In Vitro Proof-of-Concept Study: Lidocaine and Epinephrine Co-Loaded in a Mucoadhesive Liquid Crystal Precursor System for Topical Oral Anesthesia
Abstract
1. Introduction
2. Results
2.1. Development and Structural Characterization of Formulations
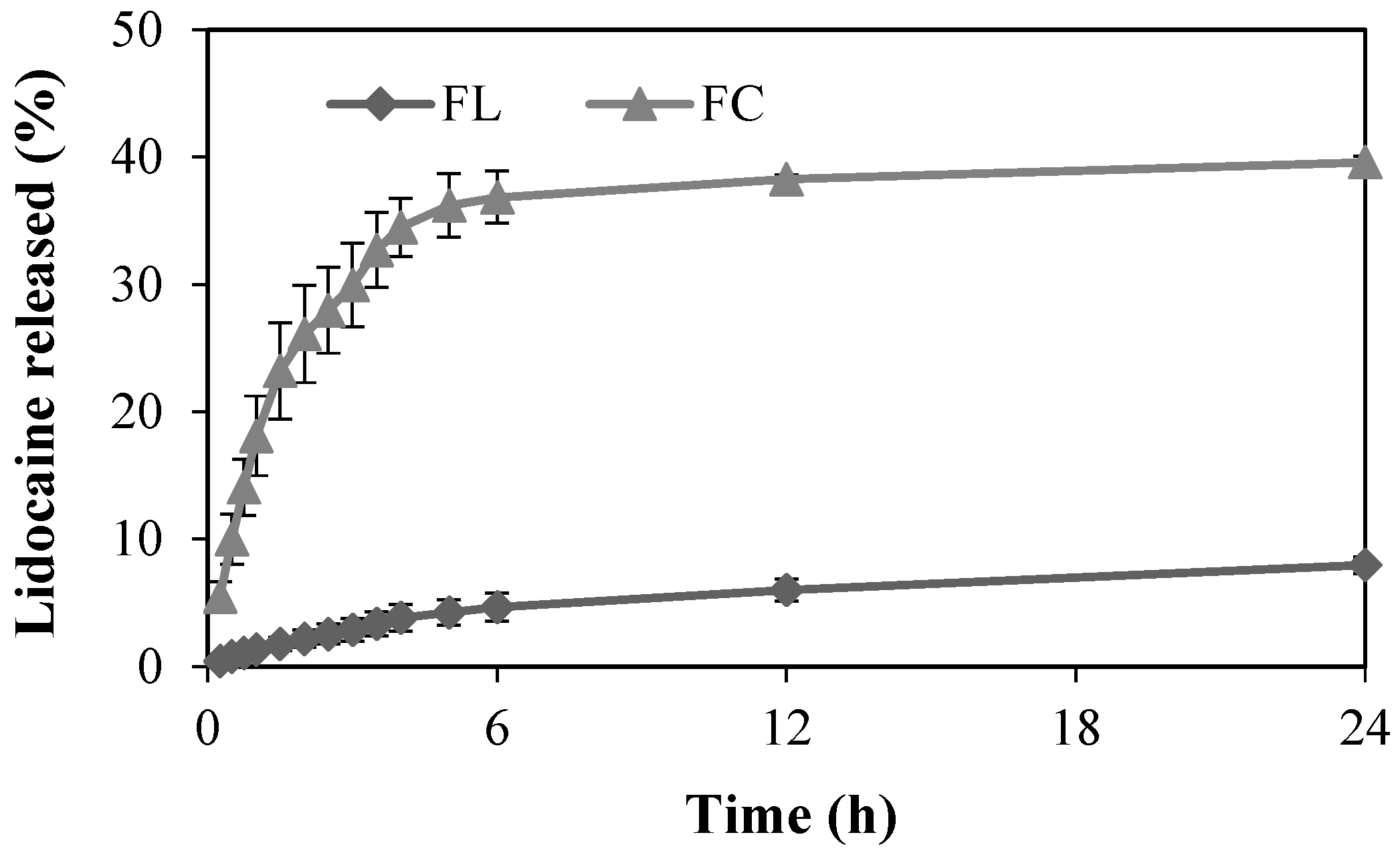
2.2. In Vitro Release Study
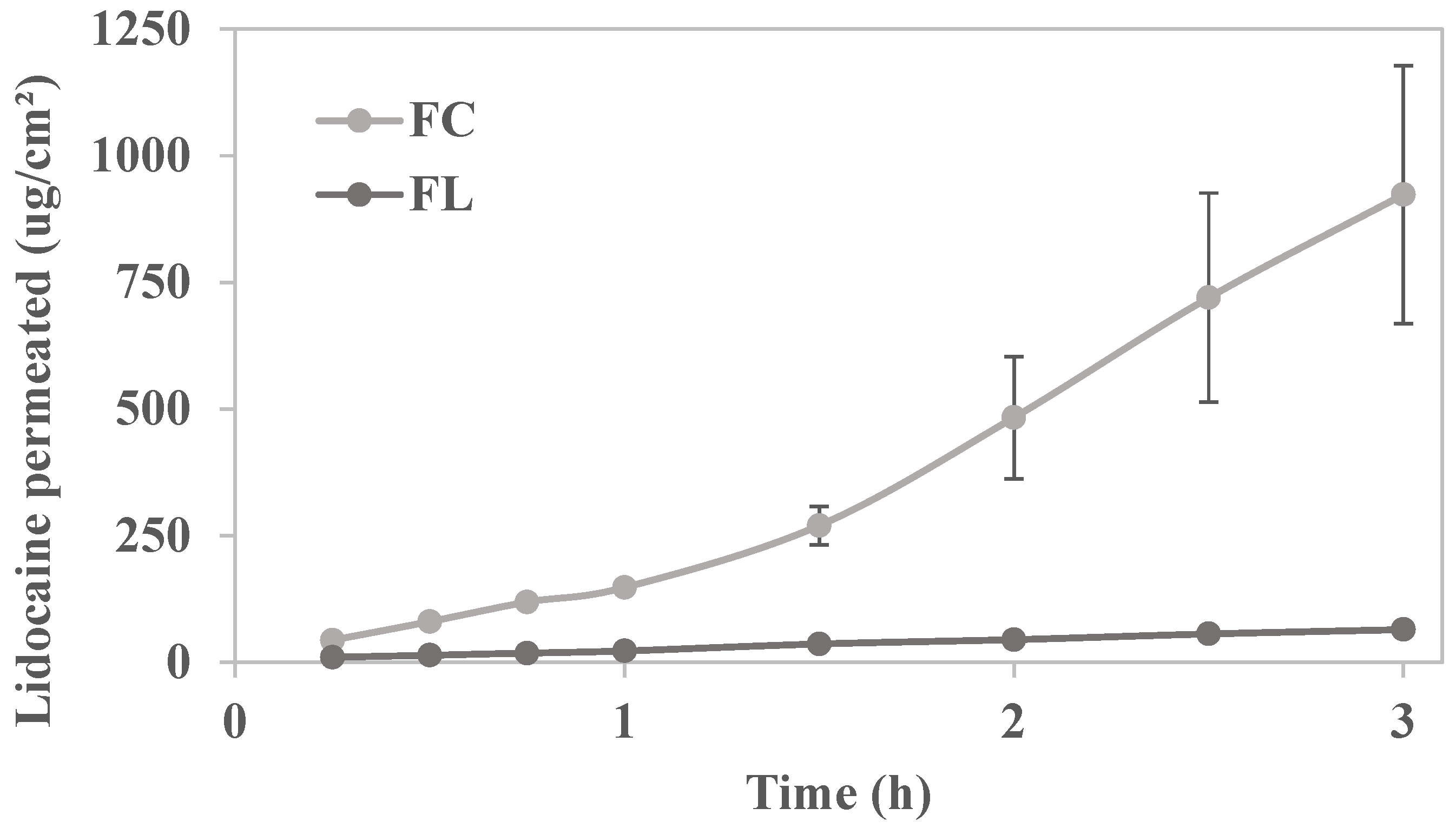
2.3. In Vitro Permeation Study
2.4. In Vivo Chicken Chorioallantoic Membrane (CAM) Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Liquid Crystal Precursor System
4.3. Structural Characterization of Formulations
4.3.1. Polarized Light Microscopy (PLM)
4.3.2. Small-Angle X-Ray Scattering (SAXS)
4.3.3. Rheological Analysis
- Continuous rheological analysis
- Oscillatory rheological analysis
4.4. Texture Profile Analysis
4.5. In Vitro Evaluation of the Mucoadhesive Force
4.6. In Vitro Release Study
4.7. In Vitro Permeation Study
4.8. In Vivo Chicken Chorioallantoic Membrane (CAM) Toxicity Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.-Z.; Shi, R.-J.; Ke, B.-W.; Tang, Y.-L.; Liang, X.-H. Paresthesia in Dentistry: The Ignored Neurotoxicity of Local Anesthetics. Heliyon 2023, 9, e18031. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Villines, D.C.; Baker, S. Efficacy of a Glycopolymer-Based Oral Rinse Upon Pain Associated with Ulcerative and Erosive Lesions of the Oral Mucosa: A within-Subject Pilot Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S.F. Handbook of Local Anesthesia, 7e: South Asia Edition-E-Book; Elsevier: Gurgaon, India, 2019. [Google Scholar]
- Akimoto, T.; Hashimoto, S.; Sunada, K. Dexmedetomidine (12.5 Μg/Ml) Improves Tissue Distribution, Anesthetic Action, and Hemodynamic Effects of Lidocaine after Palatal Infiltration in Rats. Odontology 2016, 104, 390–396. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Ribeiro, L.N.