The Antimicrobial Activity of Silver Nanoparticles Biosynthesized Using Cymbopogon citratus Against Multidrug-Resistant Bacteria Isolated from an Intensive Care Unit
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization, Antioxidant Activity, and Total Phenolic Content of Cymbopogon citratus Essential Oil
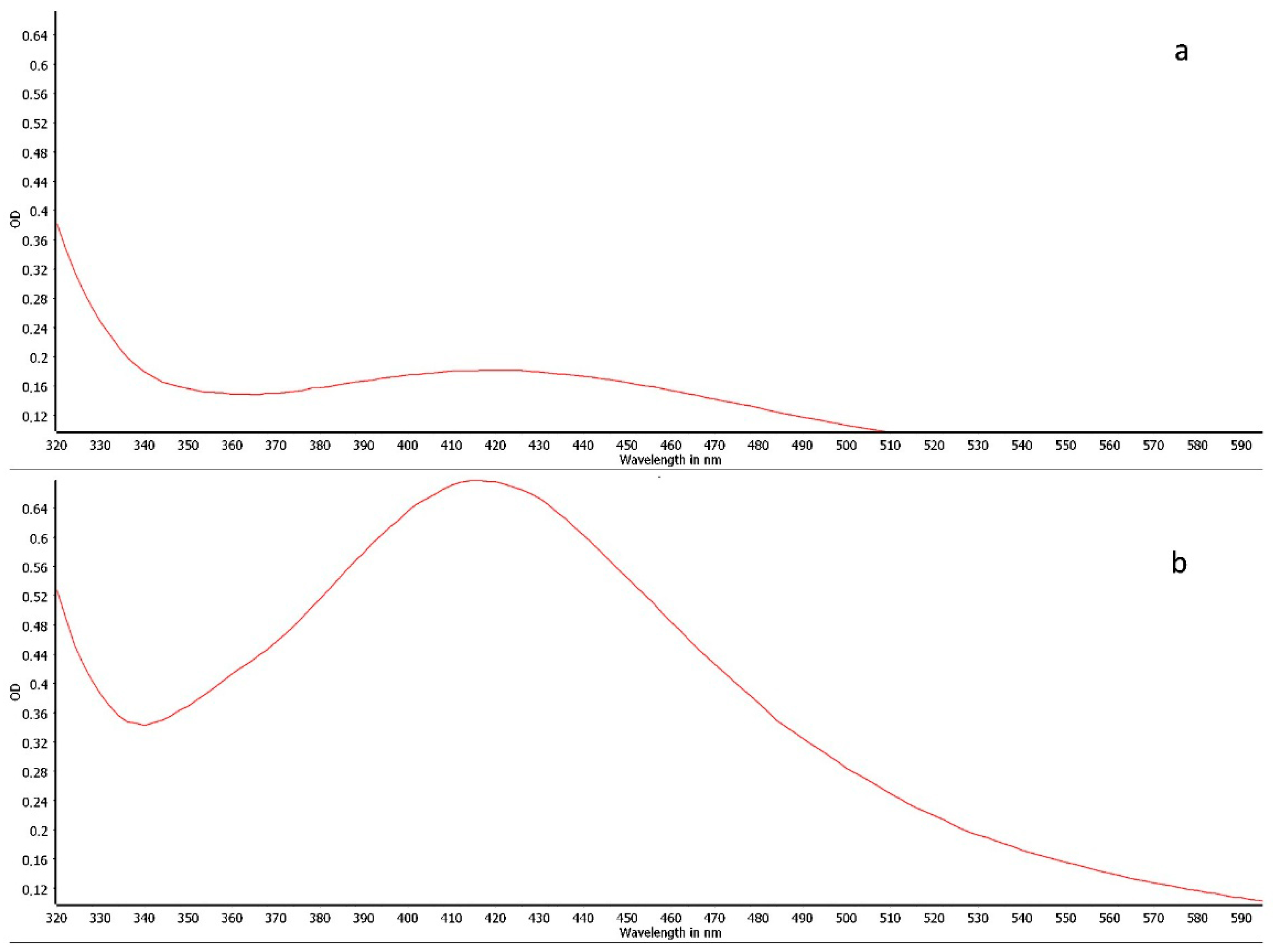
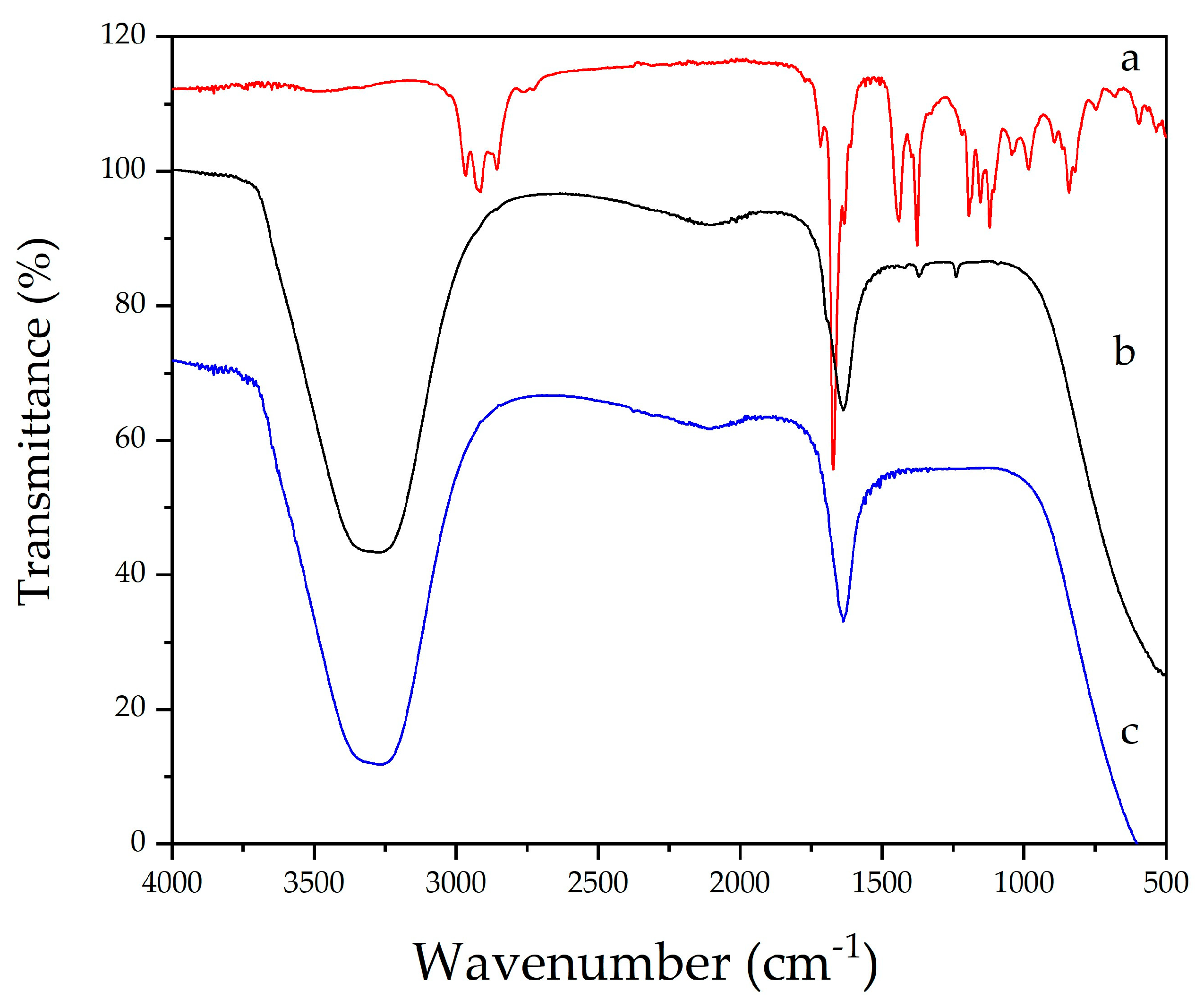
2.2. Synthesis and Physicochemical Characterization of Silver Nanoparticles
2.3. Microbiological Analysis, Isolate Selection, and Antimicrobial Susceptibility
2.4. In Vitro Antimicrobial Activity of Silver Nanoparticles Synthesized with Cymbopogon citratus Essential Oil
3. Materials and Methods
3.1. Materials
3.2. Essential Oil Extraction, Chemical Characterization, Antioxidant Activity, and Total Phenolic Content of Cymbopogon citratus
3.3. Synthesis and Characterization of AgNPs via Bioreduction with Cymbopogon citratus Essential Oil
3.4. Bacterial Isolation for Antimicrobial Efficacy Testing
3.5. Evaluation of the Antimicrobial Activity of AgNPs
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 April 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef]
- Fish, D.N.; Ohlinger, M.J. Antimicrobial Resistance: Factors and Outcomes. Crit. Care Clin. 2006, 22, 291–311. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Morgan, D.J.; Rogawski, E.; Thom, K.A.; Johnson, J.K.; Perencevich, E.N.; Shardell, M.; Leekha, S.; Harris, A.D. Transfer of Multidrug-Resistant Bacteria to Healthcare Workers’ Gloves and Gowns after Patient Contact Increases with Environmental Contamination*. Crit. Care Med. 2012, 40, 1045–1051. [Google Scholar] [CrossRef]
- Rose, G.K.; Thakur, B.; Soni, R.; Soni, S.K. Biosynthesis of Silver Nanoparticles Using Nitrate Reductase from Aspergillus Terreus N4 and Their Potential Use as a Non-Alcoholic Disinfectant. J. Biotechnol. 2023, 373, 49–62. [Google Scholar] [CrossRef]
- Qais, F.A.; Samreen; Ahmad, I. Green Synthesis of Metal Nanoparticles: Characterization and Their Antibacterial Efficacy. In Antibacterial Drug Discovery to Combat MDR: Avenues for New Therapeutics; Ahmad, I., Ahmad, S., Rumbaugh, K.P., Eds.; Springer: Singapore, 2019; pp. 635–680. [Google Scholar] [CrossRef]
