UPLC-MS/MS Metabolomics Reveals Babao Dan’s Mechanisms in MASH Treatment with Integrating Network Pharmacology and Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Network Pharmacology
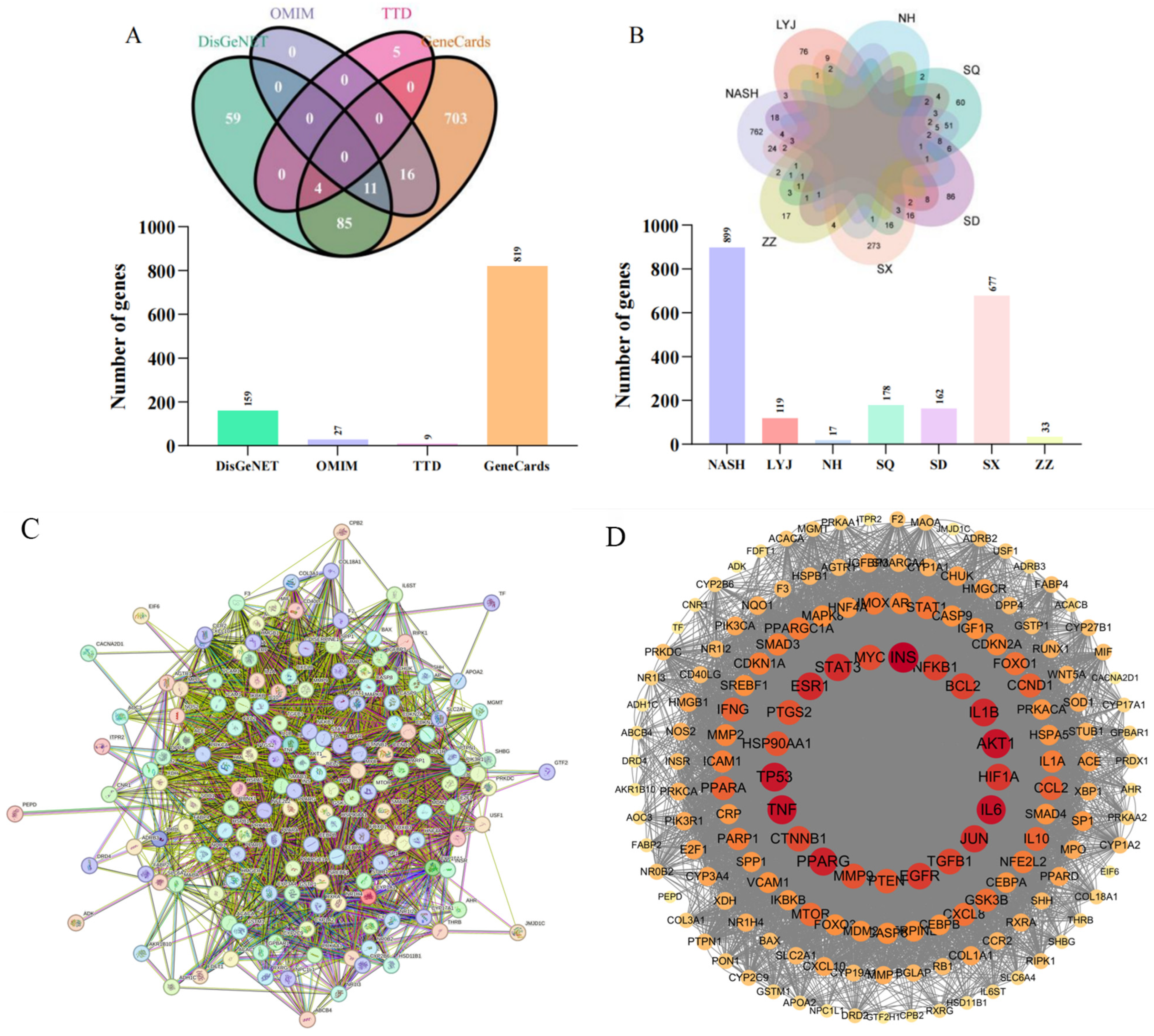
2.1.1. BBD and MASH Common Target Identification
2.1.2. PPI Network and Core Targets
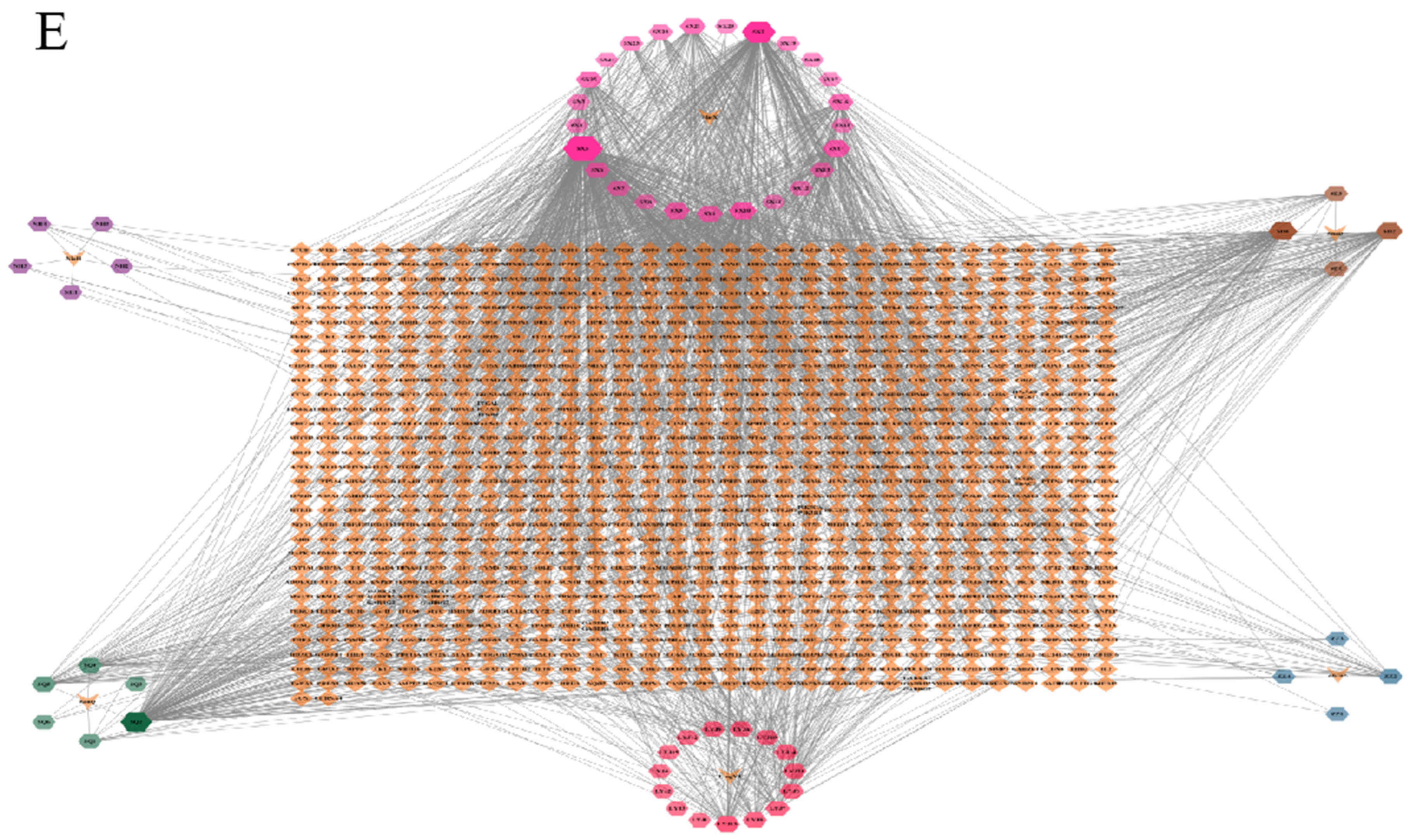
2.1.3. Drug Active Ingredient Target Map
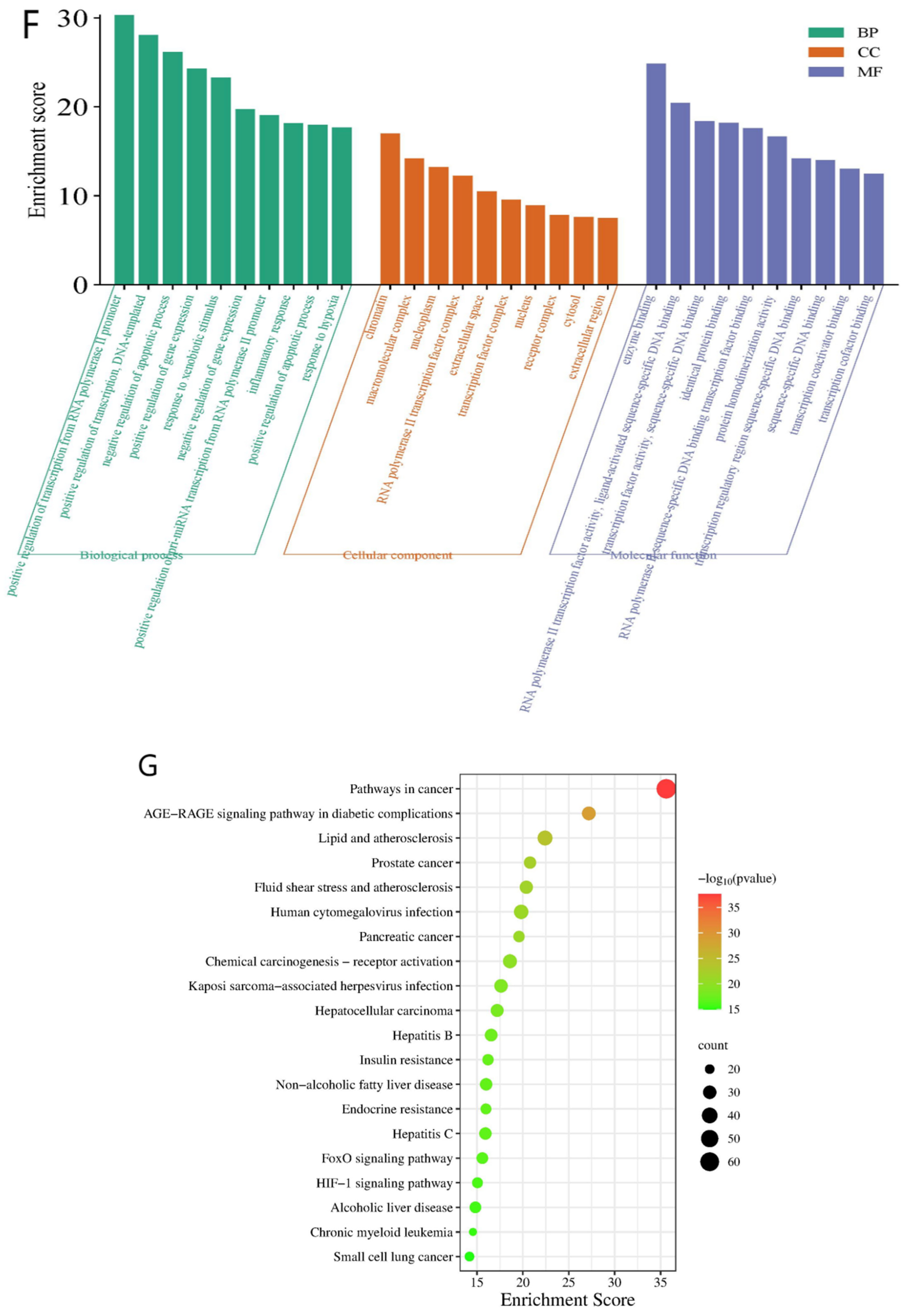
2.1.4. Enrichment Analysis
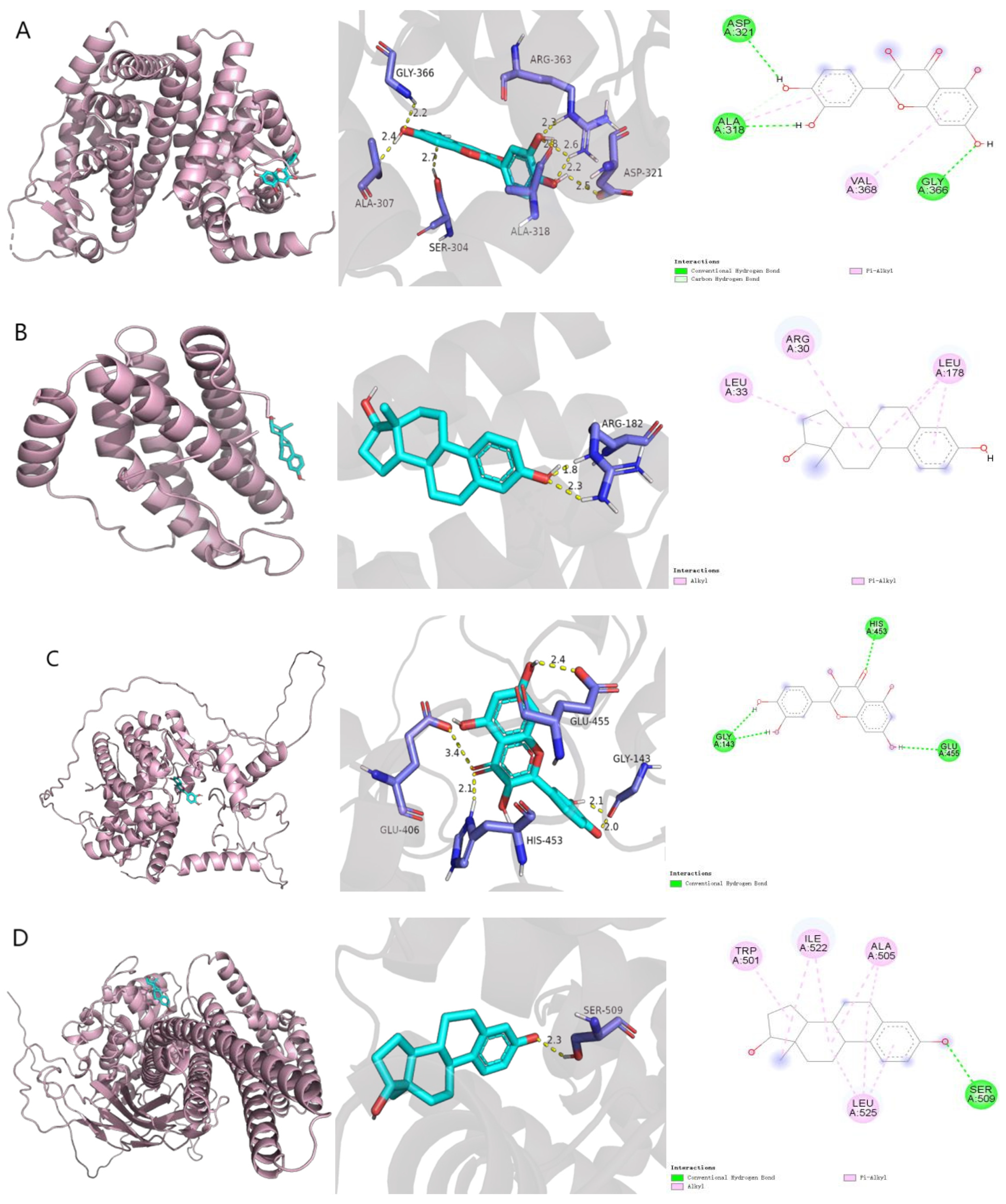
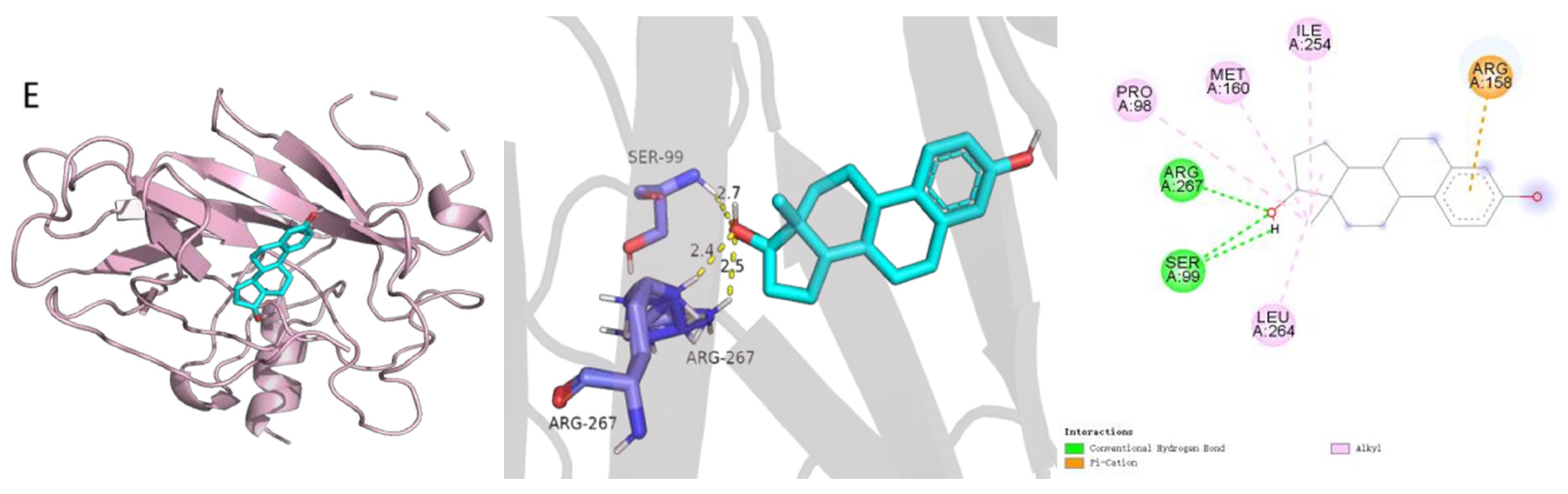
2.2. Molecular Docking
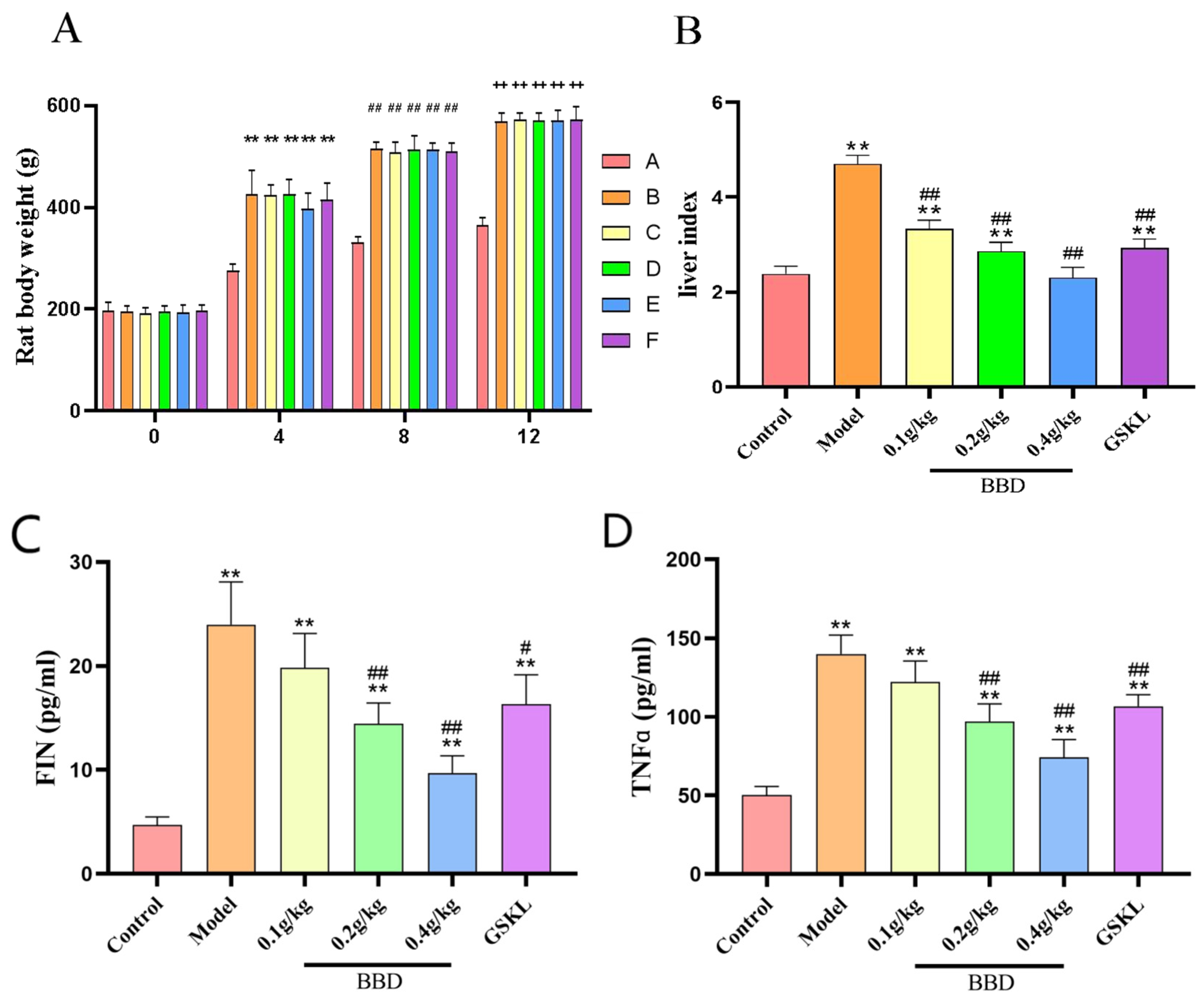
2.3. Changes in Body Weight and Liver Index in Rats
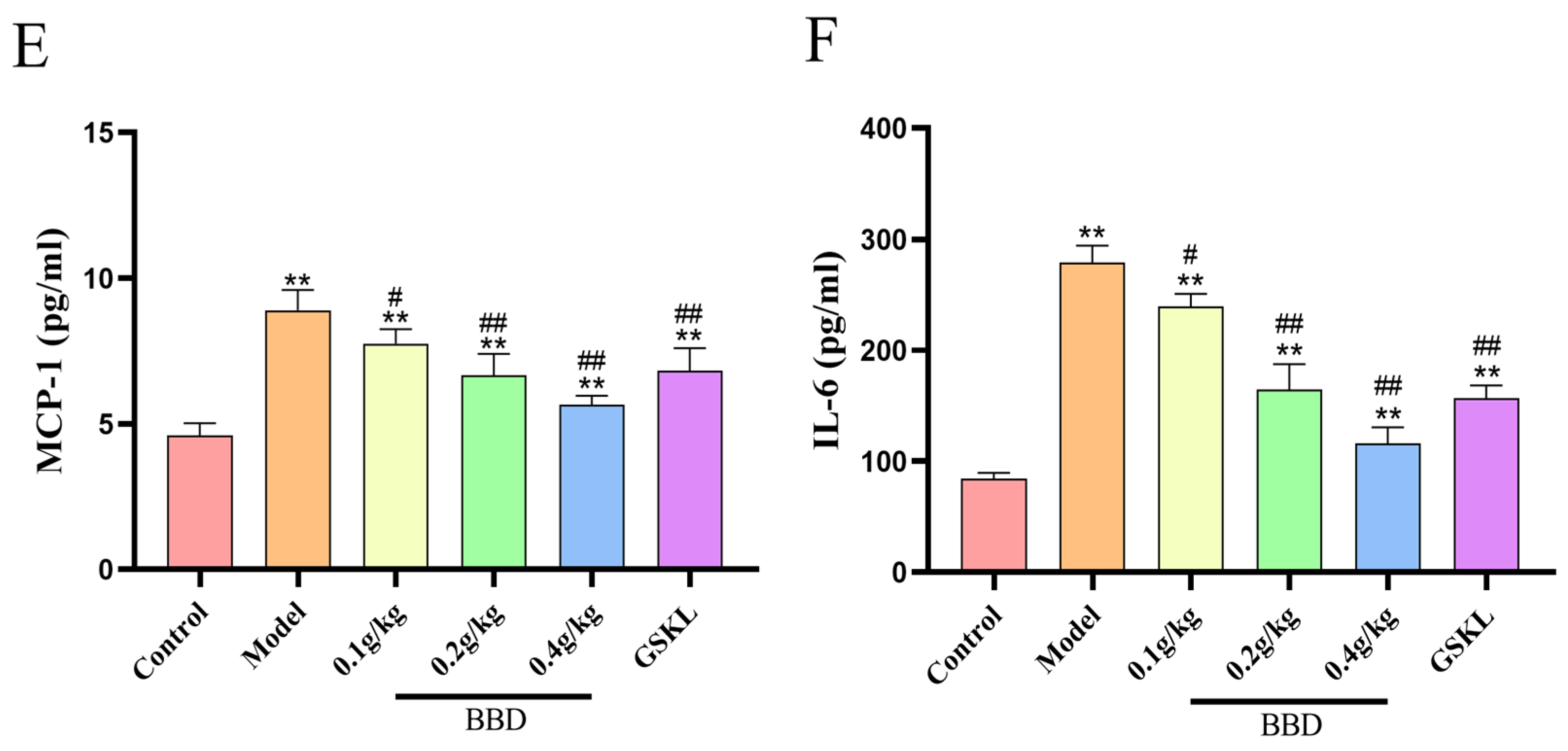
2.4. ELISA Results
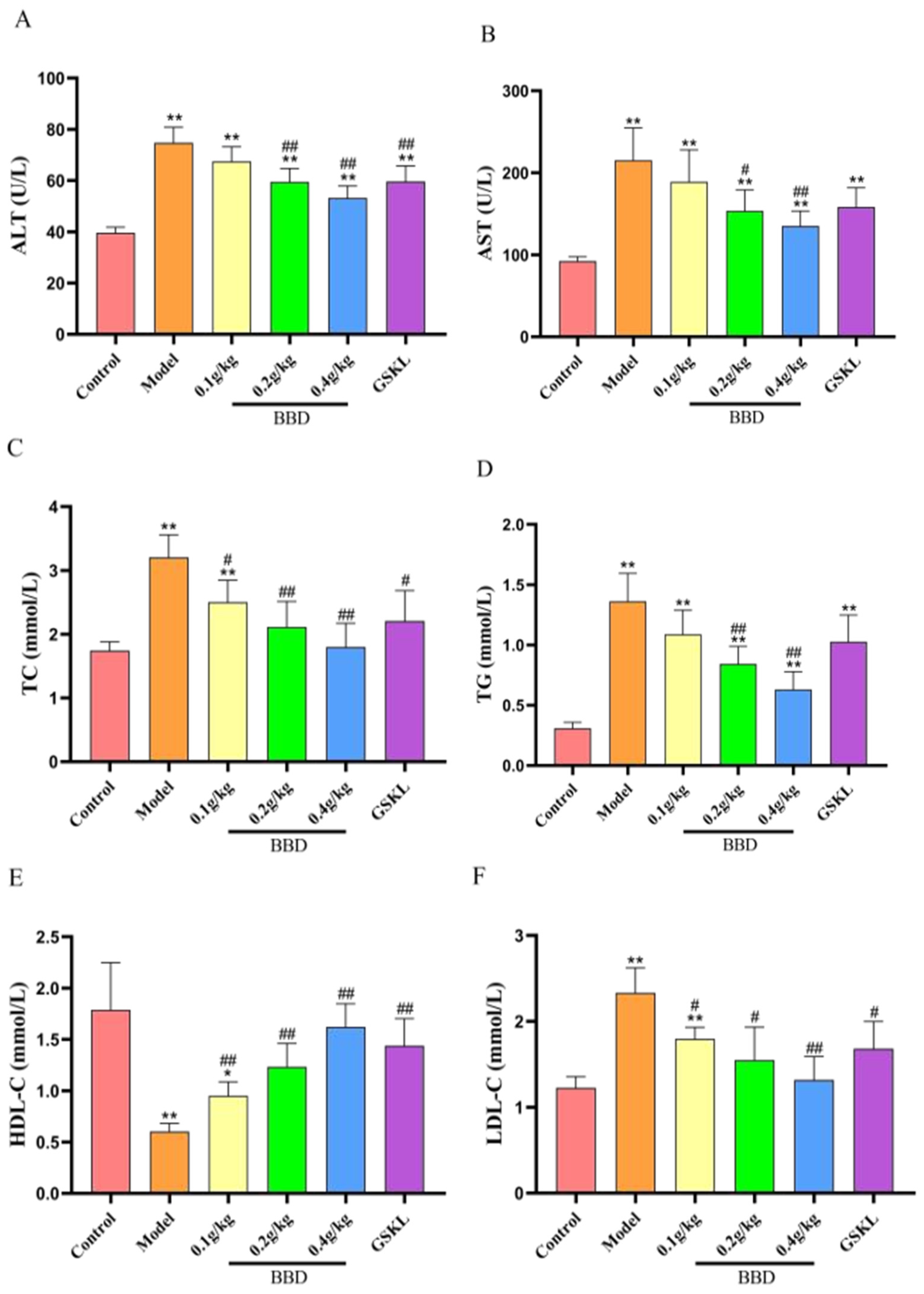
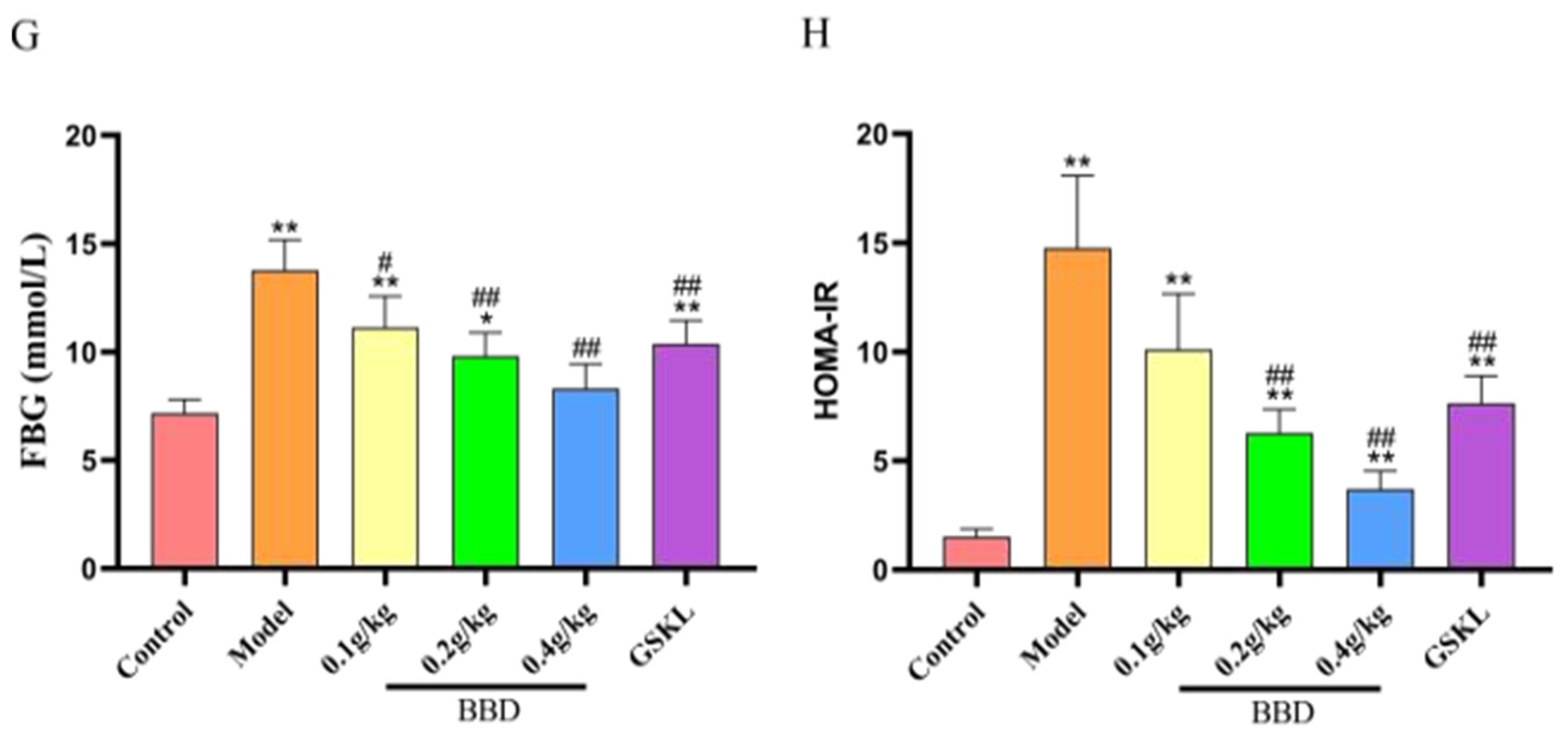
2.5. Biochemical Index Testing Results
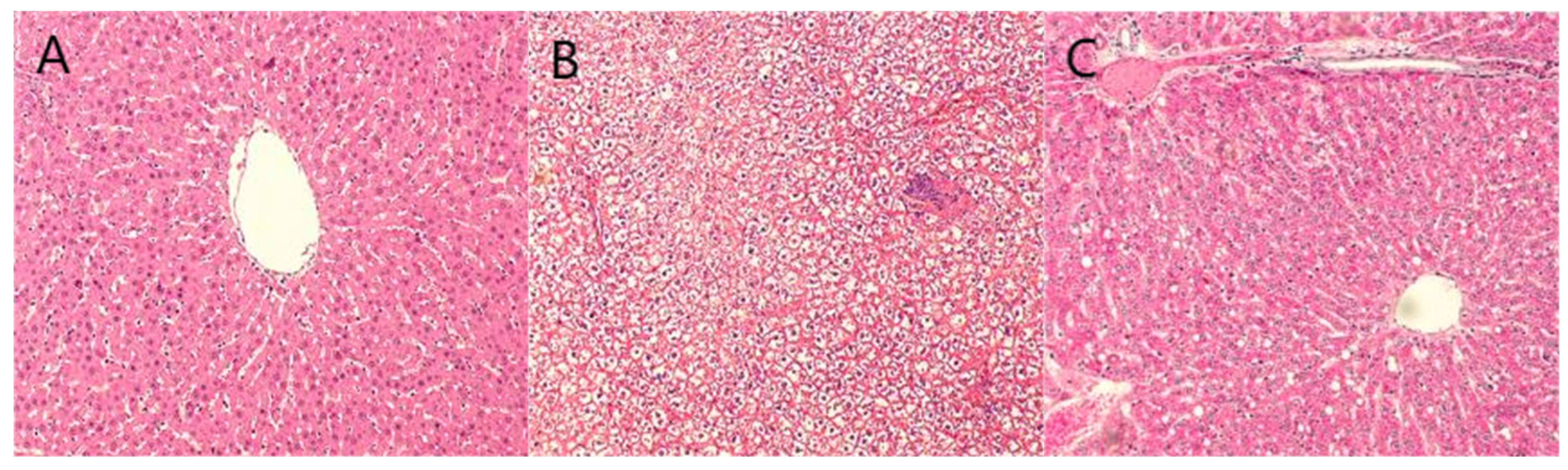
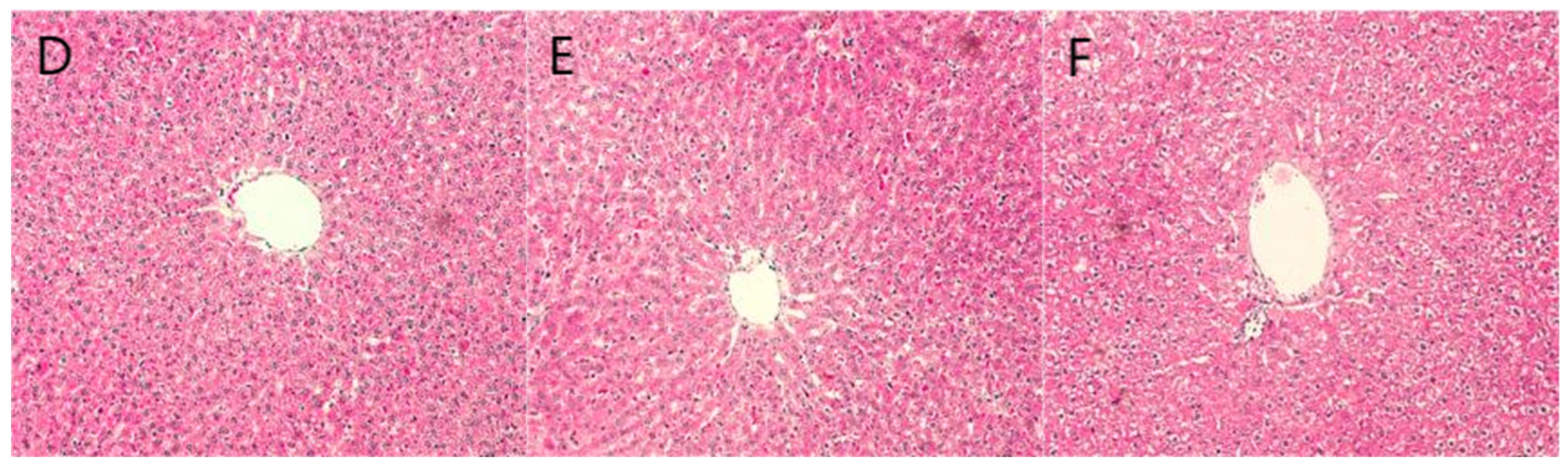
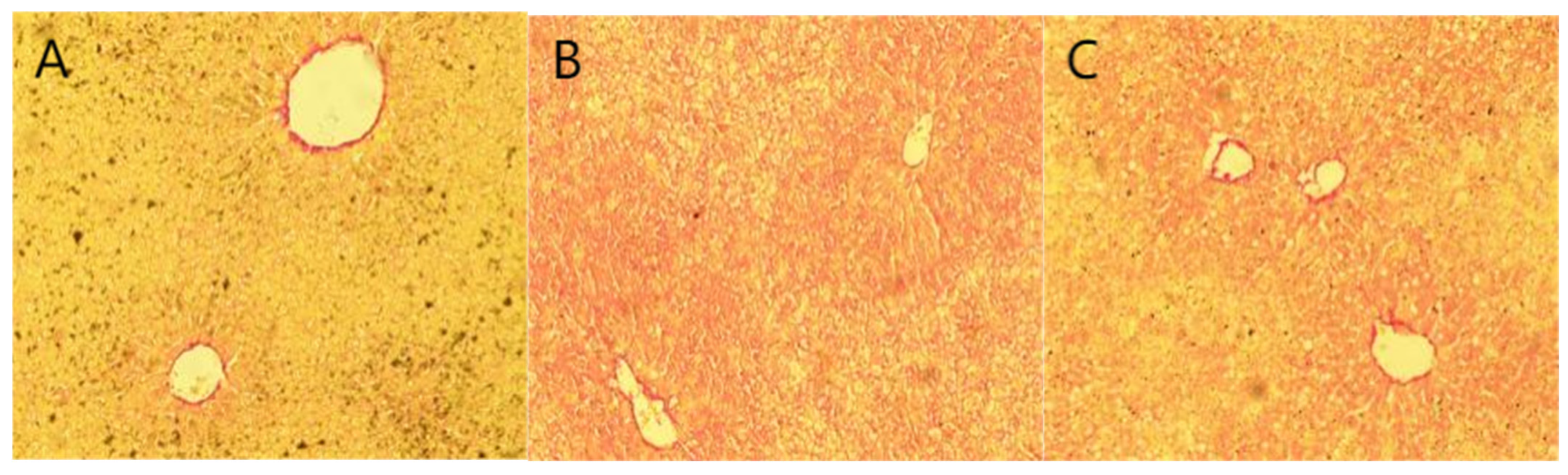
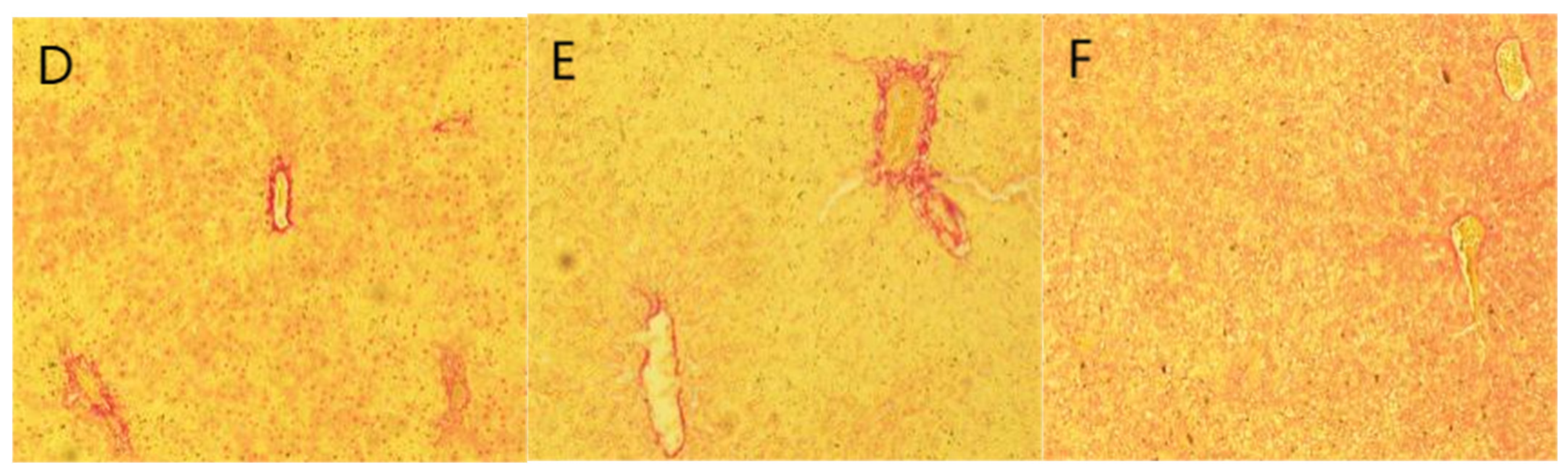
2.6. Staining Results
2.7. Metabolomics
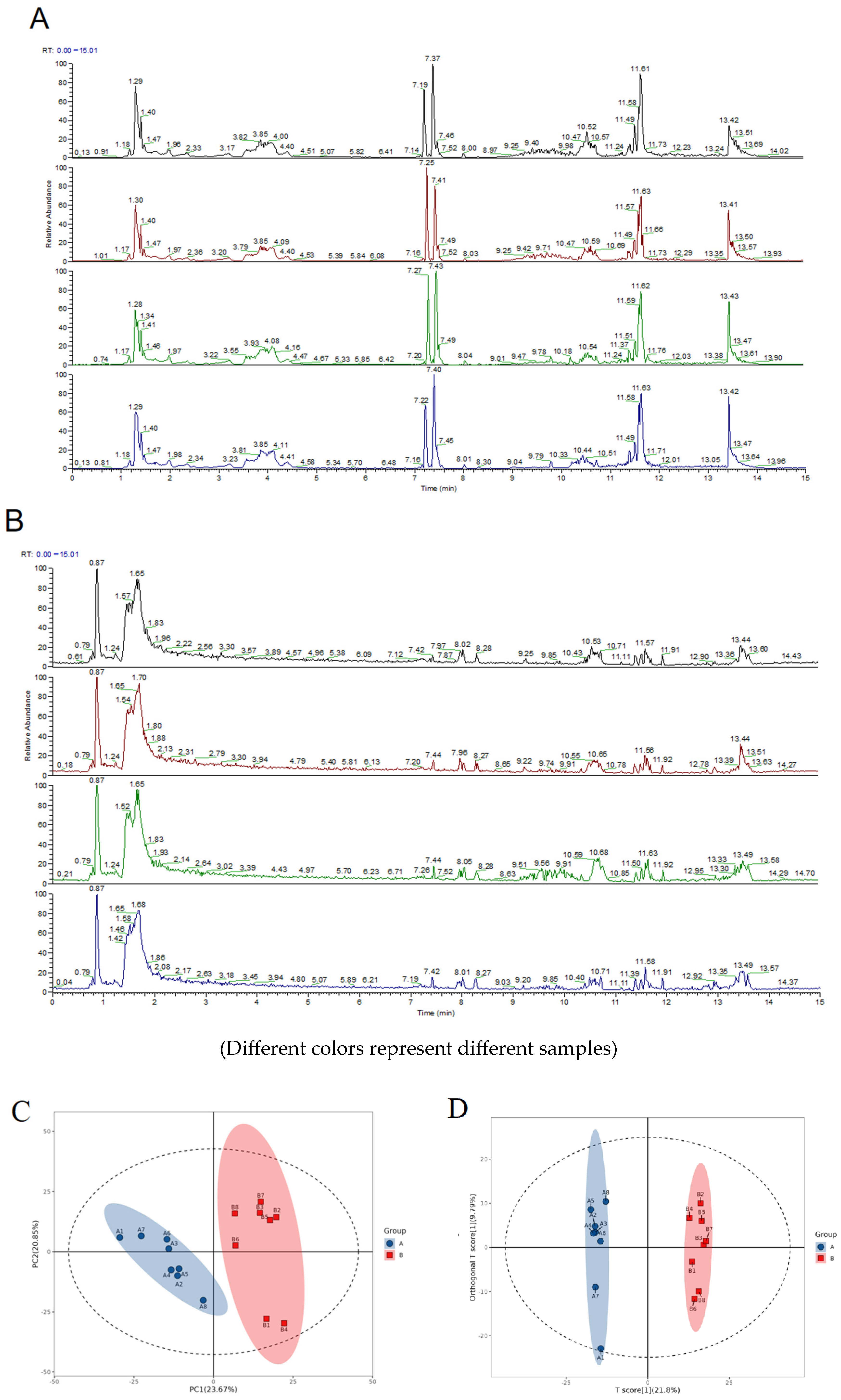
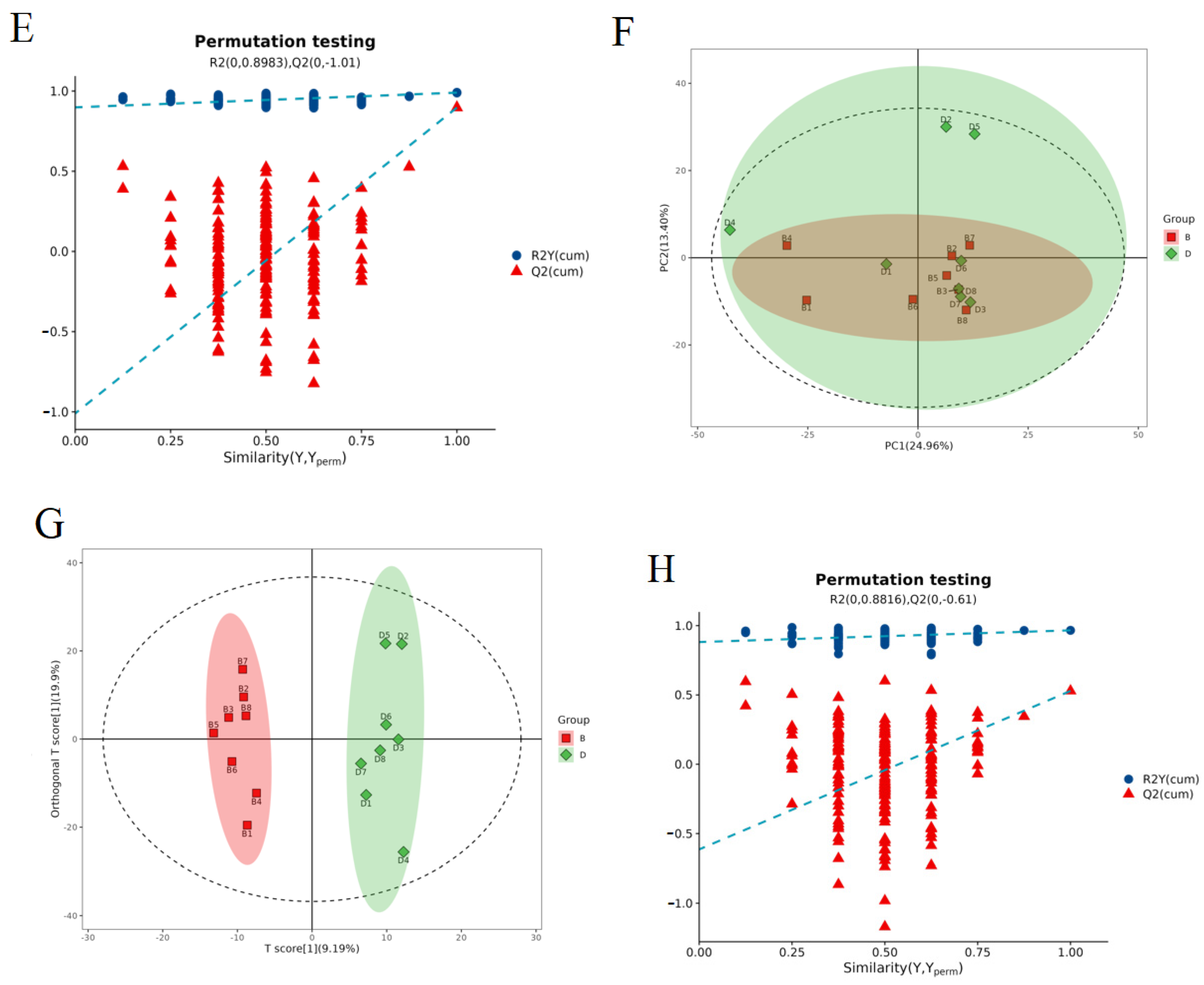
2.7.1. Evaluation of the Quality of the Experiments
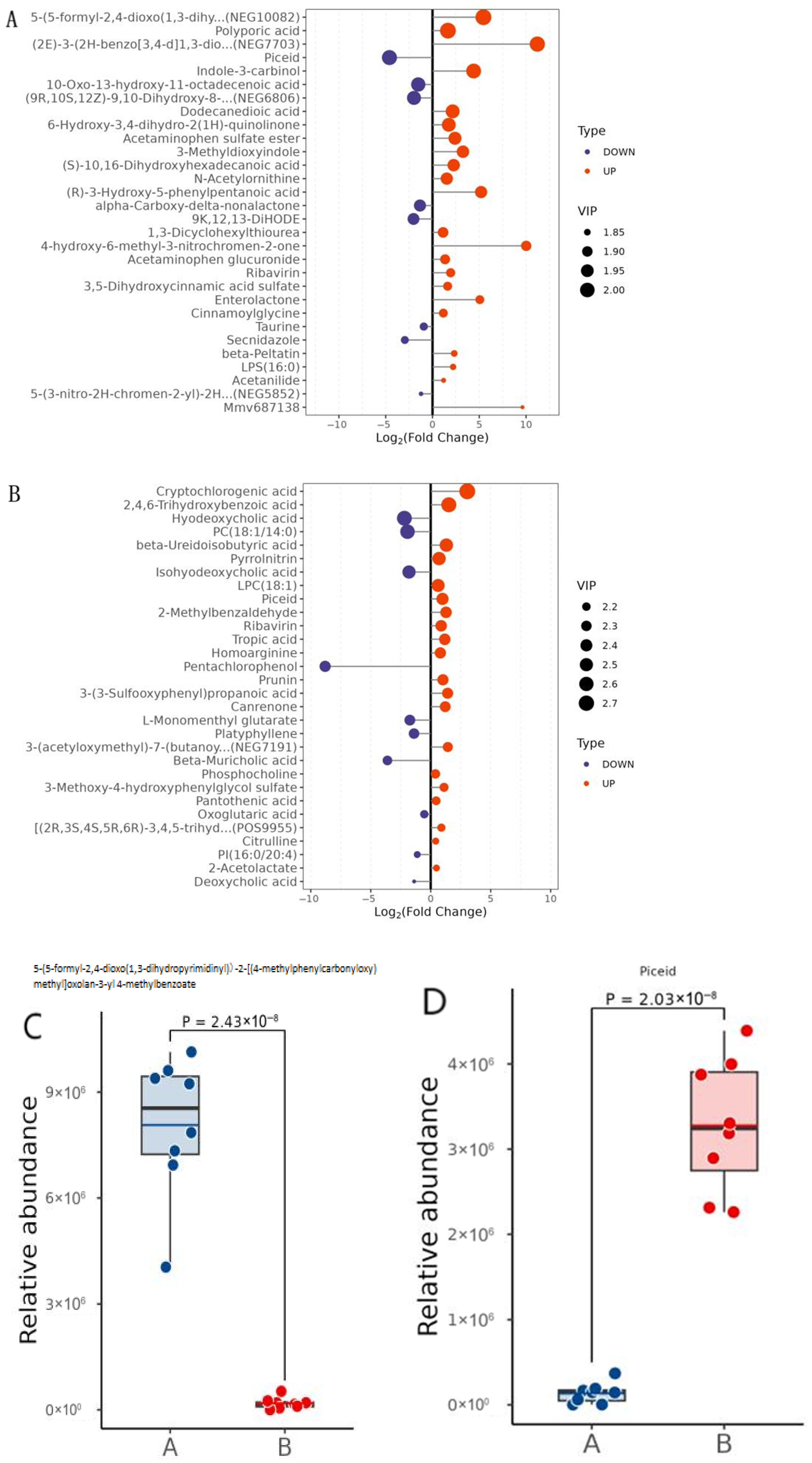
2.7.2. Significantly Different Metabolite Statistics
2.7.3. Differential Metabolite Significance Analysis
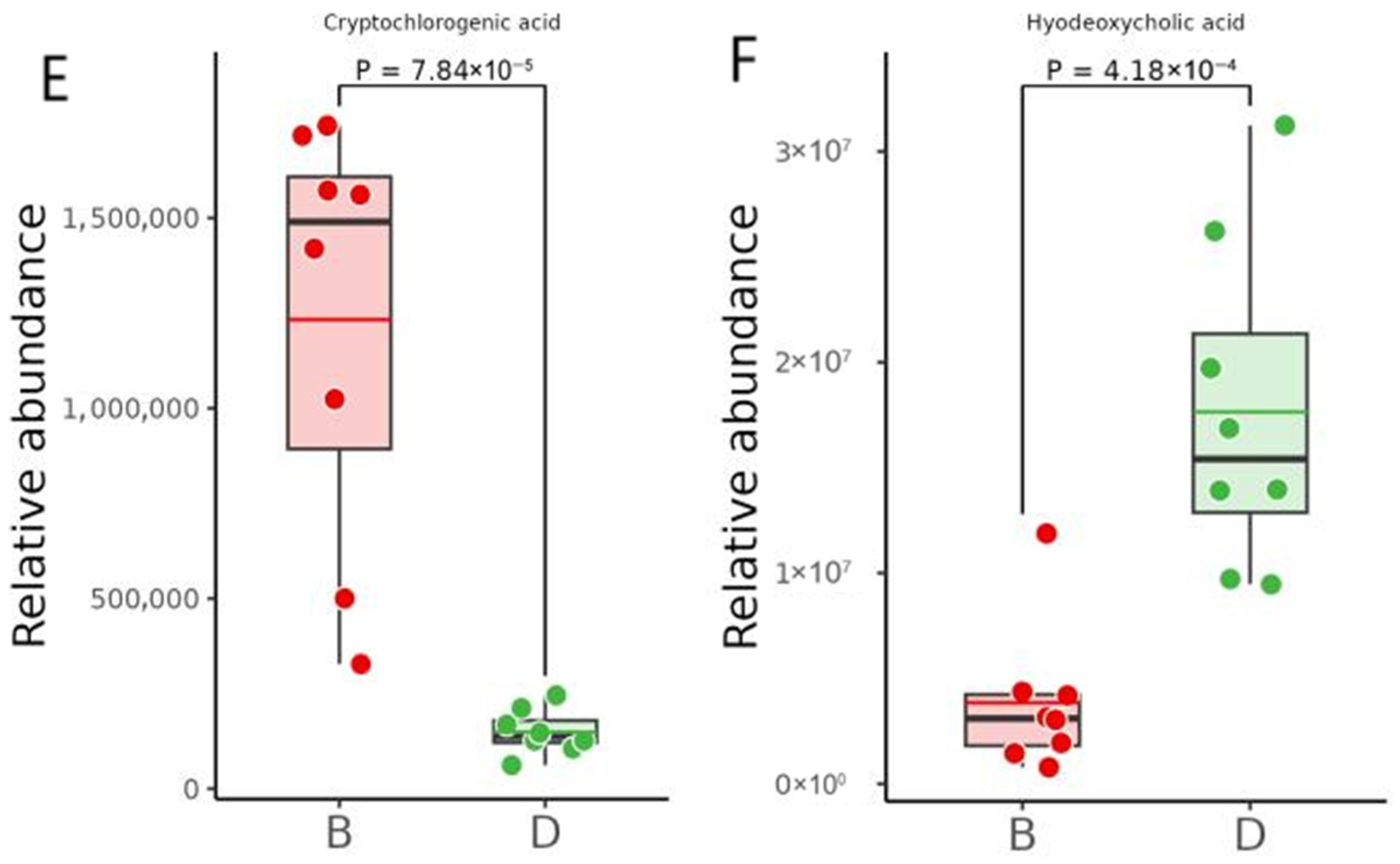
2.7.4. Differential Metabolite Abundance Analysis
2.8. Metabolomic Bioinformatics Analysis
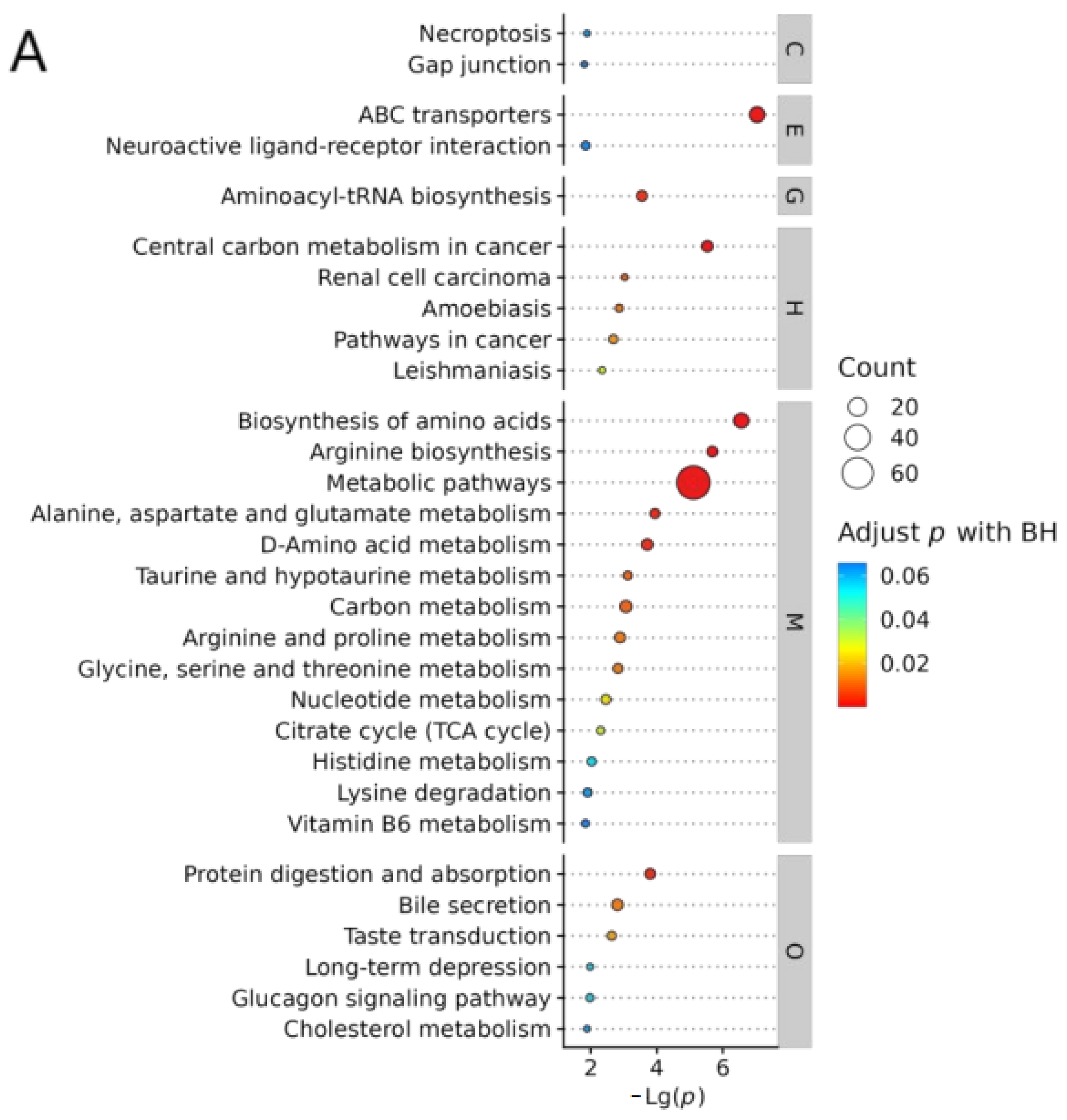
2.8.1. KEGG
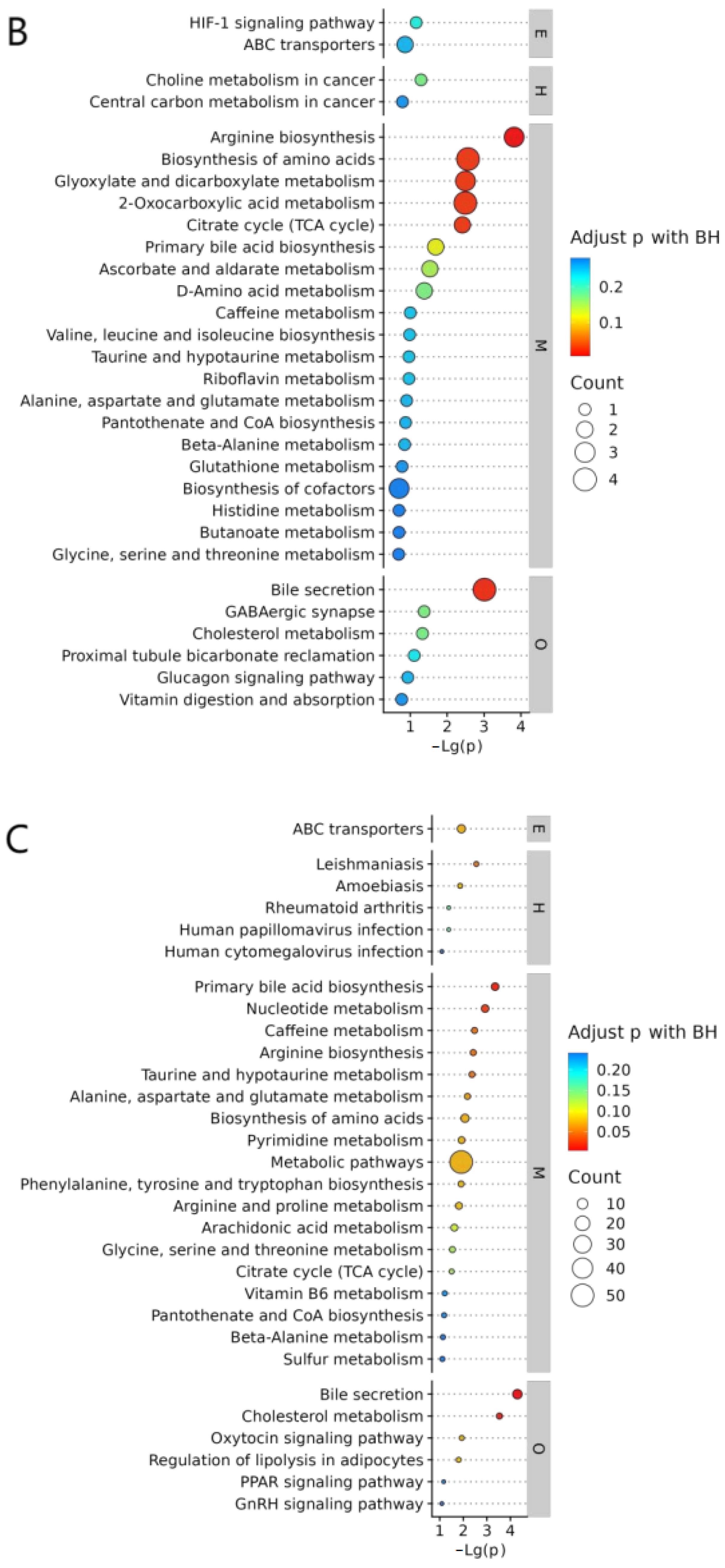
2.8.2. Functional Interaction Network Diagrams for Pathways
3. Discussion
3.1. Network Pharmacology
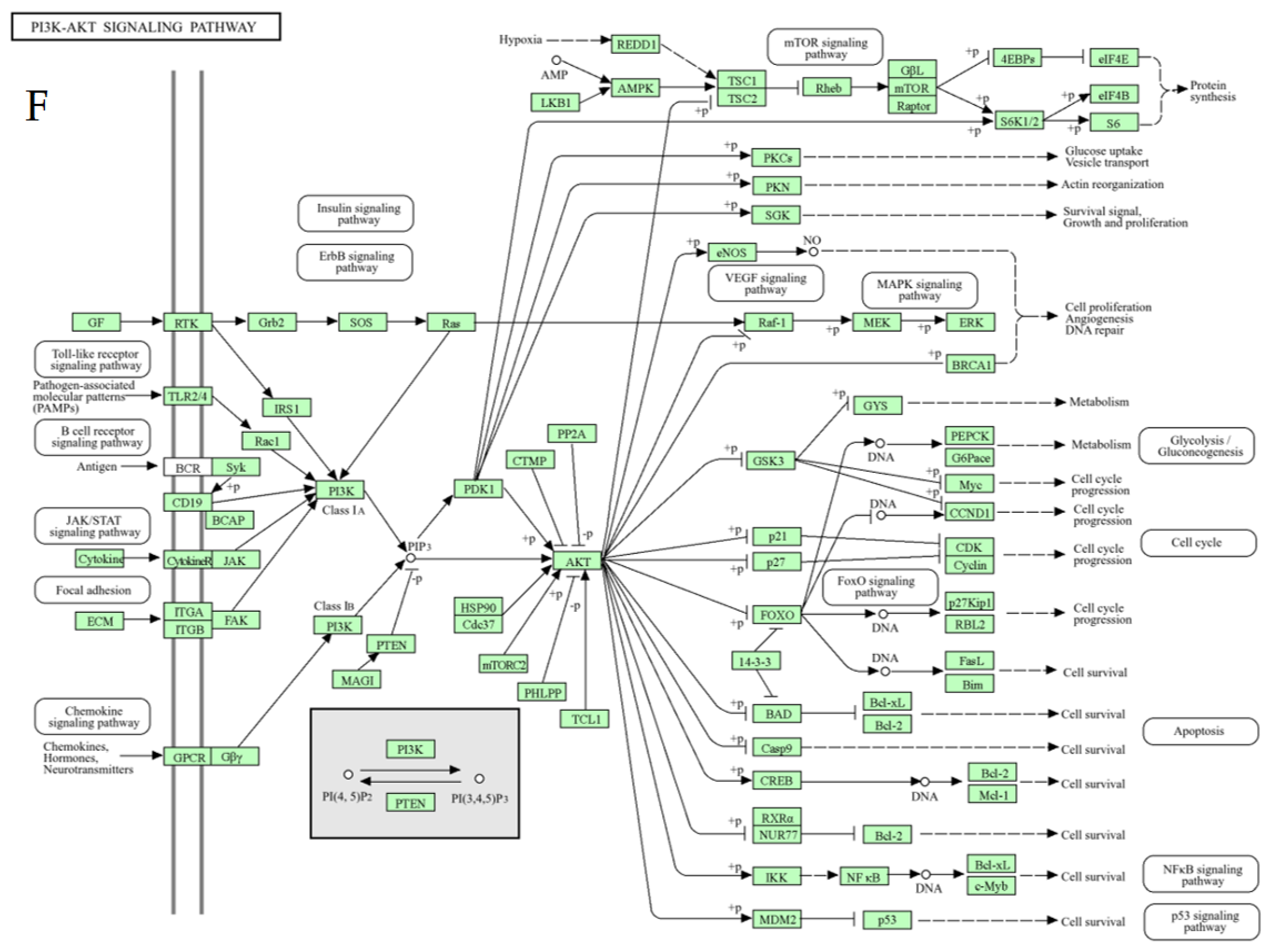
3.1.1. PI3K-AKT Signaling Pathway