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; de Araujo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent Advances and Perspectives in Topical Oral Anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P. Scientific Evidence on the Links between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Peng, C.-H.; Yang, Y.-S.; Chan, K.-C.; Kornelius, E.; Chiou, J.-Y.; Huang, C.-N. Periodontal Treatment and the Risks of Cardiovascular Disease in Patients with Type 2 Diabetes: A Retrospective Cohort Study. Intern. Med. 2017, 56, 1015–1021. [Google Scholar] [CrossRef]
- Wambier, L.M.; de Geus, J.L.; Boing, T.F.; Chibinski, A.C.R.; Wambier, D.S.; Rego, R.O.; Loguercio, A.D.; Reis, A. Intrapocket Topical Anesthetic Versus Injected Anesthetic for Pain Control During Scaling and Root Planing in Adult Patients: Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2017, 148, 814–824.e2. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Serpe, L.; Martinelli, C.C.M.; da Silva, C.B.; Dos Santos, C.P.; Novaes, P.D.; Volpato, M.C.; de Paula, E.; Lopez, R.F.V.; Groppo, F.C. Evaluation of Different Pig Oral Mucosa Sites as Permeability Barrier Models for Drug Permeation Studies. Eur. J. Pharm. Sci. 2016, 81, 52–59. [Google Scholar] [CrossRef]
- Jimson, S.; Ranjani, S.S.; Lenka, S.; Jimson, S. Comparative Effects of Clonidine and Adrenaline with Lignocaine During Maxillary Infiltration Anaesthesia for Dental Extraction. J. Clin. Diagn. Res. JCDR 2015, 9, ZC85. [Google Scholar] [CrossRef]
- Boyce, R.A.; Kirpalani, T.; Mohan, N. Updates of Topical and Local Anesthesia Agents. Dent. Clin. 2016, 60, 445–471. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Xylocaine®® (Lidocaine Hydrochloride and Epinephrine) Injection, Usp—Prescribing Information; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2004. [Google Scholar]
- European Medicines Agency (EMA). Guideline on Clinical Development of Fixed Combination Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2017; Ema/Chmp/158268/2017. [Google Scholar]
- Relação Nacional de Medicamentos Essenciais; RENAME, Ministério da Saúde (Brasil): Brasilia, Brazil, 2024.
