Gallic Acid and Taurine Attenuate Thiamethoxam-Induced Hepatotoxicity in Rats by Modulating SIRT-1/PGC-1α, NF-κB/iNOS, and p53/Bax/Caspase-3 Pathways
Abstract
1. Introduction
2. Results
2.1. TAU and/or GA Improve the Body Weight and Hepatosomatic Index in TMX-Exposed Rats
2.2. TAU and/or GA Mitigate Hepatic Impairment Caused by TMX
2.3. TAU and/or GA Ameliorate TMX-Induced Oxidative Insult in Liver Tissues
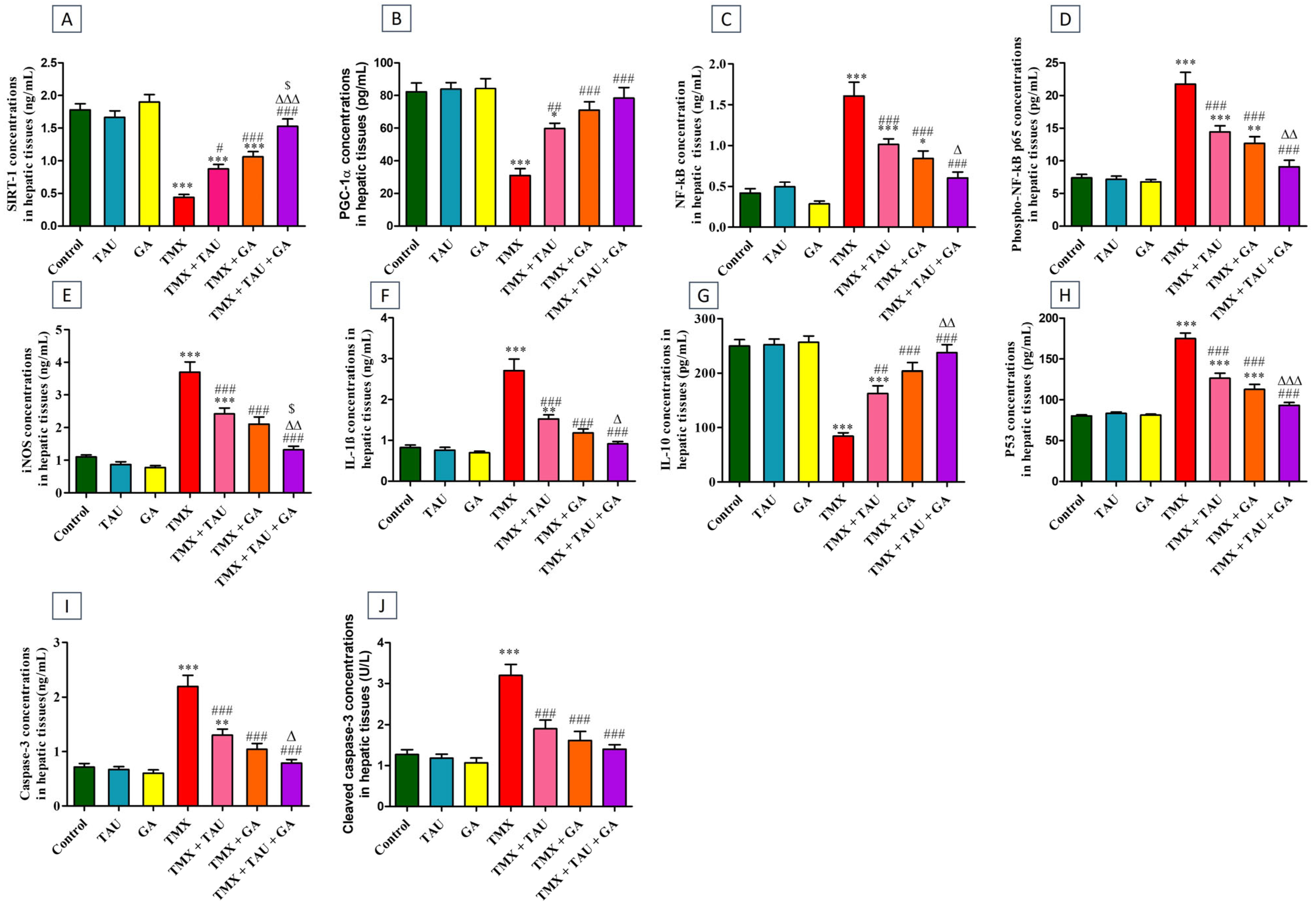
2.4. TAU and/or GA Modulate the Hepatic Concentrations of SIRT-1, PGC-1α, NF-κB, Phospho-NF-κB p65, iNOS, IL-1β, IL-10, p53, Caspase-3, and Cleaved Caspase-3 in TMX-Exposed Rats
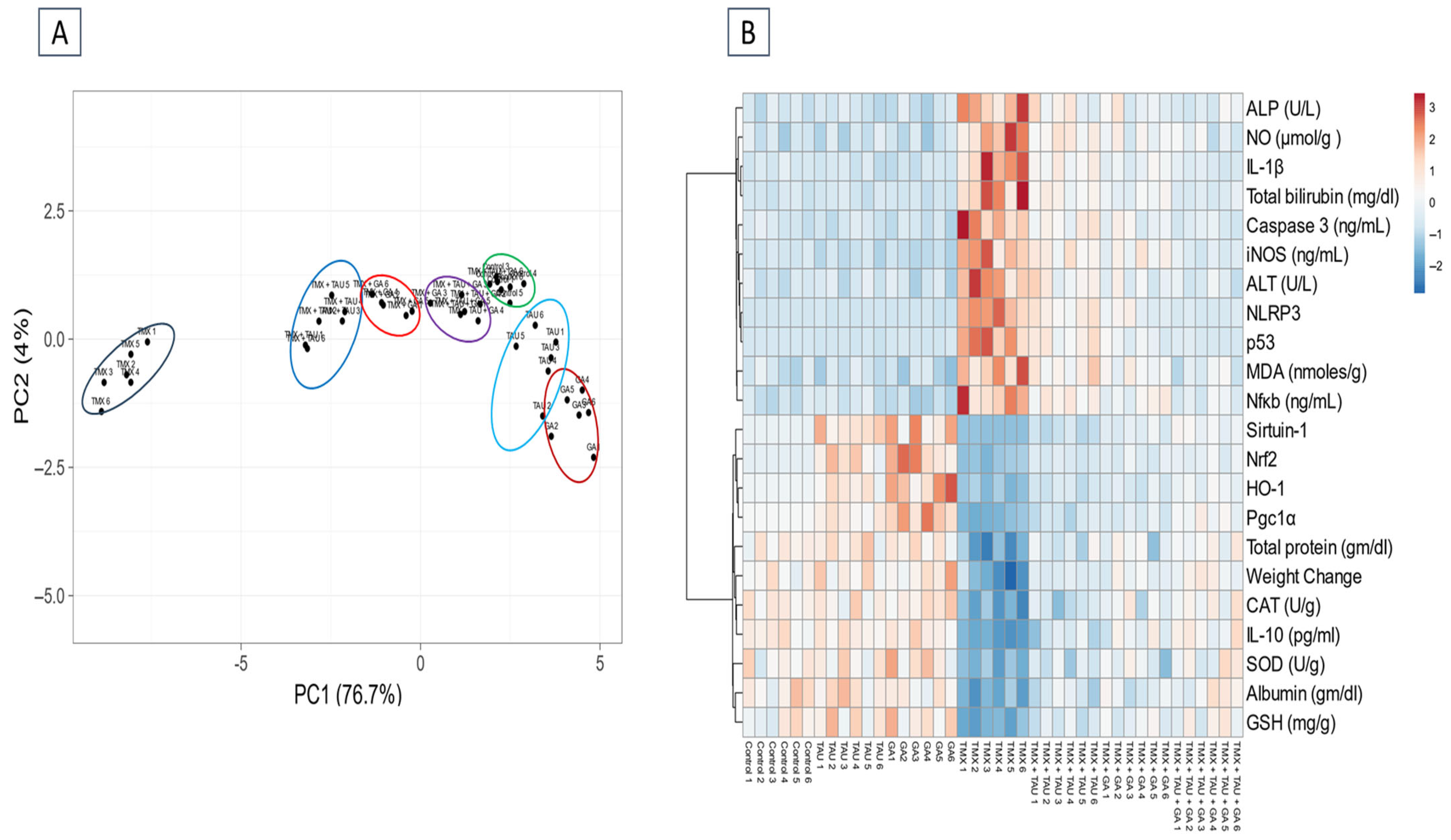
2.5. TAU and/or GA Regulate the Transcription Levels of SIRT-1, PGC-1α, Nrf2, HO-1, NLRP3, and p53 in Liver Tissues of TMX-Administered Rats
2.6. Multivariate Analysis
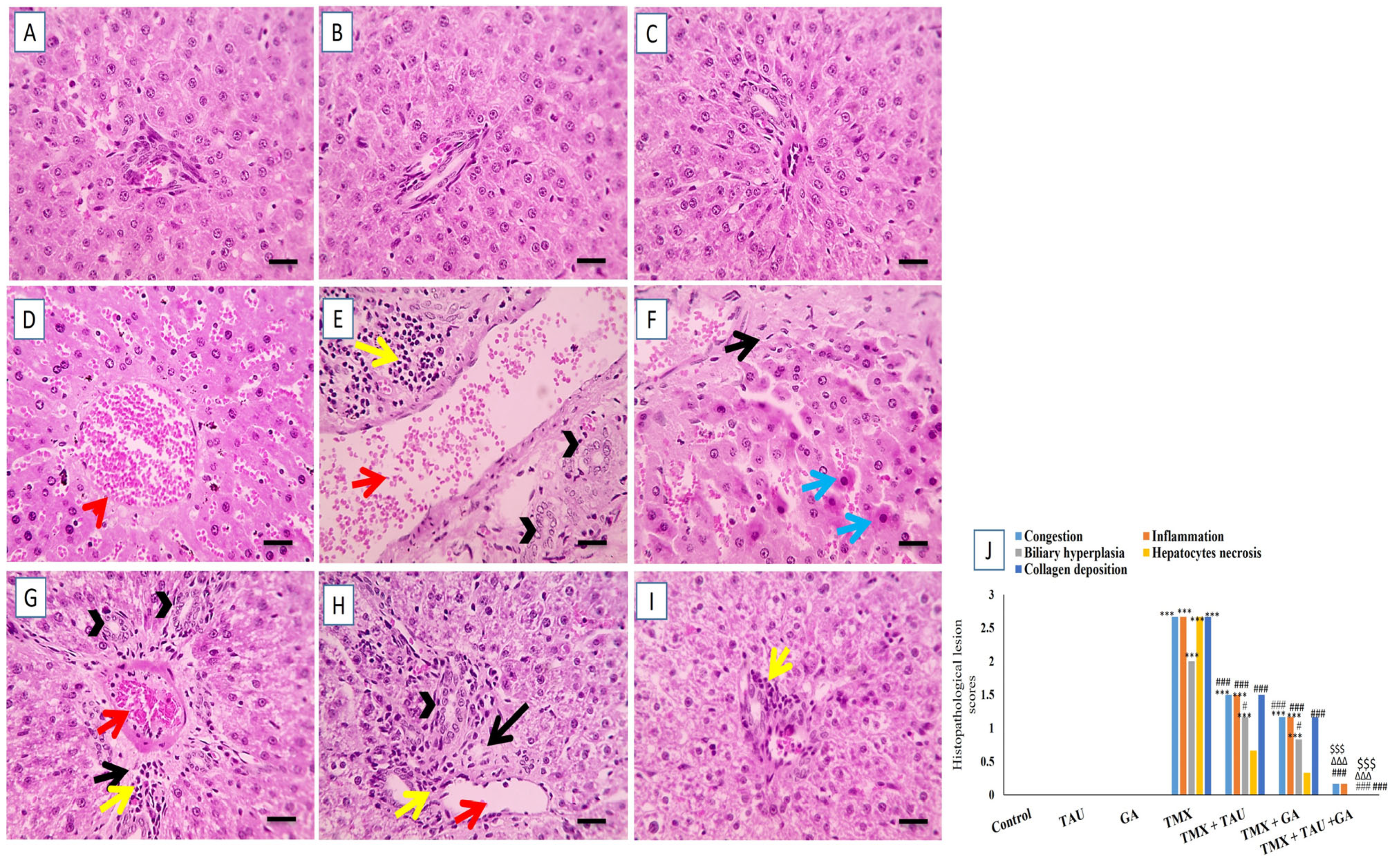
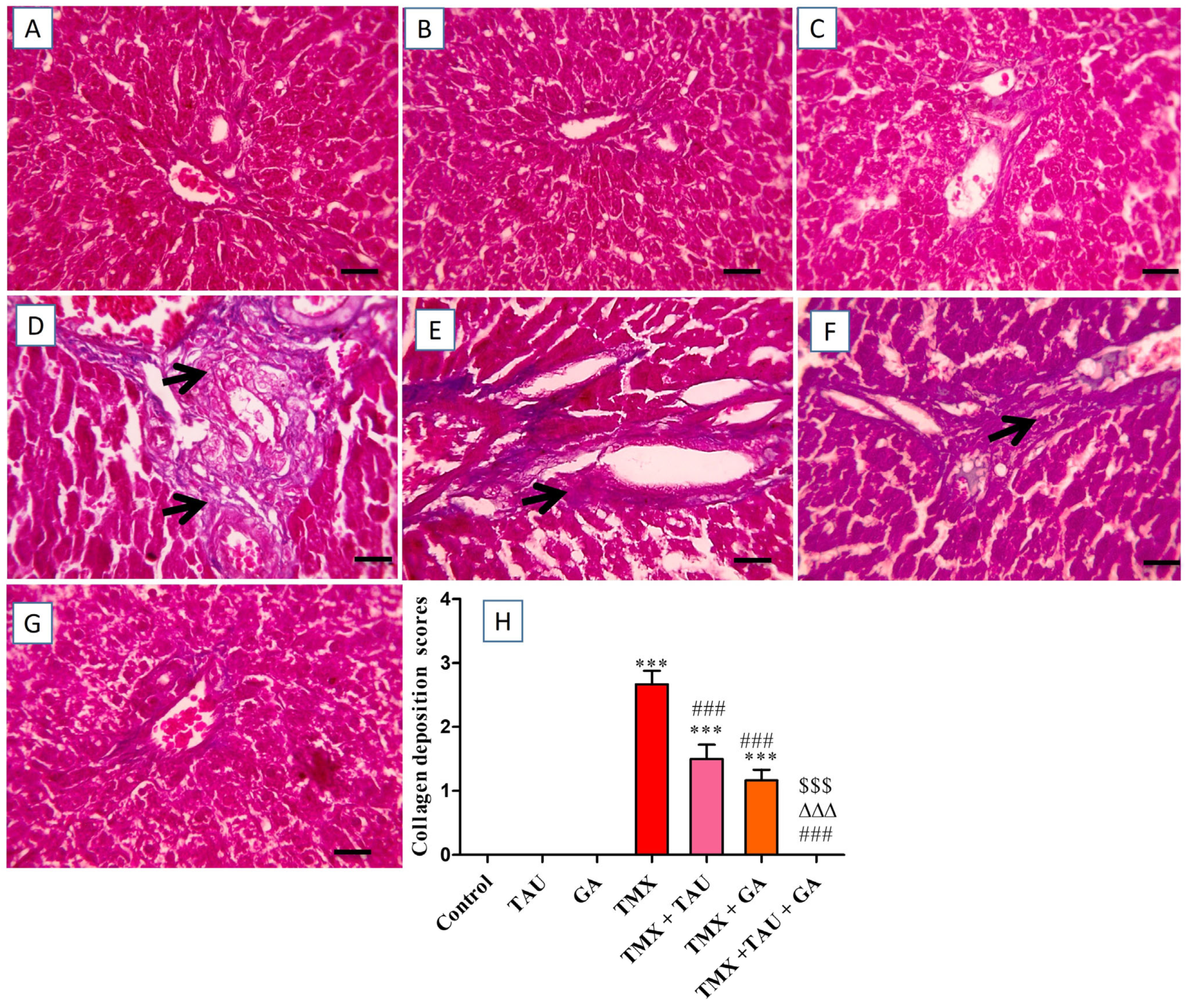
2.7. TAU and/or GA Alleviate TMX-Induced Hepatic Histological Lesions
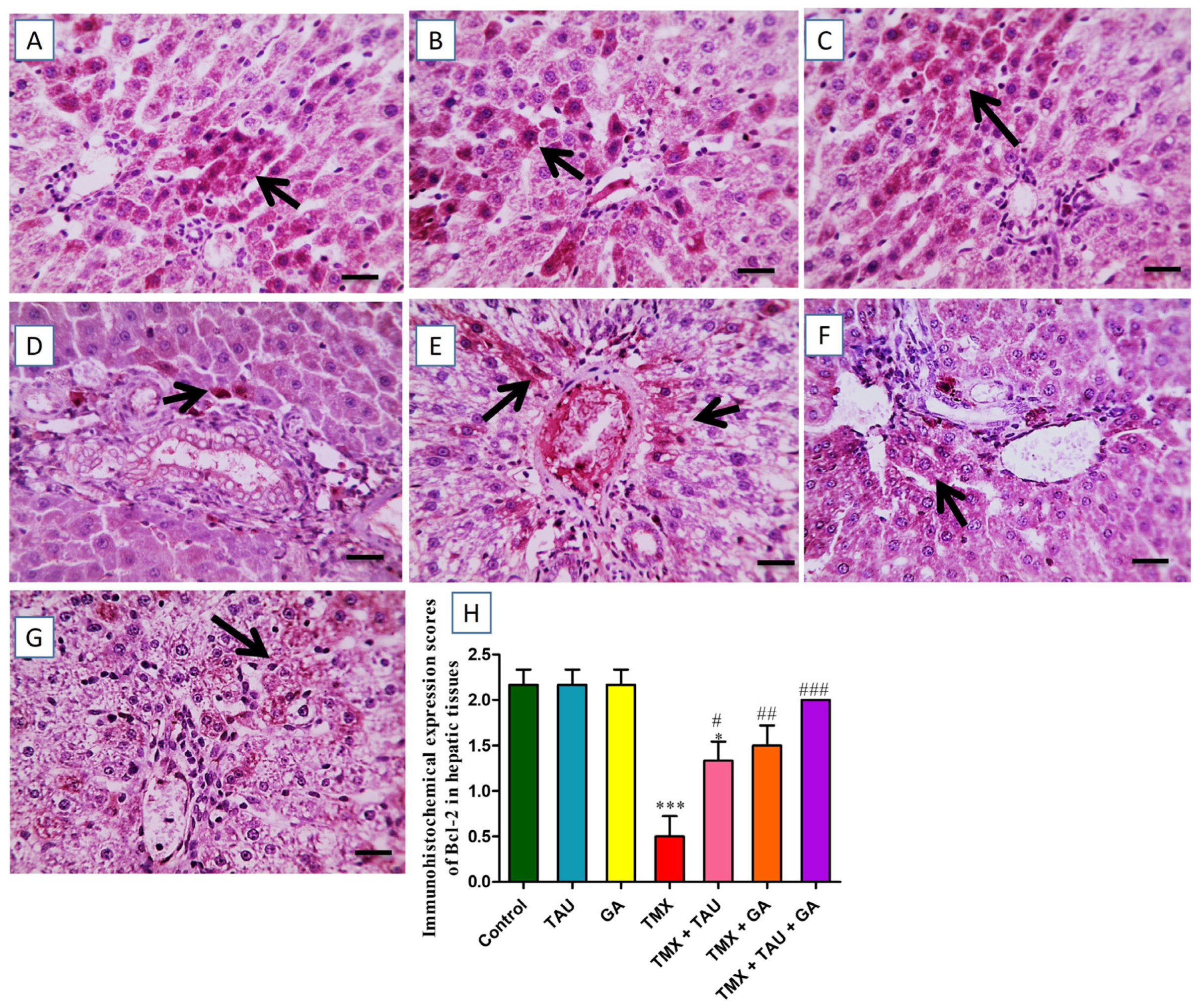
2.8. TAU and/or GA Modulate the Immunoexpression of TNF-α, Bax, and Bcl-2 in the Liver of TMX-Treated Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Sample Size Calculation
4.4. Experimental Plan
4.5. Samples Collection
4.6. Biochemical Investigations
4.6.1. Serum Biomarkers of Hepatic Function
4.6.2. Determination of Oxidative Stress Indices in Hepatic Tissues
4.6.3. ELISA Analysis
4.7. qRT-PCR for the Transcription Levels of SIRT-1, PGC-1α, Nrf2, HO-1, NLRP3, and p53 in Liver Specimens
4.8. Histopathological Scrutinization of Liver Samples
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferases |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| CAT | Catalase |
| GA | Gallic acid |
| GSH | Reduced glutathione |
| HO-1 | Heme oxygenase-1- |
| IL-1β | Interleukin-1β |
| IL-10 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MnSOD | Manganese superoxide dismutase |
| nAChRs | Nicotinic acetyl choline receptors |
| NF-κB | Nuclear factor kappa |
| NO | Nitric oxide |
| NLRP3 | Nucleotide-binding domain, leucine-rich-containing family, pyrin domain–containing-3 |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| PGC-1α | Peroxisome proliferator-activated receptor (PPAR) gamma coactivator 1 alpha |
| SIRT1 | Sirtuin-1 |
| TAU | Taurine |
| TMX | Thiamethoxam |
| TNF-α | Tumor necrosis factor alpha |
| WHO | World Health Organization |
References
- Li, L.; Liang, H.; Zhao, T.; Liu, Y.; Yan, S.; Zhu, W. Differential effects of thiamethoxam and clothianidin exposure on their tissue distribution and chronic toxicity in mice. Chem. Biol. Interact. 2022, 366, 110149. [Google Scholar] [CrossRef]
- Gui, T.; Jia, G.F.; Xu, J.; Ge, S.J.; Long, X.F.; Zhang, Y.P.; Hu, D.Y. Determination of the residue dynamics and dietary risk of thiamethoxam and its metabolite clothianidin in citrus and soil by LC-MS/MS. J. Environ. Sci. Health 2019, 54 Pt B, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.T.; Khan, A.; Ahmad, M.; Anwar, M.F.; Khatoon, A.; Saleemi, M.K.; Akram, M.N. Effect of sub lethal doses of thiamethoxam (a pesticide) on hemato-biochemical values in cockerels. Pak. Vet. J. 2017, 37, 135–138. [Google Scholar] [CrossRef]
