Saccharomyces boulardii CNCM I-745 Supernatant Improves Markers of Gut Barrier Function and Inflammatory Response in Small Intestinal Organoids
Abstract
1. Introduction
2. Results
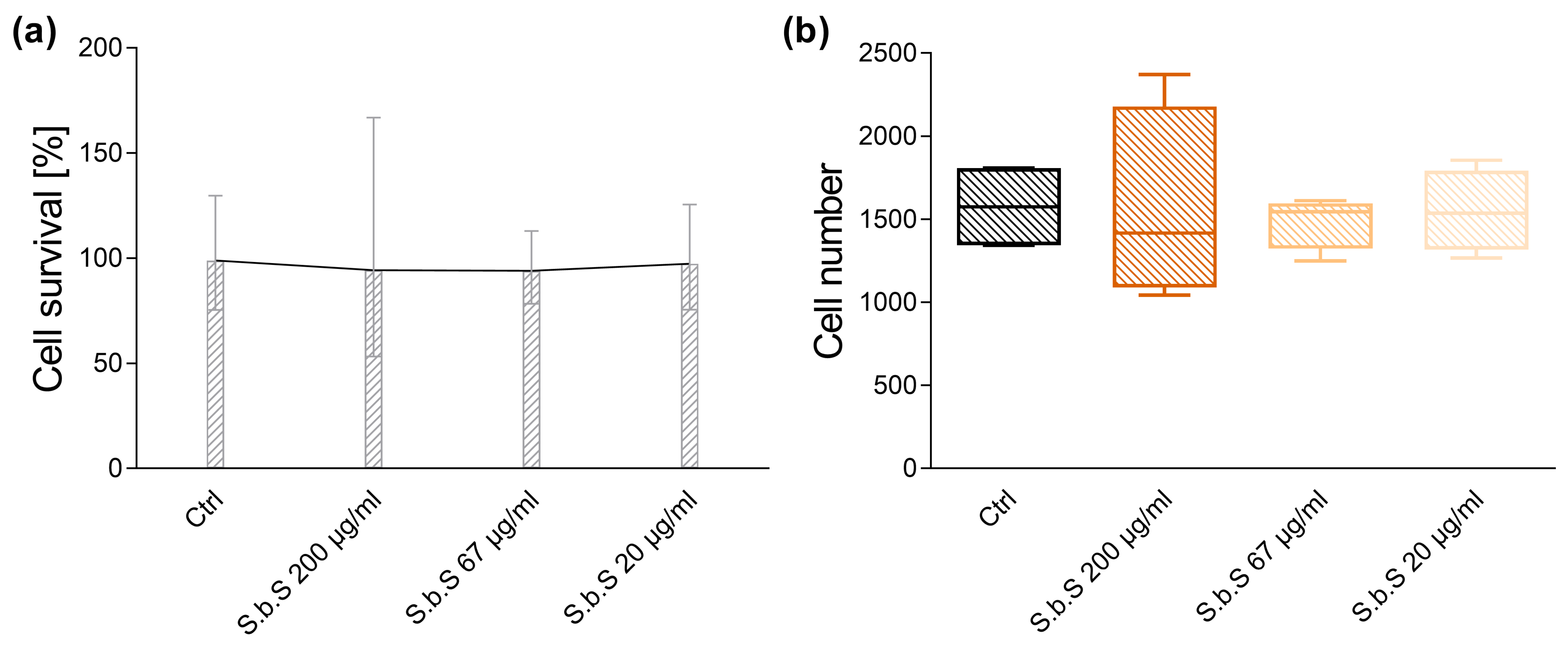
2.1. Determination of S.b.S Concentration
2.2. S.b.S Improves GFRed- and LPS-Dependent Disturbances of GI Barrier Function
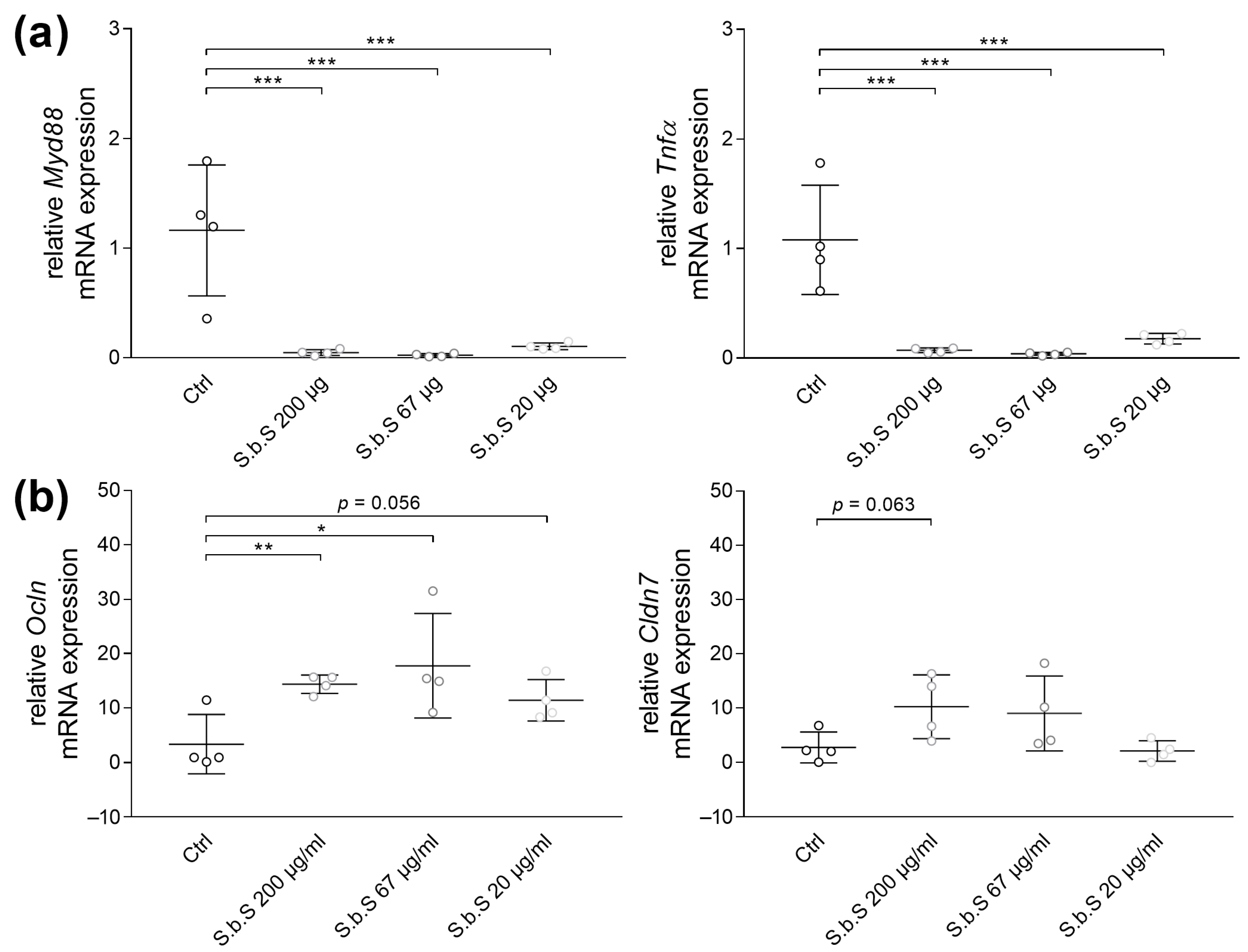
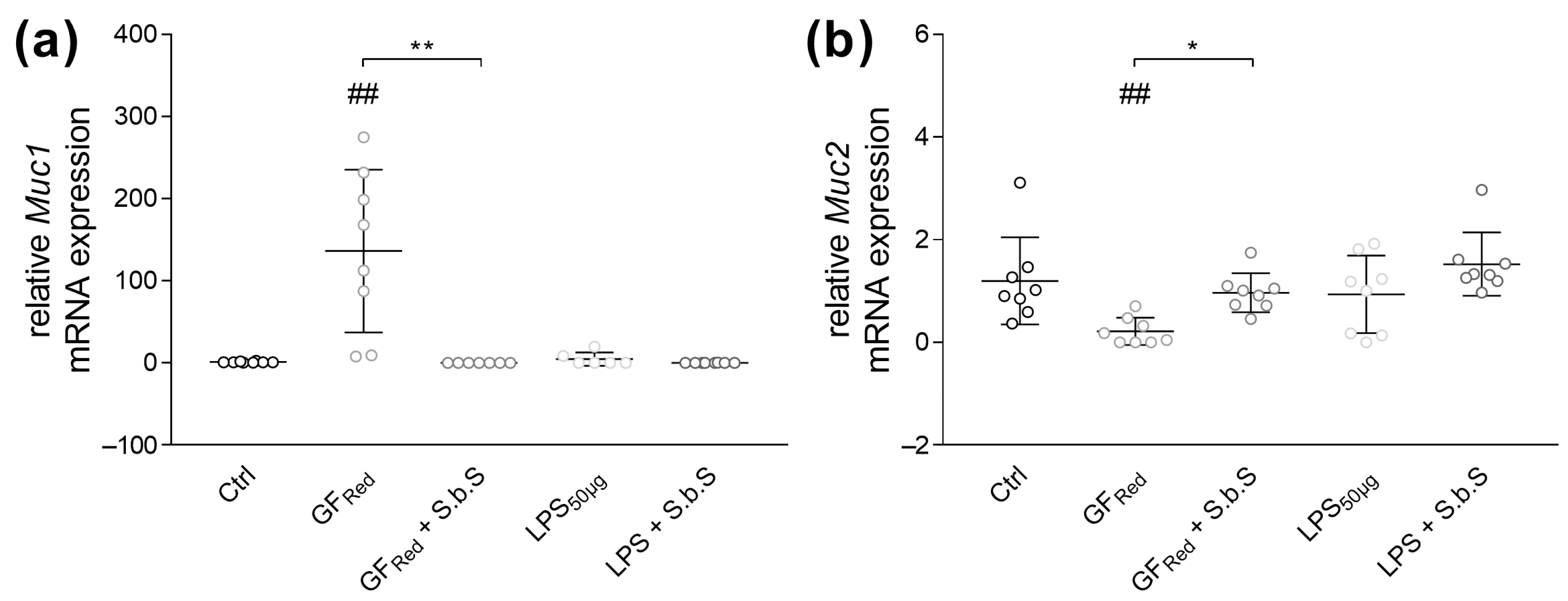
2.2.1. S.b.S Exposition Regulates Stress-Induced Changes in TJ and Muc Transcripts Expression
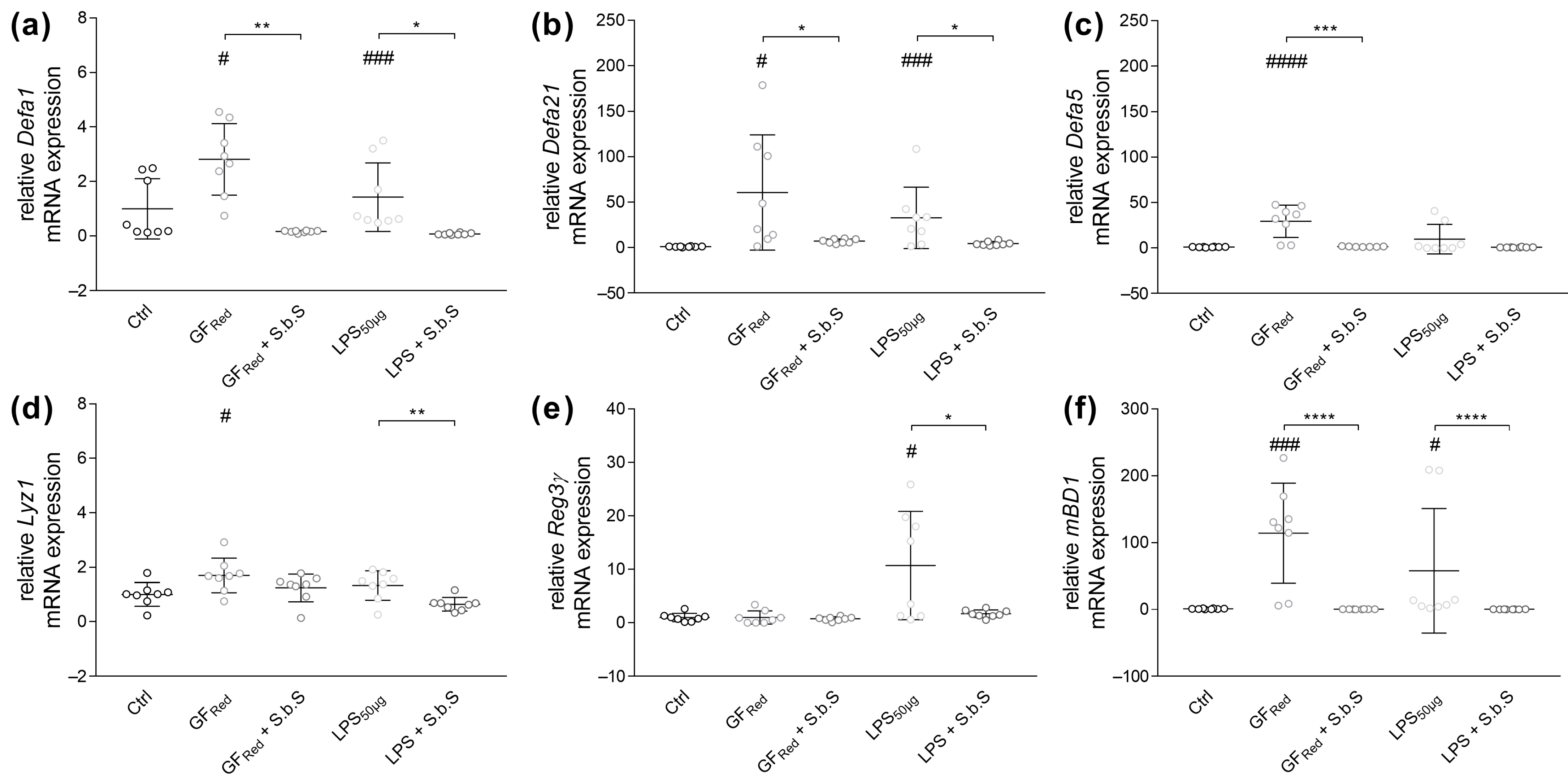
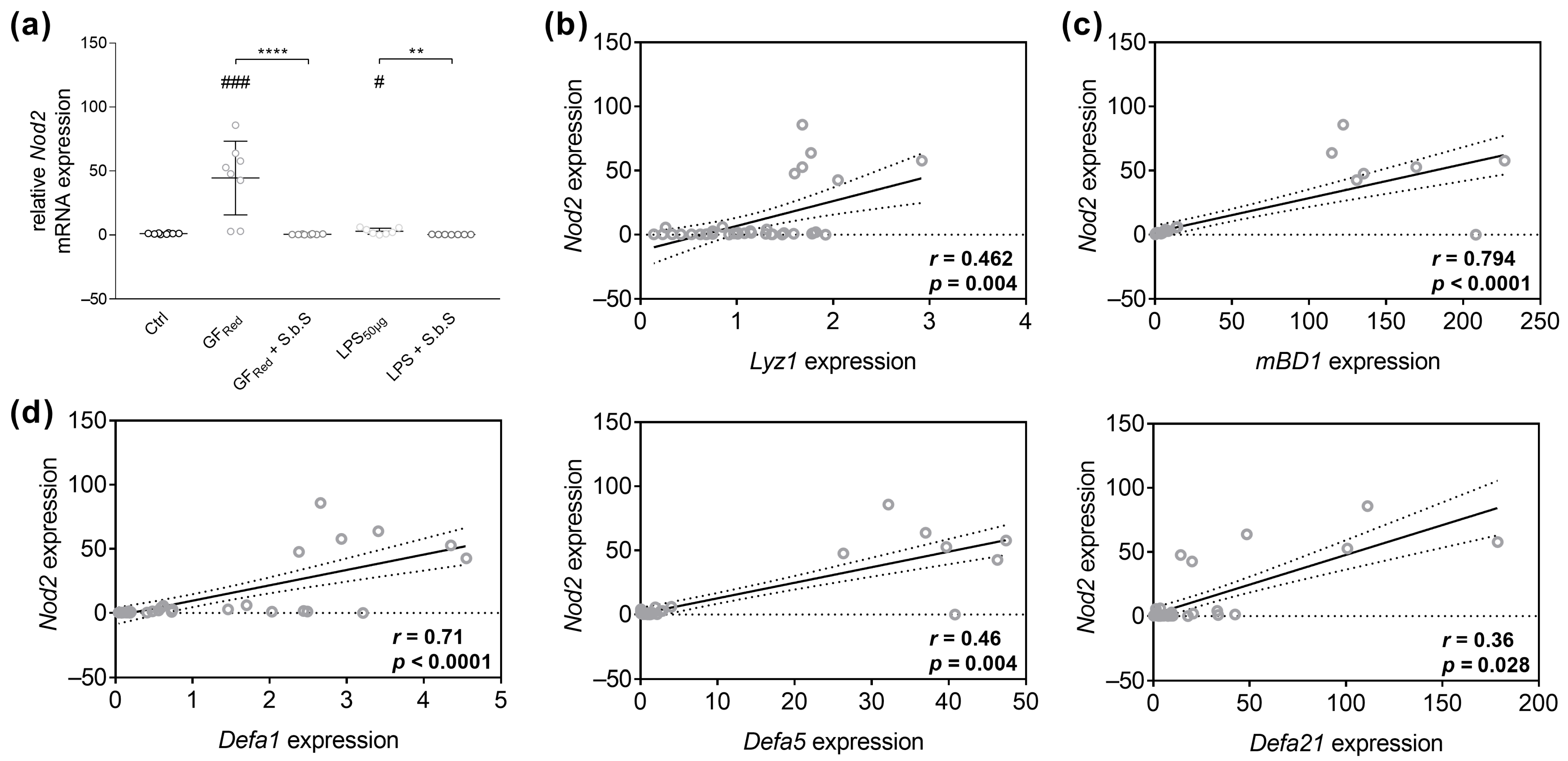
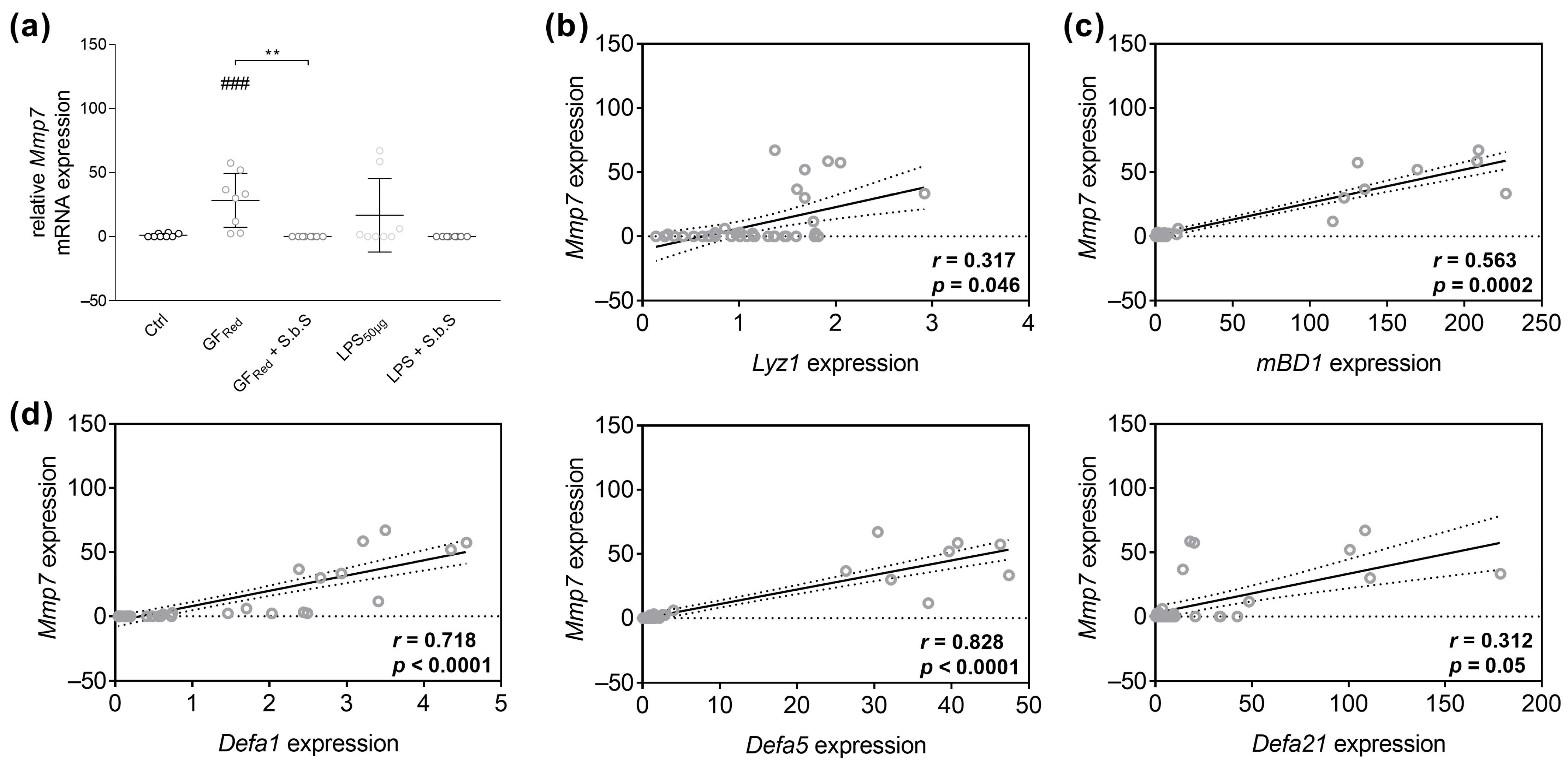
2.2.2. S.b.S Normalizes Antimicrobial Peptide Defense in GFRed- and LPS-Treated Organoids
2.3. S.b.S Reduces Myd88 and Proinflammatory Cytokine Transcript Expression in Stressed Organoids
3. Discussion
4. Materials and Methods
4.1. Generation of Saccharomyces boulardii Supernatant
4.2. BCA Protein Assay
4.3. Organoid Cell Culture
4.3.1. Isolation and Cultivation
4.3.2. Media Change and Cell Passage
4.4. Dose-Finding Studies
4.4.1. MTT-Assay
4.4.2. RT-PCR
4.5. Stimulation
4.6. RNA Isolation, Generation of Standard Plasmids, and RT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S.b.S | Saccharomyces boulardii supernatant |
| GI | Gastrointestinal |
| LPS | Lipopolysaccharide |
| GFRed | Cell culture medium with reduced growth factors |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| TJ | Tight junction |
| AJ | Adherent junction |
| Muc | Mucin |
| Ocln | Occludin |
| Cldn | Claudin |
| ZO-1 | Zonula occludens 1 |
| JAM-A | Junctional adhesion molecule A |
| Nod | Nucleotide binding oligomerization domain |
| Mmp7 | Matrix metalloproteinase-7 |
| Myd88 | Myeloid differentiation primary response 88 |
| IL | Interleukin |
| Tnfα | Tumor necrosis factor α |
| S. boulardii | Saccharomyces boulardii |
| C. difficile | Clostridioides difficile |
| E. coli | Escherichia coli |
| IBD | Inflammatory bowel disease |
| AMP | Antimicrobial peptide |
| CD | Crohn’s disease |
| Ctrl | RPMI control |
| ANOVA | One-way analysis of variance |
| SEM | Standard error of the mean |
| Defa | α-defensin |
| Lyz1 | Lysozyme |
| mBD1 | Murine β-defensin 1 |
| Reg3γ | Regenerating islet-derived protein 3 gamma |
| PRR | Pattern recognition receptor |