- Becker, D.E.; Reed, K.L. Local Anesthetics: Review of Pharmacological Considerations. Anesth. Prog. 2012, 59, 90–102. [Google Scholar] [CrossRef]
- Kalra, G.; Makkar, S.; Menrai, N.; Kalia, V.; Suri, N.; Gupta, S. Comparative Evaluation of the Efficacy and Onset of Local Anesthesia Using Buffered 2% Lidocaine with 1: 100,000 Adrenaline, Non-Buffered 2% Lidocaine with 1: 100,000 Adrenaline, Buffered 4% Articaine with 1: 100,000 Adrenaline and Non-Buffered 4% Articaine with 1: 100,000 Adrenaline in Dental Extraction. J. Maxillofac. Oral Surg. 2024, 23, 1255–1260. [Google Scholar] [PubMed]
- Depieri, L.V.; Borgheti-Cardoso, L.N.; Campos, P.M.; Otaguiri, K.K.; de Carvalho Vicentini, F.T.M.; Lopes, L.B.; Fonseca, M.J.V.; Bentley, M.V.L.B. Rnai Mediated Il-6 in Vitro Knockdown in Psoriasis Skin Model with Topical Sirna Delivery System Based on Liquid Crystalline Phase. Eur. J. Pharm. Biopharm. 2016, 105, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Borgheti-Cardoso, L.N.; Depieri, L.V.; Kooijmans, S.A.; Diniz, H.; Calzzani, R.A.; Vicentini, F.T.; van der Meel, R.; Fantini, M.C.; Iyomasa, M.M.; Schiffelers, R.M.; et al. An in Situ Gelling Liquid Crystalline System Based on Monoglycerides and Polyethylenimine for Local Delivery of Sirnas. Eur. J. Pharm. Sci. 2015, 74, 103–117. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W. Liquid Crystalline Phases for Enhancement of Oral Bioavailability. AAPS PharmSciTech 2021, 22, 81. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and Chitosan Polymer-Based Mucoadhesive Liquid Crystalline Systems Intended for Buccal Drug Delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Franz-Montan, M.; Baltazar, F.; Chorilli, M. Innovative Mucoadhesive Precursor of Liquid Crystalline System Loading Anti-Gellatinolytic Peptide for Topical Treatment of Oral Cancer. J. Biomed. Nanotechnol. 2021, 17, 253–262. [Google Scholar] [CrossRef]
- Balian, G.M.F.C.; Luiz, M.T.; Di Filippo, L.D.; Chorilli, M. Mucoadhesive Liquid Crystal Precursor System for Photodynamic Therapy of Oral Cancer Mediated by Methylene Blue. Photodiagnosis Photodyn. Ther. 2023, 44, 103739. [Google Scholar] [CrossRef]
- Rodero, C.F.; Fioramonti Calixto, G.M.; Cristina Dos Santos, K.; Sato, M.R.; Aparecido Dos Santos Ramos, M.; Miro, M.S.; Rodriguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef]
- Amin, M.K.; Boateng, J.S. Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery. Mar. Drugs 2022, 20, 156. [Google Scholar] [CrossRef]
