Coronary Artery Calcium Score as a Predictor of Anthracycline-Induced Cardiotoxicity: The ANTEC Study
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Associations Between Atherosclerosis and Clinical Parameters
2.3. Incidence of CTRCD
2.4. Univariable and Multivariable Analysis
2.5. Validation of the HFA-ICOS Risk Score
2.6. Deaths
3. Discussion
3.1. Incidence of Atherosclerosis
3.2. Impact of Atherosclerosis on Cardiovascular Complications
3.3. Impact of Atherosclerosis on CTRCD
3.4. Validation of the HFA-ICOS Risk Tool
3.5. Strengths and Limitations
4. Materials and Methods
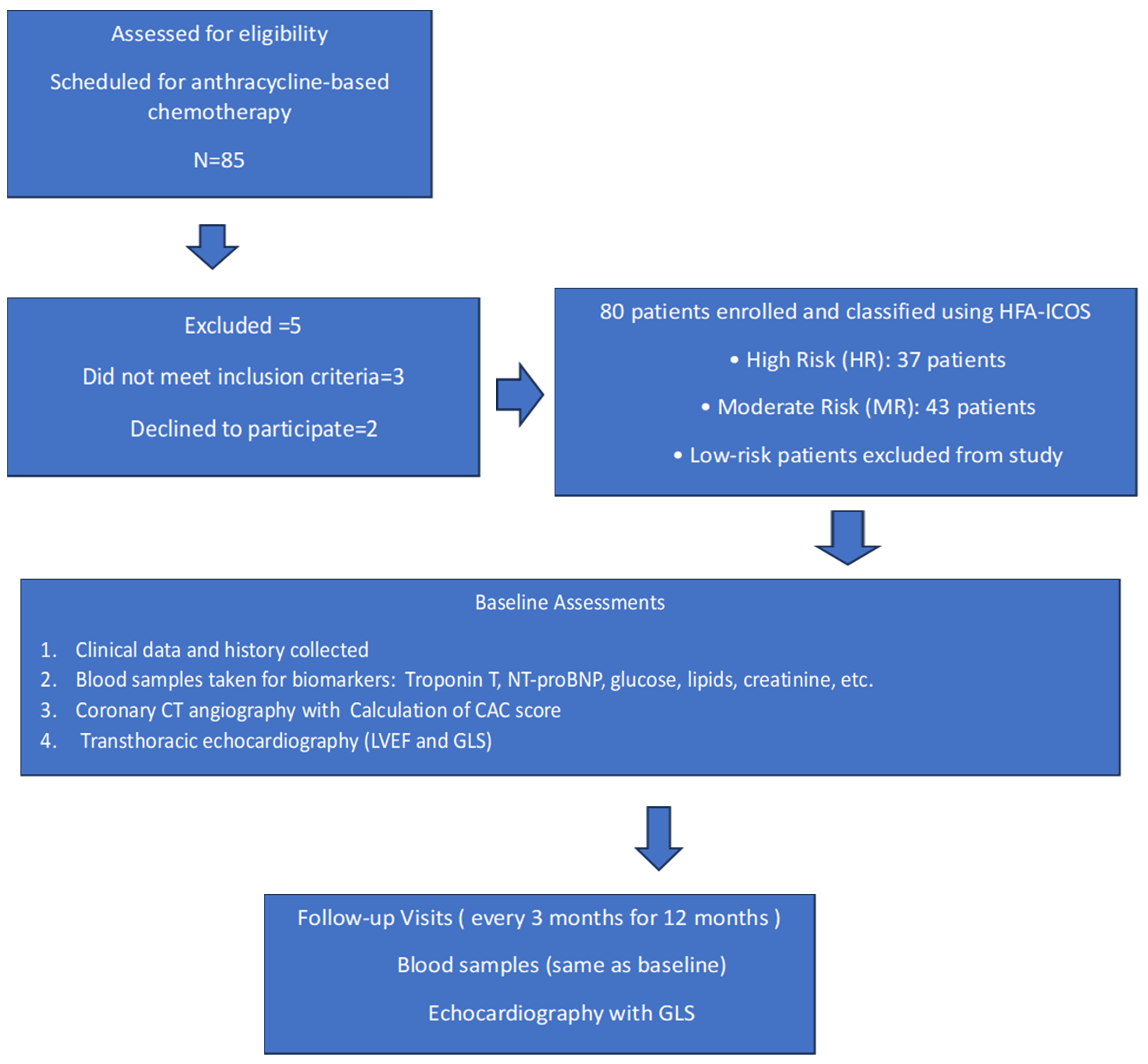
4.1. Study Structure
4.2. Patients Classification, CCTA and Echocardiography
4.3. Data Acquisition and Statistical Analysis
4.3.1. Data Acquisition and Quality Control
4.3.2. Sample Size Calculations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTRCD | Cancer therapy-related cardiac dysfunction |
| CTR-CVT | Cancer therapy-related cardiovascular toxicity |
| HFA-ICOS | Heart Failure Association–International Cardio-Oncology Society |
| CCTA | Coronary computed tomography angiography |
| CAC score | Coronary artery calcium score |
| GLS | Left ventricular peak systolic global longitudinal strain |
| hs-cTnT | High-sensitivity cardiac troponin T |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| eCRF | Electronic case report forms |
| HIS | Hospital Information System |
| LVEF | Left ventricle ejection fraction |
| CVD | Cardiovascular disease |
References
- Mukai, M.; Komori, K.; Oka, T. Mechanism and Management of Cancer Chemotherapy-Induced Atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Hooks, M.; Sandhu, G.; Maganti, T.; Chen, K.H.A.; Wang, M.; Cullen, R.; Velangi, P.S.; Gu, C.; Wiederin, J.; Connett, J.; et al. Incidental coronary calcium in cancer patients treated with anthracycline and/or trastuzumab. Eur. J. Prev. Cardiol. 2022, 29, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Franco, F.X.; McDonald, M.; Rivera, C.; Perez-Villa, B.; Collier, P.; Moudgil, R.; Gupta, N.; Sadler, D.B. Use of computed tomography coronary calcium score for prediction of cardiovascular events in cancer patients: A retrospective cohort analysis. Cardiooncology 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.W.; Dudum, R.; Jain, S.S.; Maron, D.J.; Patel, B.N.; Khandwala, N.; Eng, D.; Chaudhari, A.S.; Sandhu, A.T.; Rodriguez, F. Association of Coronary Artery Calcium Detected by