1. Introduction
The genus Sideritis (Lamiaceae) includes aromatic, annual and perennial herbaceous plants, and small shrubs diversified in terms of morphology and chemistry, growing at different altitudes and in different habitats. Wild populations of Sideritis develop on sunny, steep slopes and pastures, from a few to over 3000 m above sea level [
1,
2,
3]. In recent years, in-depth studies have focused on the Sideritis genus, particularly regarding its botanical, phytochemical, and pharmacological characteristics. All of its species have been found to contain significant amounts of terpenes, phenolic compounds, and essential oils, i.e., chemical constituents responsible for their multifaceted pharmacological activity [
2,
3]. Due to its unique medicinal properties, the interest in
Sideritis spp. herbs is constantly growing, both in the European and global markets. One of the representatives of the Sideritis genus is
Sideritis scardica Griseb. (synonyms:
S. florida (Boiss. & Heldr.),
S. raeseri subsp.
florida (Boiss. & Heldr.; Papan. & Kokkini)) [
4], endemic to the Balkan Peninsula, known in the southeastern part of Europe for traditional uses: alleviating colds, strengthening the body, and as a sedative. The species occurs in rocky areas and in the alpine regions of northern Greece up to the Olympus mountain range and Pelion [
4,
5,
6]. It is a perennial plant, with a simple or branched stem, lanceolate leaves covered with white tomentose, with yellow, bell-shaped, densely haired flowers [
3]. The above-ground parts of sideritis (referred to as sideritis herb) are collected mainly from the wild during flowering. They are very popular in Mediterranean countries, widely available in shops and at local markets. Known as “mountain tea” or “shepherd′s tea,” they have been used in traditional medicine since ancient times for their antimicrobial, analgesic, antispasmodic, carminative, and antidiabetic effects [
3,
7]. Today, infusions and decoctions of the Sideritis herb are prepared for medicinal purposes and used internally to aid digestion; strengthen the immune system; treat flu, sinusitis, allergies, pain, and anxiety; as well as locally in the form of compresses or therapeutic baths [
2].
Sideritis species are considered promising therapeutic agents in the treatment of upper respiratory tract and peptic ulcer/inflammatory diseases [
8]. The European Medicines Agency (EMA) has recognized
Sideritis spp. as medicinal plants within the framework of traditional use. Infusions of
S. scardiaca Griseb.,
S. clandestina (Bory & Chaub.) Hayek,
S. raeseri Boiss. & Heldr., and
S. syriaca L. are acknowledged for their use in the treatment of colds, relieving coughs, and mild gastrointestinal disorders [
1,
9]. Extracts of
S. scardica represent a rich source of polyphenols and exhibit pronounced antioxidative properties [
10]. The total polyphenol content in sideritis herb reaches 240 g per kg of dry weight, contributing to its multiple health benefits [
3]. The broad-spectrum biological effects of
S. scardica extracts are believed to result from the presence of polyphenolic compounds, particularly phenolic acids [
11,
12,
13]. In a study conducted by Behrendt et al. [
5], supplementation with
S. scardica extract combined with B-group vitamins over a six-week period was shown to reduce the effects of mental stress and improve stress resilience, cognitive efficiency, and visual attention under acute stress conditions. According to the authors, these findings may be of particular relevance to individuals performing cognitive tasks in high-conflict or noisy environments (e.g., open-plan offices or while driving). The pharmacological profile of
S. scardica extracts suggests potential applications in phytotherapeutic approaches for mental health conditions such as anxiety disorders, major depression, attention deficit hyperactivity disorder (ADHD), intellectual disability, or neurodegenerative diseases [
14,
15]. Furthermore, certain compounds isolated from Sideritis spp. have demonstrated antiproliferative, cytotoxic, and anti-HIV activities [
1,
16].