- Victorelli, F.D.; Calixto, G.M.F.; dos Santos, K.C.; Buzza, H.H.; Chorilli, M. Curcumin-Loaded Polyethyleneimine and Chitosan Polymer-Based Mucoadhesive Liquid Crystalline Systems as a Potential Platform in the Treatment of Cervical Cancer. J. Mol. Liq. 2021, 325, 115080. [Google Scholar] [CrossRef]
- Stribeck, N. Saxs Data Analysis of a Lamellar Two-Phase System. Layer Statistics and Compansion. Colloid Polym. Sci. 1993, 271, 1007–1023. [Google Scholar] [CrossRef]
- ECVAM; EURL. DB-ALM Method Summary No 96: Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM); ECVAM: Ispra, Italy, 2010. [Google Scholar]
- ECVAM. DB-ALM and Protocol No. 96: Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM). In EURL ECVAM DB-ALM: Protocol; EURL ECVAM DB-ALM: Ispra, Italy, 2019. [Google Scholar]
- Lenze, M.; Benedetti, M.D.; Roco, J.; Ramírez, P.G.; Blanco, R.; Yaceszen, S.; Corrales, C.; Wikinski, S.; Gutiérrez, M.L. Advancing Ocular Safety Research: A Comprehensive Examination of Benzocaine Acute Exposure without Animal Testing. Toxicol. Lett. 2024, 394, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes in-Situ Gel for Ocular Delivery: Optimization, in-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Veerman, E.C.; Nieuw Amerongen, A.V. Biochemical Composition of Human Saliva in Relation to Other Mucosal Fluids. Crit. Rev. Oral Biol. Med. 1995, 6, 161–175. [Google Scholar] [CrossRef]
- Marena, G.D.; Fonseca-Santos, B.; dos Santos, R.M.A.; dos Santos, K.C.; Bauab, T.M.; Chorilli, M. Incorporation of Ursolic Acid in Liquid Crystalline Systems Improves the Antifungal Activity against Candida sp. J. Pharm. Innov. 2021, 16, 576–586. [Google Scholar] [CrossRef]
- de Oliveira, R.N.; Campos, P.M.; Pinto, R.M.C.; Mioduski, J.; Santos, R.D.; Justus, B.; de Paula, J.d.F.P.; Klein, T.; Boscardin, P.M.D.; Corrêa, S.d.A.P. The Promising Antischistosomal Activity of Oleic Acid-Loaded Polymeric Nanocapsules for Oral Administration. J. Drug Deliv. Sci. Technol. 2021, 63, 102429. [Google Scholar] [CrossRef]
- Cereda, C.M.S.; Mecatti, D.S.; Papini, J.Z.B.; Bueno, D.V.; Franz-Montan, M.; Rocha, T.; Pedrazzoli Junior, J.; de Paula, E.; de Araújo, D.R.; Grillo, R. Bupivacaine in Alginate and Chitosan Nanoparticles: An in Vivo Evaluation of Efficacy, Pharmacokinetics, and Local Toxicity. J. Pain Res. 2018, 11, 683–691. [Google Scholar] [CrossRef]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. A Curcumin-Loaded Liquid Crystal Precursor Mucoadhesive System for the Treatment of Vaginal Candidiasis. Int. J. Nanomed. 2015, 10, 4815. [Google Scholar] [CrossRef]