- Godakhindi, V.; Kravitz, E.; Vivero-Escoto, J.L. Light-Activable Silver Nanoparticles for Combatting Antibiotic-Resistant Bacteria and Biofilms. Molecules 2025, 30, 626. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Solomon, M.; Holban, A.M.; Bălăceanu-Gurău, B.; Dițu, L.M.; Alberts, A.; Grumezescu, A.M.; Manolescu, L.S.C.; Mihai, M.M. Silver Nanoparticles Functionalized with Polymeric Substances to Reduce the Growth of Planktonic and Biofilm Opportunistic Pathogens. Int. J. Mol. Sci. 2025, 26, 3930. [Google Scholar] [CrossRef]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M. Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective. Appl. Biochem. Biotechnol. 2024, 196, 3636–3669. [Google Scholar] [CrossRef]
- Bawazeer, S. Green Synthesis: An Eco-Friendly Approach for the Synthesis of Silver Nanoparticles Functionalized with Operculina turpethum and It’s In Vitro and in Vivo Biological Activities. Int. J. Nanomed. 2025, 20, 2991–3005. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, A.H.; Bhat, M.Y.; Siddiqui, S.; Alamri, S.A.M.; Alrumman, S.A. Fungal-Mediated Synthesis of Silver Nanoparticles: A Novel Strategy for Plant Disease Management. Front. Microbiol. 2024, 15, 1399331. [Google Scholar] [CrossRef]
- Bharose, A.A.; Hajare, S.T.; H. P., G.; Soni, M.; Prajapati, K.K.; Singh, S.C.; Upadhye, V. Bacteria-Mediated Green Synthesis of Silver Nanoparticles and Their Antifungal Potentials against Aspergillus flavus. PLoS ONE 2024, 19, e0297870. [Google Scholar] [CrossRef]
- Gwada, C.A.; Ndivhuwo, P.S.; Matshetshe, K.; Aradi, E.; Mdluli, P.; Moloto, N.; Otieno, F.; Airo, M. Phytochemical-Assisted Synthesis, Optimization, and Characterization of Silver Nanoparticles for Antimicrobial Activity. RSC Adv. 2025, 15, 14170–14181. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Govindarajan, D.K.; Selvaraj, V.; Selvaraj, A.S.J.M.; Shahul Hameed, S.; Pandiarajan, J.; Veluswamy, A. Green Synthesis of Silver Micro- and Nano-Particles Using Phytochemical Extracts of Cymbopogon citratus Exhibits Antibacterial Properties. Mater. Today Proc. 2023, 76, 103–108. [Google Scholar] [CrossRef]
- Ramasamy, M.; Karuppiah, P.; Ranganathan, H.; Djearamane, S.; Muthukrishnan, E.; Kayarohanam, S.; Arumugam, N.; Almansour, A.I.; Shing Wong, L. Nanomedicine Potential of Cymbopogon citratus Linn. Biogenic Synthesized Silver Nanoparticles: A Study on Antimicrobial and Anticancer Efficacy. J. King Saud Univ. Sci. 2024, 36, 103533. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A. The in Vitro Antimicrobial Activity of Cymbopogon Essential Oil (Lemon Grass) and Its Interaction with Silver Ions. Phytomedicine 2015, 22, 657–665. [Google Scholar] [CrossRef]