- Cartereau, A.; Martin, C.; Thany, S.H. Neonicotinoid insecticides differently modulate acetycholine-induced currents on mammalian α7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU first and second watch lists in the water environment. Clean–Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- Main, A.R.; Headley, J.V.; Peru, K.M.; Michel, N.L.; Cessna, A.J.; Morrissey, C.A. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS ONE 2014, 9, e92821. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- European Food Safety Authority. Reasoned opinion on the modification of the existing MRLs for thiamethoxam and clothianidin in various crops. EFSA J. 2012, 10, 2990. [Google Scholar] [CrossRef]
- Ramanathan, S.; Sanjeevi, G.; Narayanan, B.; Kurien, A.A. Thiamethoxam, a neonicotinoid poisoning causing acute kidney injury via a novel mechanism. Kidney Int. Rep. 2020, 5, 1111–1113. [Google Scholar] [CrossRef]
- Abdel-Razik, R.K.; Mosallam, E.M.; Hamed, N.A. The deterrent effect of acetylcysteine against hepatic and renal damage in thiamethoxam exposed rats. Egypt. J. Chem. 2022, 65, 251–266. [Google Scholar] [CrossRef]
- El Okle, O.S.; Lebda, M.A.; Tohamy, H.G. Thiamethoxam-induced biochemical, hormonal and histological alterations in rats. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 320–325. [Google Scholar]
- Khaldoun-Oularbi, H.; Bouzid, N.; Boukreta, S.; Makhlouf, C.; Derriche, F.; Djennas, N. Thiamethoxam Actara® induced alterations in kidney, liver, cerebellum and hippocampus of male rats. J. Xenobiot. 2017, 7, 7149. [Google Scholar] [CrossRef] [PubMed]
- El Okle, O.S.; El Euony, O.I.; Khafaga, A.F.; Lebda, M.A. Thiamethoxam induced hepatotoxicity and pro-carcinogenicity in rabbits via motivation of oxidative stress, inflammation, and anti-apoptotic pathway. Environ. Sci. Pollut. Res. 2018, 25, 4678–4689. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.S.A.; Bhatnagar, U.; Subramanian, V. Developmental Neurotoxicity of Thiamethoxam in Wistar Rats. Int. J. Vet. Med. 2023, 2, 1–8. [Google Scholar]
- Li, X.; Zhao, H.; Gong, M.; Zhang, F.; Liu, S.; Zhang, Z.; He, Y.; Hollert, H.; Zhang, X.; Shi, W.; et al. Thiamethoxam at environmentally relevant concentrations induces neurotoxicity in zebrafish larvae through binding with multiple receptors. Eco-Environ. Health 2025, 4, 100133. [Google Scholar] [CrossRef]
- El-Din, M.A.E.D.S.; Ghareeb, A.E.W.E.; El-Garawani, I.M.; El-Rahman, H.A.A. Induction of apoptosis, oxidative stress, hormonal, and histological alterations in the reproductive system of thiamethoxam-exposed female rats. Environ. Sci. Pollut. Res. 2023, 30, 77917–77930. [Google Scholar] [CrossRef]
- Rada, P.; Pardo, V.; Mobasher, M.A.; García-Martínez, I.; Ruiz, L.; González-Rodríguez, Á.; Sanchez-Ramos, C.; Muntané, J.; Alemany, S.; James, L.P.; et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxid. Redox Signal. 2018, 28, 1187–1208. [Google Scholar] [CrossRef]
- Farghali, H.; Kemelo, M.K.; Canová, N.K. SIRT1 modulators in experimentally induced liver injury. Oxidative Med. Cell Longev. 2019, 2019, 8765954. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhang, M.; Kageyama, S.; Ke, B.; Fujii, T.; Sosa, R.A.; Reed, E.F.; Datta, N.; Zarrinpar, A.; Busuttil, R.W.; et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J. Hepatol. 2017, 67, 1232–1242. [Google Scholar] [CrossRef]