3.1.2. Relationship of ESR1, IL6, PPARG, STAT3, and TP53 to the PI3K-AKT Signaling Pathway
3.1.3. Quercetin and 17-Beta-Estradiol
3.2. Animal Experimentation
3.2.1. GanShuangKeLi
3.2.2. Indicators
3.3. Metabolomics
3.3.1. Significantly Different Metabolites
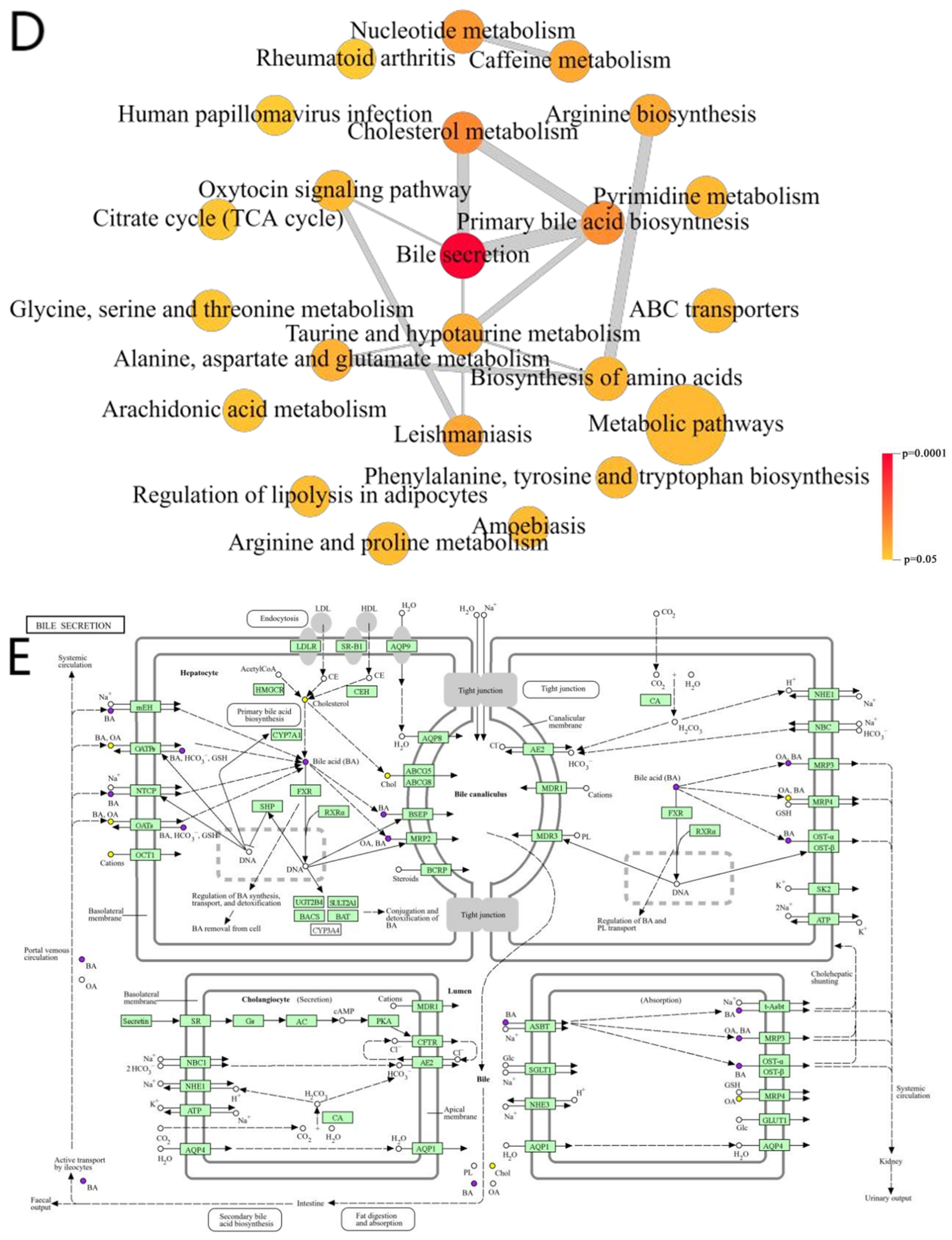
3.3.2. Bile Secretion Pathway
3.3.3. Relationship of ESR1, IL6, PPARG, STAT3, and TP53 to the Bile Secretion Pathway
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation Process, Quality Control Data, and Chemical Fingerprint of BBD
4.3. Network Pharmacology
4.4. Molecular Docking
4.5. Selection and Grouping of Experimental Animals
4.6. Biochemical Indicator Testing
4.7. Serum Inflammatory Factor Levels and Fasting Insulin Tests
4.8. Tissue Embedding Sectioning and Staining
4.9. Data Analysis
4.10. Metabolomics
4.10.1. Extraction of Metabolites
4.10.2. LC-MS/MS Analysis
Mass Spectrometry Acquisition
4.10.3. Data Preprocessing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| BATMAN-TCM | Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine |
| BBD | Babao Dan |
| CYP27A1 | Sterol 27-hydroxylase |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| CYP8B1 | Sterol 12α-hydroxylase |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| DisGeNet | Database of gene–disease associations |
| DL | Drug likeness |
| ESR1 | Estrogen receptor 1 |
| FBG | Fasting blood glucose |
| FINS | Fasting serum insulin |
| GO | Gene Ontology |
| GOT/AST | Glutamic oxaloacetic transaminase |
| GSKL | GanShuangKeLi |
| HDL-C | High-density lipoprotein cholesterol |
| HE | Hematoxylin–eosin |
| IL-6 | Interleukin-6 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDL-C | Low-density lipoprotein cholesterol |
| MCP-1 | Rat monocyte chemokine protein-1 |