- Cherian, T.; Ali, K.; Musarrat, J. Green Functionalization of Silver Nanoparticles Using Leaf Extract of Cymbopogon citratus and Assessment of Their Biological Activities. Next Nanotechnol. 2025, 8, 100216. [Google Scholar] [CrossRef]
- Santhana Lakshmi, V.; Ranjani, S.; Hemalatha, S. Anti-Bacterial Activity of Cymbopogon citratus Nanoparticles against Vibrio Species Infecting Aquatic Organisms. Aquat. Toxicol. 2023, 260, 106583. [Google Scholar] [CrossRef]
- Tazi, A.; Zinedine, A.; Rocha, J.M.; Errachidi, F. Review on the Pharmacological Properties of Lemongrass (Cymbopogon citratus) as a Promising Source of Bioactive Compounds. Pharmacol. Res.—Nat. Prod. 2024, 3, 100046. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K.; Muhury, R. An Update on Bioactive Potential of a Monoterpene Aldehyde Citral. J. Biol. Act. Prod. Nat. 2012, 2, 186–199. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ferreira, A.L.A.; Rosa, G.B.; Azevedo, M.S.; Ferrareze, J.P.; Komatsu, R.A.; Nunes, M.R.; Da Rosa, C.G.; Schmit, R.; Costa, M.D.; et al. Feijoa [Acca sellowiana (Berg) Burret] Accessions Characterization and Discrimination by Chemometrics. J. Sci. Food Agric. 2020, 100, 5373–5384. [Google Scholar] [CrossRef]
- Rakib-Uz-Zaman, S.M.; Apu, E.H.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.; Khan, M.A.R.; Shuborna, N.S.; Shams, S.M.; et al. Biosynthesis of Silver Nanoparticles from Cymbopogon citratus Leaf Extract and Evaluation of Their Antimicrobial Properties. Challenges 2022, 13, 18. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Konfo, T.R.C.; Djouhou, F.M.C.; Koudoro, Y.A.; Dahouenon-Ahoussi, E.; Avlessi, F.; Sohounhloue, C.K.D.; Simal-Gandara, J. Essential Oils as Natural Antioxidants for the Control of Food Preservation. Food Chem. Adv. 2023, 2, 100312. [Google Scholar] [CrossRef]