Routine Ungated CT Imaging With Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2023, 82, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Rossi, A.; Guglielmo, M.; Dweck, M.R.; Gaemperli, O.; Nieman, K.; Pugliese, F.; Maurovich-Horvat, P.; Gimelli, A.; Cosyns, B.; et al. Clinical applications of cardiac computed tomography: A consensus paper of the European Association of Cardiovascular Imaging-part II. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e136–e161. [Google Scholar] [CrossRef] [PubMed]
- Acar, Z.; Kale, A.; Turgut, M.; Demircan, S.; Durna, K.; Demir, S.; Meriç, M.; Ağaç, M.T. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Chotenimitkhun, R.; D’Agostino, R., Jr.; Lawrence, J.A.; Hamilton, C.A.; Jordan, J.H.; Vasu, S.; Lash, T.L.; Yeboah, J.; Herrington, D.M.; Hundley, W.G. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can. J. Cardiol. 2015, 31, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Obasi, M.; Abovich, A.; Vo, J.B.; Gao, Y.; Papatheodorou, S.I.; Nohria, A.; Asnani, A.; Partridge, A.H. Statins to mitigate cardiotoxicity in cancer patients treated with anthracyclines and/or trastuzumab: A systematic review and meta-analysis. Cancer Causes Control 2021, 32, 1395–1405. [Google Scholar] [PubMed]
- Kim, J.; Nishimura, Y.; Kewcharoen, J.; Yess, J. Statin Use Can Attenuate the Decline in Left Ventricular Ejection Fraction and the Incidence of Cardiomyopathy in Cardiotoxic Chemotherapy Recipients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3731. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Fiala, M.A.; Gage, B.F.; Wildes, T.M.; Sanfilippo, K. Statins Reduce Mortality in Multiple Myeloma: A Population-Based US Study. Clin. Lymphoma Myeloma Leuk. 2020, 20, e937–e943. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Janbabai, G.; Esmailian, J.; Yazdani, J. Effect of Rosuvastatin in Preventing Chemotherapy-Induced Cardiotoxicity in Women With Breast Cancer: A Randomized, Single-Blind, Placebo-Controlled Trial. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Yamani, N.; Ali, A.; Kumar, P.; Figueredo, V.; Unzek, S.; Mookadam, F. Meta-analysis Evaluating the Use of Statins to attenuate Cardiotoxicity in Cancer Patients receiving Anthracyclines and Trastuzumab-based Chemotherapy. Am. J. Cardiol. 2021, 156, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Statins and the Prevention of Heart Disease. JAMA Cardiol. 2017, 2, 464. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; D’Agostino, R.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Houbois, C.; Marwick, T.H.; Kei, T.; Saha, S.; Runeckles, K.; Huang, F.; Shalmon, T.; Thorpe, K.E.; Pezo, R.C.; et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Brann, A.M.; Bai, C.J.; Hibbeln, J.F.; Williams, K.A.; Okwuosa, T.M. A comparative assessment of coronary artery calcification on chest CT scans of patients referred to a cardio-oncology clinic. Cardiooncology 2016, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Gernaat, S.A.; Išgum, I.; De Vos, B.D.; Takx, R.A.; Young-Afat, D.A.; Rijnberg, N.; Grobbee, D.E.; van der Graaf, Y.; De Jong, P.A.; Leiner, T.; et al. Automatic Coronary Artery Calcium Scoring on Radiotherapy Planning CT Scans of Breast Cancer Patients: Reproducibility and Association with Traditional Cardiovascular Risk Factors. PLoS ONE 2016, 11, e0167925. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Lian, Y.; Yin, J.; Zhu, M.; Yang, C.; Tu, C.; Peng, Y.; Li, X.; Zhang, J. Cardiovascular Risk Stratification by Automatic Coronary Artery Calcium Scoring on Pretreatment Chest Computed Tomography in Diffuse Large B-Cell Lymphoma Receiving Anthracycline-Based Chemotherapy: A Multicenter Study. Circ. Cardiovasc. Imaging 2023, 16, e014829. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Chung, S.Y.; Oh, C.; Cho, I.; Kim, K.H.; Byun, H.K.; Yoon, H.I.; Oh, J.; Chang, J.S. Automated coronary artery calcium scoring in patients with breast cancer to assess the risk of heart disease following adjuvant radiation therapy. Breast 2022, 65, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Xu, T.; Lopez-Mattei, J.; Pan, T.; Lu, Y.; Irizarry-Caro, J.A.; Mohan, R.; Zhang, X.; Meng, Q.H.; Lin, R.; et al. Coronary artery calcium score on standard of care oncologic CT scans for the prediction of adverse cardiovascular events in patients with non-small cell lung cancer treated with concurrent chemoradiotherapy. Front. Cardiovasc. Med. 2022, 9, 1071701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasmussen, T.; Køber, L.; Abdulla, J.; Pedersen, J.H.; Wille, M.M.W.; Dirksen, A.; Kofoed, K.F. Coronary artery calcification detected in lung cancer screening predicts cardiovascular death. Scand. Cardiovasc. J. 2015, 49, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Reiter–Brennan, C.; Dardari, Z.; Marshall, C.H.; Nasir, K.; Miedema, M.D.; Berman, D.S.; Rozanski, A.; Rumberger, J.A.; Budoff, M.J.; et al. Association between coronary artery calcium and cardiovascular disease as a supporting cause in cancer: The CAC consortium. Am. J. Prev. Cardiol. 2020, 4, 100119. [Google Scholar] [CrossRef] [PubMed]