Extracts from sideritis herb contain three main classes of active compounds: essential oils (0.02–0.83%), diterpenes (0.33%), and polyphenols (3.19–12.38 mg GAE·g
−1 DM) [
17]. Mróz et al. [
18] conducted a comparative analysis of the phytochemical profiles of various extracts of
S. raeseri and
S. scardica and classified the major groups of identified secondary metabolites as flavonoids, terpenoids, phenylethanoid glycosides, and phenolic acids. The principal antioxidants in these extracts were derivatives of isoscutellarein and hypolaetin, as well as verbascoside and chlorogenic acid. Among the tested solvents, 70% ethanol proved to be the most effective extractant for various classes of phytochemicals, including antioxidants. Chemical characterization of n-hexane extracts obtained from the aerial flowering parts of
S. scardica (Macedonia) and
S. raeseri (Macedonia and Albania) revealed the presence of over 90 components, predominantly diterpenes and hydrocarbons. The most abundant components were hentriacontane, nonacosane, heptacosane, and two unidentified compounds, presumed to be diterpenes [
19]. The total polyphenol content and antioxidant activity, as determined using the DPPH method, were 50.8 mg GA/g and 3.2–8.9 mg/mL for
S. scardica, and 48.9 mg GA/g and 7.6–12.6 mg/mL for
S. raeseri, respectively. The high cross-pollination capacity within the genus Sideritis contributes to the emergence of numerous phenotypes. A phenotypic assessment of
S. scardica populations from six sites located in northern Greece revealed considerable morphological variability in leaf and inflorescence traits, with a coefficient of variation higher than 15% [
4]. High phenotypic variability in sideritis plants may also be related to chemical variability, and this consequently causes fluctuations in the quality of herbal products. Sarrou et al. [
20] showed a comparable polyphenolic profile across sideritis extracts, with chlorogenic acid and verbascoside being the dominant constituents, at concentrations ranging from 134.88 to 230.00 and from 1511.51 to 2234.32 mg/100 g, respectively. Similarly, Stanoeva et al. [
21] investigated the polyphenolic profile, including the phenolic acids, phenylethanoid glycosides, and flavonoids, in 42 samples representing four Sideritis species from various regions of the Balkan Peninsula in order to reveal correlations between their taxonomy, geographical location, and the profile and content of polyphenolic compounds. They demonstrated that the differences observed in the phenolic profile depended more on the geographical location and climate than on the species. The aim of the present study was to evaluate the phytotherapeutic potential of
S. scardica extracts derived from commercial raw materials originating from Albania, Bulgaria, Macedonia, and Türkiye. The evaluation was based on the analysis of their phytochemical profiles using high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (HPLC/ESI-QTOF-MS), as well as the quantification of biologically active compounds—including total polyphenols, flavonoids, and phenolic acids—and the assessment of their antioxidant and antimicrobial activities. To the best of our knowledge, this is the first study to evaluate the phytotherapeutic value of sideritis raw materials available through official retail channels (herbal/medical stores), taking into account their chemical composition and biological activity in the context of potential geographical origins.
3. Discussion
Extraction—the basic process of separating active substances from inert/inactive material—requires the use of an appropriate solvent and extraction procedure. Extraction parameters are selected depending on the type of raw material and bioactive substance. Yanchev et al. [
10] used different extraction procedures for
S. scardica and obtained the highest values of total polyphenols and total flavonoids in 70% ethanol extracts, followed by water/ethanol mixtures and aqueous decoctions. The highest antioxidant potential was found in extractions with water and 70% ethanol solution, where the amount of polyphenols was also sufficient. The results obtained in this study regarding total polyphenol content (4.0–32.2 mg GAE/g dry plant material) and flavonoid content (2.15–6.18 mg QE/g dry plant material) in the aerial parts of
S. scardica are consistent with previous literature data, where flavonoid levels were reported in the range of 1.33–5.14 mg QE/g dry material.
In comparison, methanol extracts of
S. rubriflora from Türkiye exhibited significantly higher flavonoid content, reaching 155.7 mg/g, while extracts from
S. congesta and
S. vuralii showed lower, yet still higher values than in our study (31.7 and 14.2 mg/g, respectively) [
22]. Notably, among the samples analyzed in the present research, the highest flavonoid content (6.18 mg/g) was observed in the sample originating from Albania (Sh4), whereas the sample from Türkiye contained 2.5 mg/g. This value is substantially lower than those reported for other Turkish Sideritis species. Similarly, low levels of phenolic compounds were noted in tea prepared from hybrid plants (
S. scardica × S. syriaca), where the flavonoid content was 9.6 mg/g and the total polyphenol content reached 32.2 mg/g [
23]. In a study conducted by Irakli et al. [
24], the total polyphenol content of
S. scardica aerial parts was determined using an ultrasound-assisted extraction method with water infusion. The obtained value was 32.2 mg GAE/g of dry plant material, which is consistent with the results reported by Alipieva et al. [
23], who found 29.20 mg GAE/g in a methanolic extract. These findings suggest that ultrasound-assisted extraction may facilitate the efficient extraction of polyphenolic compounds.