| TLR | Toll-like receptor |
| EPEC | Enteropathogenic Escherichia coli |
| EHEC | Enterohemorrhagic Escherichia coli |
| hBD | Human β-defensin |
| NF-κB | Nuclear factor k-light-chain-enhancer of activated B cells |
| TNBS | 2,4,6-Trinitrobenzenesulfonic acid |
| DSS | Dextran sulfate sodium |
| SCFA | Short chain fatty acid |
| UC | Ulcerative colitis |
| DC | Dendritic cell |
| mDC | Myeloid DC |
| IFN-γ | Interferon-γ |
| RPMI | Roswell Park Memorial Institute |
| CIB | Crypt isolation buffer |
| CCM | Cell culture medium |
References
- Surawicz, C.M.; Elmer, G.W.; Speelman, P.; McFarland, L.V.; Chinn, J.; van Belle, G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: A prospective study. Gastroenterology 1989, 96, 981–988. [Google Scholar] [CrossRef]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am. J. Gastroenterol. 1995, 90, 439–448. [Google Scholar]
- Kotowska, M.; Albrecht, P.; Szajewska, H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: A randomized double-blind placebo-controlled trial. Aliment. Pharmacol. Ther. 2005, 21, 583–590. [Google Scholar] [CrossRef]
- Shan, L.-S.; Hou, P.; Wang, Z.-J.; Liu, F.-R.; Chen, N.; Shu, L.-H.; Zhang, H.; Han, X.-H.; Han, X.-X.; Cai, X.-X.; et al. Prevention and treatment of diarrhoea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Benef. Microbes 2013, 4, 329–334. [Google Scholar] [CrossRef]
- Efremova, I.; Maslennikov, R.; Zharkova, M.; Poluektova, E.; Benuni, N.; Kotusov, A.; Demina, T.; Ivleva, A.; Adzhieva, F.; Krylova, T.; et al. Efficacy and Safety of a Probiotic Containing Saccharomyces boulardii CNCM I-745 in the Treatment of Small Intestinal Bacterial Overgrowth in Decompensated Cirrhosis: Randomized, Placebo-Controlled Study. J. Clin. Med. 2024, 13, 919. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Effect of Saccharomyces boulardii on cAMP- and Ca2+ -dependent Cl- secretion in T84 cells. Dig. Dis. Sci. 1999, 44, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Sandré, C.; Barc, M.-C. Saccharomyces boulardii modulates dendritic cell properties and intestinal microbiota disruption after antibiotic treatment. Gastroenterol. Clin. Biol. 2010, 34, S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Przesdzing, I.; Metzke, D.; Schmitz, J.; Radbruch, A.; Baumgart, D.C. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin. Exp. Immunol. 2009, 156, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Metzke, D.; Schmitz, J.; Dörffel, Y.; Baumgart, D.C. Anti-inflammatory effects of Saccharomyces boulardii mediated by myeloid dendritic cells from patients with Crohn’s disease and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1083–G1092. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Antonioli, L.; Benvenuti, L.; Pellegrini, C.; Di Salvo, C.; Calvigioni, M.; Panattoni, A.; Ryskalin, L.; Natale, G.; Banni, S.; et al. Use of Saccharomyces boulardii CNCM I-745 as therapeutic strategy for prevention of nonsteroidal anti-inflammatory drug-induced intestinal injury. Br. J. Pharmacol. 2023, 180, 3215–3233. [Google Scholar] [CrossRef]
- Martins, F.S.; Silva, A.A.; Vieira, A.T.; Barbosa, F.H.F.; Arantes, R.M.E.; Teixeira, M.M.; Nicoli, J.R. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch. Microbiol. 2009, 191, 623–630. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Y.; Zhang, Y.; Hamza, T.; Yu, H.; Saint Fleur, A.; Galen, J.; Yang, Z.; Feng, H. A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Sci. Transl. Med. 2020, 12, eaax4905. [Google Scholar] [CrossRef]
- Castagliuolo, I.; LaMont, J.T.; Nikulasson, S.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect. Immun. 1996, 64, 5225–5232. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Floyd, J.L.; Prasad, R.; Dupont, M.D.; Adu-Rutledge, Y.; Anshumali, S.; Paul, S.; Li Calzi, S.; Qi, X.; Malepati, A.; Johnson, E.; et al. Intestinal neutrophil extracellular traps promote gut barrier damage exacerbating endotoxaemia, systemic inflammation and progression of diabetic retinopathy in type 2 diabetes. Diabetologia 2025, 68, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Weber, S.; Vos, M.; Krämer, S.; Volynets, V.; Kaserouni, S.; McClain, C.J.; Bischoff, S.C. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: Role of endotoxin. J. Hepatol. 2008, 48, 983–992. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Xu, C.; Hao, M.; Li, H.; Ding, L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology 2021, 10, 1923910. [Google Scholar] [CrossRef]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Volynets, V.; Küper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Königsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef]
