Tailoring Treatment in Complex Regional Pain Syndrome: A Comparative Study of Therapeutic Approaches in Complex Rehabilitation
Abstract
1. Introduction
2. Results
2.1. General Assessment
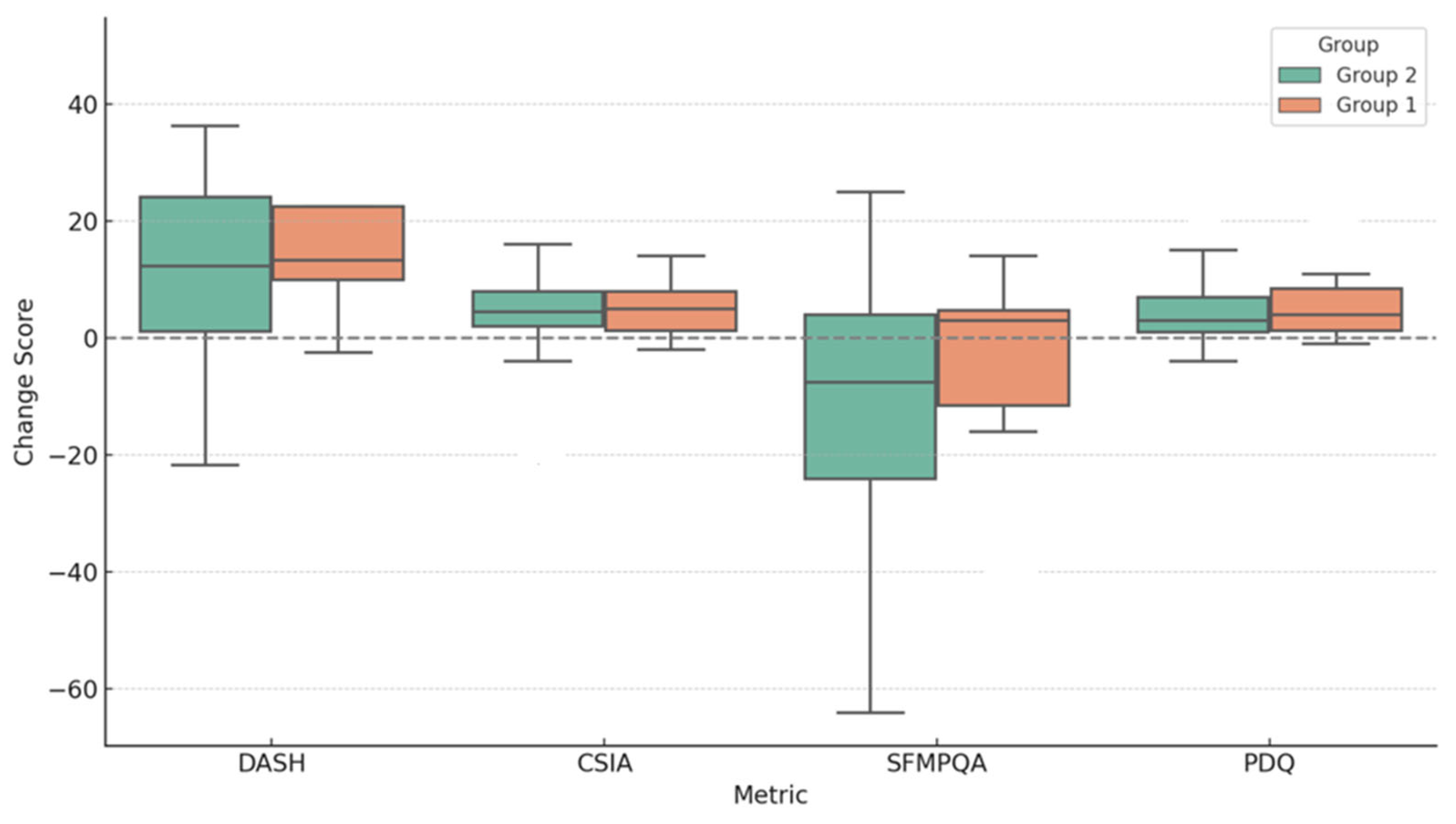
2.2. Comparison Analysis of Treatment Plan
2.3. Comparison Subanalysis of Anticonvulsants Effectiveness
3. Discussion
4. Materials and Methods
4.1. Study Design
Eligibility Criteria
4.2. Assessment
- Numerical Rating Scale (NRS);
- Short-Form McGill Pain Questionnaire (SF-MPQ);
- PainDETECT Questionnaire (PDQ);
- Central Sensitization Inventory (CSI).
4.3. Rehabilitation Program
4.4. Treatment
- Constant Analgesic Therapy Group (Group 1)
- 2.