According to the literature, the total content of phenolic compound in sideritis herb can also vary significantly depending on the species [
8,
13,
17]. In the study conducted by Sevindik et al. [
22], which investigated several Sideritis species including
S. rubriflora,
S. libanotica subsp.
violasens,
S. brevidens,
S. erythrantha var.
cedretorum,
S. congesta, and
S. vuralii from Türkiye, the total phenolic content ranged from 35.5 to 366.9 mg/g. The highest values were recorded for extracts obtained from
S. erythrantha subsp.
cedretorum and
S. rubriflora, reaching 366.9 and 328.3 mg/g dry weight, respectively. These are substantially higher levels compared to the present study, in which the total phenolic content in sideritis herb of Türkiye origin (sample Sh2), as determined by the Folin–Ciocalteu method, was 0.46 mg GAE/g of raw material. This was among the highest values recorded in our study, surpassed only by the sample from Albania (Sh4), which exhibited a total phenolic content of 1.75 mg GAE/g. In the study by Tadić et al. [
11], the total polyphenol content in ethanol extracts of
S. scardica Griseb. reached 188.5 mg/g, while Nakiboğlu et al. [
25] reported a value of 0.089 μg GAE/μg extract for methanol extracts of
S. spylea. Additionally, Uysal et al. [
26] determined a total polyphenol content of 129.75 mg/g and a total flavonoid content of 111.47 mg/g in
S. libanotica subsp.
kurdica from Iraq.
A positive correlation was found between polyphenol content and antioxidant activity. In the study conducted by Uysal et al. [
26], the ethanol extract of
S. libanotica subsp.
kurdica from Iraq (Duhok) demonstrated high antioxidant activity, with a DPPH radical scavenging effect of 75.15% ± 1.45 at a concentration of 2 mg/mL. Similarly, the methanol extracts analyzed in the present study exhibited strong DPPH radical scavenging capacity, ranging from 63.8% to 87.9%. The highest antioxidant activity, as measured by DPPH inhibition, was observed in the extracts from Türkiye and Macedonia at 87.9% and 87.5%, respectively. In contrast, Sevindik et al. [
22] reported significantly lower DPPH scavenging activity for extracts of
S. congesta and
S. brevidens, with values of 39.1% and 38.9%, respectively.
In the case of antioxidant activity measured using the FRAP method, various Sideritis species originating from Türkiye demonstrated activity levels ranging from 970.8 to 1500.2 μmol/g [
22]. Similarly, high antioxidant activity assessed by ferric ion reducing ability (FRAP), ranging from 14.5 to 29.5 mg Trolox/g, was observed in the present study. The highest activity was recorded for the sample Sh4 (Albania). These findings indicate that the content of polyphenolic compounds, flavonoids, as well as antioxidant activity in Sideritis species is highly variable and depends both on the geographical origin and the specific species studied. This also indicates the need to standardize the sideritis raw material based on the polyphenol content or specific compounds from this group.
Our research demonstrates not only the strong antioxidant and antimicrobial activity of Sideritis extracts but also the considerable variability of both the raw material and the and the resulting extracts. The differences in the chemical composition of
S. scardica and
S. raeseri originating from various geographical regions may be attributed to environmental factors, as well as to the natural tendency of some Sideriris species to interbreed [
27].
