplatin, oxaliplatin) often induce severe side effects and drug resistance [
27,
28,
29]. With advances in nanotechnology and chemical synthesis, novel platinum-based drugs and their targeted modifications undoubtedly offer promising prospects for improving current anticancer therapies [
30]. Platinum(IV) complexes, serving as prodrugs for divalent platinum agents, may address this clinical challenge through rational targeted modifications.
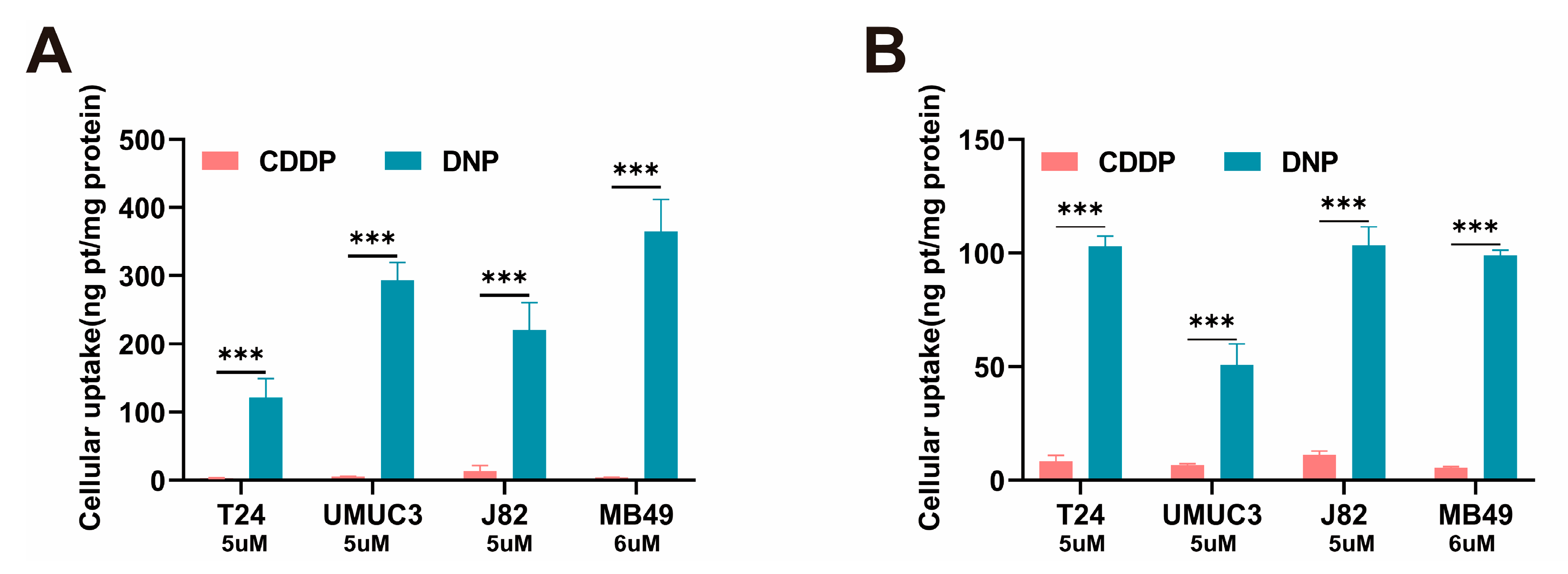
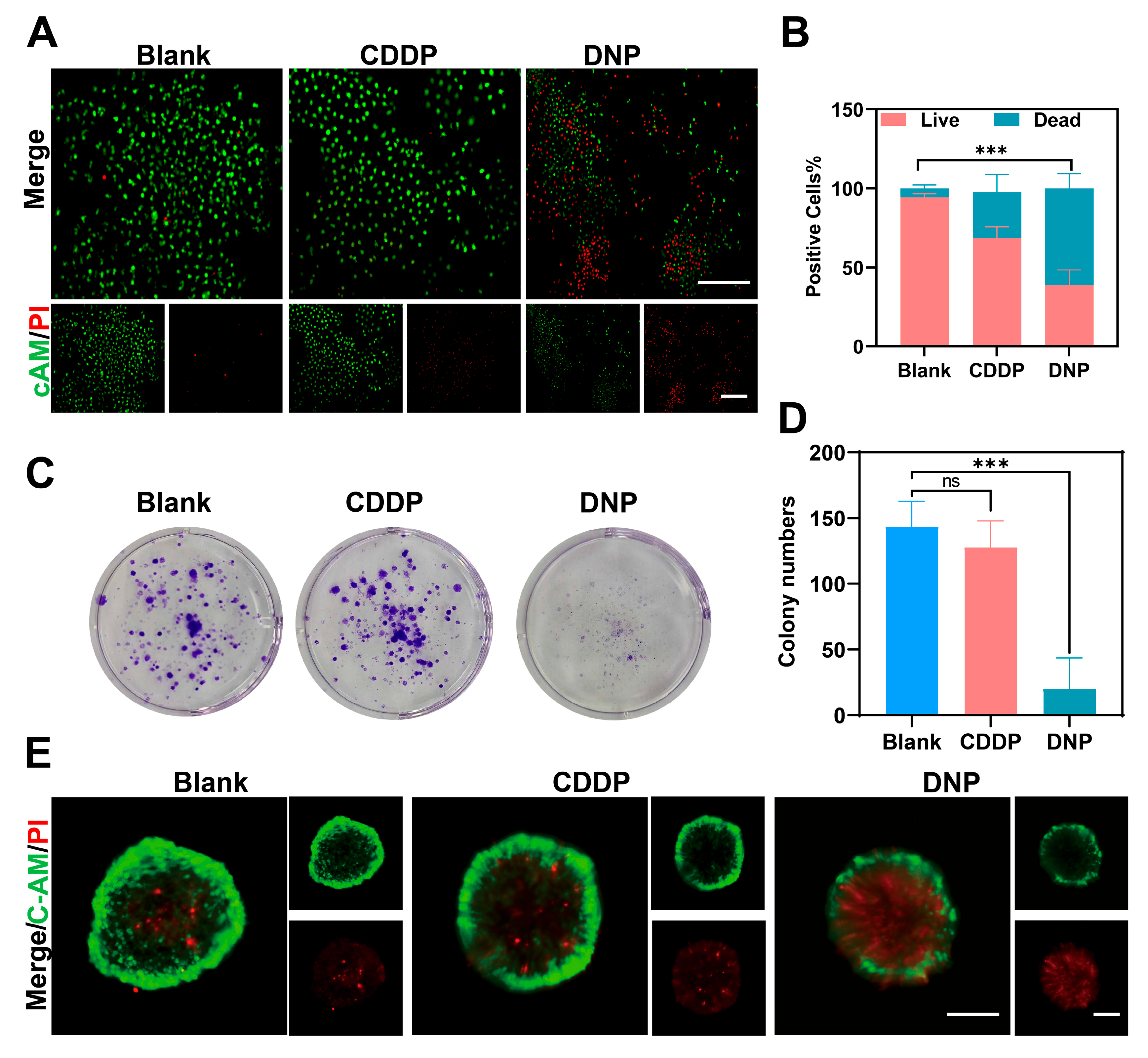
We demonstrated that DNP exhibited significantly stronger cytotoxicity than cisplatin. Previous studies have reported that DNP exhibits significantly higher lipophilicity (logPO/W = 1.46) than cisplatin (logPO/W = −2.35) [
18]. Given that the lipophilicity of compounds is directly correlated with their passive diffusion efficiency across membranes, the enhanced hydrophobicity of DNP facilitates its penetration through the cellular membrane barrier, leading to a significant increase in intracellular platinum accumulation. This observation aligns well with our subsequent findings: compared to cisplatin, DNP demonstrates significantly higher platinum uptake in cancer cells and greater platinum content bound to cellular DNA. Furthermore, DNP exhibited significant growth inhibition not only in monolayer-cultured cancer cells but also in three-dimensional (3D) tumor spheroids. This benefit can undoubtedly be attributed to the enhanced lipophilicity of DNP, which facilitates its penetration through the outer layers of tumor spheroids. Moreover, colony formation assays revealed that cancer cells treated with DNP exhibited nearly complete growth inhibition.