- Ruple, H.K.; Haasis, E.; Bettenburg, A.; Maier, C.; Fritz, C.; Schüle, L.; Löcker, S.; Soltow, Y.; Schintgen, L.; Schmidt, N.S.; et al. The gut microbiota predicts and time-restricted feeding delays experimental colitis. Gut Microbes 2025, 17, 2453019. [Google Scholar] [CrossRef]
- Li, F.; Armet, A.M.; Korpela, K.; Liu, J.; Quevedo, R.M.; Asnicar, F.; Seethaler, B.; Rusnak, T.B.; Cole, J.L.; Zhang, Z.; et al. Cardiometabolic benefits of a non-industrialized-type diet are linked to gut microbiome modulation. Cell 2025, 188, 1226–1247.e18. [Google Scholar] [CrossRef]
- Men, X.; Shi, X.; Xu, Q.; Liu, M.; Yang, H.; Wang, L.; Men, X.; Xu, H. Exploring the pathogenesis of chronic atrophic gastritis with atherosclerosis via microarray data analysis. Medicine 2024, 103, e37798. [Google Scholar] [CrossRef]
- Filipe Rosa, L.; Petersen, P.P.; Görtz, L.F.; Stolzer, I.; Kaden-Volynets, V.; Günther, C.; Bischoff, S.C. Vitamin A- and D-Deficient Diets Disrupt Intestinal Antimicrobial Peptide Defense Involving Wnt and STAT5 Signaling Pathways in Mice. Nutrients 2023, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Filipe Rosa, L.; Rings, A.; Stolzer, I.; Koeninger, L.; Wehkamp, J.; Beisner, J.; Günther, C.; Nordkild, P.; Jensen, B.A.H.; Bischoff, S.C. Human α-Defensin 51-9 and Human β-Defensin 2 Improve Metabolic Parameters and Gut Barrier Function in Mice Fed a Western-Style Diet. Int. J. Mol. Sci. 2023, 24, 13878. [Google Scholar] [CrossRef]
- Wehkamp, J.; Chu, H.; Shen, B.; Feathers, R.W.; Kays, R.J.; Lee, S.K.; Bevins, C.L. Paneth cell antimicrobial peptides: Topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006, 580, 5344–5350. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; López-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Satchell, D.P.; Pesendorfer, P.; Tanabe, H.; Wilson, C.L.; Hagen, S.J.; Ouellette, A.J. Activation of Paneth cell alpha-defensins in mouse small intestine. J. Biol. Chem. 2002, 277, 5219–5228. [Google Scholar] [CrossRef]
- Weis, S.; King, I.L.; Vivas, W. To sense or not to sense, Paneth cell regulation of mucosal immunity. Cell Host Microbe 2024, 32, 1648–1650. [Google Scholar] [CrossRef]