- Pang, J.; Yin, L.; Jiang, W.; Wang, H.; Cheng, Q.; Jiang, Z.; Cao, Y.; Zhu, X.; Li, B.; Qian, S.; et al. Sirt1-mediated deacetylation of PGC-1α alleviated hepatic steatosis in type 2 diabetes mellitus via improving mitochondrial fatty acid oxidation. Cell. Signal. 2024, 124, 111478. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Wang, X.; Li, A.; Shan, A.; Ma, J. The promotion of fatty acid β-oxidation by hesperidin via activating SIRT1/PGC1α to improve NAFLD induced by a high-fat diet. Food Funct. 2024, 15, 372–386. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, Y.; Liu, J.; Li, P.; Luo, X.; Zhang, B. Activating SIRT1 deacetylates NF-κB p65 to alleviate liver inflammation and fibrosis via inhibiting NLRP3 pathway in macrophages. Int. J. Med. Sci. 2023, 20, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, T.; Ye, M.; Qiu, Y.; Pei, L.; Jiang, R.; Pang, N.; Huang, Y.; Liang, B.; Ling, W.; et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018, 17, 89–98. [Google Scholar] [CrossRef]
- El Shaffei, I.; Abdel-Latif, G.A.; Farag, D.B.; Schaalan, M.; Salama, R.M. Ameliorative effect of betanin on experimental cisplatin-induced liver injury; the novel impact of miRNA-34a on the SIRT1/PGC-1α signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, 1–14. [Google Scholar] [CrossRef]
- Ojo, O.A.; Rotimi, D.E.; Ojo, A.B.; Ogunlakin, A.D.; Ajiboye, B.O. Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats. Sci. Rep. 2023, 13, 1577. [Google Scholar] [CrossRef]
- Falahi, S.; Falahi, S.; Zarejousheghani, M.; Ehrlich, H.; Joseph, Y.; Rahimi, P. Electrochemical sensing of gallic acid in beverages using a 3D bio-nanocomposite based on carbon nanotubes/spongin-atacamite. Biosensors 2023, 13, 262. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Houshmand, G.; Goudarzi, M.; Sezavar, S.H.; Mehrzadi, S.; Mansouri, E.; Kalantar, M. Ameliorative effect of gallic acid on sodium arsenite-induced spleno-, cardio-and hemato-toxicity in rats. Life Sci. 2019, 217, 91–100. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225. [Google Scholar]
- Sarkaki, A.; Farbood, Y.; Gharib-Naseri, M.K.; Badavi, M.; Mansouri, M.T.; Haghparast, A.; Mirshekar, M.A. Gallic acid improved behavior, brain electrophysiology, and inflammation in a rat model of traumatic brain injury. Can. J. Physiol. Pharm. 2015, 93, 687–694. [Google Scholar] [CrossRef]
- Omobowale, T.O.; Oyagbemi, A.A.; Ajufo, U.E.; Adejumobi, O.A.; Ola-Davies, O.E.; Adedapo, A.A.; Yakubu, M.A. Ameliorative effect of gallic acid in doxorubicin-induced hepatotoxicity in Wistar rats through antioxidant defense system. J. Diet. Suppl. 2018, 15, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, M.; Heidarian, E.; Shaghaghi, M.; Roshanmehr, H.; Najafi, M.; Moradi, A.; Nouri, A. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats. Pharm. Biol. 2020, 58, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Anwar, M.M.; Farghaly, H.S.; Kandeil, M.A. Gallic acid and ferulic acid protect the liver from thioacetamide-induced fibrosis in rats via differential expression of miR-21, miR-30 and miR-200 and impact on TGF-β1/Smad3 signaling. Chem. Biol. Interact. 2020, 324, 109098. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Mahmoud, A.B.; Al-Salahy, M.B.; Mohamed Moustafa, F.A. Morphological, immunohistochemical, and biochemical study on the ameliorative effect of gallic acid against bisphenol A-induced nephrotoxicity in male albino rats. Sci. Rep. 2023, 13, 1732. [Google Scholar] [CrossRef]