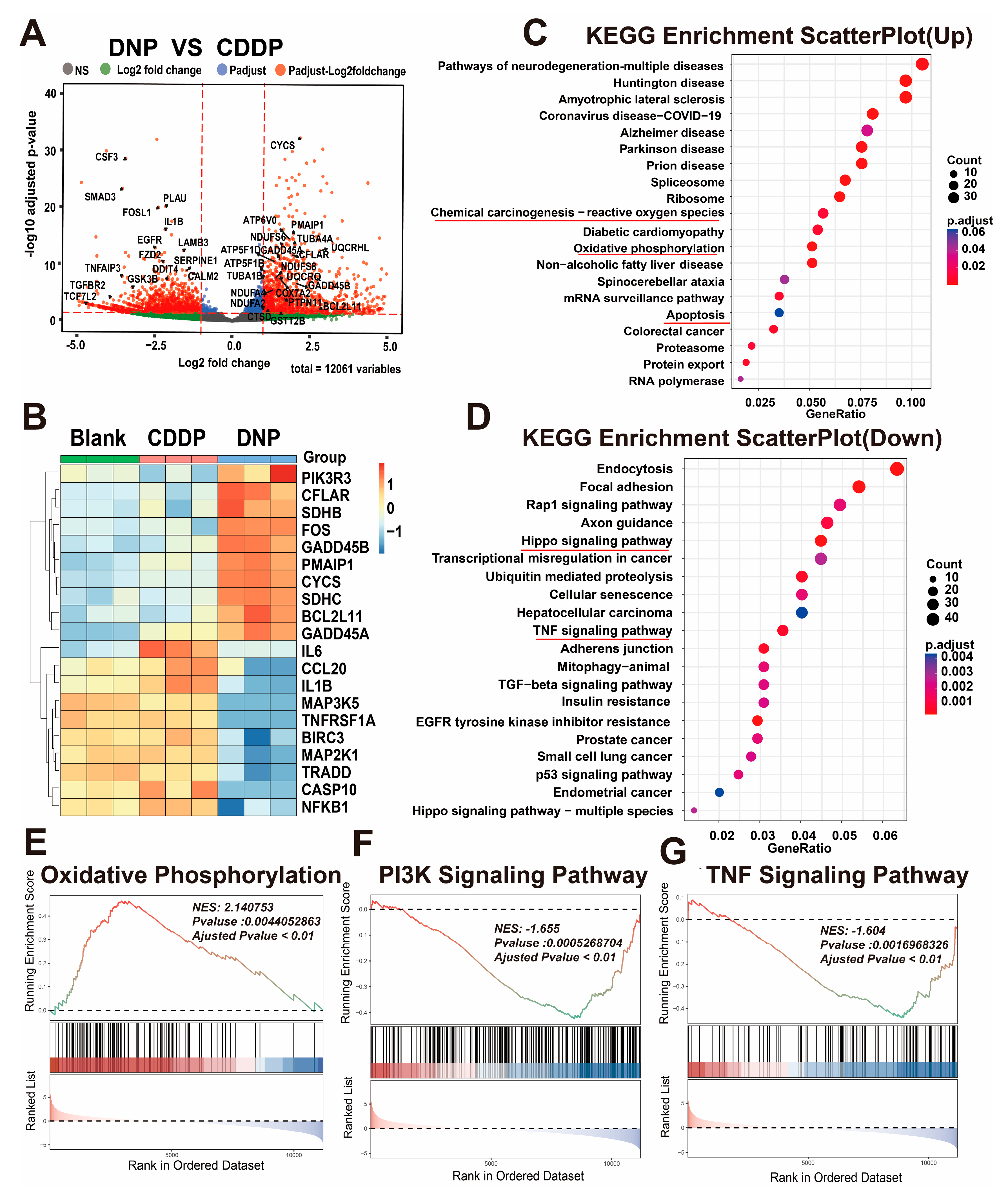
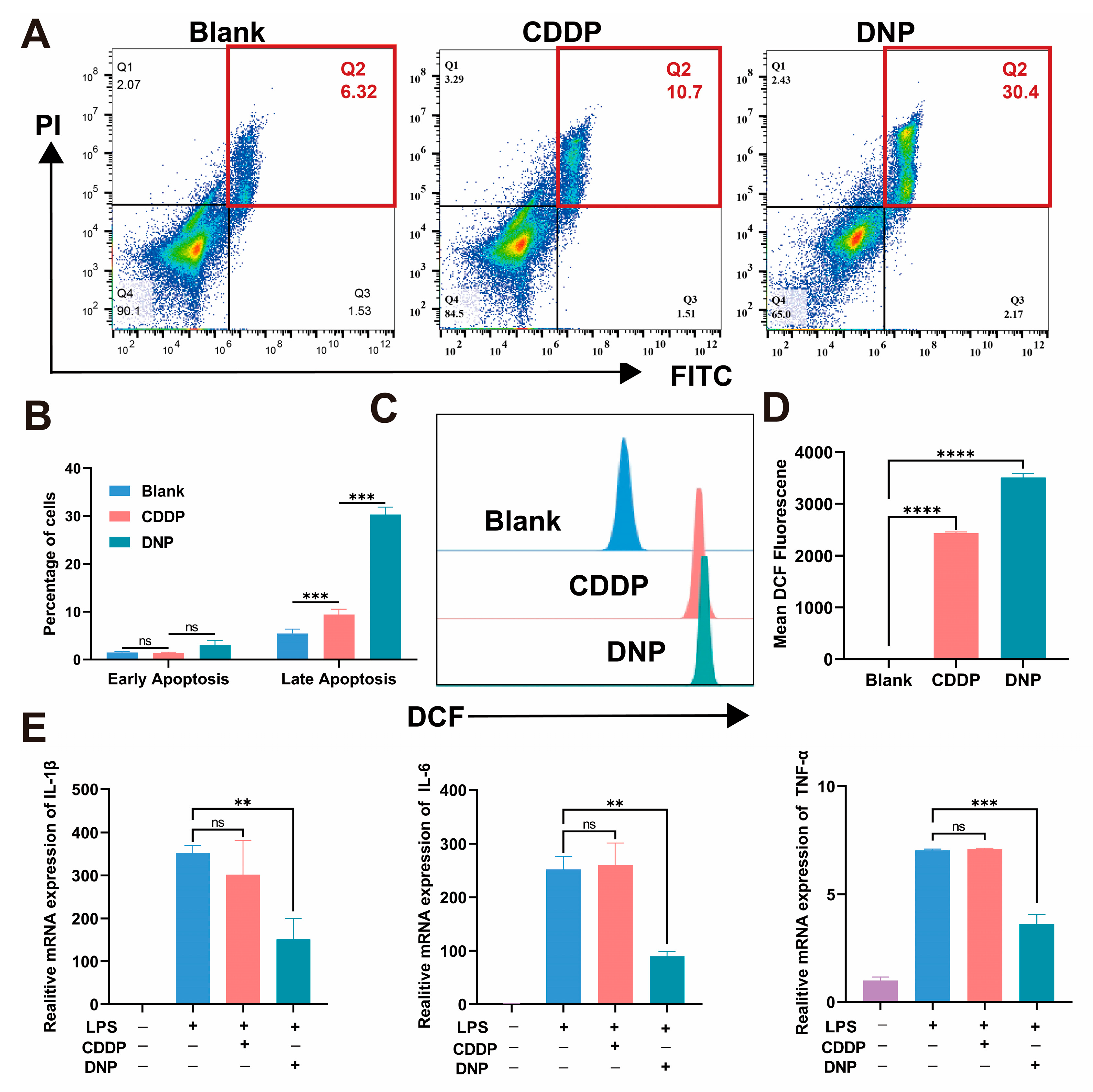
Moreover, we used RNA-seq technology to study T24 cells treated with DNP and cisplatin and found that DNP has a unique multi-target mechanism. Both KEGG and GSEA analyses consistently showed that DNP triggered pathways related to ROS (like oxidative phosphorylation and apoptosis) and also blocked TNF signaling, which is an inflammatory pathway. This finding provides direct evidence supporting the “chemo-anti-inflammatory” dual-functional design concept for Pt(IV) prodrugs. Subsequent apoptosis assays revealed that DNP exhibited significantly higher pro-apoptotic efficiency than cisplatin. This improvement might come from DNP’s special redox properties, which greatly raise ROS levels and directly activate the mitochondrial apoptosis pathway, as noted in earlier studies [
23]. Furthermore, DNP demonstrated significant anti-inflammatory activity in the inflammatory model, which may be due to its axial ligand NPX blocking prostaglandin synthesis. However, the study has the following limitations: the quantitative relationship between ROS levels and apoptosis rates needs to be validated, and the precise mechanism of the axial ligand NPX needs to be clarified. In summary, DNP exerts its antitumor effects through a unique dual mechanism involving both pro-apoptotic and anti-inflammatory actions. This distinctive property suggests its potential to overcome the drug resistance and toxicity limitations associated with conventional platinum-based agents.