- On-Demand Analgesic Therapy Group (Group 2)
- 3.
- No Pharmacological Pain Treatment Group (Group 3)
4.5. Outcome Measures
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Wen, B.; Xu, J.; Zhang, Y.; Xu, L.; Huang, Y. Efficacy and Safety of Pharmacological Treatment in Patients with Complex Regional Pain Syndrome: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harnik, M.A.; Kesselring, P.; Ott, A.; Urman, R.D.; Luedi, M.M. Complex Regional Pain Syndrome (CRPS) and the Value of Early Detection. Curr. Pain. Headache Rep. 2023, 27, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Guthmiller, K.B.; Varacallo, M. Complex Regional Pain Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Dutton, L.K.; Rhee, P.C. Complex Regional Pain Syndrome and Distal Radius Fracture: Etiology, Diagnosis, and Treatment. Hand Clin. 2021, 37, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, E.C.O.; Dempsey, B.; Romero, L. Complex Regional Pain Syndrome. Am. Fam. Physician 2021, 104, 49–55. [Google Scholar] [PubMed]
- Bean, D.J.; Tuck, N.L.; Magni, N.; Aamir, T.; Pollard, C.; Lewis, G.N. The efficacy of an interdisciplinary pain management program for complex regional pain syndrome compared to low back pain and chronic widespread pain: An observational study. Pain. Med. 2025, 26, 180–188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fassio, A.; Mantovani, A.; Gatti, D.; Rossini, M.; Viapiana, O.; Gavioli, I.; Benini, C.; Adami, G. Pharmacological treatment in adult patients with CRPS-I: A systematic review and meta-analysis of randomized controlled trials. Rheumatology 2022, 61, 3534–3546. [Google Scholar] [CrossRef] [PubMed]
- Varenna, M.; Braga, V.; Gatti, D.; Iolascon, G.; Frediani, B.; Zucchi, F.; Crotti, C.; Nannipieri, F.; Rossini, M. Intramuscular neridronate for the treatment of complex regional pain syndrome type 1: A randomized, double-blind, placebo-controlled study. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211014020. [Google Scholar] [CrossRef] [PubMed]
- Parinder, A.; Lyckegård Finn, E.; Dahlin, L.B.; Nyman, E. Associated factors, triggers and long-term outcome in Complex Regional Pain Syndrome (CRPS) in the upper limb—A descriptive cross-sectional study. PLoS ONE 2025, 20, e0320263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pique, B.; Atalla, M.A.; Van de Winckel, A.; Walsh, N.E.; Lewis, J.S. Rehabilitation Interventions for Adults with Complex Regional Pain Syndrome: A Scoping Review Protocol. Musculoskelet. Care 2024, 22, e1956. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Wang, R.; Su, X.; Wang, X.Q. Effect and mechanisms of exercise for complex regional pain syndrome. Front. Mol. Neurosci. 2023, 16, 1167166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafiee, E.; MacDermid, J.; Packham, T.; Walton, D.; Grewal, R.; Farzad, M. The Effectiveness of Rehabilitation Interventions on Pain and Disability for Complex Regional Pain Syndrome: A Systematic Review and Meta-analysis. Clin. J. Pain. 2023, 39, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Hyun, S.E.; Park, J.; Lim, J.Y. Understanding the Rehabilitation Needs of Korean Patients With Complex Regional Pain Syndrome. Ann. Rehabil. Med. 2020, 44, 218–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falcone, G. Efficacy of an intensive multimodal rehabilitation program in adult patients affected by complex regional pain syndrome type 1 (CRPS 1): A randomized controlled trial. Int. Phys. Med. Rehab J. 2024, 9, 91–97. [Google Scholar] [CrossRef]
- Mangnus, T.J.P.; Bharwani, K.D.; Dirckx, M.; Huygen, F.J.P.M. From a Symptom-Based to a Mechanism-Based Pharmacotherapeutic Treatment in Complex Regional Pain Syndrome. Drugs 2022, 82, 511–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, M.C.; Park, D. Algorithm for multimodal medication therapy in patients with complex regional pain syndrome. J. Yeungnam Med. Sci. 2023, 40 (Suppl.), S125–S128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norton, K.F.; Furnish, T.J. Perspectives on the pharmacological management of complex regional pain syndrome. Expert. Opin. Pharmacother. 2023, 24, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Snichelotto, F.; Moretti, A. An update on the pharmacotherapeutic options for complex regional pain syndrome. Expert. Rev. Neurother. 2024, 24, 177–190. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, N.E.; Wand, B.M.; McAuley, J.; Marston, L.; Moseley, G.L. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst. Rev. 2013, 4, CD009416. [Google Scholar] [CrossRef] [PubMed]