- Luan, X.; Ou, W.; Hu, J.; Lu, J. Gallic acid alleviates lipopolysaccharide-induced renal injury in rats by inhibiting cell pro-death and inflammatory response and its mechanism. Cell. Mol. Biol. 2023, 69, 31–35. [Google Scholar] [CrossRef]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress. Mol. Med. Rep. 2021, 24, 1–19. [Google Scholar] [CrossRef]
- Gürer, H.; Özgünes, H.; Saygin, E.; Ercal, N. Antioxidant effect of taurine against lead-induced oxidative stress. Arch. Environ. Contam. Toxicol. 2001, 41, 397–402. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673. [Google Scholar]
- Dincer, S.İ.B.E.L.; Özenirler, S.; Öz, E.; Akyol, G.; Özoğul, C. The protective effect of taurine pretreatment on carbon tetrachloride-induced hepatic damage–a light and electron microscopic study. Amino Acids 2002, 22, 417–426. [Google Scholar] [CrossRef]
- Hagar, H.H. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol. Lett. 2004, 151, 335–343. [Google Scholar] [CrossRef]
- Ince, S.; Arslan-Acaroz, D.; Demirel, H.H.; Varol, N.; Ozyurek, H.A.; Zemheri, F.; Kucukkurt, I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed. Pharmacother. 2017, 96, 263–268. [Google Scholar] [CrossRef]
- Gedikli, E.; Barış, V.Ö.; Yersal, N.; Dinçsoy, A.B.; Müftüoğlu, S.F.; Erdem, A. Taurine protects doxorubicin-induced hepatotoxicity via its membrane-stabilizing effect in rats. Life 2023, 13, 2031. [Google Scholar] [CrossRef]
- Liu, J.; Ai, Y.; Niu, X.; Shang, F.; Li, Z.; Liu, H.; Li, W.; Ma, W.; Chen, R.; Wei, T.; et al. Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1–p53 activation. Chem. Biol. Interact. 2020, 317, 108972. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, T.V.; Aksoylu, E.; Wayne Goodman, D. Nonoxidative activation of methane. Catal. Rev. 2003, 45, 151–203. [Google Scholar] [CrossRef]
- Hamed, I.A.; Sherif, R.M.; El-Sheikh, E.S.A.; Aldawek, A.M.; Shalaby, A.A. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J. 2023, 13, 1334–1345. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 5th ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- El-Sheikh, E.S.A.; Hamed, I.A.; Alduwish, M.A.; Momenah, M.A.; Melebary, S.J.; Alsolmy, S.A.; Alghamdi, M.S.; Alharbi, A.A.; Sherif, R.M.; Shalaby, A.A. The Ameliorative Effect of Vitamin C against Sub-chronic Thiamethoxam Toxicity in Male Rats. Pak. Vet. J. 2024, 44, 803–811. [Google Scholar]
- Timbrell, J.A.; Seabra, V.; Waterfield, C.J. The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. Vasc. Syst. 1995, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Taziki, S.; Khori, V.; Jahanshahi, M.; Seifi, A.; Babakordi, F.B.; Nikmahzar, E.N. Protective role of taurine against hepatotoxicity induced by pyrazinamide in rats. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 824–828. [Google Scholar] [CrossRef]
- Abd-Allah, E.M.; Hilal, M.A.E.H.; Mohamed, D.S.; Hassan, A.O.; Said, A.M. Protective Effect of Taurine Against Bisphenol A-Induced Hepatotoxicity in Albino Rats. Egypt. J. Hosp. Med. 2022, 89, 6226–6230. [Google Scholar] [CrossRef]
- Dorğru-Abbasoğlu, S.; Kanbağli, Ö.; Balkan, J.; Cevikbaş, U.; Aykaç-Tokerl, G.; Uysall, M. The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum. Exp. Toxicol. 2001, 20, 23–27. [Google Scholar] [CrossRef]