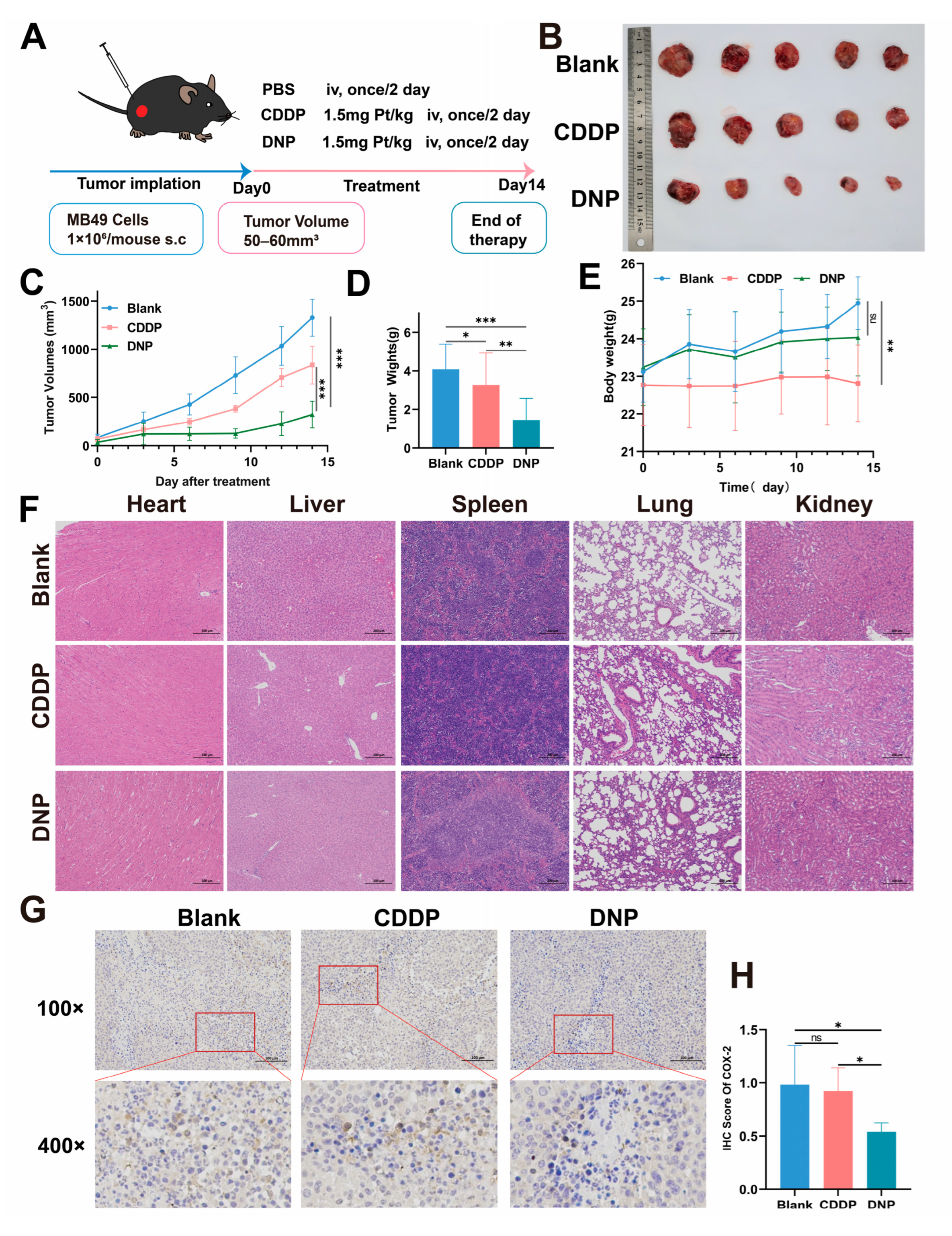
Finally, we established an orthotopic bladder cancer animal model to evaluate the therapeutic potential of the novel platinum(IV) complex DNP. The findings provide important guidance for the clinical translation of platinum-based drugs. DNP demonstrated significantly superior antitumor efficacy compared to cisplatin, which may be attributed to its unique mechanism of action. The enhanced lipophilicity of the Pt(IV) prodrug likely improves tumor tissue penetration and retention effects. Additionally, the axial NPX ligand may potentiate the platinum core’s cytotoxicity by downregulating COX-2 expression. Histopathological examination of organ tissues demonstrated superior safety profiles of DNP, as evidenced by the absence of cisplatin-typical renal tubular vacuolization [
31] and well-preserved histological integrity in major organs. Interestingly, no significant changes in body weight were observed in model mice. Our study provides critical evidence for the further development of DNP, but future investigations are still required to characterize its pharmacokinetic profile and safety in vivo.
4. Materials and Methods
4.1. Reagents and Chemicals
Cisplatin was purchased from MCE. Hydrogen peroxide (30%, H2O2), nitric acid, and hydrochloric acid were purchased from Sinopharm Chemical Reagent. The remaining chemical reagents came from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China), while dimethyl sulfoxide (DMSO) was purchased from Sigma (St. Louis, MO, USA).