- Battisti, N.M.L.; Andres, M.S.; Lee, K.A.; Ramalingam, S.; Nash, T.; Mappouridou, S.; Senthivel, N.; Asavisanu, K.; Obeid, M.; Tripodaki, E.S.; et al. Incidence of cardiotoxicity and validation of the Heart Failure Association-International Cardio-Oncology Society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res. Treat. 2021, 188, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Suntheralingam, S.; Fan, C.-P.S.; Calvillo-Argüelles, O.; Abdel-Qadir, H.; Amir, E.; Thavendiranathan, P. Evaluation of Risk Prediction Models to Identify Cancer Therapeutics Related Cardiac Dysfunction in Women with HER2+ Breast Cancer. J. Clin. Med. 2022, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Santana, B.; Saldaña-García, J.; Caro-Codón, J.; Zamora, P.; Moliner, P.; Martínez Monzonis, A.; Zatarain, E.; Álvarez-Ortega, C.; Gómez-Prieto, P.; Pernas, S.; et al. Anthracycline-induced cardiovascular toxicity: Validation of the Heart Failure Association and International Cardio-Oncology Society risk score. Eur. Heart J. 2024, 46, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.C.; Espinola-Zavaleta, N.; Barac, A.; Asch, F.; Antonio-Villa, N.E.; Espinosa-Fernandez, J.R.; Luna-Alcala, S.; Martinez-Dominguez, P.; Proano-Bernal, L.; Aparicio-Ortiz, A.D.; et al. Validation of the HFA-ICOS risk assessment tool with real-world data from a prospective cohort of breast cancer patients in treatment with anthracyclines and trastuzumab. Eur. Heart J. 2024, 45, ehae666.3188. [Google Scholar] [CrossRef]
- Borowiec, A.; Ozdowska, P.; Rosinska, M.; Jagiello-Gruszfeld, A.; Jasek, S.; Waniewska, J.; Kotowicz, B.; Kosela-Paterczyk, H.; Lampka, E.; Makowka, A.; et al. Prognostic value of coronary atherosclerosis and CAC score for the risk of chemotherapy-related cardiac dysfunction (CTRCD): The protocol of ANTEC study. PLoS ONE 2023, 18, e0288146. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, T.; Asch, F.M.; Davidson, B.; Delgado, V.; DeMaria, A.; Dilsizian, V.; Gaemperli, O.; Garcia, M.J.; Kamp, O.; Lee, D.C.; et al. Non-Invasive Imaging in Coronary Syndromes: Recommendations of The European Association of Cardiovascular Imaging and the American Society of Echocardiography, in Collaboration with The American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Comput. Tomogr. 2022, 16, 362–383. [Google Scholar] [CrossRef] [PubMed]