- Olayinka, E.T.; Ore, A.; Ola, O.S.; Adeyemo, O.A. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med. Sci. 2015, 3, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Ojeaburu, S.I.; Oriakhi, K. Hepatoprotective, antioxidant and, anti-inflammatory potentials of gallic acid in carbon tetrachloride-induced hepatic damage in Wistar rats. Toxicol. Rep. 2021, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Apaydın, F.G.; Kalender, S.; Baş, H.; Kalender, Y. Protective role of gallic acid against fenitrothion-induced hepatotoxicity and nephrotoxicity via oxidative stress, histopathological and biochemical alterations. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Wang, X.; Anadón, A.; Wu, Q.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Mechanism of neonicotinoid toxicity: Impact on oxidative stress and metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef]
- Yan, S.H.; Wang, J.H.; Zhu, L.S.; Chen, A.M.; Wang, J. Thiamethoxam induces oxidative stress and antioxidant response in zebrafish (D anio R erio) livers. Environ. Toxicol. 2016, 31, 2006–2015. [Google Scholar] [CrossRef]
- Katić, A.; Kašuba, V.; Kopjar, N.; Lovaković, B.T.; Čermak, A.M.M.; Mendaš, G.; Micek, V.; Milic, M.; Pavicic, I.; Želježić, D.; et al. Effects of low-level imidacloprid oral exposure on cholinesterase activity, oxidative stress responses, and primary DNA damage in the blood and brain of male Wistar rats. Chem. Biol. Interact. 2021, 338, 109287. [Google Scholar] [CrossRef]
- Abd Elkader, H.T.A.E.; Hussein, M.; Mohammed, N.A.; Abdou, H.M. The protective role of l-carnitine on oxidative stress, neurotransmitter perturbations, astrogliosis, and apoptosis induced by thiamethoxam in the brains of male rats. N-S Arch. Pharmacol. 2024, 397, 4365–4379. [Google Scholar] [CrossRef]
- Duzguner, V.; Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pestic. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- Abduh, M.S.; Alruhaimi, R.S.; Alqhtani, H.A.; Hussein, O.E.; Abukhalil, M.H.; Kamel, E.M.; Mahmoud, A.M. Rosmarinic acid mitigates chlorpyrifos-induced oxidative stress, inflammation, and kidney injury in rats by modulating SIRT1 and Nrf2/HO-1 signaling. Life Sci. 2023, 313, 121281. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef]
- Huss, J.M.; Kelly, D.P. Nuclear receptor signaling and cardiac energeties. Circ. Res. 2004, 95, 568–578. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; Günay, A.; Parlak, V.; Yeltekin, A.C.; Ozgeris, F.B.; Turkez, H.; Alak, G.; Atamanalp, M. Modulatory role ulexit against thiamethoxam-induced hematotoxicity/hepatotoxicity oxidative stress and immunotoxicity in Oncorhynchus mykiss. Mar. Environ. Res. 2024, 193, 106294. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Lombardini, J.B.; Azuma, J. Interaction between the actions of taurine and angiotensin II. Amino Acids 2000, 18, 305–318. [Google Scholar] [CrossRef]
- de Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Wang, N.; Tong, J.; Sun, W.; Yang, J.; Wu, G. Alleviation of taurine on liver injury of type 2 diabetic rats by improving antioxidant and anti-inflammatory capacity. Heliyon 2024, 10, e28400. [Google Scholar] [CrossRef]
- Sanjay, S.; Girish, C.; Toi, P.C.; Bobby, Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signalling cascades in Wistar Rats. J. Pharm. Pharmacol. 2021, 73, 473–486. [Google Scholar] [CrossRef]
- Chang, W.T.; Huang, S.C.; Cheng, H.L.; Chen, S.C.; Hsu, C.L. Rutin and gallic acid regulates mitochondrial functions via the SIRT1 pathway in C2C12 myotubes. Antioxidants 2021, 10, 286. [Google Scholar] [CrossRef]