- Vinicius De Oliveira Brisola Maciel, M.; Da Rosa Almeida, A.; Machado, M.H.; Elias, W.C.; Gonçalves Da Rosa, C.; Teixeira, G.L.; Noronha, C.M.; Bertoldi, F.C.; Nunes, M.R.; Dutra De Armas, R.; et al. Green Synthesis, Characteristics and Antimicrobial Activity of Silver Nanoparticles Mediated by Essential Oils as Reducing Agents. Biocatal. Agric. Biotechnol. 2020, 28, 101746. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Castro, L.E.N.; Da Rosa, C.G.; Almeida, A.D.R.; Maciel-Silva, F.W.; Kempe, P.R.G.; De Oliveira, A.L.R.; Forster-Carneiro, T.; Bertoldi, F.C.; Barreto, P.L.M.; et al. Production of Nanocomposite Films Functionalized with Silver Nanoparticles Bioreduced with Rosemary (Rosmarinus officinalis, L.) Essential Oil. J. Agric. Food Res. 2023, 11, 100479. [Google Scholar] [CrossRef]
- Basera, P.; Lavania, M.; Agnihotri, A.; Lal, B. Analytical Investigation of Cymbopogon citratus and Exploiting the Potential of Developed Silver Nanoparticle Against the Dominating Species of Pathogenic Bacteria. Front. Microbiol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Nunes, M.R.; De Souza Maguerroski Castilho, M.; De Lima Veeck, A.P.; Da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and Antimicrobial Methylcellulose Films Containing Lippia alba Extract and Silver Nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Longo, M.; De Oliveira, J.L.; Da Rosa, C.G.; De Lima Veeck, A.P.; De Aquino, R.S.; Masiero, A.V.; Bertoldi, F.C.; Barreto, P.L.M.; Nunes, M.R. Nanocomposite Poly (Ethylene Oxide) Films Functionalized with Silver Nanoparticles Synthesized with Acca Sellowiana Extracts. Colloids Surf. Physicochem. Eng. Asp. 2020, 602, 125125. [Google Scholar] [CrossRef]
- De Melo, A.P.Z.; De Oliveira Brisola Maciel, M.V.; Sganzerla, W.G.; Da Rosa Almeida, A.; De Armas, R.D.; Machado, M.H.; Da Rosa, C.G.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antibacterial Activity, Morphology, and Physicochemical Stability of Biosynthesized Silver Nanoparticles Using Thyme (Thymus vulgaris) Essential Oil. Mater. Res. Express 2020, 7, 015087. [Google Scholar] [CrossRef]
- Narciso, A.M.; Da Rosa, C.G.; Nunes, M.R.; Sganzerla, W.G.; Hansen, C.M.; De Melo, A.P.Z.; Paes, J.V.; Bertoldi, F.C.; Barreto, P.L.M.; Masiero, A.V. Antimicrobial Green Silver Nanoparticles in Bone Grafts Functionalization for Biomedical Applications. Biocatal. Agric. Biotechnol. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Loaiza, W.M.; Ruiz, A.K.R.; Patiño, C.C.O.; Vivas, M.C. Bacterial Resistance in Hospital-Acquired Infections Acquired in the Intensive Care Unit: A Systematic Review. Acta Medica Hradec Kral. Czech Repub. 2023, 66, 1–10. [Google Scholar] [CrossRef]