- Parise, G.K.; Ferranti, K.N.; Grando, C.P. Sais Anestésicos Utilizados Na Odontologia: Revisão De Literatura. J. Oral Investig. 2017, 6, 75–84. [Google Scholar] [CrossRef]
- Yajurvedi, P.; Shah, N.; Mahajan, A.M.; Sanghvi, D.; Shah, Y. Comparative Evaluation of the Efficacy of Lidocaine with Adrenaline and Lidocaine with Clonidine in Maxillary Infiltration Anesthesia. Int. J. Innov. Res. Dent. Sci. 2017, 2, 174–181. [Google Scholar]
- Parish, H.G.; Morton, J.R.; Brown, J.C. A Systematic Review of Epinephrine Stability and Sterility with Storage in a Syringe. Allergy Asthma Clin. Immunol. 2019, 15, 7. [Google Scholar] [CrossRef]
- Mueller-Goymann, C.C.; Frank, S.G. Interaction of Lidocaine and Lidocaine-Hcl with the Liquid Crystal Structure of Topical Preparations. Int. J. Pharm. 1986, 29, 147–159. [Google Scholar] [CrossRef]
- Engström, S.; Engström, L. Phase Behaviour of the Lidocaine-Monoolein-Water System. Int. J. Pharm. 1992, 79, 113–122. [Google Scholar] [CrossRef]
- Cid, Y.P.; Pedrazzi, V.; de Sousa, V.P.; Pierre, M.B.R. In Vitro Characterization of Chitosan Gels for Buccal Delivery of Celecoxib: Influence of a Penetration Enhancer. AAPS PharmSciTech 2012, 13, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.M.D.; Gremiao, M.P.D.; Cury, B.S.F. Mucin-Polysaccharide Interactions: A Rheological Approach to Evaluate the Effect of Ph on the Mucoadhesive Properties. Int. J. Biol. Macromol. 2020, 149, 234–245. [Google Scholar] [CrossRef]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive Systems Composed of Poloxamer 407 and Hpmc or Nacmc: Mechanical, Rheological and Sol-Gel Transition Analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Batistuzzo, J.; Itaya, M.; Eto, Y. Formulário Médico Farmacêutico, 3rd ed.; Editora Pharmabooks: São Paulo, Brazil, 2006; p. 585. [Google Scholar]
- Tamburic, S.; Craig, D.Q. A Comparison of Different in Vitro Methods for Measuring Mucoadhesive Performance. Eur. J. Pharm. Biopharm. 1997, 44, 159–167. [Google Scholar] [CrossRef]