| Characteristics of the Patients, n = 77 | Number of Patients, % | |
|---|---|---|
| Age group (years) | <65 | 41 (53.2%) |
| ≥65 | 36 (46.8%) | |
| Cancer | breast | 59 (76.6%) |
| lymphoma | 7 (9.1%) | |
| sarcoma | 11 (14.3%) | |
| Risk group | High Risk | 37 (48.1%) |
| Moderate Risk | 40 (51.9%) | |
| BMI | <25 | 24 (31.2%) |
| 25–30 | 28 (36.4%) | |
| ˃30 | 25 (32.5%) | |
| Coexisting Conditions * | Hypertension | 60 (77.9%) |
| Hyperlipidemia | 58 (75.3%) | |
| Diabetes | 11 (14.3%) | |
| Chronic kidney disease | 12 (15.6%) | |
| Ever smoke | 37 (48.1%) | |
| NYHA scale | 1 | 54 (70.1%) |
| 2 | 23 (29.9%) | |
| ECOG score | 0 | 55 (71.4%) |
| 1 | 21 (27.3%) | |
| 2 | 1 (1.3%) | |
| Medications * | Beta-blockers | 33 (42.9%) |
| ACE-I | 34 (44.2%) | |
| Statins | 23 (29.9%) | |
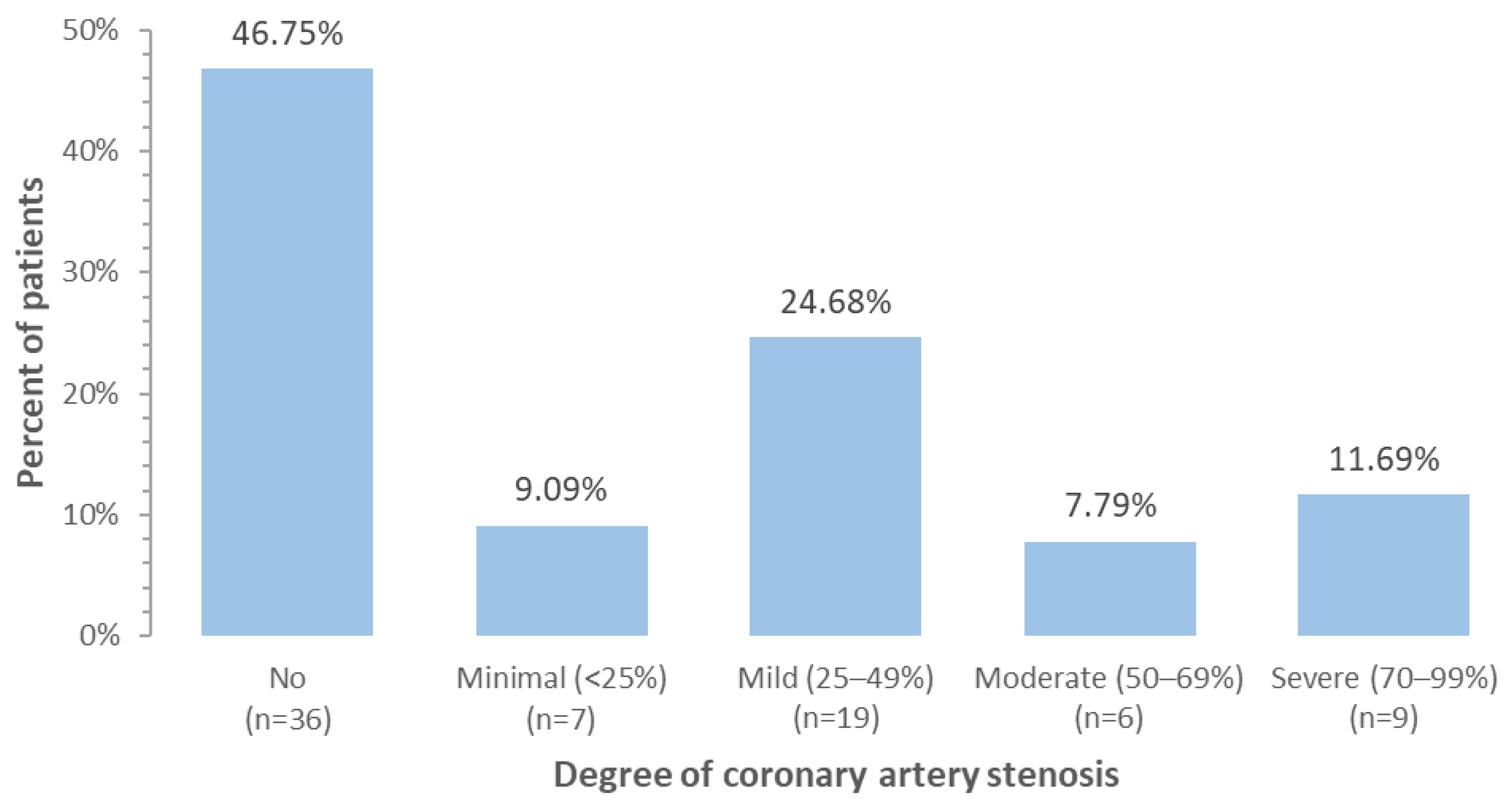
| Coronary artery stenosis | No | 36 (46.8%) |
| Minimal (<25%) | 7 (9.1%) | |
| Mild (25–49%) | 19 (24.7%) | |
| Moderate (50–69%) | 6 (7.8%) | |
| Severe (70–99%) | 9 (11.7%) | |
| Coronary artery calcium (CAC) score | 0 | 36 (46.8%) |
| 1–99 | 24 (31.2%) | |
| 100–399 | 14 (18.2%) | |
| 400+ | 3 (3.9%) | |
| Anthracycline dose (mg/m2) | <250 | 62 (80.5%) |
| ≥250 | 15 (19.5%) | |
| Troponin T (ng/L) | ≤14 | 71 (92.2%) |
| >14 | 6 (7.8%) | |
| NT-proBNP (pg/mL) | <125 | 48 (62.3%) |
| ≥125 | 29 (37.7%) | |
| Left ventricle ejection fraction, LVEF (%) | ≥55% | 73 (94.8%) |
| 50–54% | 4 (5.2%) | |
| Factor | CAC Score = 0 n = 36 | CAC Score > 0 n = 41 | p-Values | |
|---|---|---|---|---|
| Age group | <65 | 28 (77.8%) | 13 (31.7%) | <0.001 |
| 65 | 8 (22.2%) | 28 (68.3%) | ||
| Cancer | breast | 26 (72.2%) | 33 (80.5%) | 0.389 |
| lymphoma | 5 (13.9%) | 2 (4.9%) | ||
| sarcoma | 5 (13.9%) | 6 (14.6%) | ||
| Anthracycline dose (mg/m2) | <250 | 28 (77.8%) | 34 (82.9%) | 0.569 |
| ≥250 | 8 (22.2%) | 7 (17.1%) | ||
| Risk group | HR | 6 (16.7%) | 31 (75.6%) | <0.001 |
| MR | 30 (83.3%) | 10 (24.4%) | ||
| BMI | <25 | 12 (33.3%) | 12 (29.3%) | 0.712 |
| 25–30 | 14 (38.9%) | 14 (34.1%) | ||
| ≥30 | 10 (27.8%) | 15 (36.6%) | ||
| Hypertension | No | 12 (33.3%) | 5 (12.2%) | 0.026 |
| Yes | 24 (66.7%) | 36 (87.8%) | ||
| Hyperlipidemia | No | 14 (38.9%) | 5 (12.2%) | 0.007 |
| Yes | 22 (61.1%) | 36 (87.8%) | ||
| Ever smoke | No | 20 (55.6%) | 20 (48.8%) | 0.553 |