| NASH | Nonalcoholic steatohepatitis |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MAFLD | Metabolic-associated fatty liver disease |
| OB | Oral bioavailability |
| OMIM | Online Mendelian Inheritance in Man |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PPI | Protein–protein interaction |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TC | Triglyceride |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform |
| TG | Total cholesterol |
| TNFα | Serum tumor necrosis factor alpha |
| TP53 | Tumor protein p53 |
| TTD | Therapeutic Target Database |
References
- National Workshop on Fatty Liver and Alcoholic Liver Disease; Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee; Chinese Medical Doctor Association. Guideline of prevention and treatment for nonalcoholic fatty liver disease. J. Pract. Hepatol. 2018, 21, 177–186. [Google Scholar]
- Xue, R.; Fan, J. Current situation and prospect of FXR agonist in the treatment of nonalcoholic steatohepatitis. Liver 2021, 26, 835–837. [Google Scholar]
- Yuan, P.; Chen, W. Insights to new medicine development for treatment of patients with nonalcoholic steatohepatitis. J. Pract. Hepatol. 2021, 24, 305–308. [Google Scholar]
- Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Torimura, T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol. Res. 2021, 52, 422–432. [Google Scholar] [CrossRef]
- Liu, P. Clinical efficacy of Babaodan capsules in the treatment of jaundice type viral hepatitis. Clin. Med. Res. Pract. 2018, 3, 138–139. [Google Scholar]
- Lv, H.; Yin, X. Clinical Observation of the Combination of Babaodan Capsules and Ganxile Granules with Western Medicine in the Treatment of Acute Jaundice Virus Hepatitis. Chin. Med. Emerg. 2017, 26, 328–330. [Google Scholar]