S. scardica extracts and their component verbascoside have shown cognitive enhancement, stress-protective, neuroprotective, neurogenetic, anxiolytic, anti-aging, anti-inflammatory, antimicrobial, gastroprotective, glycemic, anti-obesity, antioxidant, and anticancer effects [
28,
29]. We confirmed that the main detected phytochemicals of
S. scardica herb were phenylethanoid glycosides, especially verbascoside (also known as acteoside). The extract obtained from the Bulgarian raw material contained the largest number of identified compounds (20), with verbascoside being the dominant one. Moreover, this extract was the only one to contain citric acid. The extracts from Türkiye and Macedonian sideritis contained 18 compounds each, while in the extract from Albanian Sideritis, 19 compounds were identified including numerous flavonoids (e.g., apigetrin) and organic acids, including malic acid and chlorogenic acid. The sideritis extract from Türkiye showed relatively higher concentrations of chlorogenic acid compared to the others. Kaparakou et al. [
30], analyzing hydroalcoholic extracts from Sideritis species (
S. raeseri,
S. scardica, and
S. syriaca) originating from Greece using LC-MS/MS-QTOF analysis, identified the presence of 23 secondary metabolites, including 17 flavonoids, 4 phenylethanoid glycosides, 1 phenolic acid, and 1 fatty acid. Among the identified compounds, verbascoside (a phenylethanoid glycoside) and selected flavonoids such as apigenin 7-O-glucoside and isoscutellarein 7-O-[6″-O-acetyl]-allosyl(1→2)-glucoside were found in all samples. The presence of verbascoside as the main active compound in Sideritis herb was also confirmed in the present study, along with flavonoids as the dominant class of compounds. Bardakci et al. [
31] analyzed the extracts of
S. congesta P.H.Davis & Hub.-Mor. and demonstrated that the ethyl acetate fraction contained the highest polyphenol content, the strongest antioxidant activity, as well as the highest content of verbascoside and martinoside, before the water fraction. The R-H
2O fraction contained seven compounds, including the phenylethanoid glycoside, verbascoside, two flavonoids, stachyspinoside, isoscutellarein 7-O-(6‴-O-acetyl)-β-allopyranosyl-(1→2)-β-glucopyranoside, the phenolic compound chlorogenic acid, the iridoid glycoside ajugoside, and a mixture of monoterpenoid glucosides: betulalbuside A and 1-hydroxylinaloyl 6-O-β-D-glucopyranoside.
Gioran et al. [
28] showed that sideritis extracts exhibit not only antioxidant activity but also other bioactivities relevant to neuroprotection. Their study demonstrated that
S. clandestina subsp.
peloponnesiaca, although a weaker antioxidant compared to other Sideritis spp., has antiaggregation activity. The authors identified two pure compounds—sideridiol and verbascoside—as responsible for these beneficial effects. The results support the potential use of mountain tea in the elderly population for dementia treatment and demonstrate the activity of sideridiol against Aβ aggregation, which can be used for drug development. Importantly, treatment with sideridiol did not induce adverse effects in animal models, consistent with its lack of toxicity. Phenotypic analysis of the animals treated with verbascoside indicated a low but still existing toxic potential. Overall, no significant changes were observed in the measured traits (except at specific concentrations of verbascoside), which is consistent with the lack of toxicity of the extracts and compounds tested.
Sideritis extracts represent promising herbal remedies with a broad spectrum of biological potential. These extracts inhibit amyloid-β aggregation and toxicity in
Caenorhabditis elegans, which was used as a model of Alzheimer′s disease. Midpolar extracts (40 and 50% ethanol) were the most active, reducing plaque count by 21% and delaying amyloid-β-induced paralysis by up to 3.5 h [
15]. Evaluation of hexane extracts from wild
S. scardica and the cultivated
S. scardica ×
S. syriaca hybrid showed that the main components of all extracts were diterpenes and n-alkanes. None of them were active against Gram-positive
E. coli and the fungus
C. albicans, but all demonstrated good activity against
Staphylococcus aureus. Interestingly, the antibacterial activity of wild and cultivated plants did not differ significantly. The weakest antibacterial activity was observed in the sample with the lowest diterpene concentration [
32]. In this study, the Sh2 extract, which exhibited the strongest and most effective broad-spectrum activity, was particularly effective against both Gram-positive and Gram-negative bacteria, as well as against fungal strains such as
Candida auris and
C. glabrata. These results may indicate the high content of phenolic acids and chlorogenic acid in this extract, compounds known for their antimicrobial efficacy [
10,
13]. Verbascoside, the main component detected in all extracts, especially Sh1 and Sh2, is widely known for its bactericidal and antifungal activity through mechanisms such as cell membrane disruption and inhibition of bacterial enzymes. Furthermore, the presence of isoacetoside and forsythoside B—phenylethanoid glycosides structurally related to verbascoside—may enhance the observed synergistic antimicrobial activity [
31]. The moderate to strong activity of the Sh2 extract against MRSA and
Acinetobacter baumannii, which are known multidrug-resistant pathogens, suggests the potential of
S. scardica extracts as a complementary strategy for treating resistant infections. Similar results have been previously reported for other Sideritis species, where high levels of polyphenols, particularly flavonoids and phenolic acids, correlated with antimicrobial potential [
33]. Interestingly, although the Sh1 extract was less active against Gram-negative strains and fungi, it exhibited targeted bactericidal activity against
Enterococcus faecalis, suggesting possible extract- or compound-specific interactions. This confirms previous observations that variability in the antimicrobial efficacy of Sideritis extracts may depend on geographic origin and phytochemical composition [