- Collins, L.M.; Dawes, C. The Surface Area of the Adult Human Mouth and Thickness of the Salivary Film Covering the Teeth and Oral Mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A Food Perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Alawdi, S.; Solanki, A.B. Mucoadhesive Drug Delivery Systems: A Review of Recent Developments. J. Sci. Res. Med. Biol. Sci. 2021, 2, 50–64. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Campos, M.L.; Peccinini, R.G.; Gremião, M.P.D. Nasal Administration of Liquid Crystal Precursor Mucoadhesive Vehicle as an Alternative Antiretroviral Therapy. Eur. J. Pharm. Biopharm. 2013, 84, 219–227. [Google Scholar] [CrossRef]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; de Morais Ribeiro, L.N.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C. Hybrid Hydrogel Composed of Polymeric Nanocapsules Co-Loading Lidocaine and Prilocaine for Topical Intraoral Anesthesia. Sci. Rep. 2018, 8, 5005. [Google Scholar] [CrossRef]
- Barboza, A.d.S.; Ribeiro de Andrade, J.S.; Ferreira, M.L.; Peña, C.L.D.; da Costa, J.S.; Fajardo, A.R.; Lund, R.G. Propolis Controlled Delivery Systems for Oral Therapeutics in Dental Medicine: A Systematic Review. Dent. J. 2023, 11, 162. [Google Scholar] [CrossRef]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef]
- Emileigh, G.; Vought, K.; Patel, K.; Suzuki, H.; Usuda, K.; Shiramizu, A.; Koplowitz, L.P.; Koplowitz, B.; Maibach, H.I.; Lissin, D. Biorelevant in Vitro Skin Permeation Testing and in Vivo Pharmacokinetic Characterization of Lidocaine from a Nonaqueous Drug-in-Matrix Topical System. AAPS PharmSciTech 2021, 22, 215. [Google Scholar]
- Li, W.-Q.; Wu, J.-Y.; Xiang, D.-X.; Luo, S.-L.; Hu, X.-B.; Tang, T.-T.; Sun, T.-L.; Liu, X.-Y. Micelles Loaded with Puerarin and Modified with Triphenylphosphonium Cation Possess Mitochondrial Targeting and Demonstrate Enhanced Protective Effect against Isoprenaline-Induced H9c2 Cells Apoptosis. Int. J. Nanomed. 2019, 18, 8345–8360. [Google Scholar] [CrossRef]
- Garcia, M.T.J.; de Vasconcellos, F.L.L.; Raffier, C.P.; Roberts, M.S.; Grice, J.E.; Benson, H.A.E.; Leite-Silva, V.R. Alternative Methods to Animal Studies for the Evaluation of Topical/Transdermal Drug Delivery Systems. Curr. Top. Med. Chem. 2018, 18, 287–299. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.A.C. Avaliação Da Toxicidade De Anestésicos Tópicos De Uso Odontológico Por Meio Do Modelo Da Membrana Corioalantóica Do Embrião De Galinha. Master’s Dissertation, Universidade Estadual de Campinas (UNICAMP), Piracicaba, SP, Brazil, 2023. Available online: https://hdl.handle.net/20.500.12733/13469 (accessed on 28 July 2025).