- Chitneni, A.; Patil, A.; Dalal, S.; Ghorayeb, J.H.; Pham, Y.N.; Grigoropoulos, G. Use of Ketamine Infusions for Treatment of Complex Regional Pain Syndrome: A Systematic Review. Cureus 2021, 13, e18910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alorfi, N.M. Pharmacological Methods of Pain Management: Narrative Review of Medication Used. Int. J. Gen. Med. 2023, 16, 3247–3256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Rosa, F.; Giannatiempo, B.; Charlier, B.; Coglianese, A.; Mensitieri, F.; Gaudino, G.; Cozzolino, A.; Filippelli, A.; Piazza, O.; Dal Piaz, F.; et al. Pharmacological Treatments and Therapeutic Drug Monitoring in Patients with Chronic Pain. Pharmaceutics 2023, 15, 2088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonezzi, C.; Fornasari, D.; Cricelli, C.; Magni, A.; Ventriglia, G. Pharmacological Management of Adults with Chronic Non-Cancer Pain in General Practice. Pain. Ther. 2020, 9 (Suppl. S1), 17–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budge, C.; Taylor, M.; Mar, M.; Hansen, C.; Fai, F. Chronic pain: Good management of practical pain control strategies is associated with being older, more health activated and having better mental health. J. Prim. Health Care 2020, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Merrill, J.O.; Rutter, C.M.; Sullivan, M.; Campbell, C.I.; Weisner, C. Time-scheduled vs. pain-contingent opioid dosing in chronic opioid therapy. Pain 2011, 152, 1256–1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webster, L.; Gudin, J.; Raffa, R.B.; Kuchera, J.; Rauck, R.; Fudin, J.; Adler, J.; Mallick-Searle, T. Understanding Buprenorphine for Use in Chronic Pain: Expert Opinion. Pain. Med. 2020, 21, 714–723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, R.P.; Ho, C.H.; Cheung, L.P. Scheduled analgesic regimen improves rehabilitation after hip fracture surgery. Orthop. Relat. Res. 2013, 471, 2349–2360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caradonna, A.J.; Lee, D.; Caparó, M. Early Neuropathic Treatment May Prevent the Chronic Stage of Complex Regional Pain Syndrome Type II (CRPS II). Cureus 2023, 15, e36861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van de Vusse, A.C.; Stomp-Van Den Berg, S.G.; Kessels, A.H.; Weber, W.E. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type 1 [ISRCTN84121379]. BMC Neurol. 2004, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Johnston, B.; Amaria, K.; Watkins, J.; Campbell, F.; Pehora, C.; McGrath, P. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scand. J. Pain 2016, 13, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, J.; Gaines, C. Gabapentin for chronic neuropathic pain in adults. Br. J. Community Nurs. 2019, 24, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Abdi, S. Use of anticonvulsants and antidepressants for treatment of complex regional pain syndrome: A literature review. Pain. Manag. 2021, 11, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Saltık, S.; Sözen, H.G.; Basgul, S.; Karatoprak, E.Y.; Içağasıoğlu, A. Pregabalin Treatment of a Patient with Complex Regional Pain Syndrome. Pediatr. Neurol. 2016, 54, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Lima Pessôa, B.; Netto, J.G.M.; Adolphsson, L.; Longo, L.; Hauwanga, W.N.; McBenedict, B. Complex Regional Pain Syndrome: Diagnosis, Pathophysiology, and Treatment Approaches. Cureus 2024, 16, e76324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harden, R.N.; McCabe, C.S.; Goebel, A.; Massey, M.; Suvar, T.; Grieve, S.; Bruehl, S. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain. Med. 2022, 23 (Suppl. S1), S1–S53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goebel, A.; Birklein, F.; Brunner, F.; Clark, J.D.; Gierthmühlen, J.; Harden, N.; Huygen, F.; Knudsen, L.; McCabe, C.; Lewis, J.; et al. The Valencia consensus-based adaptation of the IASP complex regional pain syndrome diagnostic criteria. Pain 2021, 162, 2346–2348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curran, P. Practical Hydrotherapy, a Manual for Students and Practitioners. Sagwan Press: Hillside, NJ, USA, 2018. [Google Scholar]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain. Med. Malden Mass. 2020, 21, S6–S12. [Google Scholar] [CrossRef] [PubMed]