- Teltschik, Z.; Wiest, R.; Beisner, J.; Nuding, S.; Hofmann, C.; Schoelmerich, J.; Bevins, C.L.; Stange, E.F.; Wehkamp, J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology 2012, 55, 1154–1163. [Google Scholar] [CrossRef]
- Dahan, S.; Dalmasso, G.; Imbert, V.; Peyron, J.-F.; Rampal, P.; Czerucka, D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect. Immun. 2003, 71, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Vallance, B.A.; Boyer, L.; Bergstrom, K.S.B.; Walker, J.; Madsen, K.; O’Kusky, J.R.; Buchan, A.M.; Jacobson, K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G295–G306. [Google Scholar] [CrossRef] [PubMed]
- Garcia Vilela, E.; Lourdes Abreu Ferrari, M.d.; Da Oswaldo Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Da Sales Cunha, A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef]
- Fan, S.; Weight, C.M.; Luissint, A.-C.; Hilgarth, R.S.; Brazil, J.C.; Ettel, M.; Nusrat, A.; Parkos, C.A. Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation. Mol. Biol. Cell 2019, 30, 566–578. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Kakogiannos, N.; Ferrari, L.; Giampietro, C.; Scalise, A.A.; Maderna, C.; Ravà, M.; Taddei, A.; Lampugnani, M.G.; Pisati, F.; Malinverno, M.; et al. JAM-A Acts via C/EBP-α to Promote Claudin-5 Expression and Enhance Endothelial Barrier Function. Circ. Res. 2020, 127, 1056–1073. [Google Scholar] [CrossRef]
- Johnson, A.H.; Frierson, H.F.; Zaika, A.; Powell, S.M.; Roche, J.; Crowe, S.; Moskaluk, C.A.; El-Rifai, W. Expression of tight-junction protein claudin-7 is an early event in gastric tumorigenesis. Am. J. Pathol. 2005, 167, 577–584. [Google Scholar] [CrossRef]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Nunes, D.P.; Troxler, R.F.; Offner, G.D. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J. Dent. Res. 2003, 82, 883–887. [Google Scholar] [CrossRef]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J. Gastroenterol. 2019, 25, 2188–2203. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Dahan, S.; Mograbi, B.; Rossi, B.; Rampal, P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect. Immun. 2000, 68, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Mumy, K.L.; Chen, X.; Kelly, C.P.; McCormick, B.A. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G599–G609. [Google Scholar] [CrossRef] [PubMed]