- Silva, A.C.S.; Miotto, A.R.; Bernardelli, S.N.Ú.; Pereira, V.C. Staphylococcus saprophyticus: Fatores de Virulência, Susceptibilidade Antimicrobiana e Infecções Do Trato Urinário. Braz. J. Health Rev. 2023, 6, 27895–27906. [Google Scholar] [CrossRef]
- Osman, A.-H.; Darkwah, S.; Kotey, F.C.N.; Odoom, A.; Hotor, P.; Dayie, N.T.K.D.; Donkor, E.S. Reservoirs of Nosocomial Pathogens in Intensive Care Units: A Systematic Review. Environ. Health Insights 2024, 18, 11786302241243239. [Google Scholar] [CrossRef]
- Sahu, M.K.; George, N.; Rastogi, N.; Bipin, C.; Singh, S.P. Uncommon Pathogens Causing Hospital-Acquired Infections in Postoperative Cardiac Surgical Patients. J. Card. Crit. Care TSS 2019, 3, 089–096. [Google Scholar] [CrossRef]
- Cattoir, V. The Multifaceted Lifestyle of Enterococci: Genetic Diversity, Ecology and Risks for Public Health. Curr. Opin. Microbiol. 2022, 65, 73–80. [Google Scholar] [CrossRef]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of Enterococci. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI: Wayne, PA, USA, 2024; ISBN 978-1-68440-220-5. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-Acquired Infections Caused by Enterococci: A Systematic Review and Meta-Analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillance 2021, 26, 2001628. [Google Scholar] [CrossRef]
- Geraldes, C.; Tavares, L.; Gil, S.; Oliveira, M. Enterococcus Virulence and Resistant Traits Associated with Its Permanence in the Hospital Environment. Antibiotics 2022, 11, 857. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef]
- Hussien, A.M.; Taher, H.S.; Abdulla, H.M. Biosynthesized Silver Nanoparticles as Promising Antimicrobial Agents and Their Mode of Action. Adv. Environ. Life Sci. 2024, 6, 1–11. [Google Scholar] [CrossRef]
- Kiełtyka-Dadasiewicz, A.; Esteban, J.; Jabłońska-Trypuć, A. Antiviral, Antibacterial, Antifungal, and Anticancer Activity of Plant Materials Derived from Cymbopogon citratus (DC.) Stapf Species. Pharmaceuticals 2024, 17, 705. [Google Scholar] [CrossRef]
- Khosakueng, M.; Taweechaisupapong, S.; Boonyanugomol, W.; Prapatpong, P.; Wongkaewkhiaw, S.; Kanthawong, S. Cymbopogon citratus L. essential oil as a potential anti-biofilm agent active against antibiotic-resistant bacteria isolated from chronic rhinosinusitis patients. Biofouling 2024, 40, 26–39. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Version 7.0; StatSoft, Inc.: Tulsa, OK, USA, 2004. [Google Scholar]