| Variable | W-Statistic | p-Value | Change | 95% CI |
|---|---|---|---|---|
| DASH | 50.0 | 0.00001 | −13.87 | [−19.22; −8.52] |
| LEFS | 261.0 | 0.53246 | +1.66 | [−2.93; 6.25] |
| NRS | 71.0 | 0.0001 | −1.78 | [−2.52; −1.04] |
| CSIA | 93.0 | 0.00027 | −4.19 | [−5.78; −2.60] |
| SFMPQA | 186.0 | 0.02005 | 10.76 | [3.53; 17.99] |
| SFMPQB1 | 25.0 | 0.0 | −33.64 | [−41.93; −25.35] |
| PDQ | 93.0 | 0.00079 | −3.67 | [−6.24; −1.10] |
| Parameters | Group 1 (n = 15) | Group 2 (n = 6) | Group 3 (n = 11) | |||
|---|---|---|---|---|---|---|
| M [CI 95%] | p | M [CI 95%] | p | M [CI 95%] | p | |
| DASH | −14.3 [−23.68, −4.91] | 0.0056 | −18.71 [−29.92, −7.50] | 0.0078 | −10.64 [−21.36, 0.08] | 0.0515 |
| LEFS | 1.14 [−6.43, 8.70] | 0.7543 | −1.79 [−19.57, 16.00] | 0.8065 | 4.12 [−3.26, 11.50] | 0.2447 |
| NRS | −1.69 [−3.07, −0.32] | 0.0185 | −1.92 [−2.88, −0.95] | 0.0037 | −1.83 [−3.11, −0.56] | 0.0089 |
| CSIA | −1.72 [−5.22, 1.78] | 0.3136 | −7.00 [−13.01, −0.99] | 0.0303 | −6.50 [−10.30, −2.70] | 0.0031 |
| SFMPQA | 8.75 [−3.47, 20.97] | 0.1492 | 5.50 [−13.20, 24.20] | 0.4837 | 16.42 [3.32, 29.52] | 0.0186 |
| SFMPQB1 | −29.67 [−44.42, −14.91] | 0.0006 | −33.33 [−63.24, −3.43] | 0.0352 | −39.75 [−49.81, −29.69] | 0.0000 |
| PDQ | −1.56 [−5.53, 2.42] | 0.4209 | −6.17 [−12.65, 0.32] | 0.0583 | −5.58 [−10.76, −0.40] | 0.0370 |
| Group | Participants (n) | Mean Age (years, M(SD)) | Male (n) | Female (n) | DASH | LEFS | NRS | CSIA | SFMPQA | SFMPQB | PDQ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 49.53 (13,2) | 3 | 12 | 51.29 | 53.86 | 5.42 | 35.06 | 18.00 | 62.89 | 18.33 |
| 2 | 6 | 61.67 (11,7) | 1 | 5 | 69.21 | 47.20 | 4.83 | 37.00 | 21.00 | 63.67 | 21.00 |
| 3 | 11 | 55.10 (14,2) | 3 | 8 | 55.68 | 64.94 | 4.46 | 32.42 | 15.00 | 48.58 | 18.17 |
| Medication Class | Agents Used | Daily Dosage Information | Route |
|---|---|---|---|
| Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | Ketoprofen, Ketonal | 100–200 mg | oral |
| Ibuprofen | 400–800 mg | oral | |
| Paracetamol | 500–1000 mg | oral | |
| Anticonvulsants | Pregabalin | 75–150 mg | oral |
| Gabapentin | 600–1200 mg | oral | |
| Opioid Analgesics | Tramadol | 50–100 mg | oral |
| Oxycodone | 5–10 mg | oral | |
| Combination Therapy | Tramadol + Paracetamol | 75/325 mg | oral |
| Tramadol + Dexketoprofen | 75/25 mg | oral | |
| Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) | Duloxetine | 30–60 mg | oral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreieva, I.; Tarnacka, B.; Zalewski, A.; Wiśniowska, J. Tailoring Treatment in Complex Regional Pain Syndrome: A Comparative Study of Therapeutic Approaches in Complex Rehabilitation. Pharmaceuticals 2025, 18, 1114. https://doi.org/10.3390/ph18081114
Andreieva I, Tarnacka B, Zalewski A, Wiśniowska J. Tailoring Treatment in Complex Regional Pain Syndrome: A Comparative Study of Therapeutic Approaches in Complex Rehabilitation. Pharmaceuticals. 2025; 18(8):1114. https://doi.org/10.3390/ph18081114
Chicago/Turabian StyleAndreieva, Iana, Beata Tarnacka, Adam Zalewski, and Justyna Wiśniowska. 2025. "Tailoring Treatment in Complex Regional Pain Syndrome: A Comparative Study of Therapeutic Approaches in Complex Rehabilitation" Pharmaceuticals 18, no. 8: 1114. https://doi.org/10.3390/ph18081114
APA StyleAndreieva, I., Tarnacka, B., Zalewski, A., & Wiśniowska, J. (2025). Tailoring Treatment in Complex Regional Pain Syndrome: A Comparative Study of Therapeutic Approaches in Complex Rehabilitation. Pharmaceuticals, 18(8), 1114. https://doi.org/10.3390/ph18081114