- Li, Y.; Lu, Y.; Yang, Z.; Chen, Y.; Liu, J.; Zhang, M.; Chen, X.; Huang, Y. A randomized controlled study on the protective effect of Babaodan capsules on liver function after liver cancer resection. J. Integr. Tradit. West. Med. Hepatol. 2024, 34, 2022–2206. [Google Scholar]
- Sheng, D.; Zhao, S.; Gao, L.; Zheng, H.; Liu, W.; Hou, J.; Jin, Y.; Ye, F.; Zhao, Q.; Li, R.; et al. BabaoDan attenuates high-fat diet-induced non-alcoholic fatty liver disease via activation of AMPK signaling. Cell Biosci. 2019, 9, 77. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.C.; Lam, H.Y.; Yap, K.H.; Jacot, W.; Jones, R.H.; Eng, H.Y.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Zhang, F.; Liu, F.; Zhao, J.; Huang, X.; Luo, D.; Guo, J. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Biomed. Pharmacother. 2023, 161, 114545. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Zhang, Z.; Chen, J.; Fan, Y.; Guo, J.; Su, Y.; Wang, H.; Zhang, X.; Wu, X.; Jiang, Q.; et al. High-fat diet promotes liver tumorigenesis via palmitoylation and activation of AKT. Gut 2024, 73, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Levine, K.M.; Bahreini, A.; Wang, P.; Chu, D.; Park, B.H.; Oesterreich, S.; Lee, A.V. Upregulation of IRS1 enhances IGF1 response in Y537S and D538G ESR1 mutant breast cancer cells. Endocrinology 2018, 159, 285–296. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Aprile, M.; Melillo, D.; Mucel, I.; Giorgetti-Peraldi, S.; Cormont, M.; Italiani, P.; Blüher, M.; Tanti, J.F.; Ciccodicola, A.; et al. TNFα mediates inflammation-induced effects on PPARG splicing in adipose tissue and mesenchymal precursor cells. Cells 2021, 11, 42. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Wang, Z.; Zuo, H.; Wang, J.; Xing, Y.; Jin, C.; Xu, G.; Piao, L.; Piao, H.; et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine 2020, 68, 153172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Lin, M.; Zhu, W.; Liang, Y.; Hong, X.; Zhao, Y.; Young, K.H.; Hu, W.; Feng, Z. Glutaminase 2 negatively regulates the PI3K/AKT signaling and shows tumor suppression activity in human hepatocellular carcinoma. Oncotarget 2014, 5, 2635–2647. [Google Scholar] [CrossRef]
- Katsaros, I.; Sotiropoulou, M.; Vailas, M.; Kapetanakis, E.I.; Valsami, G.; Tsaroucha, A.; Schizas, D. Quercetin’s Potential in MASLD: Investigating the Role of Autophagy and Key Molecular Pathways in Liver Steatosis and Inflammation. Nutrients 2024, 16, 3789. [Google Scholar] [CrossRef]
- Kravchenko, L.; Unhurian, L.; Tiuzhinska, S.K.; Ivanova, Y.; Obrazenko, M.; Zahorodnya, L.; Yamilova, T. Increasing the efficiency of hypolipidemic therapy with the combined use of quercetin in patients with non-alcoholic fatty liver disease on the background of the metabolic syndrome. Ceska Slov. Farm. 2023, 72, 296–302. [Google Scholar] [CrossRef]
- Kilanczyk, E.; Ruminkiewicz, D.; Banales, J.M.; Milkiewicz, P.; Milkiewicz, M. DHEA Protects Human Cholangiocytes and Hepatocytes against Apoptosis and Oxidative Stress. Cells 2022, 11, 1038. [Google Scholar] [CrossRef]
- Magri, A.; Barbaglia, M.N.; Foglia, C.Z.; Boccato, E.; Burlone, M.E.; Cole, S.; Giarda, P.; Grossini, E.; Patel, A.H.; Minisini, R.; et al. 17,β-Estradiol inhibits hepatitis C virus mainly by interference with the release phase of its life cycle. Liver Int. 2017, 37, 669–677. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, P.; Bi, Y.; Wang, B.; Wang, Z.; Li, Z.; Chen, Y.; Duan, Z. Mechanism of Ganshuang granule extract in alleviating N-acetyl-p-aminophenol-induced hepatocellular injury. J. Clin. Hepatol. 2021, 37, 120–125. [Google Scholar]