| Yes | 16 (44.4%) | 21 (51.2%) | ||
| Diabetes | No | 33 (91.7%) | 33 (80.5%) | 0.162 |
| Yes | 3 (8.3%) | 8 (19.5%) | ||
| Chronic kidney disease | No | 35 (97.2%) | 30 (73.2%) | 0.004 |
| Yes | 1 (2.8%) | 11 (26.8%) | ||
| NT-proBNP | <125 | 28 (77.8%) | 20 (48.8%) | 0.009 |
| ≥125 | 8 (22.2%) | 21 (51.2%) | ||
| GLS | <16% | 2 (5.6%) | 7 (17.1%) | 0.117 |
| ≥16% | 34 (94.4%) | 34 (82.9%) | ||
| LVEF | ≥55% | 35 (97.2%) | 38 (92.7%) | 0.370 |
| 50–54% | 1 (2.8%) | 3 (7.3%) |
| n CTRCD/n Total | CTRCD Incidence, 95% CI | Univariable Risk Ratio for CTRCD, 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Age group | <65 | 23/41 | 56.1% (40.6–70.5%) | - | 0.228 |
| ≥65 | 25/36 | 69.4% (52.5–82.4%) | 1.2 (0.9–1.8) | ||
| Cancer | Breast | 37/59 | 62.7% (49.6–74.2%) | - | 0.440 |
| Lymphoma | 3/7 | 42.9% (14.1–77.4%) | 0.7 (0.3–1.6) | ||
| Sarcoma | 8/11 | 72.7% (40.9–91.1%) | 1.2 (0.8–1.8) | ||
| Risk group | HR | 29/37 | 78.4% (62.1–88.9%) | 4.0 (1.5–10.9) | 0.005 |
| MR | 19/40 | 47.5% (32.5–63.0%) | - | ||
| BMI | <30 | 31/54 | 57.4% (43.8–70.0%) | - | 0.171 |
| ≥30 | 17/23 | 73.9% (52.4–87.9%) | 1.3 (0.9–1.8) | ||
| Hypertension | No | 8/17 | 47.1% (25.2–70.1%) | - | 0.141 |
| Yes | 40/60 | 66.7% (53.7–77.5%) | 1.4 (0.8–2.4) | ||
| Hyperlipidemia | No | 11/19 | 57.9% (35.3–77.6%) | - | 0.645 |
| Yes | 37/58 | 63.8% (50.6–75.2%) | 1.1 (0.7–1.7) | ||
| Ever smoke | No | 29/40 | 72.5% (56.6–84.2%) | - | 0.056 |
| Yes | 19/37 | 51.4% (35.4–67.0%) | 0.7 (0.5–1.0) | ||
| Diabetes | No | 41/66 | 62.1% (49.7–73.1%) | - | 0.924 |
| Yes | 7/11 | 63.6% (33.4–85.9%) | 1.0 (0.6–1.7) | ||
| Chronic kidney disease | No | 38/65 | 58.5% (46.0–69.9%) | - | 0.102 |
| Yes | 10/12 | 83.3% (51.7–95.9%) | 1.4 (1.0–2.0) | ||
| NYHA scale | 1 | 32/54 | 59.3% (45.6–71.6%) | - | 0.393 |
| 2 | 16/23 | 69.6% (48.1–84.9%) | 1.2 (0.8–1.7) | ||
| ECOG score | 0 | 32/55 | 58.2% (44.7–70.6%) | - | 0.417 |
| 1 | 15/21 | 71.4% (48.9–86.7%) | 1.2 (0.9–1.7) | ||
| 2 | 1/1 | 100.0% | - | ||
| Beta-blockers | No | 27/44 | 61.4% (46.2–74.6%) | - | 0.839 |
| Yes | 21/33 | 63.6% (46.0–78.3%) | 1.0 (0.7–1.5) | ||
| ACE-i | No | 26/43 | 60.5% (45.1–74.0%) | - | 0.703 |
| Yes | 22/34 | 64.7% (47.3–78.9%) | 1.1 (0.8–1.5) | ||
| Statins | No | 32/54 | 59.3% (45.6–71.6%) | - | 0.393 |
| Yes | 16/23 | 69.6% (48.1–84.9%) | 1.2 (0.8–1.7) | ||
| CAC score | 0 | 18/36 | 50.0% (34.0–66.0%) | - | 0.036 |
| >0 | 30/41 | 73.2% (57.5–84.6%) | 1.5 (1.0–2.1) | ||