34]. Overall, the results support the ethnopharmacological use of
S. scardica and highlight the potential of its polyphenol-rich extracts as natural antimicrobials.
Antimicrobial and anti-inflammatory properties have been attributed to verbascoside [
29]. Our research confirms these associations and further suggests a possible role for chlorogenic acid. The most active and broadest-spectrum extract, Sh2, was characterized not only by the presence of verbascoside but also by the highest chlorogenic acid content. Chlorogenic acid exhibits diverse antimicrobial effects, making it a potential preservative and food additive. This phenolic compound also exerts an inhibitory effect on multidrug efflux systems of multidrug-resistant bacteria and their biofilm formation, as well as an antifungal effect against
C. albicans by having an impact on the fungi′s cell membrane [
35].
In summary, our results indicate that the Sh2 extract (Türkiye) demonstrates the greatest therapeutic potential, particularly against Gram-positive pathogens and Candida species, acting primarily through bactericidal and fungistatic mechanisms. The observed variability in antimicrobial activity among the extracts highlights the influence of phytochemical composition, which is likely affected by factors such as solvent type, polarity, and extraction conditions. Further studies involving detailed phytochemical profiling and investigations into mechanisms of action are necessary to identify the active constituents responsible for this antimicrobial activity and to evaluate their potential synergy with conventional antibiotics or antifungal agents. Given the observed variability, future research should focus on the identification and quantification of specific bioactive compounds. Although considerable research has been conducted on
S. scardica extracts, the precise compounds responsible for the reported biological effects remain insufficiently characterized. Among the identified candidates, sideridiol (from ethyl acetate fractions) and verbascoside (from methanol extracts) appear particularly promising [
28].
In conclusion, our findings on the chemical composition and biological activity of methanolic extracts from S. scardica of different geographical origins provide valuable insights that may support the development of herbal medicinal products and dietary supplements.
4. Materials and Methods
4.1. Plant Material
The research material was the herb of
Sideritis scardica Griseb. originating from Bulgaria (Sh1), Türkiye (Sh2), Macedonia (Sh3), and Albania (Sh4), which was suitably crushed and intended for further studies to determine the content of secondary metabolites (phenolic acids, flavonoids, polyphenols) and to determine their biological activity, including microbiological activity and antioxidant activity. The raw material for research was purchased in herbal-medical stores located in Lublin (Poland). Sample designations, origins of the raw material, and their producers are given in