- Moghadam, D.; Zarei, R.; Vakili, S.; Ghojoghi, R.; Zarezade, V.; Veisi, A.; Sabaghan, M.; Azadbakht, O.; Behrouj, H. The effect of natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: Role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol. Biol. Rep. 2023, 50, 77–84. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Rubartelli, A. Redox control of NLRP3 inflammasome activation in health and disease. J. Leukoc. Biol. 2012, 92, 951–958. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Beigi, T.; Safi, A.; Satvati, M.; Kalantari-Hesari, A.; Ahmadi, R.; Meshkibaf, M.H. Protective role of ellagic acid and taurine against fluoxetine induced hepatotoxic effects on biochemical and oxidative stress parameters, histopathological changes, and gene expressions of IL-1β, NF-κB, and TNF-α in male Wistar rats. Life Sci. 2022, 304, 120679. [Google Scholar] [CrossRef]
- Zheng, J.; Qiu, G.; Zhou, Y.; Ma, K.; Cui, S. Hepatoprotective effects of taurine against cadmium-induced liver injury in female mice. Biol. Trace Elem. Res. 2023, 201, 1368–1376. [Google Scholar] [CrossRef]
- Singla, E.; Dharwal, V.; Naura, A.S. Gallic acid protects against the COPD-linked lung inflammation and emphysema in mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The interplay of oxidative stress and inflammation: Mechanistic insights and therapeutic potential of antioxidants. Oxidative Med. Cell Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Oren, M. Relationship of p53 to the control of apoptotic cell death. In Seminars in Cancer Biology; Elsevier Science Ltd.: London, UK, 1994; Volume 5, pp. 221–227. [Google Scholar]
- Shi, Y.; Chen, J.; Weng, C.; Chen, R.; Zheng, Y.; Chen, Q.; Tang, H. Identification of the protein–protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003, 305, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: The roads to ruin. Cell 1998, 94, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, F.E.; El-Rahman, G.I.A.; Behairy, A.; Abd-Elhakim, Y.M.; Saber, T.M.; Metwally, M.M.; El-Fatah, S.S.A.; Samaha, M.M.; Saber, T.; Aglan, M.A. Thymol’s modulation of cellular macromolecules, oxidative stress, DNA damage, and NF-kB/caspase-3 signaling in the liver of imidacloprid-exposed rats. Environ. Toxicol. Pharmacol. 2024, 109, 104492. [Google Scholar] [CrossRef]
- Rashid, K.; Das, J.; Sil, P.C. Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem. Toxicol. 2013, 51, 317–329. [Google Scholar] [CrossRef]
- Durgun, C.; Deveci, E. Gallic acid treatment protects intestinal tissue against ischaemia-reperfusion. Folia Morphol. 2023, 82, 633–640. [Google Scholar] [CrossRef]
- Ong, A.L.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef]
- Ramkumar, K.; Vijayakumar, R.; Vanitha, P.; Suganya, N.; Manjula, C.; Rajaguru, P.; Sivasubramanian, S.; Gunasekaran, P. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum. Exp. Toxicol. 2014, 33, 638–649. [Google Scholar] [CrossRef]
- Kaur, S.; Muthuraman, A. Ameliorative effect of gallic acid in paclitaxel-induced neuropathic pain in mice. Toxicol. Rep. 2019, 6, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, H.A.C.; Dymock, J.F. The determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beutler, E Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888.
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Smythe, G.; Sachdev, P.; Guillemin, G.J. Differential expression of sirtuins in the aging rat brain. Front. Cell. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef]