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Jones, D.; Woolfson, A.; Brown, A. Textural, Viscoelastic and Mucoadhesive Properties of Pharmaceutical Gels Composed of Cellulose Polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; O’Neill, M.J. Mucoadhesive, Syringeable Drug Delivery Systems for Controlled Application of Metronidazole to the Periodontal Pocket: In Vitro Release Kinetics, Syringeability, Mechanical and Mucoadhesive Properties. J. Control. Release 1997, 49, 71–79. [Google Scholar] [CrossRef]
- de Araujo, J.S.M.; Volpato, M.C.; Muniz, B.V.; Xavier, G.G.A.; Martinelli, C.C.M.; Lopez, R.F.V.; Groppo, F.C.; Franz-Montan, M. Resistivity Technique for the Evaluation of the Integrity of Buccal and Esophageal Epithelium Mucosa for in Vitro Permeation Studies: Swine Buccal and Esophageal Mucosa Barrier Models. Pharmaceutics 2021, 13, 643. [Google Scholar] [CrossRef]
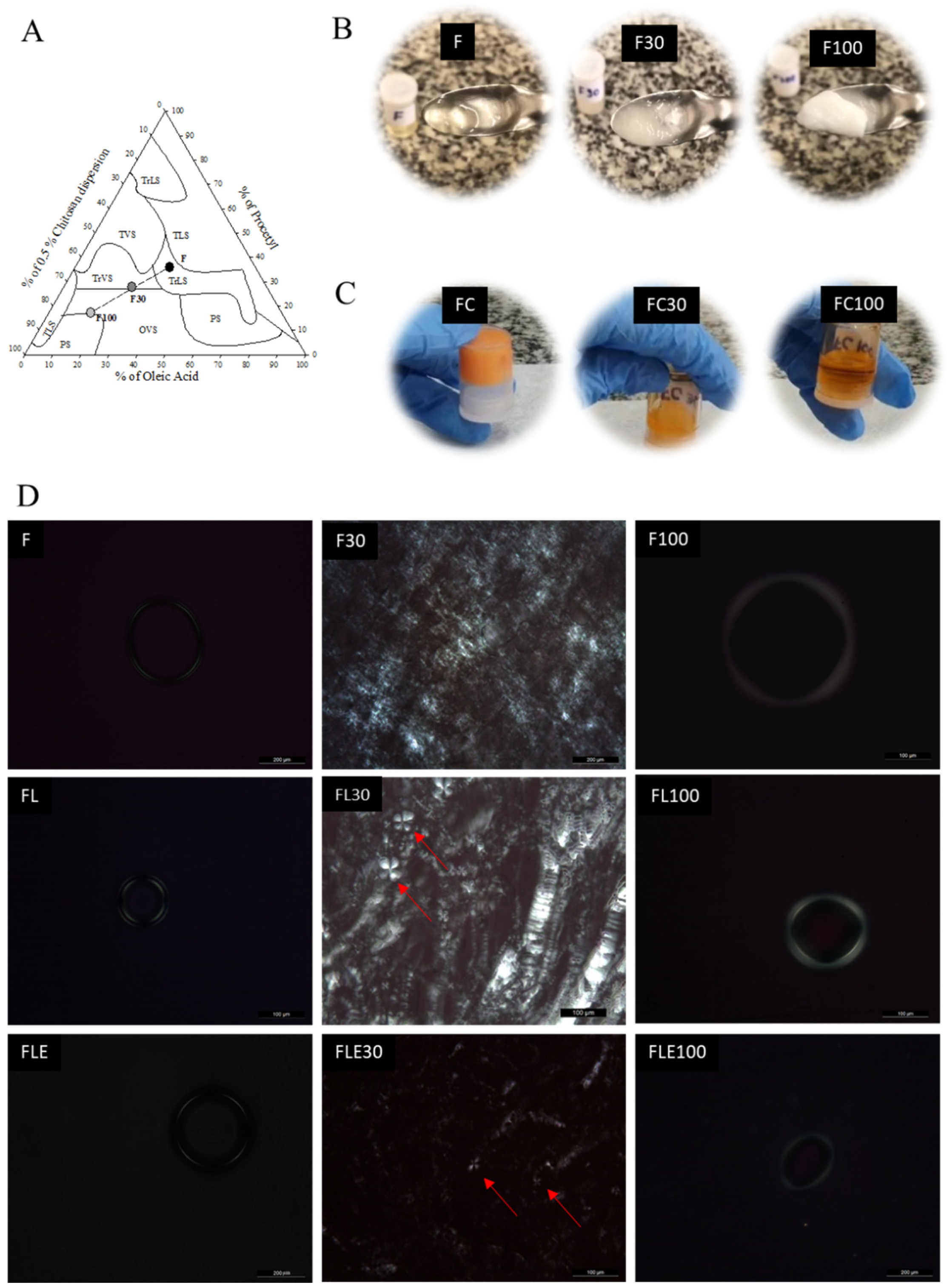

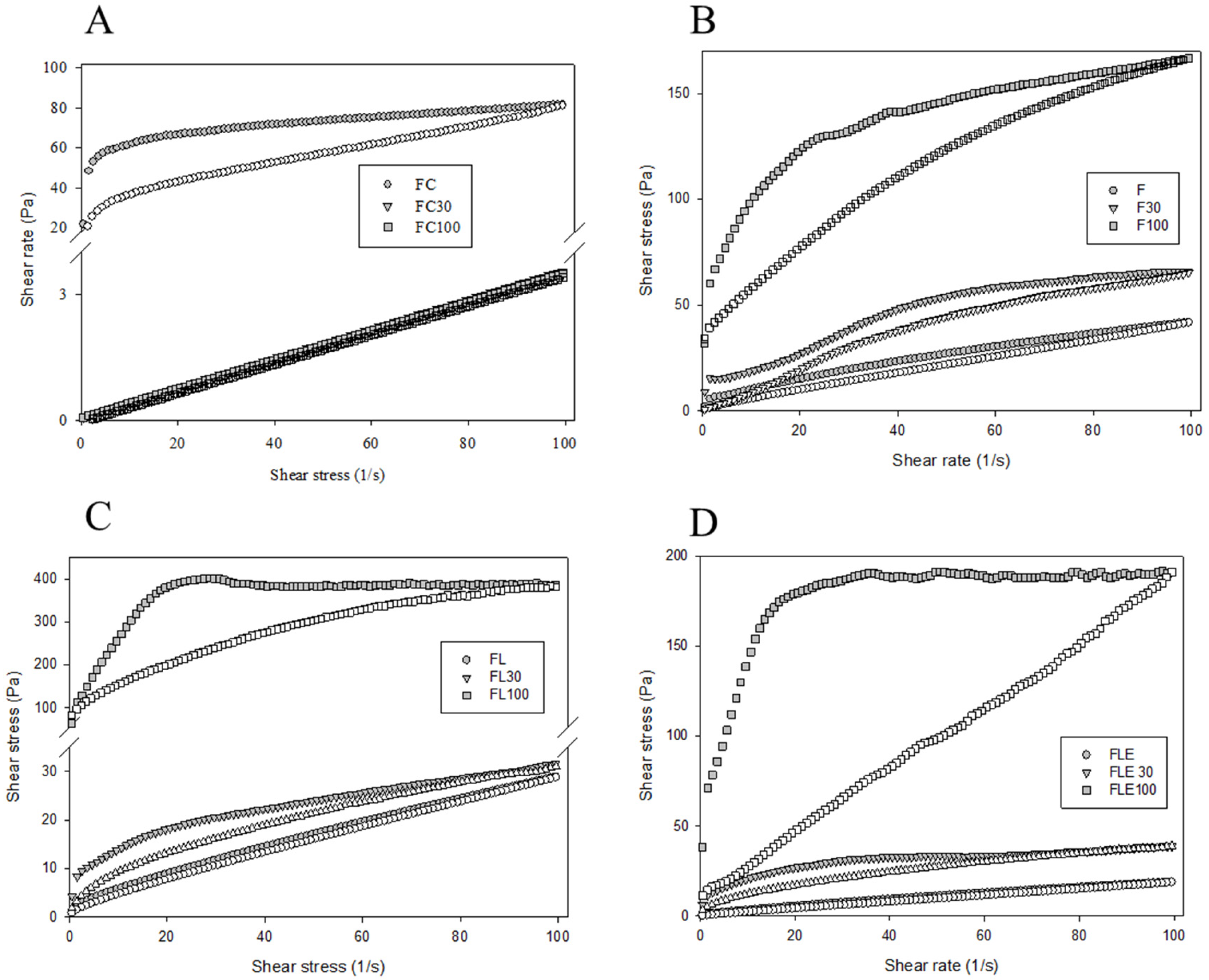
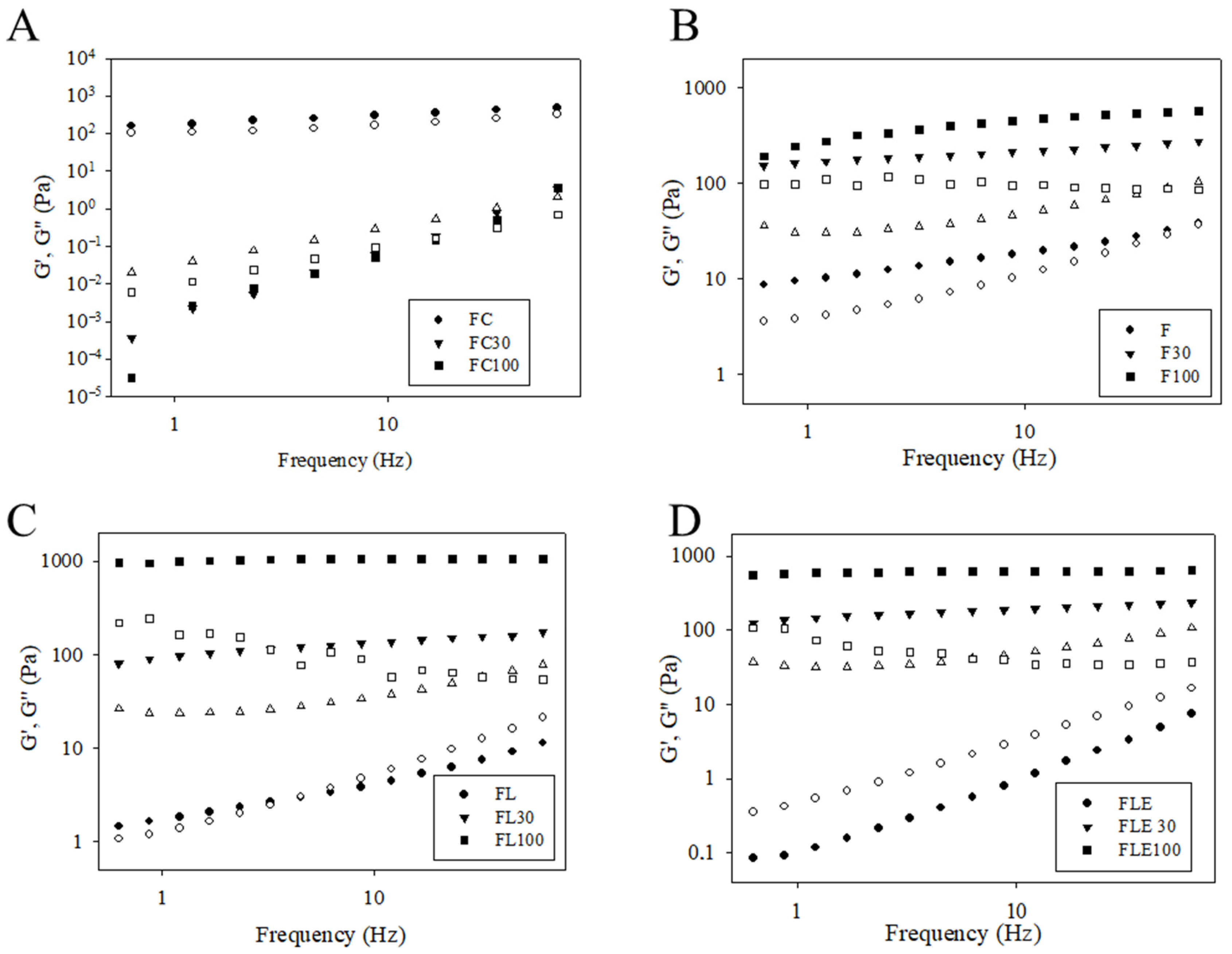

| Formulations | Mechanical Properties | Mucoadhesive Properties | ||||
|---|---|---|---|---|---|---|
| Hardness (mN) | Compressibility (mN·s) | Adhesiveness (mN·s) | Cohesion (Dimensionless) | Peak of Mucoadhesive Strength (mN) | Mucoadhesive Strength Work (mN·s) | |
| F | - * | - * | - * | - * | 680 ± 73 g | 189 ± 28 d |
| F30 | 49 ± 2 b | 147 ± 7 b | 81 ± 10 b | 0.687 ± 49 a | 466 ± 123 ef | 223 ± 29 d |
| F100 | 138 ± 9 c | 478 ± 40 d | 464 ± 43 e | 0.803 ± 17 b | 144 ± 23 a | 174 ± 18 cd |
| FL | - * | - * | - * | - * | 238 ± 24 d | 84 ± 13 a |
| FL30 | 33 ± 1 a | 111 ± 5 a | 30 ± 5 a | 0.821 ± 21 b | 183 ± 22 bc | 119 ± 22 bc |
| FL100 | 179 ± 11 d | 681 ± 52 e | 596 ± 44 e | 0.742 ± 15 a | 218 ± 24 cd | 111 ± 7 bc |
| FLE | - * | - * | - * | - * | 420 ± 51 e | 120 ± 18 bc |
| FLE30 | 34 ± 1 a | 98 ± 8 a | 54 ± 5 b | 0.793 ± 26 b | 407 ± 178 e | 210 ± 24 d |
| FLE100 | 191 ± 24 d | 756 ± 111 e | 638 ± 95 e | 0.711 ± 26 a | 154 ± 18 ab | 296 ± 21 e |
| FC | 357 ± 32 e | 1005 ± 131 f | 1276 ± 262 f | 0.933 ± 52 c | 992 ± 284 h | 313 ± 79 e |
| FC30 | - * | - * | - * | - * | 600 ± 87 f | 83 ± 13 a |
| FC100 | - * | - * | - * | - * | 640 ± 140 fg | 99 ± 17 ab |
| Kinetic Models | Formulations | |
|---|---|---|
| FC | FL | |
| Korsmeyer-Peppas | ||
| R2 | 0.8209 | 0.9704 |
| n | 0.2765 | 0.5105 |
| Higuchi | ||
| R2 | 0.3968 | 0.9723 |
| K | 1.5917 | 1.6935 |
| First order | ||
| R2 | 0.000 | 0.4965 |
| K | 0.0009 | 0.0046 |
| Weibull | ||
| R2 | 0.9979 | 0.9943 |
| b | 0.7457 | 0.7780 |
| Parameters | Formulations | |
|---|---|---|
| FC | FL | |