Table 3.
4.2. Identification of Compounds with the HPLC/ESI-QTOF-MS Method
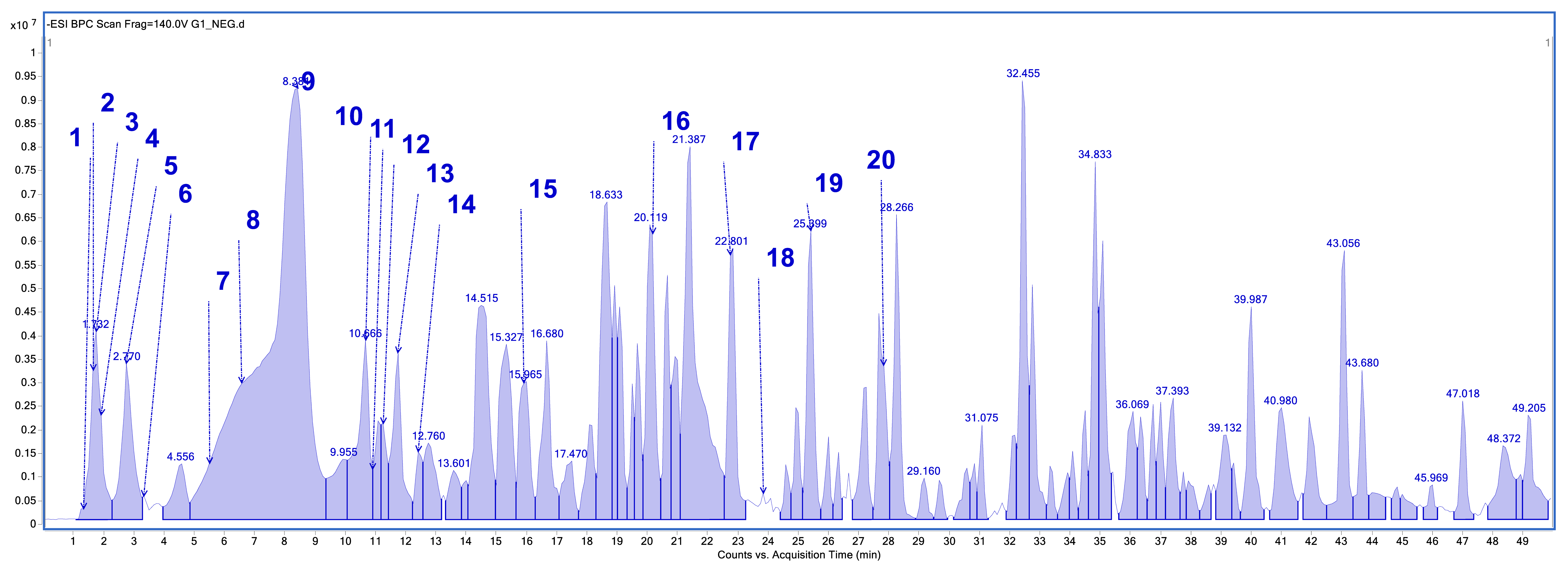
The plant material underwent triple extraction (30 min each) using 20 g of ground S. scardica herb and 100 mL of methanol. The resulting extracts were filtered and subsequently evaporated using a rotary evaporator (Rotary Evaporator 05-ST; IKA-Werke GmbH & Co., Staufen, Germany). The resulting dense extracts were then used to prepare samples for compound identification via the HPLC/ESI-QTOF-MS technique. For this purpose, approximately 0.1 g of the dense extract was weighed and dissolved in 10 mL of methanol using an ultrasonic bath.
The purified samples were analyzed qualitatively by an HPLC/ESI-QTOF-MS system in negative ion mode utilizing the 6530B Accurate-mass-QTOF-MS (Agilent Technologies, Inc., Santa Clara, CA, USA) mass spectrometer with an ESI-Jet Stream ion source system. The Agilent 1260 chromatograph was equipped with a DAD detector, autosampler, binary gradient pump, and column oven. Gradients of solvents, water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B), were used as the mobile phases. The following gradient procedure was adopted: 0–7 min, 15–35% of B; 7–20 min, 35–40% B; 20–42 min 40–65% B, 42–43 min 65–95% B, 43–50 min 95% B; the post time was 10 min. Total time of analysis was 60 min, with a stable flow rate at 0.300 mL/min. For the stationary phase Phenomenex Luna Omega Polar C18 (Phenomenex, Torrance, CA, USA), 100 × 2.1 mm, dp 3 um was used. The injection volume for extracts was 10 μL. ESI-QTOF-MS analysis was performed according to the following parameters of the ion source: Dual spray jet stream ESI; positive and negative ion mode; gas (N2) flow rate: 12 L/min.; nebulizer pressure: 35 psig; vaporizer temp.: 300 °C; m/z range 100–1000 mass units, with acquisition Mode Auto MS/MS; collision-induced dissociation (CID): 10 and 30 eV with MS scan rate 1 spectrum per s, 2 spectra per cycle; skimmer: 65 V; fragmentor: 140 V; and octopole RF Peak: 750 V.
The identification was performed on the basis of MS/MS spectra. Identification was performed with use of open access MS-Dial 5.1.230719 software [
36], and this was compared with literature data.
4.3. Phenolic Acid Content
The content of phenolic acids in the tested extracts was determined using a spectrophotometric method, based on the procedure described in the Polish Pharmacopoeia X [
37]. Samples of approximately 1 g of
S. scardica herb were prepared and extracted three times (30 min each) with methanol (Chempur, Pol-Aura, Warsaw, Poland) as the solvent. The combined extracts were then filtered, the solvent was evaporated, and 20 mL of hot distilled water was added. The samples were subsequently stored at 4 °C for 12 h.