- Terciolo, C.; Dobric, A.; Ouaissi, M.; Siret, C.; Breuzard, G.; Silvy, F.; Marchiori, B.; Germain, S.; Bonier, R.; Hama, A.; et al. Saccharomyces boulardii CNCM I-745 Restores intestinal Barrier Integrity by Regulation of E-cadherin Recycling. J. Crohn’s Colitis 2017, 11, 999–1010. [Google Scholar] [CrossRef]
- Altınok, Ö.; Baş, M.; Gelenli Dolanbay, E.; Kolgazi, M.; Mert, T.; Uslu, Ü. Collagen Peptides and Saccharomyces boulardii CNCM I-745 Attenuate Acetic Acid-Induced Colitis in Rats by Modulating Inflammation and Barrier Permeability. Food Sci. Nutr. 2025, 13, e70189. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Sun, J.; Xu, H.; Wang, M.; Zuo, X.; Fu, Q.; Guo, Y.; Chen, Z.; Zhang, P.; et al. Saccharomyces boulardii Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating NF-κB and Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 1622375. [Google Scholar] [CrossRef]
- Jones, E.J.; Matthews, Z.J.; Gul, L.; Sudhakar, P.; Treveil, A.; Divekar, D.; Buck, J.; Wrzesinski, T.; Jefferson, M.; Armstrong, S.D.; et al. Integrative analysis of Paneth cell proteomic and transcriptomic data from intestinal organoids reveals functional processes dependent on autophagy. Dis. Models Mech. 2019, 12, dmm037069. [Google Scholar] [CrossRef]
- Wilson, S.S.; Tocchi, A.; Holly, M.K.; Parks, W.C.; Smith, J.G. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol. 2015, 8, 352–361. [Google Scholar] [CrossRef]
- Chelliah, R.; Kim, E.-J.; Daliri, E.B.-M.; Antony, U.; Oh, D.-H. In Vitro Probitotic Evaluation of Saccharomyces boulardii with Antimicrobial Spectrum in a Caenorhabditis elegans Model. Foods 2021, 10, 1428. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Naimah, A.K.; Al-Manhel, A.J.A.; Al-Shawi, M.J. Isolation, Purification and Characterization of Antimicrobial Peptides Produced from Saccharomyces boulardii. Int. J. Pept. Res. Ther. 2018, 24, 455–461. [Google Scholar] [CrossRef]
- Huang, F.-C.; Lu, Y.-T.; Liao, Y.-H. Beneficial effect of probiotics on Pseudomonas aeruginosa-infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation. Innate Immun. 2020, 26, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef] [PubMed]