RPMI 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM) (high glucose), fetal bovine serum (FBS) and penicillin-Streptomycin were purchased from Gibco (Oakland, CA, USA) Trypsin without EDTA (FMS20231201001) was purchased from Fcmacs Biotech Co., Ltd (Nanjing, China). The Cell Counting Kit-8 (CCK-8, KTA1020-1000T) was purchased from Abbkine Scientific Co., Ltd (Wuhan, China). The Calcein/PI Cell Viability and Cytotoxicity Assay Kit (C2015S), the BCA Protein Assay Kit (P0010S), and the Reactive Oxygen Species Detection Kit (S0033S) were purchased from Beyotime Biotechnology (Shanghai, China). The genomic DNA extraction kit (DC102-01) for cells and tissues, CellCounting-Lite 3D Luminescent Assay (Vazyme #DD1102) as well as the qPCR reagents, were purchased from Vazyme Biotech Co., Ltd (Nanjing, China). Additionally, the Annexin V-FITC/PI apoptosis detection reagent (K2003) was purchased from APExBIO Technology LLC (Houston, TX, USA).
4.2. Cell Culture
The human bladder cancers (T24, UMUC3, J82) were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China). The murine bladder cancer cell line MB49 was generously gifted by Professor Haibo Shen (Shanghai Jiao Tong University, Shanghai, China). RAW264.7 cells were obtained from Nanjing University School of Life Sciences. The MB49 were cultured in RPMI-1640 supplemented with 10% FBS and 1× penicillin-Streptomycin, whereas the other cell lines were cultured in DMEM also enriched with 10% FBS. Cells were grown at 37 °C in an incubator (Thermo Fisher Scientific, Waltham, MA, USA) containing 5% CO2.
4.3. Animals
Six- to eight-week old male C57BL/6 mice (18–23 g) were purchased from GemPharmatech Co., Ltd (Nanjing, China). All animal experimental protocols followed the regulations of the People’s Republic of China on the Administration of Laboratory Animals, and it use protocol was reviewed and approved by the Nanjing University Animal Ethical and Welfare Committee (Approval No: IACUC-2412011). All animals were housed under a pathogen-free condition in groups of five mice per cage. The temperature was kept at 21 ± 2 °C, relative humidity varied between 40% and 70%, and a 12 h light/dark cycle was maintained.
4.4. Compound Synthesis·and·Characterization
The synthetic route and characterization data for compound DNP are available in the literature procedure [
18]. Oxoplatin was stirred with NPX, TEA, and TBTU in DMF under dark conditions for 48 h. The resulting mixture was treated with ethanol and water to yield a yellow precipitate, which was subsequently dissolved in methanol and precipitated with diethyl ether. After several repetitions of this precipitation procedure, the product was dried under vacuum to afford a pale yellow powder.
The structural integrity and purity of the synthesized complexes were systematically characterized using an integrated analytical approach. Nuclear magnetic resonance (NMR) spectra (1H, 13C, and 195Pt) were acquired on a Bruker DRX-400 spectrometer (BRKR, Billerica, USA) at 298 K, with DMSO-d6 as the solvent and tetramethylsilane (TMS) as an internal reference. High-resolution Mass Spectrometry (HR-MS) data were measured in negative ion mode using a Thermo Q Exactive Orbitrap (Thermo Fisher Scientific, Waltham, MA, USA) instrument. Isotopic distribution patterns were simulated using ISOPRO 3.0 to confirm molecular composition. Compound purity was analyzed on a Thermo Scientific™ (Thermo Fisher Scientific, Waltham, MA, USA) Vanquish Core system equipped with a Thermo Scientific Acclaim120 C18 column (5 μm, 4.6 × 250 mm). The mobile phase comprised water (A) and acetonitrile (B) at 1.0 mL/min. Gradient elution: 0–2 min: 5% B, 2–30 min: 5–95% B, 30–35 min: 95% B. Detection at 254 nm with column temperature maintained at 40 °C. DNP was dissolved in acetonitrile (with trace DMSO for solubility), filtered through a 0.22 μm membrane, and injected (10 μL).
4.5. Cell Uptake
Four bladder tumor cell lines (T24, UMUC3, J82, and MB49) were seeded into 6-well plates and incubated overnight. The day after, cells were treated with DNP or cisplatin (CDDP) 12 h, with PBS-treated cells as controls. Post-treatment, cells were washed twice with ice-cold PBS, pelleted by centrifugation, and digested sequentially with nitric acid (95 °C, 2 h), hydrogen peroxide (1 h), and hydrochloric acid (1 h). Platinum content in the digested lysates was quantified via ICP-MS after filtration.