After incubation, the solutions were filtered into volumetric flasks and diluted with distilled water to a final volume of 100 mL. From the resulting extracts, 1 mL was taken and mixed sequentially with 1 mL of distilled water, 1 mL of 0.5 M hydrochloric acid, and 1 mL of Arnow’s reagent (Chempur, Piekary Śląskie, Poland). After 6 min, 1 mL of 1 M sodium hydroxide solution (Chempur, Piekary Śląskie, Poland) and 5 mL of distilled water were added. The final mixture was transferred to a cuvette, and the absorbance was measured at 490 nm using a Hitachi U-2900 UV-Vis model spectrophotometer, (Hitachi High-Tech Corporation, Ibaraki, Japan) against a control sample (reagent mixture without extract).
The phenolic acid content was expressed as milligrams of caffeic acid equivalent per gram of extract (mg/g).
4.4. Flavonoid Content
The flavonoid content was determined spectrophotometrically according to the methodology described in the Polish Pharmacopoeia V [
38], using solutions obtained after extraction of the dried plant material. The extraction was performed with a solvent mixture consisting of acetone (Chempur, Piekary Śląskie, Poland), hydrochloric acid (250 g·L
−1; Chempur, Piekary Śląskie, Poland), and methenamine (5 g/L; Merck, Poznań, Poland). For the analysis, 1 g of crushed
S. scardica herb was used and extracted three times.
Absorbance was measured at 425 nm using a Hitachi U-2900 UV-Vis model spectrophotometer, (Hitachi High-Tech Corporation, Ibaraki, Japan) against an appropriate reference solution. The results were expressed as total flavonoids (mg·g−1 dry weight), calculated as quercetin equivalents.
4.5. Biological Activity of the Sideritis Herba
4.5.1. Polyphenols Content
The total polyphenol content in
S. scardica herb was determined using the Folin–Ciocalteu method, with minor modifications, according to the procedures described by Singleton and Rossi [
39] and Turkmen et al. [
40]. One gram of crushed herb was weighed and placed in a flat-bottomed flask, followed by the addition of 50 mL of methanol (Chempur, Piekary Śląskie, Poland). The prepared plant material was extracted under a reflux condenser in a water bath for 30 min. The extraction was repeated three times, each time using 50 mL of methanol.
To determine phenolic content, 0.1 mL of the methanol extract was mixed with 6 mL of distilled water and 0.5 mL of Folin–Ciocalteu reagent (Chempur, Piekary Śląskie, Poland). After thorough mixing, the solution was left to stand for 3 min. Then, 1.5 mL of saturated sodium carbonate (Chempur, Piekary Śląskie, Poland) solution and 1.9 mL of distilled water were added. The mixture was incubated at 40 °C for 30 min in a thermostat. Absorbance was measured at 765 nm against a reagent blank (without extract) using a Hitachi U-2900 UV-Vis model spectrophotometer, (Hitachi High-Tech Corporation, Ibaraki, Japan).
The results were calculated based on a standard calibration curve prepared using gallic acid and expressed as milligrams of phenolic compounds per gram of dry weight (mg GAE/g DW).
4.5.2. Assessment of Antioxidant Activity by the DPPH Radical Scavenging Assay
The antioxidant activity of methanol extracts from dried Sideritis herb was determined using the DPPH method, which involves a colorimetric measurement of the degree of reduction of DPPH (2,2-diphenyl-1-picrylhydrazyl; Merck, Poznań, Poland) free radicals. The absorbance was measured at 517 nm using a Hitachi U-2900 UV-Vis model spectrophotometer, (Hitachi High-Tech Corporation, Ibaraki, Japan) and methanol as a reference solution. Each analysis was performed in triplicate. A 1% solution of ascorbic acid ((vitamin C; Sigma-Aldrich, St. Louis, MO, USA)) in methanol was used as a control. To prepare the extract, approximately 2 g of the sample was poured with 50 mL of methanol and then left for 24 h in a dark place. After this time, the solution was filtered and used for further analyses. A blank sample was prepared by replacing the extract with water. The analytical procedure was performed according to the method described by Yen and Chen [
41]. The results were expressed as % inhibition of DPPH radicals according to the formula proposed by Rossi et al. [
42]:
At—absorbance of the test sample,
Ar—absorbance of blank test.