- Šplíchal, I.; Fagerhol, M.K.; Trebichavský, I.; Šplíchalová, A.; Schulze, J. The effect of intestinal colonization of germ-free pigs with Escherichia coli on calprotectin levels in plasma, intestinal and bronchoalveolar lavages. Immunobiology 2005, 209, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, S.; Tao, H.; Grillo, A.; Elia, C.; Gasbarrini, G.; Sepulveda, A.R.; Gasbarrini, A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig. Liver Dis. 2005, 37, 320–329. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Liang, X.; Zhu, L.; Fang, Y.; Dong, L.; Zheng, Y.; Xu, X.; Li, M.; Cai, T.; et al. A decrease in Flavonifractor plautii and its product, phytosphingosine, predisposes individuals with phlegm-dampness constitution to metabolic disorders. Cell Discov. 2025, 11, 25. [Google Scholar] [CrossRef]
- Plein, K.; Hotz, J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn’s disease with special respect to chronic diarrhea--a pilot study. Z. Gastroenterol. 1993, 31, 129–134. [Google Scholar]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P.A. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef]
- Guslandi, M.; Giollo, P.; Testoni, P.A. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 697–698. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Feizizadeh, S.; Salehi-Abargouei, A.; Akbari, V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics 2014, 134, e176–e191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, H.-J.; Guan, L.; Zhang, Y.-N.; Li, Y.; Sun, M.-J. Mechanism and therapeutic effects of Saccharomyces boulardii on experimental colitis in mice. Mol. Med. Rep. 2018, 18, 5652–5662. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Zhao, Q.; Mo, Q.; Liu, T.; Cao, H. Saccharomyces boulardii Alleviates Colitis by Regulating FXR-NLRP3 Mediated Macrophage Pyroptosis. J. Inflamm. Res. 2025, 18, 3161–3176. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, Y.W.; Chi, S.-G.; Joo, Y.-S.; Kim, H.J. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig. Dis. Sci. 2009, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, H.; Shi, L.; Li, R.; Luo, Y.; Deng, Y.; Li, S.; Li, R.; Liu, Z. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 2022, 13, 102–112. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhou, T.; Wu, J.; Feng, F.; Wang, S.; Chi, Q.; Sha, Y.; Zha, S.; Shu, S.; et al. Gut microbiota-derived butyric acid regulates calcific aortic valve disease pathogenesis by modulating GAPDH lactylation and butyrylation. iMeta 2025, e70048. [Google Scholar] [CrossRef]