4.6. DNA Platination
Following the same cell culture and treatment protocol as above, genomic DNA was extracted using the cell/tissue genomic DNA extraction kit (Vazyme Biotech Co., Ltd., Nanjing, China). DNA concentration was quantified via NanoDrop (Thermo NanoDrop 1000) spectrophotometry (260 nm). DNA samples underwent sequential digestion with nitric acid (95 °C, 2 h), hydrogen peroxide (1 h), and hydrochloric acid (1 h). DNA-bound platinum content was analyzed by ICP-MS post-filtration.
4.7. Cytotoxicity of DNP
Cell viability was tested using the T24, UMUC3, J82, and MB49 cell lines. CDDP was dissolved in sterile PBS, DNP was dissolved in DMSO, and the stock solutions were then diluted to the desired concentration gradient using culture medium, ensuring that the DMSO was limited to less than 0.1%. Tumor cells were seeded at the density of 3000 cells/well in 96-well plates. Cells were treated with graded concentrations of CDDP or DNP for 72 h after wall attachment. The drug-containing medium was then replaced with a mixed solution of medium and CCK8 (9:1) and incubated for 1 h. Finally, the optical density (OD) was measured at 450 nm using a Synergy multifunction microplate reader. IC
50 values were calculated using GraphPad Prism software (version 9.40) with six parallel wells for each sample. The half-maximal inhibitory concentration (IC
50) was calculated as follows:
4.8. Live/Dead Cell Staining Method
The Calcein AM/PI cell viability detection kit was used to determine the proliferative activity of T24 cells. Tumor cells were seeded at a density of 3000 cells/well in a 96-well plate. When the cell density reached approximately 70%, they were treated with CDDP (0.2 µM), DNP (0.2 µM) for 24 h. Then, we incubated the cells with calcein-AM/propidium iodide for 30 min and imaged using a fluorescence microscope (LeicaWetzlar, Germany).
4.9. Colony Formation Assay
T24 cells were seeded in 6-well plates at a density of 1000 cells/well and allowed to adhere for 24 h. Cells were then exposed to CDDP (0.2 µM) or DNP (0.2 µM) for a duration of 24 h, followed by replacement with fresh DMEM. Medium was refreshed every 3 days until colonies had formed (7–10 days). Subsequent to this, the medium was discarded, and the cells were washed twice with PBS. The cells were then fixed with 4% paraformaldehyde for 15 min, after which they were stained with 0.5% crystal violet and photographed for subsequent analysis.
4.10. Three-Dimensional Tumor Spheroid Viability Assay
T24 cells were seeded at a density of 1000 cells/well in ultra-low-attachment 96-well plate (Corning, Glendale, AZ, USA). After the cells form stable spheroids, they are intervened with CDDP (5 µM) and DNP (5 µM) for 5 days. Then, the cell spheroids were washed 2–3 times with PBS and stained with Calcein AM/PI detection reagent. Finally, images were taken using a fluorescence microscope (LeicaWetzlar, Germany).
4.11. Bulk Sequencing
T24 cells were seeded at a density of 3 × 105 in 6 cm culture dishes. When the cell density reaches 80%, the cells are treated with DNP (2 µM) and CDDP (2 µM) for 24 h. Later, the cell samples were collected, and total RNA was extracted using Trizol reagent according to the instructions. The RNA samples were then quantified using a NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA) to ensure that the samples selected for library construction were of adequate quality. Lastly, the transcriptome sequencing analysis was performed by Singleron Biotech Co., Ltd Nanjing, China. Genes with a false discovery rate (FDR) parameter less than 0.05 and an absolute multiple change of at least 2 are considered to be differentially expressed genes (DEGs). The identification of DEGs between the CDDP group and the DNP group was facilitated by the utilization of volcano plots and heat maps. Subsequent to this, the identification of DEGs was subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and gene set enrichment analysis (GSEA).
4.12. Cell Apoptosis Assay
T24 cells were seeded at a density of 2 × 105 cells/well in 6-well plates. When the cell density reached 70%, the cells were incubated with DNP (0.1 µM) and CDDP (0.1 µM) for 48 h. The cells were then collected using trypsin without EDTA (Fcmacs) and washed twice with ice-cold PBS. The apoptosis rate was detected using the Annexin V-FITC/PI Apoptosis Kit (APExBIO Technology LLC (Houston, TX, USA). Following the kit instructions to add Annexin V-FITC and PI, cells were incubated in the dark for 15 min. After washing twice with PBS, the resuspended cells were prepared for flow cytometry analysis (Beckman Coulter, Inc, Brea, CA, USA).