- Zhou, X.; Yang, X.; Hu, R. Observation of the therapeutic effect of Ganshuang granules on patients with liver fibrosis and its impact on liver function. Guizhou Med. J. 2023, 47, 197–198. [Google Scholar]
- Ma, J.; Gao, L.; Zhang, M.; Gao, Q.; Tao, X.; Fan, Y.; Yang, J. Study on the Improvement Effect and Mechanism of Lycium barbarum Polysaccharides Combined with Aerobic Exercise on Non alcoholic Fatty Hepatitis Rat Model. J. Clin. Hepatol. 2021, 37, 1348–1353. [Google Scholar]
- Wang, X.; Wang, Y.; Shang, M.; Liu, Y.; Chen, G. Experimental study on the activation of IRS-1/PI3K/Akt signaling pathway by Di’ao Xinxuekang to improve insulin resistance in non-alcoholic fatty liver disease mice. Chin. J. Clin. Pharmacol. Ther. 2024, 29, 121–129. [Google Scholar]
- Deng, Y.; Ji, G.; Wang, Y.; Jiang, Z. Intervention effect of resveratrol on non-alcoholic steatohepatitis in mice. Chin. J. Public Health 2015, 31, 88–91. [Google Scholar]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef]
- Zhang, J. Study on the Composition and Quality Control of Bile Acids in Traditional Chinese Medicine Snake Gallbladder. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2019. [Google Scholar]
- Tan, Q.; Du, Y.; Huang, H.; Xu, J.; Wu, Y. The role of intestinal microecology/bile acid metabolism/FXR pathway in the development of NAFLD. Chin. J. Integr. Tradit. West. Med. Liver Dis. 2023, 33, 467–470. [Google Scholar]
- Zheng, W.; Wang, X.; Han, H.; Jing, Z.; Zhao, W.; Xue, B.; Zhang, Y. Study on the regulation of bile acid metabolism in sleep deprived mice by Jiaotai Pill based on LC-MS. Chin. J. Tradit. Chin. Med. 2021, 46, 5382–5392. [Google Scholar]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ye, S.; Jing, R.; Zhu, W.; Chu, X.; Li, Y.; Zhang, W. Estrogen receptor alpha-mediated mitochondrial damage in intrahepatic bile duct epithelial cells leading to the pathogenesis of primary biliary cholangitis. Environ. Toxicol. 