- Belviranlı, M.; Okudan, N. Exercise training protects against aging-induced cognitive dysfunction via activation of the hippocampal PGC-1α/FNDC5/BDNF pathway. Neuromolecular Med. 2018, 20, 386–400. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueyama, T.; Nishi, T.; Yamamoto, Y.; Kawakoshi, A.; Sunami, S.; Iguchi, M.; Tamai, H.; Ueda, K.; Ito, T.; et al. Nrf2-inducing anti-oxidation stress response in the rat liver-new beneficial effect of lansoprazole. PLoS ONE 2015, 9, e97419. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, X.; Gong, X.; Zhou, H.; Cai, C. Effects of hyperoxia exposure on the expression of Nrf2 and heme oxygenase-1 in lung tissues of premature rats. Mol. Cell. Probes 2020, 51, 101529. [Google Scholar] [CrossRef]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef]
- Deng, W.G.; Fu, Y.; Li, Y.L.; Sugiyama, T. Potential role of p53 mutation in chemical hepatocarcinogenesis of rats. World J. Gastroenterol. 2004, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Messaoudi, I.; Said, L.; El Heni, J.; Kerkeni, A.; Said, K. Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch. Environ. Contam. Toxicol. 2010, 59, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Chu, L.; Li, P.; Song, T.; Han, X.; Zhang, X.; Song, Q.; Liu, T.; Zhang, Y.; Zhang, J. Protective effects of tannic acid on pressure overload-induced cardiac hypertrophy and underlying mechanisms in rats. J. Pharm. Pharmacol. 2017, 69, 1191–1207. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y.; Xue, Y.; Han, X.; Zhang, X.; Ma, Z.; Sun, S.; Chu, X.; Cheng, J.; Guan, S.; et al. Crocin attenuates isoprenaline-induced myocardial fibrosis by targeting TLR4/NF-κB signaling: Connecting oxidative stress, inflammation, and apoptosis. N-S Arch. Pharmacol. 2020, 393, 13–23. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Zheng, B.; Liang, Y.; Shi, J.; Zhang, J.; Gao, Y. Ameliorative effects and mechanism of crocetin in arsenic trioxide-induced cardiotoxicity in rats. Mol. Med. Rep. 2020, 22, 5271–5281. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]




| Experimental Groups | Body Weight (gm) | Body Weight Changes (%) | ||||
|---|---|---|---|---|---|---|
| 0 day | 7th day | 14th day | 21st day | 28th day | ||
| Control | 172.53 ± 3.03 | 183.55 ± 3.69 | 190.26 ± 4.75 | 196.76 ± 5.40 | 204.35 ± 7.61 | 18.25 ± 2.52 |
| TAU | 170.60 ± 2.45 | 182.45 ± 3.15 | 192.73 ± 3.90 | 201.45 ±4.47 | 207.93 ± 8.20 | 21.70 ± 3.36 |
| GA | 171.08 ± 2.91 | 184.33 ± 4.23 | 195.43 ± 5.45 | 205.02 ± 5.13 | 211.28 ± 9.29 | 23.24 ± 3.48 |
| TMX | 173.32 ± 2.39 | 176.16 ± 3.44 | 167.25 ± 5.41 * | 161.53 ± 6.11 ** | 158.10 ± 8.48 ** | −9.01 ± 3.69 *** |
| TMX + TAU | 170.16 ± 2.96 | 173.55 ± 3.03 | 177.60 ± 3.68 | 180.73 ± 3.88 | 184.72 ± 3.53 # | 8.53 ± 0.54 ## |
| TMX + GA | 174.28 ± 2.41 | 176.72 ± 2.81 | 179.25 ± 2.69 | 187.15 ± 3.43 # | 196.43 ± 6.02 # | 12.59 ± 2.05 ### |
| TMX + TAU + GA | 171.76 ± 2.34 | 175.81 ± 2.99 | 183.71 ± 3.65 | 194.02 ± 4.12 ## | 201.51 ± 6.32 ## | 17.18 ± 2.11 ### |
| Experimental Groups | ALT (U/L) | ALP (U/L) | Total Protein (g/dL) | Albumin (g/dL) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|---|
| Control | 27.25 ± 2.21 | 193.42 ± 8.97 | 8.60 ± 0.35 | 4.86 ± 0.37 | 0.25 ± 0.02 |
| TAU | 28.18 ± 2.53 | 191.53 ± 7.27 | 9.10 ± 0.54 | 5.08 ± 0.34 | 0.27 ± 0.03 |