| Anthracycline dose (mg/m2) | <200 | 9/13 | 69.2% (40.5–88.2%) | - | 0.793 |
| 200–400 | 33/55 | 60.0% (46.4–72.2%) | 0.9 (0.6–1.3) | ||
| ˃400 | 6/9 | 66.7% (32.8–89.1%) | 1.0 (0.5–1.7) | ||
| Troponin T | <14 | 44/71 | 62.0% (50.0–72.6%) | - | 0.820 |
| ≥14 | 4/6 | 66.7% (26.3–91.8%) | 1.1 (0.6–1.9) | ||
| NT-proBNP | <125 | 27/48 | 56.2% (41.9–69.7%) | - | 0.156 |
| ≥125 | 21/29 | 72.4% (53.4–85.7%) | 1.3 (0.9–1.8) | ||
| LVEF (%) | ≥55 | 45/73 | 61.6% (49.9–72.2%) | - | 0.591 |
| 50–54 | 3/4 | 75.0% (23.1–96.8%) | 1.2 (0.7–2.2) |
| Multivariable Risk Ratio for CTRCD, 95% CI | p-Value | ||
|---|---|---|---|
| Adjustment for age group, hypertension and hyperlipidemia | |||
| CAC score > 0 | No | Reference | |
| Yes | 1.53 (1.06–2.21) | 0.0247 | |
| Age group | 24–49 | Reference | |
| 50–64 | 1.55 (0.85–2.83) | 0.1496 | |
| 65–80 | 1.2 (0.65–2.22) | 0.5524 | |
| Hypertension | No | Reference | |
| Yes | 1.48 (0.83–2.63) | 0.1805 | |
| Hyperlipidemia | No | Reference | |
| Yes | 1.03 (0.66–1.61) | 0.9094 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiec, A.; Ozdowska, P.; Rosinska, M.; Zebrowska, A.M.; Jasek, S.; Kotowicz, B.; Waniewska, J.; Kosela-Paterczyk, H.; Lampka, E.; Pogoda, K.; et al. Coronary Artery Calcium Score as a Predictor of Anthracycline-Induced Cardiotoxicity: The ANTEC Study. Pharmaceuticals 2025, 18, 1102. https://doi.org/10.3390/ph18081102
Borowiec A, Ozdowska P, Rosinska M, Zebrowska AM, Jasek S, Kotowicz B, Waniewska J, Kosela-Paterczyk H, Lampka E, Pogoda K, et al. Coronary Artery Calcium Score as a Predictor of Anthracycline-Induced Cardiotoxicity: The ANTEC Study. Pharmaceuticals. 2025; 18(8):1102. https://doi.org/10.3390/ph18081102
Chicago/Turabian StyleBorowiec, Anna, Patrycja Ozdowska, Magdalena Rosinska, Agnieszka Maria Zebrowska, Sławomir Jasek, Beata Kotowicz, Joanna Waniewska, Hanna Kosela-Paterczyk, Elzbieta Lampka, Katarzyna Pogoda, and et al. 2025. "Coronary Artery Calcium Score as a Predictor of Anthracycline-Induced Cardiotoxicity: The ANTEC Study" Pharmaceuticals 18, no. 8: 1102. https://doi.org/10.3390/ph18081102
APA StyleBorowiec, A., Ozdowska, P., Rosinska, M., Zebrowska, A. M., Jasek, S., Kotowicz, B., Waniewska, J., Kosela-Paterczyk, H., Lampka, E., Pogoda, K., Nowecki, Z., & Walewski, J. (2025). Coronary Artery Calcium Score as a Predictor of Anthracycline-Induced Cardiotoxicity: The ANTEC Study. Pharmaceuticals, 18(8), 1102. https://doi.org/10.3390/ph18081102