2023, 38, 2803–2818. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, X.; Zhang, N.; Yu, Y.; Gong, P.; Zhang, X.; Ma, Y.; Li, X.; Li, J. Clonorchis sinensis aggravates biliary fibrosis through promoting IL-6 production via toll-like receptor 2-mediated AKT and p38 signal pathways. PLoS Negl. Trop. Dis. 2023, 17, e0011062. [Google Scholar] [CrossRef] [PubMed]
- Wren, S.N.; Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. A Villin-driven Fxr transgene modulates enterohepatic bile acid homeostasis and response to an n-6-enriched high-fat diet. Int. J. Mol. Sci. 2020, 21, 7829. [Google Scholar] [CrossRef] [PubMed]
- Vageli, D.P.; Doukas, P.G.; Siametis, A.; Judson, B.L. Targeting STAT3 prevents bile reflux-induced oncogenic molecular events linked to hypopharyngeal carcinogenesis. J. Cell. Mol. Med. 2022, 26, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Xie, S.; Yan, Z.; Li, Z.; Li, Y.; Wang, L.; Jiao, F. p53 status and its prognostic role in extrahepatic bile duct cancer: A meta-analysis of published studies. Dig. Dis. Sci. 2011, 56, 655–662. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Tong, X.; Peng, W.; Wei, S.; Sun, T.; Wang, Y.; Zhang, B.; Li, W. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of Huai Hua San against ulcerative colitis. Drug Des. Dev. Ther. 2021, 15, 3255–3276. [Google Scholar] [CrossRef]
- GB/T35892-2018; Laboratory Anima—Guideline for Ethical Review of Animal Welfare. National Standard of the People’s Republic of China: Beijing, China, 2018.