| GA | 25.40 ± 2.77 | 187.26 ± 9.67 | 8.95 ± 0.41 | 4.60 ± 0.25 | 0.22 ± 0.02 |
| TMX | 74.56 ± 5.94 *** | 372.88 ± 23.49 *** | 3.85 ±0.45 *** | 1.76 ± 0.19 *** | 1.33 ± 0.17 *** |
| TMX + TAU | 48.38 ± 4.62 ** ### | 264.61 ± 20.96 * ### | 6.70 ± 0.48 ## | 3.18 ± 0.27 ** # | 0.68 ± 0.04 ** ### |
| TMX + GA | 35.85 ± 3.80 ### | 241.11 ± 16.73 ### | 7.15 ± 0.57 ### | 3.61 ± 0.26 ## | 0.53 ± 0.05 ### |
| TMX + TAU + GA | 31.00 ± 3.10 ### ∆ | 215.30 ± 12.36 ### | 8.01 ± 0.34 ### | 4.40 ± 0.34 ### | 0.34 ± 0.03 ### ∆ |
| Experimental Groups | MDA (nmole/g Tissue) | NO (µmol/g Tissue) | SOD (U/g Tissue) | CAT (U/g Tissue) | GSH (mg/g Tissue) |
|---|---|---|---|---|---|
| Control | 46.40 ± 4.25 | 63.18 ± 4.43 | 42.61 ± 3.60 | 6.28 ± 0.27 | 16.98 ± 1.78 |
| TAU | 43.76 ± 3.79 | 62.56 ± 4.90 | 44.10 ± 3.35 | 6.11 ± 0.43 | 19.78 ± 1.91 |
| GA | 38.68 ± 4.46 | 59.45 ± 3.96 | 48.21 ± 4.48 | 6.65 ± 0.35 | 20.65 ± 1.86 |
| TMX | 110.32 ± 8.78 *** | 142.60 ± 12.17 *** | 17.23 ± 1.58 *** | 2.13 ± 0.31 *** | 4.31 ± 0.59 *** |
| TMX + TAU | 81.08 ± 6.31 ** # | 103.61 ± 5.01 ** ## | 29.05 ± 2.45 # | 3.83 ± 0.30 *** # | 11.46 ± 1.07 # |
| TMX + GA | 69.82 ± 3.96 ### | 87.32 ± 6.44 ### | 33.08 ± 3.56 # | 5.01 ± 0.45 ### | 14.11 ± 1.15 ### |
| TMX + TAU + GA | 54.13 ± 5.92 ### ∆ | 76.60 ± 6.75 ### | 40.15 ± 3.92 ### | 5.75 ± 0.30 ### ∆ | 16.00 ± 1.63 ### |
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | References |
|---|---|---|---|
| SIRT-1 | CACCAGAAAGAACTTCACCACCAG | ACCATCAAGCCGCCTACTAATCTG | Braidy et al. [100] |
| PGC-1α | AATGAATGCAGCGGTCTTAG | GTCTTTGTGGCTTTTGCTGT | Belviranlı and Okudan, [101] |
| Nrf2 | CACATCCAGACAGACACCAGT | CTACAAATGGGAATGTCTCTGC | Yamashita et al. [102] |
| HO-1 | GGCTTTAAGCTGGTGATGGC | GGGTTCTGCTTGTTTCGCTC | Chu et al. [103] |
| NLRP3 p53 | CAGACCTCCAAGACCACGACTG GTCGGCTCCGACTATACCACTAT | CATCCGCAGCCAATGAACAGAG CTCTCTTTGCACTCCCTGGGGG | Samra et al. [104] Deng et al. [105] |
| β-actin | TCCTCCTGAGCGCAAGTACTCT | GCTCAGTAACAGTCCGCCTAGA | Banni et al. [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elazab, S.T.; Safhi, F.A.; Al-Akeel, R.K.; Deraz, R.H.; Sarkar, S.; Gamal Eldin, R.E.A. Gallic Acid and Taurine Attenuate Thiamethoxam-Induced Hepatotoxicity in Rats by Modulating SIRT-1/PGC-1α, NF-κB/iNOS, and p53/Bax/Caspase-3 Pathways. Pharmaceuticals 2025, 18, 1112. https://doi.org/10.3390/ph18081112
Elazab ST, Safhi FA, Al-Akeel RK, Deraz RH, Sarkar S, Gamal Eldin REA. Gallic Acid and Taurine Attenuate Thiamethoxam-Induced Hepatotoxicity in Rats by Modulating SIRT-1/PGC-1α, NF-κB/iNOS, and p53/Bax/Caspase-3 Pathways. Pharmaceuticals. 2025; 18(8):1112. https://doi.org/10.3390/ph18081112
Chicago/Turabian StyleElazab, Sara T., Fatmah A. Safhi, Rasha K. Al-Akeel, Raghda H. Deraz, Souvarish Sarkar, and Rania Essam Ali Gamal Eldin. 2025. "Gallic Acid and Taurine Attenuate Thiamethoxam-Induced Hepatotoxicity in Rats by Modulating SIRT-1/PGC-1α, NF-κB/iNOS, and p53/Bax/Caspase-3 Pathways" Pharmaceuticals 18, no. 8: 1112. https://doi.org/10.3390/ph18081112
APA StyleElazab, S. T., Safhi, F. A., Al-Akeel, R. K., Deraz, R. H., Sarkar, S., & Gamal Eldin, R. E. A. (2025). Gallic Acid and Taurine Attenuate Thiamethoxam-Induced Hepatotoxicity in Rats by Modulating SIRT-1/PGC-1α, NF-κB/iNOS, and p53/Bax/Caspase-3 Pathways. Pharmaceuticals, 18(8), 1112. https://doi.org/10.3390/ph18081112