| RIC | RIL | Composition (%) | |
|---|---|---|---|
| Geranial | 1268 | 1271 | 41.6 |
| Neral | 1239 | 1240 | 31.8 |
| β-Mirceno | 989 | 989 | 19.3 |
| Geraniol | 1250 | 1249 | 2.3 |
| 6-Methyl-5-heptene-2-one | 984 | 986 | 1.7 |
| 2-Undecanone | 1292 | 1292 | 0.7 |
| Total Identified | 97.5 |
| Sample | DPPH Assay (µg TEs/mL) | ABTS Assay (µg TEs/mL) | TPC (mg GEs/mL) |
|---|---|---|---|
| Lemongrass EO | 282.0 ± 5.6 | 456.6 ± 11.2 | 1.2 ± 0.1 |
| Sample | Z-Average (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| AgNPs (3 mM precursor) | 87.0 ± 1.1 b | 0.14 ± 0.006 b | −23.0 ± 0.4 a |
| AgNPs (6 mM precursor) | 147.3 ± 1.5 a | 0.91 ± 0.006 a | −10.4 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusso, B.P.; Almeida, A.R.; Nunes, M.R.; Becker, D.; Hotza, D.; da Rosa, C.G.; dos Santos, V.V.; da Silva, B.F. The Antimicrobial Activity of Silver Nanoparticles Biosynthesized Using Cymbopogon citratus Against Multidrug-Resistant Bacteria Isolated from an Intensive Care Unit. Pharmaceuticals 2025, 18, 1120. https://doi.org/10.3390/ph18081120
Gusso BP, Almeida AR, Nunes MR, Becker D, Hotza D, da Rosa CG, dos Santos VV, da Silva BF. The Antimicrobial Activity of Silver Nanoparticles Biosynthesized Using Cymbopogon citratus Against Multidrug-Resistant Bacteria Isolated from an Intensive Care Unit. Pharmaceuticals. 2025; 18(8):1120. https://doi.org/10.3390/ph18081120
Chicago/Turabian StyleGusso, Bianca Picinin, Aline Rosa Almeida, Michael Ramos Nunes, Daniela Becker, Dachamir Hotza, Cleonice Gonçalves da Rosa, Vanessa Valgas dos Santos, and Bruna Fernanda da Silva. 2025. "The Antimicrobial Activity of Silver Nanoparticles Biosynthesized Using Cymbopogon citratus Against Multidrug-Resistant Bacteria Isolated from an Intensive Care Unit" Pharmaceuticals 18, no. 8: 1120. https://doi.org/10.3390/ph18081120
APA StyleGusso, B. P., Almeida, A. R., Nunes, M. R., Becker, D., Hotza, D., da Rosa, C. G., dos Santos, V. V., & da Silva, B. F. (2025). The Antimicrobial Activity of Silver Nanoparticles Biosynthesized Using Cymbopogon citratus Against Multidrug-Resistant Bacteria Isolated from an Intensive Care Unit. Pharmaceuticals, 18(8), 1120. https://doi.org/10.3390/ph18081120