| Ingredient | Number | Bioactive Compound | Molecular Formula | Relative Molecular Mass | Key Effects |
|---|---|---|---|---|---|
| Ling Yang Jiao | LYJ1 | Alanine | C3H7NO2 | 89.09 g/mol | Antipyretic, sedative, and liver-clearing |
| LYJ2 | Arginine | C6H14N4O2 | 174.2 g/mol | ||
| LYJ3 | Aspartic acid | C4H7NO4 | 133.1 g/mol | ||
| LYJ4 | Cysteine | C3H7NO2S | 121.16 g/mol | ||
| LYJ5 | Glutamic acid | C5H9NO4 | 147.13 g/mol | ||
| LYJ6 | Histidine | C6H9N3O2 | 155.15 g/mol | ||
| LYJ7 | Isoleucine | C6H13NO2 | 131.17 g/mol | ||
| LYJ8 | Leucine | C6H13NO2 | 131.17 g/mol | ||
| LYJ9 | L-Valine | C5H11NO2 | 117.15 g/mol | ||
| LYJ10 | Methionine | C5H11NO2S | 149.21 g/mol | ||
| LYJ11 | Phenylalanine | C9H11NO2 | 165.19 g/mol | ||
| LYJ12 | Proline | C5H9NO2 | 115.13 g/mol | ||
| LYJ13 | Tryptophan | C11H12N2O2 | 204.22 g/mol | ||
| LYJ14 | Tyrosine | C9H11NO3 | 181.19 g/mol | ||
| LYJ15 | Threonine | C4H9NO3 | 119.12 g/mol | ||
| Niu Huang | NH1 | 12α-Trihydroxy-5β-cholestane-24-oic acid methyl ester | C25H42O5 | 422.6 g/mol | Antispasmodic and neuroprotective |
| NH2 | Deoxycholic acid methyl ester | C25H42O4 | 406.6 g/mol | ||
| NH3 | Deoxycholic acid | C24H40O4 | 392.6 g/mol | ||
| NH4 | ZINC01280365 | C21H30O3 | 330.5 g/mol | ||
| NH5 | Cholesterol | C27H46O | 386.7 g/mol | ||
| San Qi | SQ1 | Glycyrrhizin | C15H12O4 | 256.25 g/mol | Hemostatic and anti-fibrotic |
| SQ2 | Quercetin | C15H10O7 | 302.23 g/mol | ||
| SQ3 | Diisooctyl phthalate | C24H38O4 | 390.6 g/mol | ||
| SQ4 | β-Sitosterol | C29H50O | 414.7 g/mol | ||
| SQ5 | Soy sterols | C29H48O | 412.7 g/mol | ||
| SQ6 | Ethyl linoleate | C20H36O2 | 308.5 g/mol | ||
| She Dan | SD1 | Bile acids | C24H40O5 | 408.6 g/mol | Hepatoprotective and antimicrobial |
| SD2 | Glycocholic acid | C26H43NO6 | 465.6 g/mol | ||
| SD3 | α-Usodeoxycholic acid | C24H40O6 | 424.6 g/mol | ||
| SD4 | Taurocholic acid | C26H45NO7S | 515.7 g/mol | ||
| She Xiang | SX1 | Androst-4-ene-3,17-dione | C19H26O2 | 286.4 g/mol | Neurostimulant and cardioprotective |
| SX2 | Testosterone | C19H28O2 | 288.4 g/mol | ||
| SX3 | 3,5-Dihydroxybenzoic acid | C7H6O4 | 154.12 g/mol | ||
| SX4 | Musenin | C51H82O21 | 1031.2 g/mol | ||
| SX5 | 17β-Estradiol | C18H24O2 | 272.4 g/mol | ||
| SX6 | Allantoin | C4H6N4O3 | 158.12 g/mol | ||
| SX7 | 3-Methylcyclotridecan-1-one | C14H26O | 210.36 g/mol | ||
| SX8 | Musk pyridine | C16H25N | 231.38 g/mol | ||
| SX9 | SCHEMBL2197370 | C15H26O | 222.37 g/mol | ||
| SX10 | Decylamine | C30H40Cl2N4 | 527.6 g/mol | ||
| SX11 | Musk lactone A1 | C8H18O5S | 226.29 g/mol | ||
| SX12 | Methylpiperonylphenol | C10H12O | 148.2 g/mol | ||
| SX13 | Cyclotetradecan-1-one | C14H26O | 210.36 g/mol | ||
| SX14 | 3β-hydroxy-5α-androstan-17-one | C19H30O2 | 290.4 g/mol | ||
| SX15 | α-Estradiol | C18H24O2 | 272.4 g/mol | ||
| SX16 | Androstenone | C19H30O2 | 290.4 g/mol | ||
| SX17 | Cyclopentadienone | C26H46N2O | 402.7 g/mol | ||
| SX18 | Cholesterol | C27H46O | 386.7 g/mol | ||
| SX19 | Musk ketone | C16H30O | 238.41 g/mol | ||
| SX20 | 2,6-decamethylene pyridine | C15H23N | 217.35 g/mol | ||
| SX21 | 3α-hydroxy-5α-androstan-17-one | C19H30O2 | 290.4 g/mol | ||
| SX22 | Diethyltoluamide | C12H17NO | 191.27 g/mol | ||
| SX23 | Musk ketone | C15H28O | 224.38 g/mol | ||
| SX24 | 2,6-Ninomethylidenepyridine | C14H21N | 203.32 g/mol | ||
| SX25 | 5-cis-cyclotetradecen-1-one | C14H24O | 208.34 g/mol | ||
| Zhen Zhu | ZZ1 | Aluminum | Al | 26.981 g/mol | Sedative and corneal repair |
| ZZ2 | Copper | Cu | 63.55 g/mol | ||
| ZZ3 | Iron | Fe | 55.84 g/mol | ||
| ZZ4 | Silicon | Si | 28.085 g/mol |
| Betweenness unDir | Closeness unDir | Degree unDir | Name |
|---|---|---|---|
| 1301.063994 | 0.005291005 | 250 | INS |
| 827.1577515 | 0.005076142 | 234 | TNF |
| 648.0910967 | 0.005050505 | 234 | AKT1 |
| 724.0959497 | 0.005076142 | 234 | IL6 |
| 763.3984781 | 0.005000000 | 230 | TP53 |
| 682.0782369 | 0.004878049 | 220 | PPARG |
| 482.4419534 | 0.004854369 | 216 | IL1B |
| 595.3456063 | 0.004784689 | 214 | ESR1 |
| 542.9045489 | 0.004739336 | 210 | STAT3 |
| 325.8217509 | 0.004739336 | 208 | JUN |
| Protein | Ligand | Binding Energy (kcal/mol) |
|---|---|---|
| INS | quercetin | −6.6 |
| TNF | quercetin | −6.8 |
| AKT1 | quercetin | −6.3 |
| IL6 | 17-beta-estradiol | −7.2 |
| TP53 | 17-beta-estradiol | −7.7 |
| PPARG | quercetin | −7.1 |
| IL1B | DFV | −7.0 |
| ESR1 | quercetin | −7.3 |
| STAT3 | 17-beta-estradiol | −7.4 |
| JUN | glycocholic acid | −5.6 |
| Comparison Group | R2X (cum) | R2Y (cum) | Q2 (cum) | RMSEE |
|---|---|---|---|---|
| A vs. B | 0.316 | 0.99 | 0.897 | 0.0543 |
| B vs. D | 0.291 | 0.966 | 0.529 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Su, Y.; Han, A.; Qi, H.; Zhao, J.; Qiu, X. UPLC-MS/MS Metabolomics Reveals Babao Dan’s Mechanisms in MASH Treatment with Integrating Network Pharmacology and Molecular Docking. Pharmaceuticals 2025, 18, 1111. https://doi.org/10.3390/ph18081111
Zhang S, Su Y, Han A, Qi H, Zhao J, Qiu X. UPLC-MS/MS Metabolomics Reveals Babao Dan’s Mechanisms in MASH Treatment with Integrating Network Pharmacology and Molecular Docking. Pharmaceuticals. 2025; 18(8):1111. https://doi.org/10.3390/ph18081111
Chicago/Turabian StyleZhang, Shijiao, Yanding Su, Ao Han, He Qi, Jiade Zhao, and Xiangjun Qiu. 2025. "UPLC-MS/MS Metabolomics Reveals Babao Dan’s Mechanisms in MASH Treatment with Integrating Network Pharmacology and Molecular Docking" Pharmaceuticals 18, no. 8: 1111. https://doi.org/10.3390/ph18081111
APA StyleZhang, S., Su, Y., Han, A., Qi, H., Zhao, J., & Qiu, X. (2025). UPLC-MS/MS Metabolomics Reveals Babao Dan’s Mechanisms in MASH Treatment with Integrating Network Pharmacology and Molecular Docking. Pharmaceuticals, 18(8), 1111. https://doi.org/10.3390/ph18081111