- Dalmasso, G.; Cottrez, F.; Imbert, V.; Lagadec, P.; Peyron, J.-F.; Rampal, P.; Czerucka, D.; Groux, H.; Foussat, A.; Brun, V. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology 2006, 131, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]

| Myd88 | Tnfα | IL-6 | IL-1β | |
|---|---|---|---|---|
| Ctrl | 2.44 ± 0.71 | 1.49 ± 0.28 | 1.21 ± 0.22 | 4.41 ± 1.72 |
| GFRed | 58.05 ± 12.69 ### | 46.58 ± 12.02 ### | 134.4 ± 36.97 ## | 66.39 ± 16.45 ### |
| GFRed ± S.b.S | 11.76 ± 2.98 ** | 0.3 ± 0.1 *** | 0.79 ± 0.37 ** | 5.88 ± 1.22 *** |
| LPS | 23.05 ± 14.18 | 20.87 ± 12.37 | 42.24 ± 25.97 | 42.8 ± 28.17 |
| LPS ± S.b.S | 14.5 ± 1.8 $ | 0.6 ± 0.17 | 0.79 ± 0.13 $ | 4.38 ± 0.61 |
| CCM | GFRed | |
|---|---|---|
| GlutaMaxTM | 2 mM | 2 mM |
| Hepes | 10 mM | 10 mM |
| R-Spondin | 1 µg/mL | 0.5 µg/mL |
| Noggin | 100 ng/µL | 50 ng/µL |
| B-27™ supplement | 20 µL/mL | 20 µL/mL |
| N-Acetylcysteine | 1.63 mg/mL | 1.63 mg/mL |
| Primocin | 0.1 mg/mL | 0.1 mg/mL |
| mEGF | 50 ng/mL | 50 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipe Rosa, L.; Gonda, S.; Roese, N.; Bischoff, S.C. Saccharomyces boulardii CNCM I-745 Supernatant Improves Markers of Gut Barrier Function and Inflammatory Response in Small Intestinal Organoids. Pharmaceuticals 2025, 18, 1167. https://doi.org/10.3390/ph18081167
Filipe Rosa L, Gonda S, Roese N, Bischoff SC. Saccharomyces boulardii CNCM I-745 Supernatant Improves Markers of Gut Barrier Function and Inflammatory Response in Small Intestinal Organoids. Pharmaceuticals. 2025; 18(8):1167. https://doi.org/10.3390/ph18081167
Chicago/Turabian StyleFilipe Rosa, Louisa, Steffen Gonda, Nadine Roese, and Stephan C. Bischoff. 2025. "Saccharomyces boulardii CNCM I-745 Supernatant Improves Markers of Gut Barrier Function and Inflammatory Response in Small Intestinal Organoids" Pharmaceuticals 18, no. 8: 1167. https://doi.org/10.3390/ph18081167
APA StyleFilipe Rosa, L., Gonda, S., Roese, N., & Bischoff, S. C. (2025). Saccharomyces boulardii CNCM I-745 Supernatant Improves Markers of Gut Barrier Function and Inflammatory Response in Small Intestinal Organoids. Pharmaceuticals, 18(8), 1167. https://doi.org/10.3390/ph18081167