4.5.3. Assessment of Antioxidant Activity by the FRAP (Ferric Reducing Antioxidant Power) Assay
The antioxidant activity of the methanol extracts of the tested plant material was determined using the FRAP (Ferric Reducing Antioxidant Power) method, following the procedure described by Thaipong et al. [
43] and Mulugeta et al. [
44], with slight modifications. For the analysis, 2 g of Sideritis herb was weighed and extracted with 50 mL of methanol (Chempur, Pol-Aura, Warsaw, Poland). The extract was left to stand for 24 h. After this period, the solution was filtered, and 100 μL of the extract was taken for further analysis.
The FRAP reagent was freshly prepared before use by mixing acetate buffer (0.3 M), TPTZ solution, and iron(III) chloride hexahydrate solution (20 mM) in a volume ratio of 10:1:1 (all reagents from Sigma-Aldrich, St. Louis, MO, USA or Chempur, Pol-Aura, Poland). For the assay, 3 mL of the freshly prepared FRAP reagent and 100 μL of the sample extract were combined in test tubes and incubated at 37 °C for 10 min. After incubation, absorbance was measured at 593 nm against a blank (FRAP reagent without extract) using a Hitachi U-2900 UV-Vis model spectrophotometer, (Hitachi High-Tech Corporation, Ibaraki, Japan).
The antioxidant capacity was calculated based on a standard curve prepared using Trolox, and the results were expressed as Trolox equivalents (mg Tr/g dry weight).
4.5.4. The Antimicrobial Activity of the Sideritis Herb Extracts
The antimicrobial activity of the
Sideritis scardica Griseb. extracts was evaluated using the broth microdilution method, in accordance with the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [
45], as previously described [
46]. For antimicrobial testing, the methanol extract was first concentrated by evaporating methanol under reduced pressure. The resulting thick extract was then dissolved in dimethyl sulfoxide (DMSO), which was used as the solvent for preparing test solutions.
Serial dilutions of the S. scardica extracts were prepared to achieve final concentrations of 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06, and 0.03 mg/mL. This method was employed to determine the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), or minimum fungicidal concentration (MFC) of each essential oil against twenty-three microbial strains under in vitro conditions.
The antimicrobial efficacy of the S. scardica extracts was assessed against reference strains obtained from the American Type Culture Collection (ATCC), comprising twelve Gram-positive bacteria strains (Staphylococcus aureus ATCC 29213, ATCC 6538P, ATCC 25923—methicillin-sensitive; Staphylococcus aureus ATCC 43300, ATCC BAA1707—methicillin-resistant; Staphylococcus epidermidis ATCC 12228; Enterococcus faecalis ATCC 29212, ATCC 51299; Enterococcus faecium ATCC 19434; Micrococcus luteus ATCC 10240; Bacillus subtilis ATCC 6633; Bacillus cereus ATCC 10876); eleven Gram-negative bacteria strains (Salmonella enteritidis ATCC13076; Salmonella Typhimurium ATCC 14028; Proteus mirabilis ATCC 12453; Bordetella bronchiseptica ATCC 4617; Escherichia coli ATCC 25922 and ATCC 35218; Klebsiella pneumoniae ATCC 13883 and ATCC BAA2146; Enterobacter aerogenes ATCC 13048; Pseudomonas aeruginosa ATCC 27853; Acinetobacter baumanii ATCC 19606); and twelve yeast strains (Candida albicans ATCC 2091, ATCC 10231, 14053; Candida auris CDC B11903; Candida glabrata ATCC 90030, ATCC 15126; Candida parapsilosis ATCC 22019; Candida krusei ATCC 14243; Candida lusitaniae ATCC 34449; Candida tropicalis ATCC 1369; Geotrichum candidum ATCC 34614; Candida glabrata ATCC 66032).
All assays were performed in triplicate. Standard antimicrobial agents were used as positive controls: fluconazole (0.06–16 µg/mL) for yeasts, ciprofloxacin (0.015–16 µg/mL) for Gram-negative bacteria, and vancomycin (0.06–16 µg/mL) for Gram-positive bacteria. The MIC values obtained for these reference compounds were 1 µg/mL fluconazole for C. albicans ATCC 10231, 1 µg/mL vancomycin for S. aureus ATCC 29213, and 0.015 µg/mL ciprofloxacin for E. coli ATCC 25922.
4.6. Statistical Analysis
The obtained results are presented as the means and were statistically analyzed by ANOVA, and the averages were compared using Tukey′s HSD test at the probability level α = 0.05. Statistical analyses were calculated with Statistica 13.3 PL software (StatSof Inc., Tulsa, OK, USA).