| Flow (µg.cm−2·h−1) | 635.0 ± 161.4 * | 41.4 ± 1.01 |
| Lag time (h) | 0.33 ± 0.03 * | - |
| Sample | Group | Group Score | Sample Toxicity Classification |
|---|---|---|---|
| Positive control | 1 | 14 | Irritant |
| 2 | 16 | Irritant | |
| 3 | 15 | Irritant | |
| Negative control | 1 | 0 | Non-irritant |
| 2 | 0 | Non-irritant | |
| 3 | 0 | Non-irritant | |
| F | 1 | 7 | Moderate irritant |
| 2 | 6 | Moderate irritant | |
| 3 | 6 | Moderate irritant | |
| FL | 1 | 6 | Moderate irritant |
| 2 | 5 | Slight irritant | |
| 3 | 6 | Moderate irritant | |
| FLE | 1 | 5 | Slight irritant |
| 2 | 5 | Slight irritant | |
| 3 | 3 | Slight irritant | |
| FC | 1 | 7 | Moderate irritant |
| 2 | 6 | Moderate irritant | |
| 3 | 7 | Moderate irritant |
| Score | Classification | Representative Image |
|---|---|---|
| - | Non-irritant | 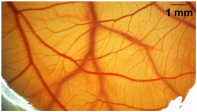 |
| <6 | Slight irritant |  |
| 6 ≤ S ≤ 12 | Moderate irritant | 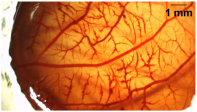 |
| 12 ≤ S ≤ 16 | Irritant |  |
| ≥16 | Severe irritant |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, G.M.F.; Pestana, A.M.; Bezerra, A.A.C.; Luiz, M.T.; Duarte, J.L.; Chorilli, M.; Franz-Montan, M. In Vitro Proof-of-Concept Study: Lidocaine and Epinephrine Co-Loaded in a Mucoadhesive Liquid Crystal Precursor System for Topical Oral Anesthesia. Pharmaceuticals 2025, 18, 1166. https://doi.org/10.3390/ph18081166
Calixto GMF, Pestana AM, Bezerra AAC, Luiz MT, Duarte JL, Chorilli M, Franz-Montan M. In Vitro Proof-of-Concept Study: Lidocaine and Epinephrine Co-Loaded in a Mucoadhesive Liquid Crystal Precursor System for Topical Oral Anesthesia. Pharmaceuticals. 2025; 18(8):1166. https://doi.org/10.3390/ph18081166
Chicago/Turabian StyleCalixto, Giovana Maria Fioramonti, Aylla Mesquita Pestana, Arthur Antunes Costa Bezerra, Marcela Tavares Luiz, Jonatas Lobato Duarte, Marlus Chorilli, and Michelle Franz-Montan. 2025. "In Vitro Proof-of-Concept Study: Lidocaine and Epinephrine Co-Loaded in a Mucoadhesive Liquid Crystal Precursor System for Topical Oral Anesthesia" Pharmaceuticals 18, no. 8: 1166. https://doi.org/10.3390/ph18081166
APA StyleCalixto, G. M. F., Pestana, A. M., Bezerra, A. A. C., Luiz, M. T., Duarte, J. L., Chorilli, M., & Franz-Montan, M. (2025). In Vitro Proof-of-Concept Study: Lidocaine and Epinephrine Co-Loaded in a Mucoadhesive Liquid Crystal Precursor System for Topical Oral Anesthesia. Pharmaceuticals, 18(8), 1166. https://doi.org/10.3390/ph18081166