4.13. RT-qPCR Analysis
RAW264.7 cells were seeded at a density of 5 × 104 cells/well in 12-well plates and stimulated with lipopolysaccharide (LPS, 100 ng/mL) for 18 h. Then, RAW264.7 cells were treated with CDDP (0.4 µM) and DNP (0.4 µM) for 18 h. The treated cells were collected, and whole RNA was extracted and detected according to the above method. Subsequently, HiScript II Q RT SuperMix for qRT-PCR (+gDNA wiper) (Vazyme Biotech Co., Ltd, Nanjing, China) was utilized for reverse transcription, and ChamQ Blue Universal SYBR qPCR Master Mix (Vazyme, Q312) for PCR amplification. The reaction conditions were as follows: Initial denaturation at 95 °C for 30 s; then 95 °C for 10 s, 60 °C for 30 s for a total of 40 cycles; then 95 °C for 15 s, 60 °C for 1 min; finally 95 °C for 15 s. Utilizing GAPDH as the reference gene, the mRNA expression levels of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the cells were measured employing the 2-ΔΔCT method.
4.14. ROS Production Assay
T24 cells were seeded at a density of 2 × 105 cells/well in 6-well plates. When the cell density reaches 70%, the cells are incubated with DNP (0.2 µM) and CDDP (0.2 µM) for 24 h. Tumor cells were collected and washed twice with PBS. The level of ROS produced in T24 cells was detected using a reactive oxygen detection kit. The cells were incubated with 10 µM DCF-DA probe in the dark for 30 min, washed 2–3 times with FBS-free medium, and resuspended for flow cytometry analysis.
4.15. Establishment and Treatment of Subcutaneous Tumor Model
The design of the in vivo treatment experiment is shown in
Figure 6A. 1 × 10
6 MB49 cells were injected into the right flank. The tumor volume and body weight were evaluated every two days. Until the tumors grew to approximately 50 mm
3, mice were randomized, and the first treatment was administered. All animals were divided into three groups (
n = 5) and treated with PBS (control group), CDDP (1.5 mg Pt/kg), or DNP (1.5 mg Pt/kg). The drug was administered intravenously every two days until the tumor volume of the control group reached 1500 mm
3. After the treatment, all mice were executed and the tumor tissue as well as vital organs (heart, liver, spleen, lungs, and kidneys) was surgically excised.
4.16. Hematoxylin and Eosin Staining
The microtome (Leica, Wetzlar, Germany) was utilized to achieve 5-micron-thick sections from the paraffin-embedded mouse tumor tissues. The tissue sections should then be placed in an oven (GNP-9050, Shanghai, China) at 65 °C for 1 h. The deparaffinization of the sections was then conducted using xylene, followed by hydration with a series of ethanol solutions, ranging from 100% to 30%. The next step is to stain the sections with hematoxylin solution for 5 min, differentiate them in 0.25% hydrochloric acid in ethanol for 5 s, and then rinse them in distilled water. Following this, the sections should be stained with eosin solution for 5 s and then rehydrated through a series of progressively higher alcohols (from 75% to 100%) and xylene. Finally, the slides should be photographed using a fluorescence microscope (Leica, Wetzlar, Germany).
4.17. Immunohistochemistry (IHC)
Firstly, the mouse tumor tissues must be deposited in an oven that has been calibrated to 65 °C for a period of 20 min. Subsequently, the sections should be deparaffinised using xylene and hydrated through a graded series of alcohol (from 100% to 70%), followed by rinsing with distilled water. The next step is to place the slides in an antigen retrieval solution at 95 °C and incubate for 10 min to complete antigen retrieval. The slides were then left at room temperature for ten minutes to soak in 3% hydrogen peroxide, which inhibited the activity of endogenous peroxidase. The slides were then washed thrice with PBS and subsequently blocked with a 5% bovine serum albumin (BSA) solution at ambient temperature for a period of one hour. The main antibody against COX-2 was then incubated with the slides for one hour at 37 °C in a humidified box. This was followed by three washes with PBST. Then, an appropriate dilution of secondary antibody was incubated with the slides for 30 min at 37 °C in a humidified box. Staining was then performed with 3,3′-diaminobenzidine (DAB) and hematoxylin. Finally, the samples should be photographed using a microscope (Leica, Wetzlar, Germany).
4.18. Statistical Analysis
Data analysis was conducted with GraphPad Prism 8.0, with results presented as mean ± standard deviation. Intergroup comparisons utilized one-way ANOVA, with statistical significance thresholds defined as * p < 0.05, ** p < 0.01, and *** p < 0.001, **** p < 0.0001.